Abstract
Importance
Major depressive disorder (MDD) frequently emerges during adolescence and can lead to persistent illness, disability and suicide. The maturational changes that take place in the brain during adolescence underscore the importance of examining neurobiological mechanisms during this time period of early illness. However, neural mechanisms of depression in adolescents have been understudied. Prior research has implicated the amygdala in emotion processing in mood disorders, and adult depression studies have suggested amygdala-frontal connectivity deficits. Resting-state functional magnetic resonance imaging (rsfMRI) is an advanced tool that can be used to probe neural networks and identify brain-behavior relationships.
Objective
To examine amygdala resting-state functional connectivity (RSFC) in adolescents with and without MDD using rsfMRI, and to examine how amygdala RSFC relates to a broad range of symptom dimensions.
Design
Cross-sectional rsfMRI study.
Setting
Depression research program at an academic medical center.
Participants
41 girls and boys aged 12–19 years with MDD and 29 healthy adolescents (frequency matched on age and sex) with no psychiatric diagnoses.
Main Outcome Measure
Using a whole-brain functional connectivity approach, we examined correlation of spontaneous fluctuation of blood-oxygen-level-dependent (BOLD) signal of each voxel in the whole brain with that of the amygdala.
Results
Adolescents with MDD showed lower positive RSFC between amygdala and hippocampus, parahippocampus and brain stem; this connectivity was inversely correlated with general depression, dysphoria, and lassitude, and positively correlated with well-being. Patients also showed greater (positive) amygdala-precuneus RSFC (in contrast to negative amygdala-precuneus RSFC in controls.)
Conclusion
Impaired amygdala-hippocampal/brainstem and amygdala-precuneus RSFC has not previously been highlighted in depression and may be unique to adolescent MDD. These circuits are important for different aspects of memory and self-processing, and of modulation of physiological responses to emotion. The findings suggest potential mechanisms underlying both mood and vegetative symptomatology, potentially via impaired processing of memories and visceral signals that spontaneously arise during rest, contributing to the persistent symptoms experienced by adolescents with depression.
Introduction
Major depressive disorder (MDD) is a leading cause of disability and global disease burden1 and frequently emerges during adolescence.2 Many adolescents do not respond to evidence-based treatments,3, 4 highlighting the need to better understand the pathophysiology. Current theory holds that fronto-limbic neural networks underlying emotion processing are abnormal in MDD.5, 6 However, neurobiological research in adolescents has lagged behind that of adults. Due to the significant brain maturational changes that occur during adolescence,7 pathophysiology in adolescent MDD could be different than in adults. Developmental changes may contribute to the increased risk of disease onset during adolescence, while also providing a potential window for intervention to restore developmental trajectories. These considerations underscore the importance of advancing understanding of the neurobiology of adolescent MDD.
The amygdala, an important area for processing threat and orchestrating a complex set of emotional and physiological responses,8 has been centrally implicated in depression.9 Amygdala networks are involved in critical functions relevant to depression including emotion regulation (through connections to frontal and insular areas), modulation of sensory information (through connections with visual, auditory, taste and olfactory cortices), and processing of visceral information in relation to emotional stimuli (through connections with the brain stem).10 Based on the importance of amygdala in emotion systems and its implication in MDD, the current study is focused on examining amygdala networks in adolescents with MDD.
Resting-state functional magnetic resonance imaging (rsfMRI) is an excellent tool for probing neural networks. This approach measures resting-state functional connectivity (RSFC) indexed by the correlation between brain regions in the pattern of spontaneous fluctuation of blood oxygen level dependent (BOLD) signal during rest.11 Positive and negative correlations are understood to reflect synchrony in regions subserving similar and opposite goals, respectively.12 Prior studies have shown that rsfMRI can reliably map RSFC in adults13 and children.14 Research in adults has suggested that MDD involves a deficit in amygdala-frontal connectivity.15 In the first-published rsfMRI study on adolescent depression, we failed to find amygdala RSFC abnormalities in 12 (mostly medicated) adolescents with MDD compared to healthy 14 controls (HC), but documented abnormally low RSFC in a subgenual anterior cingulate cortex (ACC)-based network.16 Since then, several studies have reported abnormal RSFC in children or adolescents with MDD.17–21 However, the only study focusing on amygdala reported that children at risk for depression (due to personal and/or maternal history) had lower negative amygdala RSFC than HC with a dorsal cognitive control networks, and lower positive RSFC with an inferior limbic network.20
The primary goal of this study was to examine amygdala RSFC in adolescents with MDD and HC. To extend beyond prior work, we examined a larger sample of unmedicated adolescents with fully-syndromal MDD and no substance-abuse disorders. Taking into account recent concerns in rsfMRI research,22–24 we incorporated robust methods to address physiological noise and subject motion during scanning, without global signal removal. We predicted that, like adults with MDD,15 adolescents with MDD would show diminished amygdala-frontal RSFC. Given the continuing development of amygdala-frontal projections into adulthood25 and sexual dimorphism in adolescent brain development (e.g.26), we explored group-by-sex and group-by-age interactions. Finally, we explored how amygdala RSFC related to overall depression severity as well as broad set of depression and anxiety symptom dimensions.
