Abstract
Enucleation of a recipient oocyte is one of the key processes in the procedure of somatic cell nuclear transfer (SCNT). However, especially in bovine species, lipid droplets spreading in the ooplasm hamper identification and enucleation of metaphase II (MII) chromosomes, and thereby the success rate of the cloning remains low. In this study we used a new experimental system that enables fluorescent observation of chromosomes in living oocytes without any damage. We succeeded in visualizing and removing the MII chromosome in matured bovine oocytes. This experimental system consists of injecting fluorescence-labeled antibody conjugates that bind to chromosomes and fluorescent observation using a conventional halogen-lamp microscope. The cleavage rates and blastocyst rates of bovine embryos following in vitro fertilization (IVF) decreased as the concentration of the antibody increased (p<0.05). The enucleation rate of the conventional method (blind enucleation) was 86%, whereas all oocytes injected with the antibody conjugates were enucleated successfully. Fusion rates and developmental rates of SCNT embryos produced with the enucleated oocytes were the same as those of the blind enucleation group (p>0.05). For the production of SCNT embryos, the new system can be used as a reliable predictor of the location of metaphase plates in opaque oocytes, such as those in ruminant animals.
Introduction
Mammalian cloning by somatic cell nuclear transfer (SCNT) has been achieved in various species and provides opportunities for multireplication of elite animals, basic and applied research in developmental biology, and the conservation of endangered species. However, the efficiency of SCNT remains low due to high rates of embryonic, perinatal, and neonatal loss (Han et al., 2003; Wilmut et al., 2002).
A difficult step in the production of SCNT embryos is the removal of all genetic material from matured oocytes to avoid chromosomal abnormalities. The metaphase II (MII) chromosomes in oocytes of experimental animals such as mice and rabbits are visible under a conventional microscope, e.g., differential interference contrast, which makes them easy to remove. However, the chromosomes of the oocytes of domestic animals such as cattle and pigs are difficult to remove because they are obscured by numerous lipid granules (Tatham et al., 1995). Two main methods have been used to remove chromosomes from recipient oocytes of these domestic animals (Fulka et al., 2004; Li et al., 2004). One is the method in which MII chromosomes are visualized by staining the oocytes with Hoechst 33342 dye, and the chromosomes are removed under visible light and ultraviolet (UV) irradiation (Critser and First, 1986; Smith, 1993). In the second method, the chromosomes along with a small volume of cytoplasm are removed by guiding a position of the first polar body without any staining of chromosomes (McGrath and Solter, 1983; Prather et al., 1989). However, there are disadvantages of these methods. In the first method, exposure to UV irradiation is detrimental to oocytes. For the second method, the volume of removed cytoplasm increases and enucleation rates vary widely among laboratories (40–90%) depending on the operator's skill (Dominko et al., 2000; Jeon et al., 2011; Prather et al., 1989; Tani et al., 2006). Hence, establishing an experimental method in which the MII chromosomes of oocytes from domestic animals could be visible under the manipulator without any detrimental effect to development would be a powerful tool for animal production.
Recent advances in fluorescence microscopy and the development of new vital fluorescent probes have provided useful tools for investigations in the biological field, such as live-cell imaging in which molecules and cellular components are observed in living conditions (Kimura et al., 2010). Moreover, a new vital fluorescent probe to the metaphase chromosomes, namely a phycoerythrin-labeled antibody against phosphorylated serine-10 residue of histone H3 (H3S10ph), has been developed. It has been reported that phosphorylation of histone H3S10 occurs specifically in chromosomes of somatic cells and oocytes at the metaphase stage (Bui et al. 2004; Swain et al. 2007; Van Hooser et al. 1998; Wei et al. 1998). Recently, we demonstrated that the metaphase plate of the second meiotic division in mouse and cattle oocytes is clearly visible after injection of an antibody under a halogen-lamp microscope (Yamagata et al., 2012).
In this study, we first determined the optimal concentration of the antibody (phycoerythrin-labeled anti-histone H3S10ph antibody) for detection of the chromosomes in matured bovine oocytes. Furthermore, we examined effects of this antibody injected into oocytes on early development of bovine embryos following in vitro fertilization and SCNT embryos with enucleated oocytes after injection of the antibody.
Materials and Methods
Ethics
All experimental procedures involving animals were approved by the Animal Research Ethics Committee of Kinki University (#KAAT-21-001).
Setup of microscope with a transmission fluorescence filter system
The microscope setup with a transmission fluorescence filter system used for manipulation and observation was described previously (Yamagata et al., 2012). Briefly, an inverted microscope (IX-70, Olympus, Tokyo, Japan) equipped with a 100 W halogen lamp was used for manipulation and observation. The filter adaptor previously made by us was placed on the top of the condenser [numerical aperture (NA)=0.5] of the IX-70 (Yamagata et al., 2012). The optical axis of the condenser was adjusted before setting the adaptor to the condenser. To observe phycoerythrin, a bandpass filter (480–555 nm; Olympus) was inserted into the filter adapter and a 580-nm barrier filter was set in the filter cube without a dichroic mirror (U-MWIG 3; Olympus). In this study, an Olympus objective lens (UPlanSApo; NA=0.75; 20×) was used.
