Abstract
Protection of motoneurons is an important goal in the treatment of spinal cord injury (SCI). We tested whether neuroprotective microRNAs (miRs) like miR-206, miR-17, miR-21, miR-7-1, and miR-106a could enhance efficacy of estrogen receptor (ER) agonists such as 1,3,5-tris (4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT, ERα agonist), Way200070 (WAY, ERβ agonist), and estrogen (EST, ERα and ERβ agonist) in preventing apoptosis in the calcium ionophore (CI) insulted VSC4.1 motoneurons. We determined that 200 nM CI induced 70% cell death. Treatment with 50 nM PPT, 100 nM WAY, and 150 nM EST induced overexpression of ERα, ERβ, and both receptors, respectively, at mRNA and protein levels. Treatment with ER agonists significantly upregulated miR-206, miR-17, and miR-7-1 in the CI insulted VSC4.1 motoneurons. Transfection with miR-206, miR-17, or miR-7-1 mimic potentiated WAY or EST to inhibit apoptosis in the CI insulted VSC4.1 motoneurons. Overexpression of miR-7-1 maximally increased efficacy of WAY and EST for down regulation of pro-apoptotic Bax and upregulation of anti-apoptotic Bcl-2. A search using miRDB indicated that miR-7-1 could inhibit expression of L-type Ca2+ channel protein alpha 1C (CPα1C). miR-7-1 overexpression and WAY or EST treatment down regulated CPα1C but upregulated p-Akt to trigger cell survival signaling. The same therapeutic strategy increased expression of the Ca2+/calmodulin-dependent protein kinase II beta (CaMKIIβ) and the phosphorylated cAMP response element binding protein (p-CREB) so as to promote Bcl-2 transcription. Whole cell membrane potential and mitochondrial membrane potential studies indicated that miR-7-1 highly potentiated EST to preserve functionality in the CI insulted VSC4.1 motoneurons. In conclusion, our data indicated that miR-7-1 most significantly potentiated efficacy of EST for functional neuroprotection and this therapeutic strategy could be used in the future to attenuate apoptosis of motoneurons in SCI.
Keywords: apoptosis, CI, CPα1C, CAMKIIβ, ER agonists, miR-7-1, VSC4.1 motoneurons
INTRODUCTION
Current understanding of the crucial mechanisms underlying progressive pathogenesis in spinal cord injury (SCI) is limited and traditional therapeutic agents cannot effectively prevent excessive neuroinflammation and nonstop neurodegeneration, especially in motoneurons. Effective treatment of SCI to protect motoneurons will require a multifaceted therapeutic strategy that can provide functional neuroprotection and promote cell regeneration. To accomplish this, it is imperative to uncover the upstream regulators responsible for coordinating apoptosis and inflammatory responses (Grau et al., 2004; Grau et al., 2006; Hook et al., 2009).
Calcium ionophore (CI) is extensively used to imitate the effects of physiological stimuli related to free Ca2+ in the cells (Martina et al., 1994; Wang, 1994). CI induces synthesis of nitric oxide through activation of calmodulin-dependent nitric oxide synthase in cell culture (Knowles and Moncada, 1992). It is also known to uncouple oxidative phosphorylation and inhibit ATPase activity in mitochondria (Reed and Lardy, 1972). CI potentiates response to glutamate receptors and induces apoptosis (Rodriguez-Tarduchy et al., 1990; Caron-Leslie et al., 1994).
MicroRNAs (miRs) may be critical to the pathogenesis of several neurodisorders (Eacker et al., 2009; Saugstad, 2010) including SCI (Yan et al., 2012). miRs are capable of regulating hundreds of genes simultaneously, either post-transcriptionally or through promoter interaction (Li et al., 2006; Breving and Esquela-Kerscher, 2010). It is highly likely that miRs play significant roles in induction of many secondary inflammatory processes following SCI and thus modulation of their expression can be an attractive therapeutic strategy in SCI. Currently, miRs are considered to be a novel class of therapeutic tools for treatment of SCI, especially because of their small size. There are only scanty reports describing the potential roles of miRs in SCI, most of which have performed expression analysis. Deregulation of miR-206, miR-17, miR-21, miR-7-1, and miR-106a is known to be correlated with different pathophysiological processes like inflammation, oxidative stress, apoptosis, glial scar formation, and axonal degeneration (Kusuda et al., 2011; Liu and Xu, 2011; Yan et al., 2012).
Estrogen (EST) and other EST receptor (ER) agonists provide neuroprotection in traumatic brain injury, SCI, and ischemic injury and also in many neurodegenerative diseases (Sribnick et al., 2003; Nilsen and Brinton 2004; Gerstner et al., 2009). EST and other ER agonists act as powerful anti-oxidant and also as anti-inflammatory agent (Sribnick et al., 2005). Recent studies indicated that ER agonists could prevent increases in intracellular free Ca2+, activation of calpain, and apoptosis (Sur et al., 2003, Sribnick et al., 2004). The protective effects of EST and other ER agonists are mediated via ER alpha (ERα) and ER beta (ERβ). As demonstrated in a number of earlier studies, EST and its structural analogs acted as promising neuroprotectants due to their anti-oxidant properties in a number of model systems (Behl et al., 1997; Green and Simpkins, 2000). Other previous studies demonstrated that EST or ER agonists could protect hippocampal neurons (Wu et al., 2005; Zhao et al., 2005) and primary cortical neurons (Cordey and Pike, 2005) after activation of mitogen-activated protein kinase (MAPK) signaling cascades.
The ventral spinal cord 4.1 (VSC4.1) motoneuron cell line was generated by fusion of embryonic rat ventral spinal cord neuron with mouse N18TG2 neuroblastoma cell (Crawford et al., 1992; Smith et al., 1994). It has not yet been investigated whether overexpression of neuroprotecive miR can enhance efficacy of any ER agonist or EST in VSC4.1 motoneurons. Our results demonstrated that transfection with miR-7-1 followed by treatment with EST could upregulate cell survival factors and inhibit apoptosis to protect functional VSC4.1 motoneurons from supraphysiological CI toxicity. Therefore, miR-7-1mediated augmentation of efficacy of EST can be a novel therapeutic strategy for functional protection of motoneurons in SCI.
MATERIALS AND METHODS
Cell culture
The VSC4.1 motoneurons were grown in monolayer to subconfluency in 75-cm2 flasks containing 10 ml of DMEM/F12 medium with 15 mM HEPES, pyridoxine, and NaHCO3 (Sigma Chemical, St. Louis, MO, USA), supplemented with 2% Sato’s components, 1% penicillin, and 1% streptomycin (Invitrogen, Carlsbad, CA, USA), and 10% heat-inactivated fetal bovine serum (FBS). The media and FBS were purchased from BioAbChem (Ladson, SC, USA). CI, 1,3,5-tris (4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT, the ERα agonist), Way200070 (WAY, the ERβ agonist), and EST (the ERα and ERβ agonist) were procured from Sigma Chemical. All anti-miR and miR mimics were purchased from Dharmacon (Chicago, IL, USA). Cells from all treatment groups were used to determine cell viability, levels of mRNA and protein of specific factors regulating apoptosis, and biochemical features of apoptosis.
Determination of residual cell viability using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay
The VSC4.1 motoneurons were seeded into 96-well microculture plates at 1×104 cells/well and allowed to attach overnight. The next day, cells were exposed to different concentrations (25, 50, 100, 200 and 500 nM) of CI in DMEM/F12 medium supplemented with 2% FBS and incubated for 24 h. The medium was replaced with fresh medium containing MTT (0.2 mg/ml) and the plates were incubated for another 3 h. Then, dimethyl sulfoxide (DMSO) was added to dissolve the MTT formazan crystals and absorbance of the color was measured at 570 nm with background subtraction at 630 nm. Final concentration of DMSO in each treatment was maintained at < 0.01% that did not affect cell viability or death. Cell viability was calculated as percentage of viable cells in the total population. Another set of cell viability studies was performed to optimize neuroprotective efficacy of ER agonists (PPT, WAY, and EST) following exposure of VSC4.1 motoneurons to CI insult. First, cells were exposed to 200 nM CI for 24 h and post-treated with different doses (ranging from 0 to 175 nM) of PPT, WAY, and EST. After 24 h incubation with ER agonists, the MTT assay was performed as described above.
