Abstract
Importance
Mental-stress-induced myocardial ischemia (MSIMI) is an intermediate surrogate endpoint representing the pathophysiological link between psychosocial risk factors and adverse outcomes of coronary heart disease (CHD). However, pharmacological interventions aimed at reducing MSIMI have not been well studied.
Objective
To examine the effects of 6 weeks of escitalopram treatment vs. placebo on MSIMI and other psychological stress-related biophysiological and emotional parameters.
Design, Setting, and Participants
The REMIT study is a randomized, double-blind, placebo-controlled trial of patients with clinically stable CHD and laboratory MSIMI. Enrollment occurred from 7/24/2007–8/24/2011 at a tertiary medical center.
Interventions
Eligible participants were randomized 1:1 to receive escitalopram (dose began at 5 mg with titration to 20 mg/day in 3 weeks) or placebo over 6 weeks.
Main Outcome Measure
Occurrence of MSIMI, defined as (1) development or worsening of regional wall motion abnormality; (2) left ventricular ejection fraction reduction ≥8%; and/or (3) horizontal or downsloping ST-segment depression ≥1mm in ≥2 leads lasting for ≥3 consecutive beats during ≥1 of 3 mental tasks.
Results
127 participants were randomized to escitalopram (n=64) or placebo (n=63); 112 (96.1%) completed endpoint assessments (n=56 in each arm). At the end of 6 weeks, more patients taking escitalopram (34.2% [95% CI, 25.4 to 43.0]) had absence of MSIMI during the 3 mental stressors compared with patients taking placebo (17.5% [95% CI, 10.4 to 24.5]) based on unadjusted multiple imputation model for intention-to-treat analysis. A significant difference favoring escitalopram was observed (OR=2.62 [95% CI, 1.06 to 6.44]). Rates of exercise-induced ischemia were slightly lower at 6 weeks in the escitalopram group (45.8% [95% CI, 36.6 to 55.0]) than in patients receiving placebo (52.5% [95% CI, 43.3 to 61.7]), compared with baseline escitalopram (49.2% [95% CI, 39.9 to 58.5]) vs placebo (56.7% [95% CI, 47.5 to 65.9]), but this difference was not statistically significant.
Conclusion and relevance
Among patients with stable CHD and baseline MSIMI, 6 weeks of escitalopram versus placebo resulted in lower prevalence of MSIMI. There was no difference in exercise-induced ischemia.
Trial Registration
ClinicalTrials.gov Identifier: NCT00574847
INTRODUCTION
A robust body of evidence has identified emotional stress as a potential triggering factor in coronary heart disease (CHD) and other cardiovascular events.1–4 Recognition of the role played by acute emotional stressors in triggering episodes of acute coronary syndromes led to provocation experiments aimed at inducing emotional distress in a controlled setting and documenting reversible impairment of cardiac function. This ultimately resulted in the formal concept of mental stress-induced myocardial ischemia (MSIMI). Over the last 3 decades, the association of emotional distress and myocardial ischemic activity in the laboratory has been well studied.5 MSIMI in the laboratory occurs in up to 70% of patients with clinically stable CHD and is associated with increased risk of death and cardiovascular events.5,6
Few studies have examined therapeutics that effectively modify MSIMI,7–9 perhaps due to the mechanistic complexity underlying this phenomenon, which encompass a wide range of central and peripheral physiological changes associated with emotions and behaviors. However, recent evidence suggests that selective serotonin reuptake inhibitors (SSRIs) may reduce mental-stress-induced hemodynamic response, metabolic risk factors,10,11 and platelet activity.12,13 We therefore conducted the Responses of Mental Stress Induced Myocardial Ischemia to Escitalopram Treatment (REMIT) trial (NCT00574847) to investigate whether SSRI treatment can improve MSIMI.
METHODS
The REMIT trial was a randomized, double-blind trial comparing the SSRI escitalopram versus placebo in participants with clinically stable CHD and MSIMI. The study was conducted at the Duke University Health System in the United States. The study protocol was reviewed and approved by the Duke institutional review board, and all participants provided voluntary written informed consent.
Eligibility and Recruitment
A complete description of the trial methodology has been published.14 Briefly, we screened all patients with CHD who visited Duke cardiology clinics. Adult patients aged ≥21 years with CHD, as documented by angiographic findings of coronary artery stenosis ≥70%, history of myocardial infarction, or history of cardiac revascularization were eligible for participation. Exclusion criteria included significant cognitive impairment, life-threatening comorbidity (estimated 50% mortality within 1 year), active suicidal ideation, and psychiatric conditions precluding SSRI use.14 Patients who exhibited MSIMI during baseline screening were qualified for the trial intervention.
Race and ethnicity of the REMIT participants was obtained via self-report using the following categories: Hispanic Ethnicity: Yes or No; Race: White, Black, Native American, Asian or Pacific Islander, Other (Specify).
Overall Design
REMIT tested the hypothesis that the SSRI escitalopram would reduce MSIMI to a greater extent than placebo in patients with clinically stable CHD and MSIMI at enrollment. Participants underwent mental and exercise stress testing during baseline screening. Participants who demonstrated MSIMI were randomly allocated in a 1:1 ratio, divided in blocks of 10, in double-blind fashion to escitalopram or matching placebo for 6 weeks. Randomization was conducted by Duke University Investigation Drug Service Pharmacy. All randomization information was secured and accessible only to authorized individuals working within the Investigational Drug Service. The initial dose of study drug was 5 mg/day, increasing to 10 mg/day in 1 week and to 20 mg/day at week 3 and for the remainder of the study period. If participants were unable to tolerate higher doses, the dose could be decreased to 5 mg/day. Mental and exercise stress testing were repeated at the end of the 6-week intervention. Adherence to study drug was assessed via weekly inquiries and pill counts upon return of medication bottles. Study medication was tapered following endpoint assessments. Participants who preferred to continue on study medication were provided a prescription for 1 month’s supply of escitalopram and required to follow up with their primary cardiologist or primary care physician.
Stress-Induced Myocardial Ischemia
All stress tests were conducted at the Duke Cardiac Diagnostic Unit in the morning following 24–48 hours withholding of beta-blockers.14 Three mental stress tasks—mental arithmetic, mirror trace, and public speaking with anger recall—delivered in the same sequence throughout the study were used to assess MSIMI; each test was followed by a 6-minute rest.
After mental stress testing, participants underwent a treadmill exercise test using the standard Bruce protocol. Exercise testing was terminated according to American College of Sports Medicine guidelines. Testing was repeated at the 6-week endpoint visit; an alternative mental arithmetic task was used to prevent habituation. Echocardiography and electrocardiography (ECG) were used to assess ischemia. Left ventricular ejection fraction (LVEF) was calculated from a 3–5 beat loop. Wall motion assessments were determined from systole from 1 cardiac cycle at a frame rate of 30–40 frames/second using the 16-segment model recommended by the American Society of Echocardiography. These assessments were blinded to randomization and stress tests.
To minimize variation and temporal drift and to enhance reliability, all echo images of participants who completed both baseline and endpoint stress testing were batch read by 2 experienced cardiologists (E.V. and Z.S.) after completing the 6-week endpoint assessments.15 Blood pressure, heart rate, and standard 12-lead ECG were measured simultaneously during acquisition of echo images. MSIMI was defined by presence of ≥1 ischemic markers: compared to rest measurements, (1) any development or worsening of wall motion; (2) reduction of LVEF ≥8%; and/or (3) deviation of ST-segment in ≥2 leads lasting for ≥3 consecutive beats, occurring during ≥1 of the 3 mental stress tasks. Exercise-induced myocardial ischemia (ESIMI) was defined as development of the above during exercise testing. Continuing presence or absence of any of 3 ischemic markers was evaluated at the 6-week endpoint visit for both MSIMI and ESIMI.
