Abstract
Objectives
The primary focus of this study was to examine associations between depressive symptoms and mental stress induced myocardial ischemia (MSIMI) in patients with coronary heart disease (CHD).
Methods
Adult patients with documented CHD were recruited for baseline mental stress and exercise stress screening testing as a part of the enrollment process of the REMIT trial. Patients were administered the Beck Depression Inventory II (BDI-II) and the Center for Epidemiologic Studies Depression Scale (CESD). Following a 24-48-hour Beta-blocker withdrawal, consented patients completed three mental stress tests followed by a treadmill exercise test. Ischemia was defined as 1) any development or worsening of any wall motion abnormality (WMA), 2) reduction of left ventricular ejection fraction (LVEF) ≥ 8% by transthoracic echocardiography, and/or ischemic ST-segment change by electrocardiography during stress testing. MSIMI was considered present when ischemia occurred in at least one mental test. Data were analyzed using logistic regression adjusting for age, gender, and resting left ventricular ejection fraction.
Results
One hundred twenty five (44.2 %) of 283 patients were found to have MSIMI and 93 (32.9%) had ESIMI. Unadjusted analysis showed that BDI-II scores were positively associated with the probability of MSIMI (OR = .1.30: 95% CI 1.06 – 1.60, p = .013) and number of MSIMI positive tasks (all p < .005). These associations were still significant after adjustment for covariates (ps ≤ .05).
Conclusions
In CHD patients, depressive symptoms were associated with a higher probability of MSIMI. These observations may enhance our understanding of the mechanisms contributing to the association of depressive symptoms to future cardiovascular events.
Keywords: Mental stress induced myocardial ischemia, exercise induced myocardial ischemia, depressive symptoms, coronary heart disease
Several large-scale epidemiological studies have reported that high levels of depressive symptoms (1-3) are associated with an elevated risk of incident coronary heart disease (CHD), and with a poorer prognosis in patients with CHD. The mechanisms responsible for these associations are not well understood, but psychological stress induced physiological changes have been posited as possible explanatory mechanisms. One potentially harmful consequence of mental stress in patients with CHD is mental stress-induced myocardial ischemia (MSIMI), a transient condition whose occurrence is particularly prevalent in this patient population (4, 5). The presence of MSIMI in CHD patients has repeatedly been found to be associated with a poorer prognosis (6-9), independent of established risk factors.
To date, only a small number of studies have investigated the association between depressive symptoms and MSIMI. One study (10) of 135 CHD patients with a positive exercise stress test reported a curvilinear relationship between the Center for Epidemiologic Studies Depression Scale (CESD) scores and the probability of MSIMI. For patients with mild- to- moderate levels of depressive symptoms, CESD scores were associated with a greater likelihood of developing MSIMI, whereas an inverse association emerged between depressive symptoms and the probability of MSIMI in patients with more severe levels of depression. A more recent study (11) of 184 CHD patients with a positive exercise stress test reported a non-significant association between the Beck Depression Inventory (BDI-TOT) and MSIMI, but unlike the previous study (10) they did not test for nonlinear effects. The mixed results of the two existing studies make it difficult to draw firm conclusions about the association of depressive symptoms and MSIMI. Another limitation in this area of study stems from the fact that CHD patients with no exercise stress test induced myocardial ischemia (ESIMI) were usually not studied, limiting the generality of these findings.
In order to better characterize the association between depressive symptoms and MSIMI, and to make the findings more representative of the entire CHD population, additional studies utilizing larger samples of patients both with and without ESIMI are needed. In an effort to help address some of the aforementioned limitations in the literature, the REMIT (Responses of Myocardial Ischemia to Escitalopram Treatment) trial recruited clinically stable CHD patients to examine the associations between depressive symptoms, as measured by the BDI and CESD, and MSIMI (occurrence and severity), and ESIMI. In addition, the study set out to examine the associations of MSIMI and ESIMI to hostility, anxiety, and perceived social stress; psychological constructs as these have also been associated with a poorer prognosis in CHD patients in previous studies (1-3). The inclusion of these measures allowed us to examine whether any of the associations between depression and MSIMI could be accounted for by a broader disposition to experience negative affect and distress.
Methods
Participants (N = 310) were male and female adult CHD patients, age 21 year or older, who were recruited systematically from the Cardiology outpatient clinics of the Duke University Health System between July 2007 and September 2011 for screening for the REMIT study (NCT00574847) (12). The presence of CHD was documented by an angiographic finding of coronary artery stenosis ≥ 70%, history of myocardial infarction [MI], and/or prior revascularization procedures, such as coronary artery bypass graft surgery [CABG] or percutaneous coronary intervention (PCI). Inclusion criteria required that patients CHD be clinically stable defined as no recent (< 3 months) MI, coronary artery bypass graft surgery (CABG), or any other revascularization procedures such as percutaneous transluminal coronary angioplasty with/out stenting, free of unstable angina, and no plan for revascularization procedures. Patients were excluded if they had significant medical problems (i.e. cardiac, pulmonary, metabolic, renal, hepatic disease, or malignancy) or psychiatric problems (i.e. bipolar spectrum mood disorders, psychotic disorders, current substance abuse/dependence or history within 6 months, and have active suicidal ideation) that interfere with the participation in the trial intervention. Patients were also excluded if they had severe psychiatric symptoms and use of antidepressants was another exclusionary criteria related to the trial intervention. Inclusion in the trial also required that patients be able to read and speak English. The protocol was reviewed and approved by the Duke University Health System Institutional Review Board. All participants provided written, voluntary, informed consent before participating in any further assessment.
Study Procedures
A complete description of the study procedures has been detailed previously by Jiang et al. (12). Briefly, study subjects underwent two sessions of assessment and testing. On the first day, participants were administered an interview designed for the collection of demographic and clinical characteristics data, followed by a series of psychometric tests and a psychiatric assessment (12). Participants completed the mental stress and exercise testing protocols on the second day.
Mental Stress and Exercise Testing
The stress testing was conducted between 8 and 11am at the Duke Cardiac Diagnostic Unit. Prior to stress testing, beta-blockers were withheld upon agreement of patients’ cardiologist for 24-48 hours, depending on the half-life of the specific medication. Other cardiac medications were not withheld during the stress test. Following a 20-minute rest period, participants underwent three mental stress tasks in sequence: mental arithmetic, mirror trace, and anger recall in fixed order. A rest period of 6 minutes followed every stress test. Patients completed the mental stress protocol while in a left lateral position to allow for echocardiography, which was conducted during the final three minutes of each rest period and during each mental stress task. Measures of blood pressure and heart rate were obtained once a minute during the last three minutes of each rest period and during each of the mental stress tasks. After the mental stress testing, patients completed a treadmill exercise test using the standard Bruce protocol (13). The exercise testing was terminated according to the guidelines of the American College of Sports Medicine. Echocardiography was conducted immediately following the cessation of the exercise.
Assessment of Myocardial Ischemia
Transthoracic echocardiography (14) and electrocardiography (ECG) were used to assess the presence of ischemia. Images (parasternal long- and short-axis views and apical 4- and 2-chamber views) were acquired using a 3-MHz transducer while in the harmonic imaging mode of the Philips iE33 system (Philips Ultrasound, Bothell, Washington). The American Society of Echocardiography 16-segment model was used to assess left ventricular wall motion (15). Each segment was graded and scored as normal (1 = normal or hyperdynamic, score) or abnormal (2 = hypokinetic, 3 = akinetic, 4 = dyskinetic, or 5 = aneurysmal) wall motion. A wall motion score index (WMSI) was calculated as the sum of the segmental wall motion scores divided by the total number of the scored segments. Separate scores were calculated for the wall motion data collected at rest, during each of the three mental stress tests, and during the exercise stress test. The Kappa value for the intra/inter variability of wall motion analysis in this study ranges between 0.80 and 0.87. LVEF was calculated using the biplane Simpson's method (15).
