Abstract
Heart failure treatment guidelines provide no recommendations regarding the intake of protein, though it has been proposed that increasing protein intake may result in clinical improvement. High protein intake mightimprove protein synthesis and cell function, and prevent deterioration in mitochondrial and left ventricular function. We assessed the effects of a high protein diet on the development of heart failure characterized by cardiac hypertrophy, impaired mitochondrial oxidative metabolism and contractile dysfunction induced by transverse aortic constriction in rats. A standard diet with 18% of energy intake from protein was compared to a high protein diet (30% of energy intake). First we evaluated the effects of protein intake on the development of heart failure during 14 weeks of aortic constriction, and found similar cardiac hypertrophy, contractile dysfunction, ventricular dilation, and decreased cardiac mitochondrial oxidative capacity with both 18% and 30% protein. We then assessed more advanced heart failure, with 22 weeks of aortic constriction. We again saw no difference in cardiac mass, left ventricular volume, mitochondrial oxidative capacity or resistance to permeability transition between the 18% and 30% protein diets. There was a modest but significant decrease in survival with heart failure with the 30% protein diet compared to 18% protein (p<0.003). In conclusion, consumption of a high protein diet did not affect cardiac mass, left ventricular volumes or ejection fraction, or myocardial mitochondrial oxidative capacity in rats with pressure overload induced heart failure, but significantly decreased survival.
Keywords: congestive heart failure, dietary protein, mitochondria, nutrition, mitochondrial permeability transition
Introduction
Heart failure is a major public health problem in the industrialized worldand despite aggressive treatment with current pharmacotherapies, prognosis for these patients remains poor. It has recently been suggested that the amount and composition of dietary macronutrientsmay affect the symptoms and outcomes in heart failurepatients (Chess & Stanley, 2008;Kalantar-Zadeh et al., 2008;Ershow & Costello, 2006). Specifically, it has been suggested that a high protein diet might be beneficial in the advanced stages of heart failure. Current heart failure treatment guidelinesprovide no recommendations regarding the intake of protein, fat and carbohydrate (Lindenfeld et al., 2010;Dickstein et al., 2008). In the advanced stages of heart failure patients frequently experience a progressive loss of muscle and fat mass, termed “cardiac cachexia”, which is a strong predictor of worsening heart failure and poor clinical outcome(Anker et al., 2003). It has been proposed that increasing protein and/or total energy intake incachexic heart failure patients may prevent or reverse cachexia, but this remains to be definitively established(Rozentryt et al., 2010;Aquilani et al., 2008).
Less is known about the impact of protein intake on thedevelopment and progression of heart failure. This is particularly relevant to heart failure due to arterial hypertension, where there is cardiac hypertrophy and a transient net protein accumulation in the heart. Healthy people subjected to resistance exercise training exhibit greater skeletal muscle hypertrophy when given dietary protein supplements (Cermak et al., 2012). While this may be beneficial for skeletal muscle, a similar response in the myocardium to high protein intake in hypertension would be detrimental, as hypertension-induced left ventricle (LV) hypertrophy is a long established predictor of new onset heart failureand poor outcomes (Levy et al., 1996). Advanced heart failure due to hypertension induced LV hypertrophy is associated with disruption of cellular processes essential to maintain normal cardiac function, specifically impairment ofmitochondrial function and decreased capacity formyocardial oxygen consumption and ATP generation(Mann & Bristow, 2005;Neubauer, 2007;Stanley et al., 2005). High protein intake could be detrimental if it accelerates net protein synthesis in the myocardium and increases the extent of cardiac hypertrophy. On the other hand, a high protein diet could maintain optimal protein synthesis and prevent deterioration in mitochondrial and LV function. Epidemiological and interventional studies suggest that a high intake of protein under conditions of stable body mass has either minor effects or no effect on cardiovascular disease risk factors (e.g. serum lipids and blood pressure) in people without heart failure (He et al., 2011). On the other hand, a recent epidemiological study in middle age women found that high protein intake was associated with a greater incidence of cardiovascular disease over a 16 year follow-up period(Lagiou et al., 2012;Lagiou et al., 2007), though this was not observed in a similar analysis in a population of men(Preis et al., 2010). The effects of high protein intake on the development and progression of heart failure have not been reported.
In the present investigation we assessed the effects of a high protein diet on the development of cardiac hypertrophy, LV chamber remodeling, contractile dysfunction and survival in response to chronic aortic pressure overload induced by constriction of the thoracic aorta in rats. We hypothesized that high protein intake (30% of energy intake from protein) would accelerate LV hypertrophy and the development of heart failure, and decrease survival compared to a normal protein intake (18% of energy intake from protein). Further, the adverse effects of high protein intake in heart failure would manifest in a lower capacity for cardiac energy transfer, a hallmark of heart failure. This latter effect would result in impaired mitochondrial function, and increased susceptibility of mitochondria to undergo permeability transition, a catastrophic event associated with loss of ATP production and cell death. Studies were performed in a well characterized rat model of heart failure caused by constriction of the transverse aorta to generate in LV hypertrophy, LV chamber expansion, and mitochondrial and contractile dysfunction (Bugger et al., 2010;Doenst et al., 2010;Garnier et al., 2003). We compared a standard diet with 18% of energy intake from protein to a high protein diet (30% of energy intake). Two series were performed: first we evaluated the effects of protein intake during the initial 14 weeks following aortic constriction, and then in a subsequent study we assessed more advanced heart failure out to 22 weeks following constriction.
Methods
Experimental design
Experiments were conducted according to the Guideline for the Care and Use of Laboratory Animals (NIH publication 85-23) and were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee. Two experimental protocols were performed, and in both protocols the dietary treatment was initiated three days after surgery. In Protocol 1 the effects of protein intake (18% or 30% of energy intake as protein) on left ventricular mass, chamber size, contractile function, and mitochondrial physiology were assessed. Protocol 1 was a 2 by 2 design comparing sham or aortic constriction surgery and either a normal protein or high protein diet. Dietary treatment was initiated three days following sham or aortic constriction surgery to induced heart failure, and continued for 14 weeks. At the time of assignment to diet the group sizes were 15 and 20 for the sham and heart failure groups, respectively, for animals receiving18% of energy intake as protein, and 14 and 20 for the sham and heart failure animals with 30% protein.
In Protocol 2 we assessed the effects of more prolonged dietary treatment (22 weeks) to allow for development of more advanced failure. Protocol 2 had a single sham group that was fedthe 18% protein diet, and two groups with heart failure fed either 18% or 30% protein. At the time of assignment to diet the group sizes were 16 for the sham group, and 32 and 20 for heart failure animals fed 18% or 30% protein, respectively.
Transverse Aortic Constriction Surgery
Heart failure was induced by constricting the transverse aorta with a surgically implanted tantalum clip. Male Sprague-Dawley rats (7-8 weeks old, 70-100 g, Harlan, Indianapolis, IN) were anesthetized with isoflurane (5%), intubated, and mechanically ventilated with 1.5-2.5% isoflurane in oxygen to effect. A partial median sternotomy was performed and the thymus was resected. After dissection of the aortic arch, a tantalum clip (0.50 mm internal diameter hemostasis clip) was placed on the aorta between the brachiocephalic trunk and the left common carotid artery as previously described in detail(Zaha et al., 2003). Age-matched Sham-operated animals underwent the same procedure without clip application.
