Abstract
Melanoma is the most lethal form of human sk in cancer. However, only limited chemotherapy is currently available for the metastatic stage of the disease. Since chemotherapy, radiation and sodium arsenite treatment operate mainly through induction of the intrinsic mitochondrial pathway, a strongly decreased mitochondrial function in metastatic melanoma cells, could be responsible for low efficacy of the conventional therapy of melanoma. Another feature of metastatic melanoma cells is their proinflammatory phenotype, linked to endogenous expression of the inflammatory cytokines, such as TNFα IL6 and IL8, their receptors, and constitutive NF-κB- and STAT3-dependent gene expression, including cyclooxygenase-2 (PTGS2/COX2). In the present study, we treated melanoma cells with immunological (monoclonal antibody against TNFα or IL6), pharmacological (small molecular inhibitors of IKKβ-NF-κB and JAK2-STAT3) or genetic (specific RNAi for COX-2) agents that suppressed the inflammatory response in combination with induction of apoptosis via TRAIL. As a result of these combined treatments, exogenous TRAIL via interactions with TRAIL-R2/R1 strongly increased levels of apoptosis in resistant melanoma cells. The present study provides new understanding of the regulation of TRAIL-mediated apoptosis inmelanoma and will serve as the foundation for the potential development of a novel approach for a therapy of resistant melanomas.
Keywords: MELANOMA, NF-κB, STAT3, COX-2, TRAIL, APOPTOSIS
Scientific observations indicate that the incidence of melanoma has significantly increased over the last 40 years in the USA and worldwide, especially among young Caucasian women. The probability of an American developing melanoma jumped from 1 in 1,500 in 1960, to 1 in 68 in 2000, and is projected to increase to 1 in 50 by the year 2010 [Rigel et al., 1996; Rigel, 2002]. In the USA approximately 60,000 new cases were diagnosed and 8,420 deaths occurred in 2008 (ACS, 2008). To date, the most effective singleagent chemotherapies for melanoma treatment are the alkylating drugs dacarbazine and temozolomide [Gogas et al., 2007]. However, advanced melanomas respond poorly to chemo- and radiotherapy and no effective therapy exists to inhibit the metastatic spread of this cancer [Perlis and Herlyn, 2004; Chin et al., 2006]. Numerous epidemiological studies have assessed risk factors associated with melanomas. Even though UV exposure has been identified as one of the major risk factors, the general etiology of melanoma remains unclear.
The tumor-promoting effects of inflammation, which could be a result of microbial infection, strong physical or chemical injury, and obesity, are now widely recognized [Coussens and Werb, 2002; Karin et al., 2006]. The inflammatory cytokines, TNFα, IL1β IL6, and IL8, produced by the tumor microenvironment affect the development and progression of the early stage melanomas in vivo by regulating the expression of specific genes, which controls proliferation and cell survival. In addition, as a result of Darwinian selection, metastatic cancer cells (including melanomas) also acquired the proinflammatory phenotype that was linked with endogenous NF-κB-directed gene expression of proinflammatory cytokines, their corresponding receptors and, finally, with constitutive expression and strong enzymatic activity of cycloox-ygenase-2 (COX-2), one of the critical enzymes involved in the inflammatory response directing prostaglandin synthesis [Subbaramaiah and Dannenberg, 2003; Karin, 2009]. There is also a link between chronic inflammation and deficiency of mitochondrial function, which was observed during cancer progression [Pelicano et al., 2006], as well as in neurodegenerative diseases [Beal, 2004]. A deficiency of normal mitochondrial respiratory function, but increased production of reactive oxygen species are also known to be one of the characteristic features of the inflammatory response [Alexeyev et al., 2004] and might be frequently found in cancer cells, which have a tendency to change their energetic metabolisms from oxidative phosphorylation to oxidative glycolysis (“Warburg effect”) [Pelicano et al., 2006]. This critical link between inflammation and tumorigenesis presents further challenges for treatment of melanoma as establishing a connection between proinflammatory phenotype and suppression of apoptosis in metastatic melanoma cells.
Since the identification of TRAIL in 1995, this cytokine has been intensively investigated, due to its strong and specific antitumor effects through induction of apoptosis. In contrast to Fas, surface expression of TRAIL-Receptors is mostly restricted to cancer, but not to normal cells. However, many melanoma cell lines demonstrated resistance to TRAIL based on numerous protective mechanisms including downregulation of the endogenous death receptor gene expression and strong upregulation of antiapoptotic protein expression [Schaefer et al., 2007; Ashkenazi et al., 2008]. Furthermore, the genetic background of such resistance may include upregulation of gene expression of growth factors, cytokines, and their corresponding receptors controlling general cell survival and antiapoptotic activity.
The main goal of the present study is to further elucidate the mechanism of TRAIL-resistance and to increase sensitivity to TRAIL-induced apoptosis in metastatic melanoma cells by suppression of their proinflammatory response. This study is ultimately aimed to translate the results to the clinic, where this investigation could lead to much needed improvement of melanoma therapy. Our former investigations demonstrated that ionizing radiation upregulated death receptor (FAS, TRAIL-Receptor1/DR4, and TRAIL-Receptor2/DR5) total and surface expression in human melanoma cells and increased TRAIL/TRAIL-R-mediated apoptosis [Ivanov et al., 2007]. In contrast, numerous investigations have indicated profound antiapoptotic effects of active STAT3 in different types of cancer cells, including melanomas [Ivanov et al., 2001; Kusaba et al., 2007]. In the present study, we focused attention on pharmacological, immunological or genetic inhibition of the TNFα/TNFR–NF-κB–IL-6/IL-6R–STAT3 signaling cascade [Grivennikov and Karin, 2008] to further suppress expression of its target proteins, including antiapoptotic cFLIP, Bcl-xL, Mcl-1, Survivin, and COX-2 [Kreuz et al., 2001; Ivanov et al., 2003; Subbaramaiah and Dannenberg, 2003; Fulda and Debatin, 2006], and protein regulators of cell proliferation, including Cyclin D1 [Darnell, 2002; Leslie et al., 2006]. We expect that as a result of such treatment, exogenous TRAIL/TRAIL-R interactions will induce apoptosis in death-ligand-resistant melanoma cells. Furthermore, we anticipate that suppression of COX-2, which is highly expressed in metastatic melanoma cells [Ivanov and Hei, 2006a], will be especially important for upregulation of TRAIL-induced apoptosis.
MATERIALS AND METHODS
MATERIALS
Human Killer-TRAIL was purchased from Alexis. PI3K inhibitor LY294002, IKK inhibitor BMS-345541, STAT3 inhibitor-6 S3I-201 (also known as NSC 74859) and ATM inhibitor KU-55933 were purchased from Calbiochem.
CELL LINES
Normal human fibroblasts TIG-3 were obtained from the Health Science Research Resource Bank (Osaka, Japan). TIG-3 fibroblasts and human melanoma cell lines LU1205 (also known as 12051u), WM9, WM35, WM793, WM852 [Satyamoorthy et al., 1997], A375, FEMX, and HHMSX were maintained in DMEM medium supplemented with 10% fetal bovine serum (FBS), L-glutamine, and antibiotics.
FACS ANALYSIS OF DR5 AND FAS LEVELS
Surface levels of DR4, DR5, and Fas were determined by staining with the PE-labeled mAbs from eBioscience and from BD Biosciences. A FACS Calibur flow cytometer (Becton Dickinson) combined with the CellQuest program was used to perform flow cytometric analysis.
TRANSFECTION AND LUCIFERASE ASSAY
The NF-κB luciferase reporter containing two κB binding sites, and the STAT-Luc reporter containing three repeats of GAS sites were used to determine NF-κB and STAT transactivation, respectively, in transiently transfected cell lines.
APOPTOSIS STUDIES
Cells were exposed to soluble TRAIL (50 ng/ml) alone or in combination with cycloheximide (2 µg/ml). Different variations of combined treatment were used in the presence or in the absence of specific inhibitors of signaling pathways followed by TRAIL treatment. After treatment, cell nuclei were stained by PI. Apoptosis was then assessed by quantifying the percentage of hypodiploid nuclei using FACS analysis. Annexin-FITC plus PI staining, which allowed us to distinguish apoptotic and necrotic cells, was performed according to the manufacture’s protocol (BD Pharmingen).
CLONOGENIC SURVIVAL ASSAY
Cells were exposed to sodium arsenite (1–5 µM), to soluble TRAIL (50 ng/ml) alone or in combination with CHX (1 µg/ml), BMS-345541 (10 µM), STAT3 inhibitor-6 (50 µM) alone or in combination with TRAIL for 24 h. Treated cells (500/dish) were then placed in duplicate on 10-cm dishes for analysis of clonogenic survival 12 days after treatment. Colonies were stained using crystal violet solution. The percentage of colony-formation (in relation to values for untreated control cells) was calculated.
WESTERN BLOT ANALYSIS
Total cell lysates (50 µg protein) were resolved on SDS–PAGE, and processed according to standard protocols. The monoclonal antibodies used for Western blotting included: anti-β-Actin (Sigma); anti-FLIP (NF6) (Axxora); anti-COX-2 (Cayman Chemical). The polyclonal antibodies used included: anti-DR5 (Axxora); anti-phospho-AKT (Ser473) and anti-AKT; anti-STAT3 and anti-phospho-STAT3 (Tyr705) (Cell Signaling). The secondary Abs were conjugated to horseradish peroxidase; signals were detected using the ECL system (Amersham).
