Abstract
We sought to determine the relationships among intrarenal and systemic inflammation and renal disease in HIV. We compared paired serum and urinary levels (normalized to urine creatinine) of monocyte chemotactic protein-1 (MCP-1), regulated on activation normal T cell expressed and secreted (RANTES), interferon-γ-induced protein-10 (IP-10), interleukin-8 (IL-8), and β2-microglobulin (B2M) between two groups of HIV-infected subjects not receiving antiretroviral therapy (ART) [A: not expecting to initiate ART immediately due to having CD4 cell counts ≥350/μl, N=26; B: about to initiate ART, N=19], a group of HIV-infected subjects receiving virologically suppressive antiretroviral therapy [C, N=30], and a group of HIV-uninfected, healthy volunteers [D, N=45]. We then correlated these inflammatory biomarker levels with urine protein/creatinine ratios (uPCR), urine albumin/creatinine ratios (uACR), and estimated glomerular filtration rates (eGFR). Urine inflammatory biomarker levels were highest in Group B. When combining all four study groups, statistically significant positive correlations included uPCR with urine IL-8, urine MCP-1, urine IP-10, and serum IP-10 and uACR with urine IL-8, urine B2M, serum IP-10, and serum B2M. eGFR was statistically significantly negatively correlated with serum MCP-1 and serum B2M. Paired serum and urine levels of IP-10 and B2M (but not IL-8, RANTES, or MCP-1) were significantly correlated with each other in the overall group. The levels of urine inflammatory markers tested differed by HIV status and use of virologically suppressive ART. These urine and serum inflammatory markers were differentially correlated with uPCR, uACR, and eGFR, suggesting that different intrarenal and systemic inflammatory pathways may contribute to different measures of nephropathy.
Introduction
Kidney disease remains a major complication of human immunodeficiency virus (HIV) infection. Compared to HIV-uninfected individuals, HIV-infected persons have significantly greater risks of proteinuria,1 albuminuria,2 and lower estimated glomerular filtration rates (eGFR).3 In addition, proteinuria,4 albuminuria,5,6 and impaired renal function prior to antiretroviral therapy (ART) are independent predictors of both progression to AIDS4 and overall mortality.4,7,8 As such, HIV-related renal disease may be part of a systemic inflammatory or immunological process.
We previously found in a study of HIV-infected subjects who were not receiving ART that dipstick proteinuria ≥1+was significantly associated with greater T cell activation as measured by percentages of peripheral blood CD8+CD38+HLA-DR+ cells.9 In a recent pilot study of HIV-infected patients not on ART, we investigated the contribution of intrarenal inflammation to renal injury by assessing the relationships between urinary inflammatory biomarkers and quantitative proteinuria and albuminuria.10 We found that urinary levels of monocyte chemotactic protein-1 (MCP-1) and regulated on activation normal T cell expressed and secreted (RANTES), but not interleukin-8 (IL-8) or interferon-γ-induced protein-10 (IP-10), were significantly associated with both proteinuria and albuminuria.10 In addition, proteomic analysis from this pilot study suggested that β2-microglobulin (B2M) was universally detected in the urine samples analyzed.
To further characterize the systemic and intrarenal inflammatory profiles and their relationships with kidney disease in HIV, we measured paired urinary and serum levels of MCP-1, RANTES, IL-8, IP-10, and B2M along with quantitative proteinuria, albuminuria, and renal function in HIV-infected persons not receiving antiretroviral therapy, HIV-infected persons receiving virologically suppressive ART, and HIV-uninfected controls.
Materials and Methods
Study participants
This study included 75 HIV-seropositive patients and 45 HIV-seronegative subjects who completed other local studies by our group (ClinicalTrials.gov NCT00796822, NCT00864916, NCT00919724, and NCT01270802) and who consented to have their sera and urine samples stored and available for future analysis. Samples from all participants from these previously conducted trials were used for the current analysis. Participants were recruited from the HIV outpatient clinics associated with the Indiana University Health medical system. All were over the age of 18 years.
The 75 HIV-positive subjects were classified into three groups based on antiretroviral history, CD4 count, and plasma HIV RNA levels. Groups A and B were HIV-infected patients who enrolled into trials assessing the utility of pentoxifylline to reduce inflammation and improve endothelial function as measured by flow-mediated dilation of the brachial artery. The samples assayed in the current analysis were from the baseline visits of these two trials. Group A included 26 HIV-seropositive patients who had a CD4 cell count ≥350/μl, were not considered nonadherent to ART, and were not known to be long-term nonprogressors, had no receipt of ART within 6 months of sample acquisition, and were not expected to initiate ART for at least 3 months during the trial period.11 Group B included 19 HIV-seropositive patients who had no receipt of ART within 6 months of sample acquisition but were about to initiate ART per their HIV provider; there was no CD4 count eligibility criterion.
Group C included 30 HIV-seropositive patients who had received tenofovir/emtricitabine/efavirenz (TDF/FTC/EFV) as their initial ART regimen for at least 1 year and were virologically suppressed (defined as having an HIV-1 RNA level <50 copies/ml) at study screening.12 Group D included 45 HIV-seronegative persons without medical comorbidities and matched with subjects in Group A and Group B based on age (±10 years), sex, and smoking status (current vs. not current); the choice of these matching characteristics was based on demographic factors known to be associated with HIV-related endothelial function measured using the flow-mediated dilation technique. Major exclusion criteria in all groups included known cardiovascular disease, diabetes mellitus, uncontrolled hypertension, thyroid abnormalities, systemic inflammatory disease other than hepatitis B or C coinfection, pregnancy or breastfeeding during the study, creatinine clearance <50 ml/min, hemoglobin <9.0 g/dl, alanine (ALT) or aspartate (AST) aminotransferase >3 times the upper limit of normal, total bilirubin >2.5 times the upper limit of normal, or ongoing fever or active infection/malignancy requiring treatment during the study visit.
