Abstract
Purpose
Pantoprazole sodium (Protonix) is a proton pump inhibitor (PPI) widely used to treat peptic ulcer and gastroesophageal reflux due to its ability to inhibit gastric acid secretion. Therefore, a large group of the population exposed to total body irradiation (TBI) in the event of a nuclear disaster would be on this or similar medications. We investigated the effect of pantoprazole on TBI-induced lethality in mice.
Methods and Materials
Male CD2F1 mice were exposed to various doses of uniform TBI using a 137Cs irradiator. Pantoprazole was administered by twice daily subcutaneous injection in saline from 4 days before to 5 days after irradiation. Effects on gastric pH, and gastrointestinal (GI) and hematopoietic toxicity were evaluated.
Results
Pantoprazole administration significantly exacerbated 30 day lethality and gastrointestinal toxicity. Median survival after 9.0Gy TBI was reduced from 22 days to 12 days (p=0.006). Pantoprazole adversely effected intestinal crypt survival and mucosal surface area. In contrast, equivalent doses of a histamine type−2(H2) receptor blocker (cimetidine) did not alter TBI-induced lethality.
Conclusion
The adverse effect of pantoprazole on TBI-induced lethality is highly important because of the widespread use of PPI in the general population, as well as use of these drugs for acid suppression in individuals exposed to radiation. Further studies of the mechanisms underlying the adverse effect of PPI after exposure to TBI are clearly warranted. Until results from such studies are available, other acid-suppressing strategies should be preferred in the context of radiation exposure.
Keywords: Pantoprazole, Radiation lethality, Total body irradiation, Proton pump inhibitor, Gastro-intestinal injury, Accidents - radiation, Atom Bomb effects, Intestine, Radiation accidents
INTRODUCTION
Proton pump inhibitors (PPI), H2-receptor antagonists and antacids are commonly used to reduce gastric acid secretion in conditions such as gastroesophageal reflux disease (GERD) and peptic ulcer disease (Palileo and Kaunitz 2011). Studies have shown the superiority of PPI over histamine 2 (H2)-blockers in preventing peptic ulcers (Lai et al 2003) due to their greater ability to suppress gastric acid secretion. PPI are currently used by a large segment of the population often for prolonged periods of time. In fact, PPI are the third most prescribed drugs in the United States with annual sales exceeding $14 billion.
PPI work by binding irreversibly to the H+/K+ ATPase proton pump of gastric parietal cells causing prolonged acid suppression, far longer than their plasma half-life. Restoration of acid production occurs by protein turnover and neutralization of acid-inhibition by reducing agents such as glutathione (Shin and Sachs 2004). While PPI are generally considered safe with negligible side effects, prolonged use is thought to be associated with the development of gastrointestinal carcinoma in patients with Helicobacter pylori infection (Graham and Genta 2008). More recently PPI have been associated with the development of community-associated Clostridium difficile infection in patients not exposed to antibiotics (Chitnis 2013). The main pathway of metabolism of PPI is through hepatic cytochrome P450 (CYP) 2C19 enzyme system. Genetic CYP2C19 deficiency is associated with prolonged elimination half-life up to 2- to 4-fold (Baker 2006). PPI can also directly inhibit the enzyme CYP2C19 (Zyvaga 2012). Proton pump inhibitors are commonly also considered part of the supportive therapy regimen for radiation-induced side effects such as gastrointestinal ulceration (Steer and Harper 2002) and esophagitis (Berkey 2010).
Nuclear disaster, such as a massive radiation leak or the result of a nuclear device, is a present threat throughout the world. Because so much of the population is routinely taking PPI, it is critical to determine how these drugs may affect outcome in the face of a radiation disaster. We have determined in a murine model that PPI. in contrast to what is assumed, increase the lethality after exposure to total body irradiation (TBI). These experiments bring into question the safety of PPI in the general population and first responders in the event of a nuclear catastrophe.
METHODS AND MATERIALS
Chemicals
Pantoprazole sodium (Protonix) was purchased from Pfizer (formerly Wyeth Pharmaceuticals, Philadelphia, PA, USA). All other chemicals, unless otherwise mentioned, were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Animals
Animal handling and experimental protocols for this study were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Central Arkansas Veterans Healthcare System (CAVHS) and University of Arkansas for Medical Sciences. Male CD2F1 mice (Harlan Sprague Dawley, Indianapolis, IN, USA) were used in this study. Mice were housed in conventional cages with controlled temperature and humidity under a 12–12 hr day-night light cycle and had free access to water and chow (Harlan Teklad laboratory diet 7012, Purina Mills, St. Louis, MO, USA) during the entire period of handling.