Preparation of donor cells for nuclear transfer
Bovine fibroblast cells were obtained from ear skin samples from a 5-month-old Japanese Black male calf. The cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM; Nissui Pharmaceutical, Tokyo, Japan) supplemented with 10% (vol/vol) fetal bovine serum (FBS; BioWest, Paris France, 10% FBS-DMEM) at 37°C in 5% CO2 in air with high humidity. The cells were used at passages 10–13, and synchronized at the G0/G1 phase of the cell cycle by culturing to 80–90% confluence.
Oocytes collection and in vitro maturation
Bovine oocytes were matured as reported previously (Saeki et al., 1998). Briefly, bovine ovaries were obtained from a local slaughterhouse and were transported in saline at 20–25°C. Cumulus–oocyte complexes (COCs) were collected from the ovaries, and then they were washed with 25 mM HEPES-buffered tissue culture medium-1999 (TCM-199) with Hanks' salts (199H; Gibco, Invitrogen Life Technologies, Tokyo, Japan) supplemented with 5% (vol/vol) FBS and 25 μL/mL gentamicin (FBS199H). The washed COCs were matured for 18–21 h in 50 μL of 25 mM HEPES-buffered TCM-199 with Earle's salts (199E; Gibco) supplemented with 5% FBS, 0.5 mM sodium pyruvate, 25 μg/mL gentamicin, 0.02 AU/mL follicle-stimulating hormone (FSH) (Antrin; Kyoritsu Pharmaceutical, Tokyo, Japan), and 1 μg/mL estradiol-17β and covered with paraffin oil at 39°C in 5% CO2 in air in high humidity (10 COCs/droplet).
Injection of antibody labeled with phycoerythrin
To detect chromosomes of matured bovine oocytes, phycoerythrin-labeled anti-histone H3S10ph antibody (Hayashi-Takanaka et al., 2009; Yamagata et al., 2012) was injected into the oocytes. The original concentration of the antibody was 1500 μg/mL; the antibody was dissolved in Milli-Q water at 1:5 (300μg/mL), 1:10 (150μg/mL), 1:20 (75μg/mL), and 1:100 (15μg/mL) dilutions. For injection, matured bovine oocytes were placed in a 5-μL droplet of FBS199H covered with paraffin oil. The antibody was then injected into the cytoplasm of the oocytes using an injection pipette (5 μm diameter) with a piezo-driven manipulator. The injection volume was approximately 25 pL and estimated from the displacement of the meniscus of the mercury in the pipette. After the injection, oocytes were transferred into a 50-μL droplet of FBS199E, covered with paraffin oil, and incubated at 39°C in 5% CO2 in air with high humidity.
In vitro fertilization
Frozen–thawed spermatozoa were washed twice by centrifugation at 700×g for 7 min in IVF 100 medium (Research Institute for the Functional Peptides, Yamagata, Japan). The sedimented spermatozoa were resuspended with IVF 100 medium. Matured oocytes injected with the antibody were transferred into fertilization droplets under mineral oil. The spermatozoa were then introduced into fertilization droplets containing oocytes. The oocytes and spermatozoa (4×106 sperm/mL and 10 COCs/100 μL droplet) were co-cultured for 6 h at 39°C and 5% CO2 in air with high humidity. Six hours after insemination, the surrounding cumulus cells and spermatozoa were completely removed from the oocytes.
Somatic cell nuclear transfer
SCNT was carried out essentially as described previously (Iwamoto et al., 2012). Recipient oocytes were enucleated under a halogen-lamp microscope using fluorescence imaging as follows. The surrounding cumulus cells were removed by pipetting from COCs at 18–21 h postmaturation in FBS199H containing 0.25% (wt/vol) hyaluronidase. Phycoerythrin-labeled antibody was injected into the denuded oocytes with the polar body. The location of chromosomes was revealed by fluorescence of the phycoerythrin (Fig. 1), and the zona pellucida proximately above the fluorescence was then cut. The cytoplasm, including the fluorescent chromosomes, was removed by pressing the oocyte with a glass needle. For a control, the zona pellucida above the first polar body of an oocyte that had not been injected with the fluorescence-labeled antibody was cut using a fine glass needle. The cytoplasm beneath the first polar body was removed by pressing the oocyte with the glass needle. For both groups, enucleation was confirmed by visualizing the maternal chromosomes under UV light after staining the karyoplast with 20 μg/mL Hoechst 33342.
FIG. 1.
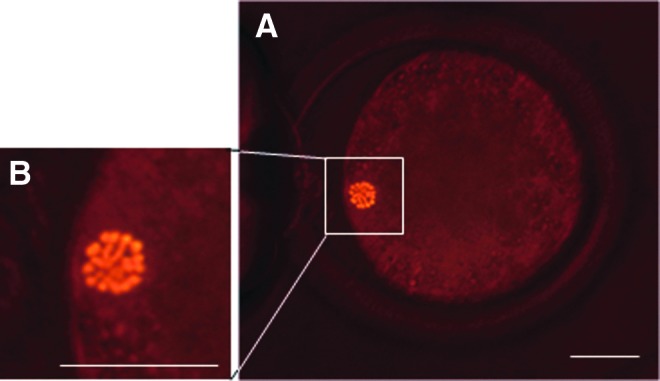
Fluorescence images of bovine oocyte injected with phycoerythrin-labeled antibody. (A) Fluorescence image of bovine oocyte injected with phycoerythrin-labeled antibody. (B) A magnified image shows individual chromosomes of the oocyte were detected clearly above the fluorescence under the halogen light. Scale bar, 30 μm. Color images available online at www.liebertpub.com/cell
Cultured fibroblast cells synchronized at the G0/G1 phase were injected into the perivitelline space of the enucleated oocytes. These couplets were held by two needle electrodes and were electrically fused by two direct current pulses of 2.72 kV/cm for 11 μsec each using an Electro Cell Manipulator (ECM-200; BTX, San Diego, CA, USA) in Zimmerman fusion medium. Fused couplets were activated with 5 μM ionomycin for 5 min and then treated with 10 μg/mL cycloheximide in CR1aa (Rosenkrans et al., 1993) supplemented with 5% (vol/vol) FBS (5%FBS/CR1aa) until 6 h post fusion (hpf) at 39[°C in 5% CO2, 5% O2, and 90% N2 in high humidity.