Other Study Outcomes
Other study outcomes included biophysiological and psychological measurements obtained at baseline and repeated at 6 weeks. Biophysiological measures included resting platelet serotonin receptor transporter number (5HTT), measures of serotonin uptake into platelets (binding affinity [Kd]), and platelet serotonin density of 5HT uptake sites (Vmax). Measures of resting and mental-stress-induced LVEF and hemodynamic responses including systolic blood pressure (SBP) and diastolic blood pressure (DBP), heart rate, rate-pressure product, exercise capacity (measured via duration of treadmill exercise test, peak heart rate, attainment of target heart rate), ESIMI, occurrence of chest pain, chest discomfort, shortness of breath, and other physical discomfort during stress testing were obtained. Psychological measures included symptoms of depression via Beck Depression Inventory scale,16 state and trait anxiety via Spielberger State-Trait Anxiety Scales,17 hostility via Ho scale,18 and social stress via perceived stress scale.19 Two clusters of emotional response to mental and exercise stress testing were obtained via a visual scale of 0–100: (1) positive emotions including calm and being in control, and (2) negative emotions including frustration, tension, and sadness.14
Statistical Analysis
Bivariate analyses by treatment assignment were conducted on demographic and clinical variables. Resting LVEF20 and sex21 have epidemiological and clinical relevance to the primary outcome and were included in the adjusted model. The primary outcome of presence of MSIMI at 6 weeks and its association with treatment assignment was examined under intention-to-treat (ITT) principle using logistic regression. We used multiple imputation techniques22 to compensate for potential bias introduced by missing endpoint data. The imputed model to predict the outcome consisted of age, baseline resting EF, sex, and the treatment variable. For our primary outcome, unadjusted and adjusted imputed logistic regression models provided odds ratios and 95% confidence intervals (CIs) for the association of the study intervention with MSIMI. The Hosmer-Lemeshow statistic was reported as an index of goodness of fit for this model.
For the primary outcome, per-protocol analysis was also conducted on participants who completed both baseline and endpoint assessments. Secondary outcomes were classified into biophysiological and psychological outcomes. A general linear model was used for continuous variables and logistic regression for dichotomized outcomes; these models were controlled for sex and baseline corresponding values. To enhance reliability of mental stress assessments under various domains and reduce the number of statistical tests, we averaged the 3 mental stress measurements. Analysis was also conducted on participants who completed both baseline and endpoint assessments (per-protocol sample) for secondary outcomes. Because the results of the ITT and the per-protocol sample were similar, we chose to present the results of the ITT analyses. All tests performed were two-sided.
Primary results were examined at a significance level of .05. For secondary outcomes, alpha was fixed at .05, and Bonferroni adjustment was used for the number of variables in each domain. This resulted in a critical P value of .05/9=.006 for the analysis of the biophysiological outcomes and a critical P value of .1/7=.007 for the analysis of the psychological outcomes. All analyses were performed in SAS version 9.3.
The study was designed to have at least 80% power to detect between-group differences on MSIMI improvement or no MSIMI at the end of the 6-week treatment period. For a sample of 60 participants in each of treatment arm, we estimated to observe 30% improvement in MSIMI in the placebo group and over 60% MISMI improvement in the escitalopram group. With these estimates and a 2-sided alpha=.05, the study had >80% power with up to 10 dropouts. Because we experienced more dropouts than anticipated, with the approval of the study Data and Safety and Monitoring Board and the Duke IRB we increased total enrollment from 120 to 127 to ensure that at least 110 participants completed both baseline and 6-week assessments.
RESULTS
Participant Characteristics and Baseline Assessments
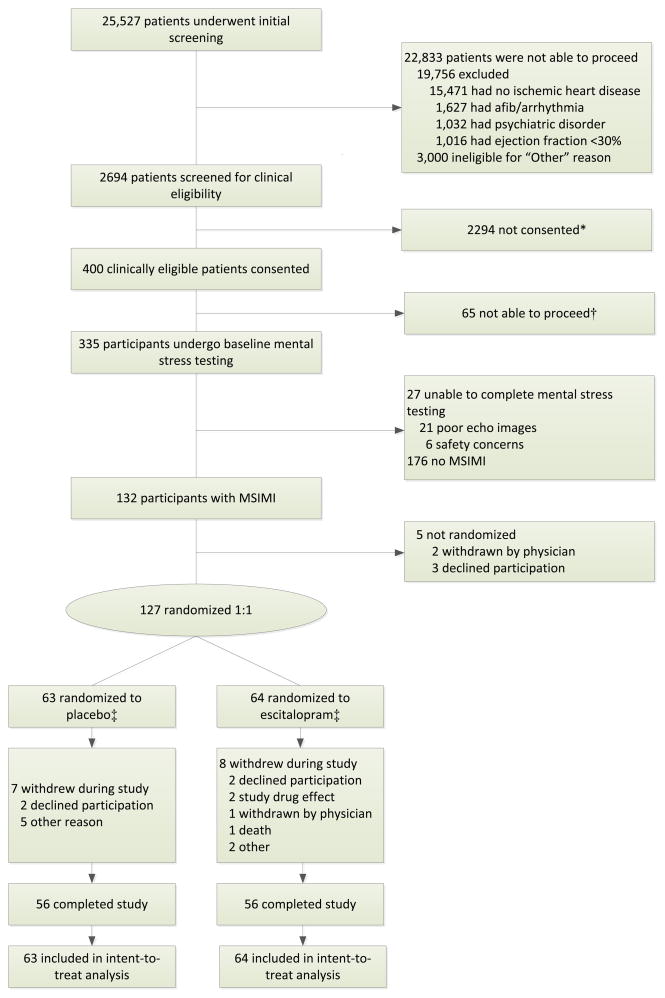
A total of 127 participants with clinically stable CHD and MSIMI at baseline screening were randomized to the trial intervention; 112 (56 in each group) completed endpoint assessments. Study participant disposition is shown in Figure 1. Demographic and clinical characteristics and baseline cardiovascular and emotional measures by treatment group are summarized in Tables 1 and 2, respectively. Participants allocated to escitalopram were significantly older than those assigned to placebo (66.5 vs. 61.4 years, P=.01). Participants allocated to placebo were more likely to be female and had higher rates of resting abnormal wall motion and lower LVEF, although differences between groups were not statistically significant. The majority of participants were taking aspirin, statins, and beta-blockers; approximately 45% were taking other antiplatelet agents; and nearly 80% were taking ACE inhibitors or angiotensin-receptor blockers (Table 1). No differences in medication use by treatment group were noted.
Figure 1.
Study flow for REMIT Trial.
*Reasons given for patients not consented to REMIT (n=2294): Lack of interest (n=529); living too far away (n=75); lack of approval of primary clinical provider or study PI because of medical comorbidities (n=457); other (wrong contact information, coumadin) (n=127); awaiting phone screening (n=239); currently taking antidepressants that could not be discontinued (n=91); would contact later due to recent procedures or patient preference (n=127); awaiting approval of primary clinical providers (n=322); attempted but failed to contact (left message and/or no answer, etc.) (n=327).
†Reasons for patients who provided consent but did not proceed with baseline assessments: taking antidepressant that could not be discontinued (n=5); safety concern of primary cardiologists (n=2); Beck Depression Inventory score <4 (initial exclusion criteria that was eliminated rapidly via IRB approval) (n=11); dropped out due to medical conditions (n=7); family-related issues (n=8); changed mind about the study medication or job changes (n=11); and no reason provided (n=21).
‡Two patients randomized to escitalopram declined to take study medication; 5 patients randomized to placebo did not take study medication.
Table 1.
