Abstract
Background
In metastatic renal cell carcinoma (mRCC) patients treated with targeted agents and their primary tumor (PT) in situ, early PT decrease in size correlates with improved overall PT response, but the effect on overall survival (OS) is unknown.
Objective
To evaluate whether early PT size reduction is associated with improved OS in patients with mRCC undergoing treatment with sunitinib.
Design, setting, and participants
We reviewed the clinical and radiographic data of all mRCC patients seen at our institution between January 2004 and December 2009 without prior systemic treatment who received sunitinib with their PT in situ.
Measurements
Two independent reviewers measured the diameter of the PT and metastatic disease at baseline and subsequent scans to assess response. Early minor response was defined as ≥10% decrease within 60 d of treatment initiation. Univariate and multivariate analyses were used to calculate a hazard ratio (HR) corresponding to the risk of death based on clinical and pathologic factors as well as PT response.
Results and limitations
We identified 75 consecutive patients with a median follow-up of 15 mo. All patients were intermediate or poor risk by common risk stratification systems. Median initial PT diameter was 9.7 cm. Median maximum PT size reduction was −10.2% overall and −36.4% in patients who had early minor PT response.
Median OS for patients with <10% decrease in PT, ≥10% decrease in PT after 60 d, and ≥10% decrease in PT within the first 60 d was 10.3, 16.5, and 30.2 mo, respectively. On multivariate analysis, early minor response was an independent predictor of improved OS (HR: 0.26; p = 0.031). Other significant predictors included venous thrombus, multiple bone metastases, lactate dehydrogenase above the upper limit of normal, symptoms at presentation, and more than two metastatic sites.
Conclusions
Early minor PT response is associated with improved OS. Future studies should evaluate this prognostic factor to identify patients with prolonged OS.
Keywords: Renal cell carcinoma, Sunitinib, Targeted therapy, Primary tumor response, Metastatic, Overall survival
1. Introduction
The natural history of metastatic renal cell carcinoma (mRCC) is variable with most patients surviving <2 yr. Several studies have identified prognostic factors for overall survival (OS) and separated patients into risk groups based on expected survival [1–3]. However, it remains difficult to estimate individual patient survival or predict response to newer therapies.
Over the last decade, agents that target angiogenesis or cell growth pathways have replaced immunotherapy as the first-line systemic treatment for most mRCC patients [4]. Although the traditional approach of cytoreductive nephrectomy before systemic therapy for mRCC remains an accepted paradigm, randomized trials are underway to investigate the optimal role and timing of surgery with targeted therapy [5,6]. The available data for the treatment of mRCC patients with targeted agents and their primary tumor (PT) in situ come from retrospective series [7–9] or small feasibility trials [10–12] that demonstrate dramatic decreases in the PT size are possible but occur rarely (6–16%). Most patients experience some decrease in PT size or no growth while on treatment, but how this affects survival is unknown. In studies with RCC and other metastatic solid tumors, several investigators have questioned whether using Response Evaluation Criteria in Solid Tumors (RECIST) definitions is an appropriate metric because targeted agents frequently elicit responses that do not meet radiologic criteria for partial response, although patients have apparent survival benefit [13–15].
In a prior study of mRCC patients treated with targeted agents, we identified early minor PT response as a predictor of improved overall PT response as well as an increased likelihood of response in metastatic sites [7]. However, no study to date has examined whether a response in the PT may correlate with OS, which is an important factor to consider in patients when initial cytoreductive nephrectomy is deferred. Therefore, the purpose of this study was to evaluate whether an early minor PT response was associated with improved OS in patients with mRCC undergoing treatment with sunitinib.
2. Material and methods
2.1. Patients
After institutional review board approval, medical records were collected to identify all treatment-naive patients with mRCC and primary kidney tumor in place treated with sunitinib from November 1, 2004, to December 31, 2009. Eligible patients for the study included those who had computed tomography (CT) imaging at baseline before treatment initiation and at least one follow-up CT scan while on treatment with sunitinib. OS times were calculated using the Kaplan-Meier method and corresponded to the time of treatment initiation to time of death or last known follow-up, whichever occurred earlier. Patients undergoing delayed cytoreductive nephrectomy were censored at the time of surgery.