Mice of 6–7 weeks of age and weighing 22–25 g were selected at the time of initiation of experiments. All experiments were performed with different doses of single TBI. Groups comprised of 4 to 8 mice were sacrificed humanely at set time points (0, 3.5, 7, 14 and 21 days post TBI) as per the experimental requirements. In lethality studies, mice were observed twice daily during the experimental period and those appearing clearly moribund (more than 25% weight loss, lethargy, huddling, shivering, hunched posture or vocalization) were euthanized immediately by Co2 asphyxiation followed by cervical dislocation, in accordance with American Veterinary Medical Association (AVMA) 2007 Guidelines on Euthanasia. Animals in the lethality experiments that survived to 30 days were euthanized on the next day.
Irradiation and Dosimetry
Un-anesthetized mice were irradiated in a Shepherd Mark I model−25 137Cs irradiator (J. L. Shepherd & Associates, San Fernando, CA, USA). Mice were exposed to uniform TBI at a dose rate of 1.35 Gy per minute, after correction for decay.
Pantoprazole and Cimetidine Treatment
Pantoprazole was administered twice daily as a subcutaneous injection in physiological saline at a dose of 16 mg/kg body weight, four days before through five days after irradiation. This dose has previously been shown to raise the gastric pH to 4–5, consistent with the results of typical oral treatment in humans (Stiefel 2006). Cimetidine, a histamine H2-receptor antagonist used as a reference control, was administered through oral gavage at a dose of 50mg/Kg body weight, similar to the pantoprazole schedule. Vehicle (control) group received physiological saline alone as subcutaneous injection.
Gastric Acidity
Mice treated with pantoprazole, cimetidine and physiological saline (vehicle) for the same period as the TBI groups were fasted overnight. Mice were anesthetized with isoflurane vapor, gastric content exposed and acidity measured with narrow range pH strips.
Survival Analysis
Post-irradiation survival analysis with and without PPI treatment was performed by exposing mice to graded doses of TBI or 9.0 Gy. The mice were monitored up to 30 days following TBI, and the number of dead/moribund mice recorded twice daily.
Intestinal Mucosal Surface Area
Mice were sacrificed exactly at 3.5 day post TBI (9.0 Gy) and a segment of proximal jejunum extracted, fixed and stained with hematoxylin and eosin. Using a projection/cycloid method as described previously by our group (Langberg et al 1996), mucosal surface area was measured.
Intestinal crypt colony
The surviving fraction of intestinal crypt colonies was measured in proximal jejunum (Withers and Elkind 1970). Mice were killed exactly 3.5 days after TBI (0, 8, 10, 13 and 16 Gy), and segments of proximal jejunum were obtained, fixed, embedded so as to obtain four transverse sections per specimen, cut at 3–5 µm, and stained with hematoxylin and eosin. A crypt containing 10 or more adjacent chromophilic non-Paneth cells was counted as a surviving crypt. The average of four circumferences was considered as a single value per mouse for statistical purposes.
Plasma Citrulline
Blood samples were collected exactly at 3.5 days post TBI at different radiation doses. Samples were centrifuged at 12,000×g for 10 minutes and plasma collected. Citrulline concentrations were determined using a high-throughput hydrophilic interaction liquid chromatography (HILIC)-MS/MS method, as previously described (Gupta et al 2011).
Hematological Analysis
Whole blood was collected via retro-orbital bleeding using heparinized micro-hematocrit capillary tubes into Ethylenediaminetetraacetic acid (EDTA) coated microcentrifuge tubes at 0, 3.5, 7, 14 and 21 days after TBI (9.0 Gy). White blood cell (WBC), red blood cell (RBC) and platelet count was measured using a veterinary hemocytometer (Hematrue System, Heska Corporation, Loveland, CO, USA) according to the manufacturer’s instructions.
Spleen colonies
Spleens were collected at day 12 post TBI at 9.0 Gy and fixed in Bouin’s solution. Spleen colonies were clearly visible as yellowish nodules against a dark, smooth background and were counted by two independent observers under magnification.
Statistical Methods
Statistical analyses were performed Prism Graphpad 5.0 (La Jolla, CA, USA). Data are presented as mean±Standard error of the mean (SEM), except for survival analysis. Two-tailed t-tests were used throughout at P<0.05. Survival curves were constructed using the Kaplan-Meier method and were compared using the log-rank test. Survival curves for the crypt colony assay were compared using regression analysis with radiation dose and treatment group as independent variables.