Baseline Demographic and Clinical Characteristics
| Variable | Escitalopram (n = 64) | Placebo (n = 63) | Total (N = 127) |
|---|---|---|---|
| Mean (SD) age, ya | 66.5 (9.3) | 61.4 (11.5) | 64.0 (10.7) |
|
| |||
| Race, white, n (%) | 53 (82.8) | 47 (74.6) | 100 (78.7) |
|
| |||
| Female sex, n (%) | 11 (17.2) | 15 (23.8) | 26 (20.5) |
|
| |||
| Living alone, n (%) | 14 (21.9) | 13 (20.6) | 27 (21.3) |
|
| |||
| Unmarried, n (%) | 18 (28.1) | 22 (34.9) | 40 (31.5) |
|
| |||
| Smoking, n (%) | |||
| Current | 13 (20.3) | 8 (12.7) | 21 (16.5) |
|
| |||
| Past | 27 (42.2) | 35 (55.6) | 62 (48.8) |
|
| |||
| Never | 24 (37.5) | 20 (31.8) | 44 (34.6) |
|
| |||
| Medications at baseline, n/N (%) | |||
| ASAb | 60/63 (95.2) | 61 (96.8) | 121/126 (96.0) |
|
| |||
| Other antiplatelet agent | 28/63 (44.4) | 29 (46.0) | 57/126 (45.2) |
|
| |||
| ACEI | 40/63 (63.5) | 39 (61.9) | 79/126 (62.7) |
|
| |||
| ARB | 11/63 (17.5) | 10 (15.9) | 21/126 (16.7) |
|
| |||
| Beta-blocker | 55/63 (87.3) | 54 (85.7) | 109/126 (86.5) |
|
| |||
| Calcium channel blocker | 14/63 (22.2) | 11 (17.5) | 25/126 (19.8) |
|
| |||
| Statin | 62/63 (98.4) | 57/62 (91.9) | 119/125 (95.2) |
|
| |||
| Other lipid lowering agent | 17/63 (27.0) | 9/61 (14.8) | 26/124 (21.0) |
|
| |||
| Chest pain, n (%) | |||
| At rest | 5 (7.8) | 3 (4.8) | 8 (6.3) |
|
| |||
| On exertion | 9 (14.1) | 7 (11.1) | 16 (12.6) |
|
| |||
| No. diseased coronary arteries, n/N (%)c | |||
| 1 | 22/63 (34.9) | 26/63 (41.3) | 48/126 (38.1) |
|
| |||
| 2 | 9/63 (14.3) | 12/63 (19.0) | 21/126 (16.7) |
|
| |||
| 3 | 19/63 (30.2) | 17/63 (27.0) | 36/63 (28.6) |
|
| |||
| 4 | 13/63 (20.6) | 8/63 (12.7) | 21/63 (16.7) |
|
| |||
| Medical history | |||
| Myocardial infarction, n/N (%) | |||
| Yes | 30 (46.9) | 30 (47.6) | 60 (47.2) |
|
| |||
| No | 34 (53.1) | 32 (50.8) | 66 (52.0) |
|
| |||
| Uncertain | 0 (0) | 1 (1.6) | 1 (0.8) |
|
| |||
| Prior CABG, n (%) | 31 (48.4) | 31 (49.2) | 62 (48.8) |
|
| |||
| Prior PTCA/stenting, n (%) | 37 (57.8) | 39 (61.9) | 76 (59.8) |
|
| |||
| History of DM, n (%) | 21 (32.8) | 14 (22.2) | 92 (72.4) |
|
| |||
| Hypertension, n (%) | 50 (78.1) | 48 (76.2) | 98 (77.2) |
|
| |||
| Hyperlipidemia, n (%) | 62 (96.9) | 57 (90.5) | 119 (93.7) |
|
| |||
| Heart failure, n (%) | 2 (3.1) | 5 (7.9) | 7 (5.5) |
|
| |||
| NYHA class, n/N (%) | |||
| I | 60 (93.8) | 57/62 (91.9) | 117/126 (92.9) |
|
| |||
| II | 3 (4.7) | 4/62 (6.4) | 7/126 (5.6) |
|
| |||
| III | 1 (1.6) | 1/62 (1.6) | 2/126 (1.6) |
|
| |||
| History of depression, n (%) | 11 (17.2) | 10 (15.9) | 21 (16.5) |
p = .01
Significant coronary stenosis ≥70% or status post revascularization in the 4 epicardial coronary arteries (left main, left anterior descending, left circumflex, and right coronary) documented prior to enrollment.
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; DM, diabetes mellitus; NYHA, New York Heart Association; PTCA, percutaneous coronary angiography; SSRI, selective serotonin reuptake inhibitor
Table 2.
Baseline Cardiovascular and Emotional Measurements
| Variablea | Escitalopram (n = 64) | Placebo (n = 63) | Total (N = 127) |
|---|---|---|---|
| Resting WMA, n (%) | 32 (50.0) | 34 (54.0) | 66 (52.0) |
| Resting ejection fraction |
n = 60 56.3 (10.4) |
n = 58 53.6 (13.2) |
n = 118 55.0 (11.9) |
| Ejection fraction, mean of 3 change scores |
n = 59 −2.1 (5.2) |
n = 58 −2.6 (5.0) |
n = 117 −2.3 (5.1) |
| Resting SBP, mm HG |
n = 62 125.9 (18.5) |
n = 56 127.5 (16.4) |
n = 118 126.6 (17.5) |
| SBP, mean of 3 change scores, mm HG |
n = 62 25.9 (12.2) |
n = 56 26.8 (13.1) |
n = 118 26.3 (12.6) |
| Resting DBP, mm HG |
n = 62 71.3 (12.4) |
n = 56 73.2 (9.9) |
n = 118 72.2 (11.3) |
| DBP, mean of 3 change scores, mm HG |
n = 62 14.3 (8.7) |
n = 56 15.0 (8.6) |
n = 118 14.6 (8.7) |
| Resting heart rate, BPM |
n = 61 86.5 (16.3) |
n = 56 84.2 (14.1) |
n = 117 85.3(15.2) |
| Heart rate, mean of 3 change scores, BPM |
n = 61 9.3 (8.0) |
n = 56 9.5 (7.8) |
n = 118 9.4 (7.9) |
| Weight, kg |
n = 60 190.6 (36.0) |
n = 62 185.7 (31.1) |
n = 122 188.1 (33.5) |
| BMI |
n = 60 28.3 (4.8) |
n = 62 27.9 (3.4) |
n = 122 28.1 (4.2) |
| RPP, mean of 3 change scores |
n = 61 3257.2 (1963.7) |
n = 56 3358.6 (1998.0) |
n = 118 3305.8 (1972.3) |
| VMAX, p/107 platelets/5 min |
n = 50 211.3 (103.3) |
n = 50 274.9 (238.1) |
n = 100 243.1 (185.4) |
| KD100, nM (SD) |
n = 50 330.9 (115.4) |
n = 50 401.0 (220.7) |
n = 100 365.9 (178.7) |
| 5HTT, fm/mg protein |
n = 48 165.0 (60.1) |
n = 50 156.9 (56.5) |
n = 98 160.9 (58.1) |
| Beck Depression Inventoryb | 9.4 (7.0) | 8.5 (6.3) | 9.0 (6.7) |
| Perceived stress scalec | 23.1 (7.1) |
n = 62 23.2 (7.8) |
n = 126 23.1 (7.4) |
| Hostilityd | 9.7 (5.0) |
n = 62 10.4 (4.6) |
n = 126 10.1 (4.8) |
| Hostile affecte | 1.6 (1.4) |
n = 62 1.7 (1.3) |
n = 126 1.6 (1.4) |
| Trait anxietyf | 34.7 (0.9) | 34.1 (0.9) | 34.4 (10.7) |
| State anxietyg | 30.9 (10.8) | 28.0 (9.0) | 29.4 (10.0) |
| Resting negative affecth | 30.4 (17.1) |
n = 62 34.2 (17.6) |
n = 126 32.2 (17.4) |
| Negative affect, mean of 3 change scores (SD) | 25.4 (17.8) |
n = 62 26.0 (20.5) |
n = 126 25.7 (19.1) |
| Resting positive affecti | 63.9 (20.0) |
n = 62 54.9 (19.14) |
n = 126 59.5 (20.0) |
| Positive affect, mean of 3 change scores | −26.8 (23.5) |
n = 62 −28.6 (22.8) |
n = 126 −27.7 (23.1) |
| ESIMI, n/N (%) | 29/59 (49.2) | 34/60 (56.7) | 63/119 (52.9) |
| Exercise capacity Peak heart rate, BPM |
n = 59 137.0 (21.0) |
n = 60 144.1 (18.9) |
n = 119 140.6 (20.2) |
| Met target heart rate, n (%) |
n = 59 46 (78.0) |
n = 60 51 (85.0) |
n = 119 97 (81.5) |
| Duration, min |
n = 59 6.3 (2.5) |
n = 60 6.8 (3.1) |
n = 119 6.6 (2.8) |
Values are given as mean (SD) unless otherwise noted.