2.2. Assessment of treatment response
To evaluate the response of the PT to treatment with sunitinib, two independent reviewers measured the diameter of the primary and metastatic tumors at baseline imaging and subsequent scans according to RECIST v.1.1. A minor response was defined as ≥10% decrease in the PT or sum of longest diameters (SLD) for metastatic sites. Responses within 60 d of treatment initiation were defined as early responses. Pathologic data (eg, histology, Fuhrman nuclear grade, presence of sarcomatoid dedifferentiation) were obtained from biopsy results of the PT unless the patient had undergone later cytoreductive nephrectomy, in which case these data were obtained from the final surgical specimen.
2.3. Statistical analyses
Univariate and multivariate Cox proportional hazards regression analyses were used to calculate a hazard ratio (HR) corresponding to the risk of death based on clinical and pathologic factors as well as PT response. Factors considered in analyses included age, gender, race, laboratory results at treatment initiation (lactate dehydrogenase, serum calcium, corrected serum calcium, albumin, white cell count, absolute neutrophil count, absolute lymphocyte count, monocyte count, platelet count, eosinophil count, alkaline phosphatase, creatinine), tumor size, Fuhrman grade, histology, stage, presence of venous thrombus, symptoms at presentation (metastatic, local, and systemic), smoking status, number of metastatic sites, site of metastases, and number of metastases within the organ site. A p value (two sided) <0.05 was considered significant. For all analyses, Stata software v.10.1 (StataCorp, College Station, TX, USA) was used.
3. Results
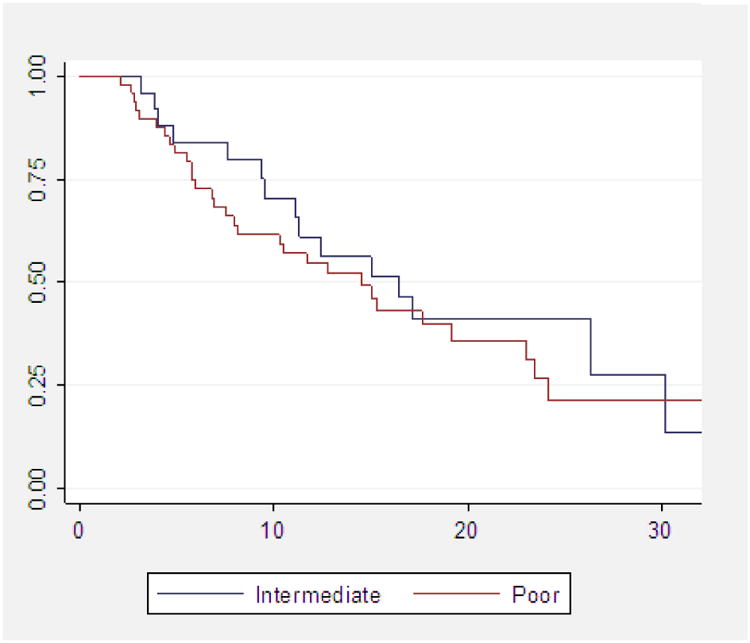
We identified 75 patients who met the inclusion criteria for the study. Median follow-up time was 15 mo (95% confidence interval [CI], 10.5–23.0). Table 1 lists the characteristics of the patient population. Decision to start sunitinib as primary treatment and to defer cytoreductive nephrectomy was at the discretion of the treating physician. Clinical rationale to defer surgery included doctor/patient preference in 24 patients (32.0%), widespread metastatic disease in 19 patients (25.3%), brain metastasis, non– clear cell or sarcomatoid dedifferentiation on biopsy in 13 patients (17.3%), poor Eastern Cooperative Oncology Group performance status or significant comorbidity in 11 patients (14.7%), or unresectable PT in 8 patients (10.7%). Using prognostic criteria described at Memorial Sloan-Kettering Cancer Center (MSKCC) by Motzer et al [16], 26 (34.7%) and 49 (65.3%) were intermediate or poor risk, respectively. Using prognostic criteria described by Heng et al [2], 38 (50.7%) and 37 (49.3%) were intermediate or poor risk, respectively. Table 2 shows MSKCC criteria based on time and intensity of response. MSKCC risk grouping (intermediate vs poor) did not correlate with OS on univariate (HR: 1.31; 95% CI, 0.70–2.44; p = 0.393) or multivariate analysis. There is no correlation between MSKCC risk group and minor PT response (odds ratio [OR]: 1.04; 95% CI, 0.40– 2.70, p = 0.933).