RESULTS
Pantoprazole and Gastric pH
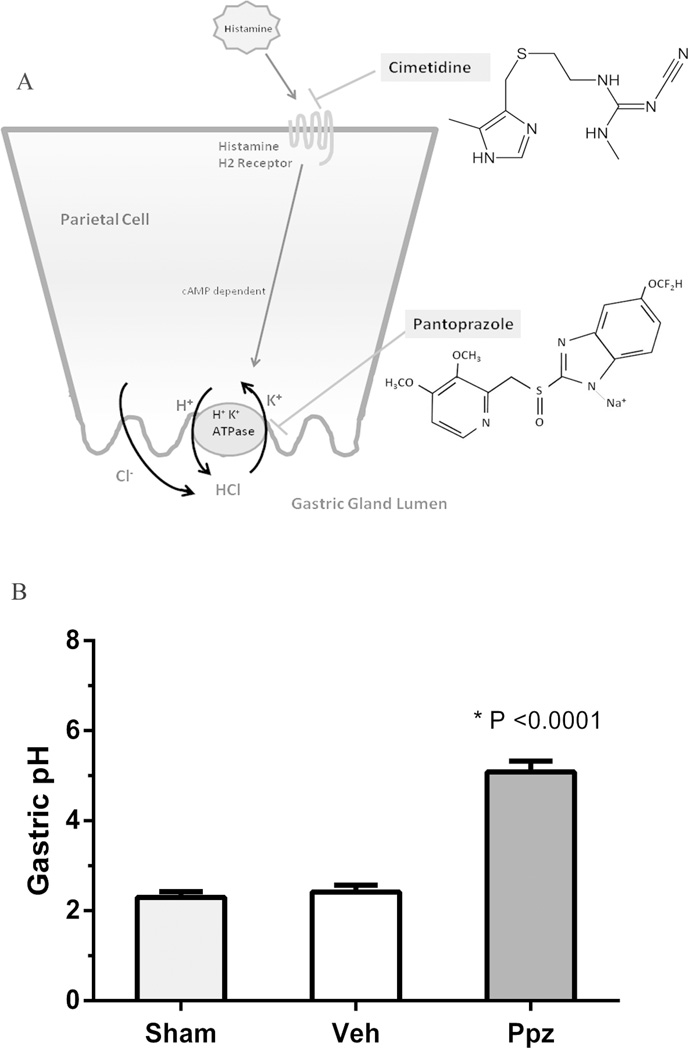
The ability of pantoprazole to suppress gastric acid was evaluated after twice-daily administration for 10 days. A cartoon of the mechanism of acid suppression by PPI and H2 receptor blockers is shown in Figure 1A. Significant elevation in gastric pH was observed post-pantoprazole treatment (Figure 1B). Mean pH of the stomach was raised to 5.0 by pantoprazole compared with no change (pH mean 2.4) in the control animals (p<0.0001). However, mean pH was not significantly elevated by cimetidine (pH mean 2.9) compared to controls. Mean pH was significantly elevated by pantoprazole compared to cimetidine (p<0.0001).
Figure 1.
Mechanism of action of pantoprazole (Proton pump inhibitor) and cimetidine (H2RA) in suppression of gastric acidity (A). Effect of pantoprazole (Ppz) treatment on gastric acidity in mice (B). Mice were treated with twice daily subcutaneous injection of Ppz at 16 mg/kg body weight for 10 days before gastric pH measurement. Ppz treatment elevated gastric pH significantly compared to vehicle and untreated. Values are displayed as mean±SEM of each group. Ppz = Pantoprazole treated, Veh = Vehicle (saline) treated and Sham = Untreated. n = 6 for each group
Survival Analysis
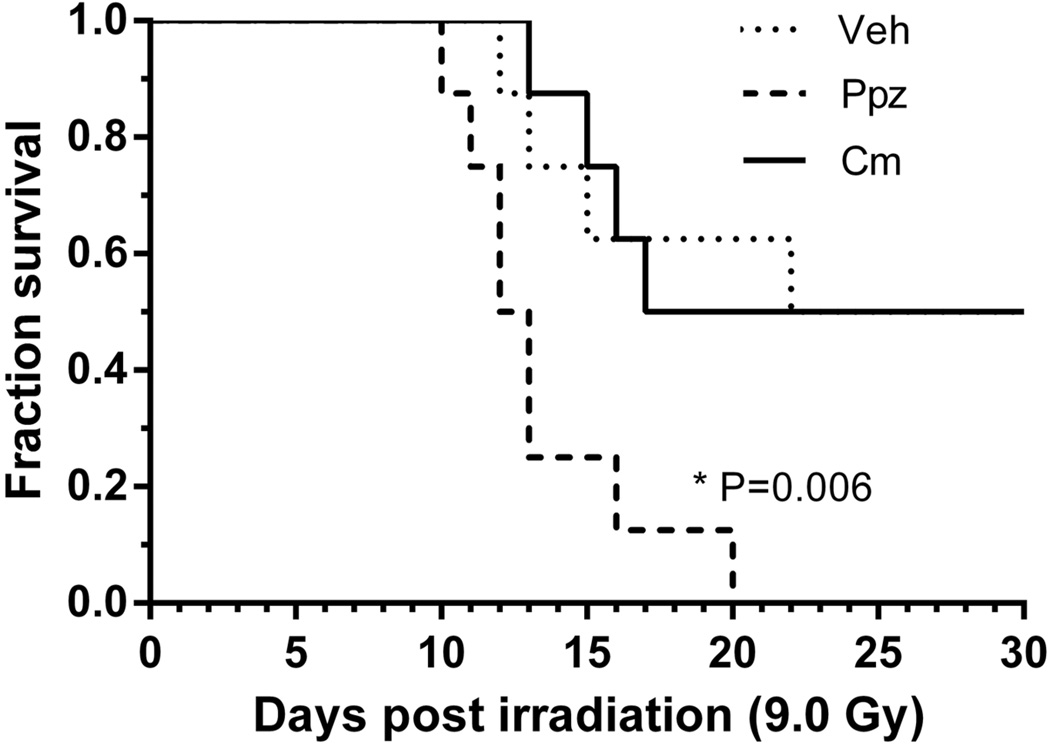
The lethal dose of 50% of animals at 30 days post-TBI (LD50/30) for CD2F1 mice was assessed as 9.0 Gy from our previous studies and was selected as the radiation dose for evaluation of the influence of pantoprazole on TBI lethality. Cimetidine was used as a reference control to evaluate the effect of an alternative antacid on survival (Figure 2). Survival analysis showed no significant difference between control and cimetidine-treated mice after TBI, while pantoprazole treatment resulted in significant mortality (P=0.006). Therefore, enhancement of radiation lethality by pantoprazole appears to be via a pH-dependent mechanism.
