Abstract
Exosomes are small membrane bound vesicles that carry biological macromolecules from the site of production to target sites either in the microenvironment or at distant sites away from the origin. Exosomal content of cells varies with the cell-type that produces them as well as environmental factors that alter the normal state of the cell such as viral infection. Human DNA and RNA viruses alter the composition of host proteins as well as incorporate their own viral proteins and other cargo into the secreted exosomes. While numerous viruses can infect various cell types of the CNS and elicit damaging neuropathologies, very little have been studied for their exosomal composition, content and function on recipient cells. Therefore, there is a pressing need to understand how DNA and RNA viral infections in CNS control exosomal release. Some of the more recent studies including HIV-1, HTLV-1 and EBV infected B cells indicate that exosomes from these infections have been shown to contain viral miRNAs, viral transactivators and a host of cytokines that can control the course of infection. Finally, because exosomes can serve as vehicles for the cellular delivery of proteins and RNA and given that the blood-brain barrier is a formidable challenge in delivering therapeutics to the brain, exosomes may be able to serve as ideal vehicles to deliver protein or RNA-based therapeutics to the brain.
Keywords: Exosomes, Virus, Retrovirus, Neurovirology, Neuropathology, Extracellular Signaling, CNS
Introduction
Exosomes are small membrane bound vesicles ranging from 50 to 90 nm. The definition of exosomes has undergone considerable evolution since the word was first coined by Johnstone et al., (Johnstone, Mathew et al. 1991). Initial studies by Johnstone stated that exosomes were simply a means of removing cellular waste from the cytosol in maturing reticulocytes (Johnstone et al., 1991). Studies to date have found that exosomes play a significant role in cell-cell signaling, cancer progression (Luga et al., 2012), HIV particle release (Izquierdo-Useros et al., 2010), host immune responses (Aline et al., 2004; Colino et al., 2007; Giri et al., 2010), and even as carriers of prions (Leblanc et al., 2006). In the nervous system, exosomes are implicated in normal physiological conditions as well as in mediating pathological states. Oligodendrocytes secrete exosomes in response to neuronal signals such as is triggered by the neurotransmitter glutamate through activation of ionotropic glutamate receptors (Fruhbeis, Frohlich et al. 2013). Secreted exosomes can travel to distant sites in the body via the circulatory system or taken up by cells in the immediate microenvironment itself. At the target site, they are internalized by recipient cells through endocytosis (Tian et al., 2010). Finally, the release of exosomal contents in the recipient cells can trigger a variety of responses in the target cell.
The biogenesis of exosomes is still being debated. While many studies show that they are of endosomal origin formed upon fusion of endosomal multivesicular bodies (MVBs) with the plasma membrane, a study by Booth et al., showed that exosomes can bud directly from the plasma membrane (Booth, 2006). Given their small size, evaluation of the purity of exosome preparations as well as their characterization is best determined by electron microscopy. Differences in density of exosomes from other membrane-derived vesicles can be exploited to prepare exosomal preparations from crude lysates using a sucrose gradient. Exosomes have been found to float at densities ranging from 1.15 to 1.19 g/ml on continuous sucrose gradients. By comparison, vesicles purified from the endoplasmic reticulum float at 1.18 to 1.25 g/ml, and vesicles from the Golgi at 1.05 to 1.12 g/ml (Thery, Amigorena et al. 2006). Further refinements of isolation methods indicated that a ratio of ≥ 3×1010 particles/μg of protein implies purity of the exosomes (Webber and Clayton 2013).
Similar to plasma membranes, exosomes are organized in a bilayer but unlike plasma membranes, the exosomal membrane is relatively rigid at pH 7. The rigidity may be the reason why exosomes are protected from lipolytic or proteolytic degradation while in circulation. Exosomes enclose cargos that contain proteins, various classes of RNA and lipids. Because of the existence of multiple mechanisms for protein sorting into exosomes, secreted exosomes are heterogeneous in their size and cargo, even when they are derived from the same cell (Colombo, Moita et al. 2013). Further heterogeneity in size and content comes from cell type, microenvironmental stimuli e.g. virally infected or uninfected cells. Common exosomal protein markers, whether involved in the biogenesis of the exosomes or not include Alix and TSG101, which are components of the ESCRT machinery (Henne, Buchkovich et al. 2011), and the tetraspanin CD63 (Beatty 2008).
The presence of lipids, especially the sphinogolipid ceramide in exosomes is a result of raft-based microdomain-dependent method of exosomal budding from the endosomal membrane (Trajkovic, Hsu et al. 2008). The microdomains may contain high concentrations of sphingolipids from which ceramides are formed. Ceramide can induce the coalescence of small microdomains into larger domains, which promotes domain-induced budding. This pathway of exosomal biogenesis is well-accepted as the use of a cell-permeable, potent, specific, non-competitive inhibitor of neutral sphingomyelinase, GW4869, can inhibit exosome production (Kulshreshtha, Ahmad et al. 2013). The presence of other types of lipids in exosomes differ depending upon the cell type and the nature of infection [reviewed in (Subra, Laulagnier et al. 2007)]. The most striking difference between exosomes and parental cells is the balance between sphingomyelin and phosphatidylcholine. Exosomes have twice the amount of sphingomyelin and lower levels of phosphatidylcholine compared to parental cells (Laulagnier, Motta et al.). In the same study, a lipid analysis of exosomes showed that lipid composition of these vesicles is more similar to each other than to the parental cell. For example, exosomes from RBL-2H3 cells, a rat basophile leukemia cell line, and bone-marrow-derived dendritic cells of human origin were similar to each other than their parental cells. In addition, this lipid composition seems to be independent of the secretion pattern: exosomes from dendritic cells from the CNS are constitutively secreted, whereas exosomes from RBL-2H3 cells are released following stimulation with the calcium ionophore ionomycin (Laulagnier, Motta et al. 2004). Below we describe the multitude of CNS cell types that secrete exosomes and control a multilevel communication pathway, and the role of exosomes in the neuropathology associated with viral infections.
