Abstract
The discovery of beneficial neuroprotective effects of the angiotensin converting enzyme 2–angiotensin-(1-7)–Mas axis [ACE2–Ang-(1-7)–Mas] in ischemic and hemorrhagic stroke has spurred interest in a more complete characterization of its mechanisms of action. Here, we summarize findings that describe the protective role of the ACE2–Ang-(1-7)–Mas axis in stroke, along with a focused discussion on the potential mechanisms of neuroprotective effects of Ang-(1-7) in stroke. The latter incorporates evidence describing the actions of Ang-(1-7) to counter the deleterious effects of angiotensin II (AngII) via its type 1 receptor, including anti-inflammatory, anti-oxidant, vasodilatory, and angiogenic effects, and the role of altered kinase–phosphatase signaling. Interactions of Mas with other receptors, including bradykinin receptors and AngII type 2 receptors are also considered. A more complete understanding of the mechanisms of action of Ang-(1-7) to elicit neuroprotection will serve as an essential step toward research into potential targeted therapeutics in the clinical setting.
Keywords: Angiotensin converting enzyme 2, Angiotensin-(1-7), Mas, Angiotensin type 1 receptor, Angiotensin type 2 receptor, Renin-angiotensin system, Stroke, Neuroprotection, Neuron, Microglia, Ischemia, Intracerebral hemorrhage, Inflammation
Introduction
Since the development of the first orally active angiotensin converting enzyme (ACE) inhibitor, captopril, in 1975, the renin-angiotensin system (RAS) has been a primary therapeutic target for the treatment of hypertension and related diseases. The original view of the RAS holds that conversion of angiotensin I into angiotensin II (AngII) by ACE leads to activation of the angiotensin type 1 receptor (AT1R) in multiple tissues to induce various effects that act in concert to defend and increase blood pressure, elicit electrolyte and water retention by the kidney, and increase thirst via central mechanisms. In the case of prolonged overstimulation of AT1Rs, however, AngII signaling increases inflammation, fibrosis, and cellular hypertrophy, which contribute to disease pathophysiology. High blood pressure and cerebrovascular health are inextricably linked, with hypertension as the leading modifiable risk factor for stroke. Studies in both animals and humans have shown that in addition to having efficacy in treating hypertension, ACE inhibitors and AT1R blockers are protective in stroke [1–3]. Furthermore, recent work from our group and from others has shown that signaling through the AngII type 2 receptor (AT2R) is neuroprotective in preclinical models of ischemic [4–7, 8••] and hemorrhagic stroke [9]. The classical view of the RAS has been further expanded with the discovery of an alternative pathway for angiotensin peptide metabolism and signaling in which AngII is hydrolyzed by angiotensin converting enzyme 2 (ACE2) to form angiotensin-(1-7) [Ang-(1-7)]. This heptapeptide binds to and activates a unique G-protein coupled receptor known as Mas to exert effects that are largely in direct opposition to those of AT1R activation. Here, we review evidence to date for the neuroprotective actions of Ang-(1-7) in stroke, followed by an in-depth look into specific mechanisms that may account for its protective effects.
The Angiotensin-(1-7)–Mas Axis and Stroke
As a leading cause of death and disability worldwide, stroke is an exceptionally important disease for continued translational research [10]. To date, tissue plasminogen activator, a thrombolytic drug designed to reestablish tissue perfusion, remains the only FDA-approved medical treatment for ischemic stroke [11]. Unfortunately, the vast majority of stroke patients are not eligible to receive this treatment due mainly to its narrow therapeutic time window and high risk of bleeding. Although testing of many potential treatments has been performed in a large number of animal and human trials, the discovery and successful clinical testing of other efficacious drugs has proven difficult [12]. Despite their failure to identify a clinically validated drug target, these studies have provided vastly improved insight into the pathophysiology of stroke and helped to define characteristics that may determine successful testing of future targets [13]. The ACE2–Ang-(1-7)–Mas pathway has many characteristics that lend promise to its potential as a target for treatments to successfully induce stroke neuroprotection. First, the Ang-(1-7)–Mas axis undergoes dynamic changes in stroke [14], which may allow targeted treatments to act synergistically with endogenous mechanisms. Second, its effects are robust; neuroprotection has been observed in various models of ischemic and hemorrhagic stroke from different laboratories, as detailed below. Last, it is hypothesized that drug targets with multiple therapeutic effects such as those described below for the Ang-(1-7)–Mas axis, as opposed to a single mechanism of action, will prove more likely to translate [15]. For all of these and other reasons, it has been recently suggested that the Ang-(1-7)–Mas axis in stroke represents an especially promising candidate for targeted stroke therapy [16].
Stroke and the Brain RAS
Importantly for stroke, the cells of the CNS express all of the components of the ACE2–Ang-(1-7)–Mas [17]. Until recently, it was not entirely clear whether expression levels of components of this protective axis were altered following stroke. Studies had shown that levels of AngII are increased after stroke in the ventral cortex [18] and the rostral ventrolateral medulla (RVLM) [19]. In addition, AT1R expression was shown to be decreased in the cerebral cortex following transient middle cerebral artery occlusion (MCAO) [18], but increased in the RVLM [19]. In a broader study, Lu et al. recently demonstrated that ischemic stroke resulted in increased levels of Ang-(1-7), ACE2, and Mas in samples of rat ischemic cortex in the 48 h following stroke [14]. Within the RVLM, stroke resulted in decreased Ang-(1-7) levels for up to 3 days post-stroke and an initial decrease in Mas 1 day following stroke, followed by significant increases 3 and 7 days after MCAO in rats [19]. Gene array results from this study indicated an increase in ACE2 expression 1 day following stroke. Another study using samples from human stroke patients found that serum ACE2 levels were significantly higher among cardioembolic strokes and concluded that changes in ACE2 might be useful in diagnosing stroke subtype and predicting outcome [20]. Further research in this area is needed to substantiate the changes observed in these studies and to clarify ways in which the Ang-(1-7)–Mas axis can be targeted to act in synergy with endogenous post-stroke alterations in the components of this system.
Ang–(1-7) and Stroke Neuroprotection
A growing number of studies have now demonstrated neuroprotective effects of Ang-(1-7) in both ischemic and hemorrhagic stroke, some of which have been reviewed previously [17, 21–23]. Our group first used an ischemic stroke model of endothelin-1 (ET-1)-induced MCAO and found that rats that were infused centrally via the intracerebroventricular (ICV) route with Ang-(1-7) performed better on neurological function testing and had an ~50 % reduction in infarct sizes [24••, 25], which was prevented by co-administration of the Mas antagonist A-779, findings that have subsequently been verified in models of permanent MCAO [26, 27••]. In a study using AngII-overexpressing mice subjected to permanent focal ischemic stroke, neuronal ACE2 overexpression resulted in similar cerebroprotection in vivo [28] and in vitro [29]. Additionally, lentiviral ACE2 priming of endothelial progenitor cells enhanced the ability of these cells to reduce infarct size and improve neurological function [30•]. These findings are especially relevant from a translational perspective, as systemic treatment infusions were not started until 2 h after stroke. Importantly, the protective effects of the Ang-(1-7)–Mas system in stroke are preserved and may even be enhanced in aged animals [31•].
The protective effects of this axis have also been demonstrated in models of hemorrhagic stroke, including the use of stroke-prone spontaneously hypertensive rats (spSHR) fed a high-salt diet, which models the human disease condition in that spSHRs develop chronic vascular pathology and hypertension leading to intracerebral hemorrhages [25]. Our group found that chronic central administration of Ang-(1-7) in spSHRs increased lifespan, decreased the number of hemorrhages, and improved neurological function [25]. Similar Ang-(1-7)-induced cerebroprotection has been demonstrated in a second model of collagenase-induced intracranial hemorrhage [17, 32]. In summary, there is a growing body of evidence that activation of the central Ang-(1-7)–Mas axis can exert profound protective effects in stroke. As discussed in the following sections, there are likely to be multiple mechanisms and sites of action for these beneficial effects of Ang-(1-7).