Table 1. Patient and disease characteristics.
| Age, yr, median (range) | 60.0 (23–80) |
| Tumor diameter, cm, median (range) | 9.7 (1.8–21.7) |
| Body mass index, median (range) | 28.0 (17.8–45.6) |
| Gender, n (%) | |
| Male | 54 (72.0) |
| Female | 21 (28.0) |
| Race, n (%) | |
| White | 55 (73.3) |
| African American | 6 (8.0) |
| Hispanic | 9 (12.0) |
| Asian | 4 (5.3) |
| Other | 1 (1.3) |
| Histology, n (%) | |
| Clear cell | 55 (73.3) |
| Papillary | 2 (2.7) |
| Unclassified | 9 (12.0) |
| Unknown | 9 (12.0) |
| Sarcomatoid dedifferentiation | 5 (6.7) |
| Fuhrman nuclear grade, n (%) | |
| II | 12 (16.0) |
| III | 20 (26.7) |
| IV | 4 (5.3) |
| Unknown | 32 (42.7) |
| High grade | 7 (9.3) |
| ECOG PS, n (%) | |
| 0/1 | 52 (69.3) |
| 2 | 16 (21.3) |
| 3 | 7 (9.3) |
| Metastatic sites, n (%) | |
| 1 | 25 (33.3) |
| 2 | 25 (33.3) |
| ≥3 | 25 (33.3) |
| Side | |
| Right | 34 (45.3) |
| Left | 37 (49.3) |
| Bilateral | 4 (5.3) |
| Clinical T category, n (%) | |
| T1 | 14 (18.7) |
| T2 | 16 (21.3) |
| T3 | 34 (45.3) |
| T4 | 11 (14.7) |
| Symptoms at presentation, n (%) | |
| Local | 35 (46.7) |
| Systemic | 25 (33.3) |
| Metastatic | 35 (46.7) |
ECOG PS = Eastern Cooperative Oncology Group Performance Status.
Table 2. Common prognostic criteria based on time and intensity of primary tumor response.
| Total, n (%) | Early minor response, n (%) | Late minor response, n (%) | No response, n (%) | p | |
|---|---|---|---|---|---|
| Hgb < LLN | 52 (69.3) | 2 (28.6) | 23 (74.2) | 27 (73.0) | 0.049 |
| LDH > ULN | 7 (9.3) | 0 (0) | 2 (6.5) | 5 (13.5) | 0.409 |
| Corrected calcium >10 | 48 (64.0) | 4 (57.2) | 22 (71.0) | 22 (59.5) | 0.569 |
| Time from diagnosis to treatment <12 mo | 75 (100) | 7 (100) | 31 (100) | 34 (100) | – |
| ECOG PS >1 | 23 (30.7) | 1 (14.3) | 8 (25.8) | 14 (37.8) | 0.346 |
| Plt > ULN | 14 (18.7) | 2 (14.3) | 4 (12.9) | 8 (21.6) | 0.511 |
| ANC > ULN | 19 (25.3) | 3 (42.9) | 6 (19.4) | 10 (27.0) | 0.411 |
Hgb = hemoglobin; LLN = lower limit of normal; LDH = lactate dehydrogenase; ULN = upper limit of normal; ECOG PS = Eastern Cooperative Oncology Group Performance Status; Plt = platelet count; ANC = absolute neutrophil count.
The median tumor size at baseline was 9.7 cm (range: 1.8–21.7 cm). Median maximum overall change in PT size was −10.2% (range: −53.4 to 54.0) occurring at a median of 120 d (range: 76–202 d) following initiation of treatment with sunitinib (Fig. 1). A total of 38 patients (50.7%) had a maximum overall decrease in PT size of ≥10% at a median of 178 d (range: 89–251 d) following initiation of therapy with sunitinib. Seven of these patients (18.4%) reached a ≥10% decrease in PT size within the first 60 d of treatment with sunitinib. The median overall decrease in PT size was greater in these patients (−36.4%; range: −24.7 to −53.4) compared with those who did not reach a ≥10% decrease within the first 60 d (−17.1%; range: −10.2 to 37.3; p = 0.012).
Fig. 1. Waterfall plot of maximum primary tumor response to sunitinib.
