Abstract
We compared the serologic response to HIV infection in Ugandan women with HIV subtype A (N=82) and D (N=32) infection using a limiting antigen avidity assay (LAg-Avidity assay); 2,614 samples were analyzed. Study participants were followed a median of 6.6 years after HIV seroconversion. Samples were classified as assay positive if they had a LAg-Avidity assay result <1.5 normalized optical density units (OD-n). Women with subtype D infection were more likely to have delayed antibody maturation. During the first 2 years after seroconversion, the mean time that women had an assay-positive result (mean duration of recent infection, MDRI) was longer for women with subtype D infection than women with subtype A infection (267.9 days, 95% CI: 231.2–308.2 vs. 167.3 days, 95% CI: 151.8–185.9 days, p<0.01). The MDRI was also longer for women with subtype D infection after excluding low viral load samples and samples from women on antiretroviral therapy (ART). Women infected for >2 years were also more likely to be misclassified as recently infected in they had subtype D infection. Women with subtype D infection were also more likely to have antibody waning compared to women with subtype A infection. These findings may be related to the higher pathogenicity of subtype D HIV infection and are relevant to use of the LAg-Avidity assay for cross-sectional HIV incidence estimation in populations where subtype D infection is prevalent.
Introduction
Accurate HIV incidence estimates are needed to monitor the HIV/AIDS epidemic and evaluate the impact of interventions for HIV prevention.1,2 HIV incidence can be estimated from cross-sectional surveys by measuring biomarkers that evolve during the course of HIV infection.2 Many assays used for cross-sectional incidence estimation measure antibody maturation as a marker of duration of HIV infection (reviewed by Murphy and Parry3 and Guy et al.4). One limitation of this approach is that some individuals have delayed antibody maturation or have reduced anti-HIV antibody responses later in infection.5,6 Delayed antibody maturation and antibody waning can lead to misclassification of long-standing infections as recent infections. This type of misclassification has been observed at higher rates in infected with subtype D HIV compared to other subtypes.7–11
Previous reports have also demonstrated an association between subtype D HIV infection and disease progression.12,13 In Eastern Africa, subtype D infection is associated with a faster decline in CD4 cell count and shorter survival, compared to subtype A infection.14 The reason for increased pathogenicity of subtype D HIV is not well understood. Some studies have suggested that humoral immune responses to HIV infection may be weaker in individuals with subtype D infection. Previous studies have evaluated the avidity of the anti-HIV antibody response in individuals with subtype A vs. D infection using a modified version of the Genetic Systems 1/2+ O ELISA (Bio-Rad-Avidity assay).7–9,11 In those studies, lower antibody avidity was observed in both early and established subtype D infection.7
The United States Centers for Disease Control recently developed a limiting antigen avidity assay (LAg-Avidity assay) for cross-sectional HIV incidence estimation.15,16 This assay was developed to reduce the rate at which individuals with long-standing infection are misclassified as having recent infection. This assay measures the strength of antibody binding to an immunodominant region of HIV displayed on a multisubtype recombinant target antigen (rIDR-M) that is present at limiting concentrations.15 In this report, we compare the serologic responses to subtype A and D HIV infection using the LAg-Avidity assay.
Materials and Methods
Study population
The Genital Shedding (GS) Study evaluated the relationship between hormonal contraceptive use, genital shedding of HIV, and HIV disease progression among 303 Ugandan and Zimbabwean women with known dates of HIV seroconversion.17 We analyzed samples from a subgroup of Ugandan women in this study, aged 18–45 who were infected with HIV subtype A (N=82) or subtype D (N=32). These women were followed for at least 1 year after HIV seroconversion and had samples available from three or more study visits after HIV seroconversion (2,614 samples; median: 23 samples per woman; range: 3–41 samples; years of sample collection: 2001–2009, see Table 1). The median duration of follow-up was 6.6 years (range: 1.0–9.2 years). During follow-up, 38 women started antiretroviral therapy (ART). CD4 cell count and viral load data were reported previously.18 HIV subtypes were determined previously based on phylogenetic analysis of the gp120 C2–V3 region of the HIV env gene.17 The estimated date of seroconversion for each woman was defined as the midpoint between the last negative HIV antibody test and the first positive HIV antibody test, or 15 days after a visit documenting acute (HIV RNA-positive/antibody-negative) HIV infection.17
Table 1.