Figure 2.
Effect of pantoprazole (Ppz) treatment (16 mg/kg bodyweight, s.c) and Cimetidine (Cm) treatment (50 mg/kg body weight, oral) was assayed on 30 day survival following 9 Gy TBI. Kaplan-Meier analysis show a significant difference (P=0.006) following Ppz treatment while Cm treatment showed no significant difference compared to untreated (Sham). n = 8 for each group
Effect on Gastro-intestinal Injury
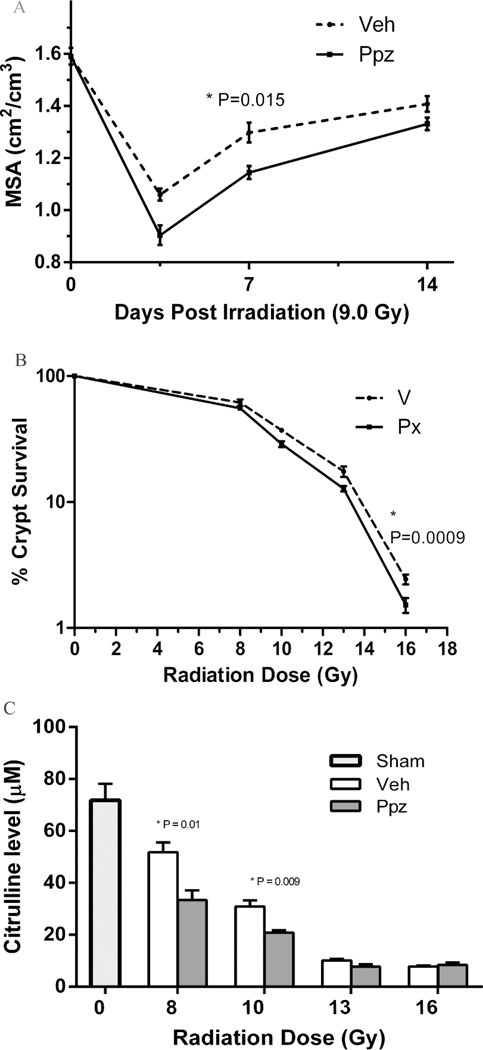
Effect of pantoprazole on radiation induced gut damage was assessed by evaluation of intestinal mucosal surface area on day 3.5, 7 and 14, and surviving intestinal crypt colony and plasma citrulline level at exactly 3.5 days post TBI. Pantoprazole treatment significantly increased mucosal erosion (Figure 3A) on day 3.5 and 7 (p = 0.015) as compared to vehicle-treated irradiated animals. Crypt colony survival after TBI was significantly reduced by pantoprazole treatment (p=0.0009) as compared to vehicle treated mice (Figure 3B). Plasma citrulline levels were significantly reduced after 8 Gy and 10 Gy of TBI (p = 0.01 and p= 0.009) in pantoprazole treated groups as compared to untreated irradiated animals (Figure 3C).
Figure 3.
