Abstract
Background
In patients with metastatic renal cell carcinoma (mRCC), the timing of systemic targeted therapy in relation to cytoreductive nephrectomy (CN) is under investigation.
Objective
To evaluate postoperative complications after the use of presurgical targeted therapy prior to CN.
Design, setting, and participants
A retrospective review of all patients who underwent a CN at The University of Texas M.D. Anderson Cancer Center from 2004 to 2010 was performed. Inclusion in this study required documented evidence of mRCC, with treatment incorporating CN.
Interventions
Patients receiving presurgical systemic targeted therapy prior to CN were compared to those undergoing immediate CN.
Measurements
Complications were assessed using the modified Clavien system for a period of 12 mo postoperatively.
Results and limitations
Presurgical therapy was administered to 70 patients prior to CN (presurgical), while 103 patients had an immediate CN (immediate). A total of 232 complications occurred in 57% of patients (99 of 173). Use of presurgical systemic targeted therapy was predictive of having a complication >90 d postoperatively (p = 0.002) and having multiple complications (p = 0.013), and it was predictive of having a wound complication (p < 0.001). Despite these specific complications, presurgical systemic targeted therapy was not associated with an increased overall complication risk on univariable or multivariate analysis (p = 0.064 and p = 0.237) and was not predictive for severe (Clavien ≥3) complications (p = 0.625). This study is limited by its retrospective nature. As is inherent to any retrospective study reporting on complications, we are limited by reporting bias and the potential for misclassification of specific complications.
Conclusions
Despite an increased risk for specific wound-related complications, overall surgical complications and the risk of severe complications (Clavien ≥3) are not greater after presurgical targeted therapy in comparison to upfront cytoreductive surgery.
Keywords: Cytoreductive nephrectomy, Metastatic renal cell carcinoma, Targeted therapy, Complications
1. Introduction
Cytoreductive nephrectomy (CN) is currently a common practice in the multimodality treatment of patients with metastatic renal cell carcinoma (mRCC) as a result of two prospective randomized trials demonstrating a survival benefit in patients randomized to CN followed by interferon-α (IFN-α) compared to IFN-α alone [1,2]. Over the past 6 yr, contemporary systemic “targeted” therapies have essentially replaced immunotherapies as the standard treatment for patients with mRCC. Although level I evidence supporting CN prior to contemporary systemic therapy is lacking, the use of CN has remained an integral part of treatment for mRCC.
Several aspects of CN are under evaluation, including the optimal timing of surgery in the course of systemic treatment, the safety of administrating presurgical systemic therapy, and determining how better to select patients who may derive the greatest benefit from CN [3–7]. In a phase 2 study performed at The University of Texas M.D. Anderson Cancer Center (MDACC), we evaluated the feasibility and safety of presurgical treatment with bevacizumab in patients with mRCC [4]. This was the first trial in patients with mRCC to evaluate the safety of presurgical treatment with antiangiogenesis therapy. Without a control for comparison, definitive statements could not be made regarding the relative risks of surgical morbidity associated with presurgical therapy.
As our experience with presurgical targeted therapy has grown at MDACC, we are obligated to remain critical of the outcomes associated with this treatment paradigm and to report on the safety with respect to surgical morbidity. In addition to published outcomes from other surgical series, our own experience has raised concerns regarding the potential effects of this sequence of therapy on postoperative outcomes. To validate our concerns, we assessed postoperative complications occurring with the use of presurgical systemic targeted therapy and compared these results to complications occurring after immediate CN in a contemporary series of patients.
2. Materials and methods
2.1. Patient population
After approval from the institutional review board at MDACC, we performed a retrospective review on all surgical patients with mRCC from 2004 to 2010. Inclusion criteria encompassed all patients with pathologically confirmed RCC (any histology) and preoperative findings of M1 disease. A total of 173 patients were identified and available for analysis. Presurgical systemic targeted therapy was administered to 70 patients (presurgical), while the remaining 103 patients received immediate CN (immediate). The majority of patients underwent a percutaneous biopsy to establish histology prior to receiving presurgical therapy. Several patients presenting with poor performance status (PS) or on therapy prior to evaluation at our institution may not have had a biopsy prior to initiation of therapy. Presurgical therapy was not knowingly administered to patients with non–clear-cell histology. Multiple systemic treatments were used, including a monoclonal antibody against vascular endothelial growth factor (VEGF), VEGF receptor inhibitors, mammalian target of rapamycin inhibitors, epidermal growth factor receptor inhibitors, or a combination of agents with or without cytotoxic chemotherapy. Patients receiving systemic therapy did so as a result of three scenarios: (1) inclusion in our previously reported presurgical clinical trial, (2) patients on systemic therapy prior to evaluation at MDACC, or (3) patients who did not meet clinical trial criteria to enroll in one of our protocols but were treated off protocol per patient or physician preference.