Diets
The two diets were custom manufactured using purified ingredients (Research Diets, New Brunswick, NJ, USA), and they both contained 14% of energy from fat (lard and soybean oil), and were free of sugar (Table 1). The standard normal protein diet contained 18% of total energy from protein (casein + L-cystine) and 68% from carbohydrate as ‘maltodextrin and corn starch. The high protein diet had 30% of total energy from protein and 56% from carbohydrate. Diets were matched for the content of vitamins, minerals and cellulose (Table 1).
Table 1. Composition of the diets.
| Ingredient (g per kg of diet) |
Standard Diet
(18% of energy from Protein) |
High Protein Diet
(30% of energy from Protein) |
|---|---|---|
| Casein | 174 | 291 |
| L-Cystine | 2.6 | 4.2 |
| Corn Starch | 538 | 429 |
| Maltodextrin 10 | 121 | 121 |
| Cellulose | 48 | 48 |
| Lard | 51 | 51 |
| Soybean Oil | 10 | 10 |
| Minerals and Vitamins | 55 | 55 |
Echocardiography
Left ventricular function was assessed by echocardiography with a high-resolution small animal imaging systems (VisualSonics Inc., Toronto, Canada). Animals were anesthetized (1.5% isoflurane by mask), the chest shaved, and images acquired with the rat in the supine position on a warming platform as previously described(Duda et al., 2009). In Protocol 1 we used a Vevo 770 system, with transducer model RMV 716, and in Protocol 2 we used a Vevo 2100 High-Resolution Imaging System with a MS250 transducer.
Tissue Harvest
After 14 weeks(Protocol 1) or 22 weeks (Protocol 2) of treatment, the animals were anaesthetized with 5.0% isoflurane between 3 and 6 h after initiation of the light phase while given free access to food. The thorax was opened and blood was collected from the left ventricle and immediately placed on ice, and centrifuged to obtain serum. The heart was removed, and sections of the left ventricle free wall were taken for biochemical analysis and stored at −80 °C, and the remainder was used for mitochondrial isolation as described below.
Mitochondrial Isolation
Cardiac mitochondria reside in two spatially distinct subpopulations: subsarcolemmal mitochondria (SSM) found in the outer region of the cell, and interfibrillar mitochondria (IFM) embeddedbetween the myofibrils(Palmer et al., 1977). Early studies found functional differences between IFM and SSM, with a greater maximal rate of respiration and resistance to stress-induced MPT in IFM than SSM (Palmer et al., 1986;Asemu et al., 2012;Palmer et al., 1977) thus it is important to assess the two populations separately. SSM and IFM were isolated according to the method of Palmer et al(Palmer et al., 1977)with minor modification(O’shea et al., 2009). Briefly, the LV was rinsed in ice cold Chappel-Perry buffer (100mM KCl, 50mM MOPS, 5mM MgSO4, 1mM ATP, 1mM EGTA, 2mg/ml BSA), blotted dry and then weighed. The ventricles were minced and homogenized in 1:10 (wt/vol) ice cold Chappel-Perry buffer. The homogenates were centrifuged at 700 × g for 10 min.The supernatant containing SSM was extracted and centrifuged again at 10,000 g to isolate SSM. The remaining pellet from the 700-g spin was resuspended in KCl-MOPS-EGTA buffer containing 100 mM KCl, 50 mM MOPS, and 0.5 mM EGTA at pH 7.4, and treated with trypsin (5 mg/g) for 10 minat 4°C. The samples were incubated with trypsin inhibitor and spun down at 700 g for 10 min.The IFM-containing supernatant was spun down at 10000 g for 10 min. The pellets were washed twice and spun down at 10000 g for 10 min and then resuspended in ice cold Chappel-Perry buffer.The concentration of mitochondrial protein was measured by the Lowry method using bovine serum albumin as standard.
Mitochondrial Respiration
Mitochondrial respiration was assessed in both IFM and SSM as described previously (O’shea et al., 2009).Isolated mitochondria (0.20 mg/mL) were respired in respiration buffer containing 100 mM KCl, 50 mM MOPS, 5 mM KH2PO4, 1 mM EGTA and 1mg/mL BSA/Fraction V. States III and IV were measured with glutamate + malate (10 and 5 mM, respectively), palmitoylcarnitine (40 μM) and succinate (20 mM) with rotenone (7.5 μM). Respiratory control ratio (RCR) was calculated as state 3/state 4, and the ADP/O ratio as the total oxygen used during state 3 divided by the amount of ADP added to the chamber.
Assessment of Mitochondrial Tolerance to Stress
The ability of SSM and IFM to tolerate either Ca2+ or reactive oxygen species (ROS)-induced MPT oxidative stress was evaluated using established assays as previously described(Papanicolaou et al., 2012). In brief, mitochondria (500 μg) were resuspended in 2.0 ml assay medium containing 100 mM KCl, 50 mM MOPS, 5 mM KH2PO4, 5 mM EGTA, 1 mM MgCl2, 5 mM glutamate, and 5 mM malate. Mitochondrial Ca2+ uptake was measured at 37°C from the fluorescence of the Ca2+ indicator calcium green-5N (CaGN-5N; Molecular Probes) with an excitation and emission of 488 and 530.Ca2+ uptake was taken from the fall in extramitochondrial Ca2+ following a bolus injection of 3 μL of 15 mM Ca2+ (30 nmoles Ca2+/mg mitochondrial protein). After stabilization (6 to 8 minutes) a continuous infusion of tert-butyl hydrogen peroxide (tBH: 400 mM) was initiated into the cuvette at a rate of 0.2 μL/min (53 nmols tBH · mg mitochondrial protein−1 · min−1), and the concentration of free Ca2+ in the medium was monitored. Extra-mitochondrial Ca2+ concentration was also monitored without infusion of tBH or Ca2+, which established that MPT did not occur without addition of tBH (data not shown).
Ca2+induced mitochondrial swelling, as inferred from the fall in absorbance of isolated mitochondria, was evaluated as previously described (Khairallah et al., 2010). Briefly, mitochondria were resuspended in buffer containing100 mM KCl, 50 mM MOPS, 5 mM KH2PO4, 5 mM EGTA, 1 mM MgCl2, 5 mM glutamate, and 5 mM malate.Using a 96 well plate reader, 50 μg of mitochondrial protein was added to 200 μL of calcium free buffer and monitored at 540 nm for 2 minutes to obtain a baseline, then either 100 or 500 nmoles Ca2+/mg mitochondrial protein was added and the absorbance monitored for 15 minutes.
Statistical analysis
Values are shown as mean ± standard error of the mean (SEM). Treatment effects were evaluated using a 2-way ANOVA comparing surgery and diet for Protocol 1 and a 1-way ANOVA for protocol 2, with the Bonferroni’s post hoc test to assess differences among groups. Survival between heart failure groups was compared using the Kaplan-Meier method. The assessment of mitochondrial Ca2+-induced swelling and Ca2+ uptake and tBH-induced Ca2+release were assessed with a 2-way ANOVA for repeated measures. A P value of less than 0.05 was considered significant.
Results
Protocol 1 - 14 weeks of Treatment
Neither diet or aortic banding significantly affected survival with the standard diet at 14 weeks, with 100% (15/15) and 79% (11/14) survival in sham rats fed the 18% and 30% protein diets, respectively, and 95% (19/20) and 85% (17/20) survival in the heart failure rats fed the 18% and 30% protein diets, respectively. Heart failure animals had a lower body mass than their respective sham when fed 30% proteins, but not with 18% protein. Liver mass and tibia length were similar among all groups (Table 2). The heart failure groups had cardiac hypertrophy, as seen in a significant increase in the mass of the atria and the left and right ventricles compared to sham animals, with no effect of diet (Table 2, Figure 1). Retroperitoneal and epididymal fat pad masses and kidney were lower in heart failure animals in both diet groups compared to sham animals (Table 2).The reduction in kidney mass suggests the possibility of renal dysfunction however assessment of serum creatinine concentration found no effect of diet or heart failure (Table 2).