EMSA
Electrophoretic mobility shift assay (EMSA) was performed for the detection of NF-κB DNA-binding activity as previously described. Ubiquitous NF-Y DNA-binding activity was used as an internal control [Ivanov and Hei, 2004].
REAL-TIME PCR ANALYSIS OF MITOCHONDRIAL DNA
Mitochondrial mtDNA copy number was determined by real-time PCR using SYBR Green detection on an Applied Biosystems 7300 Real-time PCR System (Applied Biosystems). Products amplified were a 188-bp fragment of the nuclear encoded 18S rRNA gene and a 171-bp fragment of the mtDNA encoded 12S rRNA gene. The primers were as follows: 18S sense, GGAGTATGGTTGCAAAGCTG; 18S antisense, CGCTCCACCAACTAAGAACG; 12S sense, AGAA-CACTACGAGCCACAGC; and 12S antisense, ACTTGCGCTTACTTTG-TAGCC. All reactions were done in triplicate. PCR conditions were as follows: 95°C for 15min followed by 40 cycles at 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s. Relative quantification of mtDNA/nDNA ratio was determined by the comparative threshold cycle (CT) method as described previously [Partridge et al., 2007].
OXYGEN CONSUMPTION
Oxygen consumption in intact cells was assayed as described previously [Partridge et al., 2007]. Briefly, 1 × 107 cells were suspended in 1.5 ml of DMEM lacking glucose, and oxygen concentration was assayed over 3 min at 37°C in a Hansatech (MA) Clark’s oxygen electrode unit.
ELISA
Antibodies pairs used in sandwich ELISA for this study was all commercially available. Kits to detect IL6 and TNFα were from Invitrogen.
STATISTICAL ANALYSIS
Data were calculated as means and standard deviations. Comparisons of results between treated and control groups were made by the Students’ t-test. A P-value of 0.05 or less between groups was considered significant.
RESULTS
PARTIAL DEPLETION OF MITOCHONDRIAL DNA AND DOWNREGULATION OF MITOCHONDRIAL FUNCTIONS IN MELANOMA CELL LINES
Multiple cell functions, including programmed cell death, are dependent on mitochondria. Consequently, numerous studies have targeted mitochondria as a probable tool for cancer therapy [Costantini et al., 2000; Fulda et al., 2010]. The biogenesis of mitochondria and general mitochondrial functions in cancer cells could be substantially different when compared to normal cells. Substantial change of mitochondrial functions is also involved in the proinflammatory response of the cells. A frequent suppression of mitochondrial respiration in cancer cells might be regulated by genetic mechanisms, depending on both mutations/deletions in mtDNA targeting critical mitochondrial proteins and general depletion (decreased copy number) of mtDNA [Pelicano et al., 2006]. These specific features of mitochondriogenesis have not been previously investigated in melanoma cells. What are the actual levels of mtDNA copy number and mitochondrial respiration in metastatic melanoma cells compared to normal cells?
To address this question, we used normal human embryonic fibroblasts TIG-3, human melanocytes and several human melanoma lines representing different phases of cancer development: WM35 is a radial-growth-phase melanoma cells; WM793 is a vertical-growth-phase melanoma cells; LU1205 (also known as 12501u) and WM9 are metastatic melanoma cells. All four melanoma lines have normal NRAS, but mutated BRAF (V600E). WM35 and WM9 express functional PTEN, endogenous inhibitor of PI3K– AKT, while in WM793 cells PTEN expression is down-regulated; finally in LU1205 cells PTEN is inactivated by mutation [Krasilnikov et al., 2003; Satyamoorthy et al., 2003]. Additionally, we used HHMSX and FEMX metastatic melanoma lines. FEMX cells have normal BRAF and PTEN, while HHMSX cells have mutated BRAF (V600E) [Krasilnikov et al., 2003; Ivanov and Hei, 2005].
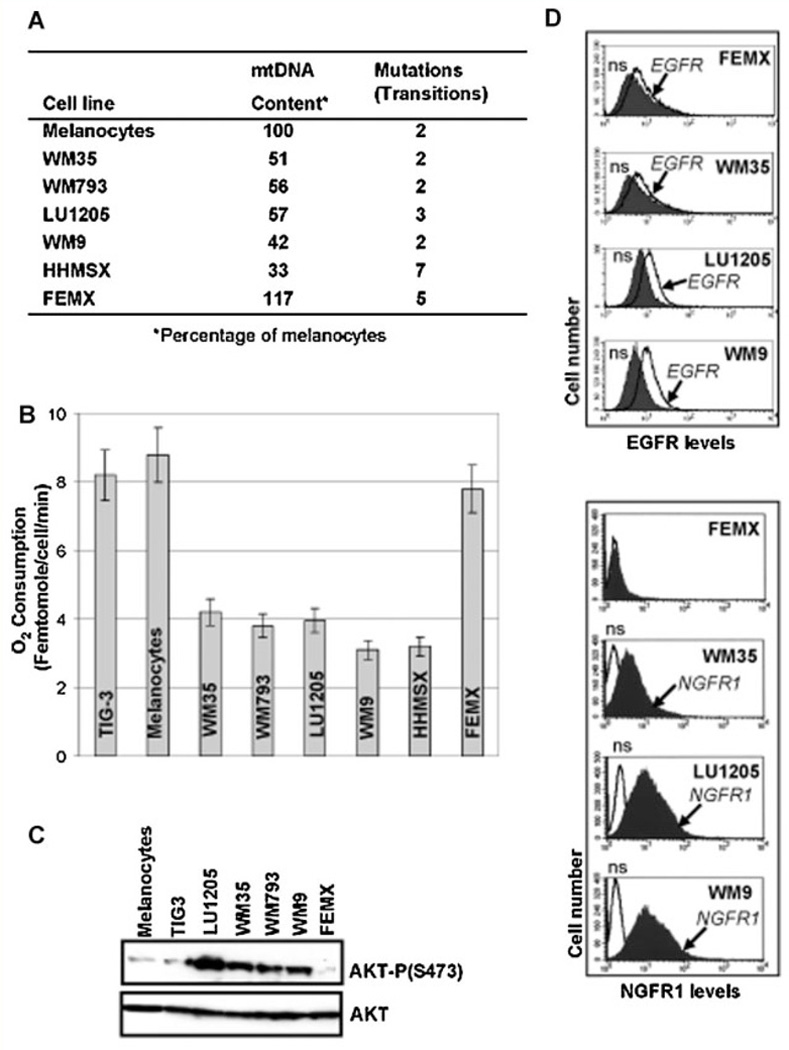
Analysis of the mtDNA by real-time PCR in these cell lines demonstrated a pronounced decrease in mtDNA copy numbers for several melanoma lines (with exception for FEMX). Sequencing mtDNA demonstrated increased levels of mutations in the D-Loop region of mtDNA from HHMSX and FEMX melanoma cells. However, these mutations were all previously reported polymorphisms (Fig. 1A). Importantly, oxygen consumption, the main reflection of mitochondrial respiration, was substantially decreased in all melanoma lines, besides FEMX, and was well correlated with down-regulation of mtDNA content (Fig. 1A,B). These observations suggested that a partial downregulation of mitochondrial function might be based on depletion of mtDNA that resulted in a decrease of mitochondrial gene expression and substantial changes of mitochondriogenesis in the corresponding melanoma cells.
Fig. 1.
Mitochondrial (mt) DNA content, mitochondrial respiration and AKT levels in human melanoma cell lines. A: Content of mitochondrial (mt) DNA was determined using the real-time PCR as described in the Materials and Methods Section. Number of mutations in the D-loop of mtDNA is indicated. All of these mutations represent previously reported polymorphisms. B: The rate of oxygen consumption for normal human fibroblasts TIG-3, human melanocytes and melanoma lines was determined using an oxygen electrode unit. C: Western blot analysis of total and phospho-AKT levels in melanocytes, TIG-3 fibroblasts and indicated melanoma lines. D: Surface levels of EGFR and NGFR1 p75 in human melanoma cells were determined by immunostaining with anti-EGFR-PE and anti-NGFR1-PE mAbs using FACS analysis; n.s., nonspecific staining.
Downregulation of mitochondriogenesis in cancer cells might also be correlated with decreased activity of the endogenous, ATP-dependent, mitochondrial apoptotic pathway due to a deficiency of ATP production by mitochondria. Additionally, upregulation of AKT activity in melanoma cells based on increased surface expression of growth factor receptor kinases, such as EGFR, IGF1R, or FGFR, in concert with a downregulation of PTEN, an endogenous inhibitor of the PI3K–AKT pathway, could also inhibit the mitochondrial apoptotic pathway [Krasilnikov et al., 2003; Wu et al., 2003; Pelicano et al., 2006]. Indeed, vertical-growth phase WM793, metastatic LU1205 (with mutated PTEN), and WM9 melanoma cells were characterized by pronounced surface expression of EGFR and substantial upregulation of the basal phospho-AKT activity compared to melanocytes and normal TIG-3 cells, while FEMX melanoma cells (with normal PTEN and low surface EGFR levels) only contained trace levels of active phospho-AKT (Fig. 1C,D). Interestingly, the early melanoma WM35 cells still contained pronounced levels of the active AKT that was phosphorylated via alternative receptor kinases (different from EGFR).