2.2. Clinical evaluation
Preoperative, operative, and postoperative characteristics were recorded for each patient. Duration of systemic therapy and time from last treatment dose to surgery were calculated. The primary end point was any postoperative complication occurring within 12 mo. Complications were captured by review of the electronic medical record, including urology clinic visits, medical oncology visits, urgent care visits, consults, review of radiographic imaging, and review of acquired medical documents from hospital and physician visits outside of our center when these were available. Complications were assessed by time and event (for those with multiple complications) and were classified using the modified Clavien system [8]. There were 45 categorized postoperative events (Table 1). Superficial wound dehiscence (at minimum, a separation of skin edges requiring dressing changes), wound infection (required intravenous or oral antibiotics), and fascial dehiscence (evisceration) were assessed independently and also subsequently grouped as wound complications.
Table 1. Two hundred thirty-two postoperative complications captured within 12 mo from surgery.
| Event | n | Event | n |
|---|---|---|---|
|
| |||
| Hematologic (n = 25): | Lymphatic (n = 14): | ||
| Bleeding | 3 | Chylous ascites | 12 |
| Retroperitoneal hematoma | 2 | Lymphocele | 2 |
| DVT | 7 | ||
| PE | 13 | ||
|
| |||
| Incisional complications (n = 40): | Pulmonary (n = 23): | ||
| Superficial wound dehiscence | 23 | Pneumonia | 6 |
| Wound infection | 12 | Respiratory failure (requiring intubation) | 12 |
| Fascial dehiscence | 2 | ||
| Incisional hernia | 3 | Pleural effusion | 5 |
|
| |||
| Genitourinary/renal (n = 28): | Cardiovascular (n = 12): | ||
| Urinary retention | 2 | MI | 2 |
| Ureteral obstruction (requiring stent placement) | 1 | Arrhythmia | 5 |
| Hypertension | 5 | ||
|
|
|||
| Acute renal failure (not requiring dialysis) | 10 | Neurologic (n = 4): | |
| CVA | 1 | ||
| Dialysis | 3 | Subdural hematoma | 1 |
| Dehydration/failure to thrive | 10 | Seizure | 1 |
| Epididymitis | 2 | Cerebral edema | 1 |
|
|
|||
| Gastrointestinal (n = 27): | |||
|
|
|||
| Pancreatitis | 2 | Miscellaneous (n = 48): | |
| Ileus | 15 | Postoperative transfusion (<30 d) | |
| Splenic laceration | 1 | Unplanned ICU stay | |
| TPN | 8 | Retained foreign object | |
| Small bowel obstruction | 1 | Prolonged drain | |
|
|
|||
| Infectious (n = 11): | Reoperation | ||
| UTI | 2 | Uncontrolled pain (requiring narcotics) | |
| Line sepsis | 3 | ||
| Intraperitoneal/retroperitoneal fluid collection | 6 | Death | |
| Addisonian crisis | |||
| Other | |||
DVT = deep vein thrombosis; PE = pulmonary embolism; MI = myocardial infarction, CVA = cerebrovascular accident; TPN = total parenteral nutrition; ICU = intensive care unit; UTI = urinary tract infection.
2.3. Statistical analysis
Summary statistics were used to describe the clinical and demographic characteristics of the study population. Student t tests (or Wilcoxon rank-sum test) were used to assess differences between patients with complications and patients without complications for continuous variables. We used the Fisher exact test to assess differences between patients with complications and patients without complication for categoric variables. We analyzed preoperative, operative, and postoperative characteristics to determine predictors of having any postoperative complications, and then more specifically for wound complications. Univariable and multivariate logistic regression models were used to determine statistically significant predictors as well as known clinical predictors of surgical complications for overall complication risk, and then more specifically for predictors of wound complications. Statistical analysis was performed using Stata/SE v.11.0 statistical software (StataCorp, College Station, TX, USA).