Definition of Stress Induced Myocardial Ischemia
MSIMI was defined as, compared to rest, the development or a worsening of any wall motion abnormality (WMA), reduction of left ventricular ejection fraction (LVEF) ≥ 8% by transthoracic echocardiography, and/or ischemic ST-segment change by electrocardiography, presented during one or more of the three mental stress tasks. These criteria were also used to score the presence of MSIMI separately for each mental stress task. These measures were used to construct an additional ischemia variable was defined as the total number of mental stress tasks that induced ischemia (i.e. MSIMI+ tasks, range from 0 – 3; 0 = no MSIMI with any mental task, 1 = MSIMI with one mental task, 2 = MSIMI with two mental tasks, and 3 = MSIMI with all three mental tasks). ESIMI was defined as the presence of any or all of the above criteria in response to ES.
Psychological Measurements
Severity of depressive symptoms was assessed by the Beck Depression Inventory II (BDI-TOT) and the Center for Epidemiologic Studies Depression Scale (CESD). The BDI-TOT (16), which consists of 21 items, has been widely used to screen for depressive symptoms in cardiac populations, and has been shown to be a good predictive tool for cardiovascular prognosis (17). The items of the BDI-TOT cover emotional, behavioral, and somatic symptoms. Cronbach's alpha for the total scale in this study was .89. Factor analytic studies (18, 19) have identified two factors labeled somatic affective (BDI-SA) and cognitive affective (BDI-CA), with prior studies having showing the SA subscale to be a better predictor of a variety of health outcomes (20).
The CESD (21) is a 20-item, questionnaire in which patients report on the frequency of depressive symptoms experienced in the past 2 weeks using a 4-point Likert scale. Cronbach's alpha for the total scale in this study was .87. Factor analyses of this scale have consistently identified four factors that have been described as positive emotions (CESD-PE), negative affect (CESD -NA), somatic symptoms (CESD -SOM), and interpersonal problems (CESD -IP) (22). This assessment was chosen as it has been used to test the association of depression and MSIMI (10) and we sought to explore whether the previous findings could be replicated in the present study.
In addition to the BDI-TOT and the CESD, patients were also administered a series of scales to assess levels of hostility, anxiety, and perceived social stress. Hostility was assessed by a 27-item abbreviated version of the Cook-Medley Hostility Scale (CMHS) (23, 24) that contained subsets of items identified in a previous study (24) as reflecting cognitive (i.e. cynicism), affective (i.e. hostile affect), and behavioral manifestations (i.e. aggressive responding) of hostility. Anxiety was assessed using the 40-item Spielberger Anxiety Scale (25), which contains two 20-item subscales measuring state (SSA) and trait (STA) manifestations of anxiety. Level of stress was measured via the 10-item Perceived Stress Scale (PSS) (26) that measures the degree to which situations in one's life are appraised as stressful. These particular scales were selected for use because they measure constructs that have been associated with the development and prognosis of CHD in previous studies and we wanted to ascertain if they were similarly associated with MSIMI.
Statistical Analysis
Logistic regression models were used to examine associations of BDI-II total scores (BDI-TOT) and CESD total scores (CESD-TOT) to the dichotomous outcomes of MSIMI and ESIMI, i.e. the presence or absence of ischemia. Initial analyses examined the bivariate relation between each depression variable and each ischemia variable followed by analyses adjusting for age, gender, resting LVEF, and resting WMSI. Because the study by Jiang et al (10) showed a nonlinear association between depressive symptoms and MSIMI, a quadratic term was computed for the total scores of the BDI-TOT and CESD-TOT with their associations with MSIMI were examined in an initial set of models. If the quadratic term was not significant, it was dropped from the model and the p-value associated with linear term was used to judge significance. Prior to analysis, the depression scores were standardized by dividing by the interquartile range of their respective distributions. Thus, an odds ratio (OR) associated with a one unit increase in a scale reflects a comparison of the 75th and 25th percentiles of its distribution. The C-Index is presented as a measure of model goodness of fit. Ordinal logistic regression was used to examine the relation between the depression measures and the number of MSIMI+ tasks. When significant, separate binary logistic models were fitted using the depression scales as predictors of each ischemia group (1, 2, or 3) versus the reference group (0). Partial Pearson correlations, adjusting for age, gender, and the appropriate hemodynamic resting value, were used to examine the associations of the BDI-TOT and CESD with the stress-induced hemodynamic responses including systolic blood pressure (SBP) and diastolic blood pressure (DBP), heart rate (HR), and rate-pressure product (RPP, SBP X HR). To enhance the reliability of mental stress hemodynamic assessments we averaged the measures obtained during the three mental stress tasks. . Additional logistic regression models were used to examine the associations of the depression subscales and the other psychosocial variables (i.e. hostility, perceived social stress and state/trait anxiety) to the study outcomes, i.e., MSIMI occurrence, frequency of MSIMI+ tasks, and ESIMI variables. A visual examination of the distributions of all of the psychological scales revealed that the measures of depression, anxiety, and the PSS were right skewed. Therefore, these variables were log transformed prior to analysis. As with the primary variables, each scale was transformed by dividing by its interquartile range and ORs were expressed as a comparison of the 75th and 25th percentiles of the distribution of each scale. We first tested the bivariate relations between each psychosocial variable and each outcome, followed by analyses adjusting for covariates. SAS version 9.1 was utilized for the analysis (SAS Incorporated, Cary NC). A p-value of < 0.05 was considered statistically significant.
Results
Descriptive statistics
Of the 310 patients with CHD who underwent stress testing, 283 had complete data for the primary endpoint (i.e. MSIMI), the psychological scales, and the covariates (i.e. age, gender, resting LVEF, and resting WMSI) that were used in the adjusted models and thus, comprised the primary analysis sample of the present study. Data for stress-induced hemodynamic responses was only available for 268 of those patients. Of the 283 patients that comprised the primary analysis sample, 125 (44.2 %) were found to have MSIMI and 93 (32.9%) had ESIMI. MSIMI occurred in 70 (24.7%) patients during mental arithmetic, 87 (30.7%) patients during mirror trace, and 96 (33.9%) patients during anger recall. Furthermore, 45 (36.0%) of the 125 patients with MSIMI showed MSIMI during only one task, 32 (25.6%) showed MSIMI during two tasks, and 48 (38.4%) showed MSIMI during all three mental stress tasks. Patients who showed MSIMI were more likely to show ESIMI (χ2(1) = 39.40, p < .001), with ESIMI occurring in 55.83% of the patients with MSIMI and 18.67% of patients who did not show MSIMI. ESIMI was also associated with the number of MSIMI episodes (χ2(1) = 46.33, p < .001), with the highest rate of ESIMI occurring in patients showing MSIMI to all three stress tasks (66.7%) compared to patients showing MSIMI to two tasks (60%), one task (40.5%), and patients who did not experience MSIMI (18.7%). Patients showing MSIMI were more likely to live alone (p = .030) and not be married (p = .016) (See Table 1), observations that have been reported previously (5) Patients showing MSIMI also had lower resting LVEF, higher resting WMSI (p = .001), and were more likely to be taking angiotensin II receptor antagonists (p = .044). The BDI-TOT was significantly and negatively associated with age (-.16, p = .009), resting LVEF (r = -.13, p = .033), duration of exercise stress test (r = -.21, p < .001) and positively and significantly associated with resting WMSI (r = .15, p = .013) (Table 2). The CESD was also negatively and significantly associated with age (-.17, p = .006) and duration of the exercise stress test (-.17, p = .006). Females scored higher on the BDI-TOT and CESD scales than did males (p = .003 and p < .001).