Methods
Participants
The University of Minnesota Institutional Review Board approved this study. Participants (or a parent if under 18) provided written informed consent. Participants aged 17 years and younger provided written assent. Adolescents with MDD and HC aged 12 to 19 years were recruited to participate through community postings and referrals from local mental health services. MDD participants were eligible if they had a primary diagnosis of MDD and had not received any psychotropic medication treatment for the past 2 months. HC participants were eligible if they had no current or past psychiatric diagnoses, and were frequency matched to the MDD group on age and sex. Exclusion criteria for both groups included the presence of a neurological or chronic medical condition, mental retardation, pervasive developmental disorder, substance use disorder, bipolar disorder, or schizophrenia.
Assessment
After the informed consent process, all participants completed a comprehensive diagnostic assessment. Interviews were conducted separately with adolescents and parents, and included Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version27 and the Children’s Depression Rating Scales—Revised (CDRS-R).28 Self-report measures assessing symptoms in the past two weeks included the Beck Depression Inventory II (BDI-II)29, 30 and the Inventory of Depression and Anxiety Symptoms (IDAS).31–33 The IDAS provides a score for the following symptom dimensions: general depression, dysphoria, lassitude, insomnia, suicidality, appetite loss, appetite gain, ill temper, well-being, social anxiety, panic, and traumatic intrusion.
MRI Data Acquisition
Data were acquired at the Center for Magnetic Resonance Research at UMN using a Siemens 3T TIM Trio scanner. A five-minute structural scan was acquired using a T1-weighted high-resolution magnetization prepared gradient echo (MPRAGE) sequence: TR = 2530ms; TE = 3.65ms; TI = 1100ms; flip angle = 7 degrees; 1mm slices, FOV = 256, voxel size 1×1×1mm; GRAPPA = 2. The six-minute rsfMRI scan (30 minutes into the overall protocol) was comprised of 180 contiguous echo planar imaging (EPI) whole brain volumes with TR = 2000ms; FOV = 256; voxel size 3.43×3.43×4mm; 34 slices; 64×64 matrix, during which participants were instructed to stay awake with their eyes closed. Physiological data (respiration and cardiac traces) were simultaneously collected. A field map was collected.
Anatomical Imaging Preprocessing
FreeSurfer Version 5.3 (surfer.nmr.mgh.harvard.edu) was used to process T1 data including brain extraction and parcellation of data into a standard set of anatomically-based regions of white and grey matter. FreeSurfer output was visually inspected; when any errors were identified (n = 2) they were manually corrected on a slice-by-slice basis. After ensuring the corrections were satisfactory, the pipeline’s remaining steps were repeated. No corrections were required in the vicinity of the amygdala. The processed T1 data was registered to the rsfMRI data using bbregister.
Resting-State fMRI Preprocessing
Image processing was conducted using tools from the FMRIB software library (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) as well as custom tools developed in MATLAB. Initial processing included brain extraction and motion correction. A denoising procedure was applied incorporating RETROICOR34 to remove physiological noise caused by cardiac and respiratory cycles as well as any linear trends. Correction for magnetic field inhomogeneity-induced geometric distortion was conducted using the field map. FreeSurfer-generated regions of interest (ROIs) for lateral ventricles (cerebrospinal fluid; CSF) and white matter (WM) were aligned to rsfMRI data using FLIRT. Mean BOLD time series within these ROIs were extracted using fslmeants. We performed a regression of each other voxel’s time series on eight nuisance variables: WM time series, CSF time series, and the six motion parameters. Data scrubbing was performed following Power and colleagues,22 excluding any volume with a value for the temporal derivative of time courses’ root mean squared head motion variance (DVARS value) exceeding 8 and/or a framewise dependent value exceeding 0.5, along with the previous volume and the two following volumes. If at least 33% of volumes were removed, participants were excluded from analyses (two MDD and three HC).
RSFC Analysis
First-level
A seed-based, whole-brain approach was used to examine RSFC stemming from left and right amygdala. To avoid misregistration errors, we used anatomically-based ROIs. FreeSurfer-based right and left amygdala ROIs were registered to the pre-processed rsfMRI data, and average time series of voxels in these regions were extracted. These time series were used as primary regressors in separate (left and right) general linear model analyses of each other voxel’s time series, resulting in whole-brain amygdala RSFC maps. We used Gaussian Random Field Theory to correct for multiple testing using a cluster threshold of p < 0.05 and z > 2.3. Additional processing steps included spatial smoothing (5mm kernel), prewhitening, and registration to anatomical data and standard space (MNI 152)35 for later group analysis.
Second-level
As noted in previous work,20 amygdala RSFC maps for left and right amygdala were highly similar to each other (see eFigures 1–2). Therefore, following others,20 to limit the number of tests and for ease of presentation, we conducted a second-level analysis to average each person’s right with their left amygdala RSFC maps.
Third-level
To address the primary study question, we conducted a voxel-wise analysis of mean amygdala RSFC comparing groups, including covariates of age and sex using Gaussian Random Field Theory to correct for multiple comparisons, with a cluster z threshold of 2.3 and p < 0.05. We also conducted exploratory analyses to examine group-by-sex and group-by-age interaction effects on amgydala RSFC throughout the brain.
Fourth-level
A series of follow-up analyses used the significant clusters resulting from group analyses as a mask to extract average z-scores from each participant’s un-thresholded amygdala RSFC map. Within the MDD group, Pearson correlations were conducted on these z-scores with symptom severity (CDRS-R and BDI-II total scores) and IDAS symptom domains. To account for multiple analyses conducted, used Holm’s stepdown Bonferroni Approach (Holm, 1979). Holm’s procedure is less conservative than Bonferroni and, similar to Bonferroni, does not require that the tests be independent. To explore whether additional clinical factors such as prior medication exposure or presence of a comorbid anxiety disorder might have influenced the results, we also compared mean amygdala RSFC within these clusters between adolescents with MDD who were and were not medication naïve, and between adolescents with MDD with and without a current anxiety disorder using an independent samples t test.