Exosome Secretion, Fusion, Composition, and Functionality within the CNS
Neurons
Exosomes isolated from rat cortical cultures have been shown to contain the adhesion protein L1, which are only expressed by neurons indicating expression of exosomes from these cell types (Faure, Lachenal et al. 2006). This was further confirmed using an engineered mouse line that overexpresses yellow fluorescent protein (YFP) in neurons; YFP was found to be localized in extracellular vesicles containing exosomal markers Alix and TSG101 (Faure, 2006). Furthermore, culturing cortical neurons in media with high KCl to depolarize cells greatly increased exosome secretion as compared to the same cells cultured in low KCl, demonstrating that depolarization induces secretion of exosomes from neurons. Additionally, exosomes secreted from axons have been shown to contain cytokine binding proteins, thereby aiding in the synaptic transfer of these important cell signaling molecules. One such example is the incorporation of the Evenness interrupted/Wntless/Sprinter (Evi/Wls/Srt) membrane protein in exosomes secreted from the presynaptic termini of neurons, which binds and transports the Wnt family member - Wingless (Wg) extra-vesicularly (Korkut, Ataman et al. 2009).
Oligodendrocytes
Oligodendrocytes play a key role in the CNS by myelinating the axons of several neurons with extensions of their plasma membranes. It has been shown that the exosomes of oligodendrocytes contain key components of the myelin sheath including myelin proteolipid protein (PLP), myelin basic protein (MBP), and myelin oligodendrocyte glycoprotein (MOG) (Kramer-Albers, Bretz et al. 2007). Additional proteomic analysis of the oligodendrocytic exosomes showed they contained numerous enzymes involved in metabolism, signaling, and cellular stress, in particular proteins that function to reduce oxidative stress (Kramer-Albers, 2007). Furthermore, secretion of exosomes containing these vital components is enhanced with increased cytoplasmic calcium levels, demonstrating induction by cellular signaling (Kramer-Albers, 2007).
Astroglia
Within the CNS, astrocytes play a key role in supporting neurons. Under normal host conditions, astrocytes provide several beneficial growth factors to neurons including glial-derived neurotrophic factor (GDNF), nerve growth factor (NGF), and brain-derived neurotrophic factor (BDNF). However, quiescent astrocytes can be reactivated under certain conditions, such as exposure to pro-inflammatory cytokines, after which they switch into an antagonistic mediator of neurodegenerative conditions.
It has been shown that the secretion of protective heat shock protein 70 (Hsp70) in astrocyte exosomes is up-regulated after thermal stress (Taylor, Robinson et al. 2007). Furthermore, the secretion of Hsp70 in exosomes was inhibited by the use of MEK1/2 and PI3K inhibitors (U0126 and LY294002, respectively) although these inhibitors did not reduce total cellular Hsp70 levels. This data indicates that the secretion of Hsp70 in exosomes is positively regulated in part through the phosphorylation of extracellular signal related kinase 1/2 (Erk1/2) and Akt signal transduction pathways. Conversely, inhibition of the phosphorylation of c-jun N-terminal kinase (JNK) actually increased exosomal secretion of Hsp70 upon thermal shock, demonstrating a feed-back inhibition role for this member of the mitogen-activated protein kinase (MAPK) pathway.
Microglia
Microglial cells are the primary immune system effector cells within the CNS. The composition of proteins secreted by murine microglial cells in exosomes includes several SNARE proteins that are present in post-Golgi and late-endosome vesicles, as well as other late-endosomal marker proteins such as Rab7, Rab11, Lamp1, Lamp2, CD9 and CD63 (Potolicchio, Carven et al. 2005). Additionally, protein markers of other intracellular compartments such as the trans-Golgi network are lacking in the microglial exosomes and the overall protein composition is comparable to that of other antigen presenting cell (APC) exosomes. Furthermore, MHC class II and other associated proteins could also be detected in microglial exosomes, consistent with their role as the APC of the CNS. A novel membrane protein on microglial exosomes that was not observed on B cell or dendritic cell exosomes was the aminopeptidase N or CD13. This peptidase has been shown to cleave the neuropeptides methionine- and leucine-enkaphalin and activity of this enzyme from microglial exosomes was verified by in vitro biochemical assays (Potolicchio, 2005).
The secretion of exosomes from primary rat microglia has been shown to be induced by Wnt3a signaling via a GSK-independent pathway (Hooper, Sainz-Fuertes et al. 2012). The addition of Wnt3a did not elicit increased production of proinflammatory cytokines from microglia such as TNFα or IL1β. In contrast to the activation of exosome secretion in microglia, Wnt3a had no measurable effect on the release of exosomes from primary rat cortical neurons, which demonstrate constitutive secretion of exosomes when cultured. This difference in behavior exemplifies the varied mechanisms by which exosome secretion is activated in the various cell types within the CNS.
Exosomes in Pathological Conditions of the CNS
Many human neurodegenerative diseases are characterized by abnormal protein turnover. The aggregation of the microtubule-associated cytosolic protein tau and its subsequent secretion into the extracellular space, enclosed within exosomes, plays a central role in the neurodegeneration seen in Alzheimer’s disease (Gendreau and Hall 2013). Also, it has recently been shown that astrocytes exposed to amyloid peptide actively secrete exosomes containing prostate apoptosis response 4 (PAR-4) and ceramide, which in turn are taken up by neighboring astrocytes and cause apoptosis (Wang, Dinkins et al. 2012). In astrocytes lacking the ceramide generating enzyme neutral sphingomyelinase 2, secretion of the pro-apoptotic exosomes could not be induced by exogenous amyloid peptide, although additional treatment with exogenous C18 ceramide again activated PAR-4 enriched exosome release from these cells. Furthermore, confocal microscopy showed colocalization of exosomal markers PAR-4 and ceramide on the processes as the site of exosome exchange.