Effect of pantoprazole (Ppz) treatment on gastro-intestinal injury parameters post 9 Gy TBI. Intestinal mucosal surface area show significant difference following Ppz treatment on day 3.5 and 7 (P = 0.015) as compared to untreated (A). Intestinal crypt colony survival show significant decrease (P = 0.0009) in count following Ppz treatment between 8 and 16 Gy TBI (B). Plasma citrulline levels show significant decrease in Ppz treated groups at 8 and 10 Gy TBI (P = 0.0132 and P = 0.009) when compared to untreated. Values are expressed as mean±SEM of each group. n = 8 for each group
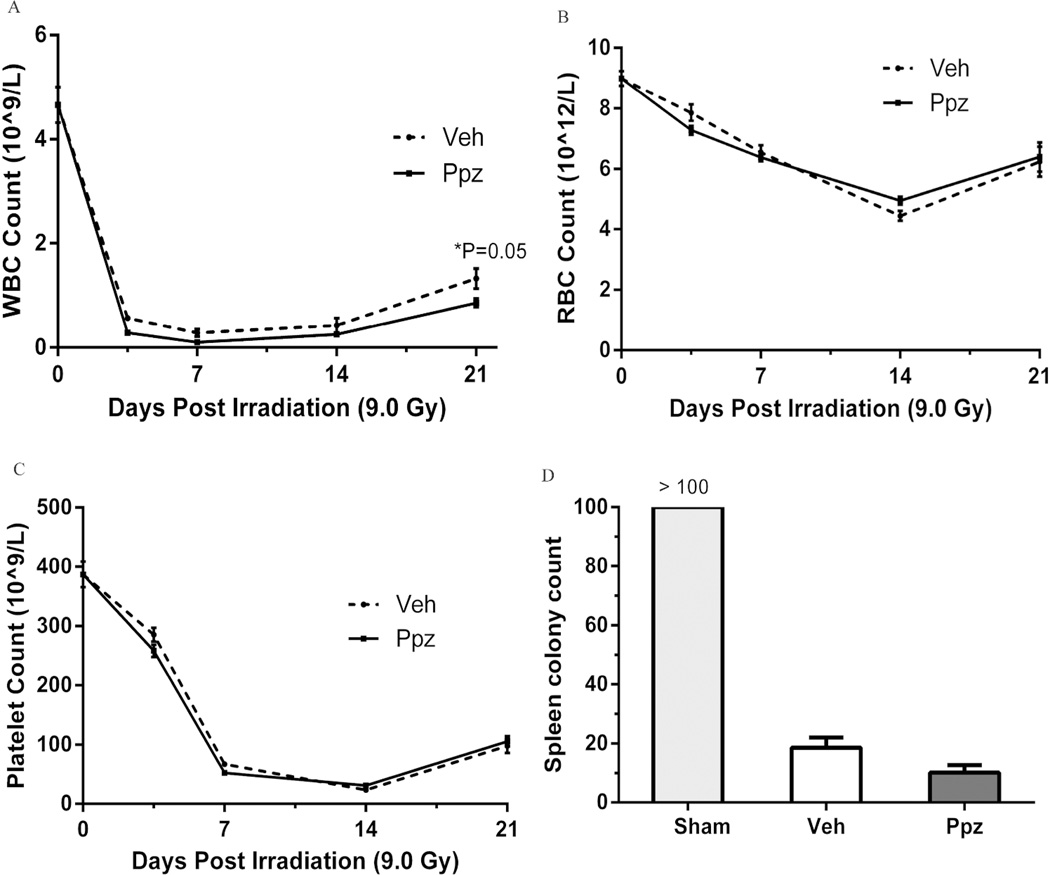
Effect on Hematopoietic Damage
Pantoprazole treatment demonstrated a non-significant reduction in WBC count at 3.5, 7 and 14 day but a significant decline on day 21 (P=0.05) as compared to untreated TBI groups (Figure 4A). RBC (Figure 4B) and platelet (Figure 4C) counts showed no significant difference following pantoprazole treatment. Surviving spleen colonies were also reduced, although non-significantly, following pantoprazole treatment (Figure 4D).
Figure 4.
Effect of pantoprazole (ppz) treatment on hematopoietic toxicity parameters post 9 Gy TBI. Ppz treatment decrease WBC count non-significantly on the early time points and show a significant decrease (P = 0.05) on day 21 (A). RBC and platelet counts show no significant difference following Ppz treatment on all time points (B and C). PPz treatment resulted in a non-significant decrease in surviving spleen colony count on day 12 (D). Values are expressed as mean±SEM of each group. n = 8 for each group.
DISCUSSION
This study demonstrates clear evidence of potentiation of radiation lethality by administration of pantoprazole during total body irradiation (TBI). The increase in lethality appears to be related to gastric pH suppression and occurs with increased GI mucosal damage. These findings are significant because of the widespread use of Proton Pump Inhibitors and thus a large segment of the population may be adversely affected in the event of a nuclear catastrophe.
Gastric proton pumps are involved in the key regulation of gastric acid production and belong to the ion transporter family using the adenosine triphosphate (ATP) phosphorylation-dephosphorylation reaction. Non-activated proton pumps are located within the cytoplasm of gastric parietal cells and, when activated, migrate to the lumen to exchange one H+ ion for a K+ ion. One CI− is also secreted at the same time to form an HCl molecule, contributing to the gastric acidity (Figure 1A). PPI are converted to their active sulfonamide derivatives in the acidic environment of gastric parietal cells and form irreversible disulfide bonds with cysteine residues of the proton pump, leading to pump inactivation (Sachs and Shin 2004).