Semi-quantative reverse transcription-polymerase chain reaction (RT-PCR) for mRNA
The VSC4.1 motoneurons were grown in six-well plates for 48 h and then exposed to 200 nM CI and incubated for another 24 h. The old medium was replaced with fresh medium and cells were treated with 50 nM PPT, 100 nM WAY, or 150 nM EST for another 24 h. Total RNA was extracted from all treatment groups using TRIzol reagent as per manufacturer’s protocol (Invitrogen). The levels of mRNA expression of ERα, ERβ, Bax, Bcl-2, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were examined using semi-quantative RT-PCR. Primers for ERα, ERβ, Bax, Bcl-2, and GAPDH genes (Table 1) were designed using Oligo software (National Biosciences, Plymouth, MN, USA). Total RNA (300 ng) was used for each set of primers for transcription and amplification using a single-step RT-PCR kit (Invitrogen) on a PCR thermal cycler (Eppendorf, Westbury, NY, USA), as we reported recently (Chakrabarti et al., 2013). The RT-PCR products were resolved on 1.5% agarose gels by electrophoresis, stained with ethidium bromide (1 μg/ml), and visualized on a UV (303 nm) transilluminator, and photographed digitally using the UVDI Compact Digimage System (Major Science, Saratoga, CA, USA). The levels of mRNA expression of the target genes were determined by calculating the optical density (OD) of the bands using Gel-Pro analyzer software (Media Cybernetics, Silver Spring, MD, USA).
Table 1.
Primers for determining levels of mRNA of ERα, ERβ, Bax and Bcl-2 and also expression of specific miRs in VSC4.1 motoneurons
| Primers for target genes | Sequences |
|---|---|
| ERβ | Forward: 5′-TCC CTC TTT GCG TTT GGA CTA-3′ Reverse: 5′-TTC CCG GCA GCA CCA GTA ACC-3′ |
| ERα | Forward: 5′-AAT TCT GAC AAT CGA CGC CAG-3′ Reverse: 5′-GTG CTT CAA CAT TCT CCC TCC TC-3′ |
| Bax | Forward: 5′-GCA GAG AGG ATG GCT GGG GAG A-3′ Reverse: 5′-TCC AGA CAA GCA GCC GCT CAC G-3′ |
| Bcl-2 | Forward: 5′-GGA TGA CTT CTC TCG TCG CTA C-3′ Reverse:5′-TGC AGA TGC CGG TTC AG-3′ |
| GAPDH (control for mRNA) | Forward: 5′-TTC ACC ACC ATG GAG AAG GC-3′ Reverse: 5′-GGC ATG GAC TGT GGT CAT GA-3′ |
| miR-206 | Forward: 5′-ACA TGC TTC TTT ATA TCC CCA-3′ Reverse: 5′-AAA CCA CAC ACT TCC TTA CAT TC-3′ |
| miR-17 | Forward: 5′-GCA GGA AAA AAG AGA ACA TCA CC-3′ Reverse: 5′-TGG CTT CCC GAG GCAG-3′ |
| miR-21 | Forward: 5′-GCT TAT CAG ACT GAT GTT GAC TG-3′ Reverse: 5′-CAG CCC ATC GAC TGG TG-3′ |
| miR-106b | Forward: 5′-TAA AGT GCT GAC AGT GCA GAT AGT G-3′ Reverse: 5′-CAA GTA CCC ACA GTG CGG T-3′ |
| miR-7-1 | Forward: 5′-TGG AAG ACT AGT GAT TTT GTT GT-3′ Reverse: 5′-AGA CTG TGA TTT GTT GTC GAT T-3′ |
| U6 RNA (control for miR) | Forward: 5′-CTC GCT TCG GCA GCA CA-3′ Reverse: 5′-AAC GCT TCA CGA ATT TGC GT-3′ |
Reverse transcription (RT) of total RNA for cDNA of specific miRs
At the end of each treatment, total RNA was extracted from 3×106 cells using TRIzol reagent as per manufacturer’s protocol (Invitrogen). The primers specific for miR-206, miR-17, miR-21, miR-7-1, and miR-106a were designed (Table 1) from the primary precursor molecule sequences from the miRBase database. The custom synthesized primers were procured from Eurofins MWG Operon (Huntsville, AL, USA). Total RNA was used for RT to cDNA using the specific primers following a procedure as we reported previously (Chakrabarti et al., 2012). Here, primers in RT reaction included a mixture of 10 μM each of the antisense primers for specific miRs and U6 RNA (Table 1).
Polymerase chain reaction (PCR) for amplification of specific miRs
The cDNA of specific miRs from each treatment group was used as template in PCR reaction. Briefly, each PCR reaction (25 μl) was performed on a PCR thermal cycler (Eppendorf, Westbury, NY, USA) after mixing 0.5 μl of 10 μM miR specific primers, 2.5 μl of 10 μM PCR buffer, 0.75 μl of 50 mM MgCl2, 0.5 μl of 10 mM dNTPs, and 0.2 μl of 5 U/μl Platinum Taq DNA polymerase (Invitrogen) and 2 μl RT product. The cycling protocol consisted of an initial inactivation of RT enzyme at 95°C 10 min followed by 35 cycles of denaturation at 95°C for 15 sec, annealing at 52°C for 30 sec, and extension at 72°C for 30 sec and also a final extension at 72°C for 7 min. Amplified specific miR products were resolved on 2.2% agarose gels by static-field electrophoresis, stained with ethidium bromide (1 μg/ml), destained the background of gels in water, visualized on a UV (303 nm) transilluminator, and photographed digitally using the UVDI Compact Digimage System (Major Science).
Real-time quantitative RT-PCR (qRT-PCR) for specific miRs
The levels of expression of specific miRs were determined using real-time qRT-PCR, as we described previously (Chakrabarti et al., 2012), with several modifications. All PCR reagents were used from the SYBR green core reagent kit (Applied Biosystems, Foster City, CA, USA). Master mix (3 μl) containing all of the PCR reaction components except the primers were dispensed into a real-time qRT-PCR plate (Life Technologies, Grand Island, NY, USA). The master mix contained 0.5 μl of 10xPCR buffer, 0.7 μl of 25 mM MgCl2, 0.1 μl of 12.5 mM dNTPs, 0.01 μl of UNG, 0.025 μl of AmpliTaq Gold DNA polymerase, 0.5 μl of dilute cDNA (1:100) and water to 3 μl. All miRs and U6 RNA were assayed in duplicate in the reaction plate. Real-time qRT-PCR was performed on an Applied Biosystems 7900HT real-time qRT-PCR instrument. For amplification of each specific miR, PCR was performed for 15 s at 95°C and 1 min at 60°C for 40 cycles followed by the thermal denaturation. The expression of each miR relative to U6 RNA control was determined using the 2−ΔCT method (Livak et al., 2001).
Transfection of VSC4.1 cells with specific miRs
The VSC4.1 motoneurons were seeded at a concentration of 5×105 cells per well in 6-well plates. On the next day, cells were transfected with an anti-miR (anti-miR-206, anti-miR-17, or anti-miR-7-1) or a miR (miR-206, miR-17, or miR-7-1) oligomeric RNA at 50 nM final concentration using 20 μl Lipofectamine 2000 reagent and Opti-MEM medium following the manufacturer’s protocol (Invitrogen). After 12 h, transfection medium was replaced by fresh growth medium containing 2% FBS and no treatment (control) or 200 nM CI. After incubation for 24 h, cells were treated with 100 nM WAY or 150 nM EST and incubated for another 24 h. The effects of transfection of cells with an anti-miR or a miR alone or in combination with drugs were studied by flow cytometry, RT-PCR analysis, Western blotting, and membrane potential analysis.
Annexin V-fluorescein isothiocyanate (FITC)/propidiun iodide (PI) double staining and flow cytometry for determining induction of apoptosis
The VSC4.1 motoneurons were harvested after the desired transfection and drug treatments and washed twice with phosphate-buffered saline (PBS, pH 7.4) before being fixed with 70% (v/v) ethanol for 15 min. Subsequently, cells were centrifuged to obtain pellets and residual ethanol was aspirated. Then, the motoneurons were digested with 2 mg/ml DNase-free RNase A for 30 min at 37°C. Annexin V-FITC/PI double staining of the motoneurons was performed as per manufacturer’s instructions (BD Biosciences, San Jose, CA, USA), and then analyzed on an Epics XL-MCL Flow Cytometer (Beckman Coulter, Fullerton, CA, USA). The motoneurons that were Annexin V negative and PI positive were considered as mechanically injured (quadrant A1), the motoneurons that were both Annexin V and PI positive (quadrant A2) were considered as late necrotic, the motoneurons that were both Annexin V and PI negative (quadrant A3) were considered as normal, and the motoneurons that were Annexin V positive and PI negative were considered as early apoptotic (quadrant A4). Flow cytometry detected the Annexin V positive cells with externalization of membrane phospholipids, an early biochemical feature of apoptosis. The Annexin V stained apoptotic motoneurons were analyzed for statistical significance.