Altered Exosome Composition and Functionality from Virally Infected Cells
The study of viral manipulation of host exosomal content and functionality is a relatively new field of study. Moreover, implications of CNS pathology of viral infections are even less well understood. To date only a select few DNA and RNA viruses have been examined in any significant detail in regards to their ability to alter the composition of host exosomes and to determine subsequent effects of these altered exosomes on recipient cells. Here, we will detail the relevant findings from experiments conducted to date and identify implications for viruses that adversely effect the normal functioning of the CNS.
DNA Viruses
While numerous DNA viruses can infect various cell types of the CNS and elicit damaging neuropathologies, none of these have yet been studied in regards to their ability to change the composition of host exosomes and the potential impact of these modified exosomes on recipient cells. Regardless, much can be elucidated from the studies of non-CNS infecting DNA viruses and the implications of these findings for the field of neurovirology are potentially significant. For instance, the herpes simplex virus 1 (HSV-1) glycoprotein B (gB) has been shown to associate with the major histocompatibility complex class II (MHCII) surface receptor, human leukocyte antigen DR (HLA-DR), and alter its trafficking through the secretory pathway (Neumann, Eis-Hubinger et al. 2003; Temme, Eis-Hubinger et al. 2010). The association of gB with HLA-DR was demonstrated to occur in post-Golgi membrane compartments where antigen (Ag) loading occurs as the gB-HLA-DR complex lacked the invariant chain, Ii, and also possessed a glycosylation profile consistent with post-Golgi maturation (Temme, Eis-Hubinger et al. 2010). Furthermore, gB prevented peptide loading into the HLA-DR complex, as well as expression of HLA-DR on the plasma membrane. In contrast to normal cell surface expression of HLA-DR, it was found that in the presence of gB, the complex was alternately trafficked through the exosomal secretion pathway and released from the cells in exosomes. The altered antigen loading and vesicular trafficking of HLA-DR by HSV-1 gB represents a novel mechanism by which HSV-1 can evade activation of CD4+ T helper cells and subsequent adaptive immune response to the virus. Based on this important mechanism of host immune evasion, it may be possible that the related varicella-zoster virus (VZV), which is part of the same Alphaherpesvirinae subfamily of Herpesviridae as HSV-1, could utilize a similar mechanism for avoiding the immune system and establishing long-lived latency in sensor neurons.
Other members of the Herpesviridae family, namely the Epstein-Barr virus (EBV) and Kaposi sarcoma herpes virus (KSHV), have also been shown to alter the composition of exosomes secreted from their host cells. A recent proteomic analysis of B cells infected with either KSHV or EBV, or a dual infection showed major changes to the exosomal protein composition as compared to the uninfected parental cell line (Meckes, Gunawardena et al. 2013). Specifically, a total of 345 proteins were identified by mass spectrometry that uniquely incorporated into the exosomes released from the infected cell lines. Of these unique proteins, 230 were common to both the KSHV and EBV infected cell lines, while 93 were specific to the EBV infected cells and 22 were found only in the KSHV cell lines. Consequently, several of the cellular pathways affected by up-regulated exosomal proteins from either EBV or KSHV infected cells were different. Specifically, the pathways affected by the KSHV up-regulated exosomal proteins were primarily related to metabolism, protein translation, and cellular migration, while those affected by the EBV up-regulated exosomal proteins included interferon and NF-κB signaling, membrane and protein trafficking, lipid raft organization, and cellular/vesicle binding. Furthermore, both viruses increased the exosomal incorporation of MHCII HLA membrane proteins but only EBV increased both the alpha and beta chains of the HLA-DR receptor, indicating that EBV may alter cell surface Ag presentation similar to HSV-1. Despite the similar increased shuttling of HLA proteins to exosomes, further experimentation needs to be conducted to determine if either virus block Ag loading into HLA receptor complexes comparable to HSV-1.
The incorporation of viral proteins into exosomes has also been shown to be important for virus survival and spread, as well as induced pathology in the host organism. In EBV infected cells, two of the viral oncoproteins, LMP1 and LMP2A, have been shown to incorporate into exosomes and secreted from the host cells (Keryer-Bibens, Pioche-Durieu et al. 2006; Ikeda and Longnecker 2007; Meckes, Shair et al. 2010). Studies have shown that LMP1 is packaged into exosomes secreted from infected nasopharyngeal carcinoma (NPC) cells and its incorporation alters the host protein composition of exosomes as compared to uninfected cells (Keryer-Bibens, Pioche-Durieu et al. 2006; Meckes, Shair et al. 2010). Specifically, an initial study demonstrated that LMP1 enriched exosomes also increased the exosome levels of galectin 9 (Keryer-Bibens, Pioche-Durieu et al. 2006). Moreover, while the presence of both proteins in exosomes contributed to reduced proliferation of recipient peripheral T cells, LMP1 alone had a much lower IC50 of 0.17 nM as compared to 46 nM for galectin 9. Therefore, the immunosuppressive functionality of the LMP1 containing exosomes was primarily attributable to the LMP1 itself. In contrast, a subsequent study of LMP1 negative exosomes derived from a different EBV infected NPC clone showed that galectin 9 significantly increased apoptosis in EBV-reactive cytotoxic CD4+ T cells isolated from healthy EBV carriers (Klibi, Niki et al. 2009). These two differing mechanisms of action by LMP1 and galectin 9 integrated into exosomes from EBV infected cells may indicate a multifaceted immunosuppressive defense orchestrated by EBV. First, LMP1 seems to illicit a broad anti-proliferative effect upon all T cell populations, while galectin 9 can target cytotoxic CD4+ T cells that are reactive against EBV viral proteins. In combination, these two mechanisms allow EBV to evade immune surveillance in the host. Subsequent work showed that LMP1 integrated exosomes also boosted the incorporation of epidermal growth factor receptor (EGFR) into exosomes (Meckes, Shair et al. 2010). Functionally, exposure of bystander cells to these LMP1 and EGFR enriched exosomes leads to the cellular uptake of these vesicles and causes activation of the Erk1/2 and Akt1 signaling cascade in the recipient cells. Similar to LMP1, another related EBV viral protein, LMP2A, is integrated into exosomes