None of these variables were statistically different between the escitalopram and the placebo groups.
Beck Depression Inventory: score range, 0–63 (higher score=greater severity of depressive symptoms)
Perceived stress scale: score range, 10–50 (higher score=greater levels of perceived stress)
Hostility: score range, 0–27 (higher score= greater levels of hostility)
Hostile Affect: score range, 0–5 (higher score=greater levels of hostile affect)
Trait anxiety: score range, 20–80 (higher score=greater levels of trait anxiety)
State anxiety: score range, 20–80 (higher score=greater levels of state anxiety)
Negative affect measured by visual analogue scale (0–100; higher score=greater levels of state negative affect)
Positive affect measured by visual analogue scale (0–100; higher score=greater levels of state positive affect)
Abbreviations: BMI, body mass index (weight in kg/height in cm); BPM, beats per minute; DBP, diastolic blood pressure; EF, ejection fraction; ESIMI, exercise-stress-induced myocardial ischemia; KD, platelet serotonin binding affinity; MSIMI, mental-stress-induced myocardial ischemia; MSs, mental stressors, RPP, rate-pressure product; SBP, systolic blood pressure; WMA, wall motion abnormality; 5HTT, platelet serotonin receptor transporter number
Study Drug Treatment and Discontinuation
Fifteen participants withdrew during the intervention: 8 in the escitalopram arm and 7 in the placebo arm (Figure 1). Seven (46.7%) withdrew prior to receiving study medication; 6 (40.0%) withdrew during Week 1; 2 (13.3%) dropped out after Week 3. Of these 2 late drop-outs, 1 was due to the primary cardiologist’s request for a cardiac catheterization; the other due to death at Week 5. Most study participants took the maximum dose of the study drug (87.1% [escitalopram]; 93.4% [placebo]) (Table 3). Adherence to study medication was defined as taking at least 75% of study medication and was assessed by pill counts at study visits. Overall, 107 of 112 study participants completing endpoint assessment were considered adherent to study medication (54/56 [96%] in the escitalopram group and 53/56 [95%] in the placebo group).
Table 3.
Dropout Reasons, Dosage at Last Visit, and Side Effects
| Variable | Escitalopram (n = 64) | Placebo (n = 63) | P valuea |
|---|---|---|---|
| Patient drop-outs, n (%) | 8 (12.5) | 7 (11.1) | .81 |
| Patient withdrew | 2 (3.1) | 2 (3.2) | |
|
| |||
| Side effects | 2 (3.1) | 0 (0) | |
|
| |||
| Physician withdrawal | 1 (1.6) | 0 (0) | |
|
| |||
| Death | 1 (1.6) | 0 (0) | |
|
| |||
| Lost to follow-up | 0 (0.0) | 0 (0.0) | |
|
| |||
| Other reasonsb | 2 (3.1) | 5 (7.9) | |
|
| |||
| Dosage at last visit, n (%) | .15 | ||
| 5 mg | 2 (3.2) | 3 (4.9) | |
|
| |||
| 10 mg | 6 (9.7) | 2 (1.6) | |
|
| |||
| 15 mg | 0 (0) | 0 (0) | |
|
| |||
| 20 mg | 54 (87.1) | 57 (93.4) | |
|
| |||
| Any side effect, n (%) | 46 (71.9) | 28 (44.4) | .002 |
|
| |||
| Patients with reported side effects, n (%) | |||
| Burning sensation | 1 (1.6) | 0 (0) | |
|
| |||
| CNS symptom | 17 (26.6) | 13 (20.6) | |
|
| |||
| Constipation | 18(28.1) | 11 (17.5) | |
|
| |||
| Cramping in legs | 3 (4.7) | 2 (3.2) | |
|
| |||
| Fatigue | 19 (29.7) | 9 (14.3) | |
|
| |||
| Irregular heartbeat | 0 (0) | 2 (3.2) | |
|
| |||
| Sexual dysfunction | 10 (15.6) | 4 (6.4) | |
|
| |||
| Sinusitis | 1 (1.6) | 0 (0.0) | |
Calculated using Fisher exact test
For placebo group: brain tumor, husbands’ health, knee surgery, irregular heartbeat, & percutaneous coronary intervention; For SSRI group: found taking escitalopram at randomization, and cluster of symptoms that indicated significant dehydration.
Abbreviations: CNS, central nervous system (symptoms included dizziness, drowsiness, and headache); SSRI, selective serotonin reuptake inhibitor
Primary Outcomes
Among all participants (N=127), 64 (50.4%) were randomized to escitalopram and 63 (49.6%) to placebo. A total of 112 (96.1%; N=56 for both arms) completed endpoint assessments (Figure 1). Although all 127 participants had either new wall motion abnormalities, decrease in LVEF ≥8, or both during ≥1 of 3 mental stress tests during baseline assessment, 5 of the 112 participants who completed follow-up were reassessed by reviewers (E.V. and Z.S.) as having no MSIMI at baseline (Table 4). No patients had any mental-stress-induced ischemic ST-segment changes, either at baseline or at 6-week assessments. Of 5 participants whose baseline MSIMI was reclassified as “no MSIMI,” 4 had no MSIMI at 6 weeks; 1 participant assigned to escitalopram developed MSIMI. Intra-rater and inter-rater variability of wall motion analysis as assessed by the kappa statistic ranged from 0.80–0.87, consistent with previous results.14
Table 4.
MSIMI Defined by Wall Motion Abnormality and/or LVEF at Baseline and Endpointa
| Variable | Escitalopram | Placebo | OR (95% CI) | P value |
|---|---|---|---|---|
| Baseline, n (%) | ||||
| Overall MSIMIb | 63 (98.4) | 63 (100) | >.99c | |
| WMA only | 37 (57.8) | 42 (66.7) | ||
|
| ||||
| LVEF ≥ -8 only | 9 (14.1) | 9 (14.3) | ||
|
| ||||
| Both | 17 (26.6) | 12 (19.1) | ||
|
| ||||
| Endpoint, n (%)d | ||||
| Overall MSIMI | 37/56 (66.1) | 47/56 (83.9) | 2.68 (1.09 to 6.61) | .03e |
| Adjusted per -protocol | – | – | 2.57 (0.99 to 6.66) | .05e |
| WMA only | 22/56 (39.3) | 32/56 (57.1) | ||
| 95% CI: 30.2 to 48.3 | 95% CI: 47.9 to 66.3 | |||
|
| ||||
| LVEF ≥ -8 only | 3/56 (5.36) | 4/56 (7.1) | ||
| 95% CI: 1.2 to 9.5 | 95% CI: 2.3 to 11.9 | |||
|
| ||||
| Both | 12/56 (21.43) | 11/56 (19.6) | ||
| 95% CI: 13.8 to 29.0 | 95% CI: 12.3 to 27.0 | |||
|
| ||||
| Imputed primary endpoint, % | ||||
| No MSIMIf | 34.2% | 17.5% | ||
| 95% CI: 31.6 to 36.8 | 95% CI: 15.4 to 19.6 | 2.62 (1.06–6.44) | 0.04d | |
No mental-stress-induced ischemic ST-segment changes were observed, either at baseline or at 6-week assessments.
One participant was found to have no mental-stress-induced myocardial ischemia after randomization.
P value presented here is from the Fisher exact test.
Results presented here included only the completers.