Table 1. Demographic and clinical characteristics of patients with/without MSIMI and patients with/without ESIMI.
| Overall (N = 283) |
MSIMI Yes (N = 125) |
MSIMI No (N =158) |
ESIMI Yes (N = 93) |
ESIMI No (N =174) |
|
|---|---|---|---|---|---|
|
| |||||
| Age (Mean/SD yrs) | 62.31(9.98) | 63.93(10.84) | 62.95(10.21) | 63.87(10.92) | 62.31(9.98) |
|
| |||||
| Race (% white) | 81.63 | 78.4 | 84.18 | 80.65 | 84.48 |
|
| |||||
| Gender (% female) | 17.67 | 22.4 | 13.92 | 20.43 | 16.09 |
|
| |||||
| BMI | 29.24(4.52) | 28.26(4.30) | 29.32(5.11) | 28.06(4.26) | 29.25(4.52) |
|
| |||||
| Living arrangement (% alone) | 15.55 | 21.8 | 11.4* | 15.05 | 13.79 |
|
| |||||
| Marital Status (% not married) | 73.5 | 66.4 | 79.11* | 76.34 | 75.29 |
|
| |||||
| CESD (%≥16) | 22.58 | 27.42 | 18.71 | 26.88 | 19.88 |
|
| |||||
| BDI (%≥10) | 34.4 | 40 | 29.94 | 41.94 | 30.64 |
|
| |||||
| Smoking (%) | |||||
| Current | 14.13 | 16.8 | 12.03 | 15.05 | 12.64 |
| Past | 54.42 | 50.4 | 57.59 | 51.61 | 56.9 |
| Never | 31.45 | 32.8 | 30.38 | 33.33 | 30.46 |
|
| |||||
| Aspirin (%) | 96.09 | 95.97 | 96.18 | 97.85 | 95.35 |
|
| |||||
| ACEI (%) | 66.31 | 65.32 | 67.09 | 65.59 | 67.63 |
|
| |||||
| Angiotensin II (%) | 13.17 | 17.74 | 9.55* | 15.05 | 11.05 |
|
| |||||
| Calcium Channel Blockers (%) | 23.13 | 21.77 | 24.2 | 19.35 | 23.84 |
|
| |||||
| Other lipid lowering medications (%) | 27.84 | 22.13 | 32.45 | 26.67 | 26.95 |
|
| |||||
| Statin(%) | 91.79 | 95.12 | 89.17 | 91.3 | 91.28 |
|
| |||||
| Anti-platelet agents (%) | 43.26 | 50.81 | 38.61 | 45.16 | 40.46 |
|
| |||||
| Beta blocker (%) | 84.04 | 87.9 | 81.01 | 89.25 | 80.35 |
|
| |||||
| History of MI(%) | |||||
| Yes | 43.46 | 47.2 | 40.51 | 37.63 | 47.13 |
| No | 55.48 | 51.2 | 58.86 | 60.22 | 52.3 |
| NS | 1.06 | 1.6 | 0.63 | 2.15 | 0.57 |
|
| |||||
| PTCA (%) | 63.6 | 63.2 | 63.92 | 60.22 | 65.52 |
|
| |||||
| CABG (%) | 42.4 | 47.2 | 38.61 | 51.61 | 36.78* |
|
| |||||
| Diabetes Mellitus (%) | 72.08 | 69.6 | 74.05 | 69.89 | 73.56 |
|
| |||||
| Hyperlipidemia (%) | 93.99 | 94.4 | 93.67 | 92.47 | 94.83 |
|
| |||||
| Hypertension (%) | 81.27 | 80 | 82.28 | 78.49 | 82.76 |
|
| |||||
| NYHA Class (%) | |||||
| 1 | 91.13 | 91.94 | 90.51 | 91.4 | 91.38 |
| 2 | 6.74 | 6.45 | 6.96 | 7.53 | 5.75 |
| 3 | 1.77 | 1.61 | 1.9 | 1.08 | 2.3 |
|
| |||||
| LVEF(Mean/SD) | 56.74(10.32) | 55.22(11.66) | 57.94(10.98)* | 56.54(10.93) | 56.78(10.30) |
|
| |||||
| WMSI (Mean/SD) | 1.20(.37) | 1.28(.42) | 1.13(.32)* | 1.19(.36) | 1.20(.38) |
Values are mean (SD) or %. Statistical tests examined associations of demographic and clinical variables to MSIMI and ESIMI. Chi-square or Fisher exact tests were used for categorical variables, and t tests were used for continuous variables.
p < 0.05.
ACEI = angiotensin-converting enzyme inhibitor; ASA = acetylsalicylic acid; BMI =body mass index; CABG = coronary artery bypass graft surgery; MI= myocardial infarction; NYHA = New York Heart Association; PTCA = percutaneous transluminal coronary transluminal coronary angioplasty; LVEF= Left ventricular ejection fraction; WMSI=wall motion score index; CESD = Center for Epidemiologic Studies Depression Scale; BDI = Beck Depression Inventory
Table 2. Correlations between BDI-TOT and CESD Scales and Selected Demographic and Clinical Variables.
| BDI-TOT | CESD | |||
|---|---|---|---|---|
| r | p | r | p | |
| Age | -.16 | .009 | -.17 | .006 |
| Gender | -.18 | .003 | -.25 | <.001 |
| Resting LVEF | -.13 | .033 | -.07 | .262 |
| Resting WMSI | .15 | .013 | .08 | .180 |
| Exercise Duration | -.21 | <.001 | -.17 | .006 |
| Peak HR | -.05 | .462 | -.05 | .448 |
| Target HR | -.05 | .458 | -.12 | .052 |
Associations of psychological Measures with MSIMI and ESIMI
The mean BDI-TOT score was 8.46 ± 7 and the mean CESD-TOT score was 10.15 ± 8.26. Patients with clinically significant levels of depressive symptoms (i.e. BDI-TOT >10 or CESD >16) tended to display more MSIMI, but neither association was significant (BDI-TOT >10 (50.6%) vs. BDI-TOT <10 (40.8%), p = .13; and CESD ≥ 16 (54.1%) vs. CESD < 16 (41%), p = .07, respectively). An examination of linear effects (Table 3) revealed that BDI-TOT scores were positively associated with probability of MSIMI (OR = 1.30; 95% confidence interval [CI], 1.06-1.60; p = .013) and ESIMI (OR = 1.29; 95%CI, 1.03-1.62; p = 0.025). Adjustments for age, gender, resting LVEF, and resting WMSI index slightly attenuated the association with MSIMI (OR = 1.24; 95%CI, 1.00-1.54; p = .053) and slightly magnified the association with ESIMI (OR = 1.33; 95%CI, 1.05-1.68; p= .019). The CESD-TOT score was not significantly associated with either MSIMI or ESIMI in the unadjusted or adjusted models (Tables 3 and 4). The quadratic terms for the BDI-TOT and the CESD-TOT were not significantly associated with MSIMI (all p values > .10).