Study design
We performed a cross-sectional analysis to compare paired serum and urinary levels of IL-8, RANTES, MCP-1, IP-10, and B2M between the four study groups. Of note, we chose not to combine Groups A and B as these participants were considered sufficiently dissimilar due to their enrollment characteristics. We measured the serum levels of these markers in order to determine if serum levels compared similarly and correlated with their respective urine levels. These inflammatory markers were then analyzed in relation to spot urine protein/creatinine ratio (uPCR), spot urine albumin/creatinine ratio (uACR), and eGFR calculated using the 2012 CKD-EPI creatinine-cystatin C combined equation.13 All urine and serum samples were obtained after an 8-h fast on the morning of the study visit. The study was approved by the Indiana University institutional review board. All participants provided written, informed consents for both participation in the parent trials and for use of their specimens for future use.
Biomarker measurements
We measured urine IL-8, RANTES, MCP-1, IP-10, and B2M using a custom enzyme-linked immunosorbent assay (ELISA) multiplex (EMD Millipore, Billerica, MA).14 These urine chemokines were normalized to urinary creatinine to account for variances in concentrations. Serum MCP-1 and IP-10 and cystatin C (calibrated to the IDMS standard) were measured using individual ELISA assays. Serum RANTES, B2M, and IL-8 were separately measured using another Millipore multiplex kit. Urine protein and urine creatinine were measured using a spectrophotometric assay and using the Jaffe kinetic reaction with picric acid, respectively. Urine albumin was measured using an immunoprecipitin reaction. All samples were run in duplicate.
Statistical analysis
Categorical variables were summarized as frequencies (percentages) and were compared using either Pearson's chi-square tests or Fisher's exact tests depending on the expected cell frequencies. Since the continuous variables did not have normal distributions, nonparametric statistics were used. Medians (quartile 1, quartile 3) were used for descriptive statistics, and rank-based methods were used for comparisons. Kruskal–Wallis one-way analysis of variance and Wilcoxon rank sum test were used for unadjusted comparisons between each study group. Proportional odds ordinal logistic models were used to adjust for demographic factors for comparisons of each HIV-infected study group (Groups A, B, C) with the uninfected control group (Group D). Spearman's rank-based correlation coefficients were calculated to assess for relationships between each inflammatory marker with each renal parameter and between each paired serum and urine inflammatory marker in the overall cohort and within each study group. The Holm–Bonferroni method was used to adjust for multiple testing in all analyses. Adjusted two-sided p values <0.05 were considered as significant. All analyses were performed in R [R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. www.R-project.org/].
Results
Study cohort characteristics
The characteristics of each group are shown in Table 1. The overall median age was 37 years with no significant age differences among the four groups. More than 70% were male in each study group. The majority of participants were non-Hispanic. Among the HIV-infected groups (Groups A, B, C), more than 60% were black, whereas only 31% of the HIV-uninfected subjects in Group D were black. Active smokers comprised nearly half of the overall group of 120 participants. As expected, the median CD4 cell count was lower and the median HIV-1 RNA log10 c/ml level was higher in Group B compared to both Groups A and C. The frequencies of participants with uPCR >0.2 and uACR >0.03, which are considered abnormal pathological levels, were similar to those found previously in HIV,15,16 with the highest rates found in Group B. Only 3% of the overall group had eGFR <60 ml/min/1.732, although nearly a third had an eGFR <90 ml/min/1.732, which are similar to rates of reduced eGFR in HIV found previously.17 Of note, Group B had the highest frequencies of reduced eGFR using both cutoffs.
Table 1.