Mechanisms of Ang-(1-7)-Induced Neuroprotection
The beneficial effects of Ang-(1-7)–Mas signaling extend beyond stroke and have been demonstrated in a variety of inflammation-related disease models including arthritis, hypertensive kidney disease, atherosclerosis, asthma, and acute respiratory distress syndrome [33–37]. Similarly, the ACE2–Ang-(1-7)–Mas axis has recently been examined for its potential to be manipulated as a therapy for cardiovascular disease, where its activation has been demonstrated to have therapeutic potential for hypertension and related pathologies, myocardial infarction, heart failure, as well as several types of cancer [38–45] and other diseases [46]. The mechanisms of protection in these varied disease pathologies are likely to overlap, as many tissues, including the brain, express tissue-specific RAS components. In this section, we review the studies that have focused on the mechanisms of Ang-(1-7)-induced protection in stroke, and we supplement these data with conclusions drawn from studies in other inflammatory and related disorders to propose a multifaceted mechanistic hypothesis for the neuroprotective actions of the ACE2–Ang-(1-7)–Mas pathway in stroke (see Fig. 1).
Fig. 1.
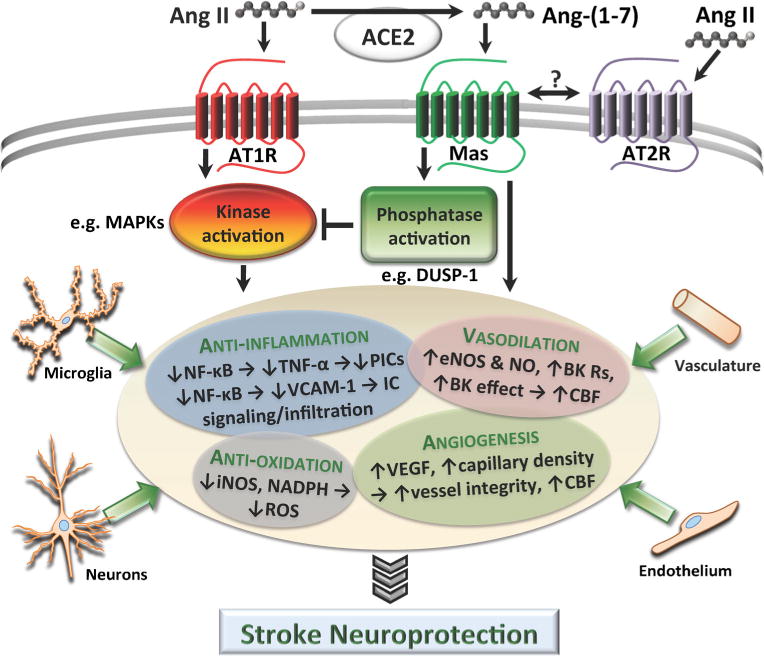
The neuroprotective effects that result from Mas activation by Ang-(1-7) are summarized here. We propose a mechanism of action in which phosphatase activation by Mas signaling leads to dephosphorylation of essential elements of the AngII–AT1R-induced kinase signaling cascade, thus inhibiting its deleterious effects. Also summarized are the beneficial actions of Mas activation that may be induced independently from AngII–AT1R signaling, with potential contributions from signaling in neurons, microglia, endothelial cells, and vasculature. Angll angiotensin II, Ang-(1-7) angiotensin-(1-7), AT1R angiotensin type 1 receptor, AT2R angiotensin type 2 receptor, MAPK mitogen-activated protein kinase, DUSP-1 dual specificity phosphatase 1, NF-κB nuclear factor kappa-light-chain-enhancer of activated B cells, TNF-α tumor necrosis factor alpha, PIC pro-inflammatory cytokine, VCAM-1 vascular cell adhesion protein 1, IC inflammatory cell, eNOS endothelial nitric oxide synthase, NO nitric oxide, BK R bradykinin receptor, CBF cerebral blood flow, iNOS inducible nitric oxide synthase, NADPH nicotinamide adenine dinucleotide phosphate-oxidase, ROS reactive oxygen species, VEGF vascular endothelial growth factor
Anti-inflammation and Anti-oxidation
Many studies, including several in stroke, have explored the specific hypothesis that Mas activation by Ang-(1-7) has antiinflammatory and anti-oxidative effects. Our group demonstrated that central administration of Ang-(1-7) during ischemic stroke attenuated the increased levels of pro-inflammatory markers within the cerebral cortex [47•]. Activation of the Ang-(1-7) axis may also decrease oxidative stress and thus limit neuronal cell death, as the levels of inducible nitric oxide synthase (iNOS), a pro-oxidant molecule that is increased in stroke, were also reduced by this peptide [48]. In an animal model of permanent cerebral ischemia, Mas activation exerted similar changes, decreasing oxidative stress and suppressing nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activity and consequent tumor necrosis factor alpha (TNF-α) signaling [27••]. NF-κB inhibition by Ang-(1-7) also occurs in other animal disease models, including hypertensive kidney disease [34], pulmonary fibrosis [49], and fatty liver disease [50]. Inhibition of nuclear translocation of NF-κB by Ang-(1-7) has also been shown to prevent the upregulation of vascular cell adhesion protein-1 by AngII [51], as well as reduced leukocyte chemotactic signaling, rolling, and adhesion [33]. These effects may be particularly relevant in the subacute period of stroke to limit excessive inflammatory cell infiltration across the compromised blood-brain barrier. In addition to reducing stroke infarct size, ACE2 overexpression in neurons of mice engineered to overproduce AngII resulted in attenuated levels of reactive oxygen species following stroke in vivo [28], in vitro [29], and in aged mice [31•], likely secondary to the observed reduction in NADPH oxidase levels. While it remains to be more clearly demonstrated, it is possible that the augmented activity of neuronal ACE2 in these mice may be exerting anti-oxidative effects indirectly through alterations in neuron-to-microglia signaling, possibly in a way that limits the activation of inflammatory microglia. In support of this possibility, we have observed in rats that administration of Ang-(1-7) in stroke resulted in attenuated cerebral cortical expression of markers of activated microglia and neuronalderived chemokines [47•], as well as decreased numbers of activated microglia [25]. In hypertension, administration of Ang-(1-7) to SHRs lowered oxidative stress, neuronal autophagy and apoptosis, levels of NADPH and iNOS, and expression of the AngII–AT1R axis while improving endogenous anti-oxidant function [26], effects that were at least partially reversible by Mas antagonism. The anti-inflammatory/anti-oxidant effects of Ang-(1-7) in stroke as well as other disease states are becoming well-established [16, 52, 53], and future work will help to clarify the specific signaling mechanisms and cell subtypes through which this peptide may be acting.
Vascular Effects and Angiogenesis
We have previously reviewed evidence which indicates that Ang-(1-7) may have a vasodilatory effect to increase regional blood flow to certain tissues, including cerebral blood flow (CBF), which might contribute to its neuroprotective efficacy [17]. This hypothesis is supported by findings from multiple groups that axis activation resulted in upregulation of endothelial NOS (eNOS) and NO production in stroke [28,29, 30•, 54, 55], as well as improved endothelial function in spSHRs [56, 57]. It was very recently demonstrated that application of Ang-(1-7) into the RVLM resulted in complete attenuation of the detrimental stroke-induced pressor response as well as preventing increased heart rate [19]. In addition to a vasodilatory role, the vasoprotection conferred to the endothelium by activation of the ACE2–Ang-(1-7)–Mas axis has been abundantly demonstrated in the context of exercise [58], aging [16], pulmonary hypertension [59, 60], atherosclerosis [61], and vascular remodeling [53], to name a few. In line with these findings, several recent lines of evidence indicate an angiogenic role for the Ang-(1-7)–Mas system in stroke. The priming of endothelial progenitor cells with ACE2 enhanced their angiogenic effect within the peri-infarct region of the mouse cerebral cortex following ischemic stroke [30•]. Infusion with Ang-(1-7) by the ICV route resulted in significantly increased brain capillary density [55], and ACE2 overexpression within neurons increased angiogenic cytokines in stroke in addition to improving levels of CBF [28]. It is clear that Ang-(1-7)-induced increases in perfusion and angiogenesis would result in great benefit in the setting of cerebral ischemia, and evidence to date points to this as one likely mechanism of protective action.