Table 3. Logistic regression analysis examining relationships of psychosocial variables to Probability of MSIMI.
| MSIMI | MSIMI (adjusted) | |||||
|---|---|---|---|---|---|---|
| OR | p | C-index | OR | p | C-index | |
| BDI-TOT | 1.30 (1.06 – 1.60) | .013 | .58 | 1.24 (1.00 - 1.54) | .053 | .66 |
| BDI-SA | 1.29(1.06 – 1.57) | .012 | .59 | 1.21(0.98 - 1.48) | .071 | .66 |
| BDI-CA | 1.36 (.81 – 2.28) | .24 | .54 | 1.41(.80 – 2.48) | .23 | .65 |
| CESD-TOT | 1.31(.92 – 1.87) | .13 | .55 | 1.22(.83 – 1.78) | .32 | .65 |
| CESD-PE | .68(.44 – 1.04) | .077 | .57 | .71(.45 – 1.12) | .14 | .66 |
| CESD-NA | 1.36 (.95 – 1.95) | .091 | .54 | 1.33 (.90 – 1.98) | .16 | .65 |
| CESD-Som | 1.21 (.84 – 1.75) | .30 | .53 | 1.07 (.73 – 1.58) | .72 | .64 |
| CESD-IP | .97 (.70 – 1.35) | .85 | .50 | 1.02 (.72 – 1.43) | .92 | .64 |
| Hostility | 1.07 (.80 – 1.43) | .64 | .52 | 1.05 (.77 – 1.42) | .77 | .64 |
| Trait anxiety | 1.11 (.78 – 1.57) | .58 | .52 | 1.09 (.74 – 1.59) | .67 | .64 |
| State anxiety | 1.19 (.81 – 1.74) | .38 | .52 | 1.21 (.80 – 1.82) | .36 | .66 |
| PSS | 1.14 (.79 – 1.65) | .48 | .53 | 1.14 (.76 – 1.72) | .53 | .65 |
BDI-CA= Beck Depression Inventory Cognitive Affective Score, BDI-SA= Beck Depression Inventory Somatic Affective Score, BDI-TOT= Beck Depression Inventory Total Score, BDI-SA= Beck Depression Inventory Somatic Affective Score, CESD-IP= Center for Epidemiologic Studies Depression Scale Interpersonal Problems Score, CESD-NA= Center for Epidemiologic Studies Depression Scale Negative Affect Score, CESD-PE= Center for Epidemiologic Studies Depression Scale Positive Emotions Score, CESD-SOM= Center for Epidemiologic Studies Depression Scale Somatic Symptoms Score, CESD-TOT =Center for Epidemiologic Studies Depression Scale Total Score, ESIMI = Exercise Induced Myocardial Ischemia, MSIMI = Mental Stress Induced Myocardial Ischemia, OR= Odds Ratio, PSS=Perceived Stress Scale.
Table 4. Logistic regression analysis examining relationships of psychosocial variables to Probability of ESIMI.
| ESIMI | ESIMI (adjusted) | |||||
|---|---|---|---|---|---|---|
| OR | p | C-index | OR | p | C-index | |
| BDI-TOT | 1.29 (1.03 - 1.62) | .025 | .60 | 1.33 (1.05 - 1.68) | .019 | .62 |
| BDI-SA | 1.30(1.05 - 1.87) | .018 | .60 | 1.32(1.05 - 1.66) | .016 | .62 |
| BDI-CA | 1.79(1.02 – 3.13) | .042 | .58 | 2.07(1.13 – 3.78) | .018 | .61 |
| CESD-TOT | 1.36(.93 – 1.98) | .12 | .55 | 1.38(.92 – 2.07) | .12 | .58 |
| CESD-PE | .57(.36 - .91) | .018 | .59 | .56(.35 - .90) | .017 | .59 |
| CESD-NA | 1.35 (.92 – 1.98) | .13 | .56 | 1.41 (.93 – 2.15) | .11 | .59 |
| CESD-Som | 1.08 (.73 – 1.60) | .70 | .51 | 1.06 (.71 – 1.59) | .78 | .55 |
| CESD-IP | 1.18 (.83 – 1.67) | .37 | .53 | 1.21 (.84 – 1.72) | .31 | .56 |
| Hostility | 92 (.68 – 1.25) | .60 | .52 | .95 (.69 – 1.31) | .75 | .55 |
| Trait anxiety | 1.32 (.91 – 1.92) | .15 | .55 | 1.40 (.94 – 2.08) | .10 | .59 |
| State anxiety | 1.45 (.97 – 2.17) | .074 | .56 | 1.50 (.98 – 2.28) | .060 | .59 |
| PSS | 1.46 (.97 – 2.18) | .068 | .57 | 1.58 (1.01 – 2.47) | .045 | .60 |
BDI-CA= Beck Depression Inventory Cognitive Affective Score, BDI-SA= Beck Depression Inventory Somatic Affective Score, BDI-TOT= Beck Depression Inventory Total Score, BDI-SOM= Beck Depression Inventory Somatic Affective Score, CESD-IP= Center for Epidemiologic Studies Depression Scale Interpersonal Problems Score, CESD-NA= Center for Epidemiologic Studies Depression Scale Negative Affect Score, CESD-PE= Center for Epidemiologic Studies Depression Scale Positive Emotions Score, CESD-SOM= Center for Epidemiologic Studies Depression Scale Somatic Symptoms Score, CESD-TOT =Center for Epidemiologic Studies Depression Scale Total Score, ESIMI = Exercise Induced Myocardial Ischemia, MSIMI = Mental Stress Induced Myocardial Ischemia, OR= Odds Ratio, PSS=Perceived Stress Scale.
Separate analysis for MSIMI in response to specific mental tasks revealed positive relations between BDI-TOT scores and probability of ischemia during mental arithmetic (OR = 1.36; 95%CI, 1.04-1.78; p = 0.024), mirror trace (OR = 1.20; 95%CI, 0.96-1.50; p = 0.10), and anger recall (OR = 1.53; 95%CI, 1.18-1.98; p = 0.001), but only the effect for anger recall remained significant after covariate adjustment (OR = 1.47; 95%CI, 1.12-1.93; p = 0.005). CESD scores were not significantly associated with probability of MSIMI in response to any mental stress task. Analysis of the depression subscales and other psychosocial variables showed that the BDI-SA subscale was positively associated with the probability of MSIMI (OR = 1.29; 95% CI, 1.06-1.57; p = .012), but this association was attenuated in the adjusted analysis (OR = 1.21; 95% CI, 0.98-1.48; padjusted = .071). BDI-SA was also positively and significantly associated with ESIMI in both unadjusted and adjusted analysis (OR = 1.30; 95% CI, 1.05-1.87; p = 0.018 and OR = 1.32; 95% CI, 1.05-1.66; padjusted = 0.016). BDI-CA scores were positively associated with probability of ESIMI (OR = 1.79; 95% CI, 1.02-3.13; p =0.042 and OR = 2.07; 95% CI, 1.13-3.78; padjusted = 0.018). Higher CESD-PE scores were negatively associated with the likelihood of ESIMI (OR = 0.57; 95% CI, 0.36-0.91;p = 0.018 and OR = 0.56; 95% CI, 0.35-0.90; padjusted = 0.017) but not MSIMI. Higher PSS scores were positively associated with the probability of having ESIMI, however this association was only significant in the adjusted model (OR = 1.46; 95% CI, 0.97-2.18; punadjusted = .068 and OR = 1.58; 95% CI, 1.01-2.47; padjusted = .045). PSS scores were not significantly associated with MSIMI. The CESD-SOM, CESD-NA, CESD-IP, CHS, STA, and the SSA, were not associated with MSIMI or ESIMI (See Table 1).