In vitro culture
The embryos produced by in vitro fertilization (IVF) or SCNT were cultured in 50 μL of 5% FBS-CR1aa (Rosenkrans et al., 1993) covered with paraffin oil at 39°C in 5% CO2, 5% O2, and 90% N2 in high humidity (20–30 embryos/droplet). Rates of cleaved embryos and blastocysts were evaluated at 168 h following the insemination or fusion.
Experimental Design
Experiment 1: Effects of antibody concentration on the intensity and duration of fluorescence
First, we determined the optimal concentration of the anti-H3S10ph antibody–phycoerythrin conjugates that allow the easy detection and enucleation of metaphase plates in matured bovine oocytes. Fluorescence signals in the oocytes after injection were measured using a conventional halogen-lamp microscope with a special fluorescence filter unit (Yamagata et al, 2012). After in vitro maturation, several concentrations of the antibody conjugates (15, 75, 150, 300, and 1500 μg/mL) were injected into cytoplasm of the oocytes, and they were incubated at 39°C and 5% CO2 in air with high humidity for 10 min. Then, fluorescence signals in the oocytes were measured using the halogen-lamp microscope. Next, we examined the effect of incubation time after antibody conjugates injection on the intensity of fluorescence signal of chromosomes in the injected oocytes. The antibody conjugates (concentrations150 and 300 μg/mL) were injected into the cytoplasm of the oocytes, and the injected oocytes were incubated for 0, 5, 10, 30, or 60 min. The fluorescence signal in the oocytes was observed using the halogen-lamp microscope. For quantitative analysis of the intensity, the fluorescent image was measured with an image analyzer system (Aqua Cosmos, Hamamatsu, Japan). Intensity of chromosomes and background fluorescence were measured by manually outlining the chromosomes and the cytoplasm of the oocyte, respectively. Mean intensity was calculated by summing intensity for each pixel within the selected region and dividing by the total number of pixels. The mean intensity of chromosomes fluorescence per pixel was subtracted from the mean intensity of background fluorescence per pixel in each oocyte.
Experiment 2: Effects of antibody concentration on early development of bovine IVF embryos
Cumulus cells were removed from matured oocytes by gentle pipetting and kept in an IVM medium until IVF. The denuded oocytes were injected with the antibody–phycoerythrin conjugates (concentrations, 0, 150, 300, and 1500 μg/mL), and the oocytes and cumulus cells were washed in IVF 100 medium. Denuded oocytes that were not injected were used as a control. The fertilization droplets also contained cumulus cells removed from matured COCs to enhance the fertilization (Saeki et al., 1994). After washing, the oocytes were inseminated with frozen–thawed spermatozoa and incubated for 6 h (39°C and 5% CO2 in air with high humidity). Following fertilization, the cumulus cells and sperm were removed from the oocytes, and the IVF embryos were cultured in 5% FBS-CR1aa (39°C in 5% CO2, 5% O2, and 90% N2 in high humidity) up to 168 h after insemination.
Experiment 3: Comparison of blind and antibody enucleation methods for production of SCNT embryos and their subsequent development
First, the percentage of oocytes that were successfully enucleated by antibody enucleation method was examined. The early development of SCNT embryos produced with the enucleated oocytes was examined 168 h after fusion with somatic cells. For enucleation, the antibody (concentration of 150 and 300 μg/mL) was injected into the cytoplasm of the denuded oocytes. After antibody injection, a small volume of cytoplasm including the fluorescent chromosomes was removed by pressing the oocyte and using phycoerythrin fluorescence under a halogen-light microscope. As a control, a small amount of cytoplasm and the first polar body were removed by pressing the uninjected oocytes without the aid of a fluorescence image. Enucleation was confirmed by visualizing the maternal chromosome under UV light after staining the removed karyoplast with 20 μg/mL Hoechst 33342. The enucleated oocytes were fused with fibroblasts to produce SCNT embryos. Following activation, the SCNT embryos were cultured in 5%FBS-CR1aa (39°C in 5% CO2, 5% O2, and 90% N2 in high humidity) up to 168 h after fusion.