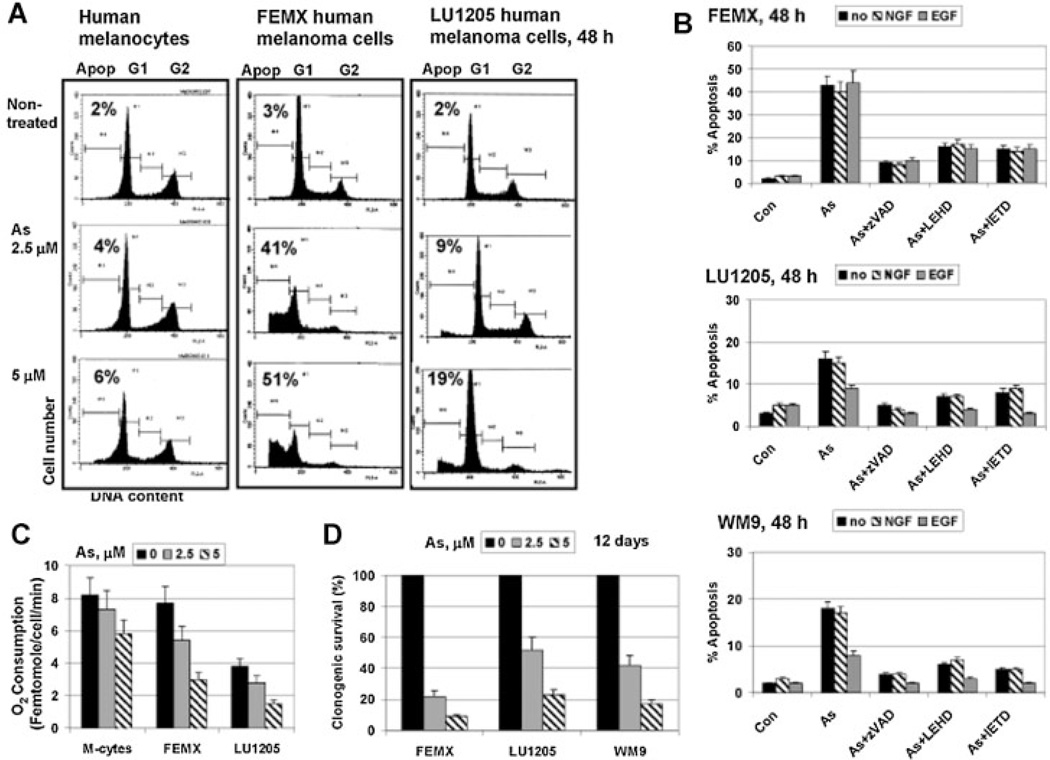
Numerous investigations demonstrated a relative resistance of normal human cells to low doses of sodium arsenite (1–5 µM), while many types of cancer cells died after such treatment [Mathas et al., 2003; Amadori et al., 2005] (Fig. 2A). The reason for this difference between normal and cancer cells is still unclear. However, among melanoma cell lines, a protective role for EGF/EGFR–PI3K–AKT signaling pathway against cytotoxic effects of sodium arsenite, which directly targeted mitochondria, was established [Ivanov and Hei, 2005; Partridge et al., 2007]. Indeed, sodium arsenite (5 µM) induced higher levels of apoptosis only in FEMX cells (Fig. 2A) that demonstrated the normal mitochondrial function and low active AKT levels before treatment (Fig. 1B,C). In contrast, sodium arsenite induced relatively low levels of apoptosis in LU1205 and WM9 cells (Fig. 2A,B), which were characterized by decreased mitochondrial respiration and high basal AKT activity before treatment (Fig. 1B,C). Furthermore, sodium arsenite (5 µM) substantially decreased mitochondrial respiration in melanoma cells, while normal human melanocytes were more resistant to arsenite treatment. As expected, melanocytes and TIG-3 normal fibroblasts were also relatively resistant to arsenite-induced apoptosis (Fig. 2A,C and Suppl. Fig. 1C). Costimulation with EGF (50 ng/ml) efficiently down-regulated arsenite-induced death of LU1205 and WM9 melanoma cells, but not FEMX (Fig. 2B). On the other hand, arsenite-induced cell death was substantially decreased by zVAD-fmk, a universal caspase inhibitor, LEHD, a caspase-9 inhibitor, and IETD, a caspase-8 inhibitor, highlighting a role of the activation of both the mitochondrial apoptotic pathway and death receptor-mediated pathway after arsenite exposure (Fig. 2B).
Fig. 2.
The mitochondrial death pathway in human melanoma cell lines. A,B: Cell cycle-apoptosis analysis of melanocytes, FEMX, LU1205, and WM9 melanoma cells, which were treated sodium arsenite (5 µM) in the presence or absence of either NGF-β (100 ng/ml) or EGF (50 ng/ml) for the next 24 h; non-treated control cells were designed as (Con). Caspase inhibitors, zVAD, LEHD, and IETD (50 µM), were additionally used. After treatment, cells were stained with PI and analyzed by FACS assay. Error bars represent mean ± SD (Student's t-test, P < 0.05). C: Effects of sodium arsenite on oxygen consumption of melanocytes (M-cytes), FEMX and LU1205 melanoma cells 24h after treatment. D: Clonogenic survival assay of melanoma cell lines 12 days after sodium arsenite treatment.
In spite of high levels of nerve growth factor receptor-1 (NGFR1 p75) surface expression on WM35, WM793, LU1205, and WM9 melanoma cells (Fig. 1D), NGF-β (100 ng/ml) in the media did not protect these cells against arsenite-induced killing indicating attenuation of NGFR1-mediated signaling in the melanoma lines used. (Fig. 2B). This was in contrast to the highly protective action of NGF-β against arsenite-induced apoptosis in differentiated neuron-like PC12 cells (Suppl. Fig. 1A,B) via AKT activation [Kimmelman et al., 2000]. On the other hand, melanoma cells displayed substantially increased cytotoxicity after sodium arsenite treatment, as compared to apoptosis (Fig. 2D), suggesting the involvement of necrotic mechanisms in sodium arsenite action. Taken together, our data demonstrated a relatively low efficacy of apoptotic pathways induced by sodium arsenite for specific killing melanoma cells with decreased mitochondrial function and permanently active AKT (most metastatic melanoma lines), but highlighted a usefulness of such an approach for killing some types of melanoma cells (FEMX) with non-suppressed mitochondrial function and low levels of the basal AKT activity.
We also screened two additional metastatic melanoma cell lines for sensitivity to arsenic. Surprisingly, A375 and WM852 melanoma cells with very low basal AKT activity [Dhawan et al., 2002; Hilmi et al., 2008] were sensitive to cytotoxic effects of sodium arsenite (5– 10 µM), and thus were killed mostly through necrotic, rather than apoptotic mechanisms (Suppl. Fig. 2). These data indicated that AKT deficiency correlated with increased levels of arsenic-induced total cell death (which could be driven either apoptotic or necrotic mechanisms). Furthermore, inhibition of PI3K–AKT activity by LY294002 strongly increases levels of arsenite-induced death in resistant melanoma lines and unfortunately, also in TIG-3 normal fibroblasts and neuron-like PC12 cells. Necrotic effects of arsenite treatment were also increased in response to AKT inhibition (Suppl. Fig. 1). Trying to avoid non-specific cytotoxicity linked to direct targeting of mitochondria in normal cells, we refocused our attention on other characteristic features of the proinflammatory response in metastatic melanoma cells and their possible role in the regulation of apoptosis.
PROINFLAMMATORY RESPONSE OF METASTATIC MELANOMA CELLS AND REGULATION OF TRAIL-INDUCED APOPTOSIS: A ROLE FOR NF-κB
Besides directly targeting mitochondria with different drugs and natural compounds, stimulation of the death receptor-dependent apoptotic pathways has been widely used for treatment of melanoma cells. However, most metastatic melanoma cell lines were relatively resistant to death ligands, FasL, and TRAIL. Numerous observations demonstrated expression and secretion of proinflammatory cytokines by cancer cells, including malignant melanoma cells in culture and in vivo [Mattei et al., 1994; Yurkovetsky et al., 2007]. We wondered whether cytokine-mediated inflammatory signaling, which could perform many functions in cell–cell communications, might also be involved in anti-apoptotic protection of melanoma cells.
To address this question, we first determined levels of secretion of TNFα and IL6, crucial proinflammatory cytokines, in cell media of several melanoma lines using specific ELISA. WM9 metastatic melanoma cells were characterized by high basal expression and secretion of TNFα and IL6, while LU1205 metastatic melanoma cells secreted substantially decreased levels of these cytokines 24 h after refreshing cell media. On the other hand, the early phase WM35 melanoma cells produced modest levels of TNFα and zero levels of IL6. Human melanocytes did not produce detectable levels of TNFα and IL6 (Fig. 3C,D). Stress exposure, such as γ-radiation at dose of 2– 5 Gy, could notably upregulate IL6 secretion by WM9 cells (1.5- to 2.0-fold increase was detected 24 h after irradiation). Furthermore, a high-level IL8 expression and secretion was previously observed in WM9 cells [Peng et al., 2007].