Anti-AT1R Effects via Altered Kinase–Phosphatase Signaling
Many of the effects mediated by Ang-(1-7) described above are in direct opposition to the action of AngII binding to AT1Rs. Indeed, Mas can hetero-oligomerize with AT1Rs and could thus act as a direct antagonist [62], which is feasible within the cells of the brain as both Mas and AT1Rs are expressed in the neurons [25, 63], microglia [25, 64], and endothelial cells [65]. Increases in AngII levels in the brain, as occurs during stroke [18], are associated with disease pathophysiology, including enhanced vascular contraction, endothelial damage, neuronal apoptosis and inflammation, and stimulation of reactive oxygen species (ROS), effects which are each counteracted by Ang-(1-7)–Mas signaling. We now turn our consideration to recent findings that specifically point toward a role for Ang-(1-7)–Mas in regulating kinase signaling to counter deleterious effects of AngII binding to the AT1R within any or all of the cellular subpopulations of the brain that could account for many of its other observed beneficial effects (see Fig. 1). Although inhibition of AT1R-coupled signaling will be the focus of this discussion, such Ang-(1-7)-induced phosphatase activity may also mediate its other beneficial effects by additionally regulating cytokine and growth factor signaling, such as by disrupting the NF-κB pathway as described earlier. Activation of the AT1R by AngII initiates intracellular kinase signaling, including mitogen-activated protein kinase (MAPK)-associated pathways, to induce regulatory effects on blood pressure and fluid balance [66]. Importantly, the AT1R-extracellular signal-regulated kinase (ERK)–p38 MAPK pathway has been shown to induce expressional upregulation of ACE and downregulation of ACE2 [67], with further suppression by AngII-induced ACE2 shedding [68]. Considering the importance of kinase signaling in the signal transduction that results from AT1R activation [66], mechanisms of anti-AngII action that involve kinase inhibition seem particularly plausible. Along these lines, it has recently been reported that the administration of an ACE2 activator [69], diminazene aceturate (Berenil®), resulted in significantly downregulated phosphorylation of MAPKs, including ERK, p38, and c-Jun N-terminal kinases, as well as the NF-κB p65 subunit in mice [70]. We have shown that administration of diminazene aceturate significantly reduces infarct size in rat ischemic stroke [24••], which may be the result of such reductions in inflammation, oxidative stress, and apoptosis secondary to the decreased phosphorylation of these pro-inflammatory mediators. In a study using vascular smooth muscle cells of the rat thoracic aorta, Ang-(1-7), via Mas activation, was shown to counteract AngII-induced decreases in ACE2 expression. Importantly, the effect of Ang-(1-7) was dependent on the activity of MAPK phosphatases, suggesting that Mas signaling may involve activation of phosphatase activity that directly counters AT1R-regulated kinase signaling [71].
Further evidence for the role of Ang-(1-7) in activating phosphatases was reported in rat primary astrocytes subjected to radiation-induced inflammation, a setting in which Ang-(1-7) treatment inhibits MAPK activation and reduces markers of inflammation in addition to increasing levels of dual specificity phosphatase 1 (DUSP-1), which is a negative regulator of the MAPK pathway [72•]. These latter findings are of particular interest in the setting of stroke, where inflammation also plays a key role in the pathophysiology of the disease. Similar enhanced negative regulation of AngII-associated MAPK signaling by Ang-(1-7) is known to occur in a variety of other tissues in both animals and humans. Interestingly, Ang-(1-7) attenuates the increased levels of ACE, AT1Rs, and AngII in addition to improving hemodynamic parameters in a rat model of vascular calcification [73]. In the setting of stroke where AngII levels are increased [74], we and others believe it is likely that the beneficial action of Ang-(1-7) may result, at least in part, from phosphatase activation and subsequent downregulation of AngII-induced intracellular kinase signaling [75], resulting in a decrease in the deleterious AngII – AT1R effects as well as disinhibition of ACE2 to further increase activity of the protective ACE2–Ang-(1-7)–Mas axis.
Interactions with Bradykinin and AT2Rs
Several lines of evidence suggest that in addition to the intracellular modifications of kinase signaling, and perhaps as a precursor to such changes, the Ang-(1-7) receptor Mas may interact with other receptors during its activation, specifically the bradykinin receptors and the AT2R, which we briefly discuss in turn here. The interplay of the RAS and the kinin–kallikrein system has been an area of research and clinical interest in the decades since the discovery that ACE acts to cleave and inactivate bradykinin. Although the precise nature of the interaction between the important vasodilatory systems is not entirely clear, Mas signaling appears to play an essential role, as the vasorelaxant effects of both bradykinin and Ang-(1-7) were completely inhibited by antagonists of the Mas receptor in human umbilical vein endothelial cells, and were absent in Mas-deficient murine microvessels. These effects appear to be mediated by altered phosphorylation of NO synthase [76]. Central infusion of Ang-(1-7) during stroke resulted in significantly increased levels of bradykinin, and at higher doses, upregulated expression of the bradykinin B1 and B2 receptors [77]. Additionally, the effects of bradykinin to dilate porcine arteries were potentiated by Ang-(1-7) [78]. Current evidence seems to indicate that activation of Ang-(1-7)–Mas augments the vasodilatory actions of brady-kinin signaling by increasing expression of kinin system components, potentiating its effects, and preventing its metabolism, but future research will be helpful in clarifying more precisely the interaction of these systems.
Accumulating evidence also suggests the possibility of an interaction between Mas and the neuroprotective AT2R which may contribute to the protective effects of Ang-(1-7) in stroke as illustrated in Fig. 1. The involvement of AT2Rs in the stimulation of Ang-(1-7) effects has been demonstrated in numerous studies through their inhibition or attenuation by AT2R antagonists such as PD 123319 and PD 123177 [79–84]. For example, Ang-(1-7)-mediated prostaglandin synthesis [85], vasodilation of coronary arteries [86], and reduction of mean arterial pressure [87] were all significantly halted by administration of PD 123319. Importantly, the cerebroprotective effects of Ang-(1-7) in stroke can be blocked by AT2R antagonism, as can the protection conferred by administration of AT2R agonist compound 21 by Mas antagonism [88]. Many studies in other disease models have demonstrated cross-inhibition of these receptors through their respective antagonists within Ang-(1-7) signaling pathways [35, 89–93], providing additional evidence to indicate the potential formation of Mas–AT2R heterodimer complexes. Further, dimerization of other RAS and related receptors have been reported, including interactions of AT1R with Mas [62], AT2R [94], bradykinin B2 receptor [95], and AT1R homodimerization [96], to name just a few. Indeed, a proposed mechanism of increasing NO production involves the dimerization of AT2R to the bradykinin B2 receptor [95]. Through direct binding, the AT2R may act as an antagonist of the AT1R by inhibiting G-protein activation and subsequent signaling [94]. Similarly, Mas serves as an AT1R antagonist through heterodimer complex formation which attenuates AngII actions [62]. These studies illustrate the common occurrence of oligomeric complexes within the RAS, making plausible the idea of Mas–AT2R heterodimerization. Future studies in this area are anticipated to clarify the nature and the functional implications of these intriguing interactions.