Results
Participants
Forty-three unmedicated adolescents with MDD and 31 HC participants completed all procedures. After excluding participants with excessive motion, 41 MDD (73% medication-naïve) and 29 HC adolescents were included in our final analyses (Table 1). There were no significant differences between groups with respect to age, sex or handedness. As expected, the groups differed significantly with respect to CDRS-R scores, BDI-II scores, and IDAS dimension scores. No group differences were detected between MDD medication-naive and medication-free participants, with the exception of IDAS scores for insomnia, panic, and social anxiety (see eTable 1). In the final sample, the number of excluded volumes was marginally different between groups (U(69) = 440.5, p = 0.053), largely because HC had fewer people with zero excluded volumes (see eFigure 3).
Table 1.
Demographic and Clinical Characteristics
| Demographic Characteristics | MDD | HC | P value1 |
|---|---|---|---|
| N | 41 | 29 | |
| Age (mean years ± SD) | 15.7 ± 2 | 16.0 ± 2 | 0.5 |
| Sex (male/female) | 9/32 (78% female) | 7/22 (76% female) | 0.8 |
| Right Handed – n (%) | 32 (91%; n = 35) | 25 (93%; n = 27) | 0.8 |
| Ethnicity – n (%) | 0.1 | ||
| Caucasian | 28 (68%) | 16 (55 %) | |
| African American | 5 (12%) | 1 (4%) | |
| Hispanic | 4 (10%) | 4 (14%) | |
| Asian | 0 | 2 (70%) | |
| Native American | 0 | 1 (3%) | |
| Other | 4 (10%) | 5 (17%) | |
| Current Comorbidities – n (%) | 27 (68%) | N/A | |
| Attention Deficit and Hyperactive Disorder | 6 (15%) | N/A | |
| Generalized Anxiety Disorder | 16 (39%) | N/A | |
| Obsessive-Compulsive Disorder | 1 (3%) | N/A | |
| Oppositional Defiant Disorder | 2 (5%) | N/A | |
| Post-Traumatic Stress Disorder | 2 (5%) | N/A | |
| Social Anxiety | 3 (8%) | N/A | |
| Dysthymia | 2 (5%) | N/A | |
| Panic Disorder | 2 (5%) | N/A | |
| Specific Phobia | 3 (8%) | N/A | |
| Social Phobia | 4 (10%) | N/A | |
| Medication History | |||
| Med-Naïve – n (%) | 30 (73%) | N/A | |
| Past Antidepressant Use –n (%) | 8 (57%) | N/A | |
| Past Stimulants Use | 4 (29%) | N/A | |
| Past Antipsychotic Use | 2 (14%) | N/A | |
| Illness History, Description, Etc | |||
| Duration of illness (mean months±SD) | 10 ± 11 (n = 39) 1 | N/A | |
| Global Assessment of Functioning (mean±SD) | 54 ± 9 | N/A | |
| Positive Family History | 28 (82%; n = 34) | N/A | |
| Clinical Severity | |||
| CDRS-R (T-scores mean±SD) | 77 ± 6 (n = 34) | N/A | |
| BDI-II Most Severe (mean±SD) | 29 ± 13 | 3 ± 4 | <0.0001 |
| IDAS Dimension scores | n = 37 | n = 28 | |
| General Depression Score | 57 ± 16 | 27 ± 4 | <0.0001 |
| Dysphoria Score | 28 ± 9 | 12 ± 3 | <0.0001 |
| Lassitude Score | 19 ± 6 | 9 ± 3 | <0.0001 |
| Insomnia Score | 5 ± 6 | 7 ± 1 | <0.0001 |
| Suicidality Score | 13 ± 7 | 6 ± 0.2 | <0.0001 |
| Loss of Appetite Score | 7 ± 3 | 3 ± 1 | <0.0001 |
| Appetite Gain Score | 6 ± 4 | 4 ± 2 | 0.008 |
| Ill Temper Score | 12 ± 6 | 6 ± 2 | <0.0001 |
| Well-being Score | 18 ± 6 | 27 ± 6 | <0.0001 |
| Social Anxiety Score | 123 ± 5 | 6 ± 1 | <0.0001 |
| Panic Score | 14 ± 7 | 8 ± 1 | <0.0001 |
| Traumatic Intrusion Score | 8 ± 4 | 4 ± 0.5 | <0.0001 |
P values resulted from chi square analyses (sex, ethnicity) or independent samples t-tests (all others).
Exact number of subjects are provided
Group Differences in Amygdala RSFC
Adolescents with MDD showed lower positive amygdala RSFC than HC with a cluster that included left hippocampus and parahippocampus, a small piece of orbitofrontal cortex and temporal pole, and also extended into the brain stem (see Figure 1 and Table 2. Also see eFigure 4 for depiction of the orbitofrontal involvement). Additionally, MDD and HC adolescents differed in amygdala RSFC with bilateral precuneus, where patients showed positive RSFC, while controls showed negative RSFC (see Figure 2 and Table 2). Follow-up analyses revealed no significant group differences between MDD medication-naïve and medication-free participants in these circuits, or between MDD participants with (n=25) versus without (n=16) a comorbid anxiety disorder (defined by the presence of any current anxiety disorder). Our whole-brain analyses to examine group-by age interaction and group-by-sex interaction in mean amygdala RSFC did not reveal any significant clusters. Additionally, when the specific regions that showed group differences were further examined (precuneus and left hippocampus/parahippocampus/brain stem), there were no significant group-by-age or group-by sex interactions.