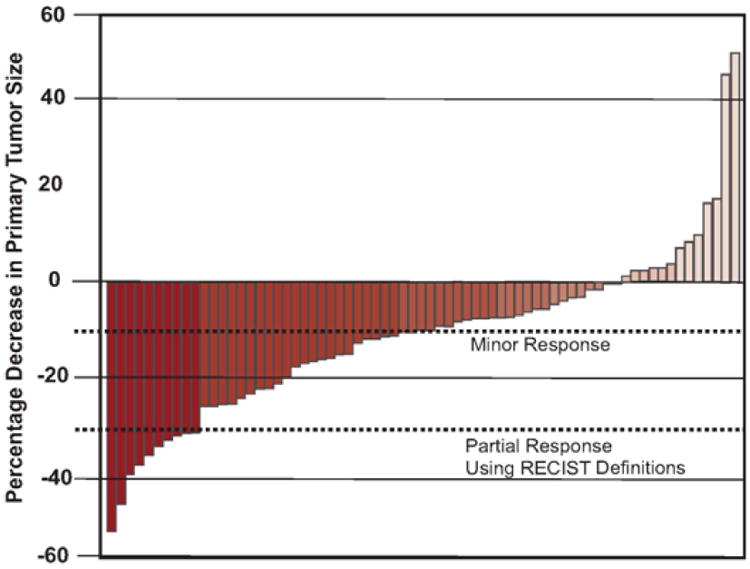
RECIST = Response Evaluation Criteria in Solid Tumors.
A total of 12 patients (16%) eventually underwent cytoreductive nephrectomy at a median time of 10.2 mo (range: 2.7–15.8 mo) following treatment initiation with sunitinib. All of these had a viable tumor in the resected specimen. Adverse events attributed to sunitinib therapy were noted in 17 patients (22.7%) requiring a reduction in dose at some point during therapy.
Median OS was higher in patients achieving ≥10% decrease in PT size as well as patients with early PT response (Fig. 4). Table 3 and 4 list the clinical and pathologic factors that were significant on univariate and multivariate stepwise Cox proportional hazards regression analysis. On multivariate analysis, achieving a ≥10% decrease in PT size was an independent predictor of OS (HR: 0.43; p = 0.007). When considering early response in multivariate analysis, achieving ≥10% decrease in PT size within the first 60 d of treatment was an even stronger independent predictor of OS (HR: 0.26; p = 0.031). Independent predictors of OS, when comparing response in the PT with response in metastatic lesions, are shown in Table 5 and 6 for univariate and multivariate analyses, respectively.
Fig. 4. Maximum primary tumor (PT) (median) response achieved and median overall survival according to level of PT response (p < 0.01).
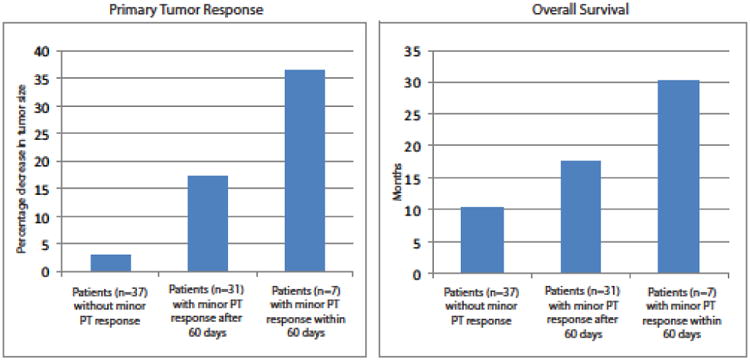
Table 3. Predictors of overall survival from univariate analysis.
| HR | 95% CI | |
|---|---|---|
| PT response, decrease in diameter | ||
| <10% | Referent | – |
| ≥10% and >60 d | 0.48 | 0.25–0.91 |
| ≥10% and ≤60 d | 0.26 | 0.08–0.82 |
| Venous thrombus | 1.44 | 1.20–1.73 |
| Radiographic retroperitoneal lymphadenopathy | 1.97 | 1.09–3.59 |
| Local symptoms at presentation | 2.08 | 1.15–3.77 |
| ECOG PS ≥2 | 2.11 | 1.11–4.00 |
| Liver metastases | 2.23 | 1.22–4.08 |
| Multiple bone metastases | 2.27 | 1.20–4.29 |
| Lactate dehydrogenase > ULN | 2.30 | 1.24–4.26 |
| Absolute lymphocyte count < LLN | 2.63 | 1.34–5.14 |
| No. of metastatic sites greater than two | 2.86 | 1.58–5.18 |
HR = hazard ratio; CI = confidence interval; PT = primary tumor; ECOG PS = Eastern Cooperative Oncology Group Performance Status; ULN = upper limit of normal value; LLN = lower limit of normal value.