In discussing the potential linkages between the Mas and AT2R signaling pathways, it is important to consider the likely localization for a direct Mas–AT2R interaction. It is expected that an interaction between Mas and AT2R would be within shared expression sites. As stated earlier, Mas expression in the brain has been reported in microglia and neurons [25], as well as endothelial cells [65], while AT2R is found primarily within neurons [97] and endothelial cells [65], with some evidence for its expression in cultured microglia [98, 99]. Following cerebral ischemia, there is an excessive activation of pro-inflammatory cells such as microglia. Additionally there are reports of an enhancement of AT2R levels in the tissue surrounding the infarcted region [100, 101]. One consequence of this upregulation may be an increased potential for Mas–AT2R interactions on neuronal or microglial cells with subsequent ligand binding and pathway activation to induce their protective responses.
Along with the evidence suggesting a direct Mas–AT2R interaction, there are several important findings that indicate their roles as independent protective pathways. Two studies have shown Ang-(1-7) induced effects in AT2R knockout mice [102, 103], and another report demonstrated successful vasodilation by an AT2R agonist in Mas-deficient mice [104]. Additionally, multiple studies have reported AT2R antagonists having no influence on Ang-(1-7) effects, suggesting that certain Mas-mediated actions may be signaled through AT2R-independent cascades [27••, 105–108]. The reasons for these different findings remain to be clarified, but could be explained by differences in species, animal models of disease, timing of drug administration, or age [90] or possibly by tissue type, as formation of receptor dimers has been shown to be tissue-specific [109]. Considering the currently available evidence, a direct Mas–AT2R interaction or dimerization seems plausible and may contribute to the protective effects in studies of stroke resulting from activation of the ACE2–Ang-(1-7)–Mas axis.
Conclusions
Accumulating evidence has demonstrated that activation of the ACE2–Ang-(1-7)–Mas axis can exert profound neuroprotective effects in both ischemic and hemorrhagic strokes, a significant addition to the beneficial actions of this protective arm of the RAS within the cardiovascular system. Though the studies on ACE2–Ang-(1-7)–Mas are as yet confined to experimental animal models, from a translational perspective, the fact that the protective actions of this axis are robust, repeatable in different stroke models, and likely occur via multiple sites of action suggest that targeting this system may become a therapeutic option for stroke. Despite the observations made so far, much work needs to be done to understand the exact mechanisms and sites of the protective action of this system, in order to uncover more favorable therapeutic targets. In this light, while the major focus of studies so far has been on actions of Ang-(1-7) within central tissues, effects within the periphery such as enhancing the function of endothelial progenitor cells [30•] may be as important. Other aspects that should be further considered are whether co-stimulation of Mas and AT2R may exert even greater beneficial actions in stroke and whether Mas activation following application of exogenous Ang-(1-7) or ACE2 activators can be optimized to coincide with post-stroke changes in RAS components in the brain, e.g., upregulation of Mas.
Acknowledgments
We gratefully acknowledge the following support for our work: the National Heart, Lung and Blood Institute (HL076803 and 2T32HL083810-06A1), the American Heart Association Greater Southeast Affiliate (12PRE11940010), and the McKnight Brain Institute.
Footnotes
Conflict of Interest Douglas M. Bennion, Emily Haltigan, Robert W. Regenhardt, U. Muscha Steckelings, and Colin Sumners declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
This article is part of the Topical Collection on Hypertension and the Kidney
Contributor Information
Douglas M. Bennion, Email: douglasbennion@ufl.edu, Department of Physiology and Functional Genomics & McKnight Brain Institute, University of Florida, 1600 SW Archer Road, PO Box 100274, Gainesville, FL 32610-0274, USA.
Emily Haltigan, Email: ehaltigan@ufl.edu, Department of Physiology and Functional Genomics & McKnight Brain Institute, University of Florida, 1600 SW Archer Road, PO Box 100274, Gainesville, FL 32610-0274, USA.
Robert W. Regenhardt, Email: rwregen@ufl.edu, Department of Physiology and Functional Genomics & McKnight Brain Institute, University of Florida, 1600 SW Archer Road, PO Box 100274, Gainesville, FL 32610-0274, USA.
U. Muscha Steckelings, Email: usteckelings@health.sdu.dk, Department of Cardiovascular and Renal Research, University of Southern Denmark, J.B. Winsløws Vej 21 3, 5000 Odense, C, Denmark.
Colin Sumners, Email: csumners@ufl.edu, Department of Physiology and Functional Genomics & McKnight Brain Institute, University of Florida, 1600 SW Archer Road, PO Box 100274, Gainesville, FL 32610-0274, USA.
References
Recently published papers of importance have been highlighted as:
• Of importance
•• Of major importance
- 1.Dai W, Funk A, Herdegen T, Unger T, Culman J. Blockade of central angiotensin AT(1) receptors improves neurological outcome and reduces expression of AP-1 transcription factors after focal brain ischemia in rats. Stroke. 1999;30(11):2391–8. doi: 10.1161/01.str.30.11.2391. [DOI] [PubMed] [Google Scholar]
- 2.Mecca AP, O’Connor TE, Katovich MJ, Sumners C. Candesartan pretreatment is cerebroprotective in a rat model of endothelin-1-induced middle cerebral artery occlusion. Exp Physiol. 2009;94(8):937–46. doi: 10.1113/expphysiol.2009.047936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong HT, Ong LM, Ho JJ. Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin-receptor blockers (ARBs) in patients at high risk of cardiovascular events: a meta-analysis of 10 randomised placebo-controlled trials. ISRN Cardiol. 2013;2013:478597. doi: 10.1155/2013/478597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joseph JP, Mecca AP, Regenhardt RW, Bennion DM, Rodriguez V, Desland F, et al. The angiotensin type 2 receptor agonist Compound 21 elicits cerebroprotection in endothelin-1 induced ischemic stroke. Neuropharmacology. 2014;81C:134–41. doi: 10.1016/j.neuropharm.2014.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Min LJ, Mogi M, Tsukuda K, Jing F, Ohshima K, Nakaoka H, et al. Direct stimulation of angiotensin II type 2 receptor initiated after stroke ameliorates ischemic brain damage. Am J Hypertens. 2014;63(3):e53–9. doi: 10.1093/ajh/hpu015. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy CA, Vinh A, Miller AA, Hallberg A, Alterman M, Callaway JK, et al. Direct angiotensin AT2 receptor stimulation using a novel AT2 receptor agonist, compound 21, evokes neuroprotection in conscious hypertensive rats. PLoS One. 2014;9(4):e95762. doi: 10.1371/journal.pone.0095762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S, Brait VH, Arumugam TV, Evans MA, Kim HA, Widdop RE, et al. Neuroprotective effect of an angiotensin receptor type 2 agonist following cerebral ischemia in vitro and in vivo. Exp Transl Stroke Med. 2012;4(1):16. doi: 10.1186/2040-7378-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.McCarthy CA, Vinh A, Broughton BR, Sobey CG, Callaway JK, Widdop RE. Angiotensin II type 2 receptor stimulation initiated after stroke causes neuroprotection in conscious rats. Hypertension. 2012;60(6):1531–7. doi: 10.1161/HYPERTENSIONAHA.112.199646. This was the first demonstration of protective AT2R effects by pharmacological activation after the 18 onset of ischemic stroke in conscious rats, providing especially important information about the clinical potential of AT2R agonism in stroke. [DOI] [PubMed] [Google Scholar]