Characteristics of the Study Population
| Subtype A (82 subjects, 1,833 samples) | Subtype D (32 subjects, 781 samples) | |
|---|---|---|
| Age at infection, mean (SD) | 26.5 (4.7) | 27.3 (5.6) |
| Duration of infection | ||
| 0–3 months | 73.2% (41/56) | 26.8% (15/56) |
| 4–6 months | 69.7% (101/145) | 30.3% (44/145) |
| 7–12 months | 71.2% (153/215) | 28.8% (62/215) |
| 13–24 months | 73.4% (282/384) | 26.6% (102/384) |
| 25–48 months | 70.2% (552/786) | 29.8% (234/786) |
| 49+ months | 68.5% (704/1,028) | 31.5% (324/1,028) |
| Sample year | ||
| 2001–2002 | 62.2% (143/230) | 37.8% (87/230) |
| 2003–2004 | 67.9% (360/530) | 32.1% (170/530) |
| 2005–2006 | 72.1% (498/691) | 27.9% (193/691) |
| 2007–2008 | 72.4% (566/782) | 27.6% (216/782) |
| >2008 | 69.8% (266/381) | 30.2% (115/381) |
| CD4 cell count (cells/μl) | ||
| >500 | 73.0% (832/1,140) | 27.0% (308/1,140) |
| 499–200 | 70.1% (808/1,153) | 29.9% (345/1,153) |
| 199–50 | 61.4% (94/153) | 38.6% (59/153) |
| <50 | 36.4% (4/11) | 63.6% (7/11) |
| No data | 60.5% (95/157) | 39.5% (62/157) |
| Viral load (copies/ml) | ||
| >50,000 | 61.5% (235/382) | 38.5% (147/382) |
| 49,999–10,000 | 76.5% (248/324) | 23.5% (76/324) |
| 9,999–400 | 77.0% (245/318) | 23.0% (73/318) |
| <400 | 47.7% (142/298) | 52.3% (156/298) |
| No data | 74.5% (963/1,292) | 25.5% (329/1,292) |
| Time on ART | ||
| Not on ART | 74.9% (1691/2,256) | 25.1% (565/2,256) |
| ART <1 year | 56.5% (87/154) | 43.5% (67/154) |
| ART >1 year | 27.0% (55/204) | 73.0% (149/204) |
ART, antiretroviral treatment; SD, standard deviation.
Laboratory methods
Samples were analyzed using the LAg-Avidity assay (Sedia Biosciences Corporation, Portland, OR).15,16 The target antigen in this assay is a recombinant protein (rIDR-M) that includes peptides of the major variants of gp41 immunodominant regions among HIV group M viruses, including subtypes A, C, D, F, G, H, J, and K, and circulating recombinant forms of HIV (AG, AB, AC, AE, AD, BF, and BG). The rIDR-M protein also contains nonviral sequences, including a polyhistidine peptide at the N-terminus and hydrophilic intervening sequences to increase solubility and yield.15 Discrimination between low-avidity and high-avidity antibodies is effected by limiting the amount of target antigen and using an acidic buffer to dissociate low-avidity antibodies. Assay results are normalized using an internal calibrator and are reported as normalized optical density units (OD-n). The package insert for the assay recommends using a cut-off value of 1.5 OD-n to define recent infection19; in this report, the term “assay positive” is used to describe samples that have LAg-Avidity assay results below this cut-off.
Statistical analysis
The following analyses were performed to assess the serologic response in women with subtype A vs. D HIV infection. Delayed antibody maturation was evaluated by determining the proportion of samples collected <1 year and <2 years after seroconversion that had a LAg-Avidity assay result <1.5 OD-n (i.e., the proportion of assay-positive samples in each time period). Antibody maturation was also evaluated by calculating the mean duration of recent infection (MDRI) for samples collected <1 year and <2 years after seroconversion, using methods described previously.20 The MDRI measures the average time after HIV infection during a specified time window (typically <1 or <2 years after seroconversion) that individuals have assay-positive test results; this measure is used to characterize HIV incidence assays.10,21 The false recent rate (FRR) of the LAg-Avidity assay was assessed by determining the proportion of samples collected >2 years after seroconversion that were assay positive (i.e., the proportion of samples from individuals known to have long-standing infection that were misclassified as recent infections).22–24 The Fisher's exact test and Chi square test were used for analysis of factors associated with having a LAg-Avidity assay result <1.5 OD-n >2 years after seroconversion. Logistic regression using general estimating equations controlled for the correlation of results within a given individual; regression analysis was performed to determine the odds of having an assay-positive result >2 years after HIV seroconversion.25 In these analyses, factors associated with a higher odds ratio in the univariate analysis (p<0.10) were included in the multivariate analysis. All analyses were performed using STATA v11 (StataCorp, College Station, TX). Antibody waning was assessed by measuring the proportion of women who had >20% decline in OD-n value from a maximum observed LAg-Avidity assay result.