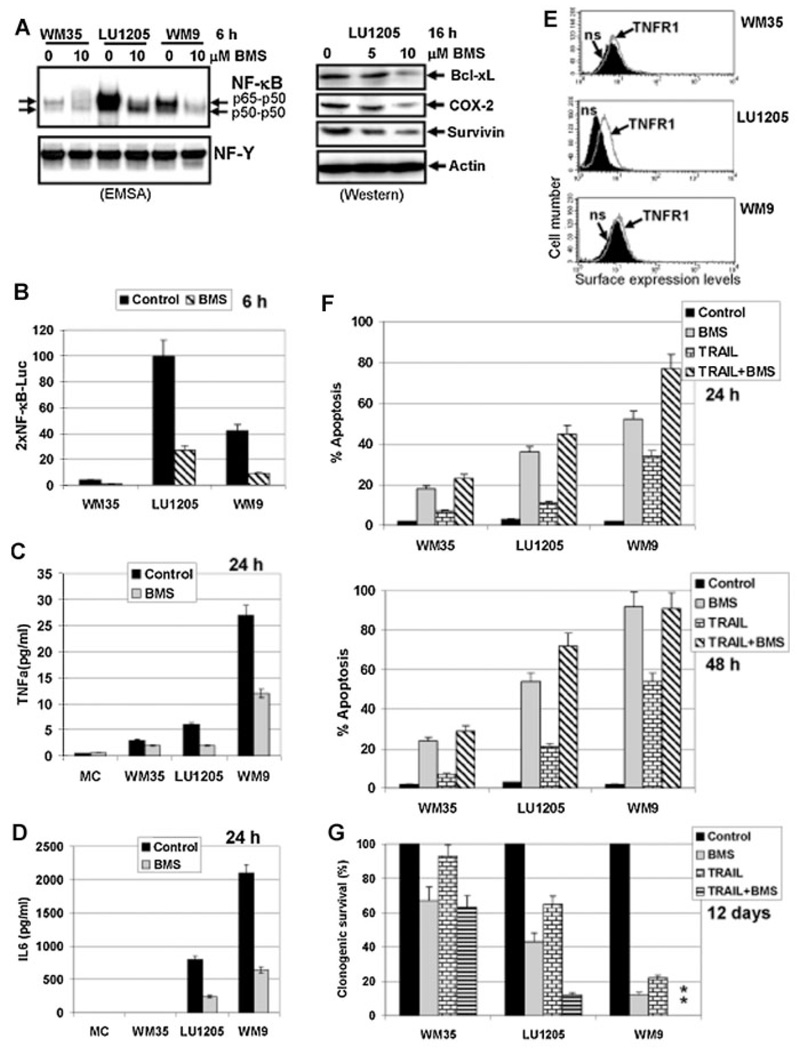
Fig. 3.
A role of NF-κB for expression and secretion of TNFα and IL6 and regulation of TRAIL-induced apoptosis in human melanoma cell lines. A: Effect of an IKK-NF-κB inhibitor BMS-345541 (10 µM) on the NF-κB DNA-binding activity determined by EMSA. Position of two main DNA-binding complexes is shown. DNA-binding activity of the ubiquitous transcription factor NF-Y was used as an internal control. Effect of BMS-345541 on expression levels of indicated proteins in LU1205 cells was determined by Western blot analysis. B: Effect of an IKK-NF-κB inhibitor BMS-345541 (10 µM) on 2×NF-κB-Luc reporter activity in transiently transfected melanoma cell lines. C,D: Effect of BMS-345541 (10 µM) on TNFα and IL6 secretion by human melanocytes (MC) and melanoma cell lines determined by ELISA. E: Surface expression of TNFR1 was determined by immunostaining with anti-TNFR1-PE mAb and FACS analysis; n.s., nonspecific staining. F: Effects TRAIL (30ng/ml) alone or in combination with BMS-345541 (10 µM) on apoptosis (24 and 48 h after exposure) in indicated melanoma lines. Apoptosis levels were determined, as described above. Error bars represent mean ± SD (Student's t-test, P < 0.05). G: Effects of TRAIL (30 ng/ml) and BMS-345541 (10 µM) on clonogenic survival melanoma cell lines.
Transcription factors NF-κB and STAT3 are the master regulators of the proinflammatory response in cells at the level of cytokine gene expression [Grivennikov and Karin, 2008; Yu et al., 2009]. As expected, BMS-345541, an inhibitor of the IKKb–NF-κB pathway, strongly decreased the nuclear NF-κB DNA-binding activity and NF-κB-dependent transcription in general (Fig. 3A,B). It significantly decreased the basal levels of secretion of TNFα and, especially, IL6 in LU1205 and WM9 cells (Fig. 3C,D) that was correlated with the critical role of NF-κB in TNF and IL6 gene expression. TNFR1 surface levels were relatively high in LU1205 cells, establishing efficient NF-κB activating loop in these cells, while WM9 cells exhibited substantially lower surface TNFR1 levels (Fig. 3E). Furthermore, suppression of nuclear NF-κB activity (Fig. 3A; EMSA), NF-κB transacting functions (Fig. 3B) and NF-κB-target gene expression (Fig. 3A, Western data) by BMS-345541 (10 µM) was accompanied by variable-level apoptosis (Fig. 3F). As a result, almost 90% of WM9 cells and 50% of LU1205 cells were killed 48 h after exposure to BMS-345541 (10 mM), while the early melanoma cells WM35 were relatively resistant.
Even though targeting NF-κB is an important tool for treatment of some metastatic melanomas [Ivanov et al., 2000; Yang et al., 2006, 2009], it appears not to be a universal approach for killing melanoma cells. Our next task was to elucidate any additional enhancing effects of IKKβ–NF-κB suppression of the apoptotic response induced by TRAIL in melanoma cell lines. Inhibition of NF-κB activation and NF-κB target gene expression resulted in down-regulation of anti-apoptotic protein levels, such as Bcl-xL, COX-2, and Survivin (Fig. 3A), in concert with other numerous NF-κB transcriptional targets. As a result, combined treatment with TRAIL (30 ng/ml) and BMS-345541 (10 µM) additively increased apoptotic levels in LU1205 cells 24–48 h after exposure (Fig. 3F). Such treatment after 48 h had no additional effects for WM9 cells that were already very sensitive to both TRAIL and BMS-345541 exposure, while still enhanced apoptotic response in these cells 24 h after treatment (Fig. 3F). WM35 cells with low sensitivity to TRAIL, due to deficiency in surface DR5/DR4 expression (see Fig. 4B), did not up-regulate levels of BMS-induced apoptosis in the presence of TRAIL (Fig. 3F). Clonogenic survival assay further confirmed additive effects of BMS-345541 and TRAIL for efficient killing LU1205 and WM9 cells (Fig. 3G).
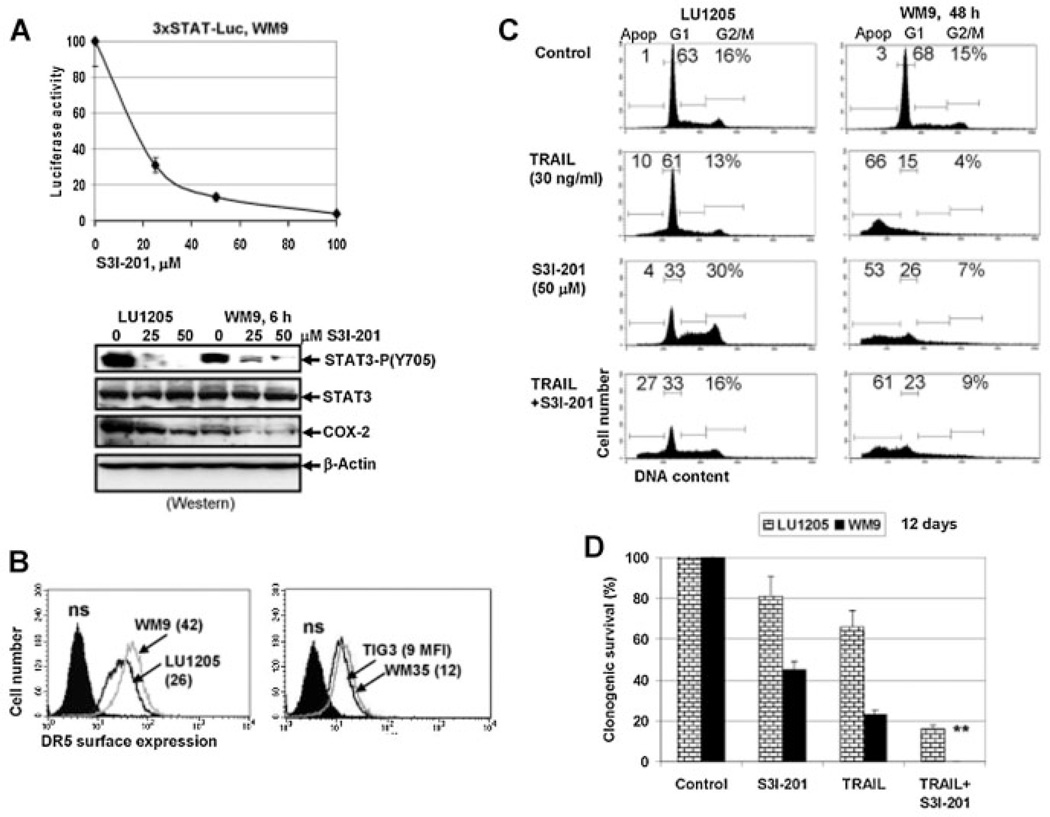
Fig. 4.