3. Results
3.1. Patient characteristics and outcomes
Baseline demographics were similar between the two groups (Table 2). The only statistically significant baseline difference was clinical N-stage (45.7% with clinically positive nodes in the presurgical group vs 29.1% in the immediate group; p = 0.035). Median follow-up for the entire cohort was 19.2 mo (range: 1.1–77.7), and median length of stay was 6 d (range: 1–107).
Table 2. Summary statistics of baseline demographic and clinical characteristics by presurgical therapy.
| No. (%) | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | All patients (n = 173) | Immediate surgery (n = 103) | Presurgical therapy (n = 70) | p value |
| Age, yr, median (range) | 60.1 (20.9–80.0) | 59.7 (26.1–80.0) | 61.4 (20.9–76.8) | 0.891 |
|
| ||||
| Gender: | 0.317 | |||
| Male | 121 (69.9) | 69 (67) | 52 (74.3) | – |
| Female | 52 (30.1) | 34 (33) | 18 (25.7) | – |
|
| ||||
| Clinical T category: | 0.090 | |||
| T1 and T2 | 91 (52.6) | 60 (58.3) | 31 (44.3) | – |
| T3 and T4 | 81 (46.8) | 43 (41.7) | 38 (54.3) | – |
| Missing | 1 (0.6) | 0 (0) | 1 (1.4) | – |
|
| ||||
| Clinical N category: | 0.035 | |||
| N0 | 111 (64.2) | 73 (70.9) | 38 (54.3) | – |
| N1 and N2 | 62 (35.8) | 30 (29.1) | 32 (45.7) | – |
|
| ||||
| Histology: | 0.608 | |||
| Clear | 149 (86.1) | 87 (84.5) | 62 (88.6) | – |
| Papillary | 11 (6.4) | 8 (7.8) | 3 (4.3) | – |
| Chromophobe | 2 (1.2) | 1 (1.0) | 1 (1.4) | – |
| Translocation | 1 (0.6) | 0 (0) | 1 (1.4) | – |
| Unclassified | 10 (5.8) | 7 (6.8) | 3 (4.3) | – |
|
| ||||
| Fuhrman grade: | 0.216 | |||
| II | 7 (4.0) | 3 (2.9) | 4 (5.7) | – |
| III | 54 (31.2) | 37 (35.9) | 17 (24.3) | – |
| IV | 112 (64.7) | 63 (61.2) | 49 (70.0) | – |
|
| ||||
| Laterality: | 0.420 | |||
| Right | 84 (48.6) | 54 (52.4) | 30 (42.9) | – |
| Left | 87 (50.3) | 48 (46.6) | 39 (55.7) | – |
| Bilateral | 2 (1.2) | 1 (1) | 1 (1.4) | – |
|
| ||||
| Follow-up, mo, median (range) | 19.2 (1.1–77.7) | 20.4 (1.1–77.7) | 19.0 (3.3–60.4) | 0.347 |
|
| ||||
| BMI>30 | 53 (30.6) | 29 (28.2) | 24 (34.3) | 0.406 |
|
| ||||
| Current smoker (yes) | 32 (18.5) | 15 (14.6) | 17 (24.3) | 0.107 |
|
| ||||
| Diabetic (yes) | 26 (15) | 15 (14.6) | 11 (15.7) | 0.832 |
|
| ||||
| Charlson: | 0.161 | |||
| Charlson <8 | 76 (43.9) | 50 (48.5) | 26 (37.1) | – |
| Charlson ≥8 | 97 (56.1) | 53 (51.5) | 44 (62.9) | – |
|
| ||||
| ECOG: | 0.229 | |||
| ECOG <2 | 161 (93.1) | 98 (95.1) | 63 (90) | – |
| ECOG ≥2 | 12 (6.9) | 5 (4.9) | 7 (10) | – |
|
| ||||
| Metastases: | 0.490 | |||
| 1 | 125 (72.30) | 72 (69.9) | 53 (75.7) | – |
| >1 | 48 (27.7) | 31 (30.1) | 17 (24.3) | – |
|
| ||||
| MSKCC risk stratification*: | 0.352 | |||
| Favorable 0 | 3 (1.7) | 3 (2.9) | 0 (0) | – |
| Intermediate 1–2 | 110 (63.6) | 63 (61.2) | 47 (67.1) | – |
| Poor >2 | 60 (34.7) | 37 (35.9) | 23 (32.9) | – |
BMI = body mass index; ECOG = Eastern Cooperative Oncology Group.