Characteristics of the Study Groups
| HIV-infected | HIV-uninfected | ||||
|---|---|---|---|---|---|
| Baseline characteristics | A (n=26) | B (n=19) | C (n=30) | D (n=45) | Overall (n=120) |
| Age (years) | 36.7 (28.6, 43.2) | 34.2 (28.5, 42.3) | 38.3 (30.2, 44.5) | 33.9 (25.7, 45.1) | 36.9 (29.0, 43.0) |
| Gender | |||||
| Female | 7 (27%) | 3 (16%) | 3 (10%) | 11 (24%) | 24 (20%) |
| Male | 19 (73%) | 16 (84%) | 27 (90%) | 34 (76%) | 96 (80%) |
| Race | |||||
| Black | 16 (62%) | 12 (63%) | 18 (60%) | 14 (31%) | 60 (50%) |
| Nonblack | 10 (38%) | 7 (37%) | 12 (40%) | 31 (69%) | 60 (50%) |
| Ethnicity | |||||
| Hispanic or Latino | 1 (4%) | 0 (0%) | 0 (0%) | 1 (2%) | 2 (2%) |
| Not Hispanic or Latino | 25 (96%) | 19 (100%) | 30 (100%) | 44 (98%) | 118 (98%) |
| Smoking status | |||||
| Nonsmoker | 15 (58%) | 8 (42%) | 12 (40%) | 23 (51%) | 58 (48%) |
| Smoker | 11 (42%) | 11 (58%) | 18 (60%) | 22 (49%) | 62 (52%) |
| CD4 count (cells/μl) | 585 (433, 639) | 186.5 (51.75, 375) | 541 (423, 960) | 880 (723.5, 1163.5) | 593 (414, 880) |
| >350 | 22 (88%) | 6 (33%) | 25 (86%) | 39 (100%) | 92 (83%) |
| HIV-1 RNA (log10 copies/ml) | 4.17 (3.69, 4.61) | 5.03 (4.46, 5.61) | 1.32 (1.32, 1.32) | NA | 3.64 (1.30, 4.63)a |
| <50 copies/ml | 1 (4%) | 0 (0%) | 28 (97%) | 0 | 29 (40%)a |
| uPCR | 0.06 (0.05, 0.08) | 0.09 (0.06, 0.18) | 0.08 (0.05, 0.11) | 0.04 (0.04, 0.06) | 0.06 (0.04, 0.10) |
| >0.3 | 1 (4%) | 4 (21%) | 0 (0%) | 0 (0%) | 5 (4%) |
| >0.2 | 2 (8%) | 4 (21%) | 3 (10%) | 1 (2%) | 10 (8%) |
| uACR | 0.0041 (0.0026, 0.0083) | 0.0114 (0.0045, 0.0304) | 0.0038 (0.0025, 0.0074) | 0.0035 (0.0025, 0.0053) | 0.0039 (0.0029, 0.0082) |
| >0.03 | 3 (12%) | 5 (26%) | 1 (3%) | 0 (0%) | 9 (8%) |
| Estimated GFR (ml/min/1.732) | 97.01 (87.6, 108.01) | 90.3 (75.35, 100.28) | 102.58 (90.18, 114.46) | 98.62 (86.03, 108.87) | 97.43 (84.86, 110.69) |
| <60 | 1 (4%) | 2 (11%) | 1 (3%) | 0 (0%) | 4 (3%) |
| <90 | 8 (31%) | 9 (47%) | 7 (24%) | 15 (34%) | 39 (33%) |
| Serum IL-8 (pg/ml) | 2.6 (1.3, 4.1) | 3.4 (2.6, 3.9) | 1.8 (1.3, 3.3) | 2.2 (1.5, 3.1) | 2.4 (1.5, 3.5) |
| Serum RANTES (pg/ml) | 28,581 (7,229, 102,655) | 9,800 (166, 23,211) | 20,475 (22, 57,118) | 7,528 (79, 35,410) | 14,336 (228, 49,823) |
| Serum MCP-1 (pg/ml) | 201 (170, 281) | 301 (220, 441) | 200 (161, 250) | 185 (155, 236) | 200 (166, 277) |
| Serum IP-10 (pg/ml) | 423 (234, 542) | 524 (435, 778) | 169 (147, 268) | 124 (95, 181) | 196 (133, 428) |
| Serum B2M (mcg/ml) | 3,205,500 (2,198,900, 3,656,775) | 4,005,500 (3,415,750, 6,319,250) | 1,528,100 (1,079,515, 1,882,200) | 1,878,900 (1,603,750, 2,229,750) | 2,128,700 (1,663,150, 3,401,450) |
| Urine IL-8/Cr (× 10−9) | 15.6 (3.2, 33.2) | 11.5 (5.7, 32.2) | 4.1 (2.0, 14.1) | 5.1 (3.0, 11.1) | 6.4 (2.6, 17.9) |
| Urine RANTES/Cr (× 10−9) | 8.5 (5.2, 14.3) | 13.0 (9.8, 17.7) | 8.1 (5.9, 11.8) | 9.5 (7.1, 14.9) | 9.7 (6.7, 14.9) |
| Urine MCP-1/Cr (× 10−7) | 3.4 (2.6, 5.8) | 5.4 (4.0, 6.1) | 4.1 (2.5, 5.4) | 3.4 (2.5, 4.9) | 3.9 (2.6, 5.5) |
| Urine IP-10/Cr (× 10−8) | 9.3 (6.2, 21.8) | 13.8 (8.3, 25.7) | 1.1 (0.16, 3.0) | 3.7 (2.7, 5.5) | 4.1 (2.6, 11.1) |
| Urine B2M/Cr | 41.7 (29.4, 63.7) | 36.7 (20.8, 46.9) | 9.2 (7.3, 12.8) | 18.3 (12.7, 25.2) | 19.5 (11.1, 36.7) |
For the HIV-infected groups only (Groups A, B, C).
Note: categorical variables presented as count (percentage) and continuous variables presented as median (quartile 1, quartile 3). Group A, HIV-infected, antiretroviral-naive, CD4 cell counts >350/μl; Group B, HIV-infected, antiretroviral-naive, initiating antiretroviral therapy; Group C, HIV-infected, virologically suppressed on tenofovir/emtricitabine/efavirenz; Group D, HIV-uninfected, healthy controls.
cr, creatinine; GFR, glomerular filtration rate; IL-8, interleukin-8; RANTES, regulated on activation normal T cell expressed and secreted; MCP-1, monocyte chemotactic protein-1; IP-10, interferon-γ-induced protein 10; B2M, β2-microglobulin; NA, not applicable; uPCR, urine protein to creatinine ratio; uACR, urine albumin to creatinine ratio.