Results
Antibody maturation
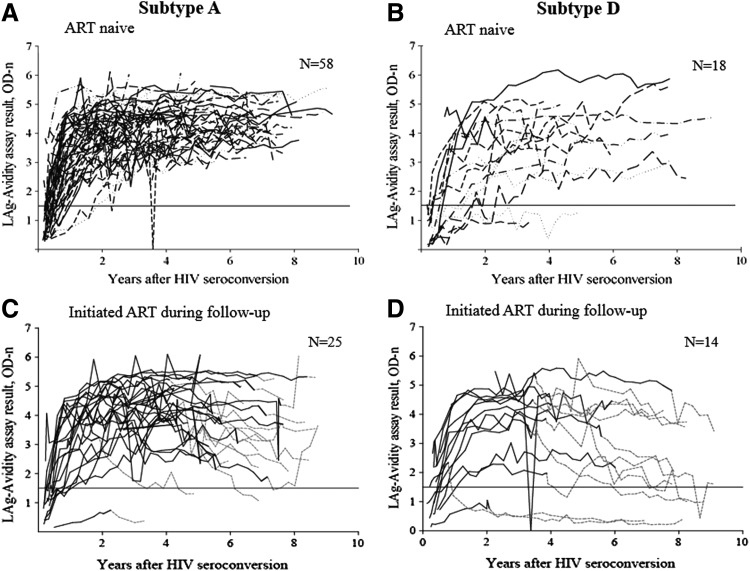
In the first step of the analysis, delayed antibody maturation was assessed by comparing the proportion of women with subtype A vs. D infection who had a LAg-Avidity assay result <1.5 OD-n (assay-positive result) in the first year or first 2 years after HIV seroconversion (Fig. 1). A higher proportion of women with subtype D infection was assay positive during the first year of infection compared to women with subtype A infection [25% (8/32) vs. 5% (4/82), p=0.002]. A similar difference was observed when the analysis was extended to include the first 2 years of infection [13% (4/32) for subtype D vs. 1% (1/82) for subtype A, p=0.008]. Furthermore, a higher proportion of women with subtype D infection was assay positive during the entire follow-up period [median: 6.6 years; 13% (4/32) for subtype D vs. 1% (1/82) for subtype A, p=0.021]. Two of the five women who remained assay positive throughout follow-up were on ART; both women initiated ART >1 year after HIV seroconversion.
FIG. 1.
The graphs show limiting antigen avidity assay (LAg-Avidity assay) results for antiretroviral treatment (ART)-naive women (A, B) and women who initiated ART during study follow-up (C, D). A horizontal line indicates an assay cutoff of 1.5 optical density units (OD-n). (A) Results for 58 women with subtype A infection who did not initiate ART during follow-up. (B) Results for 18 women with subtype D infection who did not initiate ART during follow-up. (C) Results for 25 women with subtype A infection who initiated ART during study follow-up. (D) Results for 14 women with subtype D infection who initiated ART during study follow-up. (A, B) A dashed gray line indicates the time on ART. (C, D) A solid line indicates the period prior to ART initiation; a dashed line indicates the time period after ART initiation.
Mean duration of recent infection
In the second step of the analysis, we evaluated antibody maturation by calculating the MDRI using samples collected in the first year or first 2 years after HIV seroconversion (see Materials and Methods). The MDRI was longer for women with subtype D infection than for women with subtype A infection when the analysis was limited to the first year after seroconversion (204.5 days, 95% CI: 179.9–229.5 vs. 160.3 days, 95% CI: 145.9–174.5, p<0.01) and when the analysis was extended to include the first 2 years after seroconversion (283.4 days, 95% CI: 245.7–323.1 vs. 171.9 days, 95% CI: 155.0–188.9, p<0.01).