Table 2. Body and tissue masses, and echocardiographic data at 14 weeks in Protocol 1.
| 18% Protein Diet | 30% Protein Diet | |||
|---|---|---|---|---|
|
| ||||
| Sham (n=15) |
Heart Failure (n=19) |
Sham (n=11) |
Heart Failure (n=17) |
|
| Final body mass (g) | 408 ± 8 | 390 ± 7 | 419 ± 9 | 375 ± 7* |
| Tibia Length (mm) | 39.6±0.2 | 39.4±0.2 | 39.4±0.3 | 39.1±0.2 |
| Atria mass (mg) | 57 ± 5 | 213 ± 13* | 60 ± 6 | 197 ± 18* |
| RV mass (mg) | 198 ± 6 | 413± 11* | 192 ± 6 | 402 ± 17* |
| LV mass (mg) | 876 ± 18 | 1282 ± 33* | 926 ± 24 | 1294 ± 44* |
| Retroperitoneal fat pad mass (g) | 3.63 ± 0.31 | 2.15 ± 0.16* | 3.88 ± 0.21 | 2.12 ± 0.11* |
| Epididymal fat pad mass (g) | 4.48 ± 0.28 | 3.35 ± 0.16* | 4.47 ± 0.25 | 2.97 ± 0.13* |
| Liver mass (g) | 12.0 ± 0.2 | 12.7 ± 0.3 | 12.6 ± 0.4 | 12.1 ± 0.3 |
| Kidney mass (g) | 2.53 ± 0.06 | 1.94 ± 0.04* | 2.68 ± 0.06 | 2.10 ± 0.05 * |
| Serum Creatinine (mg/dl) | 1.40 ± 0.08 | 1.37 ± 0.10 | 1.30 ± 0.08 | 1.34 ± 0.06 |
| Echocardiography: | ||||
| Heart rate (bpm) | 359 ± 7 | 342 ± 5 | 349 ± 12 | 337 ± 5 |
| End Diastolic volume (mL) | 0.50 ± 0.03 | 0.64 ± 0.05* | 0.45 ± 0.04 | 0.64 ± 0.06* |
| End Systolic volume (mL) | 0.09 ± 0.010 | 0.22 ± 0.02* | 0.09 ± 0.01 | 0.22 ± 0.03* |
p<0.05 compared to sham group within the same diet.There were no significant differences between the two diet groups.
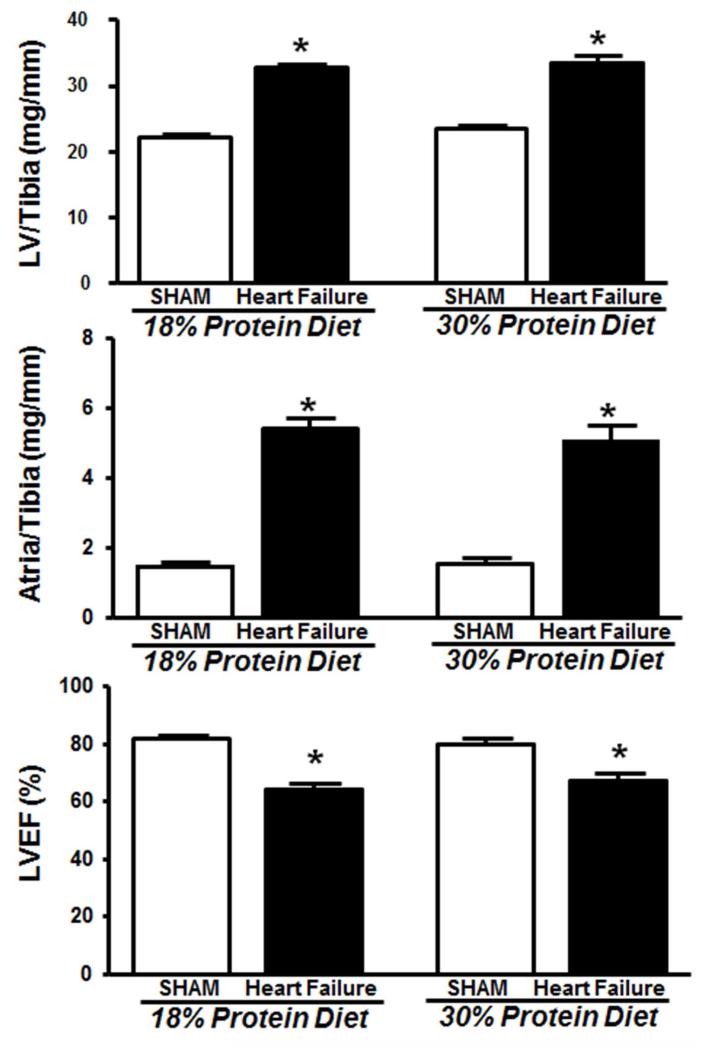
Figure 1.
Left ventricle and atria masses and LV ejection fraction (LVEF) after 14 weeks of treatment in Protocol 1. *P < 0.05 compared with respective sham. The group sizes were 15 and 19 for sham and heart failure, respectively, with 18% protein, and 11 and 16 for sham and heart failure, respectively, with 30% protein.
Cardiac Function
The heart failure groups both had increased left ventricle end systolic and end diastolic volumes and decreased ejection fraction compared to sham groups, with no differences between 18% and 30% protein intake (Table 2, Figure 1).
Mitochondrial Enzymes and Function
The expected decrease in mitochondrial oxidative capacity was observed with the 18% protein diet, as seen in the decrease in a lower yield of both SSM and IFM from the myocardium, and significant reductions in the activities of the citric acid cycle enzymes citrate synthase and aconitase, and the fatty acid β-oxidation enzyme medium-chain acyl-CoA dehydrogenase (Table 3). Increasing protein intake did not affect the heart failure-induced decline in mitochondrial oxidative capacity, as a similar decline in these parameters was seen in with the heart protein diet. We also measured the activity of these enzymes in isolated mitochondria, and observed minimal effect, finding only lower activity of medium-chain acyl-CoA dehydrogenase in the SSM subpopulation with the 18% protein diet (Table 3). The respiration of isolated SSM and IFM was generally not affected by heart failure or protein intake as assessed with a wide range of substrates, with the exception being a 23% decrease in state 3 in the SSM subpopulation with palmitoylcarnitine as substrate in animals fed 30% protein (Table 3). Taken together, these findings suggests that 1) heart failure decreased myocardial oxidative capacity by decreasing the mass of mitochondria, with minimal effect on the function of mitochondria isolated from the myocardium, and 2) these parameters were only minimally effected by high protein intake.
