Abstract
Umbilical cord blood (UCB) has the advantage of being collected and cryopreserved for years prior to use. In vitro or in murine models suggest that the duration of storage does not affect UCB progenitor cell performance, however the impact of UCB age on clinical outcomes has not been definitely defined. This study sought to determine the effect of UCB unit cryopreservation time on hematopoietic potency. We analyzed 288 single UCB units used for transplantation from 1992–2013, with unit cryopreservation time ranging from 0.08 to 11.07 years. UCB unit post thaw characteristics were examined, including percent recovery of total nucleated cells (TNC). The number of years the UCB unit spent in cryopreservation had no impact on TNC recovery nor UCB unit post-thaw viability. Duration of cryopreservation also had no impact on neutrophil or platelet engraftment in single UCB transplants. These results show that UCB units can undergo cryopreservation for at least 10 years with no impact on clinical outcomes.
Introduction
The first successful umbilical cord blood (UCB) transplant was performed in 1988[1], and since that time the ability to cryopreserve and bank UCB units has remained an essential component of their use in hematopoietic stem cell transplantation (HSCT). The use of UCB as a donor source has continued to grow, and there are currently over half a million UCB units cryopreserved in the worldwide cord blood inventory[2].
While cryopreservation is universally practiced in cord blood banking, the impact on progenitor cell function has been only partially addressed. The Broxmeyer group demonstrated that UCB units stored for up to 20 years do not lose function when used in vitro and in murine assays of progenitor cell function[3, 4], and the St Louis group reported no significant influence on clinical outcome after short term cryopreservation[5]. Parmar et al recently reported on clinical outcomes for cryopreserved units, but only documented 15 UCB units older than 5 years[6]. Hence there is still no conclusive answer to the question of whether long term cryopreservation impacts UCB transplant outcomes. Storage of UCB units comes at a financial cost to cord blood banks[7, 8], which is ultimately passed on to the patient, transplant institution and the health care system as a whole[9–11]. If long term cryopreservation is detrimental to UCB transplant outcomes, the current model of cord blood banking must be called into question. Alternatively, if the duration of cryopreservation has no impact on clinical outcomes, this provides evidence for cord blood banks to continue the current model of cryopreservation, long-term storage and distribution of UCB units, to provide a rapidly accessible donor source for transplant recipients worldwide.
In this study, we set out to determine whether duration of cryopreservation influenced single UCB transplant outcomes. We also examined the effect of cryopreservation on post-thaw UCB unit characteristics.
Methods
Study design
This was a retrospective review of 416 patients who underwent single UCB transplantation at the University of Minnesota between 1992 and 2013. Reasons for exclusion from the analysis included no available date of collection for the UCB unit (n=125), and patients who did not receive conditioning prior to receiving the UCB unit (n=3). Patients were treated on protocols approved by the University of Minnesota institutional review board, and written consent was obtained from all patients, their parents or guardians in accordance with the Declaration of Helsinki.
UCB unit processing
On delivery of UCB units to the University of Minnesota Molecular and Cellular Therapeutics facility, units were inspected, then transferred and maintained in vapor phase of liquid nitrogen storage until the day of infusion. All UCB units were thawed and washed as per the method of Rubinstein et al[12]. Prior to wash, ABO/Rh typing of the unit was performed. Following wash and prior to release for infusion, samples were taken for assessment of viability, total nucleated cell dose (TNC), CD34+ dose, and colony forming units-granulocyte-macrophage (CFU-GM). Viability was assessed using the acridine orange and propidium iodide method[13] and 7-Aminoactinomycin D (by flow cytometry). Flow cytometry was performed as per ISHAGE specifications using a dual platform, with ammonium chloride lysis for red cells followed by washing and staining.
Definitions and outcome analysis
UCB units were analyzed based on the duration of cryopreservation of the UCB unit. The TNC recovery was defined as the total TNC recovered at thaw, expressed as a percentage of the total TNC count reported prior to freezing.