The analysis was repeated after excluding samples collected from women on ART and by excluding samples with a viral load <400 copies/ml. Even after excluding those samples, the MDRI was longer for women with subtype D infection for both time intervals (for the first year after seroconversion: 202.9 days, 95% CI: 175.4–231.8 vs. 154.0 days, 95% CI: 139.5–168.7, p<0.01; for the first 2 years after seroconversion: 267.9 days, 95% CI: 231.2–308.2 vs. 167.3 days, 95% CI: 151.8–185.9, p<0.01). Furthermore, there was a greater proportional increase of MDRI for subtype D than for subtype A when results from the two time intervals were compared (24.3 vs. 8.0%). It is notable that for both subtypes and both time intervals, the MDRI values obtained in this study were longer than the MDRI that is indicated in the LAg-Avidity assay package insert (130 days, 95% CI: 118–142),20 regardless of whether low viral load samples and samples from those on ART were excluded.
Factors associated with delayed antibody maturation
As a next step, we evaluated factors associated with delayed antibody maturation. This was assessed by calculating the FFR, which was defined as the proportion of women who had an assay-positive result >2 years after seroconversion. Overall, 107 (5.9%) of the 1,814 samples collected >2 years after seroconversion were assay positive (i.e., FRR=5.9%). The following factors were associated with having an assay-positive result: older age (>28 years), longer duration of infection (>8 years), low viral load (<400 copies/ml), on ART <1 year, on ART >1 year, and subtype D infection (Table 2). In a multivariate model, the following factors were independently associated with having an assay-positive result: older age (>28 years), low viral load (<400 copies/ml), on ART >1 year, and subtype D infection (Table 2).
Table 2.
Factors Associated with Having a Limiting Antigen-Avidity Assay Result <1.5 (Optical Density Units) Among Women Infected More Than 2 Years
| Factor | % (number positive/total tested) | OR (95% CI) | aOR (95% CI) |
|---|---|---|---|
| Overall | 5.90 (107/1,814) | ||
| Age at infection | |||
| 18–24 years | 3.16 (24/759) | 1 | 1 |
| 25–28 years | 1.12 (7/623) | 0.49 (0.10–2.52) | 0.08 (0.005–1.34) |
| >28 years | 17.59 (76/432) | 8.51 (2.11–34.27)‡ | 11.90 (2.16–65.56)‡ |
| Duration of infection | |||
| 24–48 months | 5.73 (45/786) | 1 | 1 |
| 48–72 months | 4.13 (25/606) | 1.12 (0.79–1.58) | 0.25 (0.07–0.92)† |
| 72–96 months | 8.09 (30/371) | 1.99 (0.93–4.28)* | 0.20 (0.05–0.84)† |
| >96 months | 13.73 (7/51) | 3.59 (1.22–10.52)† | 0.30 (0.03–3.25) |
| Sample collection (years) | |||
| 2001–2006 | 3.94 (30/761) | 1 | 1 |
| 2007–2008 | 5.64 (39/691) | 1.51 (0.59–3.91) | 2.40 (0.48–12.13) |
| 2009–2010 | 10.50 (38/362) | 2.42 (0.86–6.79)* | 3.51 (0.72–17.27) |
| CD4 cell count (cells/μl) | |||
| ≥500 | 6.78 (49/723) | 1 | — |
| 499-200 | 5.47 (48/877) | 0.94 (0.55–1.61) | — |
| <200 | 3.97 (6/151) | 0.61 (0.31–1.21) | — |
| No data | 6.35 (4/63) | 0.67 (0.21–2.21) | — |
| Viral load (copies/ml) | |||
| >50,000 | 1.60 (3/188) | 1 | 1 |
| 49,999-400 | 1.34 (4/299) | 1.27 (0.72–2.24) | 2.13 (0.48–9.50) |
| <400 | 27.78 (75/270) | 4.08 (1.57–10.57)‡ | 9.02 (2.26–35.98)‡ |
| No data | 2.37 (25/1,057) | 1.27 (0.76–2.10) | 1.93 (0.73–5.08) |
| Time on ART | |||
| Not on ART | 1.78 (26/1,463) | 1 | 1 |
| <1 year | 8.78 (13/148) | 1.86 (1.09–3.18)† | 3.13 (0.64–15.37) |
| >1 year | 33.50 (68/203) | 4.92 (1.71–14.17)‡ | 13.63 (2.02–92.15)‡ |
| Subtype | |||
| A | 1.27 (16/1,256) | 1 | 1 |
| D | 16.31 (91/558) | 9.92 (2.04–48.27)‡ | 15.88 (2.61–96.56)‡ |
The association of having a LAg-Avidity assay result <1.5 normalized optical density units (OD-n) was examined using univariate models (OR, odds ratio) and multivariate models (aOR, adjusted odds ratio) using generalized estimating equations.