One point assigned to all presurgical patients when assessing time from diagnosis to treatment [23].
3.2. Presurgical and adjuvant systemic therapies
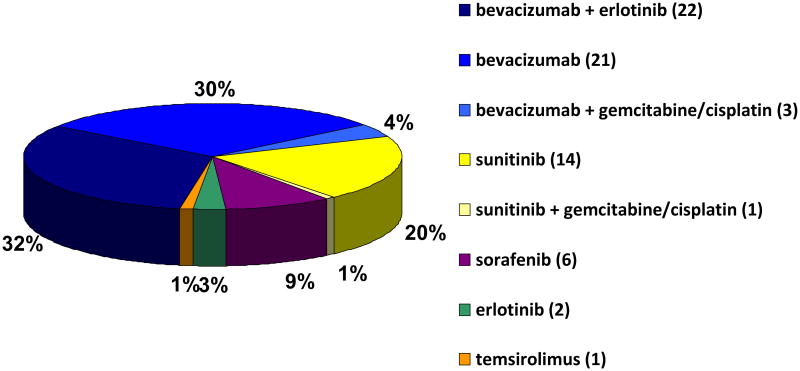
Presurgical systemic targeted therapies administered to patients prior to nephrectomy are listed in Figure 1. Duration of therapy prior to CN was a median of 1.4 mo (range: 0.2–19.6) and varied based on the reason for receiving presurgical therapy. The interval from last dose of systemic therapy to surgery was based on the specific therapy administered. The median time from last dose to surgery for bevacizumab- and sunitinib-containing regimens was 32 and 11 d, respectively. Postoperative systemic therapy was administered equally to both groups (presurgical group: 85.7%; immediate group: 74.8%; p = 0.089), although median time from surgery to start of postoperative therapy was less in the presurgical group (presurgical group: 1.2 mo; immediate group: 1.7 mo; p < 0.001).
Fig. 1. Presurgical systemic therapies administered prior to cytoreductive nephrectomy.
3.3. Surgical parameters
Surgical and perioperative parameters are listed in Table 3. Significant differences were not apparent between the two groups with regards to most surgical parameters. Patients receiving presurgical therapy were more likely to have undergone a regional lymphadenectomy (78.6% vs 61.2%; p = 0.02) and thus were more likely to be diagnosed with pathologic node-positive disease (48% vs 28.2%; p = 0.047). Although the percentage of patients undergoing laparoscopic CN was not different between groups (p = 0.225), use of laparoscopy was associated with a decreased risk of overall postoperative complications (odds ratio [OR]: 0.1; 95% confidence interval [CI], 0.0–0.3; p < 0.001).
Table 3. Summary statistics of surgical and pathologic parameters by presurgical therapy.
| No. (%) | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | All patients (n = 173) | Immediate surgery (n = 103) | Presurgical therapy (n = 70) | p value |
| Pathologic tumor size, cm, median (range) | 10 (1.7–27) | 10 (2–27) | 10 (1.7–24) | 0.397 |
|
| ||||
| Duration of surgery, min, median (range) | 185 (61–665) | 182 (61–649) | 196 (62–665) | 0.638 |
|
| ||||
| EBL, ml, median (range) | 600 (25–14 000) | 600 (25–14 000) | 700 (50–8900) | 0.464 |
|
| ||||
| Pathologic T category: | 0.674 | |||
| T1 and T2 | 27 (15.6) | 15 (14.6) | 12 (17.1) | – |
| T3 and T4 | 146 (84.4) | 88 (85.4) | 58 (82.9) | – |
|
| ||||
| Pathologic N category: | 0.047 | |||
| Nx | 55 (31.8) | 40 (38.8) | 15 (21.4) | – |
| N0 | 61 (35.3) | 34 (33) | 27 (38.6) | – |
| N1 and N2 | 57 (32.9) | 29 (28.2) | 28 (40) | – |
|
| ||||
| LND: | 0.020 | |||
| None | 55 (31.8) | 40 (38.8) | 15 (21.4) | – |
| Partial plus total | 118 (68.2) | 63 (61.2) | 55 (78.6) | – |
|
| ||||
| Matted nodes present | 15 (12.7) | 6 (9.5) | 9 (16.4) | 0.284 |
|
| ||||
| Presence of sarcomatoid features | 29 (16.8) | 14 (13.6) | 15 (21.4) | 0.214 |
|
| ||||
| Laparoscopic | 31 (17.9) | 15 (14.6) | 16 (22.9) | 0.225 |
|
| ||||
| PN | 6 (3.5) | 2 (1.9) | 4 (5.7) | 0.224 |
EBL = estimated blood loss; LND = lymph node dissection; PN = partial nephrectomy.