Differential staining of inner cell mass and trophectoderm cells in blastocysts
Inner cell mass (ICM) and trophectoderm (TE) cells of blastocysts were differentially stained as described previously (Thouas et al., 2001). Briefly, blastocysts were incubated in the first solution (TCM-199 containing 1% Triton X100 and 100 μg/mL propidium iodide [PI]) for 30 sec, and then incubated in the second solution [fixative solution of phosphate-buffered saline (PBS) containing 0.1% polyvinyl alcohol (PVA), 4% paraformaldehyde, and 25 μg/mL Hoechst 33342] for 30 min at room temperature. The fixed and stained blastocysts were washed twice in PBS containing PVA and then mounted on a drop of Vectashield and observed under an inverted fluorescence microscope (IX-71, Olympus). The number of total and TE cells was counted from Hoechst (blue) and PI (red) images. The number of ICM cells was calculated by subtracting the number of TE cells from the number of total cells.
Statistical analysis
All experiments were replicated at least three times. Data were analyzed using StatView software (v. J-4.11; Abacus Concepts, Abacus Concepts, Berkeley, CA, USA). The data obtained from luminescence activity (experiment 1) and cell number of the blastocysts (experiments 2 and 3) were analyzed with Tukey–Kramer post hoc tests for multiple comparisons following analysis of variance (ANOVA). Differences of p<0.05 were considered to be significant. The early development data of IVF and SCNT embryos (experiments 2 and 3) were analyzed with Fisher protected least significant difference (PLSD) tests following ANOVA. Differences of p<0.05 were considered to be significant.
Results
Experiment 1
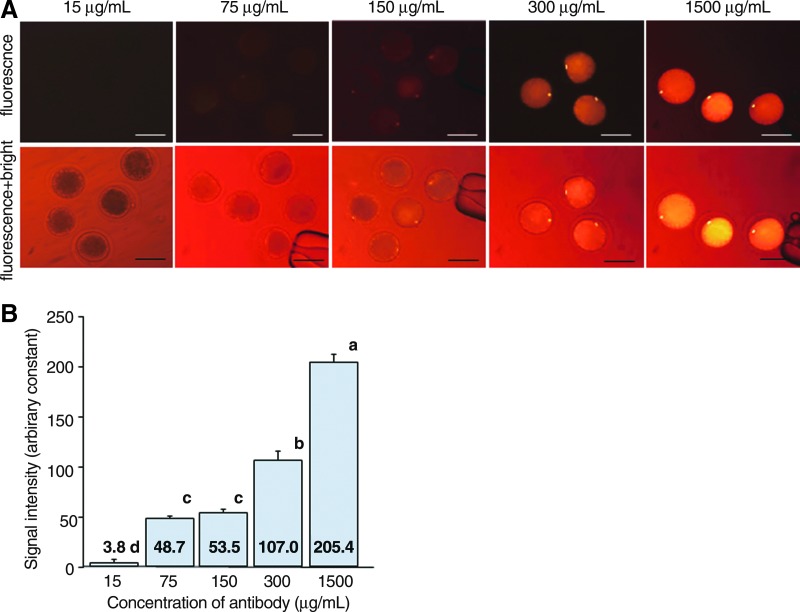
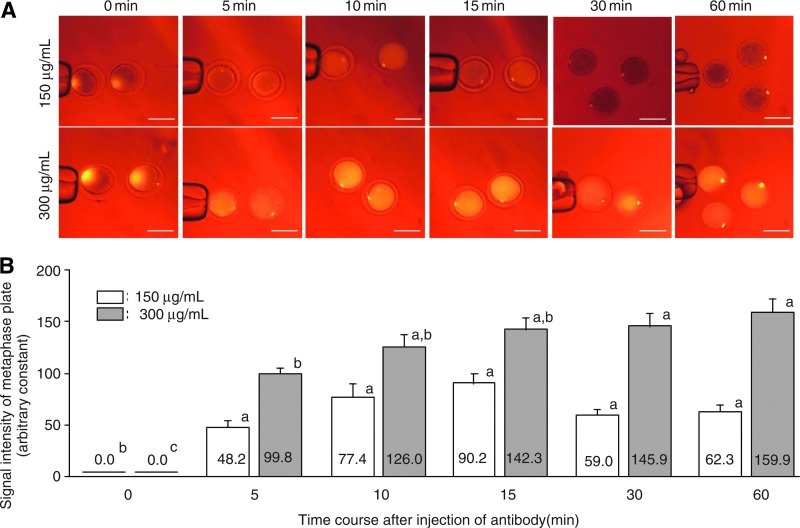
The intensity of the phycoerythrin signals of the chromosomes in bovine oocytes injected with antibody increased in a concentration-dependent manner, with 1500 μg/mL giving the highest signal (Fig. 2B). The signals of oocytes injected with the antibody conjugates less than 75 μg/mL were not detected, and those injected with 1500 μg/mL were most intense in the cytoplasm (Fig. 2A). As shown in Figure 3A, when bovine oocytes were injected with 150 and 300 μg/mL of the antibody, the signals were clearly visible 10 min after injection and sustained over 60 min after injection. The fluorescence in these images was quantitated in Figure 3B. In oocytes injected with 150 μg/mL of the antibody, the intensity of the signal reached a plateau at 15 min after injection, and was maintained until 60 min after injection. In the 300 μg/mL group, the intensity of the signal increased until 15 min after injection, and the intensity was maintained until 60 min after injection.
FIG. 2.