Neutrophil and platelet engraftment were defined as previously described[14–16]. Cox regression analysis was used to perform univariate and multivariate analysis of patient and UCB unit factors, and their influence on outcomes. The following variables were assessed for their association with neutrophil and platelet engraftment: duration of cryopreservation, post thaw TNC/kg, post thaw CD34+/kg, viability post thaw, post thaw CFU/kg, UCB unit-recipient ABO match, UCB unit-recipient HLA match, year of transplant, type of conditioning regimen used, recipient gender, recipient age, and recipient CMV status. After 2005, patients undergoing UCB transplantation at the University of Minnesota have not routinely received anti-thymocyte globulin as part of their myeloablative conditioning regimen. As such, year of transplant was examined as patients who underwent HSCT prior to 2006 compared to the more recent era.
Results
Cell recovery
There were 288 single UCB transplants eligible for analysis, with duration of cryopreservation of the UCB units ranging from 0.08–11.07 years (Figure 1). The median post-thaw values for TNC were 11.3 ×108 cells (range 0.97–38.41) and 12.9 ×106 cells (range 0.18–131.5) for CD34+ cells. The median post-thaw nucleated cell viability for the cohort was 72% (range 30–94%), and median post-thaw total CFU-GM was 1.1 ×106 (range 0–58.81). The median TNC recovery was 76% (range 30–108%). Duration of cryopreservation of the UCB unit had no significant impact on the median post-thaw TNC (p=0.22), CD34+ (p=0.28), or CFU-GM (p=0.68). Duration of cryopreservation of the UCB unit also had no impact on post-thaw nucleated cell viability and TNC recovery (Figure 2a and 2b).
Figure 1.
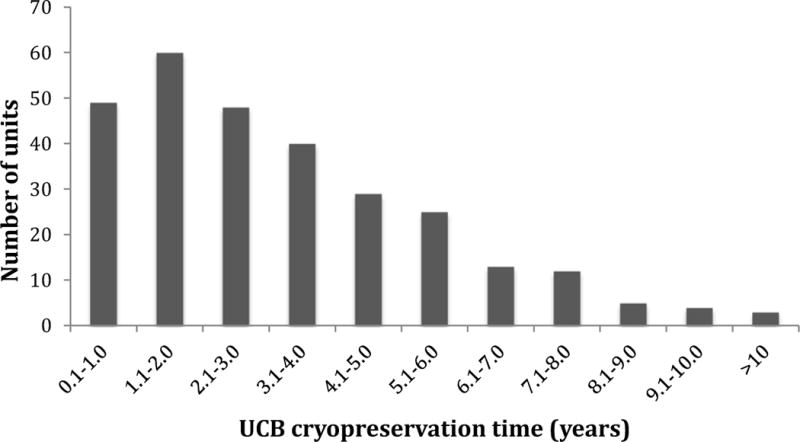
Umbilical cord blood units by duration of cryopreservation. A total of 62 umbilical cord blood units were cryopreserved for more than 5 years.
(UCB – umbilical cord blood unit)
Figure 2.