A therapeutic benefit is observed with PPI therapy for GERD, peptic ulcers and nonsteroidal anti-inflammatory drug (NSAID)-induced gastritis. PPI have also been recommended for relief of radiotherapy-induce gastritis (Sfakianakis and Sfakianaki 2007). Our present study suggests there may be adverse effects of using PPI when the intestinal tract is within the radiation field.
The effect of pantoprazole on radiation lethality may be related to the increase in gastric pH. When pantoprazole was compared with a drug that produces significantly less acid suppression by a different mechanism (cimetidine), the increased mortality was seen only with the PPI, not with the H-2 blocker. Cimetidine has a rapid onset of action but a short half-life, with detectable changes a pH noted for only up to eight hours after oral administration (Reilly et al 1998). Pantoprazole irreversibly binds to the proton pump and therefore alters pH for much longer than its plasma half-life (Shin and Sachs 2004).
We propose three mechanisms which may be contributing to the increased death from radiation seen with pantoprazole: 1) there is an increase in intestinal bacterial content in both the small and large bowel, 2) there is an increase in bacterial translocation due to a combination of mucosal injury from the radiation and bacterial transport as a direct effect of inhibition of the proton pump and 3) there is an inhibition of the immune system mitigating the effect of bacteria that have entered the bloodstream.
Proton pump inhibitors have been shown to lead to intestinal bacterial overgrowth in animal and human studies (Lewis el al. 1996, Fried et al. 1994). In a randomized human trial, a four week treatment with the PPI omeprazole resulted in significantly higher bacterial overgrowth in the duodenum than did cimetidine (Thorens et al. 1996). Gastric acidity plays a vital protective role in eliminating exogenous acid-sensitive bacteria in the stomach. This process is controlled by the parietal cells secreting hydrochloric acid to maintain gastric pH below 2. When the gastric pH is increased above 4, up to 50% of ingested bacteria will survive in the gastric environment (Tennant et al. 2008). Pantoprazole at 40 mg per day can result in a 24-hour mean gastric pH of 4 after one week of therapy (Tutuian et al. 2002). Elevation of gastric pH to 5 was seen in our experiments after 10 days of therapy with pantoprazole (figure 1B). Lower GI tract bacteria overgrowth also occurs when the gastric pH is raised (Kanno et al. 2009). Therefore, animals treated with PPI will have higher colonic bacteria when faced with total body irradiation.
Bacterial translocation may be increased with proton pump inhibitor use secondary to several changes associated with this therapy. Gastric motility has been shown to be reduced following proton pump inhibitor therapy (Parkman et al. 1998), and the viscosity of gastric mucus is also reduced with this therapy (Goddard and Spiller 1996). These factors may increase the mucosal damage that occurs with ionizing radiation. Animal studies have shown that omeprazole increases the permeability of the GI mucosa probably by widening the intraepithelial spacing (Hopkins et al. 2002). Other studies have confirmed increased bacterial translocation when the gastric pH is elevated (Dinsmore et al. 1997). Our present study shows clearly that there is increased mucosal damage in pantoprazole-treated animals. Figure 3 demonstrates by three variables the extent of small bowel injury: 1) there is a significant reduction in mucosal surface area, 2) there is a reduction in the percent of crypt survival, and 3) there is a reduction in serum citrulline levels in the proton pump inhibitor treated animals. We believe that a combination of structural damage of the mucosa and increase in gastric permeability directly from the proton pump inhibitor has a significant impact on bacterial translocation.
PPI have been shown to have an inhibitory effect on neutrophil function (Zedtwitz-Liebenstein et al. 2002, Wendall 1992). These compounds cause in inhibition in the expression of adhesion molecules involved in neutrophil-endothelial cell interactions (Yoshida et al. 2000) resulting in a diminished inflammatory response. Critical neutrophil functions, such as phagocytosis and phagolysosomal acidification, are also inhibited by PPI (Agastya et al. 2000). PPI similarly inhibit chemotaxis, superoxide generation and degranulation of neutrophils (Wendall 1992, Suzuki et al. 1996). In our experiments, only modest effects on WBC, RBC, platelet count and surviving spleen colonies were seen after PPI exposure and TBI (Figure 4). However, if the ability of the neutrophils to mitigate translocated bacteria is impaired, then the animal is at a greater risk for lethality due to gut-associated sepsis.
We conclude that pantoprazole administration along with TBI lead to increased radiation lethality. This finding assumes significance due to the widespread use of PPI in the general population and common use as a supportive treatment during radiotherapy. Further elucidation of the mechanism of the lethality potentiation by PPI needs to occur. At present, we would recommend use of non-PPI therapies in treating radiation exposure.
Acknowledgments
Financial support provided by NIH 1 RC1 A1078515-01 (AFB), U19 AI67798 (MH-J) and Veterans Administration (MH-J).