Intensity of fluorescence signals in chromosomes of bovine oocytes injected with phycoerythrin-labeled antibody. (A) Fluorescent images of bovine MII oocytes injected with various concentrations of antibody labeled with phycoerythrin with a halogen-lamp microscope equipped with a special filter unit. (Upper panels) Fluorescent images. (Lower panels) Fluorescent plus bright-field images Scale bar, 100 μm. (B) Comparison of the intensity among bovine oocytes injected with 15, 75, 150, 300, and 1500 μg/mL of the antibody. a, b, c, and d superscripts denote significant differences among concentrations of antibody (p<0.05). Color images available online at www.liebertpub.com/cell
FIG. 3.
Relationships between intensity of phycoerythrin signal and time after antibody injection. (A) Fluorescent images of bovine MII oocytes injected with 150 and 300 μg/mL of antibody labeled with phycoerythrin at various times (minutes) after injection. Scale bar,100 μm. (B) Comparison of phycoerythrin intensity in bovine oocytes injected with 150 (white bars) and 300 (gray bars) μg/mL of antibody among various times after injection. a, b, and c superscripts denote significant differences in the each group (p<0.05). Color images available online at www.liebertpub.com/cell
Experiment 2
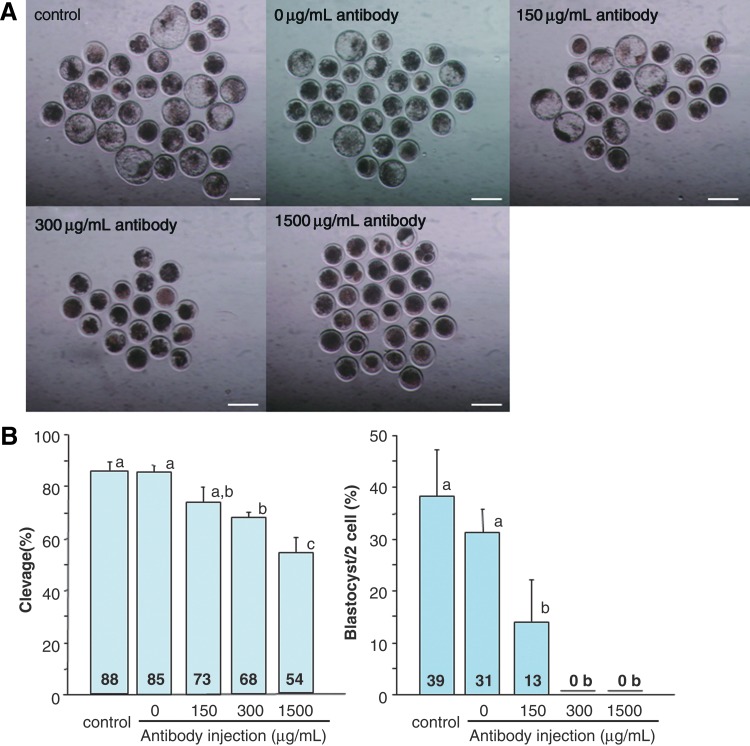
The cleavage rates of IVF embryos that were injected with the antibody decreased as the concentration of antibody increased (Fig. 4B). The blastocyst rate of the embryos injected with 150 μg/mL of the antibody was lower than that of embryos injected with 0 μg/mL of the antibody and control (Fig. 4A, B; p<0.05). No blastocysts were obtained from the embryos injected with 300 and 1500 μg/mL of the antibody (p<0.05). The ICM, TE, and number of total cells were not different among these groups (Table 1; p>0.05).
FIG. 4.
Effects of phycoerythrin-labeled antibody on early development of bovine embryos following in vitro fertilization (IVF). (A) Morphology of IVF embryos produced from bovine oocytes injected with various concentrations of antibody at 168 hpf. Scale bar, 200 μm. (B) Rates of cleavage (left) and blastocyst development (right) after IVF of the oocytes injected with various concentrations of the antibody. a, b, and c superscripts denote significant differences in the each graph (p<0.05). Color images available online at www.liebertpub.com/cell
Table 1.
Cell Number of Blastocysts Developed after In Vitro Fertilization of Oocytes with Injected Antibody Labeled with Phycoerythrina
| Cell number (mean±SEM) | |||||
|---|---|---|---|---|---|
| Method | No. embryos examined | Total | ICM | TE | % of ICM (ICM/total) |
| Control (blind enucleation) | 26 | 118±7 | 39±4 | 78±5 | 33 |
| Antibody (0 μg/mL) | 28 | 123±8 | 39±4 | 83±5 | 31 |
| Antibody (150 μg/mL) | 9 | 143±12 | 44±5 | 99±15 | 34 |
The experiment was replicated three times.
SEM, standard error of the mean; ICM, inner cell mass; TE, trophectoderm.