Table 4. Predictors of overall survival based from multivariate analysis.
| HR | 95% CI | |
|---|---|---|
| ≥10% PT response in 60 d* | 0.26 | 0.08–0.89 |
| Renal vein or IVC thrombus | 1.33 | 1.09–1.63 |
| Multiple bone metastases | 2.05 | 1.00–4.21 |
| Lactate dehydrogenase > ULN | 2.42 | 1.26–4.63 |
| Local symptoms at presentation | 3.03 | 1.57–5.84 |
| No. of metastatic sites greater than two | 3.28 | 1.65–6.52 |
HR = hazard ratio; CI = confidence interval; PT = primary tumor; IVC = inferior vena cava; ULN = upper limit of normal value.
Compared with patients either exhibiting <10% response or >10% response after 60 d (ie, whole population).
Table 5. Univariate analysis of prognostic value for overall survival of minor responses in primary tumor and metastatic sites (sum of longest diameters).
| Response (n) | HR | 95% CI | p | Median OS |
|---|---|---|---|---|
| No minor response in PT or metastases (25) | Referent | – | – | 8.1 |
| Minor response in metastases only (12) | 0.37 | 0.14–0.92 | 0.033 | 15 |
| Late minor response in PT and metastases (17) | 0.47 | 0.22–1.00 | 0.051 | 15 |
| Late PT minor response only (14) | 0.20 | 0.08–0.55 | 0.002 | 24.1 |
| Early minor response in PT and metastases (3) | 0.19 | 0.05–0.79 | 0.023 | 30.2 |
| Early PT minor response only (4) | 0.12 | 0.02–0.94 | 0.043 | 26.3 |
HR = hazard ratio; CI = confidence interval; OS = overall survival; PT = primary tumor.
Table 6. Multivariate analysis of prognostic value for overall survival of minor responses in primary tumor and metastatic sites (sum of longest diameters).
| HR | 95% CI | p | |
|---|---|---|---|
| Early PT minor response only | 0.05 | 0.01–0.50 | 0.01 |
| Early minor response in PT and metastases | 0.18 | 0.04–0.87 | 0.033 |
| Minor response in metastases only | 0.21 | 0.08–0.56 | 0.002 |
| Late minor response in PT and metastases | 0.24 | 0.11–0.54 | 0.001 |
| Late PT minor response only | 0.29 | 0.10–0.79 | 0.016 |
| Presence of liver metastases | 2.26 | 1.09–4.69 | 0.028 |
| No. of metastatic sites greater than two | 3.27 | 1.57–6.81 | 0.002 |
| LDH > ULN | 3.42 | 1.66–7.04 | 0.001 |
HR = hazard ratio; CI = confidence interval; PT = primary tumor; LDH = lactate dehydrogenase; ULN = upper limit of normal.
4. Discussion
In patients treated with sunitinib with their PT in situ, response in the PT and especially early responses in the PT predict increased overall PT response and improved OS. This prognostic factor for OS has not been previously identified and should be considered in trials with up-front targeted therapy. If these findings are validated, early PT response may be used to identify patients with improved survival to help guide future therapy. Despite the clinical benefit of targeted therapies for most patients with mRCC, the median OS in a recent multicenter series remains <2 yr [2]. Interestingly, the survival of individual mRCC patients varies considerably, with some patients surviving many years after diagnosis. This variability in disease course was observed in large retrospective studies [2,16] but also in randomized clinical trials of patients who were selected based on their known good prognostic factors [17].
In the immunotherapy era, Motzer et al identified five prognostic factors from a large cohort of mRCC patients that could divide patients into three risk groups to predict survival [16]. OS was poor and varied significantly, with a median survival of 3.9 mo in poor risk patients compared with 19.9 mo in patients with favorable risk. In patients treated with targeted agents, Heng et al validated and expanded known prognostic factors [2]. Although OS had increased, significant variability in disease course was again observed in patients treated with targeted therapy. The OS in the current study was 15 mo, which would be predicted based on risk grouping. Furthermore, our 15-mo OS is comparable with OS observed in the intermediate- and poor-risk groups included in a phase 3 trial of sunitinib, at 5.3 and 20.7 mo, respectively [18].