Table 3. Mitochondrial enzyme activities, yield and respiratory function at 14 weeks in Protocol 1.
| 18% Protein Diet | 30% Protein Diet | |||
|---|---|---|---|---|
|
Sham (n=14) |
Heart Failure (n=17) |
Sham (n=9) |
Heart Failure (n=14) |
|
| Mitochondrial Enzyme Activities in Whole Tissue | ||||
| Citrate synthase activity (μmols·g wet−1·min−1) | 160.9 ± 6.4 | 115.5 ± 6.7* | 144.2 ± 7.4 | 94.2 ± 8.7* |
| MCAD activity (μmols·g wet−1·min−1) | 12.6 ± 0.4 | 9.3 ± 0.6* | 11.4 ± 0.9 | 7.7 ± 0.6* |
| Aconitase activity (mmols·g wet−1·min−1) | 15.4 ± 0.8 | 10.2 ± 0.8* | 15.9 ± 0.6 | 10.9 ± 1.3* |
| Subsarcolemmal mitochondria | ||||
| Yield (mg mitochondrial protein/g wet mass) | 16.5 ± 0.6 | 14.1 ± 0.5* | 16.4 ± 0.4 | 14.3 ± 0.3* |
| Citrate synthase activity (μmols·mg prot.−1·min−1) | 1.9 ± 0.08 | 1.7 ± 0.05 | 1.9 ± 0.12 | 1.8 ± 0.08 |
| MCAD activity (μmols·mg prot.−1·min−1) | 0.15 ± 0.010 | 0.12 ± 0.010* | 0.15 ± 0.01 | 0.14 ± 0.010 |
| Aconitase activity (μmols·mg prot.−1·min−1) | 38.8 ± 5.2 | 42.2 ± 3.5 | 53.4 ± 6.1 | 46.5 ± 4.8 |
| Respiration: | ||||
| Glutamate + malate: State 3 | 197.4 ± 13 | 193.4 ± 10.9 | 207.4 ± 13.4 | 167.5 ± 9.2 |
| Glutamate + malate: State 4 | 29.9 ± 1.7 | 31.2 ± 2.1 | 28.9 ± 2 | 28.6 ± 1.5 |
| Glutamate + malate: RCR | 6.1 ± 0.3 | 6.4 ± 0.3 | 6.7 ± 0.4 | 6.0 ± 0.4 |
| Glutamate + malate: ADP:O | 2.2 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.3 ± 0.06 |
| Palmitoylcarnitine: State 3 | 198 ± 12 | 172 ± 8.9 | 197 ± 9.1 | 152 ± 11* |
| Palmitoylcarnitine: State 4 | 49.3 ± 3.7 | 52.7 ± 2.9 | 54.1 ± 3.2 | 46.5 ± 3.5 |
| Palmitoylcarnitine: RCR | 3.9 ± 0.3 | 3.5 ± 0.1 | 3.6 ± 0.2 | 3.4 ± 0.1 |
| Palmitoylcarnitine: ADP:O | 2.1 ± 0.1 | 2.2 ± 0.0 | 2.2 ± 0.1 | 2.3 ± 0.1 |
| Rotenone + Succinate: State 3 | 289.7 ± 15.4 | 267.3 ± 14.6 | 289.3 ± 17.4 | 299.3 ± 0.5 |
| Rotenone + Succinate: State 4 | 114.4 ± 4 | 102.9 ± 6.4 | 108 ± 7.9 | 105 ± 5 |
| Rotenone + Succinate: RCR | 2.50 ± 0.08 | 2.64 ± 0.11 | 2.69 ± 0.09 | 2.65 ± 0.08 |
| Rotenone + Succinate: P:O | 1.3 ± 0.0 | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 |
| Interfibrillar mitochondria | ||||
| Yield (mg mitochondrial protein/g wet mass) | 13.0 ± 0.7 | 10.4 ± 0.4* | 14.5 ± 0.8 | 9.5 ± 0.5* |
| Citrate synthase activity (μmols·mg prot.−1·min−1) | 2.6 ± 0.1 | 2.7 ± 0.2 | 2.7 ± 0.1 | 2.4 ± 0.1 |
| MCAD activity (μmols·mg prot.−1·min−1) | 0.18 ± 0.01 | 0.14 ± 0.01 | 0.18 ± 0.01 | 0.15 ± 0.01 |
| Aconitase activity (μmols·mg prot.−1·min−1) | 49.9 ± 5.5 | 62.7 ± 3.9 | 68.7 ± 6.2 | 52.8 ± 3.5 |
| Respiration: | ||||
| Glutamate + malate: State 3 | 204.6 ± 14 | 205 ± 11 | 207.4 ± 13.5 | 194 ± 12.6 |
| Glutamate + malate: State 4 | 35.9 ± 3.1 | 43.2 ± 2.4 | 37.6 ± 2.1 | 40.1 ± 2.7 |
| Glutamate + malate: RCR | 5.9 ± 0.3 | 4.88 ± 0.3 | 5.6 ± 0.4 | 4.99 ± 0.3 |
| Glutamate + malate: ADP:O | 2.7 ± 0.2 | 2.5 ± 0.1 | 2.5 ± 0.1 | 2.6 ± 0.1 |
| Palmitoylcarnitine: State 3 | 240.6 ± 5.4 | 231.1 ± 8.8 | 250.4 ± 20.4 | 194.5 ± 17.1 |
| Palmitoylcarnitine: State 4 | 70.8 ± 6.3 | 78.7 ± 4.3 | 71.3 ± 6.6 | 68 ± 7.4 |
| Palmitoylcarnitine: RCR | 3.4 ± 0.3 | 3 ± 0.1 | 3.3 ± 0.1 | 3 ± 0.2 |
| Palmitoylcarnitine: ADP:O | 2.2 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.4 ± 0.1 |
| Rotenone + Succinate: State 3 | 393.8±14.5 | 425.5 ± 19 | 415.5 ± 21.2 | 410 ± 17 |
| Rotenone + Succinate: State 4 | 152.8 ± 5 | 154.4 ± 6.4 | 159.3 ± 11.9 | 155.2 ± 7.4 |
| Rotenone + Succinate: RCR | 2.6 ± 0.07 | 2.76 ± 0.08 | 2.74 ± 0.13 | 2.65 ± 0.06 |
| Rotenone + Succinate: ADP:O | 1.4 ± 0.1 | 1.3 ± 0.0 | 1.4 ± 0.1 | 1.3 ± 0.1 |
p<0.05 compared to sham group within the same diet. There were no significant differences between the two diet groups
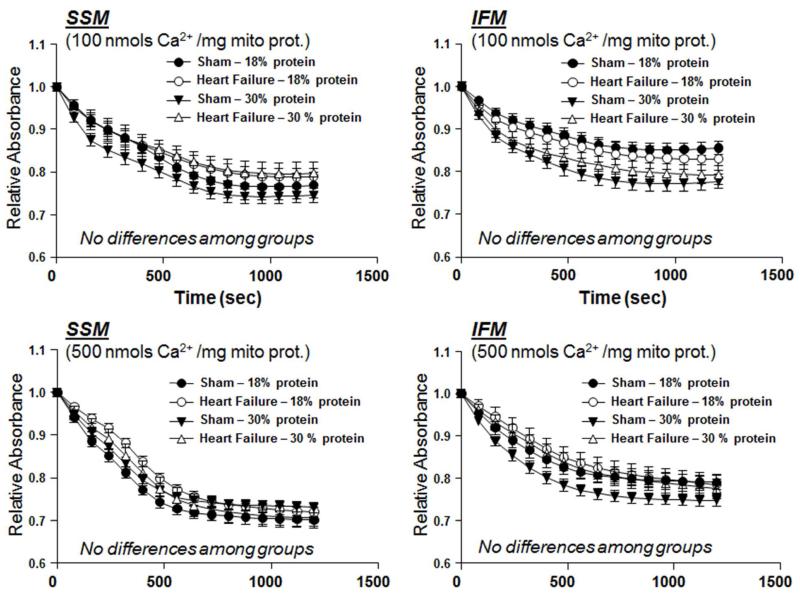
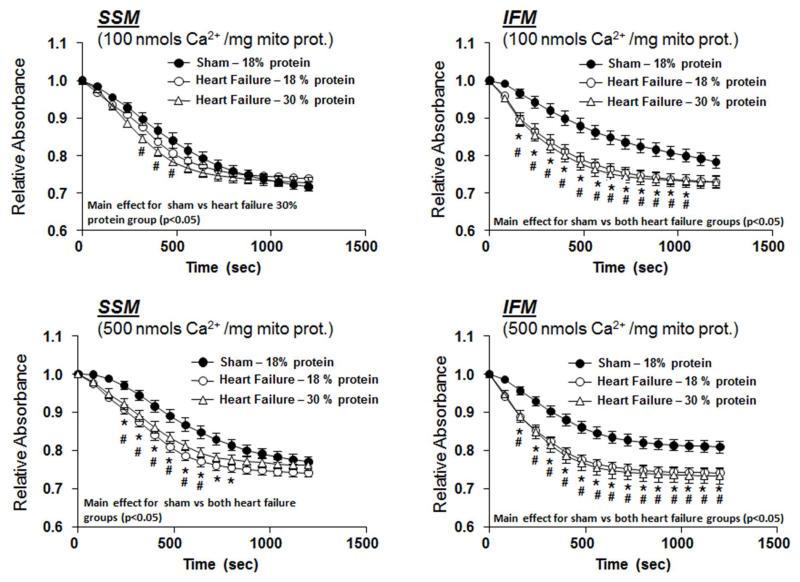
Ca2+-induced Mitochondrial Swelling
The decrease in absorbance at 540 nm following the addition of Ca2+ to isolated mitochondria, an index of mitochondrial swelling, was used as a measure of MPT. Two different Ca2+ concentrations were used to assess mitochondrial tolerance toCa2+, and both showed no difference among groups (Figure 2).