Associations of psychological measures with number of MSIMI + tasks
Of the primary depression measures, only BDI-TOT scores were significant predictors of the number of MSIMI+ tasks in both the unadjusted (p = .007) and adjusted models (p = .037). Analyses comparing each ischemia group (1, 2, or 3) to the reference group (those without any discernible MSIMI) showed that BDI-TOT (Figure 1a) scores were positively associated with the likelihood of showing ischemia in response to all three mental stress tasks (ORunadjusted = 1.71; 95%CI, 1.17 – 2.49 and ORadjusted = 1.51; 95%CI, 1.02-2.23; p = 0.038), but were not associated with ischemia to just one or two tasks. In the analysis of the depression subscales and other psychosocial variables, there was a significant association between BDI-SA scores and the number of MSIMI+ tasks (unadjusted p = .005 and adjusted p = .044). The binary logistic models revealed a pattern (See Figure 1b) similar to that seen with the BDI-TOT, i.e. BDI-SA scores were positively associated with the probability of showing ischemia to all three tasks (ORunadjusted = 1.79; 95%CI, 1.21 – 2.64; p = .004 and ORadjusted = 1.55; 95%CI, 1.05-2.30; p = 0.028), but were not associated with showing ischemia to just one or two tasks. None of the other psychological measures were significantly associated with the number of MSIMI+ tasks variable.
Figure 1a.
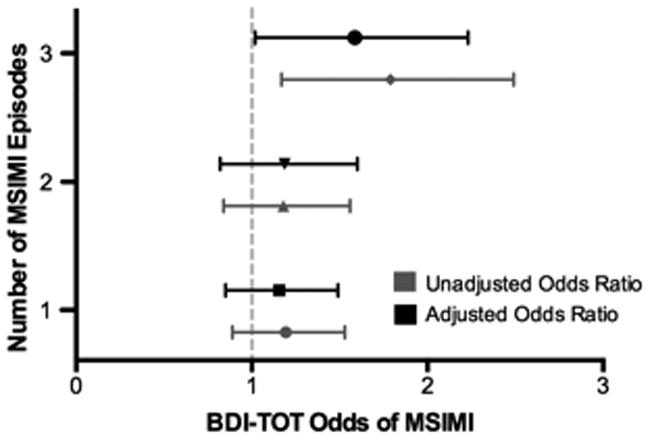
Probability of MSIMI as a function of BDI-TOT score by number of MSIMI episodes. Odd ratios (ORs) were expressed as a comparison of the 75th and 25th percentiles of the BDI-TOT scale.
Figure 1b.

Probability of MSIMI as function of BDI-SA scores by number of MSIMI episodes.
Associations of BDI-TOT and CESD scores with mental stress-induced hemodynamic changes
We next examined associations between the primary depression measures and mental stress-induced hemodynamic changes. Analysis of the BDI-TOT revealed nonsignificant relations with mental-stress-induced changes in SBP (r = .05, p = .43), DBP (r = -.03, p = .58), HR (r = -.05, p = .34), and RPP (r = -.05, p = .47). Nonsignificant relations of CESD scores with SBP (r = -.09, p = .15) and DBP (r = .03 p = .58) were found as well. However, there were significant negative associations between CESD scores and both HR (r = -.12, p = .048) and RPP (r = -.13, p = .035) changes in response to mental stress indicating that patients with higher CESD scores showed smaller changes in HR and RPP in response to the mental stress protocol.
We refitted the adjusted BDI-TOT/MSIMI logistic model in the sample with valid heomodynamic data (i.e. N = 268). The association between the BDI-TOT and MSIMI was significant (OR = 1.26; 95%CI, 1.01-1.58; p = 0.045) and remained significant after separately controlling for mental stress-induced changes in SBP (OR = 1.26; 95%CI, 1.01-1.58; p = 0.044), DBP (OR = 1.26; 95%CI, 1.00-1.57; p = 0.048), HR (OR = 1.27; 95%CI, 1.01-1.60; p = 0.04), and RPP (OR = 1.27; 95%CI, 1.01-1.60; p = 0.041).
Discussion
This study demonstrates that depressive symptoms, as measured by both the BDI-TOT and the BDI-SA scales, were associated with both the occurrence and the number of MSIMI+ tasks. Compared to patients scoring at the 25th percentile of the BDI-TOT, patients scoring at the 75th percentile of the scale were 16% more likely to exhibit signs of MSIMI to any single task, and 71% more likely to show signs of MSIMI in response to all three mental stressors. In the present study, the 75th percentile (BDI-TOT = 12) of the BDI-TOT scale reflects mild levels of depression, whereas the 25th percentile (BDI-TOT = 3) reflects minimal levels of depression. The measures of hostility, anxiety, and perceived stress were not associated with any of the MSIMI indices, suggesting that the association of depressive symptoms and MSIMI is less likely to be accounted for by an individual's general tendency to experience negative affect and distress. Overall, the results of the current study suggest that MSIMI may be a mechanistic explanation for the well-established adverse association between depression and CHD prognosis, and may also help explain the long-term impact of depression on the prognosis of cardiac patients (1-3, 20, 27).
We were not able to replicate the curvilinear association between depressive symptoms measured by CES-D and MSIMI reported by Jiang et al (10), which might be due to studying a somewhat different population, i.e. not confined to a recent positive ES and larger sample size. Nevertheless the results from our study, whose sample size almost twice that of the one used in Jiang et al's (10) earlier study do suggest a dose-response relationship across the entire distribution of BDI-II scores. It should be noted that only a small number of patients exhibited moderate- to- severe levels of depressive symptoms in either study. Using a BDI-TOT cut-off of 16, approximately 10% of the patients in the present study would be considered as manifesting moderate- to- severe levels of depression. Future studies of MSIMI, incorporating patients with more severe depression will be required to better understand the risk of MSIMI that is associated with more severe manifestations of depressive symptoms.
The association of depressive symptoms, as measured by both the BDI-TOT and BDI-SA scales, to MSIMI was particularly strong in patients who experienced MSIMI during all three stressors, but was less robust in patients showing ischemia to just one or two stressors. One possible explanation for the stronger association of depression with MSIMI in patients who experienced MSIMI during all three mental tasks is that these patients may show poorer recovery from mental stress, or they may have a lower ischemic threshold, or possibly both, and as a result are more susceptible to subsequent mental stressors. There may also be psychological reasons for these observations. Depressed individuals are more likely to engage in rumination, a cognitive process that has been shown to result in slower recovery of various stress-induced physiological parameters (28, 29) and is believed to have deleterious health effects (29).
Depressive symptoms were positively associated with the probability of showing MSIMI to each mental stress task with the strongest effect seen in the anger recall task. This might suggest that tasks requiring patients to recall negative emotions are potent elicitors of MSIMI in patients with elevated symptoms of depression. Indeed, there is evidence that people with high levels of depressive symptoms show a bias towards the recall of negative emotional material (30) and this might be expected to result in a more intense activation of pathological process contributing to MSIMI. One study reported a positive association between depressive symptoms and anger recall induced norepinephrine changes, a physiological response that has been associated with MSIMI (31). The stronger association between depressive symptoms and MSIMI in response to the anger recall task could also reflect an order effect as the mental stress tasks used in this study were presented in a fixed order-mental arithmetic, mirror trace, and anger recall. Thus, the pathophysiological impact of the mental arithmetic and mirror tracing tasks may have persisted following their cessation, resulting in a greater susceptibility of MSIMI to the anger recall task. It is important to note that it is plausible to expect that dispositional variables, such as depressive symptoms and hostility, to interact with task characteristics in increasing or decreasing the likelihood of MSIMI as evidence suggests they do with other reactivity indices (32-34). This underscores the importance of employing multiple, complementary mental stressors when assessing susceptibility to MSIMI. Previous studies have reported that MSIMI in the laboratory predicts ambulatory ischemia (8, 35, 36), and that depressive symptoms are also associated with the occurrence of myocardial ischemia during daily living (10). Additionally, individuals with elevated depressive symptoms have been shown to experience more frequent stressors than those with few or no symptoms (37, 38). Our findings suggest that CHD patients with elevated depressive symptoms likely experience more frequent episodes of myocardial ischemia during daily living than those without depressive symptoms, and that these episodes may, in part, be precipitated by the experience of negative emotions.