Comparisons of inflammatory markers between groups
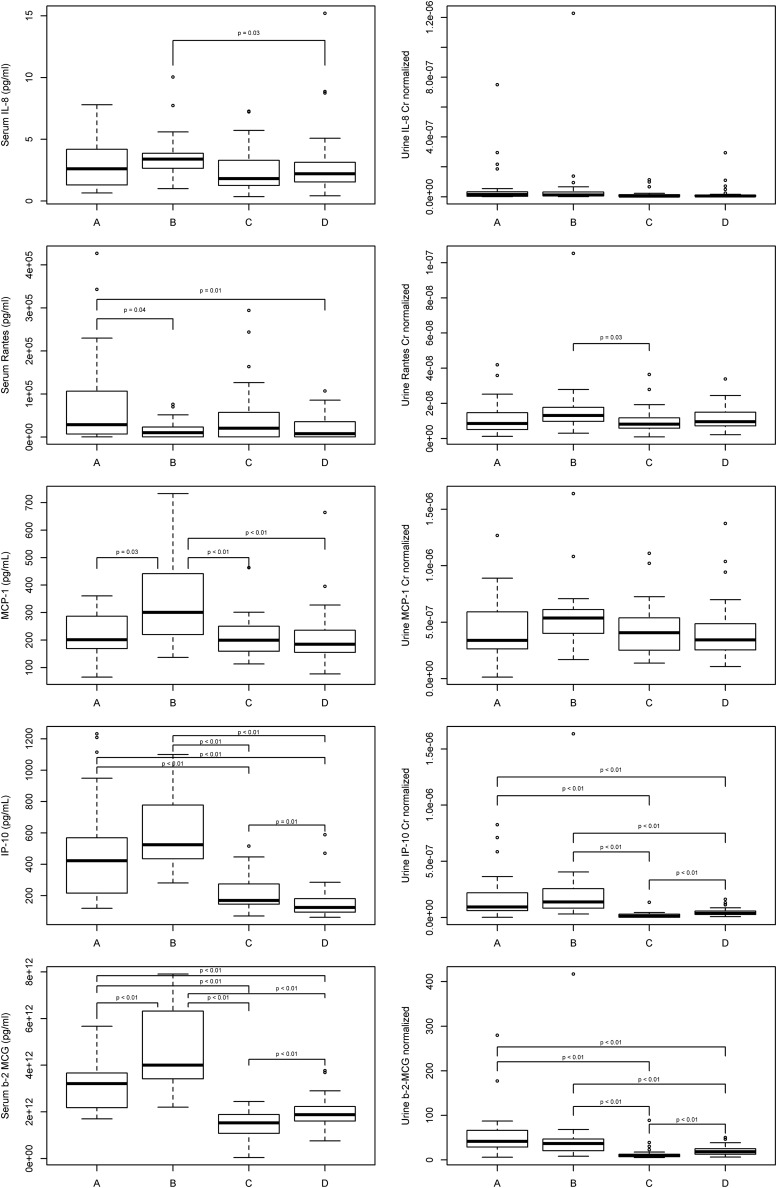
The levels of each urine and serum inflammatory marker are shown in Table 1. The statistically significant differences between groups (corrected for multiple testing) are shown in Fig. 1. Except for urine IL-8/Cr and urine MCP-1/Cr, for which no statistically significant differences were found between any of the pairwise group comparisons, we found at least one statistically significant difference in pairwise comparisons for the other eight inflammatory marker measurements. There did not appear to be similar patterns across groups when comparing serum and urine biomarker levels for IL-8, RANTES, or MCP-1. However, there did appear to be similar patterns between serum and urine IP-10 and B2M levels, with the highest levels in Group B followed by Group A. Significantly lower levels of serum and urine levels of IP-10 and B2M were found in Groups C and D compared to the untreated HIV-1-infected Groups A and B. Of note, given the marked differences in black race representation between the HIV-infected groups and the uninfected group, we specifically visually examined the distributions of each inflammatory marker by race and did not discern any obvious differences.
FIG. 1.
Pairwise comparisons of serum and urine levels of interleukin-8 (IL-8), regulated on activation normal T cell expressed and secreted (RANTES), monocyte chemotactic protein-1 (MCP-1), interferon-γ-induced protein-10 (IP-10), and β2-microglobulin (B2M) between the four study groups. Only p-values <0.05 after Holm–Bonferroni corrections are shown. The boxplots denote quartile 3 (top line of box), median (middle line within the box), and quartile 1 (bottom line of box) for each distribution of inflammatory marker. The two outermost horizontal lines represent the maximum and minimum values if no outliers were detected. Outlier values were drawn as open circles for data points smaller than [quartile 1–1.5 times the interquartile range (drawn as the lowest horizontal line)] or larger than [quartile 3+1.5 times the interquartile range (drawn as the highest horizontal line)].
To account for differences in age, race, sex, and smoking status between groups, we then constructed adjusted models accounting for these demographic variables to assess for differences in each inflammatory marker between Group D as the HIV-uninfected, healthy control reference group with each of the three HIV-infected groups (Table 2). In these adjusted comparisons, Group B was found to have significantly higher levels of urine IL-8, urine RANTES, and urine MCP-1 compared to Group D. Urine IP-10 and B2M levels were significantly higher in Groups A and B, while significantly lower in Group C, when compared to group D. Group B had significantly greater serum IL-8 and serum MCP-1 levels compared to Group D. Serum RANTES levels remained significantly higher in Group A compared to Group D. Serum IP-10 levels in each of the HIV-infected groups were significantly higher compared to the uninfected Group D. Groups A and B had significantly higher levels of serum B2M, whereas Group C had significantly lower levels, when compared to Group D.