and secreted from infected cells (Ikeda and Longnecker 2007). The exosomal incorporation of LMP2A was able to identify a potential biochemical mechanism by which this viral protein was selectively trafficked into the secreted exosomes. Specifically, it was found that cholesterol depletion from the cellular membranes using the drug methyl-beta-cyclodextrin (MCD), which disrupts lipid rafts, caused significant increases in overall cellular LMP2A levels as well as exosome integrated LMP2A. It was further shown that the MCD treatment caused marked decreases in both phosphorylation and ubiquitinylation of LMP2A in cellular lysates, but for the LMP2A that was integrated into exosomes only phosphorylation was inhibited while ubiquitinylation of the protein remained high. This indicates that the ubiquitin modification of LMP2A may be a key mechanism for sorting of this protein into secreted exosomes, while phosphorylation may signal retention within the cell. In general, these studies demonstrate the importance of the selective incorporation of both viral and host proteins into exosomes for the manipulation of signal-transduction cascades in the targeted recipient cells and identify potential mechanism by which these proteins are shuttled into host exosomes. Furthermore, the packaging of viral proteins in exosomes could serve as an important mechanism for onset of neurological disorders associated with DNA virus infections and also present novel targets for therapeutic intervention.
In addition to changes in protein composition, exosomes from EBV infected B-cells also contain viral RNA (Meckes, Shair et al. 2010; Pegtel, Cosmopoulos et al. 2010). In this regard, one study showed an enrichment of smaller 15-40 nt RNA molecules in host exosomes with a relative abundance of mature EBV miRNAs of between 102 - 105 copies per 0.5 ng of exosomal RNA (Pegtel, Cosmopoulos et al. 2010). These mature EBV miRNAs transferred into co-cultured uninfected monocyte-derived dendritic cells (MoDCs) and were further shown to down-regulate luciferase reporter systems fused with their target 3′ UTR sequences. Moreover, non-B-cells were also shown to have significant levels of mature EBV miRNAs in 60% of asymptomatic HIV-EBV co-infection patients who had elevated EBV loads. Another study also demonstrated that EBV viral miRNAs were incorporated in the exosomes of infected cells and that several viral miRNAs were selectively enriched in the exosomes at up to fourfold higher concentrations as compared to intracellular levels (Meckes, Shair et al. 2010). This study also confirmed that the viral miRNAs could be successfully transferred into uninfected recipient cells and thereby affect their target genes within these cells. Overall, the transfer of functional viral miRNAs via altered host exosomes, demonstrates yet another potent mechanism by which DNA viruses infecting the CNS may be able to dysregulate cellular processes in recipient cells and induce severe neuropathologies.
The findings to date in regards to DNA viruses being able to manipulate the host exosomes for their survival advantage demonstrate the significant potential for other viruses that infect the CNS to leverage this important cell signaling pathway. Specifically, neurotropic DNA viruses, such as the John Cunningham virus or VZV, could utilize the alteration of host exosomes to enhance spread of the virus, evade host immune surveillance, and elicit pathological effects within the host. The scarcity of publications in this field combined with the significant findings from the field of cancer-altered exosomes, as well as the studies detailing DNA virus manipulation of host exosomes, signals the possibility for high-impact novel scientific discoveries in the study of virally altered exosomes and neuropathology.
RNA Viruses
Two important retroviruses that have well known pathologies of the CNS have been examined in regards to their manipulation of host exosomes, specifically Human T-lymphotropic Virus-1 (HTLV-1) and Human Immunodeficiency Virus-1 (HIV-1). These two viruses induce CNS related pathologies including HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) and HIV-associated neurocognitive disorders (HAND) (Osame, Matsumoto et al. 1987; Roman, Spencer et al. 1987; Vernant, Buisson et al. 1987; Heaton, Franklin et al. 2011; Agrawal, Louboutin et al. 2012). The condition known as HAM/TSP was described several years prior to the identification of the causative agent, namely HTLV-1, and is defined by neuro-inflammation and partial paralysis of the limbs (Mani, Mani et al. 1969). Additionally, the spectrum of mental disease states known collectively as HAND, has continued to persist in up to 47 percent of HIV-1 patients despite the use of highly active antiretroviral therapies [HAART; (Heaton, Clifford et al. 2010)]. As these retroviruses are the causative agents of such debilitating diseases of the CNS, elucidation of the mechanisms by which they manipulate host exosomes may lead to novel targets for therapeutic intervention.
One major benefit that retroviruses gain by hijacking host exosomes is an increase in viral spread. In addition to this survival benefit to the virus, the resulting changes in recipient cells can also lead to the disease state associated with the infecting virus. To this end, HIV-1 infected Monocyte derived Macrophages (MDMs) have been shown to increase the number of exosomes and microvesicles secreted from the cell (Kadiu, Narayanasamy et al. 2012). Furthermore, the secreted exosomes were shown to contain cytokines that induce cellular migration and the release of additional pro-inflammatory cytokines from recipient cells, enhancing HIV-1 infectivity. Moreover, some virions shed from the infected MDMs were found to be associated with aggregates of exosomes secreted from those cells and the entrapped virions demonstrated enhanced infectivity toward CD4+ target cells as compared to purified virus. Additionally, the proteomic analysis of the exosomes and microvesicles from the infected MDMs identified proteins requisite for cell-free antigen presentation, T cell activation, and chemotaxis. Similar to the HIV-1 findings, we also recently identified alterations of the cytokines packaged into exosomes of HTLV-1 infected cells (Van Duyne et al., unpublished data). Again, the release of these cytokines from HTLV-1 altered exosomes could lead to enhanced infectivity in recipient cells or induce the neuroinflammation associated with HAM/TSP. In total, this data demonstrates multiple potential mechanisms by which the virus manipulated exosomes to increase viral spread and induce associated pathologies either through the activation of recipient cell signal-transduction by cytokines or by direct virion association with these secreted vesicles to enhance target cell uptake.