Of the intermediate- and poor-risk patients in our study, it is important to note that early PT responses were able to identify and stratify the patients who survived longest (Fig. 3). The widely used and validated MSKCC prognostic grouping was generally predictive of overall poor survival in our cohort but could not identify survival differences between intermediate- and poor-risk patients in our study. Possible explanations include the relatively small size of our cohort or the multiple other poor prognostic factors present in these patients including multiple sites of metastatic lesions, large volume of metastases, presence of venous thrombus, and metastatic disease to the bone, brain, or liver. Cytoreductive nephrectomy was shown to improve OS in selected patients treated with interferon α in randomized trials [19,20]. The emergence of targeted therapy has prompted a reevaluation of the benefit and optimal timing of cytoreductive surgery, and randomized clinical trials are underway to investigate these important questions [5,6,21]. Large retrospective studies have confirmed the benefit of cytoreductive nephrectomy before targeted therapy [22], which is the dominant treatment paradigm. However, initial nephrectomy may be deferred in some patients because of multiple poor prognostic factors or patient choice. With the absence of quality studies demonstrating the optimal timing of surgery, the use of presurgical targeted therapy creates a therapeutic dilemma in responding patients over who should undergo surgery and when to interrupt systemic therapy. Using the known prognostic factors and PT response, we can further stratify patients to identify those who progress early and likely would not benefit from surgery. Likewise, in patients with an early PT response, the expectation of improved OS provides more justification for interrupting systemic therapy to perform surgery. Of course these findings must be evaluated in the context of a prospective trial before changing the treatment paradigm.
Fig. 3. Kaplan-Meier estimate of overall survival based on rate and intensity of response (p < 0.05).
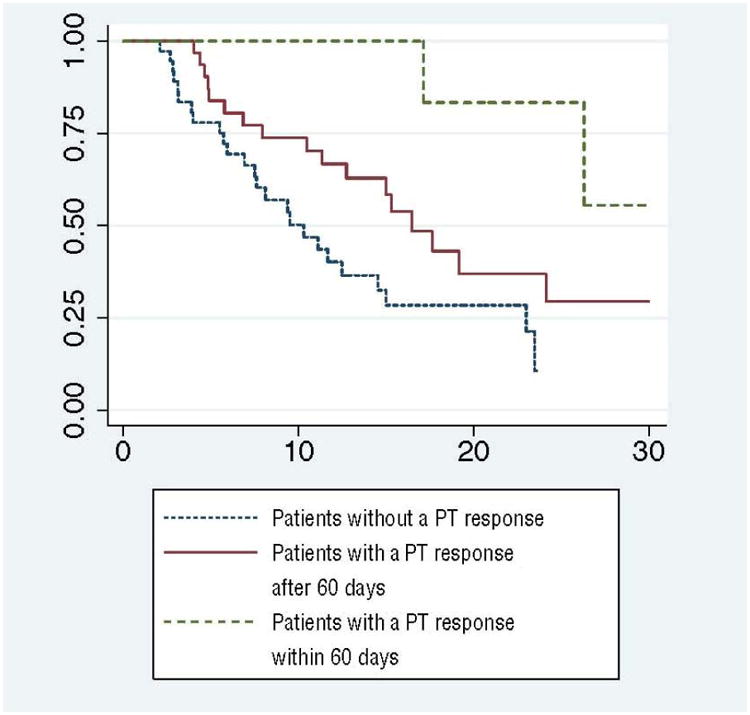
PT = primary tumor.
Many investigators have questioned whether RECIST is adequate to assess solid tumor response to targeted therapy [13–15]. In mRCC patients treated with targeted therapy, achieving a partial response is not common, but PT responses >10% can be seen in almost 40% of patients [7], which rarely improves surgical resectability[23] but may be biologically significant. Several studies have demonstrated that targeted agents frequently produce attenuation, which is not evaluated using RECIST, and has led several authors to propose alternative systems [24,25]. Thiam et al studied size variation in metastatic sites of RCC and found that a 10% decrease in the SLDs identified patients benefiting from sunitinib [5]. Recently, Krajewski et al compared RECIST and three other systems to evaluate targeted therapy response in metastatic lesions of RCC. Of the four systems evaluated, only achieving a decrease in ≥10% of the sum of tumor diameters was able to predict time to treatment failure [21].