P value presented here is from logistic regression.
Response rates were calculated from 10 imputed datasets: n = 219/640 for escitalopram and n = 110/630 for placebo groups respectively.
Abbreviations: LVEF, left ventricular ejection fraction; MSIMI, mental-stress-induced myocardial ischemia; WMA, wall motion abnormality
At the end of 6 weeks, more patients taking escitalopram (34.2% [95% CI, 25.4 to 43.0]) had absence of MSIMI during the 3 mental stressors compared with patients taking placebo (17.5% [95% CI, 10.4 to 24.5]), based on the unadjusted multiple imputation model for ITT analysis. This analysis showed that the escitalopram group had a significantly higher rate of no MSIMI compared with the placebo group (OR=2.62; 95% CI, 1.06 to 6.44; P=.04). The association between escitalopram and MSIMI improvement was no longer statistically significant following adjustment for sex and baseline resting LVEF (OR=2.53; 95% CI, 0.97 to 6.56, Padjusted=.06). For the adjusted model, the Hosmer-Lemeshow statistic was 0.56, indicating a good fit. The C-index, a measure of predictability, was 0.72. Analysis of the per-protocol population yielded similar results (OR=2.68; 95% CI, 1.09 to 6.61; P=.03). Again, the strength of the association between escitalopram use and MSIMI improvement compared with placebo was reduced after adjusting for sex and baseline resting LVEF (OR=2.57; 95% CI, 0.99–6.66; Padjusted=.05).
Physiological Outcomes
Biophysiological findings are summarized in Table 5. At 6 weeks, participants receiving escitalopram showed significant reduction in 5HTT (139.7 [95% CI, 126.1 to 153.35] vs 160.4 [95% CI, 147.0 to 173.7]) and elevation in Kd100 (4202.4 [95% CI, 3328.6 to 5076.2] vs 210.1 [95% CI, 0.0 to −1083.9]) and Vmax (404.8 [95% CI, 304.2 to 505.4] vs 182.2 [95% CI, 81.6 to 282.8]) compared with participants receiving placebo (P<.01 for all comparisons). Resting LVEF (57.4 [95% CI, 55.5 to 59.3] vs 54.8 [95% CI, 52.9 to 56.7]) and LVEF response to mental stress (−2.0 [95% CI, −3.2 to −0.7]) increased in the escitalopram group compared with the placebo group, but differences were not statistically significant (P >.10). Hemodynamic responses to mental stress were all lower in the escitalopram group; differences in heart rate and rate-pressure product between groups were significant. Weight and BMI were also reduced in the escitalopram group compared with placebo, but the reduction was not significant. Participants receiving escitalopram did not exhibit greater alteration in the rate of ESIMI in either unadjusted (OR=1.31; 95% CI, 0.64 to 2.68; P=.46) or adjusted (OR=1.24; 95% CI, 0.60 to 2.58; Padjusted=.56) analyses relative to those receiving placebo. Also, exercise capacity was not significantly altered at week 6 in participants receiving escitalopram versus those receiving placebo. Five participants reported physical symptoms during mental stress testing at baseline (3 escitalopram; 2 placebo); only 1 reported chest discomfort and 4 reported “other physical symptoms.” Five participants reported physical symptoms upon testing at 6 weeks (4 escitalopram, 1 placebo); 1 participant assigned to escitalopram reported “other physical symptom”; the others reported shortness of breath.
Table 5.
Adjusted Endpoint Analysis of Continuous and Dichotomized (%) Outcomes
| Variablea | Escitalopram (n =64) | Placebo (n = 63) | OR (95% CI) | Adjusted P valueb |
|---|---|---|---|---|
| MSIMI, % | 35.2 32.5 to 37.8 |
17.6 15.5 to 19.7 |
2.53 (0.97 to 6.56) | .06c |
|
| ||||
| Resting WMA, n/N (%) | 27/56 (48.2) 39.0 to 57.5 |
34/56 (60.7) 51.7 to 69.8 |
1.83 (0.39 to 8.73) | .45 |
|
| ||||
| Resting ejection fraction, % |
n = 60 57.4 (55.5 to 59.3) |
n = 58 54.8 (52.9 to 56.7) |
.06 | |
|
| ||||
| Ejection fraction, mean of 3 change scores |
n = 59 −2.0 (−3.2 to −0.71) |
n = 58 −2.4 (−3.7 to −1.1) |
.65 | |
|
| ||||
| Resting SBP, mm HG |
n = 62 127.58 (124.4 to 130.8) |
n = 56 127.30 (124.0 to 130.7) |
.91 | |
|
| ||||
| SBP, mean of 3 change scores, mm HG |
n = 62 19.3 (16.7 to 22.0) |
n = 56 23.6 (20.8 to 26.4) |
.03 | |
|
| ||||
| Resting DBP, mm HG |
n = 62 70.0 (67.5 to 72.4) |
n = 56 73.1 (70.5 to 75.7) |
.09 | |
|
| ||||
| DBP, mean of 3 change scores, mm HG |
n= 62 11.4 (9.5 to 13.4) |
n = 56 12.2 (10.1 to 14.2) |
.63 | |
|
| ||||
| Resting heart rate, BPM | n = 61 65.7 (64.1 to 67.4) |
n = 56 65.8 (64.1 to 67.6) |
.94 | |
|
| ||||
| Heart rate, mean of 3 change scores, BPM |
n = 61 6.34 (5.0 to 7.7) |
n = 56 9.1 (7.8 to 10.5) |
.005 | |
|
| ||||
| BMI |
n = 60 28.0 (27.8 to 28.2) |
n = 62 28.2 (28.0 to 28.3) |
.19 | |
|
| ||||
| Weight, kg |
n = 60 84.9 (84.4 to 85.4) |
n = 62 85.4 (84.8 to 85.9) |
.20 | |
|
| ||||
| RPP, mean of 3 change scores |
n = 61 2250.8 (1933.2 to 2568.3) |
n = 56 2981.3 (2649.8 to 3312.9) |
.002 | |
|
| ||||
| VMAX, p/107 platelets/5 min |
n = 50 404.8 (304.2 to 505.4) |
n = 50 182.2 (81.6 to 282.8) |
.003 | |
|
| ||||
| KD100, nM |
n = 50 4202.4 (3328.6 to 5076.2) |
n = 50 210.1 (0.0 to 1083.9) |
<.001 | |
|
| ||||
| 5HTT, fm/mg protein |
n = 48 139.7 (126.1 to 153.4) |
n = 50 160.4 (147.0 to 173.7) |
.04 | |
|
| ||||
| Beck Depression Inventoryd | 7.4 (6.3 to 8.6) | 7.0 (5.8 to 8.2) | .60 | |
|
| ||||
| Perceived Stress Scalee | 21.4 (20.3 to 22.5) |
n = 62 21.8 (20.6 to 22.9) |
.61 | |
|
| ||||
| Hostilityf | 9.9 (9.1 to 10.7) |
n = 62 10.3 (9.5 to11.1) |
.49 | |
|
| ||||
| Hostile affectg | 1.6 (1.3 to 1.8) |
n = 62 1.8 (1.6 to 2.1) |
.12 | |
|
| ||||
| Trait anxietyh | 31.2 (29.7 to 32.6) | 32.0 (30.5 to 33.4) | .44 | |
|
| ||||
| State anxietyi | 27.9 (26.4 to 29.4) | 29.5 (28.0 to 31.1) | .15 | |
|
| ||||
| Resting negative affectj | 11.7 (8.5 to 15.0) |
n = 62 13.1 (9.8 to 16.4) |
.57 | |
|
| ||||
| Negative affect, mean of 3 change scores | 23.1 (18.5 to 27.7) |
n = 62 25.1 (20.4 to 29.7) |
.56 | |
|
| ||||
| Resting positive affectk | 80.6 (76.6 to 84.6) |
n = 62 81.0 (76.9 to 85.1) |
.89 | |
|
| ||||
| Positive affect, mean of 3 change scores | −22.6 (−27.4 to −17.9) |
n = 62 −29.41 (−34.23 to −24.60) |
.05 | |
|
| ||||
| ESIMI, % |
n = 59 45.8 (36.6 to 55.0) |
n = 61 52.5 (43.3 to 61.8) |
1.24 (0.6 to 2.58) | .56 |
|
| ||||
| Exercise capacity | ||||
| Met target, n (%) |
n =59 40 (67.8) 59.2 to 76.5 |
n = 61 50 (82.0) 74.5 to 89 |
2.57 (0.81 to 8.23) | .11 |
|
| ||||
| Duration, min |
n = 59 6.8 (6.2 to 7.3) |
n = 60 6.8 (6.2 to 7.3) |
.99 | |
|
| ||||
| Peak heart rate, BPM |
n = 59 135.5 (132.4 to 138.6) |
n = 61 138.6 (135.4 to 141.7) |
.18 | |
Except where noted, all values presented in Table 5 are adjusted endpoint scores and include 95% confidence intervals.