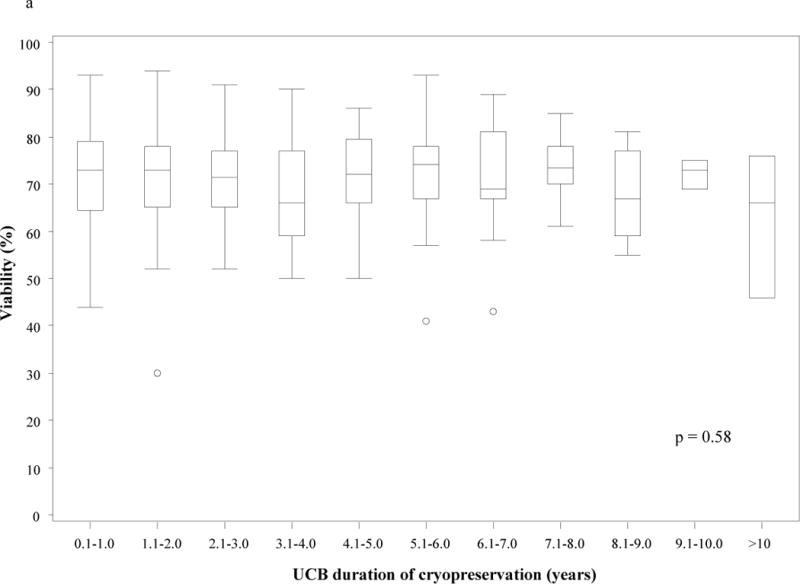
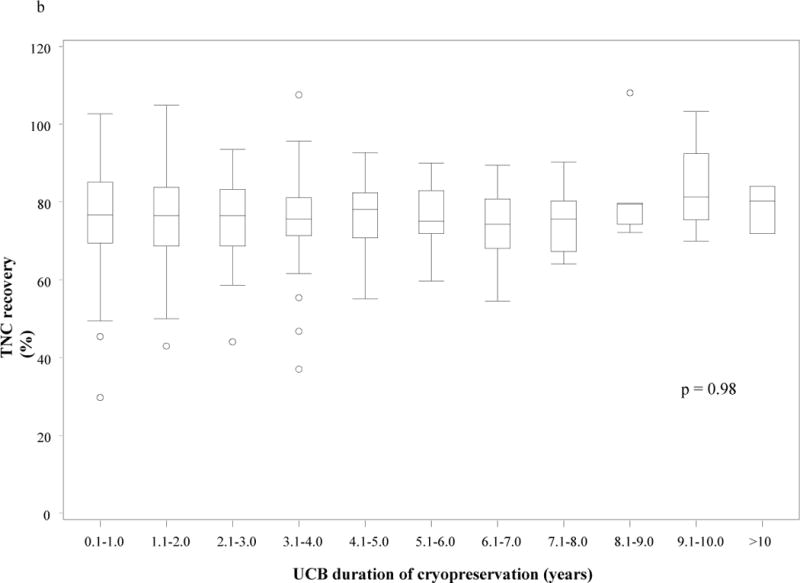
Post-thaw nucleated cell viability (a) and total nucleated cell recovery (b) based on umbilical cord blood unit duration of cryopreservation. There was no statistically significant difference in post-thaw nucleated cell viability (p = 0.58) or total nucleated cell recovery (p = 0.98) based on duration of cryopreservation.
(TNC – total nucleated cell; UCB – umbilical cord blood unit)
Neutrophil engraftment
Neutrophil engraftment for the cohort was 94% (95% CI 91–96%), with a median time to neutrophil recovery of 20 days (range 0–41). When duration of cryopreservation of the UCB unit was analyzed as a continuous variable in multivariate analysis, there was no impact on neutrophil engraftment (p=0.15, data not shown). UCB units were also analyzed in tertiles based on time spent in cryopreservation (0–2 years, 2.1–4 years, >4 years) and, tested in univariate (table 1) and multivariate analysis (table 2). There was no association of duration of cryopreservation on the probability of neutrophil engraftment. Other covariates, including CD34+ dose, CFU-GM, and year of transplantation were independently significant factors identified in multivariate analysis (Table 2). Duration of cryopreservation of the UCB unit also had no significant impact on time to neutrophil engraftment (Figure 3a).
Table 1.