In addition to altering the composition of host proteins in exosomes, both HIV-1 and HTLV-1 can also incorporate their own viral proteins into host exosomes. Recent experiments in our laboratory have shown that the viral oncoprotein Tax is incorporated into exosomes secreted from both cell lines and primary cells infected with HTLV-1 (Jaworski et al., unpublished data). The release of Tax in exosomes represents one mechanism by which the HTLV-1 virus may induce the T cell dysregulation and neuroinflammation associated with HAM/TSP. Additionally, while the mechanism of trafficking and packaging of viral proteins in exosomes has not been well characterized for most exosomal viral proteins, the incorporation of the HIV-1 protein Negative factor (Nef), represents one such biochemical pathway that is well defined. Nef has been found to contain several conserved motifs within the N-terminus that are required for its exosomal secretion (Ali, Huang et al. 2010; Campbell, Isayev et al. 2012). Moreover, use of a peptide mimicking one of these novel Nef domains termed the secretion modification region (SMR) disrupted the interaction of Nef with the cellular protein Mortalin, thereby blocking exosomal incorporation of Nef, as well as virion budding (Shelton, Huang et al. 2012). This dual inhibition also indicates the potential of a shared cellular protein trafficking mechanism between exosome protein packing and viral production. The identification of the SMR of Nef is an important finding and this exosome trafficking motif should be further studied in other exosome containing viral proteins to determine whether it is a conserved biochemical mechanism in the host.
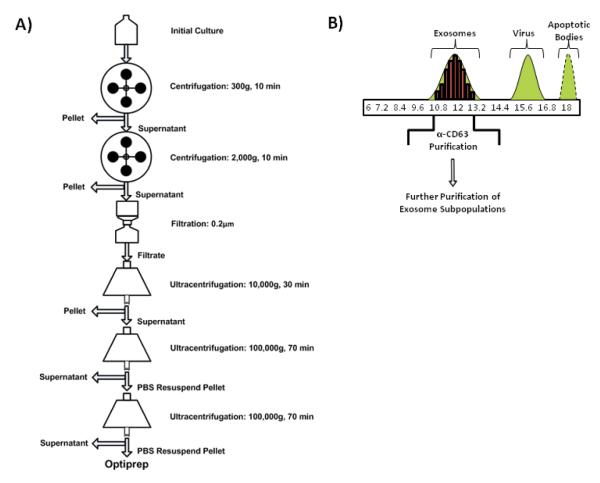
Beyond alterations in the protein composition of exosomes, HIV-1 infections have also been found to vary the RNA molecules packaged within exosomes. Our laboratory recently reported that the nascent viral trans-activating response (TAR) element transcribed from the integrated provirus is incorporated within exosomes from HIV-1 infected cell lines and found in exosomes from the serum of patients on HAART or long-term non-progressors (LNTPs) (Narayanan, Iordanskiy et al. 2013). A general diagram of how the exosomes from infeted cells were purified is depicted in Figure 1. The concentrations of the TAR RNA molecules quantified in the culture supernatant and patient sera were significant at levels between 104 - 106 and 103 copies per mL, respectively.
Figure 1. A schematic representation of the purification protocol for exosomal preparation from infected cells.
A) Exosomes are obtained from crude culture supernatants by sequential use of increasing centrifugal forces. An intermediary filtration step helps to eliminate dead cells and large debris. At the end of the centrifugations, a partially purified exosomal preparation is obtained. B) The partially purified exosomal preparation is further purified using an optiprep gradient. The exosomal fraction is usually spread among three to five fractions of the optiprep velocity gradient centrifugation. The X-axis shows the % of iodixanol in the appropriate gradients. The optiprep fractions may be further purified by using magnetic beads coated with antibodies directed against proteins exposed on exosomal membranes such as CD63. This molecule is specifically enriched in both HIV-1 and HTLV-1 exosomes (unpublished data).
Furthermore, the TAR RNA was not found to be associated with the RNA interference (RNAi) component protein Argonaute 2 (Ago2) although the microRNA biogenesis proteins Drosha and Dicer were found to be incorporated at elevated levels in the exosomes secreted from HIV-1 infected cells. Our laboratory has recently found that in contrast to HIV-1 infected cell lines, exosomes from cell lines infected with the HTLV-1 do contain viral proteins such as Tax but do not carry appreciable amounts of viral miRNAs (unpublished data). Additionally, the composition of RNAi proteins varies between HIV-1 and HTLV-1 exosomes, with HTLV-1 exosomes containing Ago2 along with cellular miRNAs but integrating only limited amounts of Drosha and Dicer (unpublished data). The increased levels of Ago2 in the presence of cellular miRNAs suggests that unlike HIV-1 altered exosomes, HTLV-1 manipulated exosomes can rapidly control mRNA translation in recipient cells upon exosome uptake. Functionally, the exosomes isolated from the supernatant of cell lines chronically infected with HIV-1 enhanced the infectivity of subsequent exposure to HIV-1 virus when incubated with uninfected cells prior to exposure to the virus (Narayanan, Iordanskiy et al. 2013). Additionally, the exosomes reduced apoptosis in target cells by down-regulating Bim and Cdk9 genes. Overall, the exosomes secreted from HIV-1 infected cells demonstrated a mechanism by which uninfected cells could become more susceptible to subsequent exposure to the virus thereby enhancing the overall infectivity within the host.