Antibodies and Western blotting
Monoclonal primary IgG antibodies against β-actin and cytochrome c oxidase subunit IV (COX-4) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and used for monitoring equal loading of cytosolic and mitochondrial proteins, respectively, in the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) experiments, as we reported recently (Chakrabarti et al., 2013). All other primary IgG antibodies were also purchased from Santa Cruz Biotechnology. Primary antibodies were diluted at a concentration of 1:1000, unless otherwise stated, for probing the blots. Secondary antibodies were horseradish peroxidase (HRP)-conjugated goat anti-mouse or anti-rabbit IgG (Santa Cruz Biotechnology) and diluted at a concentration of 1:2000 before used for detecting primary IgG antibodies. Western blotting was performed, as we described previously (Das et al., 2010a, 2010b). The isolation of cytosolic and mitochondrial fractions was performed by standard procedures. Following probing with the antibodies, Western blots were incubated with the enhanced chemiluminescence (ECL) detection reagents (GE Healthcare, Little Chalfont, Buckinghamshire, UK). The ECL treated bots were immediately exposed to X-OMAT AR films (Eastman Kodak, Rochester, NY, USA) for autoradiography. The autoradiograms were scanned on an EPSON Scanner using Photoshop software (Adobe Systems, Seattle, WA, USA). All experiments were performed in triplicates. The protein band density was quantified using Gel-Pro analyzer software (Media Cybernetics).
Electrophysiological recordings for measuring whole cell membrane potential
The whole cell membrane potentials were measured by patch-clamp electrophysiology using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA) in conjunction with AxographX software (Axograph, Sydney, NSW, Australia). After transfection or/and desired drug treatments, the motoneurons were perfused at room temperature with the extracellular recording solution containing 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 10 mM glucose, and 5 mM HEPES (pH 7.2 and osmolarity adjusted to 325 mOsM/L with sucrose). Patch electrodes (2.5 – 4.0 MOhms) were filled with an internal solution containing 150 mM KCl, 2.5 mM NaCl, 4 mM Mg-ATP, 2 mM Na-ATP, 0.3 mM Na-GTP, 5 mM Na-phosphocreatine, 10 mM HEPES (pH −7.4 and osmolarity adjusted to 310 mOsM/L with sucrose). The liquid junction potential was 4.1 mV and corrected for all recordings. After the seal formation and breakthrough in voltage-clamp (holding potential −60 mV), the amplifier was switched to current-clamp mode with zero holding current and the resulting membrane potential was recorded.
Determination of mitochondrial membrane potential
Change in mitochondrial membrane potential was measured by using the mitochondrial fluorescent probe JC-1 (Life Technologies). Control and treated the motoneurons were incubated in DMEM/F12 medium containing JC-1 (5 μg/ml) during treatment from 0 to 12 h. After staining, the motoneuron cultures were washed twice with PBS (pH 7.4). When excited at 488 nm, the fluorescence emission of JC-1 was measured at wavelengths corresponding to its monomer (530 ± 15 nm) and J aggregate (>590 nm) forms. Fluorescence was measured in a fluorescent plate reader (Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
For statistical analysis, the experiments were performed in at least in triplicates. The results were analyzed statistical significance using Minitab 16 statistical software (Minitab, State College, PA, USA). Data were expressed as mean ± standard error of mean (SEM) of separate experiments (n ≥ 3) and compared by one-way analysis of variance (ANOVA) followed by the Fisher’s post-hoc test. Difference between control (CTL, the untreated group) and a treatment was considered significant at p < 0.05.
RESULTS
Exposure to CI reduced cell viability and ER agonists protected VSC4.1 motoneurons
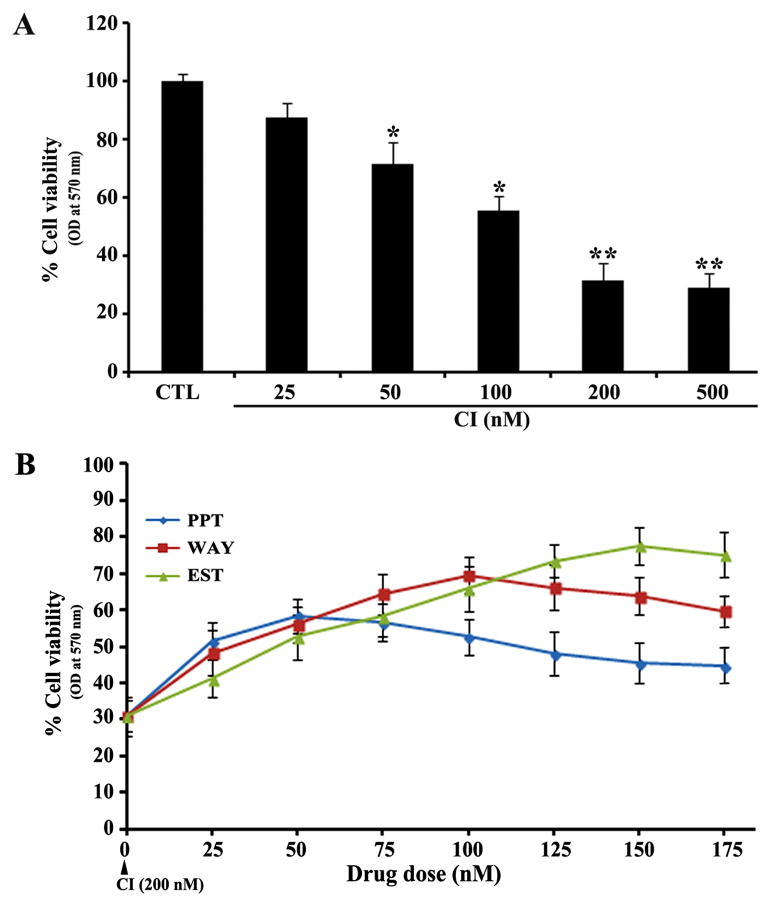
We examined the changes in residual cell viability following exposure to CI and neuroprotective abilities of ER agonists in VSC4.1 motoneurons (Fig. 1). The residual cell viability was determined by the MTT assay after treatment of VSC4.1 motoneurons with 25, 50, 100, 200, and 500 nM CI for 24 h (Fig. 1A). The MTT assay showed that CI at 25 and 50 nM concentrations was less cytotoxic, but it could cause more than 70% cell death at 200 and 500 nM concentrations (Fig. 1A). So, we selected 200 nM as the effective concentration of CI to induce cytotoxic insult in VSC4.1 motoneutons in all subsequent experiments. Our dose-response analyses with the CI insulted motoneurons indicated that 50 nM PPT, 100 nM WAY, and 150 nM EST provided maximum neuroprotection with 51%, 69%, and 77% cell viability, respectively (Fig. 1B). Therefore, we decided to use 50 nM PPT, 100 nM WAY, and 150 nM EST for neuroprtection in the CI insulted VSC4.1 motoneurons in all other experiments.
Fig. 1.
Dose response studies in VSC4.1 motoneurons. (A) Determination of residual cell viability following exposure to calcium ionophore (CI) for 24 h. Untreated cells were used as control (CTL). Cells were exposed to 25, 50, 100, 200, and 500 nM CI for 24 h and changes in cell viability were measured by the MTT assay. All experiments were conducted in triplicates and the results were analyzed for statistical significance. Difference in values between CTL and an exposure to CI was considered significant at *p<0.05 or ** p<0.01. (B) Determination of residual cell viability after treatment with different doses (0 to 175 nM) of PPT, WAY, or EST in the CI insulted VSC4.1 motoneurons. Cytoxic insult was induced with exposure to 200 nM CI for 24 h. Then, cells were treated with PPT, WAY, or EST for another 24 h and changes in cell viability were measured by the MTT assay. All experiments were conducted in triplicates.
Involvement of ERα and ERβ in neuroprotection
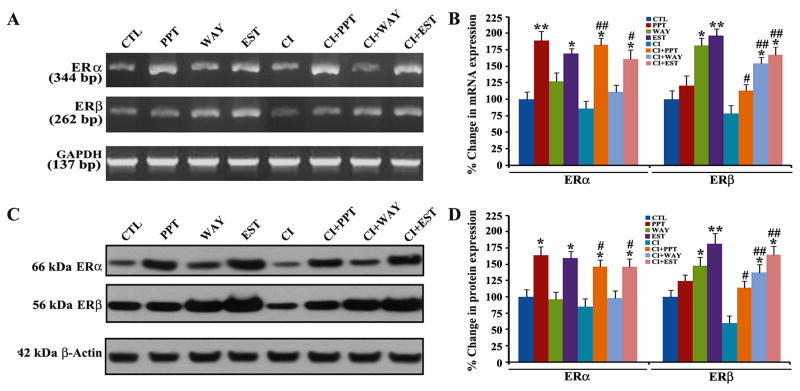
To ascertain whether involvement of both ERα and ERβ agonists could contribute to protection of VSC4.1 motoneurons against CI toxicity, we measured the expression of both ERα and ERβ at the mRNA and protein levels by semi-quantative RT-PCR and Western blotting, respectively (Fig. 2). Treatment of VSC4.1 motoneurons with one of the ER agonists changed the expression of ERα and ERβ at mRNA (Fig. 2A) and protein (Fig. 2C) levels. Compared with untreated VSC4.1 motoneurons, treatment with only PPT, WAY, or EST most significantly increased expression of ERα, ERβ, or both receptors, respectively, at mRNA (Fig. 2B) and protein (Fig. 2D) levels. Cells insulted with CI showed reduced expression of ERα and ERβ at mRNA and protein levels when compared with the untreated control cells. After the CI insult, treatment with PPT more significantly increased expression of ERα than expression of ERβ in the cells. On the other hand, treatment with WAY more significantly increased expression of ERβ than expression of ERα in the CI insulted cells. EST showed the maximum upregulation of ERβ at mRNA (Fig. 2B) and protein (Fig. 2D) levels in the CI insulted cells. Thus, our results suggested that PPT, WAY, and EST increased the expression of ERα and ERβ in varying degrees to provide neuroprotection to the CI insulted VSC4.1 motoneurons.