Figure 2.
Effect of diet on Ca2+ induced swelling of isolated mitochondria as assessed from the change in absorbance at 540nm, after 14 weeks of treatment in Protocol 1. There were no significant differences among groups. The group sizes were 15 and 19 for sham and heart failure, respectively, with the 18% protein diet, and 11 and 16 for sham and heart failure, respectively, with 30% protein.
Protocol 2 - 22 weeks of Treatment
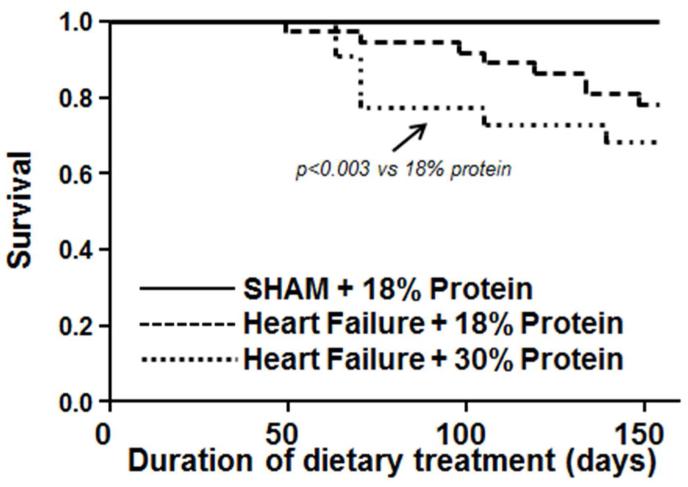
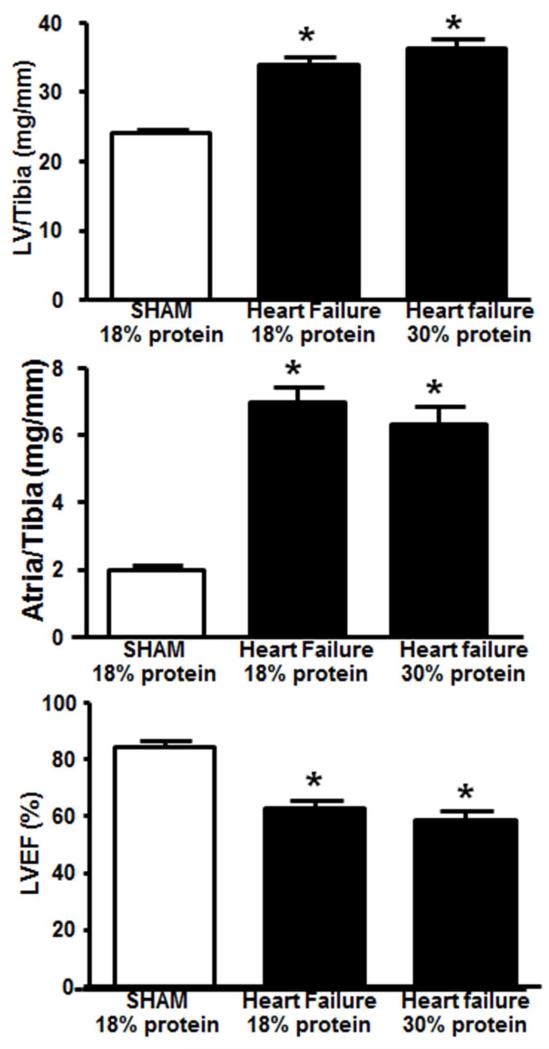
At 22 weeks of treatment there was 100% survival of the sham rats fed the 18% protein diet (Figure 3). Comparison of the two heart failure groups revealed a significantly lower survival in the 30% protein group compared to the 18% protein heart failure groups (Figure 3). There were no significant differences body mass (Table 4). Compare to the sham group there was clear cardiac hypertrophy, as seen by a significant increase in atria, left and right ventricle masses, with no difference between the 18% and 30% protein diet groups (Table 4, Figure 4). Retroperitoneal and epididymal fat pad masses were lower in heart failurecompared to the sham group, with no difference between the 18% and 30% protein diets (Table 4). Kidney mass was lower in the heart failure groups compared to sham, with no effect of dietary protein intake, and serum creatinine was not different among groups, suggesting no gross impairment in renal function.
Figure 3.
Survival plotted as a function of duration of dietary treatment for Protocol 2. Rats initiated dietary treatment 3 days after surgery. The initial group sizes were 16 for the sham group, and 32 and 15 heart failure groups with 18% and 30% protein, respectively.
Table 4. Body and tissue masses, and left ventricular volumes and function in Protocol 2 taken at 22 weeks following surgery.
| 18% Protein Diet | 30% Protein Diet | ||
|---|---|---|---|
|
Sham (n=16) |
Heart Failure (n=24) |
Heart Failure (n=13) |
|
| Final body mass (g) | 450 ± 10 | 424 ± 11 | 423 ± 9 |
| Atria mass (mg) | 81.9 ± 4.3 | 294 ± 17* | 256 ± 23.4* |
| RV mass (mg) | 210 ± 6.3 | 396 ± 22.1* | 424 ± 27* |
| LV mass (mg) | 984 ± 22 | 1411 ± 46* | 1477 ± 52* |
| Retroperitoneal fat pad mass (g) | 5.08 ± 0.26 | 3.41 ± 0.32* | 2.81 ± 0.34* |
| Epididymal fat pad mass (g) | 5.44 ± 0.39 | 4.30 ± 0.22* | 3.88 ± 0.30* |
| Liver mass (g) | 12.63 ± 0.30 | 12.72 ± 0.48 | 13.32 ± 0.38 |
| Kidney mass (g) | 2.74 ± 0.05 | 2.16 ± 0.07* | 2.33 ± 0.06* |
| Serum Creatinine (mg/dL) | 1.76 ± 0.20 | 1.24 ± 0.10 | 1.21 ± 0.20 |
| Echocardiography: | |||
| Heart rate (bpm) | 366 ± 5 | 330 ± 6* | 327 ± 7* |
| End Diastolic volume (mL) | 0.29 ± 0.03 | 0.40 ± 0.04 | 0.44 ± 0.06 |
| End Systolic volume (mL) | 0.05 ± 0.01 | 0.15 ± 0.02* | 0.18 ± 0.03* |
p<0.05 compared to the sham group. There were no significant differences between the two heart failure
Figure 4.