Univariate (a) analysis for neutrophil engraftment.
| Parameter | Engraftment rate (95% CI) | P value |
|---|---|---|
|
| ||
| UCB unit cryopreservation (years) | ||
| 0–2 | 94% (89–98) | |
| 2.1–4 | 96% (90–99) | |
| >4 | 92% (85–97) | 0.21 |
|
| ||
| TNC (×107/kg) | ||
| <2.5 | 90% (78–97) | |
| ≥2.5 | 95% (92–97) | 0.02 |
|
| ||
| CD34+ (×105/kg) | ||
| <2.5 | 89% (79–96) | |
| ≥2.5 | 96% (93–98) | <0.01 |
|
| ||
| Post thaw viability | ||
| <75% | 92% (88–96) | |
| ≥75% | 96% (91–99) | 0.86 |
|
| ||
| CFU-GM (×106/kg) | ||
| <5.0 | 92% (87–96) | |
| ≥5.0 | 97% (93–99) | <0.01 |
|
| ||
| HLA matching | ||
| 6/6 match | 93% (86–97) | |
| 5/6 or less | 95% (91–97) | 0.15 |
|
| ||
| ABO Match | ||
| Match | 92% (86–96) | |
| Minor mismatch | 96% (91–99) | |
| Major mismatch | 94% (88–98) | 0.03 |
|
| ||
| Conditioning regimen | ||
| Myeloablative | 95% (66–82) | |
| Reduced intensity | 92% (58–93) | 0.30 |
|
| ||
| Year of transplant | ||
| Prior to 2006 | 95% (90–98) | |
| 2006–2013 | 94% (89–97) | 0.08 |
|
| ||
| Recipient CMV | ||
| Positive | 92% (87–96) | |
| Negative | 95% (91–98) | 0.52 |
|
| ||
| Recipient gender | ||
| Male | 95% (91–98) | |
| Female | 93% (88–97) | 0.71 |
|
| ||
| Recipient age (years) | ||
| <18 | 94% (91–97) | |
| ≥18 | 93% (83–98) | 0.14 |
CFU-GM – colony forming unit granulocyte macrophage; CMV – cytomegalovirus; HLA – human leukocyte antigen; TNC – total nucleated cell; UCB – umbilical cord blood
Table 2.
Multivariate analysis for neutrophil engraftment.
| Parameter | Hazard Ratio (95% CI) | P value |
|---|---|---|
|
| ||
| UCB unit cryopreservation (years) | ||
| 0–2 | 1.00 | 0.33 |
| 2.1–4 | 0.90 (0.65–1.24) | |
| >4 | 0.79 (0.58–1.08) | |
|
| ||
| TNC (×107/kg) | ||
| <2.5 | 1.00 | 0.36 |
| ≥2.5 | 1.25 (0.78–1.99) | |
|
| ||
| CD34+ (×105/kg) | ||
| <2.5 | 1.00 | 0.04 |
| ≥2.5 | 1.55 (1.02–2.35) | |
|
| ||
| CFU-GM (×106/kg) | <0.01 | |
| <5.0 | 1.00 | |
| ≥5.0 | 1.58 (1.19–2.11) | |
|
| ||
| HLA matching | 0.36 | |
| 6/6 match | 1.00 | |
| 5/6 or less | 1.15 (0.85–1.55) | |
|
| ||
| Year of transplant | ||
| Prior to 2006 | 1.00 | <0.01 |
| 2006–2013 | 0.66 (0.50–0.87) | |
|
| ||
| ABO Match | ||
| Match | 1.00 | 0.51 |
| Minor mismatch | 1.16 (0.85–1.58) | |
| Major mismatch | 1.18 (0.85–1.64) | |
CFU-GM – colony forming unit granulocyte macrophage; CMV – cytomegalovirus; HLA – human leukocyte antigen; TNC – total nucleated cell; UCB – umbilical cord blood
Figure 3.
