Abstract
Background
Coronary artery disease (CAD) diagnosis by coronary computed tomographic angiography (CCTA) is useful for identification of symptomatic diabetic individuals at heightened risk for death. Whether CCTA-detected CAD enables improved risk assessment of asymptomatic diabetic individuals beyond clinical risk factors and coronary artery calcium scoring (CACS) remains unexplored.
Methods
From a prospective 12-center international registry of 27,125 individuals undergoing CCTA, we identified 400 asymptomatic diabetic individuals without known CAD. Coronary stenosis by CCTA was graded as 0%, 1–49%, 50–69%, and ≥70%. CAD was judged on a per-patient, per-vessel and per-segment basis as maximal stenosis severity, number of vessels with ≥50% stenosis, and coronary segments weighted for stenosis severity (segment stenosis score), respectively. We assessed major adverse cardiovascular events (MACE) – inclusive of mortality, nonfatal myocardial infarction (MI), and late target vessel revascularization ≥90 days (REV) – and evaluated the incremental utility of CCTA for risk prediction, discrimination and reclassification.
Results
Mean age was 60.4 ± 9.9 years; 65.0% were male. At a mean follow-up 2.4 ± 1.1 years, 33 MACE occurred (13 deaths, 8 MI, 12 REV) [8.25%; annualized rate 3.4%]. By univariate analysis, per-patient maximal stenosis [hazards ratio (HR) 2.24 per stenosis grade, 95% confidence interval (CI) 1.61–3.10, p < 0.001], increasing numbers of obstructive vessels (HR 2.30 per vessel, 95% CI 1.75–3.03, p < 0.001) and segment stenosis score (HR 1.14 per segment, 95% CI 1.09–1.19, p < 0.001) were associated with increased MACE. After adjustment for CAD risk factors and CACS, maximal stenosis (HR 1.80 per grade, 95% CI 1.18–2.75, p = 0.006), number of obstructive vessels (HR 1.85 per vessel, 95% CI 1.29–2.65, p < 0.001) and segment stenosis score (HR 1.11 per segment, 95% CI 1.05–1.18, p < 0.001) were associated with increased risk of MACE. Beyond age, gender and CACS (C-index 0.64), CCTA improved discrimination by maximal stenosis, number of obstructive vessels and segment stenosis score (C-index 0.77, 0.77 and 0.78, respectively). Similarly, CCTA findings improved risk reclassification by per-patient maximal stenosis [integrated discrimination improvement (IDI) index 0.03, p = 0.03] and number of obstructive vessels (IDI index 0.06, p = 0.002), and by trend for segment stenosis score (IDI 0.03, p = 0.06).
Conclusion
For asymptomatic diabetic individuals, CCTA measures of CAD severity confer incremental risk prediction, discrimination and reclassification on a per-patient, per-vessel and per-segment basis.
Keywords: Coronary artery disease, Major adverse cardiac events, Coronary CT angiography, Coronary artery calcium score
1. Introduction
The prevalence of diabetes mellitus is rapidly increasing worldwide, with a projected prevalence of more than 350 million individuals by 2030 [1]. While diabetics have been traditionally considered a coronary heart disease (CHD) equivalent [2], studies using coronary artery calcium scoring (CACS) have observed a high percentage of diabetic individuals to possess no coronary calcium, a finding associated with low future cardiovascular risk. These studies of CACS have also shown that this test augments prediction of CHD risk in asymptomatic diabetic individuals beyond consideration non-diabetes CHD risk factors. As an example, a high proportion of diabetics have a CAC score of 0, which is associated with an excellent prognosis. In contrast, for every increasing non-zero category of CACS, the risk is higher for a diabetic than a non-diabetic patient. Thus, current professional societal guidelines endorse the use of diagnostic testing for selected asymptomatic individuals by means of stress testing [3,4] or coronary artery calcium scoring (CACS) [5].
Coronary computed tomographic angiography (CCTA) is a non-invasive test that demonstrates high diagnostic performance for the detection and exclusion of any atherosclerosis as well as anatomically obstructive CAD [6–9]. In the general population of asymptomatic patients undergoing CCTA scanning, CCTA findings have not shown more effective risk stratification than CACS. However, whether CCTA represents a more effective method for risk assessment than CACS in selected higher risk asymptomatic patients – such as those with diabetes – is unknown.
From a consecutive cohort of individuals within a large prospective international multicenter observational cohort study, we evaluated whether CAD identified by CCTA would offer incremental risk assessment over CHD risk factors and CACS for asymptomatic diabetic individuals.