- 9.Gelosa P, Pignieri A, Fandriks L, de Gasparo M, Hallberg A, Banfi C, et al. Stimulation of AT2 receptor exerts beneficial effects in stroke-prone rats: focus on renal damage. J Hypertens. 2009;27(12):2444–51. doi: 10.1097/HJH.0b013e3283311ba1. [DOI] [PubMed] [Google Scholar]
- 10.Smith SC., Jr Reducing the global burden of ischemic heart disease and stroke: a challenge for the cardiovascular community and the United Nations. Circulation. 2011;124(3):278–9. doi: 10.1161/CIRCULATIONAHA.111.040170. [DOI] [PubMed] [Google Scholar]
- 11.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55(3):363–89. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg DA. Preclinical stroke research: gains and gaps. Stroke. 2013;44(6 Suppl 1):S114–5. doi: 10.1161/STROKEAHA.113.002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu J, Jiang T, Wu L, Gao L, Wang Y, Zhou F, et al. The expression of angiotensin-converting enzyme 2-angiotensin-1-7-Mas receptor axis are upregulated after acute cerebral ischemic stroke in rats. Neuropeptides. 2013 doi: 10.1016/j.npep.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Fisher M. Characterizing the target of acute stroke therapy. Stroke. 1997;28(4):866–72. doi: 10.1161/01.str.28.4.866. [DOI] [PubMed] [Google Scholar]
- 16.Pena Silva RA, Heistad DD. Promising neuroprotective effects of the angiotensin-(1-7)-angiotensin-converting enzyme 2-Mas axis in stroke. Exp Physiol. 2014;99(2):342–3. doi: 10.1113/expphysiol.2013.076836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regenhardt RW, Bennion DM, Sumners C. Cerebroprotective action of angiotensin peptides in stroke. Clin Sci (Lond) 2014;126(3):195–205. doi: 10.1042/CS20130324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kagiyama T, Kagiyama S, Phillips MI. Expression of angiotensin type 1 and 2 receptors in brain after transient middle cerebral artery occlusion in rats. Regul Pept. 2003;110(3):241–7. doi: 10.1016/s0167-0115(02)00223-9. [DOI] [PubMed] [Google Scholar]
- 19.Chang AY, Li FC, Huang CW, Wu JC, Dai KY, Chen CH, et al. Interplay between brain stem angiotensins and monocyte chemoattractant protein-1 as a novel mechanism for pressor response after ischemic stroke. Neurobiol Dis. 2014;S0969–9961(14):00234. doi: 10.1016/j.nbd.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Mogi M, Kawajiri M, Tsukuda K, Matsumoto S, Yamada T, Horiuchi M. Serum levels of renin-angiotensin system components in acute stroke patients. Geriatr Gerontol Int. 2013 doi: 10.1111/ggi.12167. [DOI] [PubMed] [Google Scholar]
- 21.Xu P, Sriramula S, Lazartigues E. ACE2/ANG-(1-7)/Mas pathway in the brain: the axis of good. Am J Physiol Regul Integr Comp Physiol. 2011;300(4):R804–17. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sumners C, Horiuchi M, Widdop RE, McCarthy C, Unger T, Steckelings UM. Frontiers in research: the protective arms of the reninangiotensin-system in neurological disease. Clin Exp Pharmacol Physiol. 2013 doi: 10.1111/1440-1681.12137. [DOI] [PubMed] [Google Scholar]
- 23.Jiang T, Gao L, Lu J, Zhang YD. ACE2-Ang-(1-7)-Mas axis in brain: a potential target for prevention and treatment of ischemic stroke. Curr Neuropharmacol. 2013;11(2):209–17. doi: 10.2174/1570159X11311020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24••.Mecca A, Regenhardt R, O’Connor T, Joseph J, Raizada M, Katovich M, et al. Cerebroprotection by angiotensin (1-7) in endothelin-1 induced ischemic stroke. Exp Physiol. 2011 doi: 10.1113/expphysiol.2011.058578. This was the first report of cerebroprotective effects of Ang-(1-7) in ischemic stroke. In addition to demonstrating efficacy of central administration ofAng-(1-7) administration of an ACE2 activator was also shown to induce favorable outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regenhardt RW, Mecca AP, Desland F, Ritucci-Chinni PF, Ludin JA, Greenstein D, et al. Centrally administered angiotensin-(1-7) increases the survival of stroke prone spontaneously hypertensive rats. Exp Physiol. 2013 doi: 10.1113/expphysiol.2013.075242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang T, Gao L, Shi J, Lu J, Wang Y, Zhang Y. Angiotensin-(1-7) modulates renin-angiotensin system associated with reducing oxidative stress and attenuating neuronal apoptosis in the brain of hypertensive rats. Pharmacol Res. 2013;67(1):84–93. doi: 10.1016/j.phrs.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 27••.Jiang T, Gao L, Guo J, Lu J, Wang Y, Zhang Y. Suppressing inflammation by inhibiting the NF-kappaB pathway contributes to the neuroprotective effect of angiotensin-(1-7) in rats with permanent cerebral ischaemia. Br J Pharmacol. 2012;167(7):1520–32. doi: 10.1111/j.1476-5381.2012.02105. This report provided confirmation of the neuroprotective actions of Ang-(1-7) in stroke and additionally provided early evidence regarding its anti-inflammatory effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Zhao Y, Chen S, Wang J, Xiao X, Ma X, et al. Neuronal over-expression of ACE2 protects brain from ischemia-induced damage. Neuropharmacology. 2014;79:550–8. doi: 10.1016/j.neuropharm.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng J, Li G, Chen S, Bihl J, Buck J, Zhu Y, et al. Activation of the ACE2/Ang-(1-7)/Mas pathway reduces oxygen-glucose deprivation-induced tissue swelling, ROS production, and cell death in mouse brain with angiotensin II overproduction. Neuroscience. 2014;273:39–51. doi: 10.1016/j.neuroscience.2014.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Chen J, Xiao X, Chen S, Zhang C, Chen J, Yi D, et al. Angiotensin-converting enzyme 2 priming enhances the function of endothelial progenitor cells and their therapeutic efficacy. Hypertension. 2013;61(3):681–9. doi: 10.1161/HYPERTENSIONAHA.111.00202. This translationally oriented study of the efficacy of ACE2 in stroke employed a protocol that was the first to start treatments after the induction of stroke by peripheral injections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Zheng JL, Li GZ, Chen SZ, Wang JJ, Olson JE, Xia HJ, et al. Angiotensin converting enzyme 2/Ang-(1-7)/Mas axis protects brain from ischemic injury with a tendency of age-dependence. CNS Neurosci Ther. 2014 doi: 10.1111/cns.12233. The use of aged animals in this study allowed for the discovery that the protective effects of Ang-(1-7) are preserved, and even enhanced with age, an important finding in support of future clinical testing of such therapies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Bigio MR, Yan HJ, Buist R, Peeling J. Experimental intrace-rebral hemorrhage in rats. Magnetic resonance imaging and histopathological correlates. Stroke. 1996;27(12):2312–9. doi: 10.1161/01.str.27.12.2312. discussion 2319–20. [DOI] [PubMed] [Google Scholar]