Left ventricle and atria mass, and LV ejection fraction (LVEF) after 22 weeks of treatment in Protocol 2. *P < 0.05 compared to sham. There were no significant differences between the two heart failure groups. The group sizes were 16 for the sham group, and 24 and 13 heart failure groups with 18% and 30% protein, respectively.
Cardiac Function
Heart failure decreased heart rate, increased left ventricle end systolic diameter, and decrease ejection fraction at 22 weeks post-surgery, with no effect of dietary protein intake (Table 4, Figure 4).
Mitochondrial enzymes and function
At 22 weeks, citrate synthase and medium-chain acyl-CoA dehydrogenase (MCAD) activity were reduced in failing myocardium, with no effect of diet (see online Supplemental Table). Aconitase activity was reduced in heart failure animals fed the 30% protein diet compared to the sham group, while the heart failure group on the 18% protein diet were not different from either sham or the 30% protein heart failure group (see online Supplemental Table). Mitochondrial yield was significantly decreased in both heart failure groups in IFM, and trended lower in SSM, with no effect of protein intake. Mitochondrial oxidative capacity with lipid substrate, as reflected in state 3 respiration with palmitoylcarnitine, was significantly decrease by heart failure in both IFM (25%) and SSM (20%), with no differences between the standard and high protein diets(see online Supplemental Table). Further, there were no differences in state 4 respiration, which in IFM resulted in a significant decrease in the respiratory control ratio (RCR; state 3/state4) (see online Supplemental Table).
Ca2+-induced Mitochondrial Swelling
At 22 weeks mitochondrial from heart failure animals displayed a greater decrease in absorbance than sham animals following addition of Ca2+, with no difference between 18% and 30% protein intake (Figure 5). This suggests that heart failure induces a greater susceptibility to MPT, which is unaffected by a high protein diet.
Figure 5.
Effect of diet on mitochondrial Ca2+ induced swelling, as assessed from the relative change in absorbance at 540nm, after 22 weeks of treatment in Protocol 2. * denotes p<0.05 for heart failure with 18% protein compared to sham, and # p<0.05 for heart failure with 30% protein compared to sham. The group sizes were 16 for the sham group, and 21 and 13 heart failure groups with 18% and 30% protein, respectively.
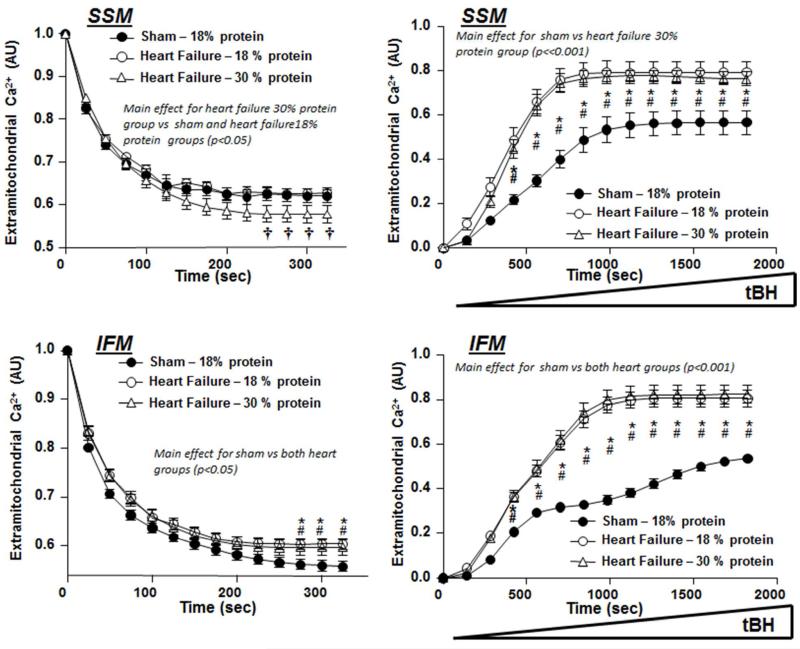
Mitochondrial Ca2+uptake and ROS-Induced MPT
ROS-induce MPT was assessed by loading isolated mitochondria with Ca2+, and then inducing MPT by exposing the mitochondria to progressively high concentration of tBH and assessing the rise in extra-mitochondrial Ca2+ concentration (Papanicolaou et al., 2012). At 22 weeks, there was no difference in tert-butyl-induced pore opening between 18 % and 30 % protein intake. However, SSM from heart failure fed 30 % protein presented small increase in extra-mitochondrial calcium uptake (Figure 6).
Figure 6.
Left Panels: Extramitochondrial Ca2+ concentration following an injection of 30 nmols Ca2+/mg mitochondrial protein at time = 0. Right Panels: Response of extramitochondrial Ca2+ concentration to a progressive rise in tert-butyl hydrogen peroxide (tBH), and index of ROS-induced MPT. Data are from Protocol 2 after 22 weeks of treatment. * denotes p<0.05 for heart failure with 18% protein compared to sham, # p<0.05 for heart failure with 30% protein compared to sham, † p,0.05 for the heart failure with 30% protein vs. both sham and 18% protein. The group sizes were 16 for the sham group, and 21 and 13 heart failure groups with 18% and 30% protein, respectively.
Discussion
High protein diets have been proposed for patients in the advanced stages of heart failure(Rozentryt et al., 2010;Aquilani et al., 2008), or as a component of a weight loss diet in obese heart failure patients (Evangelista et al., 2009), but they have not been assessed in terms of their effects on the development of cardiac hypertrophy, LV dysfunction and the progression of heart failure. Current treatment guidelines for heart failure or hypertension provide no recommendations regarding proteinintake (Lindenfeld et al., 2010;Rosendorff et al., 2007), though it has been proposed that a high protein diet could either accelerate or prevent cardiac disease in an “at risk”population (He et al., 2011;Lagiou et al., 2012;Lagiou et al., 2007;Preis et al., 2010). Thus in the present investigation we assessed the effects of a high protein diet on the development of heart failure in response to aortic pressure overload, which is characterized by cardiac hypertrophy, impaired mitochondrial oxidative metabolism and contractile dysfunction. First, we found that a high protein diet had no effect on the development of cardiac hypertrophy, contractile dysfunction and ventricular dilation, and decreased cardiac mitochondrial oxidative capacity induced by 14 weeks of aortic constriction in rats. We then assessed more prolonged heart failure with 22 weeks of aortic constriction, and again observed no difference in cardiac enlargement, contractile dysfunction, or mitochondrial abnormalities between a normal and a high protein diet. On the other hand, we observed a significant decrease in survival with heart failure with the high protein diet. Thus we provide the first evidence that a high protein diet does not prevent or accelerate development of cardiac hypertrophy or LV chamber expansion in response to pressure overload, and may decrease survival.