Of the two BDI subscales, the somatic affective subscale has been more closely linked to adverse health outcomes (20, 39). In the current study the somatic affective scale was more strongly related to manifestations of MSIMI than the cognitive affect scale. Considered collectively, the results of the current study provide additional support for a link between this dimension of depressive symptoms and cardiovascular health. At present, the mechanism for this relationship remains unclear. It may be that these symptoms of depression have a greater impact on cardiovascular health. On the other hand, these symptoms may be a reflection of the current cardiovascular disease status of the study participants. This scale contains items describing fatigue, energy depletion etc., that may be the result of experiencing frequent MSIMI. It should be noted, however, that comparisons between patients with and without MSIMI showed few differences in regard to a number of clinical variables, including resting left ventricular ejection fraction (5).
Measures of depressive symptoms were positively associated with a higher likelihood of experiencing ESIMI. It is important to note that this association was present despite evidence that depressive symptoms were associated with shorter exercise duration and a trend for a lower peak heart during exercise. This may suggest that higher levels of depressive symptoms are associated with a broader propensity to experience ischemic activity, whether due to mental or physical stress. Alternatively, this apparent susceptibility to physical stress may be the result of a carry-over effect from the mental stress protocol, as ESIMI was most prevalent in patients showing MSIMI to all three tasks and the exercise test was performed following the completion of mental tests. A recent study offered a similar explanation for the observation that CHD patients demonstrated shortened exercise durations when exercise stress followed a mental stress protocol (40). Choosing between these explanations will require further study and the utilization of a different study design. Given the high probability that differing mechanisms underlie ESIMI and MSIMI, the associations between depressive symptoms and ESIMI indices is of significant importance.
Hemodynamic changes in response to mental stress have been shown to be greater in patients showing MSIMI in some studies (41, 42) and such changes have been suggested as playing some role in ischemic responses. Depressive symptoms have also been shown to be associated with greater changes in stress blood pressure raising the possibility that the significant association between depressive symptoms and MSIMI observed in this study might be explained by concomitant stress-induced change in hemodynamic indices. However, BDI-TOT scores in the present study were not significantly associated with mental stress evoked BP, HR, or RPP reactivity and control for these reactivity variables had little effect on the associations between the BDI-TOT and MSIMI suggesting that another mechanism(s) underlies the association between depressive symptoms and MSIMI. One possible mechanism is stress-induced alterations in hemostasis (43), endothelial dysfunction, and diastolic dysfunction.
In contrast to prior observations by Jiang et al (10), we did not identify an association between the CESD scale and MSIMI. A careful examination of the CESD subscales revealed a significant association between the positive affect subscale and a lower probability of ESIMI, as well as a trend for a lower probability of MSIMI. Previous studies using the CESD have documented significant associations between the measures of positive emotions and a variety of health outcomes (44-48). Our results add to this growing body of evidence and underscore the importance of treating depressive symptoms as a multi-dimensional construct.
Hostility was not related to MSIMI in the present study. One previous study reported that CHD patients who showed MSIMI, defined as reduction in left ventricular ejection fraction of ≥ 5 in response to mental arithmetic, had higher scores on measures of anger and hostility than a group of patients showing no MSIMI (49). The apparent inconsistency between the findings of the current study and the one by Burg et al. might be explained by differences in the study definitions of MSIMI. In the current study, our definition of MSIMI was based on both WMA and LVEF changes whereas the Burg et al study relied solely on LVEF changes. Whereas a significant reduction in LVEF is believed to be a marker of global left ventricular dysfunction changes in wall motion are believed to represent the development of regional dysfunction. Thus, it may be that hostility is associated with certain indices of left ventricular function (i.e. LVEF) and not others (i.e. WMA). We plan to examine this possibility in the future.
Summary.
Depressive symptoms, specifically those describing somatic affective features, are associated with the presence and severity of MSIMI. This pattern of physiological response to acute emotional stress may explain the association between depressive symptoms and poor prognosis of CHD patients. The results of this study further emphasize the importance of undertaking clinical trials to determine whether treating depression in patients with cardiac diseases results in reduced MSIMI that, in turn, results in reduced mortality.
Acknowledgments
The authors would like to thank Dr. Kirk Adams, Professor of Medicine at Medical School of UNC, Dr. Allen Miller, Professor of Medicine at UF College of Medicine-Jacksonville, and Ms. Zhen Huang, Statistician at Duke Clinical Research Institute for their contributions as the Data Safety Monitoring Board of the REMIT study. The study was funded by National Heart, Lung, and Blood Institute, grant number R01HL085704.
Funding Source: The REMIT study was funded by the National Heart Lung and Blood Institute (NHLBI, R01HL085704), Bethesda, Maryland.
Conflict of interest disclosures: All authors received salary support through an NHLBI research grant. Dr. Redford B. Williams reports holding a U.S. patent on the 5HTTLPR L allele for use as a marker of increased cardiovascular risk in stressed persons and is a founder and major stockholder of Williams LifeSkills, Inc. Dr. Becker reports receiving research grant support from Baxter, Bristol-Myers Squibb, Johnson & Johnson, and Regado Biosciences, and consulting/ lecture fees from Baxter, Boehringer Ingelheim, Bristol-Myers Squibb, Johnson & Johnson, Regado Biosciences, and Sanofi-Aventis. Dr. Velazquez reports receiving research grants from Abbott Laboratories, Evalve, and Ikaria, and consulting fees from Boehringer Ingelheim, Gilead, and Novartis. Dr. Rogers reports receiving funding from Boston Scientific Corporation, HeartWare, and Thoratec Corporation. Dr. O’Connor reports receiving funding from the following: Actelion Pharmaceuticals Ltd., Amgen, Inc., Biscardia, LLC, Cardiology Consulting Associates, Faculty Connection, GE Healthcare, Ikaria, Neurotronik/Interventional Autonomics Corporation, Novella Clinical, Inc., Pfizer Inc., Pozen, and Roche Diagnostics.