Subsequent to direct alterations of the RNA composition of exosomes secreted from HIV-1 infected cells, other indirect mechanisms of action have been identified by which the virus may influence uninfected bystander cells. The HIV-1 transactivating protein Tat has been shown to be toxic to human neurons and, therefore, a significant contributor to HAND. Furthermore, the neuronal toxicity of Tat is potentiated by opiate drug use. One study showed that astrocytes exposed to a combination of Tat and opiate drugs secreted exosomes with elevated levels of miR-29b (Hu, Yao et al. 2012). When human neurons were subsequently exposed to the miR-29b fortified exosomes, the target gene, platelet-derived growth factor-B (PDGF-B), was repressed and neuron viability was correspondingly decreased. This demonstrates the potential for indirect exosome mediated neurotoxicity associated with a retroviral infection, and eliminates the necessity of direct viral infection to induce the associated pathology.
Other RNA viruses have also been shown to alter the composition of host exosomes. For instance, the hepatitis-C virus (HCV) alteration of host exosomes has been examined by several different laboratories. In one study, HCVRNA containing exosomes released from an HCV-infected hepatocarcinoma cell line have been shown to activate plasmacytoid dendritic cells (pDCs) to release interferon (INF-α) (Dreux, Garaigorta et al. 2012). In the same study, two sphingomylinase inhibitors, GW4869 and spiroepoxide, which inhibit exosome release, drastically reduced the levels of INF-α secreted by the co-cultured pDCs. This study demonstrates the potential of altered host cell exosomes to increase the immune system targeting of virally infected cells to the benefit of the host. In contrast to this beneficial role for host immune surveillance, another study showed that exosomes from HCV-infected hepatoma cells can also carry the viral RNA genome, proteins, and complete virions (Ramakrishnaiah, Thumann et al. 2013). Moreover, incubation of these exosomes from HCV-infected cells could establish fully productive infection in uninfected recipient hepatoma cells and the exosomes were also partially resistant to HCV neutralizing antibodies demonstrating a mechanism for evading the humoral immune system. The particular studies of HCV alteration of host exosomes suggest the likelihood of other related neurotropic viruses of the Flaviviridae family, such as the West Nile virus and the Japanese encephalitis virus, also manipulating host exosomes.
Implications for Therapeutic Intervention
Considering the ability of exosomes to deliver their cargo to recipient cells, exosomes can serve as vehicles for the cellular delivery of therapeutic proteins and RNA (Figure 2). At present, clinically validated approaches for such type of delivery are viral and synthetic carrier systems such as liposomes and nanoparticles. While these systems may have some advantages, they are simultaneously fraught with disadvantages: particles can be cleared from the body by antibodies and also have the potential for improper activation of immune responses (Skubis-Zegadlo, Stachurska et al. 2013) and nanoparticles can cause toxicity (Buzea, Pacheco et al. 2007; Becker, Herzberg et al. 2011). On the other hand, exosomes are derived from “self” and therefore rejection or toxicity is not expected.
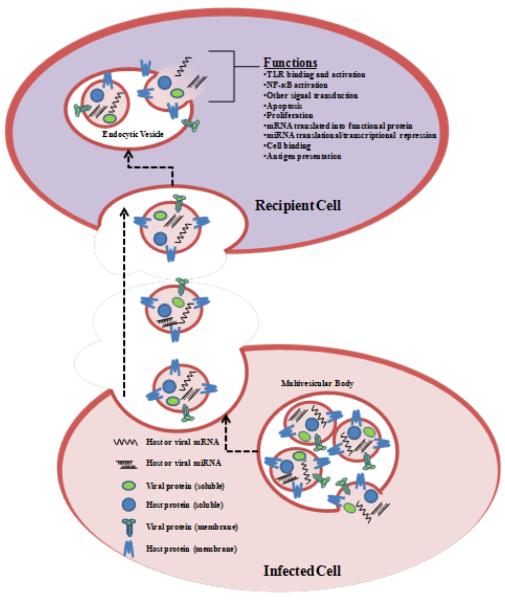
Figure 2. A hypothetical schematic view of the formation of exosomes from virally infected cells and their uptake by recipient cells.
Exosomes are formed as independent entities in the parental cell and are secreted from uninfected and/or virally infected cells. The half lives of these exosomes ranges anywhere from days to years depending on the cell type and nature of the infection. The exosomes enter recipient cells either in a receptor specific or non-specific manner. Once inside the cells, they control multiple cellular pathways including TLRs, signal transduction and transcription factors leading to control of machineries such as cell survival or apoptosis. The functional outcome of exosome activity in recipient cells is highly cell type and infection dependent.
Furthermore, since exosomal composition can be varied with cell type and environmental factors, both variables are within the control of investigators, it is possible to manipulate exosomal content in order to achieve improved therapeutic outcomes. For instance, in one study, dendritic cells (DCs) were transduced with adenoviral vector encoding IL-10, IL-4, or FasL, and exosomes obtained were used in the treatment of autoimmune disorders, and in another study, exosomes were obtained from DCs that were loaded with desired immunogens and used to treat mice with infectious diseases [reviewed in (Viaud, Thery et al. 2010)]. What remains to be elucidated is whether sufficient doses can be packaged into exosomes to achieve a therapeutic response and if purification to obtain sufficient amounts of exosomes can be performed reproducibly.