Figure 1. Lower Amygdala Connectivity in Adolescents with MDD than Controls.
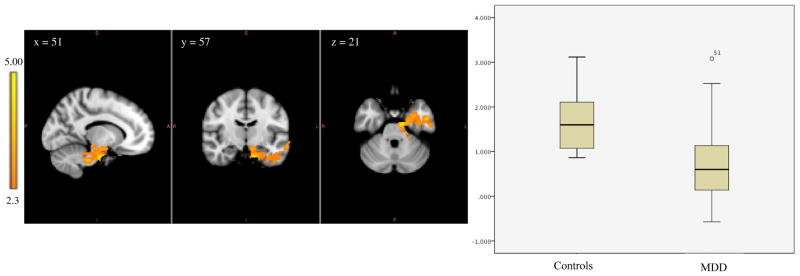
Left image depicts functional connectivity of amygdala-hippocampus and amygdala-brainstem in which the control group has higher functional connectivity compared to the MDD group. The coordinates represent the position of the voxel with the highest intensity in MNI standard space (z-stat = 5.00); Right image compares the range of functional connectivity z-scores between the amygdala and these regions for the two groups.
*Note: Analyses were repeated with MDD outlier removed and remained significant; t(67) = 5.77, p < .001
Table 2.
Size and peak z-values of the significant clusters in the group analyses
| Contrast | Brain Regions | # of Voxels | MNI Coordinates of Peak Voxel (x, y, z) | Peak z-value | Cluster mean z-value ± Standard Deviation |
|---|---|---|---|---|---|
| Controls>MDD | Total cluster | 1831 | 51, 57, 21 | 5.00 | Controls: 1.70 ± 0.67 MDD: 0.75 ± 0.83 |
| Left hippocampus | 143 | ||||
| Left parahippocampus | 274 | ||||
| Brainstem | 362 | ||||
| Left orbitofrontal cortex | 46 | ||||
| Left temporal pole | 34 | ||||
| Left temporal fusiform | 247 | ||||
| MDD> Controls | Bilateral precuneus | 682 | 43, 27, 62 | 4.30 | Controls: −0.79 ± 1.1 MDD: 0.26 ± .89 |
Figure 2. Greater Amygdala Connectivity in Adolescents with MDD than Controls.
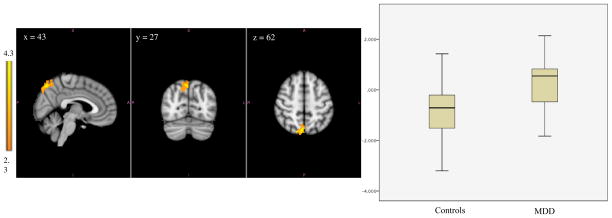
Left image depicts functional connectivity of amygdala-precuneus in which the MDD group has higher functional connectivity compared to the control group. The coordinates represent the position of the voxel with the highest intensity in MNI standard space (z-stat = 4.3). ; Right image compares the range of functional connectivity z-scores between amygdala to precuneus for the two groups.
Correlations with Symptom Domains
Within the depressed group, we used Pearson correlations to examine how amygdala RSFC scores within the clusters identified above relate to clinical severity and IDAS dimensions (Table 3). Several significant correlations were noted for the amygdala-hippocampal/brainstem circuit, where participants with lower positive RSFC in this circuit had greater IDAS general depression, dysphoria and lassitude scores, and lower IDAS well-being scores. However, the summary scores on the CDRS-R and the BDI-II were not significantly correlated with RSFC in identified amygdala networks.
Table 3.
Correlations between amygdala connectivity z-scores and symptom domains for the MDD group
| Hippocampus/parahippocampus/brainstem R (p)* | Precuneus R (p)* | |
|---|---|---|
| Clinical Severity | ||
| CDRS-R total | −0.180 (0.93) | −0.032 (1.00) |
| BDI-Total | −0.324 (0.28) | −0.101 (1.00) |
| IDAS Dimensions | ||
| General Depression Score | −0.523 (0.012) | 0.043 (1.00) |
| Dysphoria Score | −0.455 (0.050) | 0.047 (1.00) |
| Lassitude Score | −0.449 (0.050) | −0.920 (1.00) |
| Insomnia Score | −0.321 (0.30) | 0.200 (0.24) |
| Suicidality Score | −0.202 (0.92) | 0.140 (0.41) |
| Loss of Appetite Score | −0.384 (0.16) | 0.081 (0.64) |
| Appetite Gain Score | 0.093 (1.0) | 0.114 (0.50) |
| Ill Temper Score | 0.114 (1.0) | −0.019 (0.91) |
| Well-being Score | 0.470 (0.03) | 0.179 (0.29) |
| Social Anxiety Score | −0.289 (0.40) | −0.046 (0.79) |
| Panic Score | −0.184 (1.00) | 0.117 (0.49) |
| Traumatic Intrusions | −0.014 (1.00) | 0.247 (0.14) |
p values are corrected for multiple comparisons using Holm’s stepdown Bonferroni approach (Holm 1979). Results that have corrected p ≤ 0.05 are shown in bold.