Table 2.
Comparisons of Each HIV-Infected Group (Groups A, B, C) with the Uninfected Control Group for Each Inflammatory Marker After Adjustment for Demographic Characteristics
| Urine IL-8/Cr | Urine RANTES/Cr | Urine MCP-1/Cr | Urine IP-10/Cr | Urine B2M/Cr | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | Coefficient | p-value |
| Group A | 0.7178 | 0.1187 | −0.5663 | 0.231 | 0.0039 | 0.9931 | 2.1304 | 0.0001 | 2.5113 | 0.0001 |
| Group B | 1.2072 | 0.0167 | 1.0986 | 0.0207 | 1.086 | 0.0288 | 2.7256 | 0.0001 | 2.1692 | 0.0001 |
| Group C | −0.0594 | 0.8897 | −0.2577 | 0.5753 | 0.0937 | 0.8394 | −1.7248 | 0.0004 | −1.4235 | 0.0025 |
| Serum IL-8 | Serum RANTES | Serum MCP-1 | Serum IP-10 | Serum B2M | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | Coefficient | p-value |
| Group A | 0.2279 | 0.6188 | 1.3117 | 0.0035 | 0.5752 | 0.194 | 3.9128 | 0.0001 | 2.7593 | 0.0001 |
| Group B | 1.0667 | 0.0252 | −0.2258 | 0.6358 | 2.2372 | 0.0001 | 5.0374 | 0.0001 | 4.873 | 0.0001 |
| Group C | −0.4025 | 0.3808 | 0.3289 | 0.4842 | 0.1958 | 0.6446 | 1.4772 | 0.0011 | −1.2114 | 0.0123 |
Note: each group was compared to Group D as the reference group. All comparisons were adjusted for age, smoking status, race, and gender. Bolded data are those with p-values<0.05. Group A, HIV-infected, antiretroviral-naive, CD4 cell counts >350/μl; Group B, HIV-infected, antiretroviral-naive, initiating antiretroviral therapy; Group C, HIV-infected, virologically suppressed on tenofovir/emtricitabine/efavirenz; Group D, HIV-uninfected, healthy controls.
Cr, creatinine; IL-8, interleukin-8; RANTES, regulated on activation normal T cell expressed and secreted; MCP-1, monocyte chemotactic protein-1; IP-10, interferon-γ-induced protein 10; B2M, β2-microglobulin.
Correlations between serum and urinary levels of each inflammatory marker
Table 3 shows correlations between paired serum and urinary levels of each inflammatory marker in all four study groups and in the combined group. Statistically significant positive correlations were found between serum and urinary levels of IP-10 and B2M when all four study groups were combined. When correlations were analyzed separately in each study group, serum and urinary levels of IP-10 were significantly positively correlated in Group A and in Group B. Serum and urine MCP-1 levels were significantly negatively correlated in Group A. There were no significant correlations between any paired serum and urine inflammatory markers in Groups C and D.
Table 3.
Correlations Between Paired Serum and Urine Inflammatory Markers in Each Study Group and Overall
| HIV-infected | HIV-uninfected | ||||
|---|---|---|---|---|---|
| Inflammatory marker | A (n=26) | B (n=19) | C (n=30) | D (n=45) | Overall (n=120) |
| IL-8 | 0.02 (0.9102) | 0.26 (0.2761) | 0.05 (0.7945) | 0.06 (0.6974) | 0.09 (0.3559) |
| RANTES | 0.3 (0.1312) | 0.28 (0.2374) | 0.12 (0.5486) | 0.28 (0.0681) | 0.17 (0.0691) |
| MCP-1 | −0.6 (0.0013) | 0.33 (0.1726) | 0.03 (0.8833) | 0.21 (0.166) | 0.09 (0.3408) |
| IP-10 | 0.45 (0.0213) | 0.78 (0.0001) | 0.24 (0.2121) | 0.07 (0.6518) | 0.54 (<0.0001) |
| B2M | −0.19 (0.3533) | 0.25 (0.3094) | 0.06 (0.7781) | −0.26 (0.0936) | 0.44 (<0.0001) |
Note: data presented as correlation coefficient (p-value). Bolded data are those with p-values <0.05. Group A, HIV-infected, antiretroviral-naive, CD4 cell counts >350/μl; Group B, HIV-infected, antiretroviral-naive, initiating antiretroviral therapy; Group C, HIV-infected, virologically suppressed on tenofovir/emtricitabine/efavirenz; Group D, HIV-uninfected, healthy controls.
IL-8, interleukin-8; RANTES, regulated on activation normal T cell expressed and secreted; MCP-1, monocyte chemotactic protein-1; IP-10, interferon-γ-induced protein 10; B2M, β2-microglobulin.
Correlations between inflammatory markers and renal parameters
Table 4 shows the correlations between each inflammatory marker and uPCR, uACR, and eGFR in the entire study cohort of 120 participants. uPCR was positively correlated with urine IL-8/Cr, urine MCP-1/Cr, urine IP-10/Cr, and serum IP-10. uACR was positively correlated with urine IL-8, urine B2M, serum IP-10, and serum B2M. eGFR was negatively correlated with both serum MCP-1 and serum B2M. When each study group was analyzed separately (data not shown), however, only urine B2M was significantly positively correlated with uACR in Group A (rho=0.64; p=0.016), and serum MCP-1 was negatively correlated with eGFR in Group D (rho=−0.51; p=0.01). There were no other significant correlations within each study group after adjusting for multiple comparisons.