Effects of STAT3 inhibition on melanoma cell apoptosis. A: Dose-dependent effect of STAT3 inhibitor-6 (S3I-201) on STAT-dependent luciferase activity. WM9 cells were transiently transfected with 3xSTAT-Luc reporter and then treated for 6 h using S3I-201. Western blot analysis of phospho-Tyr705-STAT3 and COX-2 levels after 6-h exposure with S3I-201. Actin was used as a loading control. B: Surface expression levels of DR5 were determined for indicated cell lines using with anti-DR5-PE mAb and flow cytometry. C: Effects of S3I-201 (50 µM) alone or in combination with TRAIL on cell cycle-apoptosis in LU1205 and WM9 melanoma cells. Results of a typical experiment are presented (one from three). D: Clonogenic survival assay of LU1205 and WM9 cells 12 days after treatment with S3I-201 (50 µM), TRAIL (30 ng/ml) or their combination. Double star indicates zero survival of WM9 cells after combined treatment. Error bars represent mean ± SD (Student's t-test, P < 0.05).
A ROLE FOR STAT3 IN ANTI-APOPTOTIC PROTECTION
It was well established that the NF-κB-dependent expression and secretion of IL6 further activated the IL6/IL6R–JAK2–STAT3 pathway and STAT3-mediated gene expression via autocrine and paracrine mechanisms in inflammatory and cancer cells [Karin, 2009; Yu et al., 2009]. To determine a role for STAT3 and STAT3-transcriptional target genes in the regulation of apoptosis, we used STAT3 inhibitor-6, S3I-201 (also known as NSC 74859) (Fig. 4A), which worked through inhibition of STAT3 dimerization and activation [Lin et al., 2009]. As expected, we observed dose-dependent inhibition of STAT-reporter activity (Fig. 4A, the upper panel), suppression of Tyr705 STAT3 phosphorylation and decreased protein expression of COX-2, one of the STAT3 transcriptional targets following treatment with S3I-201 (Fig. 4A, the lower panel). WM9 cells were highly sensitive to S3I-201, as evidenced by increased apoptosis, while LU1205 cells demonstrated only pronounced changes in cell cycle distribution (with G2/M and S-phase arrest) following exposure to this inhibitor (Fig. 4C). WM9 and LU1205 cells exhibited pronounced DR5 surface expression (Fig. 4B), but displayed quite different sensitivity to TRAIL (Fig. 4C). On the other hand, levels of TRAIL-induced apoptosis were further up-regulated by S3I–201 only in LU1205 cells, while a combination of TRAIL and S3I–201 induced high levels of death of WM9 cells not only via apoptosis [that was relatively stable at 66–61% (Fig. 4C)], but probably also by necrosis. Indeed, a clonogenic survival assay demonstrated complete killing of WM9 cells by a combination of TRAIL and S3I–201 12 days after treatment (Fig. 4D). The increased resistance of LU1205 to TRAIL-induced apoptosis, compared to WM9 cells, was probably based on the substantially higher levels of NF-κB activity in LU1205 cells (see Fig. 3A).
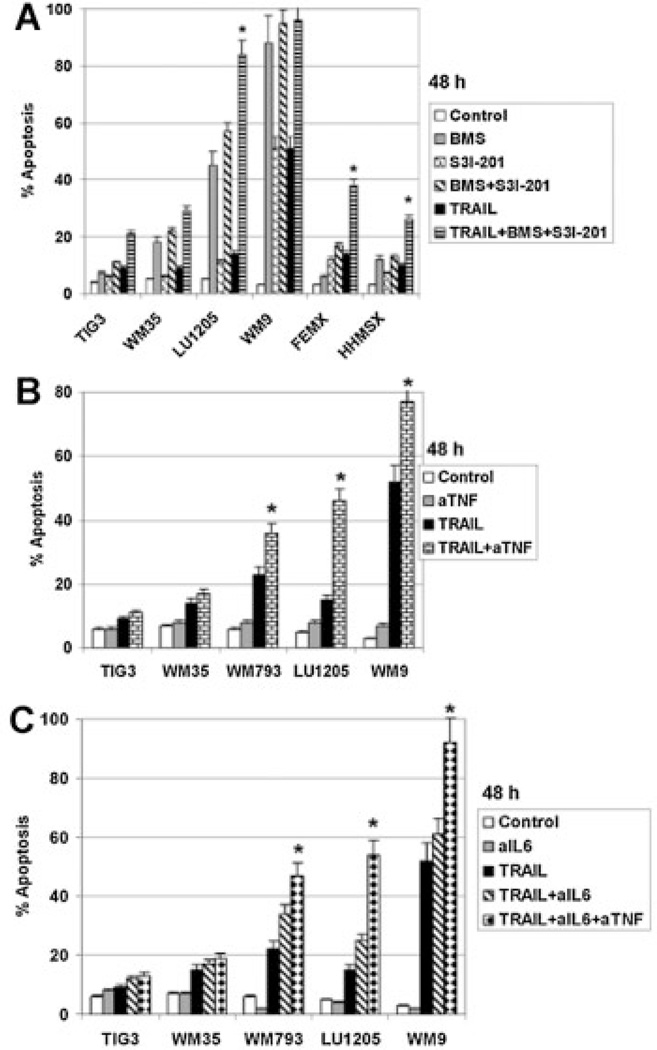
Interestingly, treatment with a combination of inhibitors for both IKK-NF-κB and STAT3 (BMS-345541 and S3I-301) demonstrated additive effects for killing LU1205 48 h after treatment (Fig. 5A). Furthermore, simultaneous inhibition of NF-κB and STAT3 notably increased levels of TRAIL-induced apoptosis for resistant FEMX and HHMSX cells (Fig. 5A). Taken together, these results further confirmed a role for the transcriptional regulators, NF-κB and STAT3, in suppression of TRAIL-induced apoptosis in melanoma cells. As a complementary approach, we used monoclonal inhibitory antibodies to block TNFα and IL6 transmission and to inhibit aurocrine/paracrine stimulation of melanoma cells by these cytokines, which further regulated NF-κB and STAT3 activation. This resulted in pronounced upregulation of TRAIL-induced apoptosis for WM793, LU1205, and WM9 cells after suppression of TNFα or IL6 transmission (Fig. 5B,C). Furthermore, the proapoptotic effect was especially strong after treatment with a combination of both inhibitory antibodies (Fig. 5C). These data are a direct demonstration of a protective role of TNFα and IL6 against TRAIL-induced apoptosis in malignant melanoma cells.
Fig. 5.
Double inhibition of NF-κB and STAT3: effects on TRAIL-induced apoptosis. A: Cells were treated with BMS-345541 (10 µM), S3I-201 (50 µM), TRAIL (30 ng/ml) individually or in combination. Apoptotic levels were determined using PI staining of DNAand flow cytometry. B,C: Effects of anti-TNFα (aTNF) (5 µg/ml), anti-IL6 (aIL6) (5 µg/ml) mAbs or their combination on TRAIL-induced apoptosis in melanoma cell lines. Error bars represent mean ± SD (Student's t-test, P <0.05)
A ROLE OF COX-2 FOR THE SUPPRESSION OF TRAIL-INDUCED APOPTOSIS IN MELANOMA CELLS
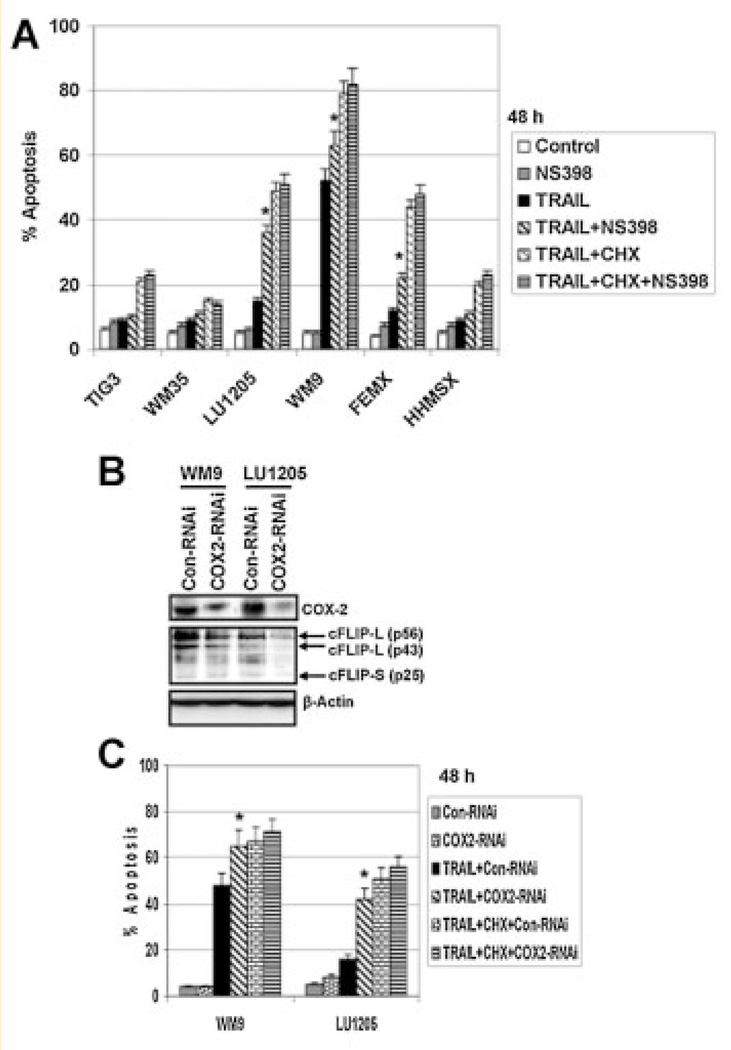
NF-κB and STAT3 are direct transcriptional regulators of numerous genes. Since one of the critical enzymatic regulators of the inflammatory response, cyclooxygenase-2 (COX-2), was a transcriptional target of both NF-κB and STAT3 [Kanekura et al., 2002; Lo et al., 2010], we further focused our attention on the significance of COX-2 in anti-apoptotic protection of human melanoma cells. Suppression of STAT3 activity, as well as NF-κB activity, was indeed accompanied by down-regulation of protein expression of COX-2 in LU1205 and WM9 melanoma cells (see Figs. 3A and 4A). On the other hand, inhibition of COX-2 enzymatic activity by a specific inhibitor NS398 (50 µM) further increased TRAIL-induced apoptotic levels in LU1205, WM9, and FEMX metastatic melanoma cells, while demonstrating no additional effects in WM35, and HHMSX melanoma cells, as well as in normal TIG-3 cells (Fig. 6A). As we previously demonstrated [Ivanov et al., 2008], TIG-3, WM35, and HHMSX cells possess low levels of DR5 surface expression and negligible DR4 surface expression (see also Fig. 4D).