Fig. 2.
RT-PCR and Western blotting for determining changes in expression of ERα and ERβ in VSC4.1 motoneurons. Treatment groups (24 h): control (CTL), 50 nM PPT, 100 nM WAY,150 nM EST, 200 nM CI, 200 nM CI + 50 nM PPT, 200 nM CI +100 nM WAY, 200 nM CI + 150 nM EST. (A) RT-PCR for alterations in mRNA expression of ERα, and ERβ. Uniform mRNA expression of GAPDH was used as a loading control in RT-PCR experiments. (B) Determination of percent changes in mRNA expression of ERα and ERβ. (C) Western blotting for alterations in protein expression of ERα and ERβ. Uniform protein expression of β-actin was used as a loading control in Western blotting. (D) Determination of percent changes in protein expression of ERα and ERβ. Difference in values between CTL and a treatment was considered significant at *p<0.05 or ** p<0.01. Difference in values between the CI treated cells and the CI + ER agonist treated cells was considered significant at #p<0.05 or ##p <0.01.
Alterations in expression of specific miRs in VSC4.1 motoneurons following treatments
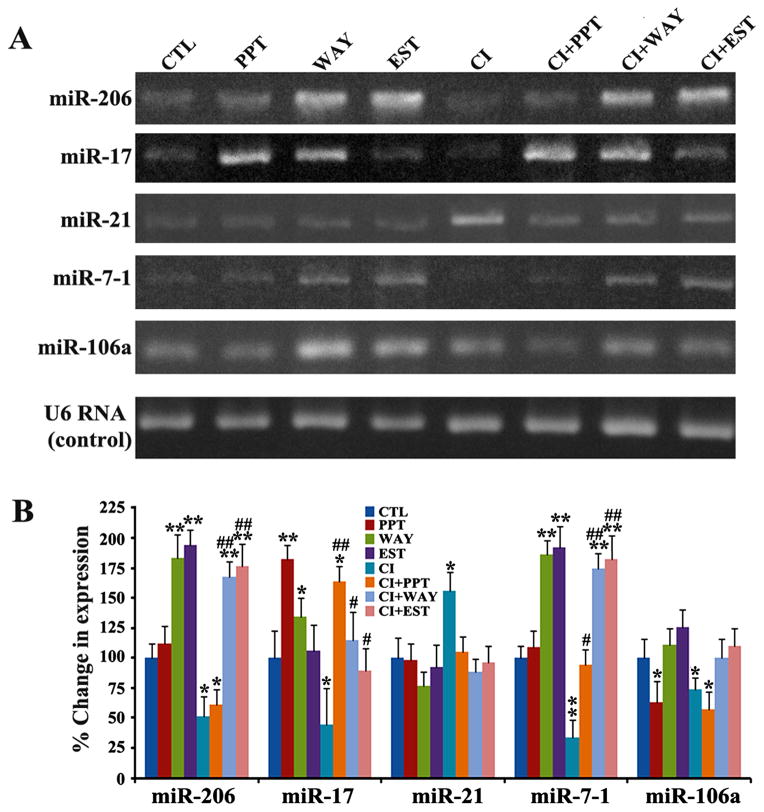
We examined the alterations in expression of five miRs (miR-206, miR-17, miR-21, miR-7-1, and miR-106a) in VSC4.1 motoneurons after the treatments with CI or/and ER agonists (Fig. 3). First, we performed qualitative RT-PCR experiments to examine the expression of these miRs and resolved the amplified RT-PCR products on the 2.2% agarose gels by electrophoresis (Fig. 3A). Then, we performed real-time qRT-PCR analyses to determine the alterations in expression of these miRs in VSC4.1 motoneurons after treatments with CI or/and ER agonists (Fig. 3B). Our real-time qRT-PCR results demonstrated that miR-206, miR-17, and miR-7-1 were upregulated whereas miR-21 were down regulated in VSC4.1 motoneurons after the treatments when compared with the untreated control cells. Alterations in expression of specific miRs in the CI insulted VSC4.1 motoneurons after the treatment with ER agonists a significant finding, as these miRs can modulate the expression of many signaling molecules in the process of neuroprotection. Because WAY and EST showed better effects than PPT in upregulating specific miRs (miR-206, miR-17, and miR-7-1) in the CI insulted VSC4.1 motoneurons, we decided to use only WAY and EST in subsequent experiments. To examine the transfection efficiency of miRs, we determined the levels of expression of all these miRs and small nuclear U6 RNA (which served as internal control) by real-time qRT-PCR analysis following transfection of the miRs. We observed increases in expression of specific miRs (miR-206, miR-17, and miR-7-1) by 2-fold to 3-fold after transfection, without alteration in expression of U6 RNA control (data not shown). Based on these results, we decided to further explore contributions of miR-206, miR-17, and miR-7-1 to neuroprotection in the CI insulted VSC4.1 motoneurons.
Fig. 3.
Qualitative RT-PCR and real-time qRT-PCR to determine levels of specific miRs in VSC4.1 motoneurons. Treatment groups (24 h): control (CTL), 50 nM PPT, 100 nM WAY,150 nM EST, 200 nM CI, 200 nM CI + 50 nM PPT, 200 nM CI +100 nM WAY, 200 nM CI + 150 nM EST. (A) Qualitative RT-PCR products of miRs were resolved by 2.2% agarose gel electrophoresis. (B) Real-time qRT-PCR products were analyzed for levels of expression of miRs after normalizing with U6 RNA. Difference in values between CTL and a treatment was considered significant at *p<0.05 or ** p<0.01. Difference in values between the CI treated cells and the CI + ER agonist treated cells was considered significant at #p<0.05 or ##p <0.01.
Transfection with miR-206, miR-17, or miR-7-1 mimic enhanced the efficacy of WAY and EST to prevent induction of apoptosis in the CI insulted VSC4.1 motoneurons
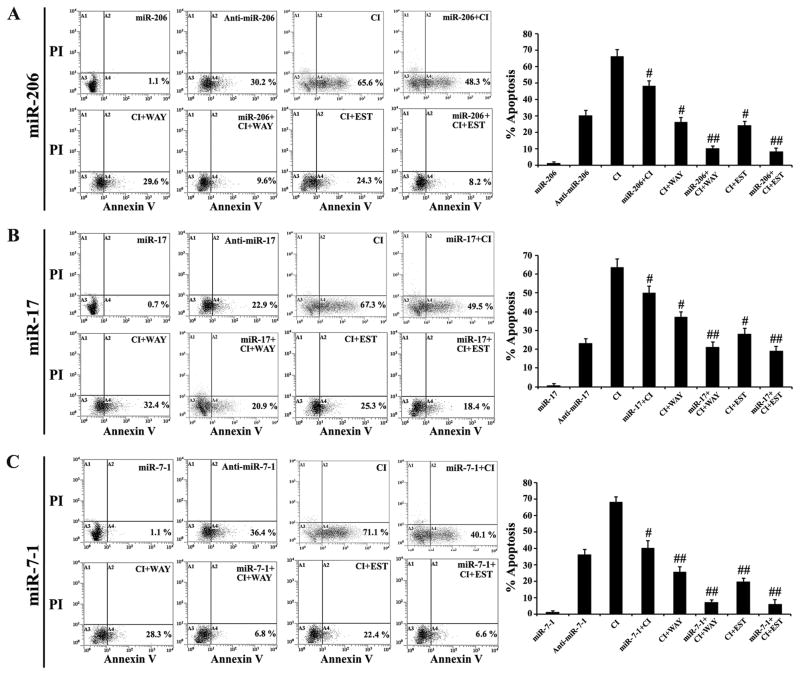
We investigated the effects of transfection of miR-206, miR-17, and miR-7-1 mimics on the neuroprotective efficacies of ER agonists in CI exposed VSC4.1 motoneurons (Fig. 4). Flow cytometric analyses of the Annexin V-FITC/PI stained cells did not show any significant difference in amounts of apoptosis in untreated control cells and cells transfected with miR-206, miR-17, or miR-7-1 mimic (data not shown). Transfection of cells with any of these miR mimics did not induce apoptosis. In contrast, we observed induction of 65–70% apoptosis in VSC4.1 motoneurons following exposure to 200 nM CI. Treatment with WAY or EST alone could protect the VSC4.1 motoneurons from CI insult to some extent, but their efficacy was very significantly enhanced when the cells were pre-transfected with miR-206 (Fig. 4A), miR-17 (Fig. 4B), or miR-7-1 (Fig. 4C) mimic. Transfection with anti-miRs could inhibit the neuroprotective effects of these endogenous miRs. Our results showed that miR-7-1 was the most potent miR in enhancing the neuroprotective efficacy of WAY (Fig. 4B) and EST (Fig. 4C) with most significant inhibition of apoptosis. In subsequent experiments, we further investigated the effects of miR-7-1 in combination with WAY or EST to determine the most successful therapeutic strategy for neuroprotection in the CI insulted VSC4.1 motoneurons.