2. Methods
The CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter) registry is an open-label, international, multicenter observational dynamic registry designed to evaluate associations between patient characteristics, CCTA findings, and incident adverse clinical events. A total of 27,125 patients who underwent CCTA at 12 centers in 6 countries (United States, Canada, Germany, Switzerland, Italy, and South Korea) were enrolled into the registry between February 2003 and December 2009. Details of the registry and data collection have been previously been published [10]. For the present study, sites with data on all-cause mortality, non-fatal myocardial infarction, and late target vessel revascularization (REV) were included, resulting in a total of 17,218 patients. From this cohort, we identified 400 patients with an established diagnosis of diabetes; who were asymptomatic; and had no history of obstructive CAD, coronary revascularization, or myocardial infarction. All patients had a CACS performed as a routine part of the CCTA examination. Diabetes was defined by established guidelines [11] and included a known history of diabetes or the use of diabetic medications. All sites had approval of their respective institutional review boards, and were compliant with the Health Insurance Portability and Accountability Act where applicable.
2.1. Data acquisition and image analysis
All CCTA performance, data acquisition, image post-processing, and interpretation in the study cohort were consistent with site-specific policies and Society of Cardiovascular Computed Tomography guidelines [12]. All CCTA studies were performed using a scanner with at least 64 detector rows, and interpreted using a 16-segment coronary vascular model.
In each coronary artery, coronary atherosclerosis was defined as any tissue structures ≥1 mm2 in size within or adjacent to the coronary artery lumen that could be discriminated from surrounding pericardial tissue, epicardial fat, or the vessel lumen itself. The luminal stenosis of coronary atherosclerotic lesions was determined by visual estimation in accordance with guidelines [12]. Maximal stenosis severity was categorized into a 4-point scale, defined as no CAD (no plaque), mild CAD (maximal stenosis 1– 49%), moderate CAD (maximal stenosis 50–69%), and severe CAD (≥70% stenosis). CAD was also assessed by the number of major epicardial vessels with obstructive (≥50% stenosis) CAD, with obstructive left main artery disease considered 3-vessel CAD; and the segment stenosis score, which measures the extent and severity of plaque by assigning each of 16 segments a score of 0–3 for absent to severe stenosis up to a maximum score of 48 [13].
2.2. Patient follow-up
Patient outcomes were determined at each institution using a dedicated physician and/or research nurse by direct interview, telephone contact, and/or review of medical records using a standardized questionnaire, as we have previously described [10]. In the United States, all-cause mortality was additionally assessed by query of the Social Security Death Index.
2.3. Statistical analysis
Our primary endpoint was major adverse cardiovascular events (MACE), as defined by a composite of all-cause mortality, non-fatal myocardial infarction, or late REV. We also examined a secondary endpoint of all-cause mortality and non-fatal myocardial infarction alone. We conducted univariate and multivariate Cox regression models for risk of MACE based on CHD risk factors (increased age per 10 years, male gender, hypertension, hyperlipidemia, and current tobacco smoking) as well as CACS and the presence of obstructive (≥50% stenosis) CAD. CACS was converted to its natural log [ln(CACS + 1)] due to its non-parametric distribution, as has previously been performed [14]. Kaplan–Meier curves were compared using the log-rank test.
To determine the incremental prognostic value of CCTA findings compared to CHD risk factors and CACS, we compared several models that assessed independent relationships between the variables and MACE using Cox regression. Only variables with a univariate p < 0.10 were added to the final multivariate models to prevent model over-fitting, with the exception of gender, which was forced into models, given the clinical role of gender differences in the prevalence, management and incidence of adverse events related to CAD [10,15].
Model A considered CAD risk factors alone, Model B added CACS, and Models C–E added maximal stenosis grade, the number of obstructive vessels, and the segment stenosis scores, respectively. Model B was compared to Model A as a baseline; Models C–E were compared to Model B as a baseline. The Harrell’s C-index was determined for each model.
As established categories do not exist for expected rates of incident MACE in the study population, patient reclassification was assessed using the integrated discrimination improvement (IDI) index [16]. The IDI index was calculated for each model, as well as stratified by CACS. All analyses were performed using SAS 9.2 (www.sas.com, Cary, NC) and SPSS 19.0 (www.spss.com, Somers, NY). A p < 0.05 was considered statistically significant.