We postulated that high protein intake could accelerate net protein synthesis in the myocardium under conditions of pressure overload, as is observed with skeletal muscle growth in humans subjected to resistance exercise training with dietary protein supplementation(Cermak et al., 2012). In contrast, we observed no effect of high protein intake on myocardial mass in either normal sham operated rats or rats with aortic pressure overload. This lack of effect occurred despite marked myocardial hypertrophy, as evidenced by increases of 1.5-fold, 2.0-fold and 3.6-fold in the masses of the LV, right ventricle and atria, respectively. Further, the high protein diet did not affect development of LV remodeling or contractile dysfunction in response to aortic pressure overload, as seen in a similar degree of enlargement of LV end systolic and diastolic volumes and fall in ejection fraction. Lastly, the deterioration in myocardial oxidative capacity and mitochondrial function was largely unaffected by protein intake. Taken together, a high protein diet did not appear to have any major effect on the development of pathological cardiac hypertrophy and heart failure in this model.
There was a modest but significant decline in survival with the 30% protein diet compared to 18% protein over 22 weeks of treatment. One should use caution in interpreting this result, and one would optimally perform this assessment with a greater sample size and more prolonged treatment to obtain near 100% mortality, as in our recent studies with other dietary interventions in heart failure (Galvao et al., 2012;Hecker et al., 2012;Galvao et al., 2013). While we observed a significant decrease in survival, at the point the study was terminated the difference between the curves appeared to be narrowing. Thus, future studies should considering caring out treatment until near complete mortality is achieved. Further, it may be preferable to assess mortality in a model that does not involve pressure overload, such as the rat infarct model infarct model(Pfeffer et al., 1985) or cardiomyopathic hamsters(Galvao et al., 2012;Hecker et al., 2012;Galvao et al., 2013).
Abnormalities in the function and structure of cardiac mitochondria are a hallmark of advanced heart failure, and are believed to contribute to clinical progression(Abel & Doenst, 2011;Rosca & Hoppel, 2010). The present of mitochondrial dysfunction in pressure overload induced hypertrophic heart failure is controversial, as there is evidence to suggest that in this situation mitochondrial density is matched to the increases in energy demand and LV mass (Nishio et al., 1995;Abel & Doenst, 2011). In the present investigation LV hypertrophy was associated with decreased myocardial oxidative capacity, as evidenced by a decrease in the activity of mitochondrial oxidative enzymes and extractable SSM and IFM per gram of tissue. The maximal rate of oxygen consumption (State III respiration) in SSM and IFM were unaffected by heart failure for non-lipid substrates and were modestly decreased for palmitoyl-carnitine, particularly at 22 weeks, but not altered by high protein intake. While we did not design the protocols allow statistical comparison between 14 and 22 weeks of aortic constriction, there was evidence to suggest mitochondrial function was worse at 22 weeks than at 14 weeks. Specifically, there was a greater susceptibility to Ca2+ induced permeability transition and a more pronounced decline in mitochondrial oxidative capacity with lipid substrates in the 22 week study than in the 14 week study. Despite the greater susceptibility to ROS or Ca2+-induced MPT with heart failure at 22 weeks, high protein intake had no effect. Thus the present study supports the notation that pressure overload induced heart failure impairs mitochondrial oxidative capacity in myocardium and reduces mitochondrial resistance to stress, with no effect of in high protein intake.
There are several issues that arise from the present investigation that need to be addressed. First, we assessed the effects of dietary protein content on prevention of heart failure induced by pressure overload and cardiac hypertrophy, and thus the ability of a high protein diet to treat established or end stage heart failure was not assessed. Previous work by other groups focused on the potential benefits of a high protein diet in the advanced stages of heart failure, where increasing protein and/or total energy intake in cachexic heart failure patients may prevent or reverse cachexia (Rozentryt et al., 2010;Aquilani et al., 2008). This important issue remains unresolved and is clearly worthy of additional investigation. Second, we assessed relatively short term treatment in young rats. Clinical heart failure is largely a disease in the elderly, thus it is important to assess the impact of dietary protein in animals with advanced age and for longer duration. This is particularly important for assessing the effects on survival, as the decrease in survival with the high protein diet in Protocol 2 was based on relative few deaths (Figure 3); this observation should be following up with prolonged treatment with complete mortality. Third, in the 22 week study we did not include a sham group treated with high protein diet as we did in the 14 week study, thus we do not know the effects of a more prolonged high protein diet on cardiac and mitochondrial function. Fourth, while the focus of this study was on the impact of high protein intake, it is also important to address the effect of low protein intake (<15% energy intake) in heart failure patients. This important topic has not been investigated in experimental studies, but is of great clinical relevance in light of the observation from the National Health and Nutrition Examination Surveys show that only 27% of heart failure patients in the United States met the dietary goal for protein intake of ≥18% of total energy intake, with an average intake of 15.8±0.2%(Lemon et al., 2010). Investigation of the impact of low protein intake on the pathophysiology and clinical progression of heart failure is warranted. Lastly, as most human heart failure presents after years of hypertension that is frequently combined with coronary artery disease and comorbidities such as obesity and diabetes, consideration should be given to evaluating the long term effects of dietary protein in a heart failure model of with myocardial infarction in combination with more modest hypertension and/or diabetes.
In conclusion, consumption of a high protein diet did not affect cardiac mass, left ventricular volumes or ejection fraction, or myocardial mitochondrial oxidative capacity in rats with pressure overload induced heart failure. There was a modest but significant decrease in survival with heart failure with the high protein diet compared to normal protein intake.
Supplementary Material
Acknowledgments
Funding:
This work was supported by the National Institutes of Health, Grant numbers HL074237, HL101434 and HL072751.