Footnotes
DECLARATION OF INTEREST
The authors report no declarations of interest.
REFERENCES
- Agastya G, West BC, Callahan JM. Omeprazole inhibits phagocytosis and acidification of phagolysosomes of norml human neutrophils in vitro. Immunopharmacology & Immunotoxicology. 2000;22:357–372. doi: 10.3109/08923970009016425. [DOI] [PubMed] [Google Scholar]
- Baker DE. Intravenous proton pump inhibitors. Reviews in Gastroenterological Disordorders. 2006;6:22–34. [PubMed] [Google Scholar]
- Berkey FJ. Managing the adverse effects of radiation therapy. American Family Physician. 2010;82:381–388. 394. [PubMed] [Google Scholar]
- Chitnis AS, Holzbauer SM, Belflower RM, Winston LG, Bamberg WM, Lyons C, Farley MM, Dumyati GK, Wilson LE, Baldavs ZG, Dunn JR, Gould H, MacCannell DR, Gerding DN, McDonald LC, Lessa FC. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA. 2013;173:1359–1367. doi: 10.1001/jamainternmed.2013.7056. [DOI] [PubMed] [Google Scholar]
- Dinsmore JE, Jackson RJ, Smith SD. The protective role of gastric acidity in neonatal bacterial translocation. Journal of Pediatric Surgery. 1997;32:1014–1016. doi: 10.1016/s0022-3468(97)90389-4. [DOI] [PubMed] [Google Scholar]
- Fried M, Siegrist H, Frei R, Froehlich F, Duroux P, Thorens J, Blum A, Bille J, Gonvers JJ, Gyr K. Duodenal bacterial overgrowth during treatment in outpatients with omeprazole. Gut. 1994;35:23–26. doi: 10.1136/gut.35.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard AF, Spiller RC. The effect of omeprazole on gastric juice viscosity, pH and bacterial counts. Alimentary Pharmacology & Therapeutics. 1996;10:105–109. doi: 10.1111/j.1365-2036.1996.tb00183.x. [DOI] [PubMed] [Google Scholar]
- Graham DY, Genta RM. Long-term proton pump inhibitor use and gastrointestinal cancer. Current Gastroenterology Reports. 2008;10:543–547. doi: 10.1007/s11894-008-0100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PK, Brown J, Biju PG, Thaden J, Deutz NE, Kumar S, Hauer-Jensen M, Hendrickson HP. Development of high-throughput HILIC-MS/MS methodology for plasma citrulline determination in multiple species. Analytical Methods. 2011;3:1759–1768. [Google Scholar]
- Hopkins AM, McDonnell C, Breslin NP, O'Morain CA, Baird AW. Omeprazole increases permeability across isolated rat gastric mucosa pre-treated with an acid secretagogue. Journal of Pharmacy & Pharmacology. 2002;54:341–347. doi: 10.1211/0022357021778583. [DOI] [PubMed] [Google Scholar]
- Kanno T, Matsuki T, Oka M, Utsonumiya H, Inada K, Magari H, Inoue I, Maekita, Ueda K, Enomoto S, Iguchi M, Yanaoka K, amai H, Akimoto S, Nomoto K, tanaka R, Ichinose M. Gastric acid reduction leads to an alteration in lower intestinal microflora. Biochemical & Biophysical Research Communications. 2009;381:666–670. doi: 10.1016/j.bbrc.2009.02.109. [DOI] [PubMed] [Google Scholar]
- Lai KC, Lam SK, Chu KM, Hue WM, Kwok KR, Wong BC, Hu HC, Wong WM, Chan OO, Chan CK. Lansoprazole reduces ulcer relapse after eradication of Helicobacter pylori in nonsteroidal anti-inflammatory drug users-a randomized trial. Alimentary Pharmacology & Therapeutics. 2003;18:829–836. doi: 10.1046/j.1365-2036.2003.01762.x. [DOI] [PubMed] [Google Scholar]
- Langberg CW, Sauer T, Reitan JB, Hauer-Jensen M. Relationship between intestinal fibrosis and histopathologic and morphometric changes in consequential and late radiation enteropathy. Acta Oncologica. 1996;35:81–87. doi: 10.3109/02841869609098484. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Franco S, Young G, O'Keefe SJ. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Alimentary Pharmacology & Therapeutics. 1996;10:557–561. doi: 10.1046/j.1365-2036.1996.d01-506.x. [DOI] [PubMed] [Google Scholar]
- Palileo C, Kaunitz JD. Gastrointestinal defense mechanisms. Current Opinion in Gastroenterology. 2011;27:543–548. doi: 10.1097/MOG.0b013e32834b3fcb. [DOI] [PubMed] [Google Scholar]