Fig. 4.
Overexpression of miR-206, miR-17, or miR-7-1 enhanced the efficacy of WAY or EST for prevention of apoptosis in the CI insulted VSC4.1 motoneurons. Treatment groups: 50 nM miR mimic for 12 h, 50 nM anti-miR mimic for 12 h, 200 nM CI for 24 h, 50 nM miR mimic for 12 h + 200 nM CI for 24 h, 200 nM CI for 24 h + 100 nM WAY for 24 h, 50 nM miR mimic for 12 h + 200 nM CI for 24 h + 100 nM WAY for 24 h, 200 nM CI for 24 h + 150 nM EST for 24 h, and 50 nM miR mimic for 12 h + 200 nM CI for 24 h + 150 nM EST for 24 h. Cells were collected for Annexin V-FITC/PI double staining and flow cytometry to determine the percentages of apoptotic cells from three independent experiments for miR-206 set (A), miR-17 set (B), and miR-7-1 set (C). Difference in values between CI insulted cells and miR mimic + CI, CI + ER agonist, or miR mimic + CI + ER agonist treated cells was considered significant at #p<0.05 or ##p <0.01.
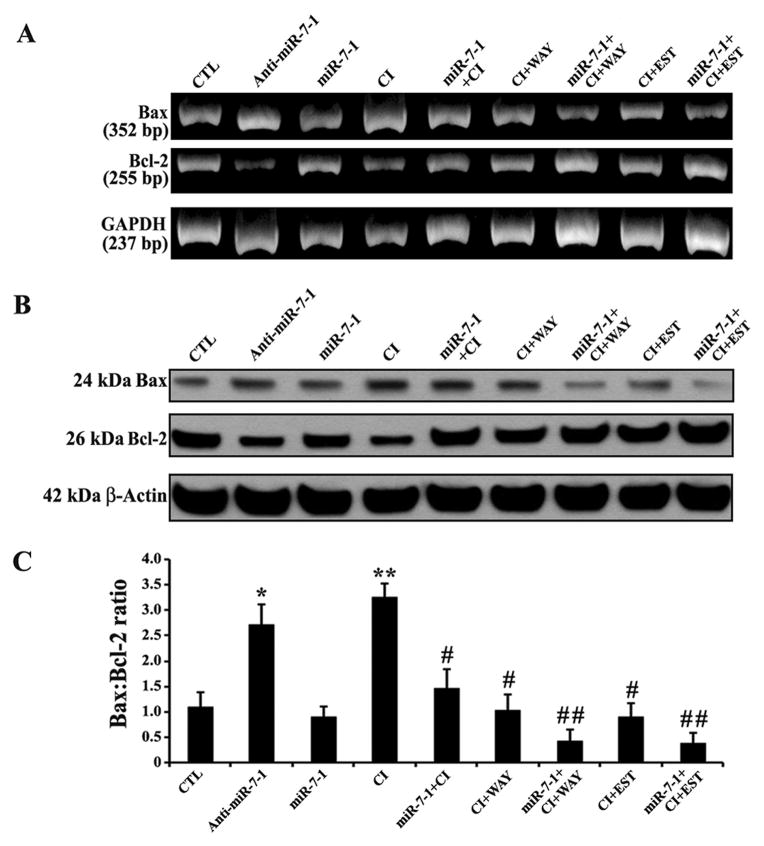
Sequential therapy with miR-7-1 and ER agonist decreased Bax:Bcl-2 ratio
Changes in expression of pro-apoptotic Bax and anti-apoptotic Bcl-2 may result in increase in Bax:Bcl-2 ratio to trigger neuronal apoptosis after a toxic insult. An effective therapeutic strategy needs to reduce Bax:Bcl-2 ratio to prevent neuronal apoptosis. Therefore, we examined the alterations in expression of Bax and Bcl-2 at the mRNA and protein levels to determine the Bax:Bcl-2 ratio in the CI insulted VSC4.1 motoneurons following sequential therapy with miR-7-1 and ER agonist (Fig. 5). We found that the cells transfected with anti-miR-7-1 mimic or exposed to CI increased Bax but decreased Bcl-2 expression at mRNA (Fig. 5A) as well as at protein (Fig. 5B) level, leading to significant increase in the Bax:Bcl-2 ratio in the cells (Fig. 5C). Monotherapy with miR-7-1, WAY, or EST could significantly decrease the Bax:Bcl-2 ratio. But sequential transfection with miR-7-1 mimic and treatment with a single ER agonist (WAY or EST) most effectively decreased the Bax:Bcl-2 ratio when compared with the CI insulted VSC4.1 motoneurons (Fig. 5C). Thus, our results clearly showed that sequential therapy with miR-7-1 and ER agonist (WAY or EST) could block an important event required for initiation of downstream apoptotic signaling cascade.
Fig. 5.
Alterations in expression of Bax and Bcl-2 at mRNA and protein levels in VSC4.1 motoneurons. Treatment groups: untreated control (CTL), 50 nM anti-miR-7-1 for 12 h, 50 nM miR-7-1 for 12 h, 200 nM CI for 24 h, 50 nM miR-7-1 for 12 h + 200 nM CI for 24 h, 200 nM CI for 24 h + 100 nM WAY for 24 h, 50 nM miR-7-1 for 12 h + 200 nM CI for 24 h + 100 nM WAY for 24 h, 200 nM CI for 24 h + 150 nM EST for 24 h, and 50 nM miR-7-1 for 12 h + 200 nM CI for 24 h + 150 nM EST for 24 h. (A) Qualitative RT-PCR experiments to show representative mRNA expression of Bax and Bcl-2. Almost uniform mRNA expression of GAPDH was used as a loading control in RT-PCR experiments. (B) Western blots to show respresentative protein expression of Bax and Bcl-2. Uniform protein expression of β-actin was used as a loading control in Western blotting. (C) Densitometric analysis to show changes in Bax:Bcl-2 ratio. Difference in values between CTL (the untreated group) and a treatment was considered significant at *p<0.05 or **p<0.01. Difference in values between the CI insulted cells and the miR-7-1 + CI, CI + ER agonist, or miR-7-1 + CI + ER agonist treated cells was considered significant at #p<0.05 or ##p <0.01.
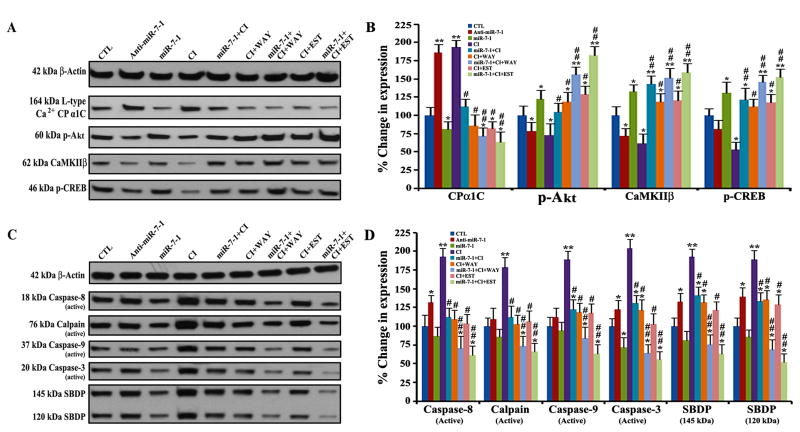
Sequential therapy with miR-7-1 and ER agonist upregulated cell survival molecules and blocked apoptosis
We examined the effects of transfection with miR-7-1 mimic or/and treatment with ER agonist (WAY or EST) on cell survival molecules and apoptotic cascade in the CI insulted VSC4.1 motoneurons by Western blotting (Fig. 6). A search using the microRNA database (miRDB) indicated that miR-7-1 plays an important role in decreasing the expression of L-type Ca2+ channel protein alpha 1C (CPα1C), a key structural and functional component of L-type voltage gated Ca2+ channels. Our results indicated that overexpression of miR-7-1 followed by treatment with ER agonist could inhibit expression CPα1C and also enhance phosphorylation of Akt (p-Akt, a survival signaling molecule) in the CI insulted VSC4.1 motoneurons (Fig. 6A). Notably, Ca2+/calmodulin dependent protein kinase II beta (CaMKIIβ) is an important factor for phosphorylation of cAMP response element binding protein (p-CREB), which is a widely known transcription factor for Bcl-2. Our Western blotting indicated that the CI insulted cells dramatically decreased the expression of CaMKIIβ and p-CREB (Fig. 6A). Transfection with miR-7-1 mimic followed by treatment with ER agonist (WAY or EST) very effectively increased expression of both CaMKIIβ and p-CREB (Fig. 6A). Our quantitative analysess of the Western blots clearly showed that miR-7-1 overexpression followed by WAY or EST treatment very significantly modulated the levels of expression of CPα1C, p-Akt, CaMKIIβ, and p-CREB proteins so as to promote cell survival signaling pathways in the CI insulted VSC4.1 motoneurons (Fig. 6B).