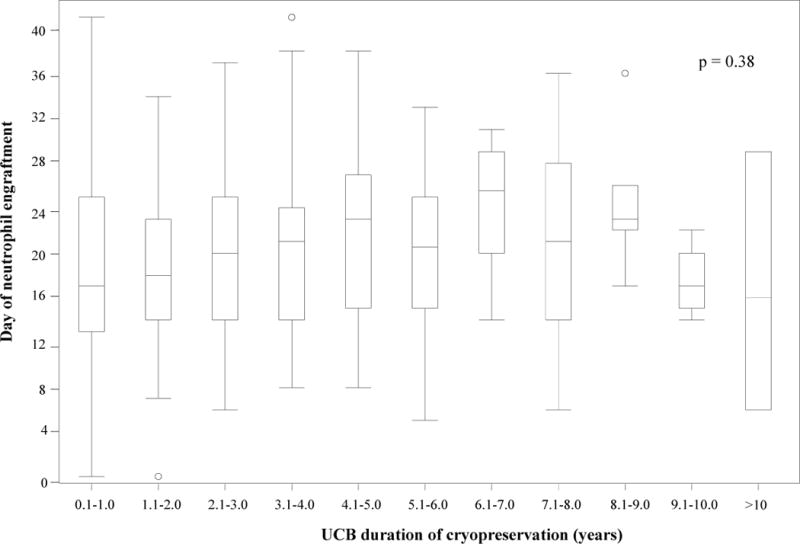
Time to neutrophil (a) and platelet (b) engraftment based on umbilical cord blood unit duration of cryopreservation. (a) There was no statistically significant difference in time to neutrophil engraftment based on duration of cryopreservation (p = 0.38). (b) Umbilical cord blood units that were cryopreserved for 4.1–5 years had a longer time to platelet engraftment than units that were cryopreserved for shorter or longer time periods (p = 0.03). (UCB – umbilical cord blood unit)
Platelet engraftment
Platelet engraftment at 1 year was 74% for the cohort (95% CI 67–81%), with a median time to platelet recovery of 48 days (range 10–224). When analyzed as a continuous variable in multivariate analysis, duration of cryopreservation of the UCB unit had no impact on platelet engraftment at 1 year (p=0.94, data not shown). Duration of cryopreservation of the UCB unit also had no significance when analyzed in tertiles in univariate and multivariate analysis (tables 3 and 4). The only covariate that was significantly associated with platelet engraftment in the multivariate analysis was CFU-GM (Table 2b). While the time to platelet engraftment was significantly different based on duration of cryopreservation of the UCB unit (p=0.03), this was driven by delayed recovery in the UCB units cryopreserved for 4.1–5 years compared to units cryopreserved for shorter or longer time periods. Thus there was no prolongation of time to platelet engraftment based on the duration of cryopreservation (Figure 3b).
Table 3.
Univariate analysis for platelet engraftment at 1 year.
| Parameter | Engraftment rate (95% CI) | P value |
|---|---|---|
|
| ||
| UCB unit cryopreservation (years) | ||
| 0–2 | 75% (64–87) | |
| 2.1–4 | 72% (59–84) | |
| >4 | 76% (63–89) | 0.89 |
|
| ||
| TNC (×107/kg) | ||
| <2.5 | 62% (43–80) | |
| ≥2.5 | 76% (69–84) | 0.10 |
|
| ||
| CD34+ (×105/kg) | ||
| <2.5 | 74% (57–92) | |
| ≥2.5 | 75% (67–83) | 0.77 |
|
| ||
| Post thaw viability | ||
| <75% | 75% (66–85) | |
| ≥75% | 73% (62–85) | 0.67 |
|
| ||
| CFU-GM (×106/kg) | ||
| <5.0 | 72% (61–82) | |
| ≥5.0 | 82% (71–92) | <0.01 |
|
| ||
| HLA matching | ||
| 6/6 match | 81% (68–95) | |
| 5/6 or less | 72% (63–80) | 0.05 |
|
| ||
| ABO Match | ||
| Match | 69% (57–81) | |
| Minor mismatch | 74% (62–86) | |
| Major mismatch | 80% (67–93) | 0.63 |
|
| ||
| Conditioning regimen | ||
| Myeloablative | 74% (66–82) | |
| Reduced intensity | 76% (58–93) | 0.02 |
|
| ||
| Year of transplant | ||
| Prior to 2006 | 71% (60–81) | |
| 2006–2013 | 77% (67–87) | 0.04 |
|
| ||
| Recipient CMV | ||
| Positive | 70% (60–80) | |
| Negative | 78% (68–88) | 0.14 |
|
| ||
| Recipient gender | ||
| Male | 74% (65–84) | |
| Female | 74% (64–85) | 0.38 |
|
| ||
| Recipient age (years) | ||
| <18 | 76% (68–84) | |
| ≥18 | 64% (46–81) | 0.76 |
CFU-GM – colony forming unit granulocyte macrophage; CMV – cytomegalovirus; HLA – human leukocyte antigen; TNC – total nucleated cell; UCB – umbilical cord blood
Table 4.