- Parkman HP, Urban JL, Knight LC, Brown KL, Trate DM, Miller MA, Maurer AH, Fisher RS. Effect of gastric acid suppressants on human gastric motility. Gut. 1998;42:243–250. doi: 10.1136/gut.42.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly TG, Grimley CE, Usselmann B, Cottrel J, Mann SG, Raskin S, Nwokolo CU. Low-dose famotidine and effervescent cimetidine in healthy subjects: a placebo-controlled overnight pH study. Alimentary Phamacology & Therapeutics. 1998;12:469–474. doi: 10.1046/j.1365-2036.1998.00323.x. [DOI] [PubMed] [Google Scholar]
- Sachs G, Shin JM. The basis of differentiation of PPI. Drugs of Today (Barcelona, Spain ) 2004;40(Suppl A):9–14. [PubMed] [Google Scholar]
- Sfakianakis G, Sfakianaki E. The sodium-iodine symporter and the proton-pump inhibitors in -related to the side effects of- the treatment of thyroid cancer with iodine-131. Hellenic Journal of Nuclear Medicine. 2007;10:2–5. [PubMed] [Google Scholar]
- Shin JM, Sachs G. Differences in binding properties of two proton pump inhibitors on the gastric H+,K+-ATPase in vivo. Biochemical Pharmacology. 2004;68:2117–2127. doi: 10.1016/j.bcp.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Steer CB, Harper PG. Gastro-oesophageal complications in patients receiving cancer therapy: the role of proton pump inhibitors. European Journal of Gastroenterology & Hepatology. 2002;14(Suppl 1):S17–S21. [PubMed] [Google Scholar]
- Stiefel U, Rao A, Pultz MJ, Jump RLP, Aron DC, Donskey CJ. Suppression of gastric acid production by proton pump inhibitor treatment facilitates colonization of the large intestine by Vancomycin-resistant Enterococcus spp. and Klebsiella pneumoniae in Clindamycin-treated mice. Antimicrobial Agents and Chemotherapy. 2006;50:3905–3907. doi: 10.1128/AAC.00522-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Mori M, Miura S, Suematsu M, Fukumura D, Kimura H, Ishii H. Omeprazole attenuates oxygen-derived free radical production from human neutrophils. Free Radical Biology & Medicine. 1996;21:727–731. doi: 10.1016/0891-5849(96)00180-3. [DOI] [PubMed] [Google Scholar]
- Tennant SM, Hartland EL, Phumoonna T, Lyras D, Rood JI, Robins-Browne RM, van Driel IR. Influence of gastric acid on susceptibility to infection with ingested bacterial pathogens. Infection & Immunity. 2008;76:639–645. doi: 10.1128/IAI.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens J, Froehlich F, Schwizer W, Saraga E, Bille J, Gyr K, Duroux P, Nicolet M, Pignatelli B, Blum AL, Gonvers JJ, Fried M. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut. 1996;39:54–59. doi: 10.1136/gut.39.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutuian R, Katz PO, Bochenek W, Castell DO. Dose-dependent control of intragastric pH by pantoprazole, 10, 20 or 40 mg, in healthy volunteers. Alimentary Pharmacology & Therapeutics. 2002;16:829–836. doi: 10.1046/j.1365-2036.2002.01232.x. [DOI] [PubMed] [Google Scholar]
- Wendall JH. Effects of omeprazole on neutrophil chemotaxis, super oxide production, degranulation, and translocation of cytochrome b-245. Gut. 1992;33:617–621. doi: 10.1136/gut.33.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers HR, Elkind MM. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. International Journal of Radiation Biology & Related Studies in Physics, Chemistry & Medicine. 1970;17:261–267. doi: 10.1080/09553007014550291. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Yoshikawa T, Tanaka Y, Fujita N, Kassai K, Naito Y, Kondo M. A new mechanism for anti-inflammatory actions of proton pump inhibitors - inhibitory effects on neutrophilendothelial cell interactions. Alimentary Pharmacology & Therapeutics. 2000;14(Suppl 1):74–81. doi: 10.1046/j.1365-2036.2000.014s1074.x. [DOI] [PubMed] [Google Scholar]
- Zedtwitz-Liebenstein K, Wenisch C, Patruta S, Parschalk B, Daxbock F, Graninger W. Omeprazole treatment diminishes intra- and extracellular neutrophil reactive oxygen production and bactericidal activity. Critical Care Medicine. 2002;30:1118–1122. doi: 10.1097/00003246-200205000-00026. [DOI] [PubMed] [Google Scholar]
- Zvyaga T, Chang SY, Chen CYang Z, Vuppugalla R, Hurley J, Thorndike D, Wagner A, Chimalakonda A, Rodrigues AD. Evaluation of six proton pump inhibitors as inhibitors of various human cytochromes P450: focus on cytochrome P450 2C19. Drug Metabolism & Disposition. 2012;40:1698–1711. doi: 10.1124/dmd.112.045575. [DOI] [PubMed] [Google Scholar]