P values presented here are adjusted value with age, sex, and the corresponding baseline measurement.
Response rates were calculated from 10 imputed datasets: n = 225/640 for escitalopram and n = 111/630 for placebo groups, respectively.
Beck Depression Inventory: score range, 0–63 (higher score=greater severity of depressive symptoms)
Perceived stress scale: score range, 10–50 (higher score=greater levels of perceived stress)
Hostility: score range, 0–27 (higher score= greater levels of hostility)
Hostile Affect: score range, 0–5 (higher score=greater levels of hostile affect)
Trait anxiety: score range, 20–80 (higher score=greater levels of trait anxiety)
State anxiety: score range, 20–80 (higher score=greater levels of state anxiety)
Negative affect measured by visual analogue scale (0–100; higher score=greater levels of state negative affect)
Positive affect measured by visual analogue scale (0–100; higher score=greater levels of state positive affect)
Abbreviations: BMI, body mass index (weight in kg/height in cm; BPM, beats per minute; ESIMI, exercise-stress-induced myocardial ischemia; KD, platelet serotonin binding affinity; MSIMI, mental-stress-induced myocardial ischemia; MSs, mental stressors; RPP, rate-pressure product; 5HTT, platelet serotonin receptor transporter number
Psychological Outcomes
Scores of symptoms of depression, trait anxiety, and perceived stress improved at 6 weeks in both intervention groups, but no differences between groups were observed (Table 5). State anxiety scores improved at week 6 in the escitalopram group and worsened in the placebo group (−2.02 [95% CI, −3.7 to −0.33] vs 0.54 [95% CI, −1.16 to 2.24]; P=.03), but this association became non-significant upon adjusting for sex and baseline state anxiety (Table 5). Hostility scores remained similar over the 6-week trial across both groups. During endpoint mental stress testing, participants in escitalopram group felt significantly more in control and calmer than those in placebo group. Negative emotional responses to mental stress were less intense in the escitalopram group than in the placebo group but the group difference was not significant. Participants in the escitalopram group also showed less reduction in positive affect during mental stress; the difference remained significant after adjusting for sex and baseline score (−22.6 [95% CI, −27.4 to −17.9], vs. −29.41 [95% CI, −34.2 to −24.6]; P<.05).
Tolerability and Safety
A significantly higher proportion of participants in the escitalopram group reported side effects compared with participants in the placebo group (46 [71.9%] vs. 28 [44.4%], P=.002) (Table 3). Side effects were relatively mild. Two participants withdrew because of side effects considered probably due to escitalopram (Figure 1).
DISCUSSION
The REMIT randomized, double-blind, controlled trial demonstrated that participants with stable CHD receiving escitalopram had an odds of 2.62 of not experiencing MSIMI at week 6, compared with those receiving placebo. Six weeks of escitalopram treatment reduced the number of platelet serotonin receptor transporters and altered the transporter binding affinity density of 5HT uptake sites, compared with the placebo group. Further, the 6-week escitalopram intervention was associated with greater improvements in hemodynamic responses to mental stress and certain measures of psychological functioning, including state anxiety and positive affect, during mental stress. Escitalopram had no effect on exercise-induced ischemia.
MSIMI in patients with clinically stable CHD has been abundantly documented.4,23 Consistent findings demonstrate that MSIMI, as documented by new wall motion abnormality, LVEF reduction ≥5 or ≥8%, and/or ischemic ECG changes, is common in patients with clinically stable CHD.5 MSIMI is not well associated with conventional cardiovascular risks,5,23 but does predict future adverse cardiovascular prognosis5,6,24 and may offer superior prognostic ability compared with ESIMI.6 However, despite the significance of MSIMI, there has been relatively little investigation of pharmacotherapeutic interventions for this condition. Two previous studies tested the effects of 4 weeks of metoprolol7 on mental-stress-induced wall motion abnormality in 19 participants and 4 weeks of nifedipine GITS vs. atenolol8 on mental-stress-induced LVEF reduction in 15 patients using a cross-control design. Rates of MSIMI prior to study intervention were 74% and 33% in these studies, respectively; due to their small sample sizes, neither study proved definitive.7,8 With a relatively large sample size, the present study demonstrates that escitalopram use is associated with a reduction in MSIMI of about 2.62 times compared with placebo. The improvement of MSIMI was seen in both mental-stress induced wall motion abnormality and LVEF decrease of ≥8 (Table 5).
Randomized trials examining cardiovascular benefits of SSRI therapy have yielded mixed results. For instance, both SADHART studies showed no benefit for sertaline vs. placebo for several cardiovascular measurements25 and composite scores.26 However, a secondary analysis of the ENRICHD trial found that depressed post-MI patients who received SSRI during the trial had lower rates of mortality than depressed patients not taking an SSRI.27
Several studies have examined the effects of SSRIs on metabolic risk factors of cardiovascular diseases. A number of investigators have reported that fluoxetine improved insulin sensitivity and glucose tolerance in small samples of patients with type 2 diabetes.28–33 Kamarck and colleagues studied the effects of citalopram on metabolic measurements and hostility in 159 healthy individuals who had high levels of hostility at baseline. The authors reported that 2 months of citalopram 40 mg/day favorably changed metabolic risk factors, including BMI, waist circumference, glucose, HDL cholesterol, triglycerides, insulin sensitivity, and resting DBP (all P<.05 vs. placebo).34 Citalopram also resulted in greater reduction of hostile affect that was a mediator in the improvement of DBP.11,34 A study of 4 weeks of paroxetine therapy in 8 healthy subjects resulted in a 10%–15% reduction in SBP and DBP responses to mental stress relative to measures obtained while participants were receiving placebo.10
Except for weight and BMI, our study did not assess metabolic risk factors. Otherwise, our findings accord with those of Kamarck and Golding et al. The observed lack of significant reductions in weight and BMI and lack of improvement in hostile affect may reflect the shorter duration of the study and the severity of disease among participants. Nonetheless, our findings support the hypothesis that short-term SSRI use improves biomarkers associated with adverse cardiovascular prognosis.
Psychosocial functioning (e.g., depression, anxiety, and hostility) was not a study selection criterion. We deliberately did not specify the potential psychological effects of escitalopram to participants during consent and intervention, as the study primarily aimed at assessing effects on MSIMI. Similar to Kamarck et al.,34 we did not find that escitalopram had a favorable effect on reducing depressive symptoms. Although state anxiety improved some among participants receiving escitalopram, especially with regard to emotional reactions to mental stress testing, trait anxiety remained unchanged. This might be due to the short-term intervention and/or reflect a study population without clinically significant depression and anxiety. However, participants receiving escitalopram reported feeling calmer and more in control during the Week 6 mental stress protocol relative to those receiving placebo. This finding is notable because positive expectations and attitudes have been shown to be associated with lower rates of mortality in CHD patients.35
Myocardial ischemia reflects an imbalance in a complex process involving both increases in the determinants of myocardial oxygen demand and decrease of coronary blood supply.36 Hemodynamic responses to mental stress differ fundamentally from exercise-induced stress, as mental stress causes little increase in heart rate and a lower-grade increase in SBP relative to physical stress.23 The underlying pathological process of MSIMI is poorly understood. Current models posit that MSIMI is due to constriction of small or micro-coronary arteries in the context of endothelial dysfunction or atherosclerosis, resulting from dysregulation of the CNS and HPA-axis system in response to emotional stress. Our study shows that escitalopram can significantly reduce MSIMI that cannot be modified by conventional anti-ischemic agents and suggests that enhancing central synaptic availability of serotonin may be an important step in CHD management.