Multivariate (b) analysis for platelet engraftment at 1 year.
| Parameter | Hazard Ratio (95% CI) | P value |
|---|---|---|
|
| ||
| UCB unit cryopreservation (years) | ||
| 0–2 | 1.00 | |
| 2.1–4 | 0.96 (0.66–1.39) | 0.81 |
| >4 | 0.87 (0.63–1.21) | 0.42 |
|
| ||
| TNC (×107/kg) | ||
| <2.5 | 1.00 | 0.10 |
| ≥2.5 | 1.53 (0.92–2.53) | |
|
| ||
| CD34+ (×105/kg) | ||
| <2.5 | 1.00 | 0.29 |
| ≥2.5 | 0.80 (0.52–1.21) | |
|
| ||
| CFU-GM (×106/kg) | ||
| <5.0 | 1.00 | 0.01 |
| ≥5.0 | 1.54 (1.13–2.11) | |
|
| ||
| HLA matching | 0.07 | |
| 6/6 match | 1.00 | |
| 5/6 or less | 0.74 (0.54–1.02) | |
|
| ||
| Year of transplant | 0.61 | |
| Prior to 2006 | 1.00 | |
| 2006–2013 | 0.92 (0.68–1.25) | |
|
| ||
| ABO Match | ||
| Match | 1.00 | |
| Minor mismatch | 0.97 (0.67–1.41) | 0.87 |
| Major mismatch | 1.19 (0.84–1.67) | 0.33 |
CFU – colony forming unit granulocyte macrophage; CMV – cytomegalovirus; HLA – human leukocyte antigen; TNC – total nucleated cell; UCB – umbilical cord blood
Discussion
In this study, we examined the engraftment capacity and kinetics of UCB units that were collected and stored for up to 12 years prior to use. We found that duration of storage, however, had no obvious impact on cellular recovery or engraftment after UCB transplantation. These results are in line with pre-clinical studies published by Broxmeyer et al[3, 4], as well a recent small clinical study[6], and support the use of cryopreserved UCB as a reliable, rapidly accessible donor source. Each UCB unit collected by cord blood banks ever increases the available donor pool, in contrast to the pool of unrelated donors, which is subject to ongoing donor attrition[17, 18]. As the pool of available UCB units grows, it will continue to make UCB transplantation more accessible, particularly for minority groups[19].
The characteristics of the UCB unit are vital to successful transplantation[20–25]. In this study, we also demonstrate that the length of cryopreservation did not significantly impact viability, TNC recovery or CFU-GM analysis in a clinical laboratory, which is supported by previous studies performed in research laboratories [3, 4]. These results question the cord bank practice of UCB units being outdated after 10 years[26] and the general practice of avoiding older UCB units for fear of poor clinical results. Thus, our study provides further evidence that long-term cryopreservation of UCB units is not detrimental to outcomes and suggests that each UCB unit should be assessed on its individual characteristics (HLA match, TNC, CD34+ etc), but not on the duration of cryopreservation of the unit.