3. Results
3.1. Patient characteristics
Baseline patient characteristics and CT characteristics are provided in Table 1. During the mean follow-up of 2.4 ± 1.1 years, there were a total of 33 MACE events (13 deaths, 8 MI’s, and 12 REV). The mean CACS was 226.2 ± 492.1, and the distribution of CACS by category is provided in Table 1. Amongst patients with a CACS of 0, no atherosclerosis was observed in 68.1% of patients, with non-obstructive and obstructive CAD noted in 21.5% and 10.5% of individuals, respectively. In the 64.0% of patients with CACS >0, obstructive CAD was present in 15.6%, 19.1%, 38.4%, and 64.3%, of those with a CACS of 1–10, 11–100, 101–400, and 400, respectively.
Table 1.
Patient demographics, calcium score, and CCTA findings.
| Variable | Estimate n = 400 |
|---|---|
| Demographics | |
| Age | 60.4 ± 9.9 |
| Male gender | 65.0% |
| Hypertension | 63.0% |
| Hyperlipidemia | 70.3% |
| Current smoking | 13.3% |
| Coronary artery calcium score (Agatston) | |
| 0 | 36.0% |
| 1–10 | 8.0% |
| 11–100 | 17.0% |
| 101–400 | 21.5% |
| ≥400 | 17.5% |
| CAD by CCTA | |
| None | 30.0% |
| Non-obstructive CAD | 42.2% |
| 1-Vessel obstructive CAD | 15.5% |
| 2-Vessel obstructive CAD | 7.0% |
| 3-Vessel obstructive CAD | 5.3% |
| Framingham Risk Score | 21.6 ± 13.3 |
| European Heart Score (DM) | 15.1 ± 16.2 |
Values provided as mean with standard deviation or percentage. Obstructive CAD is defined as ≥50% maximal diameter stenosis. CAD = coronary artery disease. CCTA = coronary computed tomography angiography.
3.1.1. Risk prediction, discrimination and reclassification
In univariable analysis, older age, higher CACS, and CAD findings by CCTA were associated with a greater risk of adverse events (Table 2). A positive relationship was noted between the number of vessels with obstructive CAD and risk of MACE (Fig. 1a) as well as for the secondary endpoint of all-cause mortality and non-fatal myocardial infarction (Fig. 1b).
Table 2.
Unadjusted variables associated with adverse events.
| Variable | Unadjusted model
|
||
|---|---|---|---|
| HR | 95% CI | p | |
| Risk factors | |||
| Increased age (per 10 years) | 1.79 | 1.21–2.65 | 0.003 |
| Male gender | 1.06 | 0.52–2.19 | 0.87 |
| Hypertension | 1.43 | 0.68–3.00 | 0.34 |
| Hyperlipidemia | 0.65 | 0.33–1.32 | 0.24 |
| Current smoking | 0.79 | 0.28–2.26 | 0.66 |
| CACS | 1.42 | 1.21–1.67 | <0.001 |
| CCTA findings | |||
| Maximal stenosis severity (per grade) | 2.24 | 1.61–3.10 | <0.001 |
| Number of vessels with obstructive CAD | 2.30 | 1.75–3.03 | <0.001 |
| Segment stenosis score (per unit) | 1.14 | 1.09–1.19 | <0.001 |
Adverse events include all-cause mortality, non-fatal myocardial infarction, and late (≥90-day) target vessel revascularization. Obstructive CAD is defined as a ≥50% stenosis. Maximal stenosis severity is graded as 0, 1–49%, 50–69%, and ≥70% diameter luminal stenosis. CACS = coronary artery calcium score, CAD = coronary artery disease, CCTA = coronary computed tomographic angiography, CI = confidence interval, HR = hazard ratio.
Fig. 1.
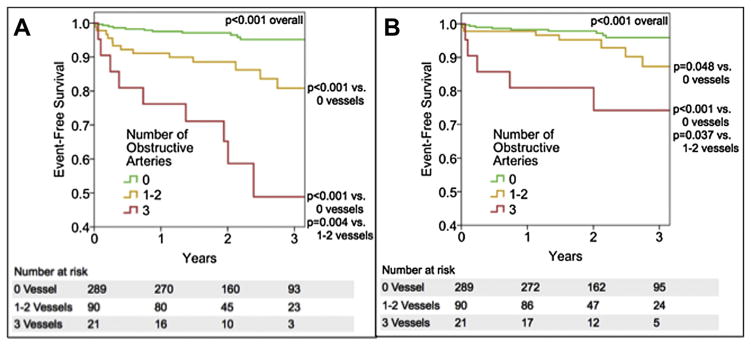
(A) Kaplan–Meier curves for event-free survival from death, nonfatal myocardial infarction, and late target vessel revascularization. (B) Kaplan–Meier curves for event-free survival from death and nonfatal myocardial infarction.