Abbreviations
- REMIT
Responses of Myocardial Ischemia to Escitalopram Treatment
- CHD
Coronary Heart Disease
- MSIMI
Mental Stress Induced Myocardial Ischemia
- ESIMI
Exercise Induced Myocardial Ischemia
- CABG
Coronary artery bypass graft surgery
- PCI
Percutaneous coronary intervention
- ECG
Electrocardiography
- WMSI
Wall Motion Score Index
- LVEF
Left Ventricular Ejection Fraction
- BDI-CA
Beck Depression Inventory Cognitive Affective Score
- BDI-SA
Beck Depression Inventory Somatic Affective Score
- BDI-TOT
Beck Depression Inventory Total Score
- BDI-SA
Beck Depression Inventory Somatic Affective Score
- CESD-IP
Center for Epidemiologic Studies Depression Scale Interpersonal Problems Score
- CESD-NA
Center for Epidemiologic Studies Depression Scale Negative Affect Score
- CESD-PE
Center for Epidemiologic Studies Depression Scale Positive Emotions Score
- CESD-SOM
Center for Epidemiologic Studies Depression Scale Somatic Symptoms Score
- CESD-TOT
Center for Epidemiologic Studies Depression Scale Total Score
- CMHS
Cook-Medley Hostility Scale
- PSS
Perceived Stress Scale
- SSA
Spielberger State Anxiety Scale
- STA
Spielberger Trait Anxiety Scale
- OR
Odds Ratio
Footnotes
- Dr. Wei Jiang received salary support through the NHLBI research grant
- Dr. Zainab Samad received salary support through the NHLBI research grant
- Dr. Stephen Boyle received salary support through the NHLBI research grant
- Dr. Richard C. Becker, received salary support through the NHLBI research grant
- Dr. Redford Williams received salary support through the NHLBI research grant
- Dr. Cynthia Kuhn received salary support through the NHLBI research grant
- Dr. Thomas L. Ortel received salary support through the NHLBI research grant
- Dr. Maragatha Kuchibhatla received salary support through the NHLBI research grant
- Dr Joseph Rogers received salary support through the NHLBI research grant
- Dr. Christopher O’Connor received salary support through the NHLBI research grant
- Dr. Eric J. Velazquez received salary support through the NHLBI research grant
References
- 1.Hemingway H, Marmot M. Evidence based cardiology: psychosocial factors in the aetiology and prognosis of coronary heart disease. Systematic review of prospective cohort studies. BMJ. 1999;318:1460–7. doi: 10.1136/bmj.318.7196.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lett HS, Blumenthal JA, Babyak MA, Sherwood A, Strauman T, Robins C, Newman MF. Depression as a risk factor for coronary artery disease: Evidence, mechanisms, and treatment. Psychosom Med. 2004;66:305–15. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- 3.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 4.Strike PC, Steptoe A. Systematic review of mental stress-induced myocardial ischaemia. Eur Heart J. 2003;24:690–703. doi: 10.1016/s0195-668x(02)00615-2. [DOI] [PubMed] [Google Scholar]
- 5.Jiang W, Samad Z, Boyle S, Becker RC, Williams R, Kuhn C, Ortel TL, Rogers J, Kuchibhatla M, O’Connor C, Velazquez EJ. Prevalence and clinical characteristics of mental stress-induced myocardial ischemia in patients with coronary heart disease. Journal of the American College of Cardiology. 2013;61:714–22. doi: 10.1016/j.jacc.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang W, Babyak M, Krantz DS, Waugh RA, Coleman RE, Hanson MM, Frid DJ, McNulty S, Morris JJ, OConnor CM, Blumenthal JA. Mental stress-induced myocardial ischemia and cardiac events. Jama-J Am Med Assoc. 1996;275:1651–6. doi: 10.1001/jama.275.21.1651. [DOI] [PubMed] [Google Scholar]
- 7.Krantz DS, Santiago HT, Kop WJ, Merz CNB, Rozanski A, Gottdiener JS. Prognostic value of mental stress testing in coronary artery disease. Am J Cardiol. 1999;84:1292–7. doi: 10.1016/s0002-9149(99)00560-3. [DOI] [PubMed] [Google Scholar]
- 8.Sheps DS, McMahon RP, Becker L, Carney RM, Freedland KE, Cohen JD, Sheffield D, Goldberg AD, Ketterer MW, Pepine CJ, Raczynski JM, Light K, Krantz DS, Stone PH, Knatterud GL, Kaufmann PG. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease - Results from the psychophysiological investigations of myocardial ischemia study. Circulation. 2002;105:1780–4. doi: 10.1161/01.cir.0000014491.90666.06. [DOI] [PubMed] [Google Scholar]
- 9.Babyak MA, Blumenthal JA, Hinderliter A, Hoffman B, Waugh RA, Coleman RE, Sherwood A. Prognosis After Change in Left Ventricular Ejection Fraction During Mental Stress Testing in Patients With Stable Coronary Artery Disease. Am J Cardiol. 2010;105:25–8. doi: 10.1016/j.amjcard.2009.08.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang W, Babyak MA, Rozanski A, Sherwood A, O’Connor CM, Waugh RA, Coleman RE, Hanson MW, Morris JJ, Blumenthal JA. Depression and increased myocardial ischemic activity in patients with ischemic heart disease. Am Heart J. 2003;146:55–61. doi: 10.1016/S0002-8703(03)00152-2. [DOI] [PubMed] [Google Scholar]
- 11.Ketterer MW, Freedland KE, Krantz DS, Kaufmann P, Forman S, Greene A, Raczynski J, Knatterud G, Light K, Carney RM, Stone P, Becker L, Sheps D. Psychological Correlates of Mental Stress-induced Ischemia in the Laboratory: The Psychophysiological Investigation of Myocardial Ischemia (PIMI) Study. Journal of health psychology. 2000;5:75–85. doi: 10.1177/135910530000500112. [DOI] [PubMed] [Google Scholar]
- 12.Jiang W, Velazquez EJ, Samad Z, Kuchibhatla M, Martsberger C, Rogers J, Williams R, Kuhn C, Ortel TL, Becker RC, Pristera N, Krishnan R, O’Connor CM, Investigators R. Responses of mental stress-induced myocardial ischemia to escitalopram treatment: Background, design, and method for the Responses of Mental Stress Induced Myocardial Ischemia to Escitalopram Treatment trial. Am Heart J. 2012;163:20–6. doi: 10.1016/j.ahj.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruce R, Lovejoy F, Jr, Pearson R, Yu P, Brothers G, Velazquez T. Normal respiratory and circulatory pathways of adaptation in exercise. J Clin Invest. 1949;28:1423–30. doi: 10.1172/JCI102207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottdiener JS, Livengood SV, Meyer PS, Chase GA. Should Echocardiography Be Performed to Assess Effects of Antihypertensive Therapy - Test-Retest Reliability of Echocardiography for Measurement of Left-Ventricular Mass and Function. Journal of the American College of Cardiology. 1995;25:424–30. doi: 10.1016/0735-1097(94)00375-z. [DOI] [PubMed] [Google Scholar]
- 15.Schiller N, Shah P, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, Silverman N, Tajik A. Recommendation for quantitation of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiog. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 16.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. San Antonio, TX: Psychological Corporation; 1996b. [Google Scholar]
- 17.Jiang W, Krishnan RRK, O’Connor CM. Depression and heart disease - Evidence of a link, and its therapeutic implications. Cns Drugs. 2002;16:111–27. doi: 10.2165/00023210-200216020-00004. [DOI] [PubMed] [Google Scholar]
- 18.Steer RA, Ball R, Ranieri WF, Beck AT. Dimensions of the Beck Depression Inventory-II in clinically depressed outpatients. J Clin Psychol. 1999;55:117–28. doi: 10.1002/(sici)1097-4679(199901)55:1<117::aid-jclp12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.Storch EA, Roberti JW, Roth DA. Factor structure, concurrent validity, and internal consistency of the beck depression inventory-second edition in a sample of college students. Depress Anxiety. 2004;19:187–9. doi: 10.1002/da.20002. [DOI] [PubMed] [Google Scholar]