Discussion
In this study we report abnormal amygdala RSFC in adolescents with MDD compared to HC. The pattern of findings has not been previously identified in the depression literature and may represent important new information about the pathophysiology of MDD in adolescents. Strengths of the present study include the relatively large sample of un-medicated adolescents with MDD (approximately twice the sample size of recent rsfMRI papers in similar populations)17, 18 and the rigorous methods used to remove noise due to physiological signals and motion. Additionally, the results of this study identify a fruitful avenue of future work by providing preliminary evidence that abnormal circuits map on to specific symptom dimensions.
Amygdala-Hippocampus/Parahippocamus RSFC
In this study, adolescents with MDD showed lower positive RSFC than HC between amygdala and a cluster involving left hippocampus and parahippocampus, and this abnormality was associated with lower sense of well-being and higher levels of general depression, dysphoria and lassitude. The amygdala is known to be richly connected with the hippocampus and parahippocampus,36, 37 and positive RSFC between amygdala hippocampus/parahippocampus has been shown in healthy adults.38 Animal models suggest that amygdala-hippocampal connections facilitate the modulation of emotional memories,39–41 and prior work using task fMRI in healthy adults has shown that amygdala-hippocampal connectivity increases during encoding and retrieval of emotional memories.42–44 A study in adults with MDD using a memory task found that patients showed greater amygdala-hippocampal connectivity than controls during successful encoding of negative emotional memories, but no group differences were found for neutral or positive memories.45 However, similar to our findings, a recent study of adults with MDD that used a whole brain, multivariate pattern classification approach identified amygdala-hippocampus as one of many connections showing lower RSFC than HC,46 and two reports in populations at risk for MDD showed similar findings.20, 47 Therefore, it could be that in patients with or at risk for depression, the circuit is under-connected during rest, potentially as a compensatory process to off-set the hyper-connectivity that may occur during processing of negative emotional memories, and/or the general hyperactivity of amygdala in depression.48, 49 These speculations require further investigation examining (a) the dynamic change of amygdala-hippocampal connections across states of rest, memory encoding and memory retrieval; (b) whether this abnormality represents a direct manifestation of illness or an adaptation due to another abnormality (e.g. excessive amygdala activation in depression); and (c) how RSFC and the related functions of this circuit might be restored as a consequence of treatment for depression.
Amygdala-Brainstem RSFC
Our findings show decreased RSFC between amygdala and brainstem in adolescents with MDD, which to our knowledge has not previously been reported in depression literature. Animal research has identified amygdala-brainstem connectivity as an important network for modulating visceral function in relation to emotional stimuli.10 Excitatory pathways extend from amygdala to brainstem centers such as periaqueductal gray, locus ceruleus, raphe nucleus, and autonomic-related brainstem nuclei; modulatory pathways from these centers project back to the amygdala.50 These pathways are important for basic functioning such as arousal and appetitive drives. In the current study, RSFC in this circuit correlated with lassitude, and, at a trend level of p ≤ 0.05, appetite loss and insomnia. These preliminary findings suggest that impaired connectivity in this circuit underlies some of the vegetative aspects of depression. Further research probing this hypothesis with experimental paradigms to assess arousal systems are needed to test this hypothesis.
Amygdala-Precuneus RSFC
In this study, adolescents with MDD had positive RSFC between amygdala and precuneus in contrast to healthy adolescents who showed negative RSFC in this circuit. The precuneus is involved in processing of self-relevant information51–57 and in episodic memory encoding and retrieval.55, 58 It is an important node within the default mode network, a group of brain regions that are more active at rest than during a task.59 Negative amygdala-precuneus RSFC has been documented in studies of healthy adults38, 60. Again, although this circuit has not previously been highlighted in depression literature, recent reports have noted a similar pattern in adults with high levels of neuroticism had positive amygdala-precuneus RSFC,61 children with personal or maternal history of MDD,20 and adults with a history of childhood maltreatment.47 Together, these findings suggest that impaired negative RSFC (or in the case of our study, the presence of positive connectivity) between two regions with opposing functions (rest versus threat) may be an important mechanism in depression. Positive synchrony between these regions during rest could underlie a failure to suppress negative self-thoughts that spontaneously emerge during rest. Alternatively, this synchrony could contribute to “disproportionate emotional coloring of self-referential or autobiographical information processing.”61 Both of these possibilities could feasibly perpetuate clinical features seen in depression such as rumination and the persistently negative mood state.
Amygdala-Frontal RSFC
We predicted that adolescents with MDD would show an amygdala-frontal RSFC deficit. However, the results revealed the deficit to be primarily in subcortical regions (e.g. hippocampus and brain stem). Only a small piece of the cluster representing lower amygdala RSFC in patients than controls extended into orbitofrontal cortex (eFigure 4). Several prior studies in adults have documented impaired amygdala-frontal RSFC, with mixed results regarding location and whether the impairment is in positive or negative RSFC.15, 62–64 Variance in findings across depression studies could arise from methodology differences, heterogeneity of illness, and/or developmental effects.65 Perhaps because the frontal lobe and its limbic connections are still developing during adolescence,7, 25 adolescents with depression show a different pattern than adults, with more prominent findings in subcortical areas that mature earlier. It may be that amygdala-frontal RSFC deficits emerge during early adulthood as the MDD versus HC gap in frontal development trajectories widen. Although the results of our age-by-group interaction analysis do not support this hypothesis, longitudinal research examining the trajectory of amygdala RSFC across development into adulthood in youth with and without MDD will be necessary to further examine this question.