Fig. 6.
Western blotting for determining the levels of molecular components in cell survival and apoptotic pathways in VSC4.1 motoneurons. Treatment groups: untreated control (CTL), 50 nM anti-miR-7-1 for 12 h, 50 nM miR-7-1 for 12 h, 200 nM CI for 24 h, 50 nM miR-7-1 for 12 h + 200 nM CI for 24 h, 200 nM CI for 24 h + 100 nM WAY for 24 h, 50 nM miR-7-1 for 12 h + 200 nM CI for 24 h + 100 nM WAY for 24 h, 200 nM CI for 24 h + 150 nM EST for 24 h, and 50 nM miR-7-1 for 12 h + 200 nM CI for 24 h + 150 nM EST for 24 h. (A) Western blotting to show expression of β-actin, CPα1C, p-Akt, CaMKIIβ, and p-CREB. (B) Densitometric analysis to show relative changes in expression of CPα1C, p-Akt, CaMKIIβ, and p-CREB. (C) Western blotting to show expression of β-actin, caspase-8, calpain, caspase-9, caspase-3, and SBDP. (D) Densitometric analysis to show relative changes in expression of caspase-8, calpain, caspase-9, caspase-3, and SBDP. Difference in values between CTL (the untreated group) and a treatment was considered significant at *p<0.05 or **p<0.01. Difference in values between the CI insulted cells and the miR-7-1 + CI, CI + ER agonist, or miR-7-1 + CI + ER agonist treated cells was considered significant at #p<0.05 or ##p <0.01.
Our Western blotting also showed that miR-7-1 overexpression followed by WAY or EST treatment markedly inhibited activation of the cysteine proteases in both the extrinsic and intrinsic pathways of apoptosis in the CI insulted VSC4.1 motoneurons (Fig. 6C & 6D). Compared with untreated control cells, the CI insulted cells dramatically increased activation of caspase-8 (a cysteine protease in extrinsic pathway of apoptosis) and calpain, caspase-9, and caspase-3 (cysteine proteases in intrinsic pathways of apoptosis). But sequential therapy with miR-7-1 mimic and ER agonist (WAY or EST) very significantly blocked activation of caspase-8, calpain, caspase-9, and caspase-3 in the CI insulted VSC4.1 motoneurons (Fig. 6C & 6D). Our results also demonstrated that sequential combination therapy significantly prevented activities of calpain and caspase-3 in fragmentation of α-spectrin to 145 kDa spectrin breakdown product (SBDP) and 120 kDa SBDP, respectively (Fig. 6C & 6D). These findings indicated that sequential combination therapy with miR-7-1 and ER agonist (WAY or EST) could prevent apoptosis and provide protection to VSC4.1 motoneurons.
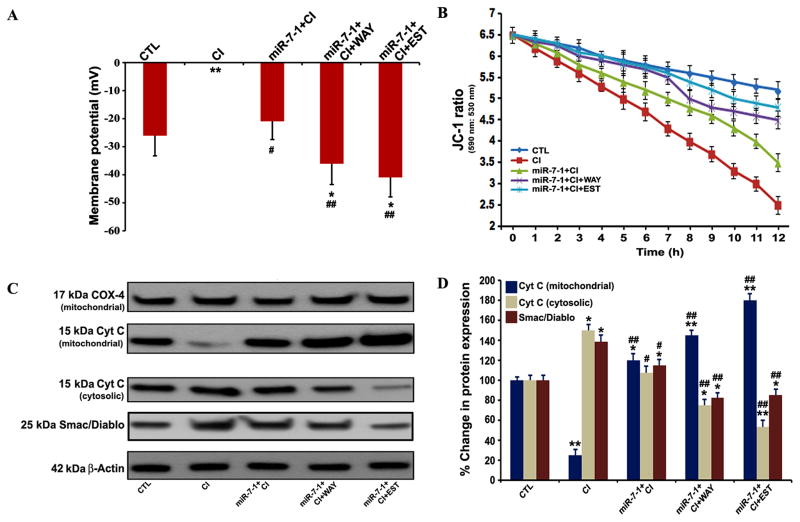
Overexpression of miR-7-1 followed by treatment with ER agonist preserved functionality in the CI insulted VSC4.1 motoneurons
We determined whole cell membrane potential and mitochondrial functionality in VSC4.1 motoneurons after all treatments (Fig. 7). The CI insulted cells committed massive cell death and thus their whole cell membrane potentials could not be recorded (Fig. 7A). Transfection with miR-7-1 mimic followed by CI insult significantly maintained whole cell membrane potential when compared with the CI insulted cells. Most significant improvement in whole membrane potential was observed when cells were subjected to sequential therapy with miR-7-1 and WAY or EST, indicating preservation of neuronal functionality.
Fig. 7.
Transfection with miR-7-1 mimic followed by treatment with WAY or EST provided functional neuroprotection in VSC4.1 motoneurons. Treatment groups: untreated control (CTL), 200 nM CI for 24 h. 50 nM miR-7-1 for 12 h + 200 nM CI for 24 h, 50 nM miR-7-1 for 12 h + 200 nM CI for 24 h + 100 nM WAY for 24 h, 50 nM miR-7-1for 12 h + 200 nM CI for 24 h + 150 nM EST for 24 h. (A) Measurement of whole cell membrane potential. (B) Determination of JC-1 ratio (590 nm: 530 nm) at different time points (0 to 12 h). (C) Western blotting to show expression of COX-4, cytochrome c (Cyt c), Smac/Diablo, and β-actin. (D) Densitometric analysis to show percent changes in the mitochondrial and cytosolic cytochrome c (Cyt c) and cytosolic Smac/Diablo. Difference in values between CTL (the untreated group) and a treatment was considered significant at *p<0.05 or **p<0.01. Difference in values between the CI insulted cells and the miR-7-1 + CI or miR-7-1 + CI + ER agonist treated cells was considered significant at #p<0.05 or ##p <0.01.
Mitochondrial swelling is correlated with the loss of mitochondrial membrane potential, which can be easily measured using JC-1 as a mitochondrial fluorescence probe. The untreated control VSC4.1 motoneurons clearly demonstrated high JC-1 ratio (590 nm:530 nm), indicating intact mitochondrial membrane potential in the cells (Fig. 7B). After exposure to CI, the cells progessively decreased the mean red and green fluorescence ratios of the mitochondria, indicating the loss of mitochondrial membrane potential (Fig. 7B). Overexpression of miR-7-1 followed by treatment with WAY or EST very effectively attenuated the loss of mitochondrial membrane potential in the cells (Fig. 7B) to prevent mitochondrial release of pro-apoptotic factors into the cytosol.
The intrinsic or mitochondrial pathway of apoptosis is mediated via the mitochondrial release of various pro-apoptotic factors like cytochrome c and Smac/Diablo into the cystosol. Smac/diablo is the second mitochondrial protein that, along with cytochrome c, promotes activation of caspases for induction of apoptosis. Our Western blot analyses indicated that indeed the loss of mitochondrial membrane potential in the CI insulted VSC4.1 motoneurons was associated with the increases in appearance of both cytochrome c and Smac/Diablo into the cytosolic fraction (Fig. 7C & 7D). Overexpression of miR-7-1 and treatment with ER agonist (WAY or EST) significantly decreased the amounts of cytosolic cytochrome c and Smac/Diablo in the CI insulted VSC4.1 motoneurons (Fig. 7C& 7D), indicating prevention of apoptosis.
DISCUSSION
Significant findings of this investigation indicate that overexpression of miR-7-1 can potentiate the therapeutic efficacy of ER agonists (WAY and EST) through down regulation of CPα1C for inhibition of Ca2+ influx and upregulation of p-Akt and p-CREB for promotion of survival signaling mechanisms in the CI insulted VSC4.1 motoneurons. Our results also demonstrate that sequential combination therapy with miR-7-1 mimic and ER agonist (especially EST) can inhibit both the extrinsic and intrinsic pathways of apoptosis in the CI insulted VSC4.1 motoneurons. Most importantly, overexpression of miR-7-1 followed by EST treatment preserved whole cell membrane potential and prevented loss of mitochondrial membrane potential so as to provide functional neuroprotection to the moneurons.