One of the limitations of this study is the heterogeneous nature of the patient population, which did not allow us to compare outcomes in relation to graft versus host disease, transplant related mortality, relapse, or survival. Our study also included relatively few UCB units that had been cryopreserved for >10 years, which makes it is difficult to extrapolate the conclusions to UCB units that have been cryopreserved for more than a decade. It must be stated, however, that there is no evidence to contradict the use of UCB units older than 10 years, and pre-clinical data suggests that these products remain viable and potent[4]. In reviewing UCB unit characteristics, it was not possible to analyze recovery of CD34+ or CFU post cryopreservation, as the heterogenous nature of measurement techniques used at different cord collection centers makes it impossible to accurately compare the different pre-freeze values. There were also a significant number of UCB units that did not have a date of collection (n=125), and so had to be omitted from the analysis. However, almost all of these units were collected and subsequently used in the earliest years of UCB transplantation, without undergoing long term cryopreservation, and so would not have contributed to the data set in a meaningful way. Our study also excluded units used in double UCB transplants, as this removed UCB unit interaction as a potential confounding factor in our analysis. Hence the impact of long term cryopreservation of the UCB unit in double UCB transplantation remains unclear.
Our study has demonstrated that the amount of time an UCB unit spends in cryopreservation up to 10 years has no significant impact on engraftment outcomes. These results support the use of UCB units that have undergone long term cryopreservation, and should provide reassurance to clinicians in the field of UCB transplantation.
Highlights.
Manuscript submission no: YBBMT-D-14-00382
“Impact of long term cryopreservation on single umbilical cord blood transplant outcomes”
Long term cryopreservation of umbilical cord blood units does not impact engraftment in single umbilical cord blood transplantation
Long term cryopreservation does not impact TNC recovery, viability, or CFU-GM of umbilical cord blood units
Cryopreservation of umbilical cord blood units can safely be performed for up to 10 years
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflict of interest to declare
Authorship statement
R.M collected and interpreted the data and wrote the manuscript, J.E.W contributed to the study design, provided patients to the study and contributed to the manuscript, C.G.B and T.C.L provided patients to the study and contributed to the manuscript, Q.C interpreted the data, performed the statistical analysis and contributed to the manuscript, D.H.M contributed to the study design, provided the data, and contributed to the manuscript, and M.R.V designed the study, provided patients to the study and wrote the manuscript.
References
- 1.Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. The New England journal of medicine. 1989;321:1174–8. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 2.Bone Marrow Donors Worldwide. http://www.bmdw.org.
- 3.Broxmeyer HE, Srour EF, Hangoc G, Cooper S, Anderson SA, Bodine DM. High-efficiency recovery of functional hematopoietic progenitor and stem cells from human cord blood cryopreserved for 15 years. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:645–50. doi: 10.1073/pnas.0237086100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broxmeyer HE, Lee MR, Hangoc G, Cooper S, Prasain N, Kim YJ, et al. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood. 2011;117:4773–7. doi: 10.1182/blood-2011-01-330514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodwin HS, Grunzinger LM, Regan DM, McCormick KA, Johnson CE, Oliver DA, et al. Long term cryostorage of UC blood units: ability of the integral segment to confirm both identity and hematopoietic potential. Cytotherapy. 2003;5:80–6. [PubMed] [Google Scholar]