Table 4.
Correlations Between Inflammatory Makers and Renal Parameters in the Overall Group (N=120)
| Inflammatory marker | uPCR | uACR | Estimated GFR |
|---|---|---|---|
| Urine IL-8/Cr | 0.31 (0.0176) | 0.33 (0.0073) | 0.06 (1) |
| Urine RANTES/Cr | 0.27 (0.0652) | 0.27 (0.0669) | −0.03 (1) |
| Urine MCP-1/Cr | 0.42 (<0.0001) | 0.26 (0.0796) | −0.04 (1) |
| Urine IP-10/Cr | 0.31 (0.0205) | 0.23 (0.2466) | −0.19 (0.4835) |
| Urine B2M/Cr | 0.18 (0.6288) | 0.41 (<0.0001) | −0.09 (1) |
| Serum IL-8 (pg/ml) | 0.22 (0.307) | 0.14 (1) | −0.09 (1) |
| Serum RANTES (pg/ml) | 0.12 (1) | 0.06 (1) | 0.12 (1) |
| Serum MCP-1 (pg/ml) | 0.19 (0.4519) | 0.12 (1) | −0.49 (<0.0001) |
| Serum IP-10 (pg/ml) | 0.39 (<0.0003) | 0.32 (0.0092) | −0.21 (0.3186) |
| Serum B2M (mcg/ml) | 0.25 (0.1208) | 0.3 (0.0205) | −0.34 (0.0065) |
Note: data presented as correlation coefficient (p-value). Bolded data are those with p-values<0.05 after adjustment for multiple testing.
Cr, creatinine; GFR, glomerular filtration rate; IL-8, interleukin-8; RANTES, regulated on activation normal T cell expressed and secreted; MCP-1, monocyte chemotactic protein-1; IP-10, interferon-γ-induced protein 10; B2M, β2-microglobulin; uPCR, urine protein to creatinine ratio; uACR; urine albumin to creatinine ratio.
Discussion
To our knowledge, this is the first study investigating urinary inflammatory markers and their potential contributions to nephropathy in HIV infection. We expanded upon our previous pilot study10 by measuring paired serum and urine markers in HIV-infected patients not receiving (Groups A and B) and receiving ART (Group C) as well as in healthy uninfected volunteers (Group D). As seen in Fig. 1, we found differing patterns of urinary inflammatory biomarker levels across these study groups. Moreover, some urinary markers did (IP-10, B2M) whereas others did not (IL-8, RANTES, MCP-1) mirror patterns of their paired serum levels across the four study groups. In addition, there were varying relationships between these urinary inflammatory markers with markers of renal injury (proteinuria and albuminuria) and renal function (eGFR).
Because of imbalances in demographic characteristics among the study groups, we also adjusted for race, sex, age, and smoking status to further assess if the inflammatory markers in the HIV-infected groups were significantly different from the uninfected controls. The differences seen in pairwise group comparisons for the serum biomarkers were confirmed in these multivariable analyses, as shown in Table 2. After multivariable adjustment, significantly higher urinary levels of IL-8, RANTES, and MCP-1 (in addition to urinary IP-10 and B2M) were now found in Group B compared to Group D. As such, it appears that untreated HIV-infected patients with lower CD4 cell counts have greater levels of renal inflammation, as assessed using all five urine biomarkers in the current study, compared to healthy controls. This may reflect greater overall inflammation and, in particular, intrarenal immune activation18 found in those with greater immunosuppression. Previous studies have suggested that HIV infection is associated with tubular or glomerular defects, therefore the urinary excretion of various proteins, including B2M, might be increased.19–21 Surprisingly, the urinary levels of IP-10 and B2M were higher in healthy volunteers than in HIV-infected patients virally suppressed with TDF/FTC/EFV. Previous observational studies suggest that the urinary B2M level would be increased in patients receiving tenofovir-based regimens due to tenofovir-induced tubulopathy.20 It is possible that some unmeasured confounding led to these unexpected results.
As shown in Table 3, urine IP-10 and B2M levels (but not IL-8, RANTES, or MCP-1) were significantly correlated with their serum counterparts in the entire study cohort, with paired IP-10 levels strongly correlated, in particular, in the untreated HIV-infected Groups A and B. The reasons for these findings are unclear. All five biomarkers studied here are low-molecular-weight proteins with similar weights ranging from ∼8 kDa to 13 kDa. So if glomerular filtration of their respective circulating serum levels was responsible for the correlations between serum and urine levels, then we would have expected significant correlations between paired serum and urine levels with all five biomarkers. The absence of correlations for some of the biomarkers suggests that there may be compartmentalization between the circulating and intrarenal markers of inflammation studied here.
The most clinically relevant analyses were those focused on the correlations between inflammatory biomarkers and markers of renal disease. In the overall cohort, we found that serum IP-10 levels and urinary levels of IL-8, MCP-1, and IP-10 were each significantly correlated with proteinuria. In contrast, urinary IL-8 and B2M and serum B2M and IP-10 were significantly correlated with albuminuria in the overall cohort. Given that proteinuria in HIV is mostly comprised of nonalbuminuric globulin proteins,15,22 which could reflect greater systemic immune activation or tubulopathy, whereas albuminuria primarily reflects glomerular disease, we expected to find different correlation patterns between these renal injury markers and the inflammatory markers assessed. IL-8 levels are associated with greater neutrophil migration and T cell activation but do not reflect monocyte activation. MCP-1, in contrast, reflects greater monocyte and T cell activation and migration.