3.4. Postoperative outcomes
3.4.1. Complications
Postoperative complications, as classified by the modified Clavien system, included 232 events occurring in 57% of patients (99 of 173) within 12 mo of CN. The majority of complications (65.1%) occurred within 30 d of CN; however, an additional 57 events (24.6%) occurred between 31 and 90 d, while another 24 events (10.3%) occurred after 90 d and were more likely in those who had received presurgical therapy (15.9% vs 3.8%; p = 0.002). Significant (Clavien ≥3) complications comprised 29.7% (69 of 232) of all events and occurred equally in both groups (presurgical group: 29.4% vs immediate group: 30.2%; p = 0.999). Although presurgical therapy was not significantly associated with an increased overall complication rate (65.7% vs 51.4%; p = 0.085), patients receiving presurgical therapy who developed a subsequent postoperative complication were more likely to have multiple events (76% vs 51%; p = 0.013). When analyzing the risk of experiencing a specific complication, presurgical therapy was associated with an increased rate of superficial wound dehiscences (24.3% vs 5.8%; p < 0.001) and wound infections (12.9% vs 2.9%; p = 0.015). Although not statistically significant, there were two facial dehiscences, both occurring in the presurgical group. Equivalent risks were noted in the remaining categorized complications, including deep vein thrombosis (DVT) or pulmonary embolism (PE) and hemorrhage.
3.4.2. Wound complications
We performed a univariable analysis to determine predictors of having a wound complication for all patients undergoing CN (Table 4). When accounting for all statistically significant covariates (body mass index [BMI] ≥30, use of presurgical therapy), baseline significant differences (clinical node status), and known risk factors for wound complications (diabetes, smoking history, and duration of surgery), a multivariate analysis confirmed that presurgical targeted therapy remained a significant predictor of having a wound complication (Table 4; OR: 4.14; 95% CI, 1.6–10.6; p = 0.003).
Table 4. Analysis of preoperative and postoperative characteristics by risk for wound complications for all patients undergoing cytoreductive nephrectomy.
| Characteristic | Univariable | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Presurgical targeted therapy* | 4.42 (1.8–10.8) | <0.001 | 4.14 (1.6–10.6) | 0.003 |
| BMI ≥30* | 2.46 (1.1–5.7) | 0.035 | 2.44 (0.96–6.2) | 0.060 |
| Diabetic* | 1.35 (0.5–4.0) | 0.564 | 1.00 (0.3–3.3) | 0.999 |
| Smoker* | 1.03 (0.4–3.0) | 0.999 | 0.73 (0.2–2.3) | 0.597 |
| Duration of surgery* (per-minute increase) | 1.00 (1.0–1.0) | 0.361 | 1.00 (1.00–1.00) | 0.929 |
| Clinical N1 or N2* | 1.84 (0.8–4.2) | 0.190 | 1.31 (0.5–3.3) | 0.563 |
OR = odds ratio; CI = confidence interval; BMI = body mass index.
Included in multivariate analysis.
3.4.3. Perioperative morbidity (all patients)
Univariable analysis was again used to determine preoperative predictors of having any complication within 12 mo of CN for all patients (Table 5). When accounting for all statistically significant variables in addition to the use of presurgical therapy, only Eastern Cooperative Oncology Group (ECOG) PS (OR: 2.40; 95% CI, 1.2–4.8; p = 0.013) and an elevated clinical T-stage (OR: 8.95; 95% CI, 1.1–79.6; p = 0.042) remained significant predictors of having a postoperative complication after CN.