Results of the multivariable analyses are shown in Table 3. Model A considered CHD risk factors alone, while Model B added CACS, which demonstrated improved risk stratification by CACS that mitigated the risk predictive ability of CHD risk factors. After adjustment for CHD risk factors and CACS, CCTA findings by maximal stenosis severity grade (Model C), the number of vessels with obstructive CAD (Model D), and the segment stenosis score (Model E) were each independently and positively associated with adverse events. From these models, number of vessels with obstructive CAD by CCTA provided the strongest relationship between incident MACE and CCTA findings of CAD.
Table 3.
Relationship between variables and adverse events.
| Model | Included variables | HR (95% CI) | χ2 | p |
|---|---|---|---|---|
| Model A | Age | 1.06 (1.02–1.10) | 8.01 | 0.005 |
| Gender | 1.08 (0.52–2.24) | 0.05 | 0.83 | |
| Model B | Age | 1.04 (0.99–1.08) | 3.54 | 0.11 |
| Gender | 0.72 (0.35–1.52) | 0.73 | 0.39 | |
| CACS | 1.43 (1.20–1.69) | 16.64 | <0.001 | |
| Model C | Age | 1.03 (0.98–1.07) | 1.30 | 0.25 |
| Gender | 0.60 (0.28–1.27) | 1.80 | 0.18 | |
| CACS | 1.24 (1.03–1.50) | 5.14 | 0.02 | |
| Maximal stenosis grade | 1.80 (1.18–2.75) | 7.49 | 0.006 | |
| Model D | Age | 1.02 (0.97–1.07) | 0.58 | 0.45 |
| Gender | 0.58 (0.27–1.24) | 1.96 | 0.16 | |
| CACS | 1.24 (1.04–1.48) | 5.59 | 0.02 | |
| # Obstructive vessels | 1.85 (1.29–2.65) | 11.24 | <0.001 | |
| Model E | Age | 1.02 (0.97–1.07) | 0.61 | 0.43 |
| Gender | 0.53 (0.24–1.17) | 2.50 | 0.11 | |
| CACS | 1.22 (1.01–1.47) | 4.41 | 0.04 | |
| Segment stenosis score | 1.11 (1.05–1.18) | 11.43 | <0.001 |
DF = 1 for all χ2 comparisons. Age is in years. Maximal stenosis is graded as absent, mild (1–49%), moderate (50–69%), and severe (≥70%) luminal stenosis. The number of obstructive vessels represents the number of arteries with stenosis ≥50%. CACS = coronary artery calcium score, CI = confidence interval, HR = hazards ratio, SSS = segment stenosis score.
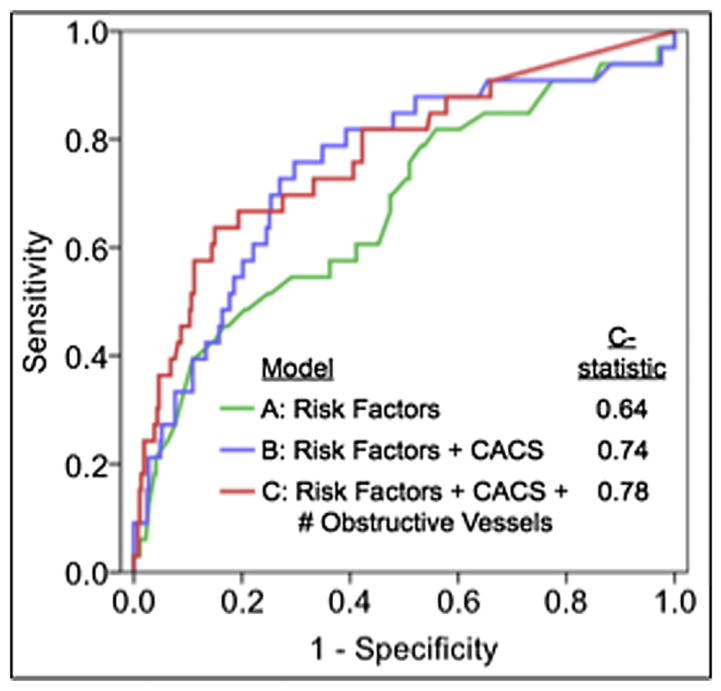
Higher predictive accuracy was observed (Table 4) when adding CACS results to CAD risk factors (Model B vs. Model A), with further improvement by the addition of CCTA findings (Models C–E). As compared to Model B (C-index 0.64), CCTA offered improved discrimination in Model D, E and F (C-index 0.77, 0.77 and 0.78, respectively). Further, the addition of CACS in Model B was associated with significantly improved risk reclassification as measured by the IDI index over Model A. When CCTA findings were also added, both maximal stenosis severity (Model C) and the number of vessels with obstructive CAD (Model D) were associated with a significant improvement in reclassification over risk factors and CACS alone, with the latter associated with the most greatest improvement in IDI. This improvement in IDI for Model D (vs. Model B) was observed both in patients with a normal CACS and a CACS >100, but not in those with a score of 1–100 (Fig. 2). In comparison to Model B, the probability of Models C–E to correctly increase the predicted probability of an event was larger than the probability to correctly decrease the predicted probability of an event (difference in mean probability to predict events and to predict non-events, respectively: Model C = 0.026 and −0.002; Model D = 0.057 and −0.005; Model E = 0.031 and −0.003). The findings of the C-index and IDI confer a ‘number needed to scan’ to identify or exclude the number of obstructive coronary vessels of 46 (Fig. 3).