Fig. 6.
Effects of downregulation of COX-2 on TRAIL-induced apoptosis. A: Effects of NS398 (50 µM), an enzymatic inhibitor of COX-2, on TRAIL (30ng/ml) or TRAIL+ CHX (1 µg/ml) induced apoptosis in fibroblasts and indicated melanoma lines. B: Effects of COX-2 suppression by specific COX-2 RNAi on COX-2 and cFLIP-L protein expression levels in WM9 and LU1205 melanoma cells. Cell cultures stably transfected by control or COX-2 RNAi were previously described. C: TRAIL-induced apoptosis in control and COX-2 deficient cell cultures. Apoptotic levels were determined 48 h after treatment using PI staining and the flow cytometry. Error bars represent mean ± SD (Student's t test, P <0.05).
Furthermore, pronounced downregulation of COX-2 protein levels by COX-2 RNAi [Ivanov and Hei, 2006b] was also accompanied by a decrease in cFLIP protein levels, a critical inhibitor of caspase-8 activity, and the subsequent upregulation of TRAIL-induced caspase-8-mediated apoptosis in LU1205 cells. For TRAIL-sensitive WM9 cells, effects of COX-2 suppression were also significant, but less pronounced (Fig. 6B). Downregulation of COX-2 levels by specific RNAi was accompanied by increased TRAIL-induced apoptosis in WM9 and LU1205 cells (Fig. 6C). An important role of cFLIP, which by itself was a transcriptional target of NF-κB and STAT3, in the negative regulation of TRAIL-induced apoptosis in melanoma cells was reported previously [Ivanov and Hei, 2006b]. Effect of pharmacological inhibition of COX-2 on downregulation of cFLIP levels in cancer cell lines was also previously reported [Liu et al., 2006]. Taken together, our results confirmed that inhibition of COX-2 (as a STAT3 and NF-κB transcriptional target in the inflammatory response) further upregulated TRAIL-induced apoptosis in melanoma cells.
ADDITIONAL INHIBITORS OF PROINFLAMMATORY GENE EXPRESSION, INCREASED THE SUSCEPTIBILITY OF MELANOMA CELLS TO TRAIL
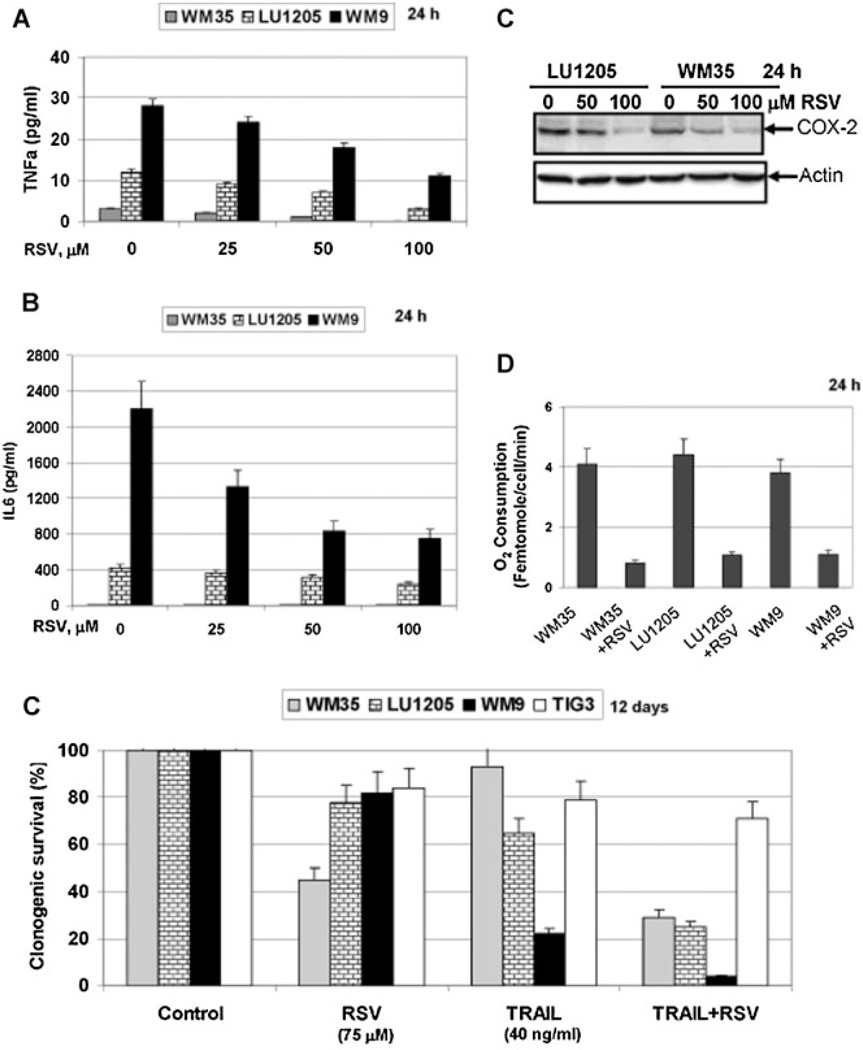
The polyphenolic phytoalexin, resveratrol (RSV), is an efficient inhibitor of both NF-κB- and STAT3-dependent transcription in melanoma lines as well as in different cancer cell lines in concert with extensive activation of ATM-p53 and MAPK pathways [Aggarwal et al., 2004; Ivanov et al., 2008]. Production and secretion of TNFα and IL6, as well as protein expression of COX-2 (Fig. 7A – C) were substantially decreased by RSV treatment of melanoma lines. RSV exposure also further decreased mitochondrial respiration in cancer cells (Fig. 7D), similar to the effects of sodium arsenite treatment (see Fig. 2). These data highlighted the general anti-inflammatory effects of RSV in melanoma cells. RSV by itself demonstrated only modest levels of melanoma cell killing determined by decreased clonogenic survival following RSV exposure (Fig. 7E). However, a combination of TRAIL and RSV was extremely effective in the induction of high levels of cell death in several melanoma lines based on results of a clonogenic survival assay (Fig. 7E), further extending previous observations on proapoptotic activity of a combination of TRAIL and RSV in cancer cells [Fulda and Debatin, 2004; Ivanov et al., 2008], while the effect of this combination on TIG-3 normal fibroblasts was only marginal. Taken together, the present results and previously published data allow us to link proapoptotic effects of RSV in a combination with TRAIL, at least partially, with suppression of anti-apoptotic functions by NF-κB and STAT3 in melanoma cells.
Fig. 7.
Resveratrol (RSV), a natural inhibitor of NF-κB and STAT3 activation, negatively regulates secretion of TNFα and IL6, COX-2 expression and increases TRAIL-induced apoptosis in melanoma lines. A,B: Effects of RSV (25–100 µM) on TNFα and IL6 secretion. C: Western blot analysis of COX-2 protein levels following RSV exposure. D: RSV (50 µM) suppressed oxygen consumption in melanoma cells. E: Effects of RSV, TRAIL, and their combination on clonogenic survival of melanoma cell lines. Error bars represent mean ± SD (Student's t-test, P < 0.05).
Among additional suppressors of inflammation, a small molecular inhibitor of the ATM-NF-κB/STAT3 signaling pathway, KU-55933 (10 µM), demonstrated pronounced anti-inflammatory effects (determined by downregulation of IL6 secretion) and upregulation of TRAIL-mediated apoptosis in human melanoma cells (Suppl. Fig. 3) confirming our recent observations on a role of ATM kinase activity in the regulation of apoptosis in melanoma cells [Ivanov et al., 2009].