Limitations
We have interpreted our amygdala RSFC group difference findings based on the clinical features of depression and what is known about the function of the implicated brain regions. However, these interpretations of our observational data should be considered preliminary and speculative. Confirmation of the hypotheses suggested here will require further research utilizing a multi-modal approach that includes behavioral methods capable of investigating the function of the circuits in question (e.g. self-processing, emotional memory, etc.). Further, our findings regarding clinical correlations between RSFC and symptom dimensions should be interpreted with caution because large number of tests that were conducted relative to the sample size. Future research is needed with larger samples to further examine the relationships with symptom dimensions.
The cross-sectional nature of this study prohibits causal interpretations of the results. It is unclear whether the abnormalities reported represent risk markers for MDD or if they emerge during the course of illness as a result of disease processes. Longitudinal research using these measures, ideally beginning with high-risk adolescents prior to illness onset and tracking the course of illness after onset, is needed to address these questions.
Similar to other adolescent depression studies (e.g. 16–18, 66) the participants in our sample had relatively high rates of current comorbid anxiety disorders. This is a limitation because the findings may not be specific depression. However, post-hoc analyses comparing patients with and without anxiety disorders on the amygdala-hippocampus and amygdala-precuneus circuits did not reveal any significant differences. Further, because there were no significant associations between the main amygdala connectivity findings with any of the anxiety dimensions from the IDAS, the abnormalities appear to be more related to depression than anxiety symptoms in these patients.
As has been recently highlighted in the literature,22, 23 subject motion during the scan can significantly impact rsfMRI findings. Several methods have been proposed outlining approaches to reduce the impact of motion artifacts, one of which we incorporated in our study.22 We removed volumes exceeding our threshold, resulting in variance across participants in the number of volumes for final analysis. As RSFC can change over time,67 this introduces the possibility that removed volumes could potentially have altered the overall RSFC measure. We and others22 believe that the potential for introducing artificial correlations from motion artifacts was a far greater risk than that of losing these short, randomly-spaced epochs of resting data.
Certain limitations arise from our seed-based approach. We used a hypothesis-driven approach for our rsfMRI data analysis, correlating the time series of a seed ROI with every voxel as an index of whole-brain functional RSFC.11 This approach limits the results based on which seed region is chosen. Data-driven approaches avoid this limitation, but results can be more difficult to interpret. Further, we used an ROI of the entire amygdala, but prior work has shown that amygdala subregions have known dissociable functional networks.38 Future research should investigate how RSFC patterns in adolescent MDD vary across amygdala subregions; such research would benefit from recent advances in acquisition methods which allow for higher spatial resolution.68 Many studies define seeds by creating a sphere in standard space around a location from published literature,16, 69 or using an atlas-defined region.15 Such approaches have limitations inherent to between-person differences in anatomy. To address this, we defined our seed regions based on each individual’s anatomy using FreeSurfer. This introduces the potential limitations of the automated approach to accurately parcellate the anatomy. However, FreeSurfer-based parcellation of the amygdala is superior in some respects compared to other automated methods.70, 71 Several studies have been recently published using FreeSurfer to investigate amygdala volume in different populations with a range of psychopathology.72–75 Further, we visually inspected each person’s FreeSurfer results to identify and correct errors, which did not occur in our regions of interest.
In summary, we report abnormal amygdala RSFC in the largest sample to date of adolescents with MDD. The findings could reflect impairments in the networks that process spontaneous memories that arise during rest and underlie persistent negative mood and vegetative symptoms in these adolescents. Future research using multi-modal approaches that incorporate experimental paradigms to probe relevant systems implicated in memory, self-processing and arousal would be ideal for further illuminating brain-behavior relationships in adolescents with MDD. Given the differences from previous findings in adults, it may be that RSFC abnormalities evolve over the course of development. Future longitudinal research is needed to understand how RSFC changes over development, course of illness, and treatment response in adolescents with MDD.
Supplementary Material
Figure 3.
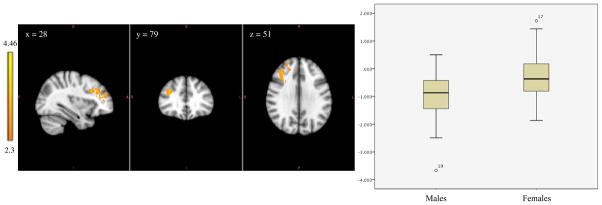
Left image shows amygdala-frontal functional connectivity in which males show greater negative functional connectivity compared to females. The coordinates represent the position of the voxel with the highest intensity in MNI standard space (z-stat = 4.46); Right image compares the range of functional connectivity z-scores between the amygdala and these regions for the two groups.
Acknowledgments
The authors would like to first and foremost thank the patients and families that contributed to this study. The study was funded by the National Institute of Mental Health (K23MH090421 to Dr. Cullen), the National Alliance for Research on Schizophrenia and Depression, the University of Minnesota Graduate School, and the Minnesota Medical Foundation. These resources supported the roles of design and conduct of the study; collection, management, and analysis of the data; and interpretation of results and preparation of the publication.