Table 4.
Effect of variables on model prediction accuracy and risk reclassification.
| Model | Included variables | IDI index (95% CI) | p | Model prediction (C-index) |
|---|---|---|---|---|
| Model A | Risk factors | – | – | 0.64 |
| Model B | Risk factors + CACS | 0.04 (0.02–0.06) vs. Model A | 0.003 | 0.74 |
| Model C | Risk factors + CACS + maximal stenosis grade | 0.03 (0.01–0.04) vs. Model B | 0.03 | 0.77 |
| Model D | Risk factors + CACS + # of obstructive vessels | 0.06 (0.04–0.08) vs. Model B | 0.002 | 0.78 |
| Model E | Risk factors + CACS + SSS | 0.03 (0.02–0.05) vs. Model B | 0.06 | 0.78 |
Age is in years. Maximal stenosis is graded as absent, mild (1–49%), moderate (50–69%), and severe (≥70%) luminal stenosis. The number of obstructive arteries represents the number of arteries with stenosis ≥50%. CACS = coronary artery calcium score, CI = confidence interval, HR = hazards ratio, IDI = integrated discrimination improvement, SSS = segment stenosis score.
Fig. 2.
Area under the receiver operating characteristics curve for future major adverse cardiac events. Model A represents age, gender and traditional CHD risk factors. Model B is inclusive of all variables in Model A plus coronary artery calcium score. Model C is inclusive of all variables in Model B plus number of obstructive coronary artery epicardial vessels.
Fig. 3.
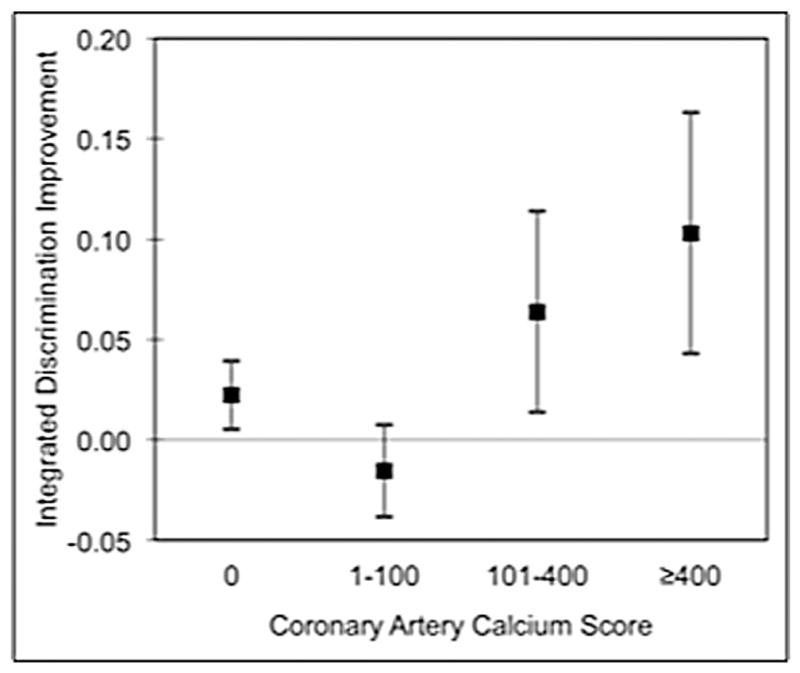
Reclassification by the number of vessels with obstructive CAD as stratified by the coronary artery calcium score. Reclassification is defined by the integrated discrimination improvement (IDI) index for patients stratified by coronary artery calcium score. The box represents the value, and the vertical bars represent the 95% confidence interval.