First, we performed dose response study using the MTT assay to optimize the CI concentration for induction of cell death in VSC4.1 motoneurons. Our results suggested that 200 nM CI would be appropriate to cause cytotoxic insult in VSC4.1 motoneurons. We performed the in situ Wright staining to monitor the mode of cell death (morphologically necrosis or apoptosis) during exposure to different concentrations of CI (data not shown) and thereby confirmed occurrence of maximum apoptosis in total cell death after treatment with 200 nM CI. So, we decided to use 200 nM CI as an effective cytotoxic insult for induction of apoptosis in VSC4.1 motoneurons. Our MTT results also suggested that 50 nM PPT, 100 nM WAY, and 150 nM EST were most potent treatments to inhibit cell death in the CI insulted VSC4.1 motoneurons. Our current results correlated well with our earlier studies that also demonstrated the maximum neuroprotection with 50 nM PPT and 150 nM EST in VSC4.1 motoneurons (Das et al., 2011). We substantiated with RT-PCR and Western blotting that PPT, WAY, and EST mediated neuroprotection mainly with involvement of ERα, ERβ, and both receptors, respectively, in the CI insulted VSC4.1 motoneurons. These results suggested that ER agonists protected VSC4.1 motoneurons from CI insult because of ER mediated activation of the cell survival mechanisms.
Alterations in expression of many neuroprotective miRs are frequently observed in SCI and other neurodisorders. To examine neuroprotective efficacy in our current investigation, we selected five miRs (miR-206, miR-17, miR-21, miR-7-1, and miR-106a) that were previously found to be highly upregulated or downregulated in course of pathogenesis in different neurodisorders including SCI (Kusuda et al., 2011; Liu et al., 2011; Yan et al., 2012). We observed significant down regulation of miR-206, miR-17, and miR-7-1 in the CI insulted VSC4.1 motoneurons but treatment with WAY or EST could significantly elevate their expression. So, we decided to further investigate the effects of overexpression of miR-206, miR-17, and miR-7-1 on the efficacy of ER agonists for neuroprotection in VSC4.1 motoneurons.
Then, we performed Annexin V-FITC/PI staining followed by flow cytometry to find out the best miR that could maximally potentiate ER agonist (WAY or EST) for prevention of apoptosis in VSC4.1 motoneurons. Our results indicated that transfection with miR-206, miR-17, or miR-7-1 mimic only provided partial neuroprotection to the CI insulted VSC4.1 motoneurons. Also monotherapy with WAY or EST only partially protected the cells from the CI insult. But the sequential miR-7-1 overexpression and WAY or EST treatment most significantly enhanced neuroprotection with the lowest residual apoptotic populations.
Next, we investigated the molecular mechanisms that provided neuroprotection. Our results showed that miR-7-1 overexpression followed by WAY or EST treatment could significantly decrease the Bax:Bcl-2 ratio to put a brake on the trigger mitochondrial pathway of apoptosis in VSC4.1 motoneurons. We performed an extensive search to find out an appropriate target of miR-7-1 in neurons using the miRDB, which indicated that miR-7-1 could decrease expression of CPα1C. CPα1C is the most important structural component of the voltage gated L-type Ca2+ channels (Soldatov, 2012). Thus, transfection of miR-7-1 mimic followed by WAY or EST treatment could inhibit the CI induced Ca2+ influx due to down regulation of CPα1C. This combination therapeutic strategy also increased expression of p-Akt to promote survival signaling in the cells. Previous in vitro studies have shown that the kinase activity of CaMKIIβ can phosphorylate Ser133 in CREB (Dash et al., 1991; Sheng et al., 1991) and phosphorylation of this Ser residue is essential for activation of CREB (Gonzalez and Montminy, 1989; Lee et al., 1990). It is widely known that p-CREB is a transcription factor for expression of Bcl-2. Signaling via CaMKIIβ activation plausibly participated in neuroprotection via upregulation of other downstream neuronal survival markers such as p-CREB and Bcl-2. Thus, our current data indicated some molecular components that resulted in strong neuroprotection in motoneurons. Moreover, combination of miR-7-1 overexpression and WAY or EST treatment significantly blocked activation of cysteine proteases in both the extrinsic and intrinsic pathways of apoptosis. Inhibition of cysteine proteases provides neuroprotection in SCI (Ray et al., 2003, 2011).
An important goal of a neuroprotective therapy is preservation of functionality in the neurons. Transfection with the miR-7-1 mimic followed by EST treatment most effectively preserved the whole cell membrane potential and reversed the loss of mitochondrial membrane potential to provide functional neuroprotection. The efficacy of this combination therapy markedly reduced mitochondrial release of cytochrome c and Smac/Diablo into the cytosol, indicating blockage of activation of caspases in the CI insulted VSC4.1 motoneurons.
In conclusion, our results demonstrated that transfection with miR-7-1 mimic most effectively enhanced efficacy of EST to promote survival mechanisms and inhibit apoptosis for functional neuroprotection in the CI insulted VSC4.1 motoneurons. This study establishes the importance of miR-7-1 in potentiation of efficacy of EST for functional neuroprotection in vitro. This therapeutic strategy can be used in the future to attenuate apoptosis of motoneurons in SCI.
Highlights.
Estrogen receptor agonists upregulated estrogen receptors in VSC4.1 cells.
Estrogen receptor agonists enhanced expression of specific neuroprotective miRs.
miR-7-1 most effectively enhanced neuroprotective functions of estrogen (EST).
Combination of miR-7-1 and EST most significantly decreased Bax:Bcl-2 ratio.
miR-7-1 powered EST for inhibition of apoptosis and preservation of functionality.
Acknowledgments
This work was supported in part by SCIRF-11-002 grant from South Carolina Spinal Cord Injury Research Foundation (Columbia, SC, USA), R01 NS65456 grant from National Institutes of Health (Bethesda, MD, USA), and I0 BX001262 grant from Department of Veterans Affairs (Baltimore, MD, USA).
Abbreviations
- ANOVA
analysis of variance
- CaMKIIβ
Ca2+/calmodulin-dependent protein kinase II beta
- CI
calcium ionophore
- COX-4
cytochrome c oxidase subunit IV
- CPα1C
L-type Ca2+ channel protein alpha 1C
- CREB
cAMP response element binding protein
- DMSO
dimethyl sulfoxide
- ECL
enhanced chemiluminescence
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor beta
- EST
estrogen
- FITC
fluorescein isothiocyanate
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HRP
horseradish peroxidase
- miRDB
microRNA database
- miRs
microRNAs
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- PI
propidiun iodide
- PPT
1,3,5-tris (4-hydroxyphenyl)-4-propyl-1H-pyrazole
- qRT-PCR
quantitative reverse transcription-polymerase chain reaction
- RT-PCR
reverse transcription-polymerase chain reaction
- SBDP
spectrin breakdown product
- SCI
spinal cord injury
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- VSC4.1
ventral spinal cord 4.1
- WAY
Way200070
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Behl C, Skutella T, Lezoualc’h F, Post A, Widmann M, Newton CJ, Holsboer F. Neuroprotection against oxidative stress by estrogens: structure–activity relationship. Mol Pharmacol. 1997;51:535–541. [PubMed] [Google Scholar]
- Breving K, Esquela-Kerscher A. The complexities of miR regulation: mirandering around the rules. Int J Biochem Cell Biol. 2010;42:1316–1329. doi: 10.1016/j.biocel.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Caron-Leslie LA, Evans RB, Cidlowski JA. Bcl-2 inhibits glucocorticoid-induced apoptosis but only partially blocks calcium ionophore or cycloheximide-regulated apoptosis in S49 cells. FASEB J. 1994;8:639–645. doi: 10.1096/fasebj.8.9.8005391. [DOI] [PubMed] [Google Scholar]