References
- 1.Ustun TB, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJ. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004 May;184:386–392. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Walters EE. Epidemiology of DSM-III-R major depression and minor depression among adolescents and young adults in the National Comorbidity Survey. Depress Anxiety. 1998;7(1):3–14. doi: 10.1002/(sici)1520-6394(1998)7:1<3::aid-da2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.Maalouf FT, Atwi M, Brent DA. Treatment-resistant depression in adolescents: review and updates on clinical management. Depress Anxiety. 2011 Nov;28(11):946–954. doi: 10.1002/da.20884. [DOI] [PubMed] [Google Scholar]
- 4.March J, Silva S, Petrycki S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. Jama. 2004 Aug 18;292(7):807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 5.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997 Summer;9(3):471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 6.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999 Jun 29;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 7.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999 Oct;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 8.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 9.Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003 Apr;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 10.Price JL. Comparative aspects of amygdala connectivity. Ann N Y Acad Sci. 2003 Apr;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- 11.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995 Oct;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 12.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005 Jul 5;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shehzad Z, Kelly AM, Reiss PT, et al. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009 Oct;19(10):2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomason ME, Dennis EL, Joshi AA, et al. Resting-state fMRI can reliably map neural networks in children. Neuroimage. 2011 Mar 1;55(1):165–175. doi: 10.1016/j.neuroimage.2010.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Y, Kong L, Wu F, et al. Decreased functional connectivity between the amygdala and the left ventral prefrontal cortex in treatment-naive patients with major depressive disorder: a resting-state functional magnetic resonance imaging study. Psychol Med. 2012 Sep;43(9):1921–1927. doi: 10.1017/S0033291712002759. [DOI] [PubMed] [Google Scholar]
- 16.Cullen KR, Gee DG, Klimes-Dougan B, et al. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci Lett. 2009 Sep 4;460(3):227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabbay V, Ely BA, Li Q, et al. Striatum-based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry. 2013 Jun;52(6):628–641. e613. doi: 10.1016/j.jaac.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connolly CG, Wu J, Ho TC, et al. Resting-State Functional Connectivity of Subgenual Anterior Cingulate Cortex in Depressed Adolescents. Biol Psychiatry. 2013 Jul 30; doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin C, Gao C, Chen C, et al. A preliminary study of the dysregulation of the resting networks in first-episode medication-naive adolescent depression. Neurosci Lett. 2011 Oct 3;503(2):105–109. doi: 10.1016/j.neulet.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Luking KR, Repovs G, Belden AC, et al. Functional connectivity of the amygdala in early-childhood-onset depression. J Am Acad Child Adolesc Psychiatry. 2011 Oct;50(10):1027–1041. e1023. doi: 10.1016/j.jaac.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaffrey MS, Luby JL, Repovs G, et al. Subgenual cingulate connectivity in children with a history of preschool-depression. Neuroreport. 2010 Dec 29;21(18):1182–1188. doi: 10.1097/WNR.0b013e32834127eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012 Feb 1;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satterthwaite TD, Wolf DH, Loughead J, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012 Mar;60(1):623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009 Feb 1;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002 Nov 11;453(2):116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- 26.Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007 Jul 15;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997 Jul;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Poznanski EO, Freman LN, Mokros HB. Children’s depression rating scale-revised. Psychopharmacology Bulletin. 1985;21:979–989. [Google Scholar]
- 29.Beck AT, Steer RA, Brown KB. Beck Depression Inventory - Revised. San Antonio, Texas: Harcourt Brace; 1996. [Google Scholar]
- 30.Osman A, Kopper BA, Barrios F, Gutierrez PM, Bagge CL. Reliability and validity of the Beck depression inventory--II with adolescent psychiatric inpatients. Psychol Assess. 2004 Jun;16(2):120–132. doi: 10.1037/1040-3590.16.2.120. [DOI] [PubMed] [Google Scholar]
- 31.Watson D, O’Hara MW, Simms LJ, et al. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS) Psychol Assess. 2007 Sep;19(3):253–268. doi: 10.1037/1040-3590.19.3.253. [DOI] [PubMed] [Google Scholar]
- 32.Watson D, O’Hara MW, Chmielewski M, et al. Further validation of the IDAS: evidence of convergent, discriminant, criterion, and incremental validity. Psychol Assess. 2008 Sep;20(3):248–259. doi: 10.1037/a0012570. [DOI] [PubMed] [Google Scholar]
- 33.Watson D, O’Hara MW, Naragon-Gainey K, et al. Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II) Assessment. 2012 Dec;19(4):399–420. doi: 10.1177/1073191112449857. [DOI] [PubMed] [Google Scholar]
- 34.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000 Jul;44(1):162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 35.Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM) Neuroimage. 1995 Jun;2(2):89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- 36.Amaral DG, Insausti R. Retrograde transport of D-[3H]-aspartate injected into the monkey amygdaloid complex. Exp Brain Res. 1992;88(2):375–388. doi: 10.1007/BF02259113. [DOI] [PubMed] [Google Scholar]
- 37.Stefanacci L, Suzuki WA, Amaral DG. Organization of connections between the amygdaloid complex and the perirhinal and parahippocampal cortices in macaque monkeys. J Comp Neurol. 1996 Nov 25;375(4):552–582. doi: 10.1002/(SICI)1096-9861(19961125)375:4<552::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 38.Roy AK, Shehzad Z, Margulies DS, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009 Apr 1;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci. 1999 Mar;2(3):289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- 40.Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997 Nov;68(3):285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- 41.Kemppainen S, Jolkkonen E, Pitkanen A. Projections from the posterior cortical nucleus of the amygdala to the hippocampal formation and parahippocampal region in rat. Hippocampus. 2002;12(6):735–755. doi: 10.1002/hipo.10020. [DOI] [PubMed] [Google Scholar]