- 20.Adams J, Kuchibhatla M, Christopher EJ, Alexander JD, Clary GL, Cuffe MS, Califf RM, Krishnan RR, O’Connor CM, Jiang W. Association of Depression and Survival in Patients with Chronic Heart Failure over 12 Years. Psychosomatics. 2012;53:339–46. doi: 10.1016/j.psym.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D Scale. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 22.Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62:123–46. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- 23.Cook WW, Medley DM. Proposed Hostility and Pharisaic - Virtue Scales for the MMPI. J Appl Psychol. 1954;38:414–8. [Google Scholar]
- 24.Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB. The Cook-Medley Hostility Scale - Item Content and Ability to Predict Survival. Psychosom Med. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Speilberger CD, Gorsuch R, Lushene R, Vagg P, Jacobs G. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists; 1983. [Google Scholar]
- 26.Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 27.Jiang W, Kuchibhatla M, Clary GL, Cuffe MS, Christopher EJ, Alexander JD, Califf RM, Krishnan RR, O’Connor CM. Congestive heart failure - Relationship between depressive symptoms and long-term mortality in patients with heart failure. Am Heart J. 2007;154:102–8. doi: 10.1016/j.ahj.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 28.Glynn LM, Christenfeld N, Gerin W. The role of rumination in recovery from reactivity: Cardiovascular consequences of emotional states. Psychosom Med. 2002;64:714–26. doi: 10.1097/01.psy.0000031574.42041.23. [DOI] [PubMed] [Google Scholar]
- 29.Brosschot JF, Pieper S, Thayer JF. Expanding stress theory: Prolonged activation and perseverative cognition. Psychoneuroendocrino. 2005;30:1043–9. doi: 10.1016/j.psyneuen.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annual review of clinical psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida K, Utsunomiya T, Morooka T, Yazawa M, Kido K, Ogawa T, Ryu T, Ogata T, Tsuji S, Tokushima T, Matsuo S. Mental stress test is an effective inducer of vasospastic angina pectoris: comparison with cold pressor, hyperventilation and master two-step exercise test. International journal of cardiology. 1999;70:155–63. doi: 10.1016/s0167-5273(99)00079-0. [DOI] [PubMed] [Google Scholar]
- 32.Suarez EC, Williams RB., Jr Situational determinants of cardiovascular and emotional reactivity in high and low hostile men. Psychosom Med. 1989;51:404–18. doi: 10.1097/00006842-198907000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Suarez EC, Williams RB., Jr The relationships between dimensions of hostility and cardiovascular reactivity as a function of task characteristics. Psychosom Med. 1990;52:558–70. doi: 10.1097/00006842-199009000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Suarez EC, Kuhn CM, Schanberg SM, Williams RB, Jr, Zimmermann EA. Neuroendocrine, cardiovascular, and emotional responses of hostile men: the role of interpersonal challenge. Psychosom Med. 1998;60:78–88. doi: 10.1097/00006842-199801000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Blumenthal JA, Jiang W, Waugh RA, Frid DJ, Morris JJ, Coleman RE, Hanson M, Babyak M, Thyrum ET, Krantz DS. Mental stress-induced ischemia in the laboratory and ambulatory ischemia during daily life. Association and hemodynamic features. Circulation. 1995;92:2102–8. doi: 10.1161/01.cir.92.8.2102. [DOI] [PubMed] [Google Scholar]
- 36.Stone PH, Krantz DS, McMahon RP, Goldberg AD, Becker LC, Chaitman BR, Taylor HA, Cohen JD, Freedland KE, Bertolet BD, Coughlan C, Pepine CJ, Kaufmann PG, Sheps DS, Grp PS. Relationship among mental stress-induced ischemia and ischemia during daily life and during exercise: The psychophysiologic investigations of myocardial ischemia (PIMI) study. Journal of the American College of Cardiology. 1999;33:1476–84. doi: 10.1016/s0735-1097(99)00075-3. [DOI] [PubMed] [Google Scholar]
- 37.Lovejoy MC, Steuerwald BL. Subsyndromal unipolar and bipolar disorders II: Comparisons on daily stress levels. Cognitive Ther Res. 1997;21:607–18. [Google Scholar]
- 38.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiat. 1999;156:837–41. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 39.Carney RM, Freedland KE. Are Somatic Symptoms of Depression Better Predictors of Cardiac Events Than Cognitive Symptoms in Coronary Heart Disease? Psychosom Med. 2012;74:33–8. doi: 10.1097/PSY.0b013e3182405ac4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stepanovic J, Ostojic M, Beleslin B, Vukovic O, Djordjevic-Dikic A, Giga V, Nedeljkovic I, Nedeljkovic M, Stojkovic S, Vukcevic V, Dobric M, Petrasinovic Z, Marinkovic J, Lecic-Tosevski D. Mental stress-induced ischemia in patients with coronary artery disease: echocardiographic characteristics and relation to exercise-induced ischemia. Psychosom Med. 2012;74:766–72. doi: 10.1097/PSY.0b013e3182689441. [DOI] [PubMed] [Google Scholar]
- 41.Specchia G, de Servi S, Falcone C, Gavazzi A, Angoli L, Bramucci E, Ardissino D, Mussini A. Mental arithmetic stress testing in patients with coronary artery disease. Am Heart J. 1984;108:56–63. doi: 10.1016/0002-8703(84)90544-1. [DOI] [PubMed] [Google Scholar]
- 42.Krantz DS, Helmers KF, Bairey CN, Nebel LE, Hedges SM, Rozanski A. Cardiovascular reactivity and mental stress-induced myocardial ischemia in patients with coronary artery disease. Psychosom Med. 1991;53:1–12. doi: 10.1097/00006842-199101000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Harrison RW, Becker RC, Ortel TL, Kuchibhatla M, Boyle SH, Samad Z, Velazquez EJ, Wilson J, Kuhn C, Williams RB, O’Connor CM, Jiang W. American College of Cardiology (ACC) 2013 Scientific Sessions. San Francisco, California: Mar 9-11, 2013. Association Between Platelet Aggregation and Mental Stress Induced Myocardial Ischemia: Results From the REMIT Trial. 2013. [Google Scholar]
- 44.Chida Y, Steptoe A. Positive psychological well-being and mortality: A quantitative review of prospective observational studies. Psychosom Med. 2008;70:741–56. doi: 10.1097/PSY.0b013e31818105ba. [DOI] [PubMed] [Google Scholar]
- 45.Brummett BH, Morey MC, Boyle SH, Mark DB. Prospective Study of Associations Among Positive Emotion and Functional Status in Older Patients With Coronary Artery Disease. J Gerontol B-Psychol. 2009;64:461–9. doi: 10.1093/geronb/gbp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fisher MN, Al Snih S, Ostir GV, Goodwin JS. Positive affect and disability among older Mexican Americans with arthritis. Arthrit Rheum-Arthr. 2004;51:34–9. doi: 10.1002/art.20079. [DOI] [PubMed] [Google Scholar]
- 47.Ostir GV, Berges IM, Ottenbacher ME, Clow A, Ottenbacher KJ. Associations between positive emotion and recovery of functional status following stroke. Psychosom Med. 2008;70:404–9. doi: 10.1097/PSY.0b013e31816fd7d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostir GV, Markides KS, Black SA, Goodwin JS. Emotional well-being predicts subsequent functional independence and survival. J Am Geriatr Soc. 2000;48:473–8. doi: 10.1111/j.1532-5415.2000.tb04991.x. [DOI] [PubMed] [Google Scholar]
- 49.Burg MM, Jain D, Soufer R, Kerns RD, Zaret BL. Role of behavioral and psychological factors in mental stress-induced silent left ventricular dysfunction in coronary artery disease. Journal of the American College of Cardiology. 1993;22:440–8. doi: 10.1016/0735-1097(93)90048-6. [DOI] [PubMed] [Google Scholar]