IP-10 is an interferon-induced protein that signals monocyte and T cell migration. Interestingly, interferons may induce a renal focal segmental glomerulosclerosis that is histologically similar to HIV-associated nephropathy (HIVAN).23 In addition, the apolipoprotein L1 (ApoL1) gene, whose polymorphisms predispose to HIVAN,24,25 itself may be induced by interferon-γ.26 Thus, IP-10 may have particular significance as a marker of HIV-related renal diseases. B2M is a general immune activation marker. In contrast to our pilot study, RANTES, a marker of T cell activation and migration, was not associated with either proteinuria or albuminuria in the current analyses. Thus, our results support both monocyte and neutrophil signals of activation and migration as potential contributors to HIV-related proteinuria and albuminuria. However, these findings should be interpreted cautiously. Of note, the only within group correlation in relation to renal injury was found between urinary B2M and uACR in Group A; we do not have an obvious reason for the lack of a similar correlation in the Group B HIV-infected participants with greater immunodeficiency.
We also noted in the overall cohort that urinary inflammation markers were not associated with renal function, although serum MCP-1 and B2M were statistically significantly and negatively correlated with eGFR. However, the relationship between MCP-1 and eGFR in the overall cohort may have been driven solely by the uninfected controls (Group D) and thus were not pertinent to HIV-infected patients. It should be noted that the significant correlations found had rho values <0.5, suggesting that the contribution of inflammation, as measured using the markers in this study, to renal disease in HIV may be modest. Our study is limited in that we did not have histological data in this study to assess whether the chemokines measured were directly related to an actual cellular influx of monocytes or neutrophils. Biopsy studies done previously from patients with HIVAN demonstrate a dense infiltrate of monocytes with increased production of MCP-1, RANTES, IL-8, and IP-10.18 To our knowledge, similar histological data are lacking in patients on ART.
Additional limitations of our study should be noted. The sample sizes of the individual study groups may have been too small to detect significant differences between groups in some of the biomarker levels and to detect significant correlations between inflammatory biomarkers and renal disease markers within study groups. Although the proportions of participants with abnormal uPCR and uACR levels were similar to those reported previously, the absolute numbers of such participants were relatively small. The cross-sectional study design does not allow us to make inferences regarding causality between inflammation and nephropathy in HIV. The results of our study as they pertain to ART-treated HIV-infected participants (Group C) are limited only to those receiving tenofovir/emtricitabine/efavirenz; it is not known if the results would differ for other treatment regimens. We also studied only five biomarkers; future studies will need to assess whether HIV-related nephropathy may be associated with other markers of inflammation or immune activation. Finally, the imbalance in black race representation between the HIV-infected and uninfected study groups may have confounded the results.
Conclusions
To our knowledge, this is the first study of its kind to assess intrarenal and systemic inflammatory markers in various groups of HIV-infected patients receiving and not receiving ART vs. uninfected controls. We found that intrarenal inflammation, as assessed in our study, is greater in untreated HIV-infected patients, especially those with low CD4 counts, when compared to both HIV-infected patients receiving virologically suppressive ART and to uninfected controls. Proteinuria and albuminuria in the overall study cohort were variably associated with different inflammatory marker profiles, possibly reflecting different inflammatory pathways. However, we did not find strong relationships between inflammatory markers and renal markers in those with HIV, whether treated with ART or not. Future studies using antiinflammatory therapies and/or antiretroviral therapy will be needed to determine whether renal inflammation is causally related to both HIVAN as well as the other various nephropathies found in those infected with HIV.
Acknowledgments
We thank the study participants for their generous donation of time and effort in the successful completion of these studies. We also thank Elisha Largent, Beth Zwickl, and Vicki Mravca-Wilkey for study coordination; Jonathon Mathews for data management; Dr. Russell Tracy and Elaine Cornell for serum MCP-1 and IP-10 measurements; and Hui Lin Chua and Artur Plett for the urine inflammation multiplex biomarker measurements and for the serum B2M, RANTES, and IL-8 measurements.
This work was supported by the NIH [R01HL095149; P30AI36219]. Additional support was provided by the Indiana Clinical and Translational Sciences Institute funded in part from the National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award [grant TR000006] and from the National Center for Research Resources [RR020128] at the National Institutes of Health. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
T.S., M.M.L., and S.K.G. contributed to the study conception. T.S., Z.L., and S.K.G. contributed to the study design and initial drafting of the manuscript. D.M. and Z.L. contributed to the statistical analysis. C.M.O. and S.K.G. contributed to the acquisition of data. All the authors contributed to the interpretation of the results and critical revision of the manuscript. All authors have seen and approved the final version of the submitted manuscript.
Author Disclosure Statement
S.K.G. has received research grants from Merck & Co., Inc., Janssen Pharmaceutics, Inc., and Gilead Sciences, Inc., and advisory/consultancy/lecture fees from Bristol-Myers Squibb and Merck & Co., Inc.