3.1.2. Exploratory secondary analyses
We also examined whether CCTA findings were independently associated with a composite endpoint of all-cause mortality and nonfatal myocardial infarction beyond CHD risk factors and CACS. In these analyses, maximal stenosis severity (HR 1.96 per grade, 95% CI 1.31–2.93, p = 0.001), the number of vessels with obstructive CAD (HR 2.10 per vessel, 95% CI 1.47–2.98, p < 0.001), and the segment stenosis score (HR 1.14 per score, 95% CI 1.08–1.21, p < 0.001) were each independently and positively associated with increased risk of events.
4. Discussion
In this prospective multicenter observational cohort study, we observed that CCTA findings of CAD extent and severity offer incremental and independent prognostic risk estimates beyond CHD risk factors and CACS for the prediction of MACE in asymptomatic diabetic individuals without a history of CAD. CCTA findings were predictive of increased risk for a composite endpoint of death, non-fatal myocardial infarction and late REV; and also for a composite endpoint inclusive of only death and non-fatal myocardial infarction. Importantly, CCTA findings of CAD extent and severity were additive to models containing CAD risk factors and CACS, and further improved model discrimination and risk reclassification. Notably, the number of vessels with obstructive CAD appeared to provide the most robust additive improvement in the predictive ability of the model and in risk reclassification.
At first glance, these findings are in apparent contrast to a recent study by our group that demonstrated no incremental value of CCTA findings to models containing CAD risk factors and CACS alone in 7590 individuals [17]. This prior study, however, included a heterogeneous group of asymptomatic individuals referred for CCTA, and did not assess potentially higher risk subsets – such as those with diabetes – which comprised only 14.6% of the study population. The present study was performed to determine whether the presence of diabetes in asymptomatic individuals requires a heightened sense of scrutiny that may be imparted by the performance of CCTA.
Given the increased risk of CHD events among patients with diabetes with coronary atherosclerosis, societal guidelines advocate for vigilant evaluation and secondary prevention measures in this population [2]. In this study, we noted by CCTA a high prevalence of CAD in asymptomatic diabetic individuals, with more than one-fourth possessing obstructive CAD and more than two-thirds possessing atherosclerosis. Prior studies examining asymptomatic individuals with diabetes have not identified an improvement in outcomes based upon an imaged-based screening strategy. In the sole randomized trial to date evaluating 1123 asymptomatic diabetes, the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study observed no reduced hazards of CHD events amongst patients undergoing prospective evaluation by myocardial perfusion single photon emission computed tomography (MPS) vs. no screening test [14]. In this study, however, almost 80% of patients randomized to the MPS arm manifested no physiologic evidence of ischemic CAD and experienced very low rates of adverse cardiac events. In contrast, the present study observed significantly higher rates of visualized CAD by CCTA, both at the anatomically obstructive and non-obstructive thresholds. As greater detection of preclinical disease has been suggested as a criterion for an ideal screening test [18], the present data may be considered hypothesis-generating for use of CCTA in this regard.
Several prior studies support the concept of CACS evaluation in asymptomatic diabetic individuals. In particular, one large study of 10,377 patients undergoing CACS and followed for an endpoint of 5-year all-cause mortality, a stepwise increase in risk of mortality was noted for diabetics compared to non-diabetics across all CACS groups, while diabetics with low or zero CACS conversely experienced a very favorable prognosis [19]. Importantly, nearly 40% of diabetics in that study exhibited a CACS of 0, a finding similar to a study by Anand and colleagues of 398 asymptomatic diabetics [20] as well as others. However, calcium represents only one component of plaque, and prior studies have reported a 6–20% prevalence of atherosclerosis in the absence of coronary calcification amongst generally symptomatic individuals with a low prevalence of diabetes [21–23]. Our study results found that nearly one third of asymptomatic diabetics with a CACS of zero indeed have atherosclerosis, and that over 10% of these individuals have obstructive CAD by CCTA. The prognostic implications of obstructive CAD by CCTA have been well examined in heterogeneous diabetic and non-diabetic cohorts [24–29], while our group has recently observed an increasing mortality risk for individuals even with non-obstructive CAD by CCTA, suggesting that the ability of CCTA to provide measures of risk stratification extend beyond that of conventional definitions of anatomically obstructive CAD [30]. In totality, these findings suggest that the consideration of CAD risk equivalent for all diabetics may not be uniformly applicable.