- Chakrabarti M, Banik NL, Ray SK. Sequential hTERT knockdown and apigenin treatment inhibited invasion and proliferation and induced apoptosis in human malignant neuroblastoma SK-N-DZ and SK-N-BE2 cells. J Mol Neurosci. 2013;51:187–198. doi: 10.1007/s12031-013-9975-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti M, Khandkar M, Banik NL, Ray SK. Alterations in expression of specific microRNAs by combination of 4-HPR and EGCG inhibited growth of human malignant neuroblastoma cells. Brain Res. 2012;1454:1–13. doi: 10.1016/j.brainres.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordey M, Pike CJ. Neuroprotective properties of selective estrogen receptor agonists in cultured neurons. Brain Res. 2005;1045:217–223. doi: 10.1016/j.brainres.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Crawford GD, Le WD, Smith RG, Xie WJ, Appel SH. A novel N18TG2!mesencephalon cell hybrid expresses properties that suggest a dopaminergic cell line of substantia nigra origin. J Neurosci. 1992;12:3392–3398. doi: 10.1523/JNEUROSCI.12-09-03392.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Banik NL, Ray SK. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer. 2010b;116:164–176. doi: 10.1002/cncr.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, McDowell M, Pava MJ, Smith JA, Reiter RJ, Woodward JJ, Varma AK, Ray SK, Banik NL. The inhibition of apoptosis by melatonin in VSC4.1 motoneurons exposed to oxidative stress, glutamate excitotoxicity, or TNF-α toxicity involves membrane melatonin receptors. J Pineal Res. 2010a;48:157–169. doi: 10.1111/j.1600-079X.2009.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Smith JA, Gibson C, Varma AK, Ray SK, Banik NL. Estrogen receptor agonists and estrogen attenuate TNF-α-induced apoptosis in VSC4.1 motoneurons. J Endocrinol. 2011;208:171–182. doi: 10.1677/JOE-10-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Karl KA, Gohcos MA, Prywes R, Kandel ER. cAMP response element binding protein is activated by Ca2+/calmodulin as well as cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1991;88:5061–5065. doi: 10.1073/pnas.88.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacker SM, Dawson TM, Dawson VL. Understanding microRNAs in neurodegeneration. Nat Rev Neurosci. 2009;10:837–841. doi: 10.1038/nrn2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner B, Lee J, DeSilva TM, Jensen FE, Volpe JJ, Rosenberg PA. 17β-Estradiol protects against hypoxic/ischemic white matter damage in the neonatal rat brain. J Neurosci Res. 2009;87:2078–2086. doi: 10.1002/jnr.22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Grau JW, Crown ED, Ferguson AR, Washburn SN, Hook MA, Miranda RC. Instrumental learning within the spinal cord: underlying mechanisms and implications for recovery after injury. Behav Cogn Neurosci Rev. 2006;5:191–239. doi: 10.1177/1534582306289738. [DOI] [PubMed] [Google Scholar]
- Grau JW, Washburn SN, Hook MA, Ferguson AR, Crown ED, Garcia G, Bolding KA, Miranda RC. Uncontrollable stimulation undermines recovery after spinal cord injury. J Neurotraum. 2004;21:1795–1817. doi: 10.1089/neu.2004.21.1795. [DOI] [PubMed] [Google Scholar]
- Green PS, Simpkins JW. Estrogens and estrogen-like non-feminizing compounds. Their role in the prevention and treatment of Alzheimer’s disease. Ann N Y Acad Sci. 2000;924:93–98. doi: 10.1111/j.1749-6632.2000.tb05566.x. [DOI] [PubMed] [Google Scholar]
- Hook MA, Moreno G, Woller S, Puga D, Hoy K, Jr, Balden R, Grau JW. Intrathecal morphine attenuates recovery of function after a spinal cord injury. J Neurotraum. 2009;26:741–752. doi: 10.1089/neu.2008.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles RG, Moncada S. Nitric oxide as a signal in blood vessels. Trends Biochem Sci. 1992;17:399–402. doi: 10.1016/0968-0004(92)90008-w. [DOI] [PubMed] [Google Scholar]
- Kusuda R, Cadetti F, Ravanelli MI, Sousa TA, Zanon S, De Lucca FL, Lucas G. Differential expression of microRNAs in mouse pain models. Mol Pain. 2011;7:7–17. doi: 10.1186/1744-8069-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GQ, Yun Y, Hoeffler JP, Habener JF. Cyclic AMP responsive transcriptional activation of CREB-327 involves interdependent phosphorylated subdomains. EMBO J. 1990;9:4455–4465. doi: 10.1002/j.1460-2075.1990.tb07896.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li LC, Okino ST, Zhao H, Pookot D, Place RF, Urakami S, Enokida H, Dahiya R. Small dsRNAs induce transcriptional activation in human cells. Proc Natl Acad Sci USA. 2006;103:17337–17342. doi: 10.1073/pnas.0607015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NK, Xu XM. MicroRNA in central nervous system trauma and degenerative disorders. Physiol Genomics. 2011;43:571–580. doi: 10.1152/physiolgenomics.00168.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Martina M, Kilić G, Cherubini E. The effect of intracellular Ca2+ on GABA-activated currents in cerebellar granule cells in culture. J Membr Biol. 1994;142:209–216. doi: 10.1007/BF00234942. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Mitochondria as therapeutic targets of estrogen action in the central nervous system. Curr Drug Targets CNS Neurol Disord. 2004;3:297–313. doi: 10.2174/1568007043337193. [DOI] [PubMed] [Google Scholar]
- Ray SK, Hogan EL, Banik NL. Calpain in the pathophysiology of spinal cord injury: neuroprotection with calpain inhibitors. Brain Res Rev. 2003;42:169–185. doi: 10.1016/s0165-0173(03)00152-8. [DOI] [PubMed] [Google Scholar]
- Ray SK, Samantaray S, Smith JA, Matzelle DD, Das A, Banik NL. Inhibition of cysteine proteases in acute and chronic spinal cord injury. Neurotherapeutics. 2011;8:180–186. doi: 10.1007/s13311-011-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed PW, Lardy HA. A23187: a divalent cation ionophore. J Biol Chem. 1972;247:6970–6977. [PubMed] [Google Scholar]
- Rodriguez-Tarduchy G, Collins M, López-Rivas A. Regulation of apoptosis in interleukin-3-dependent hemopoietic cells by interleukin-3 and calcium ionophores. EMBO J. 1990;9:2997–3002. doi: 10.1002/j.1460-2075.1990.tb07492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugstad JA. MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration. J Cereb Blood Flow Metab. 2010;30:1564–1576. doi: 10.1038/jcbfm.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Thompson MA, Greenberg ME. CREB: A Ca-regulated transcription factor phosphorylated by calmodulin- dependent kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- Smith RG, Alexianu ME, Crawford G, Nyormoi O, Stefani E, Appel SH. Cytotoxicity of immunoglobulins from amyotrophic lateral sclerosis patients on a hybrid motoneuron cell line. Proc Natl Acad Sci USA. 1994;91:3393–3397. doi: 10.1073/pnas.91.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldatov NM. Molecular Determinants of Cav1.2 Calcium Channel Inactivation. ISRN Mol Biol. 2012:10. doi: 10.5402/2012/691341. Article ID 691341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sribnick EA, Ray SK, Nowak MW, Li L, Banik NL. 17β-estradiol attenuates glutamate-induced apoptosis and preserves electrophysiologic function in primary cortical neurons. J Neurosci Res. 2004;76:688–696. doi: 10.1002/jnr.20124. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Wingrave JM, Matzelle DD, Wilford GG, Ray SK, Banik NL. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J Neurosci Res. 2005;82:283–293. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Wingrave JM, Matzelle DD, Ray SK, Banik NL. Estrogen as a neuroprotective agent in the treatment of spinal cord injury. Ann N Y Acad Sci. 2003;993:125–133. doi: 10.1111/j.1749-6632.2003.tb07521.x. [DOI] [PubMed] [Google Scholar]
- Sur P, Sribnick EA, Wingrave JM, Nowak MW, Ray SK, Banik NL. Estrogen attenuates oxidative stress-induced apoptosis in C6 glial cells. Brain Res. 2003;971:178–188. doi: 10.1016/s0006-8993(03)02349-7. [DOI] [PubMed] [Google Scholar]
- Wang X, Sada K, Yanagi S, Yang C, Rezaul K, Yamamura H. Intracellular calcium dependent activation of p72syk in platelets. J Biochem. 1994;116:858–861. doi: 10.1093/oxfordjournals.jbchem.a124607. [DOI] [PubMed] [Google Scholar]
- Wu T-W, Wang JM, Chen S, Brinton RD. 17ESTradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and Bcl-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Yan H, Hong P, Jiang M, Li H. MicroRNAs as potential therapeutics for treating spinal cord injury. Neural Regen Res. 2012;7:1352–1359. doi: 10.3969/j.issn.1673-5374.2012.17.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yune TY, Park HG, Lee JY, Oh TH. Estrogen-induced Bcl-2 expression after spinal cord injury is mediated through phosphoinositide-3-kinase/Akt-dependent CREB activation. J Neurotraum. 2008;25:1121–1131. doi: 10.1089/neu.2008.0544. [DOI] [PubMed] [Google Scholar]
- Zhao L, Chen S, Wang JM, Brinton RD. 17ESTradiol induces Ca2+ influx, dendritic and nuclear Ca2+ rise and subsequent cyclic AMP response element-binding protein activation in hippocampal neurons: a potential initiation mechanism for estrogen neurotrophism. Neuroscience. 2005;132:299–311. doi: 10.1016/j.neuroscience.2004.11.054. [DOI] [PubMed] [Google Scholar]