References
- 1.Szczech LA, Gange SJ, van der Horst C, et al. : Predictors of proteinuria and renal failure among women with HIV infection. Kidney Int 2002;61:195–202 [DOI] [PubMed] [Google Scholar]
- 2.Szczech LA, Grunfeld C, Scherzer R, et al. : Microalbuminuria in HIV infection. AIDS 2007;21:1003–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi AI, Rodriguez RA, Bacchetti P, et al. : The impact of HIV on chronic kidney disease outcomes. Kidney Int 2007;72:1380–1387 [DOI] [PubMed] [Google Scholar]
- 4.Szczech LA, Hoover DR, Feldman JG, et al. : Association between renal disease and outcomes among HIV-infected women receiving or not receiving antiretroviral therapy. Clin Infect Dis 2004;39:1199–1206 [DOI] [PubMed] [Google Scholar]
- 5.Wyatt CM, Hoover DR, Shi Q, et al. : Pre-existing albuminuria predicts AIDS and non-AIDS mortality in women initiating antiretroviral therapy. Antivir Ther 2011;16:591–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyatt CM, Hoover DR, Shi Q, et al. : Microalbuminuria is associated with all-cause and AIDS mortality in women with HIV infection. J Acquir Immune Defic Syndr 2010;55:73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner LI, Holmberg SD, Williamson JM, et al. : Development of proteinuria or elevated serum creatinine and mortality in HIV-infected women. J Acquir Immune Defic Syndr 2003;32:203–209 [DOI] [PubMed] [Google Scholar]
- 8.Choi A, Scherzer R, Bacchetti P, et al. : Cystatin C, albuminuria, and 5-year all-cause mortality in HIV-infected persons. Am J Kidney Dis 2010;56:872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta SK, Komarow L, Gulick RM, et al. : Proteinuria, creatinine clearance, and immune activation in antiretroviral-naive HIV-infected subjects. J Infect Dis 2009;200:614–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S, Heeger P, Lai X, et al. : Intrarenal Inflammation Contributes to HIV-Related Proteinuria (Abstract 736). The 17th Conference on Retroviruses and Opportunistic Infections, San Francisco, CA, 2010 [Google Scholar]
- 11.Gupta SK, Mi D, Dube MP, et al. : Pentoxifylline, inflammation, and endothelial function in HIV-infected persons: A randomized, placebo-controlled trial. PLoS One 2013;8:e60852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta SK, Mi D, Moe SM, et al. : Effects of switching from efavirenz to raltegravir on endothelial function, bone mineral metabolism, inflammation, and renal function: A randomized, controlled trial. JAIDS J Acquir Immune Defic Syndr 2013;64:279–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inker LA, Schmid CH, Tighiouart H, et al. : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris G. and Chen W: Profiling of cytokine and chemokine responses using multiplex bead array technology. Methods Mol Biol 2013;1061:265–278 [DOI] [PubMed] [Google Scholar]
- 15.Gupta SK, Shen C, Mather KJ, et al. : Neither proteinuria nor albuminuria is associated with endothelial dysfunction in HIV-infected patients without diabetes or hypertension. J Infect Dis 2011;204:1946–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta SK, Smurzynski M, Franceschini N, et al. : The effects of HIV type-1 viral suppression and non-viral factors on quantitative proteinuria in the highly active antiretroviral therapy era. Antivir Ther 2009;14:543–549 [PMC free article] [PubMed] [Google Scholar]
- 17.Mocroft A, Kirk O, Reiss P, et al. : Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS 2010;24:1667–1678 [DOI] [PubMed] [Google Scholar]
- 18.Kimmel PL, Cohen DJ, Abraham AA, et al. : Upregulation of MHC class II, interferon-alpha and interferon-gamma receptor protein expression in HIV-associated nephropathy. Nephrol Dial Transplant 2003;18:285–292 [DOI] [PubMed] [Google Scholar]
- 19.Kabanda A, Vandercam B, Bernard A, et al. : Low molecular weight proteinuria in human immunodeficiency virus-infected patients. Am J Kidney Dis 1996;27:803–808 [DOI] [PubMed] [Google Scholar]
- 20.Labarga P, Barreiro P, Martin-Carbonero L, et al. : Kidney tubular abnormalities in the absence of impaired glomerular function in HIV patients treated with tenofovir. AIDS 2009;23:689–696 [DOI] [PubMed] [Google Scholar]
- 21.Baekken M, Os I, Sandvik L, and Oektedalen O: Microalbuminuria associated with indicators of inflammatory activity in an HIV-positive population. Nephrol Dial Transplant 2008;23:3130–3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell L, Dew T, Salota R, et al. : Total protein, albumin and low-molecular-weight protein excretion in HIV-positive patients. BMC Nephrol 2012;13:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markowitz GS, Nasr SH, Stokes MB, and D'Agati VD: Treatment with IFN-{alpha}, -{beta}, or -{gamma} is associated with collapsing focal segmental glomerulosclerosis. Clin J Am Soc Nephrol: CJASN 2010;5:607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fine DM, Wasser WG, Estrella MM, et al. : APOL1 risk variants predict histopathology and progression to ESRD in HIV-related kidney disease. J Am Soc Nephrol 2012;23:343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopp JB, Nelson GW, Sampath K, et al. : APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 2011;22:2129–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sidaway P: Glomerular disease: Innate immunity–APOL1 interaction. Nat Rev Nephrol 2014;10:543–543 [DOI] [PubMed] [Google Scholar]