Experiment 3
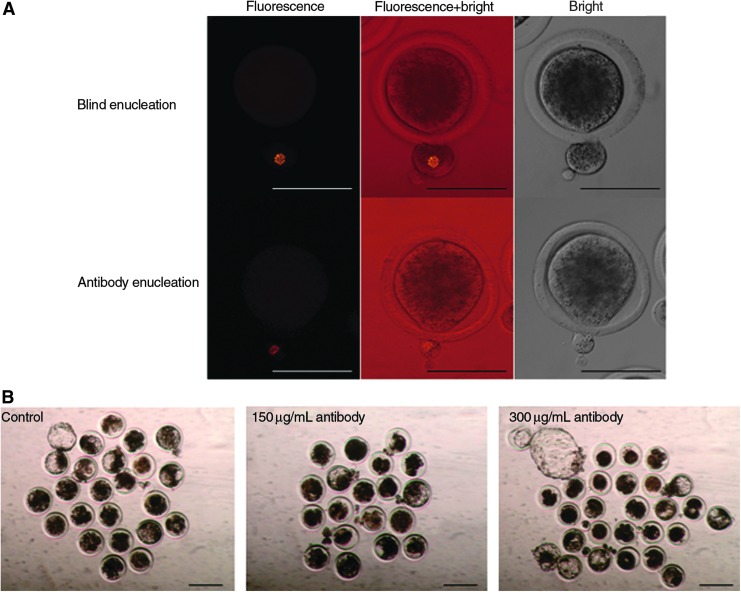
The enucleation rate of control (blind enucleation) was 86%, whereas all oocytes injected with the antibody (150 and 300 μg/mL) were successfully enucleated (Table 2). Moreover, the amount of cytoplasm removed by antibody enucleation was less than that of removed cytoplasm by blind enucleation (Fig. 5A). We produced SCNT embryos with the enucleated oocytes, and they were cultured for 168 h. There were no differences in the fusion rate and the developmental rates of these SCNT embryos (Table 3 and Fig. 5B; p>0.05). The cell numbers of ICM and TE of the SCNT embryos were the same between the blind and antibody enucleation groups (Table 4; p>0.05).
Table 2.
Enucleation of Bovine Oocytes Injected Antibody Labeled with Phycoerythrina
| Method | No. oocytes | No.(%) of oocytes survived | No. (%) of oocytes enucleated |
|---|---|---|---|
| Control (blind enucleation) | 126 | 126 (100) | 108 (86) |
| Antibody (150 μg/mL | 127 | 117 (92) | 117 (100) |
| Antibody 300 μg//mL | 119 | 95 (80) | 95 (100) |
The experiment was replicated three times.
FIG. 5.
Enucleation of bovine oocytes injected with phycoerythrin-labeled antibody and production of SCNT embryos from those enucleated oocytes. (A) Blind enucleation: Phycoerythrin-labeled antibody was injected into bovine oocytes, but enucleation was performed under a bright-field microscope, and the signals were confirmed above the fluorescence under the halogen light (fluorescence and fluorescence+bright light). Antibody enucleation: After injecting antibody into bovine oocytes, enucleation was performed while viewing the signals above the fluorescence under the halogen light. Scale bar, represents 100 μm. (B) Blastocysts of SCNT embryos produced from oocytes enucleated using phycoerythrin-labeled antibody. Scale bar, 200 μm. Color images available online at www.liebertpub.com/cell
Table 3.
Development of SCNT Embryos Produced with Oocytes Enucleated after Antibody Injectiona
| Method | No. oocytes | No.(%) of oocytes fused | No. of embryos reconstructed | No. (%) of oocytes cleaved | No. (%) of blastocyst |
|---|---|---|---|---|---|
| Control (blind enucleation) | 96 | 82 (85) | 79 | 56 (71) | 15 (27) |
| Antibody (150 μg//mL) | 97 | 70 (72) | 62 | 38 (61) | 10 (26) |
| Antibody (300 μg//mL | 87 | 73 (84) | 65 | 40 (62) | 11 (28) |
The experiment was replicated three times.
SCNT, somatic cell nuclear transfer.
Table 4.
Cell Number of Blastocysts Developed for SCNT Embryos with Enucleated Oocytes after Antibody Injectiona
| Cell number (mean±SEM) | |||||
|---|---|---|---|---|---|
| Method | No. embryos examined | Total | ICM | TE | % of ICM (ICM/total) |
| Control (blind enucleation) | 14 | 135±11 | 33±4 | 102±9 | 24 |
| Antibody (150 μg//mL) | 10 | 108±11 | 34±4 | 74±8 | 31 |
| Antibody (300 μg//mL) | 11 | 106±14 | 31±5 | 76±11 | 31 |
The experiment was replicated three times.
SCNT, somatic cell nuclear transfer; SEM, standard error of the mean; ICM, inner cell mass; TE, trophectoderm.
Discussion
In the present study, we used a phycoerythrin-labeled antibody to detect the chromosomes of MII plates in bovine oocytes with a conventional halogen-lamp microscope. Using this system, the fluorescent chromosomes of all oocytes were clearly detected and easily removed without any failure. The SCNT embryos produced with these oocytes developed to blastocysts at the same rate as SCNT embryos produced from oocytes enucleated without the fluorescent system. We demonstrated that this system assisted us in the process of removing nuclei from opaque oocytes, such as in ruminant animals, for production of SCNT embryos.
The signal in the bovine oocytes was detected with 150 μg/mL antibody, whereas we were able to detect the signal in mouse oocytes with 75 μg/mL antibody (Yamagata et al., 2012), probably because the mouse cytoplasm is more transparent than the bovine cytoplasm. The time to visualize chromosomes clearly in bovine oocytes was at least 10 min, whereas it was only 5 min in mouse oocytes (Yamagata et al. 2012). This may be because bovine oocytes are six to eight times larger in volume than mouse oocytes and contain numerous lipid granules, causing the antibody to diffuse more slowly and take more time to bind to chromosomes.