- 33.da Silveira KD, Coelho FM, Vieira AT, Sachs D, Barroso LC, Costa VV, et al. Anti-inflammatory effects of the activation of the angiotensin-(1-7) receptor, MAS, in experimental models of arthritis. J Immunol. 2010;185(9):5569–76. doi: 10.4049/jimmunol.1000314. [DOI] [PubMed] [Google Scholar]
- 34.Giani JF, Munoz MC, Pons RA, Cao G, Toblli JE, Turyn D, et al. Angiotensin-(1-7) reduces proteinuria and diminishes structural damage in renal tissue of stroke-prone spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2011;300(1):F272–82. doi: 10.1152/ajprenal.00278.2010. [DOI] [PubMed] [Google Scholar]
- 35.Tesanovic S, Vinh A, Gaspari TA, Casley D, Widdop RE. Vasoprotective and atheroprotective effects of angiotensin (1-7) in apolipoprotein E-deficient mice. Arterioscler, Thromb, Vasc Biol. 2010;30(8):1606–13. doi: 10.1161/ATVBAHA.110.204453. [DOI] [PubMed] [Google Scholar]
- 36.El-Hashim AZ, Renno WM, Raghupathy R, Abduo HT, Akhtar S, Benter IF. Angiotensin-(1-7) inhibits allergic inflammation, via the MAS1 receptor, through suppression of ERK1/2- and NF-kappaB-dependent pathways. Br J Pharmacol. 2012;166(6):1964–76. doi: 10.1111/j.1476-5381.2012.01905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wosten-van Asperen RM, Lutter R, Specht PA, Moll GN, van Woensel JB, van der Loos CM, et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J Pathol. 2011;225(4):618–27. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 38.Grobe J, Mecca A, Lingis M, Shenoy V, Bolton T, Machado J, et al. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1-7) Am J Physiol Heart Circ Physiol. 2007;292(2):H736–42. doi: 10.1152/ajpheart.00937.2006. [DOI] [PubMed] [Google Scholar]
- 39.Grobe JL, Mecca AP, Mao H, Katovich MJ. Chronic angiotensin-(1-7) prevents cardiac fibrosis in DOCA-salt model of hypertension. Am J Physiol Heart Circ Physiol. 2006;290(6):H2417–23. doi: 10.1152/ajpheart.01170.2005. [DOI] [PubMed] [Google Scholar]
- 40.Huentelman MJ, Grobe JL, Vazquez J, Stewart JM, Mecca AP, Katovich MJ, et al. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol. 2005;90(5):783–90. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- 41.Ferreira AJ, Jacoby BA, Araujo CA, Macedo FA, Silva GA, Almeida AP, et al. The nonpeptide angiotensin-(1-7) receptor Mas agonist AVE-0991 attenuates heart failure induced by myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;292(2):H1113–9. doi: 10.1152/ajpheart.00828.2006. [DOI] [PubMed] [Google Scholar]
- 42.Ferreira AJ, Shenoy V, Yamazato Y, Sriramula S, Francis J, Yuan L, et al. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179(11):1048–1054. doi: 10.1164/rccm.200811-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreira AJ, Santos RA, Almeida AP. Angiotensin-(1-7): cardioprotective effect in myocardial ischemia/reperfusion. Hypertension. 2001;38(3 Pt 2):665–8. doi: 10.1161/01.hyp.38.3.665. [DOI] [PubMed] [Google Scholar]
- 44.Santos RA, Ferreira AJ, Nadu AP, Braga AN, de Almeida AP, Campagnole-Santos MJ, et al. Expression of an angiotensin-(1-7)-producing fusion protein produces cardioprotective effects in rats. Physiol Genomics. 2004;17(3):292–9. doi: 10.1152/physiolgenomics.00227.2003. [DOI] [PubMed] [Google Scholar]
- 45.Petty WJ, Miller AA, McCoy TP, Gallagher PE, Tallant EA, Torti FM. Phase I and pharmacokinetic study of angiotensin-(1-7), an endogenous antiangiogenic hormone. Clin Cancer Res. 2009;15(23):7398–404. doi: 10.1158/1078-0432.CCR-09-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Passos-Silva DG, Verano-Braga T, Santos RA. Angiotensin-(1-7): beyond the cardio-renal actions. Clin Sci (Lond) 2013;124(7):443–56. doi: 10.1042/CS20120461. [DOI] [PubMed] [Google Scholar]
- 47•.Regenhardt RW, Desland F, Mecca AP, Pioquinto DJ, Afzal A, Mocco J, et al. Anti-inflammatory effects of angiotensin-(1-7) in ischemic stroke. Neuropharmacology. 2013;71C:154–63. doi: 10.1016/j.neuropharm.2013.03.025. This study examined many of the potential inflammatory cytokines that may be suppressed by Ang-(1-7)/Mas signaling and made contributions to our understanding of the cellular localization of Mas in the stroke brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iadecola C, Zhang F, Xu S, Casey R, Ross M. Inducible nitric oxide synthase gene expression in brain following cerebral ischemia. J Cereb Blood Flow Metab. 1995;15(3):378–84. doi: 10.1038/jcbfm.1995.47. [DOI] [PubMed] [Google Scholar]
- 49.Meng Y, Yu CH, Li W, Li T, Luo W, Huang S, et al. Angiotensinconverting enzyme 2/angiotensin-(1-7)/Mas axis protects against lung fibrosis by inhibiting the MAPK/NF-kappaB pathway. Am J Respir Cell Mol Biol. 2013 doi: 10.1165/rcmb.2012-0451OC.22. [DOI] [PubMed] [Google Scholar]
- 50.Santos SH, Andrade JM, Fernandes LR, Sinisterra RD, Sousa FB, Feltenberger JD, et al. Oral angiotensin-(1-7) prevented obesity and hepatic inflammation by inhibition of resistin/TLR4/MAPK/NF-kappaB in rats fed with high-fat diet. Peptides. 2013;46:47–52. doi: 10.1016/j.peptides.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 51.Zhang F, Ren J, Chan K, Chen H. Angiotensin-(1-7) regulates angiotensin II-induced VCAM-1 expression on vascular endothelial cells. Biochem Biophys Res Commun. 2013;430(2):642–6. doi: 10.1016/j.bbrc.2012.11.098. [DOI] [PubMed] [Google Scholar]
- 52.Gaddam RR, Chambers S, Bhatia M. ACE and ACE2 in inflammation: a tale of two enzymes. Inflamm Allergy Drug Targets. 2014 doi: 10.2174/1871528113666140713164506. IADT-EPUB-61371 [pii] [DOI] [PubMed] [Google Scholar]
- 53.Zhang Z, Chen L, Zhong J, Gao P. Oudit GY ACE2/Ang-(1-7) signaling and vascular remodeling. Sci China Life Sci. 2014;57(8):802–8. doi: 10.1007/s11427-014.4693-3. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Lu J, Shi J, Lin X, Dong J, Zhang S, et al. Central administration of angiotensin-(1-7) stimulates nitric oxide release and upregulates the endothelial nitric oxide synthase expression following focal cerebral ischemia/reperfusion in rats. Neuropeptides. 2008;42(5–6):593–600. doi: 10.1016/j.npep.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Jiang T, Yu JT, Zhu XC, Zhang QQ, Tan MS, Cao L, et al. Angiotensin-(1-7) induces cerebral ischemic tolerance by promoting brain angiogenesis in a Mas/eNOS-dependent pathway. Br J Pharmacol. 2014 doi: 10.1111/bph.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rentzsch B, Todiras M, Iliescu R, Popova E, Campos LA, Oliveira ML, et al. Transgenic angiotensin-converting enzyme 2 overexpression in vessels of SHRSP rats reduces blood pressure and improves endothelial function. Hypertension. 2008;52(5):967–73. doi: 10.1161/HYPERTENSIONAHA.108.114322. [DOI] [PubMed] [Google Scholar]