To our knowledge, the present results are the first to examine the ability of CCTA findings of CAD to improve risk stratification, discrimination and reclassification in asymptomatic individuals above and beyond risk factors and CACS. Particular to asymptomatic diabetic patients, we observed a significant improvement by CCTA findings of CAD for risk assessment and prognostication over models that incorporated clinical data and CACS. The present results were robust, and were observed using multiple measures of CAD severity including graded maximal stenosis severity, the number of vessels with obstructive CAD, and the segment stenosis score. Each of these scores was associated with improved hazards prediction and discrimination independent of CHD risk factors and CACS, with the number of vessels with obstructive CAD associated with the most robust improvement in prediction and risk reclassification. These findings suggest this metric as perhaps the most useful clinical method to identify risk in asymptomatic diabetics. Further, the magnitude of IDI index reclassification by adding CCTA to CACS and CHD risk factors exceeded the reclassification that was observed by adding CACS to CHD risk factors, suggesting that CCTA may have a potential role for better ascertainment of incident risk of CAD events. Nevertheless, the downstream clinical impact of CCTA and CACS findings in comparison to CACS was not evaluated in the current study. Future investigations evaluating the potential benefit of therapies based upon CCTA findings of CAD now appear warranted.
Germane to this finding, risk reclassification by CCTA findings of obstructive CAD resulted in a predominance of correct reclassification upwards to higher risk states, with a relatively negligible effect of downward reclassification. Significant reclassification using the number of obstructive vessels on CCTA was observed even amongst patients with a CACS of 0, suggesting that CCTA findings can correctly identify patients at increased risk even in this subgroup that has been conventionally considered at very low risk of adverse events [20]. Further, reclassification occurred across a wide range of CACS, and suggest a clinical usefulness irrespective of baseline CACS score. Importantly, however, it should be noted that the improvement in discrimination and reclassification is modest. Whether the use of CCTA for risk stratification is clinically useful and/or economically feasible remains a topic for further study.
This study is not without limitations. Importantly, while this is a prospective multicenter cohort study, these results were observed for individuals who were clinically referred for CCTA and CACS and thus are subject to all of the potential limitations intrinsic to observational design, including ascertainment, referral and treatment bias. Regarding the latter, treatment strategies were not specified based on test findings, and the results herein should be considered as a manifestation of usual medical care rather than intensified or optimal care. Yet intensified or optimal treatment of patients with CCTA CAD would likely have reduced any apparent disparity in risk between diabetic individuals with increasing extent and severity of CAD and thus, the study results may be still considered robust and generalizable. Future randomized trials should be performed to determine the potential benefit of CCTA findings in therapeutic decision making to optimize salutary outcomes. Further, there were a relatively small number of MACE which precluded adjustment for all possible confounders and, were greater numbers to be present in the study sample, generally more consistent results may be arisen (e.g., improved IDI across all CACS subgroups). By the present data, the ‘number needed to scan’ to identify or exclude obstructive coronary vessels is 46. Whether this translates to improved outcomes remains unknown and future larger studies will be with larger sample sizes are needed to more clearly determine the added benefit of CCTA performance in this population. Nevertheless, the present study represents the largest investigation to date evaluating in asymptomatic diabetic patients, and encourage the performance of larger prospective studies.
5. Conclusion
In this prospective multicenter international observational cohort study, CCTA findings improved the prediction of incident MACE in asymptomatic diabetic individuals beyond CHD risk factors and CACS, allowing for improved risk stratification, discrimination and reclassification.
References
- 1.Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S. 2005–2050. Diabetes Care. 2006;29:2114–6. doi: 10.2337/dc06-1136. [DOI] [PubMed] [Google Scholar]
- 2.Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 3.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) J Am Coll Cardiol. 2002;40:1531–40. doi: 10.1016/s0735-1097(02)02164-2. [DOI] [PubMed] [Google Scholar]
- 4.Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol. 2009;53:2201–29. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122:e525–55. doi: 10.1161/CIR.0b013e3181fcae66. [DOI] [PubMed] [Google Scholar]
- 6.van Velzen JE, Schuijf JD, de Graaf FR, et al. Diagnostic performance of non-invasive multidetector computed tomography coronary angiography to detect coronary artery disease using different endpoints: detection of significant stenosis vs. detection of atherosclerosis. Eur Heart J. 2011;32:637–45. doi: 10.1093/eurheartj/ehq395. [DOI] [PubMed] [Google Scholar]
- 7.Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–32. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135–44. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 9.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324–36. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 10.Min JK, Dunning A, Lin FY, et al. Rationale and design of the CONFIRM (COronary CT angiography EvaluatioN for clinical outcomes: an InteRnational multicenter) Registry J Cardiovasc Comput Tomogr. 2011;5:84–92. doi: 10.1016/j.jcct.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Standards of medical care in diabetes–2008. Diabetes Care. 2008;31(Suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 12.Raff GL, Abidov A, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3:122–36. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50:1161–70. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 14.Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. J Am Med Assoc. 2009;301:1547–55. doi: 10.1001/jama.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. J Am Med Assoc. 2004;291:210–5. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 16.Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. J Am Med Assoc. 2010;303:1610–6. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho I, Chang HK, Pencina MJ, et al. Coronary computed tomographic angiography and risk of all-cause mortality and non-fatal myocardial infarction in subjects without chest pain syndrome from the CONFIRM Registry (COronary CT Angiography EvaluatioN for Clinical Outcomes: an InteRnational Multi-center Registry) Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.111.081380. in press. [DOI] [PubMed]
- 18.American Medical Association Council on Scientific Affairs. [Accessed 13.08.11];Commercialized medical screening (Report A-03) Available from: http://www.ama-assn.org/resources/doc/csaph/a03csa10-fulltext.pdf.