Our finding that blastocyst rates of bovine IVF embryos decreased with increasing antibody concentration (Fig. 4B) is similar to what we found in mice, where the rate of intracytoplasmic sperm injection (ICSI) embryos decreased when the oocytes were injected with a high concentration of antibody (Yamagata et al., 2012). It also has been reported that phosphorylation of histone H3S10 is required for accurate chromosome segregation (Hayashi-Takanaka et al., 2009). Therefore, rigid binding of the antibody to the phosphorylation might raise abnormal segregation of chromosomes. In addition, this may be because the antibody disturbs the crosstalk between histone molecules, as modification of one residue that can alter modifications of the other residues (Lee et al., 2010). For example, phosphorylation of histone H3S10 accelerates acetylation of histones H3K14 and H4K16 (Cheung et al., 2000; Lo et al., 2000; Zippo et al., 2009). Therefore, injecting a high concentration of antibody into oocytes may disturb the crosstalk between histone molecules, resulting in a decrease of blastocyst development. Although developmental rates of IVF embryos following injection of the antibody conjugates decreased at a higher concentration of the antibody, developmental rates of SCNT embryos were same as those of SCNT embryos produced by traditional methods. It is supposed that most of the injected antibodies were assembled into the chromosomes of bovine oocytes after their injection and removed with the chromosomes during enucleation, resulting in successful subsequent development of SCNT embryos.
Chromosomes visualized by staining with Hoechst 33342 were able to be removed from all bovine oocytes (Dominko et al., 2000; Jeon et al., 2011), but cleavage and development rates of SCNT embryos with the enucleated oocytes were lower than those of SCNT embryos from oocytes using the blind enucleation method (Jeon et al., 2011). In this study, we used phycoerythrin as a fluorescence reagent. It has been reported that shorter wavelengths have more harmful effects on oocytes than longer wavelengths (Dominko et al. 2000; Terashita et al. 2011). Moreover, a halogen lamp produces much less intense emission at shorter wavelengths than at other wavelengths (Yamagata et al. 2012). Removal of a large amount of cytoplasm from oocytes was detrimental to the subsequent development and the quality of SCNT embryos (Hua et al., 2011; Peura et al., 1998; Westhusin et al., 1996). In the present study, we were able to greatly reduce the amount of cytoplasm removed by using the fluorescent antibody (Fig. 5A). Our finding that the blastocyst rate of SCNT embryos produced from enucleated oocytes after injecting antibody conjugates is the same as that of SCNT embryos produced by conventional method may indicate that phototoxicity using the light of the halogen lamp might be less damaging to the cytoplasm. Although we did not examine the effect of the antibody on full-term development of bovine SCNT embryos, the rate of full-term development in mouse SCNT embryos produced from enucleated oocytes using the antibody is the same as that in SCNT embryos produced using the traditional method (Yamagata et al., 2012).
In conclusion, using a fluorescent antibody against metaphase chromosomes and a halogen-lamp microscope equipped with a fluorescent filter unit, we were able to clearly detect and remove chromosomes from bovine oocytes. Blastocyst rates of SCNT embryos produced with the enucleated oocytes were the same as those of SCNT embryos produced with oocytes enucleated using a conventional blind method. The new system can be used as a reliable predictor of the location of metaphase plates in opaque oocytes, such as those in ruminant animals, for production of SCNT embryos.
Acknowledgments
We are grateful to Mr. Takaaki Sekito, Ms. Saki Nishioka, Ms. Rio Hamada, and Ms. Risa Yamamoto for their technical assistance and Ms. Naomi Backes Kamimura and Ms. Julia Walhelm-Kimura for English editing. This study was supported by a Grant-in-Aid for Scientific Research (No. 26450461) from the Japan Society for the Promotion of Science.
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Bui H.T., Yamaoka E., and Miyano T. (2004). Involvement of histone H3 (Ser10) phosphorylation in chromosome condensation without Cdc2 kinase and mitogen-activated protein kinase activation in pig oocytes. Biol. Reprod. 70, 1843–1851 [DOI] [PubMed] [Google Scholar]
- Cheung P., Tanner K.G., Cheung W.L., Sassone-Corsi P., Denu J.M., and Allis C.D. (2000). Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol. Cell, 5, 905–915 [DOI] [PubMed] [Google Scholar]
- Critser E.S., and First N.L. (1986). Use of a fluorescent stain for visualization of nuclear material in living oocytes and early embryos. Stain Technol. 61, 1–5 [DOI] [PubMed] [Google Scholar]
- Dominko T., Chan A., Simerly C., Luetjens C.M., Hewitson L., Martinovich C., and Schatten G. (2000). Dynamic imaging of the metaphase II spindle and maternal chromosomes in bovine oocytes: Implications for enucleation efficiency verification, avoidance of parthenogenesis, and successful embryogenesis. Biol. Reprod. 62, 150–154 [DOI] [PubMed] [Google Scholar]