DISCUSSION
Since many melanoma cell lines exhibit profound resistance to TRAIL treatment, we and others investigated the origin of this resistance and demonstrated an upregulation of TRAIL/TRAIL-R-mediated apoptosis in metastatic melanoma cells using different types of co-treatment for suppression of strong anti-apoptotic mechanisms in cancer cells [Hersey et al., 2006; Ivanov et al., 2007]. Melanoma has multifactorial etiology and, quite predictably, different approaches should be used for successful treatment of this disease. An interesting and potentially important difference between melanoma lines observed in the present study was the different levels of mitochondrial function that could probably predict different effects of drugs targeting mitochondria. Melanoma cells with suppressed mitochondrial function responded poorly to sodium arsenite or other mitochondrial target drugs. This was based on downregulation of the mitochondrial pathway of apoptotic death, which actively required energy produced by mitochondria, in advanced melanomas. In some cases (OM431 and SW1 melanoma cells), an additional suppression of the mitochondrial apoptotic pathway was based on strong downregulation of APAF1 levels, a critical adaptor protein of this pathway [Soengas and Lowe, 2003; Ivanov and Hei, 2006b]. We investigated a role of the proin-flammatory response of melanoma cells via TNFα/TNFR-NF-κB-IL6/IL6R–STAT3 pathways in regulation of apoptosis to consider alternative approaches for treatment of metastatic melanomas.
Although a general link between inflammation and tumorigenesis is well established [Karin, 2009], there are many different aspects to this problem. The focus of our study was the proinflammatory response of metastatic melanoma cells based on the endogenous NF-κB- and STAT3-transcriptional activation of cytokine gene expression, establishing an autocrine loop with further activation of NF-κB- and STAT3-dependent anti-apoptotic genes, including COX-2 and cFLIP. We further demonstrated that suppression of this protective loop at several points (TNFα, IKKβ– NF-κB, IL6, JAK2–STAT3, COX-2) substantially upregulated TRAIL-induced apoptosis in melanoma cells. Unfortunately, for some melanoma lines, even increasing apoptotic response to TRAIL still did not achieve high levels of apoptosis. The preliminary upregulation of TRAIL-R2/R1 (DR5/DR4) levels using different approaches might further help to upregulate TRAIL-induced apoptosis in these melanoma lines [Ivanov et al., 2007].
Numerous mechanisms that control either direct physical interaction between NF-κB RelA subunit and STAT3 [Yang et al., 2007] or extensive cross-talking between signaling pathways targeting gene expression and activation of NF-κB and STAT3 have been elucidated in the inflammatory network, including in cancer cells [Grivennikov and Karin, 2008]. One of the critical results of NF-κB and STAT3 interactions in tumorigenesis also targets the mitochondria. A deficiency of normal mitochondrial respiratory function, but increased production of reactive oxygen species, one of the characteristic features of the inflammatory response [Alexeyev et al., 2004], might be frequently found in cancer cells, which have a tendency to change their energetic metabolism from oxidative phosphorylation to oxidative glycolysis (“Warburg effect”) [Jones and Thompson, 2009]. Such downregulation of mitochondrial function could be achieved via direct depletion of mtDNA with suppression of mitochondriogenesis, as we observed in the present study. Alternatively, transcription factors, such as NF-κB and STAT3 could play multiple roles in the control of mitochondrial function. By transcriptional regulation of COX-2 and iNOS, both NF-κB and STAT3 positively control ROS and NOS production. On the other hand, via transcriptional regulation of antioxidant enzymes, such as Mn-superoxide dismutase, NF-κB could balance ROS levels and abrogate destructive effects of ROS on mitochondria [Luo et al., 2005].
The present study and our previous work [Ivanov et al., 2007, 2009] provide significant understanding of the regulation of TRAIL-mediated apoptosis in melanoma cells and will potentially foster translational development of a new therapy for treatment of currently therapy-resistant metastatic melanomas. This strategy for treatment of human melanomas, which could be further used in translational research, is based on: (i) dramatic upregulation of surface expression of death receptor (TRAIL-R1/DR4 and TRAIL-R2/ DR5) in metastatic melanomas using ionizing radiation; (ii) increasing sensitivity to apoptosis using inhibition (immunological, pharmacological or by specific RNAi) of the TNFα/TNFR– NF-κB–IL-6/IL-6R–STAT3–COX2 signaling cascade in human melanoma cells. Our study also demonstrates that dual inhibition of NF-κB and STAT3-dependent gene expression (through small molecular inhibitors or natural agents, such as resveratrol [Fulda and Debatin, 2004]) was especially efficient for increasing the TRAIL-response in some human melanoma lines.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr. M. Herlyn for melanoma cell lines and Dr. H. B. Lieberman for a critical reading of the manuscript and discussion.
Grant sponsor: Superfund; Grant number: ES 10349.
Footnotes
Additional supporting information may be found in the online version of this article.
REFERENCES
- Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004;24:2783–2840. [PubMed] [Google Scholar]
- Alexeyev MF, Ledoux SP, Wilson GL. Mitochondrial DNA and aging. Clin Sci (Lond) 2004;107:355–364. doi: 10.1042/CS20040148. [DOI] [PubMed] [Google Scholar]
- Amadori S, Fenaux P, Ludwig H, O’Dwyer M, Sanz M. Use of arsenic trioxide in haematological malignancies: Insight into the clinical development of a novel agent. Curr Med Res Opin. 2005;21:403–411. doi: 10.1185/030079904X20349. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: The potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/ TRAIL) J Clin Oncol. 2008;26:3621–3630. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondrial dysfunction and oxidative damage in Alzheimer’s and Parkinson’s diseases and coenzyme Q10 as a potential treatment. J Bioenerg Biomembr. 2004;36:381–386. doi: 10.1023/B:JOBB.0000041772.74810.92. [DOI] [PubMed] [Google Scholar]
- Chin L, Garraway LA, Fisher DE. Malignant melanoma: Genetics and therapeutics in the genomic era. Genes Dev. 2006;20:2149–2182. doi: 10.1101/gad.1437206. [DOI] [PubMed] [Google Scholar]
- Costantini P, Jacotot E, Decaudin D, Kroemer G. Mitochondrion as a novel target of anticancer chemotherapy. J Natl Cancer Inst. 2000;92:1042–1053. doi: 10.1093/jnci/92.13.1042. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE., Jr Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2:740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- Dhawan P, Singh AB, Ellis DL, Richmond A. Constitutive activation of Akt/protein kinase B in melanoma leads to up-regulation of nuclear factor-kappaB and tumor progression. Cancer Res. 2002;62:7335–7342. [PubMed] [Google Scholar]
- Fulda S, Debatin KM. Sensitization for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol. Cancer Res. 2004;64:337–346. doi: 10.1158/0008-5472.can-03-1656. [DOI] [PubMed] [Google Scholar]
- Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- Gogas HJ, Kirkwood JM, Sondak VK. Chemotherapy for metastatic melanoma: Time for a change? Cancer. 2007;109:455–464. doi: 10.1002/cncr.22427. [DOI] [PubMed] [Google Scholar]
- Gogas HJ, Kirkwood JM, Sondak VK. Chemotherapy for metastatic melanoma: Time for a change? Cancer. 2007;109:455–464. doi: 10.1002/cncr.22427. [DOI] [PubMed] [Google Scholar]
- Grivennikov S, Karin M. Autocrine IL-6 signaling: A key event in tumorigenesis? Cancer Cell. 2008;13:7–9. doi: 10.1016/j.ccr.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Hersey P, Zhuang L, Zhang XD. Current strategies in overcoming resistance of cancer cells to apoptosis melanoma as a model. Int Rev Cytol. 2006;251:131–158. doi: 10.1016/S0074-7696(06)51004-6. [DOI] [PubMed] [Google Scholar]