Another potential mechanistic explanation is that SSRIs reduce platelet aggregation.37 Reduction of platelet aggregation through inhibition of the 5HT receptor by the receptor agonist, even when initiated after onset of recurrent thrombosis, significantly improved coronary patency in a canine model.38 Escitalopram was seen to significantly alter platelet serotonin receptor transport volume and affinity in our study. Further evaluation of associations of such alterations with changes in platelet aggregation and MSIMI will provide additional mechanistic insight. Whether MSIMI improvement results from changes in cardiovascular reaction to mental stress and/or psychological modification deserves further exploration.
Limitations
A number of limitations to our study should be noted. Pre-stratification of baseline demographic and clinical characteristics for equal randomization was not applied because of the relatively small sample size. In addition, based on the multiple imputation ITT analysis, rates of MSIMI in the treatment group (65.8%) and the placebo group (82.5%) were higher than the original estimate; i.e., MSIMI rates of 40% and 70% in treatment and placebo groups, respectively. The study’s power was thus lower than expected.14 In addition, although dropout of participants was balanced between arms, the number of dropouts could potentially have had an effect on our findings.
The present study did not address whether reductions in MSIMI reduction at 6 weeks reached the ceiling effect and was not designed to test SSRI effects on major adverse cardiovascular events. In addition, we delivered the 3 mental tasks in the same sequence throughout the study for comparative consistency; thus, individual task potency could not be evaluated. Further, it is possible that an effect on MSIMI caused by 1 task might have been carried forward to affect a subsequent task, although we believe the 6-minute rest period would allow the cardiovascular system to recover from the stress. However, because the primary outcome assessed whether or not a participant had MISMI with the any of the 3 mental tasks, study findings were not adversely affected by such an arrangement. Finally, REMIT was conducted at a single, large, tertiary-care academic medical center; as such, study findings may not be generalizable to different patient populations and/or care environments.
In summary, we have demonstrated that 6-week pharmacologic enhancement of serotonergic function superimposed on the best evidence-based CHD management appears to significantly improve MSIMI occurrence. These results support and extend previous findings suggesting that that modifying central and peripheral serotonergic function could improve CHD symptoms and may have implications for understanding the pathways by which negative emotions affect cardiovascular prognosis.
Acknowledgments
Funding/support: The REMIT study was funded by the National Heart, Lung, and Blood Institute (NHLBI R01 HL085704), Bethesda, Maryland. Escitalopram and patched placebo were supplied by Forest Research Institute, Inc., Germantown, MD. Forest Research Institute had no other role in any aspect of the study.
The authors would like to thank Dr. Kirkwood Adams, Professor of Medicine at the University of North Carolina–Chapel Hill School of Medicine, Dr. Alan Miller, Professor of Medicine at University of Florida College of Medicine–Jacksonville, and Zhen Huang, MS, Statistician at the Duke Clinical Research Institute, Durham, NC, for their contributions as the Data and Safety Monitoring Board of the REMIT study. These persons received no compensation from REMIT trial funding sources for their contributions. The authors would like also to thank Jonathan McCall, MS, for his editorial assistance. Mr. McCall is an employee of the Duke Clinical Research Institute and did not receive any compensation for his work on this manuscript other than his usual salary.
Footnotes
Author contributions: Dr. Jiang had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Maragatha Kuchibhatla, PhD and Stephen Boyle, PhD were responsible for granting access to the data.
Role of the sponsor: The sponsors of this study played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Study concept and design: Wei Jiang
Acquisition of data: Wei Jiang, Eric J. Velazquez, Maragatha Kuchibhatla, Zainab Samad, Stephen Boyle, Cynthia Kuhn, Richard C. Becker, Thomas L. Ortel, Redford B. Williams Jr., Joseph G. Rogers, Christopher O’Connor
Analysis and interpretation of data: Wei Jiang, Eric J. Velazquez, Maragatha Kuchibhatla, Zainab Samad, Stephen Boyle, Cynthia Kuhn, Richard C. Becker, Thomas L. Ortel, Redford B. Williams Jr., Joseph G. Rogers, Christopher O’Connor
Drafting of the manuscript: Wei Jiang, Eric J. Velazquez, Maragatha Kuchibhatla, Zainab Samad, Stephen Boyle,
Critical revision of the manuscript for important intellectual content: Wei Jiang, Eric J. Velazquez, Maragatha Kuchibhatla, Zainab Samad, Stephen Boyle, Cynthia Kuhn, Richard C. Becker, Thomas L. Ortel, Redford B. Williams Jr., Joseph G. Rogers, Christopher O’Connor
Statistical analysis: Maragatha Kuchibhatla, Stephen Boyle,
Obtained funding: Wei Jiang
Administrative, technical, or material support: Wei Jiang
Study supervision: Wei Jiang
Conflict of interest disclosures: All authors received salary support through an NHLBI research grant. Dr. Redford B. Williams reports holding a U.S. patent on the 5HTTLPR L allele for use as a marker of increased cardiovascular risk in stressed persons and is a founder and major stockholder of Williams LifeSkills, Inc. Dr. Becker reports receiving research grant support from Baxter, Bristol-Myers Squibb, Johnson & Johnson, and Regado Biosciences, and consulting/lecture fees from Baxter, Boehringer Ingelheim, Bristol-Myers Squibb, Johnson & Johnson, Regado Biosciences, and Sanofi-Aventis. Dr. Velazquez reports receiving research grants from Abbott Laboratories, Evalve, and Ikaria, and consulting fees from Boehringer Ingelheim, Gilead, and Novartis. Dr. Rogers reports receiving funding from Boston Scientific Corporation, HeartWare, and Thoratec Corporation. Dr. O’Connor reports receiving funding from Actelion Pharmaceuticals Ltd., Amgen, Inc., Biscardia LLC, Cardiology Consulting Associates, Faculty Connection, GE Healthcare, Ikaria, Neurotronik/Interventional Autonomics Corporation, Novella Clinical Inc., Pfizer Inc., Pozen, and Roche Diagnostics.