- 19.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43:1663–9. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 20.Anand DV, Lim E, Darko D, et al. Determinants of progression of coronary artery calcification in type 2 diabetes role of glycemic control and inflammatory/vascular calcification markers. J Am Coll Cardiol. 2007;50:2218–25. doi: 10.1016/j.jacc.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 21.Hausleiter J, Meyer T, Hadamitzky M, Kastrati A, Martinoff S, Schomig A. Prevalence of noncalcified coronary plaques by 64-slice computed tomography in patients with an intermediate risk for significant coronary artery disease. J Am Coll Cardiol. 2006;48:312–8. doi: 10.1016/j.jacc.2006.02.064. [DOI] [PubMed] [Google Scholar]
- 22.Cheng VY, Lepor NE, Madyoon H, Eshaghian S, Naraghi AL, Shah PK. Presence and severity of noncalcified coronary plaque on 64-slice computed tomographic coronary angiography in patients with zero and low coronary artery calcium. Am J Cardiol. 2007;99:1183–6. doi: 10.1016/j.amjcard.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Rubinshtein R, Gaspar T, Halon DA, Goldstein J, Peled N, Lewis BS. Prevalence and extent of obstructive coronary artery disease in patients with zero or low calcium score undergoing 64-slice cardiac multidetector computed tomography for evaluation of a chest pain syndrome. Am J Cardiol. 2007;99:472–5. doi: 10.1016/j.amjcard.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 24.Chow BJ, Wells GA, Chen L, et al. Prognostic value of 64-slice cardiac computed tomography severity of coronary artery disease, coronary atherosclerosis, and left ventricular ejection fraction. J Am Coll Cardiol. 2010;55:1017–28. doi: 10.1016/j.jacc.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 25.Min JK, Lin FY, Dunning AM, et al. Incremental prognostic significance of left ventricular dysfunction to coronary artery disease detection by 64-detector row coronary computed tomographic angiography for the prediction of all-cause mortality: results from a two-centre study of 5330 patients. Eur Heart J. 2010;31:1212–9. doi: 10.1093/eurheartj/ehq020. [DOI] [PubMed] [Google Scholar]
- 26.Chow BJ, Small G, Yam Y, et al. The incremental prognostic value of cardiac CT in CAD using CONFIRM (COroNary computed tomography angiography evaluation for clinical outcomes: an InteRnational Multicenter registry) Circ Cardiovasc Imaging. 2011;4:463–72. doi: 10.1161/CIRCIMAGING.111.964155. [DOI] [PubMed] [Google Scholar]
- 27.Hadamitzky M, Freissmuth B, Meyer T, et al. Prognostic value of coronary computed tomographic angiography for prediction of cardiac events in patients with suspected coronary artery disease. JACC Cardiovasc Imaging. 2009;2:404–11. doi: 10.1016/j.jcmg.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Hadamitzky M, Hein F, Meyer T, et al. Prognostic value of coronary computed tomographic angiography in diabetic patients without known coronary artery disease. Diabetes Care. 2010;33:1358–63. doi: 10.2337/dc09-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Werkhoven JM, Cademartiri F, Seitun S, et al. Diabetes: prognostic value of CT coronary angiography–comparison with a nondiabetic population. Radiology. 2010;256:83–92. doi: 10.1148/radiol.1090600. [DOI] [PubMed] [Google Scholar]
- 30.Lin FY, Shaw LJ, Dunning AM, et al. Mortality risk in symptomatic patients with nonobstructive coronary artery disease a prospective 2-center study of 2,583 patients undergoing 64-detector row coronary computed tomographic angiography. J Am Coll Cardiol. 2011;58:510–9. doi: 10.1016/j.jacc.2010.11.078. [DOI] [PubMed] [Google Scholar]