In our analysis, an early 10% decrease in tumor diameter of the PT was predictive of improved OS. Addition of the metastatic site measurements did not offer improved ability to detect improved OS. In patients with the PT in place, assessment of the PT offers many advantages to measurement including its relative size and ease of identification. Possible explanations for the significance of the PT response compared with metastases may be the unique biology of a PT, its metastatic potential, or its relative size when compared with metastatic sites. However, these observations were made with limited patient numbers and will need to be validated in larger patient samples. Other poor prognostic factors identified in our study include decreased performance status, thrombus in inferior vena cava, presence of local symptoms, and liver metastases that are comparable with prior studies [2,16,26,27]. In addition, patients with metastatic disease in multiple sites or bulky disease are known to have worse survival [28,29].
5. Conclusions
To our knowledge this study is the first in the literature to report that the intensity and rate of PT response in mRCC patients may be used to predict OS. Limitations of our study include the sample size, its retrospective nature, as well as the significant number of patients with an unclassified histologic subtype of RCC. In addition, patients who responded to therapy were more likely to continue on therapy, which could contribute to differences in survival.
Fig. 2. Kaplan-Meier estimate of overall survival based on Memorial Sloan-Kettering Cancer Center risk groups.
Take-home message.
Patients with early primary tumor response have improved overall survival. This important prognostic factor may help select patients who could benefit from aggressive therapy and should be considered in ongoing clinical trials.
Acknowledgments
Funding/Support and role of the sponsor: This research is supported in part by the National Institutes of Health through M.D. Anderson Cancer Center Support Grant CA016672.
Footnotes
Author contributions: Christopher G. Wood had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Abel, Culp, Wood.
Acquisition of data: Abel, Culp.
Analysis and interpretation of data: Abel, Culp.
Drafting of the manuscript: Abel, Culp, Tannir, Matin, Tamboli, Wood.
Critical revision of the manuscript for important intellectual content: Abel, Culp, Tannir, Matin, Wood.
Statistical analysis: Abel, Culp, Wood.
Obtaining funding: None.
Administrative, technical, or material support: Wood.
Supervision: Abel, Culp, Tannir, Matin, Tamboli, Wood.
Other (specify): None.
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Christopher G. Wood is a consultant for Pfizer and Ethicon.
This study was presented at the Society of Urologic Oncology annual winter meeting, American Society of Clinical Oncology Genitourinary Cancer Symposium, and the American Urological Association annual meeting.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–96. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 2.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–9. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 3.Culp SH, Tannir NM, Abel EJ, et al. Can we better select patients with metastatic renal cell carcinoma for cytoreductive nephrectomy? Cancer. 2010;116:3378–88. doi: 10.1002/cncr.25046. [DOI] [PubMed] [Google Scholar]
- 4.Saylor PJ, Michaelson MD. New treatments for renal cell carcinoma: targeted therapies. J Natl Compr Canc Netw. 2009;7:645–56. doi: 10.6004/jnccn.2009.0045. [DOI] [PubMed] [Google Scholar]
- 5.Clinical Trial to Assess the Importance of Nephrectomy (CARMENA) ClinicalTrials.gov Web site. http://www.clinicaltrials.gov/ct2/show/NCT00930033.