- Hilmi C, Larribere L, Giuliano S, Bille K, Ortonne JP, Ballotti R, Bertolotto C. IGF1 promotes resistance to apoptosis in melanoma cells through an increased expression of BCL2, BCL-X(L), and survivin. J Invest Dermatol. 2008;128:1499–1505. doi: 10.1038/sj.jid.5701185. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Hei TK. Arsenite sensitizes human melanomas to apoptosis via tumor necrosis factor alpha-mediated pathway. J Biol Chem. 2004;279:22747–22758. doi: 10.1074/jbc.M314131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov VN, Hei TK. Combined treatment with EGFR inhibitors and arsenite upregulated apoptosis in human EGFR-positive melanomas: A role of suppression of the PI3K–AKT pathway. Oncogene. 2005;24:616–626. doi: 10.1038/sj.onc.1208125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov VN, Hei TK. Dual treatment with COX-2 inhibitor and sodium arsenite leads to induction of surface Fas Ligand expression and Fas-Ligand-mediated apoptosis in human melanoma cells. Exp Cell Res. 2006a;312:1401–1417. doi: 10.1016/j.yexcr.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov VN, Hei TK. Sodium arsenite accelerates TRAIL-mediated apoptosis in melanoma cells through upregulation of TRAIL-R1/R2 surface levels and downregulation of cFLIP expression. Exp Cell Res. 2006b;312:4120–4138. doi: 10.1016/j.yexcr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov VN, Kehrl JH, Ronai Z. Role of TRAF2/GCK in melanoma sensitivity to UV-induced apoptosis. Oncogene. 2000;19:933–942. doi: 10.1038/sj.onc.1203415. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Bhoumik A, Krasilnikov M, Raz R, Owen-Schaub LB, Levy D, Horvath CM, Ronai Z. Cooperation between STAT3 and c-jun suppresses Fas transcription. Mol Cell. 2001;7:517–528. doi: 10.1016/s1097-2765(01)00199-x. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Bhoumik A, Ronai Z. Death receptors and melanoma resistance to apoptosis. Oncogene. 2003;22:3152–3161. doi: 10.1038/sj.onc.1206456. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Zhou H, Hei TK. Sequential treatment by ionizing radiation and sodium arsenite dramatically accelerates TRAIL-mediated apoptosis of human melanoma cells. Cancer Res. 2007;67:5397–5407. doi: 10.1158/0008-5472.CAN-07-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov VN, Partridge MA, Johnson GE, Huang SX, Zhou H, Hei TK. Resveratrol sensitizes melanomas to TRAIL through modulation of antia-poptotic gene expression. Exp Cell Res. 2008;314:1163–1176. doi: 10.1016/j.yexcr.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov VN, Zhou H, Partridge MA, Hei TK. Inhibition of ataxia telangiectasia mutated kinase activity enhances TRAIL-mediated apoptosis in human melanoma cells. Cancer Res. 2009;69:3510–3519. doi: 10.1158/0008-5472.CAN-08-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RG, Thompson CB. Tumor suppressors and cell metabolism: A recipe for cancer growth. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekura T, Goorha S, Kirtikara K, Ballou LR. The involvement of NF-kappaB in the constitutive overexpression of cyclooxygenase-2 in cycloox-ygenase-1 null cells. Biochim Biophys Acta. 2002;1542:14–22. doi: 10.1016/s0167-4889(01)00162-8. [DOI] [PubMed] [Google Scholar]
- Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Lawrence T, Nizet V. Innate immunity gone awry: Linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Kimmelman AC, Osada M, Chan AM. R-Ras3, a brain-specific Ras-related protein, activates Akt and promotes cell survival in PC12 cells. Oncogene. 2000;19:2014–2022. doi: 10.1038/sj.onc.1203530. [DOI] [PubMed] [Google Scholar]
- Krasilnikov M, Ivanov VN, Dong J, Ronai Z. ERK and PI3K negatively regulate STAT-transcriptional activities in human melanoma cells: Implications towards sensitization to apoptosis. Oncogene. 2003;22:4092–4101. doi: 10.1038/sj.onc.1206598. [DOI] [PubMed] [Google Scholar]
- Kreuz S, Siegmund D, Scheurich P, Wajant H. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001;21:3964–3973. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaba M, Nakao K, Goto T, Nishimura D, Kawashimo H, Shibata H, Motoyoshi Y, Taura N, Ichikawa T, Hamasaki K, Eguchi K. Abrogation of constitutive STAT3 activity sensitizes human hepatoma cells to TRAIL-mediated apoptosis. J Hepatol. 2007;47:546–555. doi: 10.1016/j.jhep.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Leslie K, Lang C, Devgan G, Azare J, Berishaj M, Gerald W, Kim YB, Paz K, Darnell JE, Albanese C, Sakamaki T, Pestell R, Bromberg J. Cyclin D1 is transcriptionally regulated by and required for transformation by activated signal transducer and activator of transcription 3. Cancer Res. 2006;66:2544–2552. doi: 10.1158/0008-5472.CAN-05-2203. [DOI] [PubMed] [Google Scholar]
- Lin L, Amin R, Gallicano GI, Glasgow E, Jogunoori W, Jessup JM, Zasloff M, Marshall JL, Shetty K, Johnson L, Mishra L, He AR. The STAT3 inhibitor NSC 74859 is effective in hepatocellular cancers with disrupted TGF-beta signaling. Oncogene. 2009;28:961–972. doi: 10.1038/onc.2008.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yue P, Schonthal AH, Khuri FR, Sun SY. Cellular FLICE-inhibitory protein down-regulation contributes to celecoxib-induced apoptosis in human lung cancer cells. Cancer Res. 2006;66:11115–11119. doi: 10.1158/0008-5472.CAN-06-2471. [DOI] [PubMed] [Google Scholar]
- Lo HW, Cao X, Zhu H, Ali-Osman F. Cyclooxygenase-2 is a novel transcriptional target of the nuclear EGFR-STAT3 and EGFRvIII-STAT3 signaling axes. Mol Cancer Res. 2010;8:232–245. doi: 10.1158/1541-7786.MCR-09-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: Balancing life and death—A new approach to cancer therapy. J Clin Invest. 2005;115:2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathas S, Lietz A, Janz M, Hinz M, Jundt F, Scheidereit C, Bommert K, Dorken B. Inhibition of NF-kappaB essentially contributes to arsenic-induced apoptosis. Blood. 2003;102:1028–1034. doi: 10.1182/blood-2002-04-1154. [DOI] [PubMed] [Google Scholar]
- Mattei S, Colombo MP, Melani C, Silvani A, Parmiani G, Herlyn M. Expression of cytokine/growth factors and their receptors in human melanoma and melanocytes. Int J Cancer. 1994;56:853–857. doi: 10.1002/ijc.2910560617. [DOI] [PubMed] [Google Scholar]
- Partridge MA, Huang SX, Hernandez-Rosa E, Davidson MM, Hei TK. Arsenic induced mitochondrial DNA damage and altered mitochondrial oxidative function: Implications for genotoxic mechanisms in mammalian cells. Cancer Res. 2007;67:5239–5247. doi: 10.1158/0008-5472.CAN-07-0074. [DOI] [PubMed] [Google Scholar]
- Pelicano H, Xu RH, Du M, Feng L, Sasaki R, Carew JS, Hu Y, Ramdas L, Hu L, Keating MJ, Zhang W, Plunkett W, Huang P. Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J Cell Biol. 2006;175:913–923. doi: 10.1083/jcb.200512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng HH, Liang S, Henderson AJ, Dong C. Regulation of interleukin-8 expression in melanoma-stimulated neutrophil inflammatory response. Exp Cell Res. 2007;313:551–559. doi: 10.1016/j.yexcr.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis C, Herlyn M. Recent advances in melanoma biology. Oncologist. 2004;9:182–187. doi: 10.1634/theoncologist.9-2-182. [DOI] [PubMed] [Google Scholar]
- Rigel DS. The effect of sunscreen on melanoma risk. Dermatol Clin. 2002;20:601–606. doi: 10.1016/s0733-8635(02)00024-4. [DOI] [PubMed] [Google Scholar]
- Rigel DS, Friedman RJ, Kopf AW. The incidence of malignant melanoma in the United States: Issues as we approach the 21st century. J Am Acad Dermatol. 1996;34:839–847. doi: 10.1016/s0190-9622(96)90041-9. [DOI] [PubMed] [Google Scholar]
- Satyamoorthy K, DeJesus E, Linnenbach AJ, Kraj B, Kornreich DL, Rendle S, Elder DE, Herlyn M. Melanoma cell lines from different stages of progression and their biological and molecular analyses. Melanoma Res. 1997;(7 Suppl 2):S35–S42. [PubMed] [Google Scholar]
- Satyamoorthy K, Li G, Gerrero MR, Brose MS, Volpe P, Weber BL, Van Belle P, Elder DE, Herlyn M. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63:756–759. [PubMed] [Google Scholar]
- Schaefer U, Voloshanenko O, Willen D, Walczak H. TRAIL: A multifunctional cytokine. Front Biosci. 2007;12:3813–3824. doi: 10.2741/2354. [DOI] [PubMed] [Google Scholar]
- Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22:3138–3151. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: A molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003;24:96–102. doi: 10.1016/S0165-6147(02)00043-3. [DOI] [PubMed] [Google Scholar]
- Wu H, Goel V, Haluska FG. PTEN signaling pathways in melanoma. Oncogene. 2003;22:3113–3122. doi: 10.1038/sj.onc.1206451. [DOI] [PubMed] [Google Scholar]
- Yang J, Amiri KI, Burke JR, Schmid JA, Richmond A. BMS-345541 targets inhibitor of kappaB kinase and induces apoptosis in melanoma: Involvement of nuclear factor kappaB and mitochondria pathways. Clin Cancer Res. 2006;12:950–960. doi: 10.1158/1078-0432.CCR-05-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unpho-sphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev. 2007;21:1396–1408. doi: 10.1101/gad.1553707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zaja-Milatovic S, Thu YM, Lee F, Smykla R, Richmond A. Molecular determinants of melanoma malignancy: Selecting targets for improved efficacy of chemotherapy. Mol Cancer Ther. 2009;8:636–647. doi: 10.1158/1535-7163.MCT-08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkovetsky ZR, Kirkwood JM, Edington HD, Marrangoni AM, Velikokhatnaya L, Winans MT, Gorelik E, Lokshin AE. Multiplex analysis of serum cytokines in melanoma patients treated with interferon-alpha2b. Clin Cancer Res. 2007;13:2422–2428. doi: 10.1158/1078-0432.CCR-06-1805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.