Table 5. Analysis of preoperative and postoperative characteristics by risk of overall complications for all patients undergoing cytoreductive nephrectomy.
| Characteristic | Univariable | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| ECOG ≥2* | 9.1 (1.2–72.3) | 0.036 | 9.0 (1.1–74.6) | 0.003 |
| Clinical N1 or N2* | 2.5 (1.3–4.8) | 0.007 | 1.83 (0.96–3.5) | 0.068 |
| Clinical T3 or T4* | 2.0 (1.1–3.8) | 0.023 | 8.95 (1.1–79.6) | 0.042 |
| Presurgical targeted therapy* | 1.8 (0.97–3.4) | 0.064 | 1.50 (0.77–2.9) | 0.237 |
| BMI ≥30 | 1.5 (0.8–2.9) | 0.222 | – | – |
OR = odds ratio; CI = confidence interval; ECOG = Eastern Cooperative Oncology Group; BMI = body mass index.
Included in multivariate analysis.
3.4.4. Perioperative morbidity
Eighteen deaths (10.4%) were attributed to postoperative complications. These occurred in nine patients from each of the two groups. The majority (16 of 18) occurred within 90 d of surgery, while two occurred after 90 d—both in the presurgical group—and were associated with complications occurring as a result of the development and subsequent treatment or management of chylous ascites. Deaths resulting from disease progressions were not categorized as postoperative complications.
3.4.5. Presurgical patients only
We further evaluated the presurgical group alone to determine preoperative predictors of the overall complication rate in this subpopulation (Table 6). Using all statistically significant variables in addition to ECOG PS as a measure of clinical fitness, the multivariate analysis revealed that only a decline in serum albumin while on systemic therapy was a significant predictor of having a complication (OR: 4.20; 95% CI, 1.3–14.1; p = 0.021).
Table 6. Analysis of preoperative characteristics for risk of overall complications for patients receiving presurgical targeted therapy prior to cytoreductive nephrectomy.
| Characteristic | Univariable | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Decline in serum albumin* | 4.3 (1.3–14.1) | 0.015 | 4.20 (1.3–14.1) | 0.021 |
| BMI ≥30* | 3.8 (1.1–13.0) | 0.031 | 2.35 (0.6–8.9) | 0.208 |
| Clinical T3 or T4* | 2.7 (0.9–7.4) | 0.063 | 1.74 (0.5–5.5) | 0.352 |
| ECOG ≥2* | 3.5 (0.4–30.5) | 0.265 | 2.59 (0.3–26.2) | 0.420 |
| Charlson ≥8 | 1.3 (0.4–4.0) | 0.633 | – | – |
| Received bevacizumab | 1.4 (0.5–4.1) | 0.515 | – | – |
OR = odds ratio; CI = confidence interval; BMI = body mass index; ECOG = Eastern Cooperative Oncology Group.
Included in multivariate analysis.
4. Discussion
Although the paradigm of presurgical therapy in this population appears to hold promise in initial clinical trials, currently, the proper integration of surgery and systemic therapy in the treatment of patients with mRCC is unknown. Until the results of ongoing clinical trials are known, upfront CN followed by systemic therapy will remain an integral part of the treatment of mRCC in properly selected patients [9,10]. As in any advance in medicine, proven patient safety is of paramount importance and must be assessed before any analysis of efficacy can be made.
Existing studies that have examined the impact of targeted therapy on the primary tumor would suggest that a dramatic reduction in primary tumor size or stage is not realized with the current generation of targeted therapies [5,10,11]. That said, there is some evidence that this approach can be used as a selection criterion to identify patients suitable for CN. In our phase 2 trial of presurgical bevacizumab, 6 of the 50 patients were considered unsuitable for CN after receiving presurgical therapy [4]. Whether this is viewed as successful use of presurgical therapy as a litmus test or a missed opportunity is unknown. Similarly, there is a published prospective clinical trial showing the safety of administering 3 mo of sunitinib followed by nephrectomy in 20 patients [12]. This report did not use a valid classification system or compare perioperative morbidity with a contemporary group of patients undergoing immediate CN.