Whether the delivery vehicle of choice is a viral vector, nanoparticle, or exosome, targeted delivery to the right organ is still a work in progress. In one study involving animals, DCs were engineered to express an exosomal membrane protein, Lamp2b, fused with a neuron specific rabies viral glycoprotein (RVG) peptide (Alvarez-Erviti, Seow et al. 2011). The purified exosomes from these engineered DCs were then loaded with siRNAs using electroporation and intravenously delivered into mice. The administered exosomes demonstrated specific uptake by the cells of the CNS and functional activity of the loaded siRNAs was demonstrated by down-regulation of the target genes. This study is sufficient basis for pre-clinical studies for neuronal disorders with RNA enclosed exosomes. After all, exosomes have already been tested for cancer and demonstrated adequate safety profiles in Phase I clinical trials (Escudier, Dorval et al. 2005; Morse, Garst et al. 2005).
The blood-brain barrier is a formidable challenge in delivering therapeutics to the brain. The optimization of exosomes as delivery vehicles to carry drugs to the areas of the brain that are challenging to reach is a step in the right direction. Neurons challenged with stressful growth conditions were protected when treated with oligodendroglial exosomes that were stereotactically injected into the cerebellum and hippocampus of adult mice (Fruhbeis, Frohlich et al. 2013). It remains to be seen if such a scenario of protein transfer can be replicated with exosomes injected systemically. Exosomes from DCs have been shown to be successful delivery vehicles for systemic and targeted siRNA delivery to the brain [reviewed in (El-Andaloussi, 2013)]. The major challenge of using DCs to produce exosomes is that the primary culture does not reproducibly generate a large amount of exosomes for clinical practice.
In addition to packaging siRNAs into exosomes, the antioxidant therapeutic curcumin has also been enclosed within exosomes as a cellular deliver system (Sun, Zhuang et al. 2010). In this study, exosomes from the EL-4 murine lymphoma cell line were incubated with curcumin and then purified using a sucrose gradient. When injected intraperitoneally into mice, the curcumin loaded exosomes showed greater bioavailability and half-life as compared to a direct injection of an equal concentration of PBS-dissolved curcumin. The exosomal curcumin also demonstrated higher antioxidant effects both in vitro and in vivo by decreasing IL-6 and TNF levels. Furthermore, it also greatly increased survival rates in a lipopolysaccharide (LPS)-induced septic shock mouse model. In a separate study, exosomes encapsulating either curcumin or a signal transducer and activator of transcription 3 (Stat3) inhibitor (JSI124) were delivered intranasally to microglia cells (Zhuang, Xiang et al. 2011). This route of administration led to rapid delivery of exosome encapsulated drug(s) to the brain that was selectively taken up by microglial cells, and subsequently induced apoptosis of the microglial cells. This demonstrated that such a strategy may provide a noninvasive and novel therapeutic approach for treating inflammatory related brain diseases. Unlike the previous study with DCs, there was no engineering of the membrane proteins to target specific tissues; therefore, non-specific binding to bystander tissues would need to be examined. Nevertheless, the work does demonstrate that exosomes can be used as a delivery system for small molecule therapeutics.
Conclusions
Exosomes are small membrane bound vesicles that can transport molecules from their cellular site of production to naïve bystander cells in the microenvironment or at distant sites in the body via the circulatory/nervous system. Virally infected cells alter the host exosomal composition not only by altering the host protein/lipid/RNA content but also by incorporating their own protein or RNA into the secreted exosomes. The enclosed protein/RNA retains their functionality in target cells that can therefore alter the phenotypic characteristics of the recipient cells. Currently exosomal content is being altered by investigators with the aim of using exosomes as a delivery mechanism to transport therapeutic agents across the blood-brain barrier.
Acknowledgments
We would like to thank the members of the Kashanchi lab for assistance with the manuscript. This work was supported by NIH grant AI070740 to FK and the Geneva Foundation grant W81XWH-11-0126 to RMH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Geneva Foundation.
References
- Agrawal L, Louboutin JP, et al. HIV-1 Tat neurotoxicity: a model of acute and chronic exposure, and neuroprotection by gene delivery of antioxidant enzymes. Neurobiol Dis. 2012;45(2):657–670. doi: 10.1016/j.nbd.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Ali SA, Huang MB, et al. Genetic characterization of HIV type 1 Nef-induced vesicle secretion. AIDS Res Hum Retroviruses. 2010;26(2):173–192. doi: 10.1089/aid.2009.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- Beatty WL. Late endocytic multivesicular bodies intersect the chlamydial inclusion in the absence of CD63. Infect Immun. 2008;76(7):2872–2881. doi: 10.1128/IAI.00129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker H, Herzberg F, et al. The carcinogenic potential of nanomaterials, their release from products and options for regulating them. Int J Hyg Environ Health. 2011;214(3):231–238. doi: 10.1016/j.ijheh.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Buzea C, Pacheco II, et al. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2(4):MR17–71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- Campbell PE, Isayev O, et al. Validation of a novel secretion modification region (SMR) of HIV-1 Nef using cohort sequence analysis and molecular modeling. J Mol Model. 2012;18(10):4603–4613. doi: 10.1007/s00894-012-1452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Moita C, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013 doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- Dreux M, Garaigorta U, et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12(4):558–570. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudier B, Dorval T, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3(1):10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure J, Lachenal G, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31(4):642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Fruhbeis C, Frohlich D, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11(7):e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendreau KL, Hall GF. Tangles, Toxicity, and Tau Secretion in AD - New Approaches to a Vexing Problem. Front Neurol. 