- 57.Fraga-Silva RA, Costa-Fraga FP, Murca TM, Moraes PL, Martins Lima A, Lautner RQ, et al. Angiotensin-converting enzyme 2 activation improves endothelial function. Hypertension. 2013;61(6):1233–8. doi: 10.1161/HYPERTENSIONAHA.111.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gu Q, Wang B, Zhang XF, Ma YP, Liu JD, Wang XZ. Contribution of renin-angiotensin system to exercise-induced attenuation of aortic remodeling and improvement of endothelial function in spontaneously hypertensive rats. Cardiovasc Pathol. 2014:S1054–8807. doi: 10.1016/j.carpath.2014.05.006. (14)00060-X [pii] [DOI] [PubMed] [Google Scholar]
- 59.Shenoy V, Gjymishka A, Yagna J, Qi Y, Afzal A, Rigatto K, et al. Diminazene attenuates pulmonary hypertension and improves angiogenic progenitor cell functions in experimental models. Am J Respir Crit Care Med. 2013 doi: 10.1164/rccm.201205-0880OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li G, Liu Y, Zhu Y, Liu A, Xu Y, Li X, et al. ACE2 activation confers endothelial protection and attenuates neointimal lesions in prevention of severe pulmonary arterial hypertension in rats. Lung. 2013;191(4):327–36. doi: 10.1007/s00408-013-9470-8. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Tikellis C, Thomas MC, Golledge J. Angiotensin converting enzyme 2 and atherosclerosis. Atherosclerosis. 2013;226(1):3–8. doi: 10.1016/j.atherosclerosis.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 62.Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, et al. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation. 2005;111(14):1806–13. doi: 10.1161/01.CIR.0000160867.23556.7D. [DOI] [PubMed] [Google Scholar]
- 63.Deliu E, Brailoiu GC, Eguchi S, Hoffman NE, Rabinowitz JE, Tilley DG, et al. Direct evidence of intracrine angiotensin II signaling in neurons. Am J Physiol Cell Physiol. 2014;306(8):C736–44. doi: 10.1152/ajpcell.00131.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lanz TV, Ding Z, Ho PP, Luo J, Agrawal AN, Srinagesh H, et al. Angiotensin II sustains brain inflammation in mice via TGF-beta. J Clin Invest. 2010;120(8):2782–94. doi: 10.1172/JCI41709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bagby SP, LeBard LS, Luo Z, Speth RC, Ogden BE, Corless CL. Angiotensin II type 1 and 2 receptors in conduit arteries of normal developing microswine. Arterioscler, Thromb, Vasc Biol. 2002;22(7):1113–21. doi: 10.1161/01.atv.0000022382.61262.3e. [DOI] [PubMed] [Google Scholar]
- 66.Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20(5):953–70. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 67.Koka V, Huang XR, Chung AC, Wang W, Truong LD, Lan HY. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol. 2008;172(5):1174–83. doi: 10.2353/ajpath.2008.070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patel VB, Clarke N, Wang Z, Fan D, Parajuli N, Basu R, et al. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACEADAM-17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014;66:167–76. doi: 10.1016/j.yjmcc.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 69.Kulemina LV, Ostrov DA. Prediction of off-target effects on angiotensin-converting enzyme 2. J Biomol Screen. 2011;16(8):878–885. doi: 10.1177/1087057111413919. [DOI] [PubMed] [Google Scholar]
- 70.Kuriakose S, Uzonna JE. Diminazene aceturate (Berenil), a new use for an old compound? Int Immunopharmacol. 2014;21(2):342–5. doi: 10.1016/j.intimp.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 71.Gallagher PE, Ferrario CM, Tallant EA. MAP kinase/phosphatase pathway mediates the regulation of ACE2 by angiotensin peptides. Am J Physiol Cell Physiol. 2008;295(5):C1169–74. doi: 10.1152/ajpcell.00145.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72•.Moore ED, Kooshki M, Metheny-Barlow LJ, Gallagher PE, Robbins ME. Angiotensin-(1-7) prevents radiation-induced inflammation in rat primary astrocytes through regulation of MAP 24 kinase signaling. Free Radic Biol Med. 2013;65:1060–8. doi: 10.1016/j.freeradbiomed.2013.08.183. This study provided convincing evidence for the role of phosphatase activation and subsequent inhibition of MAPK signaling by Ang-(1-7) as part of its action to reduce inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sui YB, Chang JR, Chen WJ, Zhao L, Zhang BH, Yu YR, et al. Angiotensin-(1-7) inhibits vascular calcification in rats. Peptides. 2013;42:25–34. doi: 10.1016/j.peptides.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 74.Kagiyama T, Kagiyama S, Phillips MI. Expression of angiotensin type 1 and 2 receptors in brain after transient middle cerebral artery occlusion in rats. Regul Pept. 2003;110(3):241–7. doi: 10.1016/s0167-0115(02)00223-9. [DOI] [PubMed] [Google Scholar]
- 75.Chappell MC, Marshall AC, Alzayadneh EM, Shaltout HA, Diz DI. Update on the angiotensin converting enzyme 2-angiotensin (1-7)-Mas receptor axis: fetal programing, sex differences, and intracellular pathways. Front Endocrinol (Lausanne) 2014;4:201. doi: 10.3389/fendo.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peiro C, Vallejo S, Gembardt F, Palacios E, Novella S, Azcutia V, et al. Complete blockade of the vasorelaxant effects of angiotensin-(1-7) and bradykinin in murine microvessels by antagonists of the receptor Mas. J Physiol. 2013;591(Pt 9):2275–85. doi: 10.1113/jphysiol.2013.251413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu J, Zhang Y, Shi J. Effects of intracerebroventricular infusion of angiotensin-(1-7) on bradykinin formation and the kinin receptor expression after focal cerebral ischemia-reperfusion in rats. Brain Res. 2008;1219:127–35. doi: 10.1016/j.brainres.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 78.Raffai G, Khang G, Vanhoutte PM. Angiotensin-(1-7) augments endothelium-dependent relaxations of porcine coronary arteries to bradykinin by inhibiting ACE1. J Cardiovasc Pharmacol. 2014 doi: 10.1097/FJC.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 79.Heitsch H, Brovkovych S, Malinski T, Wiemer G. Angiotensin-(1-7)-stimulated nitric oxide and superoxide release from endothelial cells. Hypertension. 2001;37(1):72–6. doi: 10.1161/01.hyp.37.1.72. [DOI] [PubMed] [Google Scholar]
- 80.Jaiswal N, Diz DI, Chappell MC, Khosla MC, Ferrario CM. Stimulation of endothelial cell prostaglandin production by angiotensin peptides. Characterization of receptors. Hypertension. 1992;19(2 Suppl):II49–55. doi: 10.1161/01.hyp.19.2_suppl.ii49. [DOI] [PubMed] [Google Scholar]
- 81.Wiemer G, Dobrucki LW, Louka FR, Malinski T, Heitsch H. AVE 0991, a nonpeptide mimic of the effects of angiotensin-(1-7) on the endothelium. Hypertension. 2002;40(6):847–52. doi: 10.1161/01.hyp.0000037979.53963.8f. [DOI] [PubMed] [Google Scholar]
- 82.Costa MA, Lopez Verrilli MA, Gomez KA, Nakagawa P, Pena C, Arranz C, et al. Angiotensin-(1-7) upregulates cardiac nitric oxide synthase in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299(4):H1205–11. doi: 10.1152/ajpheart.00850.2009. [DOI] [PubMed] [Google Scholar]