Footnotes
Conflict of Interest: none
Reference List
- Abel ED, Doenst T. Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovasc Res. 2011;90:234–242. doi: 10.1093/cvr/cvr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker SD, Negassa A, Coats AJ, Afzal R, Poole-Wilson PA, Cohn JN, Yusuf S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet. 2003;361:1077–1083. doi: 10.1016/S0140-6736(03)12892-9. [DOI] [PubMed] [Google Scholar]
- Aquilani R, Opasich C, Gualco A, Verri M, Testa A, Pasini E, Viglio S, Iadarola P, Pastoris O, Dossena M, Boschi F. Adequate energy-protein intake is not enough to improve nutritional and metabolic status in muscle-depleted patients with chronic heart failure. Eur J Heart Fail. 2008;10:1127–1135. doi: 10.1016/j.ejheart.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Asemu G, O’Connell KA, Cox JW, Dabkowski ER, Xu W, Ribeiro RF, Shekar KC, Hecker PA, Rastogi S, Sabbah HN, Hoppel CL, Stanley WC. Enhanced Resistance to Permeability Transition in Interfibrillar Cardiac Mitochondria in Dogs: Effects of Aging and Long Term Aldosterone Infusion. Am J Physiol Heart Circ Physiol. 2012;304:H514–H528. doi: 10.1152/ajpheart.00674.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugger H, Schwarzer M, Chen D, Schrepper A, Amorim PA, Schoepe M, Nguyen TD, Mohr FW, Khalimonchuk O, Weimer BC, Doenst T. Proteomic remodelling of mitochondrial oxidative pathways in pressure overload-induced heart failure. Cardiovasc Res. 2010;85:376–384. doi: 10.1093/cvr/cvp344. [DOI] [PubMed] [Google Scholar]
- Cermak NM, Gibala MJ, van Loon LJ. Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int J Sport Nutr Exerc Metab. 2012;22:64–71. doi: 10.1123/ijsnem.22.1.64. [DOI] [PubMed] [Google Scholar]
- Chess DJ, Stanley WC. Role of diet and fuel overabundance in the development and progression of heart failure. Cardiovasc Res. 2008;79:269–278. doi: 10.1093/cvr/cvn074. [DOI] [PubMed] [Google Scholar]
- Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K, Vahanian A, Camm J, De CR, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, Mc-regor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29:2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- Doenst T, Pytel G, Schrepper A, Amorim P, Farber G, Shingu Y, Mohr FW, Schwarzer M. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovasc Res. 2010;86:461–470. doi: 10.1093/cvr/cvp414. [DOI] [PubMed] [Google Scholar]
- Duda MK, O’shea KM, Tintinu A, Xu W, Khairallah RJ, Barrows BR, Chess DJ, Azimzadeh AM, Harris WS, Sharov VG, Sabbah HN, Stanley WC. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc Res. 2009;81:319–327. doi: 10.1093/cvr/cvn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershow AG, Costello RB. Dietary guidance in heart failure: a perspective on needs for prevention and management. Heart Fail Rev. 2006;11:7–12. doi: 10.1007/s10741-006-9187-3. [DOI] [PubMed] [Google Scholar]
- Evangelista LS, Heber D, Li Z, Bowerman S, Hamilton MA, Fonarow GC. Reduced body weight and adiposity with a high-protein diet improves functional status, lipid profiles, glycemic control, and quality of life in patients with heart failure: a feasibility study. J Cardiovasc Nurs. 2009;24:207–215. doi: 10.1097/JCN.0b013e31819846b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao TF, Brown BH, Hecker PA, O’Connell KA, O’shea KM, Sabbah HN, Rastogi S, Daneault C, des RC, Stanley WC. High intake of saturated fat, but not polyunsaturated fat, improves survival in heart failure despite persistent mitochondrial defects. Cardiovasc Res. 2012;93:24–32. doi: 10.1093/cvr/cvr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao TF, Khairallah RJ, Dabkowski ER, Brown BH, Hecker PA, O’Connell KA, O’shea KM, Sabbah HN, Rastogi S, Daneault C, des RC, Stanley WC. Marine n3 Polyunsaturated Fatty Acids Enhance Resistance to Mitochondrial Permeability Transition in Heart Failure, but Do Not Improve Survival. Am J Physiol Heart Circ Physiol. 2013;73:H12–H21. doi: 10.1152/ajpheart.00657.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol. 2003;551:491–501. doi: 10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Wofford MR, Reynolds K, Chen J, Chen CS, Myers L, Minor DL, Elmer PJ, Jones DW, Whelton PK. Effect of dietary protein supplementation on blood pressure: a randomized, controlled trial. Circulation. 2011;124:589–595. doi: 10.1161/CIRCULATIONAHA.110.009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker PA, Galvao TF, O’shea KM, Brown BH, Henderson R, Jr., Riggle H, Gupte SA, Stanley WC. High-sugar intake does not exacerbate metabolic abnormalities or cardiac dysfunction in genetic cardiomyopathy. Nutrition. 2012;28:520–526. doi: 10.1016/j.nut.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantar-Zadeh K, Anker SD, Horwich TB, Fonarow GC. Nutritional and anti-inflammatory interventions in chronic heart failure. Am J Cardiol. 2008;101:89E–103E. doi: 10.1016/j.amjcard.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khairallah RJ, Sparagna GC, Khanna N, O’shea KM, Hecker PA, Kristian T, Fiskum G, des RC, Polster BM, Stanley WC. Dietary supplementation with docosahexaenoic acid, but not eicosapentaenoic acid, dramatically alters cardiac mitochondrial phospholipid fatty acid composition and prevents permeability transition. Biochim Biophys Acta. 2010;1797:1555–1562. doi: 10.1016/j.bbabio.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagiou P, Sandin S, Lof M, Trichopoulos D, Adami HO, Weiderpass E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ. 2012;344:e4026. doi: 10.1136/bmj.e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagiou P, Sandin S, Weiderpass E, Lagiou A, Mucci L, Trichopoulos D, Adami HO. Low carbohydrate-high protein diet and mortality in a cohort of Swedish women. J Intern Med. 2007;261:366–374. doi: 10.1111/j.1365-2796.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- Lemon SC, Olendzki B, Magner R, Li W, Culver AL, Ockene I, Goldberg RJ. The dietary quality of persons with heart failure in NHANES 1999-2006. J Gen Intern Med. 2010;25:135–140. doi: 10.1007/s11606-009-1139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- Nishio ML, Ornatsky OI, Craig EE, Hood DA. Mitochondrial biogenesis during pressure overload induced cardiac hypertrophy in adult rats. Can J Physiol Pharmacol. 1995;73:630–637. doi: 10.1139/y95-080. [DOI] [PubMed] [Google Scholar]
- O’shea KM, Khairallah RJ, Sparagna GC, Xu W, Hecker PA, Robillard-Frayne I, des RC, Kristian T, Murphy RC, Fiskum G, Stanley WC. Dietary omega-3 fatty acids alter cardiac mitochondrial phospholipid composition and delay Ca2+-induced permeability transition. J Mol Cell Cardiol. 2009;47:819–827. doi: 10.1016/j.yjmcc.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- Palmer JW, Tandler B, Hoppel CL. Heterogeneous response of subsarcolemmal heart mitochondria to calcium. Am J Physiol. 1986;250:H741–H748. doi: 10.1152/ajpheart.1986.250.5.H741. [DOI] [PubMed] [Google Scholar]
- Papanicolaou KN, Ngoh GA, Dabkowski ER, O’Connell KA, Ribeiro RF, Stanley WC, Walsh K. Cardiomyocyte deletion of mitofusin-1 leads to mitochondrial fragmentation and improves tolerance to ROS-induced mitochondrial dysfunction and cell death. Am J Physiol Heart Circ Physiol. 2012;302:H167–H179. doi: 10.1152/ajpheart.00833.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer MA, Pfeffer JM, Steinberg C, Finn P. Survival after an experimental myocardial infarction: beneficial effects of long-term therapy with captopril. Circulation. 1985;72:406–412. doi: 10.1161/01.cir.72.2.406. [DOI] [PubMed] [Google Scholar]
- Preis SR, Stampfer MJ, Spiegelman D, Willett WC, Rimm EB. Dietary protein and risk of ischemic heart disease in middle-aged men. Am J Clin Nutr. 2010;92:1265–1272. doi: 10.3945/ajcn.2010.29626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosca MG, Hoppel CL. Mitochondria in heart failure. Cardiovasc Res. 2010;88:40–50. doi: 10.1093/cvr/cvq240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendorff C, Black HR, Cannon CP, Gersh BJ, Gore J, Izzo JL, Jr., Kaplan NM, O’Connor CM, O’Gara PT, Oparil S. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation. 2007;115:2761–2788. doi: 10.1161/CIRCULATIONAHA.107.183885. [DOI] [PubMed] [Google Scholar]
- Rozentryt P, von HS, Lainscak M, Nowak JU, Kalantar-Zadeh K, Polonski L, Anker SD. The effects of a high-caloric protein-rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: a randomized, double-blind pilot study. J Cachexia Sarcopenia Muscle. 2010;1:35–42. doi: 10.1007/s13539-010-0008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- Zaha V, Grohmann J, Gobel H, Geibel A, Beyersdorf F, Doenst T. Experimental model for heart failure in rats--induction and diagnosis. Thorac Cardiovasc Surg. 2003;51:211–215. doi: 10.1055/s-2003-42264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.