The safety of the presurgical therapy paradigm was evaluated previously in a retrospective study at our institution. In this report, we reviewed a mixed cohort of 48 patients (metastatic and locally advanced) who received presurgical therapy and compared the surgical outcomes to a cohort of 58 patients who underwent immediate surgery. There were no statistically significant differences in the incidence of perioperative (30-d) morbidity and mortality [13]. Similar to Hellenthal et al, this study lacked a standardized classification system of postoperative complications. Recent urologic oncologic literature has demonstrated that a fair percentage of complications may not be captured within the 30-d postoperative interval and suggested that a period of 90 d may be necessary when reporting surgical outcomes [14]. After observing several late postoperative complications (>90 d) in presurgically treated RCC patients, we felt that evaluating postoperative outcomes for 12 mo would more accurately define surgical risk. In addition, significant discrepancies exist in the reporting of complications in the literature, and a standard needs to be developed to consistently assess outcomes among techniques and across institutions [15]. While an attempt to modify the Clavien system has been made by Shabsigh et al at Memorial Sloan-Kettering Cancer Center (MSKCC), this system is only minimally modified from that described by Clavien and does not address specific urologic and oncologic issues resulting from surgical intervention [9]. In the absence of a validated and standardized urologic classification system, we have used the modified Clavien system and attempted to address the 10 established basic reporting criteria as discussed in the MSKCC publication.
In line with our previously reported series, presurgical therapy was not an independent predictor for overall postoperative complication risk (p = 0.085). Importantly, its use was also not associated with significant (Clavien ≥3) complications (p = 0.625). These findings demonstrate the safety of the use of presurgical systemic therapy prior to CN. By evaluating postoperative complications from within 30 d to within 12 mo, we demonstrate that patients treated with presurgical therapy were more likely to have late complications and/or multiple events. With this added time interval, we captured an additional 81 events, which accounted for 35% of the captured postoperative complications. It should be pointed out that patients receiving presurgical therapy were more likely to undergo a lymph node dissection (LND; p = 0.02), likely because of a higher incidence of clinically positive lymph node disease. Despite this association, those patients undergoing LND were not shown to have a statistically significant increased overall complication risk (p = 0.072; data not shown in results). Wound-related complications were the most frequent complications observed in this group. When evaluating predictors of this outcome, presurgical systemic therapy was an independent risk factor for having a wound complication. This is consistent with other reports in breast and colorectal cancer describing delayed wound healing with the use of presurgical therapies [16–18]. In addition, we identified that a decline in serum albumin while on therapy was associated with having a postoperative complication (p = 0.015). Albumin is a marker of chronic nutritional status and has been shown to be a prognostic factor for morbidity and mortality in other published oncologic series [19–21]. These findings support the need for further evaluation of markers of preoperative nutritional status in surgical series.
Our study is limited, as it is a retrospective review and is subject to all the inherent biases related to reporting of postoperative complications, including inconsistent follow-up and the potential for misclassifications in the medical records. Discrepancies may be seen particularly in patients enrolled in clinical trials having shorter-interval evaluations, potential closer follow-up with more frequent wound evaluations, and more attention paid to small deviations from standard postoperative findings. In addition, although the majority of patients received presurgical therapy through enrollment in a clinical trial, there was likely selection bias when deciding which patients would receive presurgical therapy off protocol. Although this study does demonstrate equivalent postoperative surgical risks between the two groups, with an association between the use of presurgical therapy and wound complications, this study is not designed to prove causality. Consequently, because of the small numbers of patients receiving the specific agents in this study, the risks attributed to any individual therapy may be underappreciated. Several different presurgical therapies were administered, with a majority of the patients receiving bevacizumab. Bevacizumab is known to have a longer half-life (20 d) than most of the tyrosine kinase inhibitors, and this may complicate timing of surgical interventions. Nevertheless, bevacizumab was not an independent predictor of overall complication risk in our series (Table 6).
5. Conclusions
The use of presurgical therapy in patients with mRCC does not result in an increased overall complication rate or an increased risk of severe complications requiring an intervention (Clavien ≥3) when compared to immediate CN. However, there is an increased risk of wound complications and having multiple complications in patients treated with presurgical targeted therapy. Further insight into the role of nutritional status while on systemic therapy and prior to surgery may aid in identifying higher-risk surgical patients. Although it appears that the use of presurgical therapy in patients with mRCC is safe, we advocate that it be limited to patients deemed initially unresectable or to individuals enrolled in clinical trials.
Acknowledgments
Funding/Support and role of the sponsor: None.
Footnotes
Author contributions: Christopher G. Wood had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Wood, Chapin.
Acquisition of data: Chapin, Delacroix, Culp, Tamboli.
Analysis and interpretation of data: Nogueras Gonzalez, Chapin, Delacroix.
Drafting of the manuscript: Chapin, Delacroix, Wood.