- 83.Lara Lda S, Cavalcante F, Axelband F, De Souza AM, Lopes AG, Caruso-Neves C. Involvementofthe Gi/o/cGMP/PKG pathway in the AT2-mediated inhibition of outer cortex proximal tubule Na+-ATPase by Ang-(1-7) Biochem J. 2006;395(1):183–90. doi: 10.1042/BJ20051455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Souza AM, Lopes AG, Pizzino CP, Fossari RN, Miguel NC, Cardozo FP, et al. Angiotensin II and angiotensin-(1-7) inhibit the inner cortex Na+-ATPase activity through AT2 receptor. Regul Pept. 2004;120(1–3):167–75. doi: 10.1016/j.regpep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 85.Muthalif MM, Benter IF, Karzoun N, Fatima S, Harper J, Uddin MR, et al. 20-Hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1998;95(21):12701–6. doi: 10.1073/pnas.95.21.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gorelik G, Carbini LA, Scicli AG. Angiotensin 1-7 induces bradykinin-mediated relaxation in porcine coronary artery. J Pharmacol Exp Ther. 1998;286(1):403–10. [PubMed] [Google Scholar]
- 87.Walters PE, Gaspari TA, Widdop RE. Angiotensin-(1-7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Hypertension. 2005;45(5):960–6. doi: 10.1161/01.HYP.0000160325.59323.b8. [DOI] [PubMed] [Google Scholar]
- 88.Villela D, Leonhardt J, Patel N, Jospeh J, Kirsch S, Hallberg A, et al. Angiotensin AT2-receptor and receptor Mas: a complex liaison. Clin Sci (Lond) 2014 doi: 10.1042/CS20130515. In Press. [DOI] [PubMed] [Google Scholar]
- 89.Durand MJ, Raffai G, Weinberg BD, Lombard JH. Angiotensin-(1-7) and low-dose angiotensin II infusion reverse salt-induced endothelial dysfunction via different mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2010;299(4):H1024–33. doi: 10.1152/ajpheart.00328.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bosnyak S, Widdop RE, Denton KM, Jones ES. Differential mechanisms of ang (1-7)-mediated vasodepressor effect in adult and aged candesartan-treated rats. Int J Hypertens. 2012;2012:192567. doi: 10.1155/2012/192567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roks AJ, Nijholt J, van Buiten A, van Gilst WH, de Zeeuw D, Henning RH. Low sodium diet inhibits the local counter-regulator effect of angiotensin-(1-7) on angiotensin II. J Hypertens. 2004;22(12):2355–61. doi: 10.1097/00004872-200412000-00018. [DOI] [PubMed] [Google Scholar]
- 92.Pinheiro SV, Silva ACSe, Sampaio WO, de Paula RD, Mendes EP, Bontempo ED, et al. Nonpeptide AVE 0991 is an angiotensin-(1-7) receptor Mas agonist in the mouse kidney. Hypertension. 2004;44(4):490–6. doi: 10.1161/01.HYP.0000141438.64887.42. [DOI] [PubMed] [Google Scholar]
- 93.Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. Evidence for a functional interaction of the angiotensin-(1-7) receptor Mas with AT1 and AT2 receptors in the mouse heart. Hypertension. 2005;46(4):937–42. doi: 10.1161/01.HYP.0000175813.04375.8a.26. [DOI] [PubMed] [Google Scholar]
- 94.AbdAlla S, Lother H, Abdel-tawab AM, Quitterer U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J Biol Chem. 2001;276(43):39721–6. doi: 10.1074/jbc.M105253200. [DOI] [PubMed] [Google Scholar]
- 95.Abadir PM, Periasamy A, Carey RM, Siragy HM. Angiotensin II type 2 receptor-bradykinin B2 receptor functional heterodimerization. Hypertension. 2006;48(2):316–22. doi: 10.1161/01.HYP.0000228997.88162.a8. [DOI] [PubMed] [Google Scholar]
- 96.Karip E, Turu G, Supeki K, Szidonya L, Hunyady L. Cross-inhibition of angiotensin AT1 receptors supports the concept of receptor oligomerization. Neurochem Int. 2007;51(5):261–7. doi: 10.1016/j.neuint.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 97.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Distribution of angiotensin II type-2 receptor (AT2) mRNA expression in the adult rat brain. J Comp Neurol. 1996;373(3):322–39. doi: 10.1002/(SICI)1096-9861(19960923)373:3<322::AID-CNE2>3.0.CO;2-4. doi:10.1002/(SICI)1096-9861(19960923)373:3 < 322::AID-CNE2 > 3.0. CO;2-4. [DOI] [PubMed] [Google Scholar]
- 98.Miyoshi M, Miyano K, Moriyama N, Taniguchi M, Watanabe T. Angiotensin type 1 receptor antagonist inhibits lipopolysaccharide-induced stimulation of rat microglial cells by suppressing nuclear factor kappaB and activator protein-1 activation. Eur J Neurosci. 2008;27(2):343–51. doi: 10.1111/j.1460-9568.2007.06014. [DOI] [PubMed] [Google Scholar]
- 99.Valero-Esquitino V, Lucht K, Namsolleck P, Monnet-Tschudi F, Stubbe T, Lucht F, et al. Direct angiotensin AT2-receptor stimulation attenuates T-cell and microglia activation and prevents demyelination in experimental autoimmune encephalomyelitis in mice. Clin Sci (Lond) 2014 doi: 10.1042/CS20130601. CS20130601 [pii] [DOI] [PubMed] [Google Scholar]
- 100.Makino I, Shibata K, Ohgami Y, Fujiwara M, Furukawa T. Transient upregulation of the AT2 receptor mRNA level after global ischemia in the rat brain. Neuropeptides. 1996;30(6):596–601. doi: 10.1016/s0143-4179(96)90043-8. [DOI] [PubMed] [Google Scholar]
- 101.Li J, Culman J, Hortnagl H, Zhao Y, Gerova N, Timm M, et al. Angiotensin AT2 receptor protects against cerebral ischemia-induced neuronal injury. FASB J. 2005;19(6):617–9. doi: 10.1096/fj.04-2960fje. [DOI] [PubMed] [Google Scholar]
- 102.Ohshima K, Mogi M, Nakaoka H, Iwanami J, Min LJ, Kanno H, et al. Possible role of angiotensin-converting enzyme 2 and activation of angiotensin II type 2 receptor by angiotensin-(1-7) in improvement of vascular remodeling by angiotensin II type 1 receptor blockade. Hypertension. 2014;63(3):e53–9. doi: 10.1161/HYPERTENSIONAHA.113.02426. [DOI] [PubMed] [Google Scholar]
- 103.Gembardt F, van Veghel R, Coffman TM, Schultheiss HP, Danser AH, Walther T. Hemodynamic effects of vasorelaxant compounds in mice lacking one, two or all three angiotensin II receptors. Hypertens Res. 2012;35(5):547–51. doi: 10.1038/hr.2012.5. [DOI] [PubMed] [Google Scholar]
- 104.Lemos VS, Silva DM, Walther T, Alenina N, Bader M, Santos RA. The endothelium-dependent vasodilator effect of the nonpeptide Ang(1-7) mimic AVE 0991 is abolished in the aorta of masknockout mice. J Cardiovasc Pharmacol. 2005;46(3):274–9. doi: 10.1097/01.fjc.0000175237.41573.63. [DOI] [PubMed] [Google Scholar]
- 105.Brosnihan KB, Li P, Ferrario CM. Angiotensin-(1-7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension. 1996;27(3 Pt 2):523–8. doi: 10.1161/01.hyp.27.3.523. [DOI] [PubMed] [Google Scholar]
- 106.Tallant EA, Ferrario CM, Gallagher PE. Angiotensin-(1-7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol. 2005;289(4):H1560–6. doi: 10.1152/ajpheart.00941.2004. [DOI] [PubMed] [Google Scholar]
- 107.Dharmani M, Mustafa MR, Achike FI, Sim MK. Effects of angiotensin 1-7 on the actions of angiotensin II in the renal and mesenteric vasculature of hypertensive and streptozotocin-induced diabetic rats. Eur J Pharmacol. 2007;561(1–3):144–50. doi: 10.1016/j.ejphar.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 108.Silva DM, Vianna HR, Cortes SF, Campagnole-Santos MJ, Santos RA, Lemos VS. Evidence for a new angiotensin-(1-7) receptor subtype in the aorta of Sprague-Dawley rats. Peptides. 2007;28(3):702–7. doi: 10.1016/j.peptides.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 109.Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, et al. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc Natl Acad Sci U S A. 2005;102(25):9050–90550501112102. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]