Significant OR and aOR values are indicated using the following symbols: *p<0.10, †p<0.05, ‡p<0.01.
ART, antiretroviral treatment; aOR, adjusted odds ratio; OR, odds ratio.
A stratified analysis was performed to determine the association of HIV subtype and FRR. The FRR was more than 12 times higher for women with subtype D infection compared to women with subtype A infection [16.3% (91/558) vs. 1.27% (16/1256), p<0.001]. In addition, the following factors that were associated with an assay-positive result were observed in a greater proportion of women with subtype D infection: longer duration of ART [≥3 years; 40.5% (60/148) for subtype D vs. 14.6% (8/55) for subtype A], longer duration of infection [≥8 years; 29.2% (7/24) for subtype D vs. 0.0% (0/27) for subtype A], and low viral load [<400 copies/ml, 41.1% (62/151) for subtype D vs. 10.9% (13/119) for subtype A].
Antibody waning
As a final step, we assessed antibody waning in women with subtype A and D infection. For this analysis, antibody waning was defined as having a LAg-Avidity assay result >20% lower than the highest assay result obtained at a prior study visit (i.e., >20% decline in the OD-n value). Antibody waning was more common among women with subtype D infection [34% (11/32) for subtype D vs. 17% (14/82) for subtype A, p=0.045]. We also evaluated antibody waning after ART initiation. Thirty-eight of the women included in the analysis initiated ART during study follow-up (24 with subtype A infection, 14 with subtype D infection, Fig. 1). The proportion of women who had antibody waning after ART initiation was higher for women with subtype D infection than for women with subtype A infection [71% (10/14) vs. 42% (10/24), p=0.076]. Antibody waning was less common in ART-naive women (Fig. 1A and B) than in women after they initiated ART (Fig. 1C and D). The proportion of women who did not initiate ART during the study who had antibody waning was similar for women with subtype A and D infection [6.9% (4/58) for subtype A; 5.6% (1/18) for subtype D, p=0.84].
Discussion
In this report, we evaluated anti-HIV antibody responses in Ugandan women using the LAg-Avidity assay. We found different rates of antibody maturation and antibody waning in women with subtype A vs. D infection. Women infected with subtype D HIV were less likely to reach a LAg-Avidity assay cut-off value of 1.5 OD-n 1 or 2 years after HIV seroconversion and had a longer MDRI for both time intervals. These findings indicate that infection with subtype D HIV is associated with delayed antibody maturation compared to infection with subtype A HIV. The LAg-Avidity assay was also much more likely to misclassify long-term infections as recent infections in women with subtype D infection (the FFR for subtype D was 12 times higher than the FFR for subtype A). Finally, women with subtype D infection were more likely to have a decline in LAg-Avidity results more than 2 years after seroconversion, indicating a waning antibody response to HIV. This was observed for the cohort as a whole and for a subset of the cohort that excluded samples with low viral loads and samples obtained from women on ART.
One limitation of this study is that the region of the HIV genome that was sequenced for subtype determination (the gp120 C2–V3 region) is different from the region of the HIV genome represented in the target antigen of the LAg-Avidity assay (the rIDR-M peptide, which represents an immunodominant region of gp41). Some of the viral strains analyzed in this report may have been intersubtype recombinants with different subtypes in these two regions. If this were the case, it might have made it more difficult to detect subtype-specific differences in antibody responses.
It is notable that the MDRI for both subtypes in this report was substantially longer than the MDRI indicated in the LAg-Avidity assay package insert (see Results). This was also the case in a previous study that measured the MDRI during the first 2 years after seroconversion in individuals with subtype A and D infection.10 In that study, the MDRI was also longer for individuals with subtype D infection (273 days, 95% CI: 170–387) than for subtype A infection (211 days, 95% CI: 156–275). The confidence intervals for MDRI were larger in that study than in this report, which may have reflected the smaller sample size of the previous study. The FRRs in the previous study were 2.7% (95% CI: 0.1–14.2) for subtype A and 9.1% (95% CI: 0.2–41.3) for subtype D.24 In this study, the FFR for subtype A was similar, 1.3% (95% CI: 0.7–2.1), but the FFR for subtype D was higher, 16.3% (95% CI: 13.2–19.6). Differences in MDRI and FFR values obtained in the two studies may reflect differences in the study populations (e.g., this study included only women), differences in the sample sizes of the two studies (number of participants and samples assessed), or other differences in the study cohorts (e.g., duration of follow-up: median 6.6 years in this study and median of <4 years in the previous study). This report extends the previous study by examining delayed antibody maturation and antibody waning, in addition to measuring MDRI and FFR. In this study, delayed antibody maturation and a higher frequency of antibody waning were observed in subtype D infection in both ART-naive women and in women after ART initiation.