Critical revision of the manuscript for important intellectual content: Tannir, Jonasch, Culp.
Statistical analysis: Nogueras Gonzalez, Chapin, Delacroix.
Obtaining funding: Wood.
Administrative, technical, or material support: Wood.
Supervision: Wood.
Other (specify): None.
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. New Engl J Med. 2001;345:1655–9. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 2.Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966–70. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 3.Bex A, Kerst M, Mallo H, Meinhardt W, Horenblas S, de Gast GC. Interferon alpha 2b as medical selection for nephrectomy in patients with synchronous metastatic renal cell carcinoma: a consecutive study. Eur Urol. 2006;49:76–81. doi: 10.1016/j.eururo.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Jonasch E, Wood CG, Matin SF, et al. Phase II presurgical feasibility study of bevacizumab in untreated patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4076–81. doi: 10.1200/JCO.2008.21.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abel EJ, Culp SH, Tannir NM, et al. Primary tumor response to targeted agents in patients with metastatic renal cell carcinoma. Eur Urol. 2011;59:10–5. doi: 10.1016/j.eururo.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Culp SH, Tannir NM, Abel EJ, et al. Can we better select patients with metastatic renal cell carcinoma for cytoreductive nephrectomy? Cancer. 2010;116:3378–88. doi: 10.1002/cncr.25046. [DOI] [PubMed] [Google Scholar]
- 7.Bex A, Jonasch E, Kirkali Z, et al. Integrating surgery with targeted therapies for renal cell carcinoma: current evidence and ongoing trials. Eur Urol. 2010;58:819–28. doi: 10.1016/j.eururo.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 8.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55:164–74. doi: 10.1016/j.eururo.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 10.EORTC 30073. Immediate surgery or surgery after sunitinib malate in treating patients with metastatic kidney cancer [identifier NCT01099423] doi: 10.1111/bju.15625. http://clinicaltrials.gov/ct2/show/NCT01099423?term=NCT01099423&rank=1. [DOI] [PubMed]
- 11.Cost NG, Delacroix SE, Jr, Sleeper JP, et al. The impact of targeted molecular therapies on the level of renal cell carcinoma vena caval tumor thrombus. Eur Urol. 2011;59:912–8. doi: 10.1016/j.eururo.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 12.Hellenthal NJ, Underwood W, Penetrante R, et al. Prospective clinical trial of preoperative sunitinib in patients with renal cell carcinoma. J Urol. 2010;184:859–64. doi: 10.1016/j.juro.2010.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margulis V, Matin SF, Tannir N, et al. Surgical morbidity associated with administration of targeted molecular therapies before cytoreductive nephrectomy or resection of locally recurrent renal cell carcinoma. J Urol. 2008;180:94–8. doi: 10.1016/j.juro.2008.03.047. [DOI] [PubMed] [Google Scholar]
- 14.Stimson CJ, Chang SS, Barocas DA, et al. Early and late perioperative outcomes following radical cystectomy: 90-day readmissions, morbidity and mortality in a contemporary series. J Urol. 2010;184:1296–300. doi: 10.1016/j.juro.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Donat SM. Standards for surgical complication reporting in urologic oncology: time for a change. Urology. 2007;69:221–5. doi: 10.1016/j.urology.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 16.Golshan M, Garber JE, Gelman R, et al. Does neoadjuvant bevacizumab increase surgical complications in breast surgery? Ann Surg Oncol. 2011;18:733–7. doi: 10.1245/s10434-010-1366-8. [DOI] [PubMed] [Google Scholar]
- 17.Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20:1842–7. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 18.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 19.Morgan TM, Tang D, Stratton KL, et al. Preoperative nutritional status is an important predictor of survival in patients undergoing surgery for renal cell carcinoma. Eur Urol. 2011;59:923–8. doi: 10.1016/j.eururo.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun LC, Chu KS, Cheng SC, et al. Preoperative serum carcinoembryonic antigen, albumin and age are supplementary to UICC staging systems in predicting survival for colorectal cancer patients undergoing surgical treatment. BMC Cancer. 2009;9:288. doi: 10.1186/1471-2407-9-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregg JR, Cookson MS, Phillips S, et al. Effect of preoperative nutritional deficiency on mortality after radical cystectomy for bladder cancer. J Urol. 2011;185:90–6. doi: 10.1016/j.juro.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]