References
- 1.Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 2008;51(13):1237–1246. doi: 10.1016/j.jacc.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trichopoulos D, Katsouyanni K, Zavitsanos X, Tzonou A, Dalla-Vorgia P. Psychological stress and fatal heart attack: the Athens (1981) earthquake natural experiment. Lancet. 1981;1(8322):441–444. doi: 10.1016/s0140-6736(83)91439-3. [DOI] [PubMed] [Google Scholar]
- 3.Jiao Z, Kakoulides SV, Moscona J, Whittier J, Srivastav S, Delafontaine P, Irimpen A. Effect of Hurricane Katrina on incidence of acute myocardial infarction in New Orleans three years after the storm. Am J Cardiol. 2012;109:502–505. doi: 10.1016/j.amjcard.2011.09.045. [DOI] [PubMed] [Google Scholar]
- 4.Aoki T, Fukumoto Y, Yasuda S, Sakata Y, Ito K, Takahashi J, Miyata S, Tsuji I, Shimokawa H. The Great East Japan Earthquake Disaster and cardiovascular diseases. Eur Heart J. 2012;33(22):2796–2803. doi: 10.1093/eurheartj/ehs288. [DOI] [PubMed] [Google Scholar]
- 5.Strike PC, Steptoe A. Systematic review of mental stress-induced myocardial ischemia. Eur Heart J. 2003;24(8):690–703. doi: 10.1016/s0195-668x(02)00615-2. [DOI] [PubMed] [Google Scholar]
- 6.Jiang W, Babyak M, Krantz DS, et al. Mental stress-induced myocardial ischemia and cardiac events. JAMA. 1996;275(21):1651–1656. doi: 10.1001/jama.275.21.1651. [DOI] [PubMed] [Google Scholar]
- 7.Bairey CN, Krantz D, DeQuattro V, Berman D, Rozanski A. Effect of beta-blockade on low heart rate-related ischemia during mental stress. J Am Coll Cardiol. 1991;17(6):1388–1395. doi: 10.1016/s0735-1097(10)80152-4. [DOI] [PubMed] [Google Scholar]
- 8.Andrews TC, Parker JD, Jacobs S, Friedman R, Cummings N, MacCallum G, Mannting F, Tofter GH, Carlson W, Muller JE, Stone PH. Effects of therapy with nifedipine GITS or atenolol on mental stress-induced ischemic left ventricular dysfunction. J Am Coll Cardiol. 1998;32(6):1680–1686. doi: 10.1016/s0735-1097(98)00445-8. [DOI] [PubMed] [Google Scholar]
- 9.Blumenthal JA, Sherwood A, Babyak MA, et al. Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease: a randomized controlled trial. JAMA. 2005;293(13):1626–1634. doi: 10.1001/jama.293.13.1626. [DOI] [PubMed] [Google Scholar]
- 10.Golding M, Kotlyar M, Carson SW, et al. Effects of paroxetine on cardiovascular response to mental stress in subjects with a history of coronary artery disease and no psychiatric diagnoses. Psychopharmacology (Berl) 2005;182(3):321–326. doi: 10.1007/s00213-005-0075-7. [DOI] [PubMed] [Google Scholar]
- 11.Kamarck TW, Muldoon MF, Manuck SB, Haskett RF, Cheong J, Flory JD, Vella E. Citalopram improves metabolic risk factors among high hostile adults: results of a placebo-controlled intervention. Psychoneuroendocrinology. 2011;36(7):1070–1079. doi: 10.1016/j.psyneuen.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camacho A, Dimsdale JE. Platelets and psychiatry: lessons learned from old and new studies. Psychosom Med. 2000;62(3):326–336. doi: 10.1097/00006842-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Brydon L, Magid K, Steptoe A. Platelets, coronary heart disease, and stress. Brain Behav Immun. 2006;20(2):113–119. doi: 10.1016/j.bbi.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Jiang W, Velazquez EJ, Martsberger C, et al. Responses of mental stress induced myocardial ischemia to escitalopram treatment: background, design, and method for the Responses of Mental Stress Induced Myocardial Ischemia to Escitalopram Treatment trial. Am Heart J. 2012;163(1):20–26. doi: 10.1016/j.ahj.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douglas PS, DeCara JM, Devereux RB, et al. Echocardiographic imaging in clinical trials: American Society of Echocardiography Standards for echocardiography core laboratories: endorsed by the American College of Cardiology Foundation. J Am Soc Echocardiogr. 2009;22(7):755–765. doi: 10.1016/j.echo.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 17.Spielberger CD, Gorssuch RL, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- 18.Cook WW, Medley DM. Proposed hostility and pharisaic-virtue scales for the MMPI. J Appl Psychol. 1954;38:414–418. [Google Scholar]
- 19.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 20.Akinboboye O, Krantz DS, Kop WJ, et al. Comparison of mental stress-induced myocardial ischemia in coronary artery disease patients with versus without left ventricular dysfunction. Am J Cardiol. 2005;95(3):322–326. doi: 10.1016/j.amjcard.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 21.Jiang W, Samad Z, Boyle S, et al. New insight into mental stress induced myocardial ischemia: prevalence and demographic/clinical characteristics. JACC. 2013;61(7):714–722. doi: 10.1016/j.jacc.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schafer JL. Multiple Imputation: A primer. Statistical Method in Medical Research. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 23.Rozanski A, Bairey CN, Krantz DS, et al. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318(16):1005–1012. doi: 10.1056/NEJM198804213181601. [DOI] [PubMed] [Google Scholar]
- 24.Jain D, Burg M, Soufer R, Zaret BL. Prognostic implications of mental stress induced silent left ventricular dysfunction in patients with stable angina pectoris. Am J Cardiol. 1995;76(1):31–35. doi: 10.1016/s0002-9149(99)80796-6. [DOI] [PubMed] [Google Scholar]
- 25.Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 26.O’Connor CM, Jiang W, Kuchibhatla M, et al. Safety and efficacy of sertraline for depression in patients with heart failure: Results of the SADHART-CHF trial. J Am Coll Cardiol. 2010;56(9):692–699. doi: 10.1016/j.jacc.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor CB, Youngblood ME, Catellier D, et al. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62(7):792–798. doi: 10.1001/archpsyc.62.7.792. [DOI] [PubMed] [Google Scholar]
- 28.Breum L, Bjerre U, Bak JF, Jacobsen S, Astrup A. Long term effects of fluoxetine on glycemic control in obese patients with non-insulin-dependent diabetes mellitus or glucose intolerance:influence on muscle glycogen synthase and insulin receptorkinase activity. Metabolism. 1995;44(12):1570–1576. doi: 10.1016/0026-0495(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 29.Daubresse J, Kolanowski J, Krzentowski G, Kutnowski M, Scheen A, Van Gaal L. Usefulness of fluoxetine in obese non-insulin-dependent diabetics: a multicenter study. Obes Res. 1996;4(4):391–396. doi: 10.1002/j.1550-8528.1996.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 30.Gray DS, Fujioka K, Devine W, Bray GA. Fluoxetine treatment of the obese diabetic. Int J Obes Relat Metab Disord. 1992;16(3):193–198. [PubMed] [Google Scholar]
- 31.Maheux P, Ducros F, Bourque J, Garon J, Chiasson JL. Fluoxetine improves insulin sensitivity in obese patients with noninsulin-dependent diabetes mellitus independently of weight loss. Int J Obes. 1997;21(2):97–102. doi: 10.1038/sj.ijo.0800372. [DOI] [PubMed] [Google Scholar]
- 32.O’Kane M, Wiles PG, Wales JK. Fluoxetine in the treatment of obese type 2 diabetic patients. Diabet Med. 1994;11(1):105–110. doi: 10.1111/j.1464-5491.1994.tb00238.x. [DOI] [PubMed] [Google Scholar]
- 33.Potter van Loon BJ, Radder JK, Frolich M, Krans HMJ, Zwinderman AH, Meinders AE. Fluoxetine increases insulin action in obese nondiabetic and in obese non-insulin-dependent diabetic individuals. Int J Obes. 1992;16(2):79–85. [PubMed] [Google Scholar]
- 34.Kamarck TW, Haskett RF, Muldoon M, et al. Citalopram intervention for hostility: results of a randomized clinical trial. J Consult Clin Psychol. 2009;77(1):174–188. doi: 10.1037/a0014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barefoot JC, Brummett BH, Williams RB, et al. Recovery expectations and long-term prognosis of patients with coronary heart disease. Arch Intern Med. 2011;171(10):929–935. doi: 10.1001/archinternmed.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGregor M. Mechanisms of transient myocardial ischemia. Can J Cardiol. 1986;(Suppl A):53A–58A. [PubMed] [Google Scholar]
- 37.McCloskey DJ, Postolache TT, Vittone BJ, Nghiem KL, Monsale JL, Wesley RA, Rick ME. Selective serotonin reuptake inhibitors: measurement of effect on platelet function. Transl Res. 2008;151(3):168–172. doi: 10.1016/j.trsl.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Przyklenk K, Frelinger AL, 3rd, Linden MD, et al. Targeted inhibition of the serotonin 5HT2A receptor improves coronary patency in an in vivo model of recurrent thrombosis. J Thromb Haemost. 2010;8(2):331–340. doi: 10.1111/j.1538-7836.2009.03693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]