We previously analyzed samples from the same cohort using the Bio-Rad-Avidity assay.7 The target antigen in that assay includes recombinant gp160 and p24 antigens. Delayed antibody maturation was also more common in subtype D infection than in subtype A infection when the Bio-Rad-Avidity assay was used for analysis. The previous report also compared the proportion of HIV-specific IgG in this cohort using the BED capture immunoassay (BED-CEIA).7 The target antigen in the BED-CEIA is similar to the target antigen in the LAg-Avidity assay, but the recombinant peptide used in the BED-CEIA is shorter. In contrast to subtype-based differences observed for the two avidity assays (LAg-Avidity and Bio-Rad-Avidity), no significant difference was observed in the serologic response to HIV infection for women with subtype A vs. D infection using the BED-CEIA.7 This may reflect a difference in the characteristics assessed (antibody avidity vs. proportion of antibody that is HIV specific), a difference in the target antigens of the assays, or other factors.
The differences observed in the serologic response to HIV infection in individuals with subtype A vs. D HIV infection could be related to the more rapid disease progression observed in individuals with subtype D infection.12,13 Destruction of CD4-positive T cells early in infection by a more pathogenic virus could hinder B cell function and production of high-avidity antibodies. Further studies are needed to investigate the differences in antibody responses in individuals infected with different HIV subtypes.
The findings in the report also have important implications for the use of serologic assays, such as the LAg-Avidity assay, for cross-sectional HIV incidence estimation in Uganda and other countries in which subtype D HIV is prevalent. We find that the LAg-avidity assay is more likely to misclassify samples from individuals with established HIV infection as assay positive if they have subtype D infection. This suggests that the LAg-Avidity assay should not be used for HIV incidence estimation in regions in which subtype D HIV is prevalent. It should also be noted that this report included a cohort of women. A recent study found no difference in the antibody responses in men vs. women in a Ugandan population in which all infections were subtype A or D.8 Additional studies are needed to evaluate the serologic response to HIV infection in men infected with subtype A and D infection.
Acknowledgments
We wish to thank Dr. Alex Welte for his assistance with the calculation of the MDRI estimates.
This work was supported by the Centers for Disease Control and Prevention (CDC) under contract no. 200-2010-35109-00001. The research was also supported by the HIV Prevention Trials Network (HPTN) sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH), and the Office of AIDS Research, of the National Institutes of Health (NIH), Department of Health and Human Services (DHHS) (UM1AI068613), and by R01 AI095068 (NIAID). Funding was also provided by the Bill & Melinda Gates Foundation (OPP1017716). Additional funding was provided by the Division of Intramural Research, NIAID, NIH.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Brookmeyer R, Laeyendecker O, Donnell D, and Eshleman SE: Cross-sectional HIV incidence estimation in HIV prevention research. J Acquir Immune Defic Syndr 2013;63:S233–S239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Incidence Assay Critical Path Working Group: More and better information to tackle HIV epidemics: Towards improved HIV incidence assays. PLoS Med 2011;8(6):e1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy G. and Parry JV: Assays for the detection of recent infections with human immunodeficiency virus type 1. Euro Surveill 2008;13(36):pii [PubMed] [Google Scholar]
- 4.Guy R, Gold J, Calleja JM, et al. : Accuracy of serological assays for detection of recent infection with HIV and estimation of population incidence: A systematic review. Lancet Infect Dis 2009;9(12):747–759 [DOI] [PubMed] [Google Scholar]
- 5.Busch MP, Pilcher CD, Mastro TD, et al. : Beyond detuning: 10 years of progress and new challenges in the development and application of assays for HIV incidence estimation. AIDS 2010;24(18):2763–2771 [DOI] [PubMed] [Google Scholar]
- 6.Mastro TD, Kim AA, Hallett T, et al. : Estimating HIV incidence in populations using tests for recent infection: Issues, challenges and the way forward. J HIV AIDS Surveill Epidemiol 2010;2(1):1–14 [PMC free article] [PubMed] [Google Scholar]