2013;4:160. doi: 10.3389/fneur.2013.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne WM, Buchkovich NJ, et al. The ESCRT pathway. Dev Cell. 2011;21(1):77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Hooper C, Fuertes R. Sainz, et al. Wnt3a induces exosome secretion from primary cultured rat microglia. BMC Neurosci. 2012;13:144. doi: 10.1186/1471-2202-13-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Yao H, et al. Exosome-mediated shuttling of microRNA-29 regulates HIV Tat and morphine-mediated neuronal dysfunction. Cell Death Dis. 2012;3:e381. doi: 10.1038/cddis.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Longnecker R. Cholesterol is critical for Epstein-Barr virus latent membrane protein 2A trafficking and protein stability. Virology. 2007;360(2):461–468. doi: 10.1016/j.virol.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone RM, Mathew A, et al. Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins. J Cell Physiol. 1991;147(1):27–36. doi: 10.1002/jcp.1041470105. [DOI] [PubMed] [Google Scholar]
- Kadiu I, Narayanasamy P, et al. Biochemical and biologic characterization of exosomes and microvesicles as facilitators of HIV-1 infection in macrophages. J Immunol. 2012;189(2):744–754. doi: 10.4049/jimmunol.1102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keryer-Bibens C, Durieu C. Pioche, et al. Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9. BMC Cancer. 2006;6:283. doi: 10.1186/1471-2407-6-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klibi J, Niki T, et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood. 2009;113(9):1957–1966. doi: 10.1182/blood-2008-02-142596. [DOI] [PubMed] [Google Scholar]
- Korkut C, Ataman B, et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139(2):393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Albers EM, Bretz N, et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Clin Appl. 2007;1(11):1446–1461. doi: 10.1002/prca.200700522. [DOI] [PubMed] [Google Scholar]
- Kulshreshtha A, Ahmad T, et al. Proinflammatory role of epithelial cell-derived exosomes in allergic airway inflammation. J Allergy Clin Immunol. 2013;131(4):1194–1203. 1203, e1191–1114. doi: 10.1016/j.jaci.2012.12.1565. [DOI] [PubMed] [Google Scholar]
- Laulagnier K, Motta C, et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J. 2004;380(Pt 1):161–171. doi: 10.1042/BJ20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani KS, Mani AJ, et al. A spastic paraplegic syndrome in South India. J Neurol Sci. 1969;9(1):179–199. doi: 10.1016/0022-510x(69)90067-7. [DOI] [PubMed] [Google Scholar]
- Meckes DG, Jr., Gunawardena HP, et al. Modulation of B-cell exosome proteins by gamma herpesvirus infection. Proc Natl Acad Sci U S A. 2013;110(31):E2925–2933. doi: 10.1073/pnas.1303906110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckes DG, Jr., Shair KH, et al. Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci U S A. 2010;107(47):20370–20375. doi: 10.1073/pnas.1014194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse MA, Garst J, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3(1):9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan A, Iordanskiy S, et al. Exosomes Derived from HIV-1-infected Cells Contain Trans-activation Response Element RNA. J Biol Chem. 2013;288(27):20014–20033. doi: 10.1074/jbc.M112.438895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J, Hubinger A. M. Eis, et al. Herpes simplex virus type 1 targets the MHC class II processing pathway for immune evasion. J Immunol. 2003;171(6):3075–3083. doi: 10.4049/jimmunol.171.6.3075. [DOI] [PubMed] [Google Scholar]
- Osame M, Matsumoto M, et al. Chronic progressive myelopathy associated with elevated antibodies to human T-lymphotropic virus type I and adult T-cell leukemialike cells. Ann Neurol. 1987;21(2):117–122. doi: 10.1002/ana.410210203. [DOI] [PubMed] [Google Scholar]
- Pegtel DM, Cosmopoulos K, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107(14):6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potolicchio I, Carven GJ, et al. Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J Immunol. 2005;175(4):2237–2243. doi: 10.4049/jimmunol.175.4.2237. [DOI] [PubMed] [Google Scholar]
- Ramakrishnaiah V, Thumann C, et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci U S A. 2013;110(32):13109–13113. doi: 10.1073/pnas.1221899110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman GC, Spencer PS, et al. Tropical spastic paraparesis: HTLV-I antibodies in patients from the Seychelles. N Engl J Med. 1987;316(1):51. doi: 10.1056/NEJM198701013160114. [DOI] [PubMed] [Google Scholar]
- Shelton MN, Huang MB, et al. Secretion modification region-derived peptide disrupts HIV-1 Nef’s interaction with mortalin and blocks virus and Nef exosome release. J Virol. 2012;86(1):406–419. doi: 10.1128/JVI.05720-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubis-Zegadlo J, Stachurska A, et al. Vectrology of adeno-associated viruses (AAV) Med Wieku Rozwoj. 2013;17(3):202–206. [PubMed] [Google Scholar]
- Subra C, Laulagnier K, et al. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89(2):205–212. doi: 10.1016/j.biochi.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Sun D, Zhuang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AR, Robinson MB, et al. Regulation of heat shock protein 70 release in astrocytes: role of signaling kinases. Dev Neurobiol. 2007;67(13):1815–1829. doi: 10.1002/dneu.20559. [DOI] [PubMed] [Google Scholar]
- Temme S, Hubinger A. M. Eis, et al. The herpes simplex virus-1 encoded glycoprotein B diverts HLADR into the exosome pathway. J Immunol. 2010;184(1):236–243. doi: 10.4049/jimmunol.0902192. [DOI] [PubMed] [Google Scholar]
- Thery C, Amigorena S, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Chapter. 2006;3(Unit 3):22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- Trajkovic K, Hsu C, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- Vernant JC, Buisson GG, et al. Can HTLV-1 lead to immunological disease? Lancet. 1987;2(8555):404. doi: 10.1016/s0140-6736(87)92431-7. [DOI] [PubMed] [Google Scholar]
- Viaud S, Thery C, et al. Dendritic cell-derived exosomes for cancer immunotherapy: what’s next? Res. 2010;70(4):1281–1285. doi: 10.1158/0008-5472.CAN-09-3276. [DOI] [PubMed] [Google Scholar]
- Wang G, Dinkins M, et al. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD) J Biol Chem. 2012;287(25):21384–21395. doi: 10.1074/jbc.M112.340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber J, Clayton A. How pure are your vesicles? J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.19861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Xiang X, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19(10):1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]