- 6.Parmar S, de Lima M, Worth L, Petropoulos D, Lee D, Cooper L, et al. Is there an expiration date for a cord blood unit in storage? Bone marrow transplantation. 2014 doi: 10.1038/bmt.2014.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard DH, Meltzer D, Kollman C, Maiers M, Logan B, Gragert L, et al. Use of cost-effectiveness analysis to determine inventory size for a national cord blood bank. Medical decision making: an international journal of the Society for Medical Decision Making. 2008;28:243–53. doi: 10.1177/0272989X07308750. [DOI] [PubMed] [Google Scholar]
- 8.Sirchia G, Rebulla P, Tibaldi S, Lecchi L. Cost of umbilical cord blood units released for transplantation. Transfusion. 1999;39:645–50. doi: 10.1046/j.1537-2995.1999.39060645.x. [DOI] [PubMed] [Google Scholar]
- 9.Majhail NS, Mothukuri JM, Macmillan ML, Verneris MR, Orchard PJ, Wagner JE, et al. Costs of pediatric allogeneic hematopoietic-cell transplantation. Pediatric blood & cancer. 2010;54:138–43. doi: 10.1002/pbc.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blommestein HM, Verelst SG, Huijgens PC, Blijlevens NM, Cornelissen JJ, Uylde Groot CA. Real-world costs of autologous and allogeneic stem cell transplantations for haematological diseases: a multicentre study. Annals of hematology. 2012;91:1945–52. doi: 10.1007/s00277-012-1530-2. [DOI] [PubMed] [Google Scholar]
- 11.Bart T. Cost effectiveness of cord blood versus bone marrow and peripheral blood stem cells. ClinicoEconomics and outcomes research: CEOR. 2010;2:141–7. doi: 10.2147/CEOR.S11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, Migliaccio G, Migliaccio AR, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:10119–22. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mascotti K, McCullough J, Burger SR. HPC viability measurement: trypan blue versus acridine orange and propidium iodide. Transfusion. 2000;40:693–6. doi: 10.1046/j.1537-2995.2000.40060693.x. [DOI] [PubMed] [Google Scholar]
- 14.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow transplantation. 1995;15:825–8. [PubMed] [Google Scholar]
- 15.Verneris MR, Brunstein CG, Barker J, MacMillan ML, DeFor T, McKenna DH, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114:4293–9. doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693–9. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Switzer GE, Dew MA, Stukas AA, Goycoolea JM, Hegland J, Simmons RG. Factors associated with attrition from a national bone marrow registry. Bone marrow transplantation. 1999;24:313–9. doi: 10.1038/sj.bmt.1701884. [DOI] [PubMed] [Google Scholar]
- 18.Navarro WH, Switzer GE, Pulsipher M. National marrow donor program session: donor issues. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2013;19:S15–9. doi: 10.1016/j.bbmt.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Barker JN, Byam CE, Kernan NA, Lee SS, Hawke RM, Doshi KA, et al. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2010;16:1541–8. doi: 10.1016/j.bbmt.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Migliaccio AR, Adamson JW, Stevens CE, Dobrila NL, Carrier CM, Rubinstein P. Cell dose and speed of engraftment in placental/umbilical cord blood transplantation: graft progenitor cell content is a better predictor than nucleated cell quantity. Blood. 2000;96:2717–22. [PubMed] [Google Scholar]
- 21.Purtill D, Smith K, Meagher R, Lubin M, Scaradavou A, Stevens C, et al. Analysis of 402 cord blood units to assess factors influencing infused viable CD34+ cell dose: The critical determinant of engraftment. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2014;20:S59. [Google Scholar]
- 22.Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood. 2010;115:1843–9. doi: 10.1182/blood-2009-07-231068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–8. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 24.Yoo KH, Lee SH, Kim HJ, Sung KW, Jung HL, Cho EJ, et al. The impact of post-thaw colony-forming units-granulocyte/macrophage on engraftment following unrelated cord blood transplantation in pediatric recipients. Bone marrow transplantation. 2007;39:515–21. doi: 10.1038/sj.bmt.1705629. [DOI] [PubMed] [Google Scholar]
- 25.Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. The New England journal of medicine. 1998;339:1565–77. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 26.Wall DA. Regulatory issues in cord blood banking and transplantation. Best practice & research Clinical haematology. 2010;23:171–7. doi: 10.1016/j.beha.2010.05.006. [DOI] [PubMed] [Google Scholar]