- 7.Longosz AF, Morrison CS, Chen PL, et al. : Immune responses in Ugandan women infected with subtypes A and D HIV using the BED capture immunoassay and an antibody avidity assay. J Acquir Immune Defic Syndr 2014;65(4):390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longosz AF, Serwadda D, Nalugoda F, et al. : Impact of HIV subtype on performance of the limiting antigen-avidity enzyme immunoassay, the Bio-Rad avidity assay, and the BED capture immunoassay in Rakai, Uganda. AIDS Res Hum Retroviruses 2014;30(4):339–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullis CE, Munshaw S, Grabowski MK, et al. : Differential specificity of HIV incidence assays in HIV subtypes A and D-infected individuals from Rakai, Uganda. AIDS Res Hum Retroviruses 2013;29(8):1146–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kassanjee R, Pilcher CD, Keating SM, et al. : Independent assessment of candidate HIV incidence assays on specimens in the CEPHIA repository. AIDS 2014;28(16):2439–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laeyendecker O, Kulich M, Donnell D, et al. : Development of methods for cross-sectional HIV incidence estimation in a large, community randomized trial. PLoS One 2013;8(11):e78818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baeten JM, Chohan B, Lavreys L, et al. : HIV-1 subtype D infection is associated with faster disease progression than subtype A in spite of similar plasma HIV-1 loads. J Infect Dis 2007;195(8):1177–1180 [DOI] [PubMed] [Google Scholar]
- 13.Kiwanuka N, Laeyendecker O, Robb M, et al. : Effect of human immunodeficiency virus type 1 (HIV-1) subtype on disease progression in persons from Rakai, Uganda, with incident HIV-1 infection. J Infect Dis 2008;197(5):707–713 [DOI] [PubMed] [Google Scholar]
- 14.Kiwanuka N, Robb M, Laeyendecker O, et al. : HIV-1 viral subtype differences in the rate of CD4+ T-cell decline among HIV seroincident antiretroviral naive persons in Rakai district, Uganda. J Acquir Immune Defic Syndr 2010;54(2):180–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei X, Liu X, Dobbs T, et al. : Development of two avidity-based assays to detect recent HIV type 1 seroconversion using a multisubtype gp41 recombinant protein. AIDS Res Hum Retroviruses 2010;26(1):61–71 [DOI] [PubMed] [Google Scholar]
- 16.Duong YT, Qiu M, De AK, et al. : Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: Potential for HIV-1 incidence estimates and avidity maturation studies. PLoS One 2012;7(3):e33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrison CS, Chen PL, Nankya I, et al. : Hormonal contraceptive use and HIV disease progression among women in Uganda and Zimbabwe. J Acquir Immune Defic Syndr 2011;57(2):157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison CS, Demers K, Kwok C, et al. : Plasma and cervical viral loads among Ugandan and Zimbabwean women during acute and early HIV-1 infection. AIDS 2010;24(4):573–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sedia Biosciences Corporation: Sedia HIV-1 LAg-Avidity EIA. LN 6039, 04 ed. Portland, OR, 2013 [Google Scholar]
- 20.Kassanjee R, McWalter TA, Barnighausen T, and Welte A: A new general biomarker-based incidence estimator. Epidemiology 2012;23(5):721–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassanjee R, McWalter TA, and Welte A: Short Communication: Defining optimality of a test for recent infection for HIV incidence surveillance. AIDS Res Hum Retroviruses 2014;30(1):45–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sexton CJ, Costenbader EC, Vinh DT, et al. : Correlation of prospective and cross-sectional measures of HIV type 1 incidence in a higher-risk cohort in Ho Chi Minh City, Vietnam. AIDS Res Hum Retroviruses 2012;28(8):866–873 [DOI] [PubMed] [Google Scholar]
- 23.Kim AA, Hallett T, Stover J, et al. : Estimating HIV incidence among adults in Kenya and Uganda: A systematic comparison of multiple methods. PLoS One 2011;6(3):e17535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moyo S, LeCuyer T, Wang R, et al. : Evaluation of the false recent classification rates of multiassay algorithms in estimating HIV type 1 subtype C incidence. AIDS Res Hum Retroviruses 2014;30(1):29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeger SL. and Liang KY: Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42(1):121–130 [PubMed] [Google Scholar]