- 6.Immediate surgery or surgery after sunitinib malate in treating patients with metastatic kidney cancer. doi: 10.1111/bju.15625. ClinicalTrials.gov Web site. http://www.clinicaltrials.gov/ct2/show/NCT01099423. [DOI] [PubMed]
- 7.Abel EJ, Culp SH, Tannir NM, et al. Primary tumor response to targeted agents in patients with metastatic renal cell carcinoma. Eur Urol. 2011;59:10–5. doi: 10.1016/j.eururo.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas AA, Rini BI, Lane BR, et al. Response of the primary tumor to neoadjuvant sunitinib in patients with advanced renal cell carcinoma. J Urol. 2009;181:518–23. doi: 10.1016/j.juro.2008.10.001. discussion 523. [DOI] [PubMed] [Google Scholar]
- 9.van der Veldt AA, Meijerink MR, van den Eertwegh AJ, et al. Sunitinib for treatment of advanced renal cell cancer: primary tumor response. Clin Cancer Res. 2008;14:2431–6. doi: 10.1158/1078-0432.CCR-07-4089. [DOI] [PubMed] [Google Scholar]
- 10.Cowey CL, Amin C, Pruthi RS, et al. Neoadjuvant clinical trial with sorafenib for patients with stage II or higher renal cell carcinoma. J Clin Oncol. 2010;28:1502–7. doi: 10.1200/JCO.2009.24.7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellenthal NJ, Underwood W, Penetrante R, et al. Prospective clinical trial of preoperative sunitinib in patients with renal cell carcinoma. J Urol. 2010;184:859–64. doi: 10.1016/j.juro.2010.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonasch E, Wood CG, Matin SF, et al. Phase II presurgical feasibility study of bevacizumab in untreated patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4076–81. doi: 10.1200/JCO.2008.21.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamin RS, Choi H, Macapinlac HA, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760–4. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 14.Griffin N, Gore ME, Sohaib SA. Imaging in metastatic renal cell carcinoma. AJR Am J Roentgenol. 2007;189:360–70. doi: 10.2214/AJR.07.2077. [DOI] [PubMed] [Google Scholar]
- 15.Smith AD, Lieber ML, Shah SN. Assessing tumor response and detecting recurrence in metastatic renal cell carcinoma on targeted therapy: importance of size and attenuation on contrast-enhanced CT. AJR Am J Roentgenol. 2010;194:157–65. doi: 10.2214/AJR.09.2941. [DOI] [PubMed] [Google Scholar]
- 16.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–40. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 17.Lara PN, Jr, Tangen CM, Conlon SJ, Flanigan RC, Crawford ED. Predictors of survival of advanced renal cell carcinoma: long-term results from Southwest Oncology Group Trial S8949. J Urol. 2009;181:512–6. doi: 10.1016/j.juro.2008.10.021. discussion 516–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–9. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 20.Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966–70. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 21.Krajewski KM, Guo M, Van den Abbeele AD, et al. Comparison of four early posttherapy imaging changes (EPTIC; RECIST 1.0, tumor shrinkage, computed tomography tumor density, Choi criteria) in assessing outcome to vascular endothelial growth factor-targeted therapy in patients with advanced renal cell carcinoma. Eur Urol. 2011;59:856–62. doi: 10.1016/j.eururo.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 22.Choueiri TK, Xie W, Kollmannsberger C, et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol. 2011;185:60–6. doi: 10.1016/j.juro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Inman BA, George DJ. Is tumor response important for renal carcinoma? Eur Urol. 2011;59:16–7. doi: 10.1016/j.eururo.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Smith AD, Shah SN, Rini BI, Lieber ML, Remer EM. Morphology, attenuation, size, and structure (MASS) criteria: assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. AJR Am J Roentgenol. 2010;194:1470–8. doi: 10.2214/AJR.09.3456. [DOI] [PubMed] [Google Scholar]
- 25.van der Veldt AA, Meijerink MR, van den Eertwegh AJ, Haanen JB, Boven E. Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Br J Cancer. 2010;102:803–9. doi: 10.1038/sj.bjc.6605567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volpe A, Patard JJ. Prognostic factors in renal cell carcinoma. World J Urol. 2010;28:319–27. doi: 10.1007/s00345-010-0540-8. [DOI] [PubMed] [Google Scholar]
- 27.Abel EJ, Wood CG. Cytoreductive nephrectomy for metastatic RCC in the era of targeted therapy. Nat Rev Urol. 2009;6:375–83. doi: 10.1038/nrurol.2009.102. [DOI] [PubMed] [Google Scholar]
- 28.Richey SL, Culp SH, Jonasch E, et al. Outcome of patients with metastatic renal cell carcinoma treated with targeted therapy without cytoreductive nephrectomy. Ann Oncol. 2011;22:1048–53. doi: 10.1093/annonc/mdq563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halbert RJ, Figlin RA, Atkins MB, et al. Treatment of patients with metastatic renal cell cancer: a RAND Appropriateness Panel. Cancer. 2006;107:2375–83. doi: 10.1002/cncr.22260. [DOI] [PubMed] [Google Scholar]