- 42.Smith AP, Stephan KE, Rugg MD, Dolan RJ. Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron. 2006 Feb 16;49(4):631–638. doi: 10.1016/j.neuron.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 43.Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, Labar KS. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia. 2005;43(5):659–674. doi: 10.1016/j.neuropsychologia.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci U S A. 2005 Feb 15;102(7):2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatry. 2008 Jun 15;63(12):1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng LL, Shen H, Liu L, et al. Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain. 2012 May;135(Pt 5):1498–1507. doi: 10.1093/brain/aws059. [DOI] [PubMed] [Google Scholar]
- 47.van der Werff SJ, Pannekoek JN, Veer IM, et al. Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychol Med. 2013 Sep;43(9):1825–1836. doi: 10.1017/S0033291712002942. [DOI] [PubMed] [Google Scholar]
- 48.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001 Nov 1;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 49.Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002 Mar;71(3):431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- 50.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010 Jan;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kircher TT, Senior C, Phillips ML, et al. Towards a functional neuroanatomy of self processing: effects of faces and words. Brain Res Cogn Brain Res. 2000 Sep;10(1–2):133–144. doi: 10.1016/s0926-6410(00)00036-7. [DOI] [PubMed] [Google Scholar]
- 52.Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage. 2002 Oct;17(2):1080–1086. [PubMed] [Google Scholar]
- 53.Amft M, Bzdok D, Laird AR, Fox PT, Schilbach L, Eickhoff SB. Definition and characterization of an extended social-affective default network. Brain Struct Funct. 2014 Jan 8; doi: 10.1007/s00429-013-0698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007 Feb;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006 Mar;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 56.Kircher TT, Brammer M, Bullmore E, Simmons A, Bartels M, David AS. The neural correlates of intentional and incidental self processing. Neuropsychologia. 2002;40(6):683–692. doi: 10.1016/s0028-3932(01)00138-5. [DOI] [PubMed] [Google Scholar]
- 57.Lou HC, Luber B, Crupain M, et al. Parietal cortex and representation of the mental Self. Proc Natl Acad Sci U S A. 2004 Apr 27;101(17):6827–6832. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ. The mind’s eye--precuneus activation in memory-related imagery. Neuroimage. 1995 Sep;2(3):195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- 59.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001 Jan 16;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang S, Li CS. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage. 2012 Feb 15;59(4):3548–3562. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aghajani M, Veer IM, van Tol MJ, et al. Neuroticism and extraversion are associated with amygdala resting-state functional connectivity. Cogn Affect Behav Neurosci. 2013 Dec 19; doi: 10.3758/s13415-013-0224-0. [DOI] [PubMed] [Google Scholar]
- 62.Tahmasian M, Knight DC, Manoliu A, et al. Aberrant intrinsic connectivity of hippocampus and amygdala overlap in the fronto-insular and dorsomedial-prefrontal cortex in major depressive disorder. Front Hum Neurosci. 2013;7:639. doi: 10.3389/fnhum.2013.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yue Y, Yuan Y, Hou Z, Jiang W, Bai F, Zhang Z. Abnormal functional connectivity of amygdala in late-onset depression was associated with cognitive deficits. PLoS One. 2013;8(9):e75058. doi: 10.1371/journal.pone.0075058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veer IM, Beckmann CF, van Tol MJ, et al. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010:4. doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharpley CF, Bitsika V. Differences in neurobiological pathways of four “clinical content” subtypes of depression. Behav Brain Res. 2013 Aug 28;256C:368–376. doi: 10.1016/j.bbr.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 66.The Treatment for Adolescents With Depression Study (TADS): demographic and clinical characteristics. J Am Acad Child Adolesc Psychiatry. 2005 Jan;44(1):28–40. doi: 10.1097/01.chi.0000145807.09027.82. [DOI] [PubMed] [Google Scholar]
- 67.Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage. 2010 Mar;50(1):81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ugurbil K, Xu J, Auerbach EJ, et al. Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project. Neuroimage. 2013 May 21; doi: 10.1016/j.neuroimage.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007 Aug 15;37(2):579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 70.Dewey J, Hana G, Russell T, et al. Reliability and validity of MRI-based automated volumetry software relative to auto-assisted manual measurement of subcortical structures in HIV-infected patients from a multisite study. Neuroimage. 2010 Jul 15;51(4):1334–1344. doi: 10.1016/j.neuroimage.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morey RA, Petty CM, Xu Y, et al. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009 Apr 15;45(3):855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Butterworth P, Cherbuin N, Sachdev P, Anstey KJ. The association between financial hardship and amygdala and hippocampal volumes: results from the PATH through life project. Soc Cogn Affect Neurosci. 2012 Jun;7(5):548–556. doi: 10.1093/scan/nsr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rahman AS, Xu J, Potenza MN. Hippocampal and Amygdalar Volumetric Differences in Pathological Gambling: A Preliminary Study of the Associations with the Behavioral Inhibition System. Neuropsychopharmacology. 2013 Sep 27; doi: 10.1038/npp.2013.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haukvik UK, McNeil T, Lange EH, et al. Pre- and perinatal hypoxia associated with hippocampus/amygdala volume in bipolar disorder. Psychol Med. 2013 Jun 27;:1–11. doi: 10.1017/S0033291713001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chao L, Weiner M, Neylan T. Regional cerebral volumes in veterans with current versus remitted posttraumatic stress disorder. Psychiatry Res. 2013 Sep 30;213(3):193–201. doi: 10.1016/j.pscychresns.2013.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