- Fulka J., Jr., Loi P., Fulka H., Ptak G., and Nagai T. (2004). Nucleus transfer in mammals: Noninvasive approaches for the preparation of cytoplasts. Trends Biotechnol. 22, 279–283 [DOI] [PubMed] [Google Scholar]
- Han Y.M., Kang Y.K., Koo D.B., and Lee K.K. (2003). Nuclear reprogramming of cloned embryos produced in vitro. Theriogenology 59, 33–44 [DOI] [PubMed] [Google Scholar]
- Hayashi-Takanaka Y., Yamagata K., Nozaki N., and Kimura H. (2009). Visualizing histone modifications in living cells: Spatiotemporal dynamics of H3 phosphorylation during interphase. J. Cell Biol. 187, 781–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S., Zhang H., Su J.M., Zhang T., Quan F.S., Liu J., Wang Y.S., and Zhang Y. (2011). Effects of the removal of cytoplasm on the development of early cloned bovine embryos. Anim. Reprod. Sci. 126, 37–44 [DOI] [PubMed] [Google Scholar]
- Iwamoto D., Kasamatsu A., Ideta A., Urakawa M., Matsumoto K., Hosoi Y., Iritani A., Aoyagi Y., and Saeki K. (2012). Donor cells at the G1 phase enhance homogeneous gene expression among blastomeres in bovine somatic cell nuclear transfer embryos. Cell. Reprogram. 14, 20–28 [DOI] [PubMed] [Google Scholar]
- Jeon B.G., Betts D.H., King W.A., and Rho G.J. (2011). In vitro developmental potential of nuclear transfer embryos cloned with enucleation methods using pre-denuded bovine oocytes. Reprod. Domest. Anim. 46, 1035–1042 [DOI] [PubMed] [Google Scholar]
- Kimura H., Hayashi-Takanaka Y., and Yamagata K. (2010). Visualization of DNA methylation and histone modifications in living cells. Curr. Opin. Cell Biol. 22, 412–418 [DOI] [PubMed] [Google Scholar]
- Lee J.S., Smith E., and Shilatifard A. (2010). The language of histone crosstalk. Cell 142, 682–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G.P., White K.L., and Bunch T.D. (2004). Review of enucleation methods and procedures used in animal cloning: state of the art. Cloning Stem Cells, 6, 5–13 [DOI] [PubMed] [Google Scholar]
- Lo W.S., Trievel R.C., Rojas J.R., Duggan L., Hsu J-Y., Allis C.D., Marmorstein R., and Berger S.L. (2000). Phosphorylation of serine 10 in histone H3 is functionally linked in vitro and in vivo to Gcn5-mediated acetylation at lysine 14. Mol. Cell 5, 917–926 [DOI] [PubMed] [Google Scholar]
- McGrath J., and Solter D. (1983). Nuclear transplantation in the mouse embryo by microsurgery and cell fusion. Science 220, 1300–1302 [DOI] [PubMed] [Google Scholar]
- Peura T.T., Lewis I.M., and Trounson A.O. (1998). The effect of recipient oocyte volume on nuclear transfer in cattle. Mol. Reprod. Dev. 50, 185–191 [DOI] [PubMed] [Google Scholar]
- Prather R.S., Sims M.M., and First N.L. (1989). Nuclear transplantation in early pig embryos. Biol. Reprod. 41, 414–418 [DOI] [PubMed] [Google Scholar]
- Rosenkrans C.F., Jr., Zeng G.Q., GT M.C., Schoff P.K., and First N.L. (1993). Development of bovine embryos in vitro as affected by energy substrates. Biol. Reprod. 49, 459–462 [DOI] [PubMed] [Google Scholar]
- Saeki K., Nagao Y., Hoshi M., and Kainuma H. (1994). Effects of cumulus cells on sperm penetration of bovine oocytes in protein-free medium. Theriogenology 42, 1115–1123 [DOI] [PubMed] [Google Scholar]
- Saeki K., Nagao Y., Kishi M., Nagai M., and Iritani A. (1998). Timing of completion of the first meiotic division in bovine oocytes after maintenance of meiotic arrest with cycloheximide and their subsequent development. J. Vet. Med. Sci. 60, 523–526 [DOI] [PubMed] [Google Scholar]
- Smith L.C. (1993). Membrane and intracellular effects of ultraviolet irradiation with Hoechst 33342 on bovine secondary oocytes matured in vitro. J. Reprod. Fertil. 99, 39–44 [DOI] [PubMed] [Google Scholar]
- Swain J.E., Ding J., Brautigan D.L., Swain J.E., Ding J., Brautigan D.L., Villa-Moruzzi E., and Smith G.D. (2007). Proper chromatin condensation and maintenance of histone H3 phosphorylation during mouse oocyte meiosis requires protein phosphatase activity. Biol. Reprod. 76, 628–638 [DOI] [PubMed] [Google Scholar]
- Tani T., Shimada H., Kato Y., and Tsunoda Y. (2006). Demecolcine-assisted enucleation for bovine cloning. Cloning Stem Cells 8, 61–66 [DOI] [PubMed] [Google Scholar]
- Tatham B.G., Dowsing A.T., and Trounson A.O. (1995). Enucleation by centrifugation of in vitro-matured bovine oocytes for use in nuclear transfer. Biol. Reprod. 53, 1088–1094 [DOI] [PubMed] [Google Scholar]
- Terashita Y., Li C., Yamagata K., Sato E., and Wakayama T. (2011). Effect of fluorescent mercury light irradiation on in vitro and in vivo development of mouse oocytes after parthenogenetic activation or sperm microinjection. J. Reprod. Dev. 57, 564–571 [DOI] [PubMed] [Google Scholar]
- Thouas G.A., Korfiatis N.A., French A.J., Jones G.M., and Trounson A.O. (2001). Simplified technique for differential staining of inner cell mass and trophectoderm cells of mouse and bovine blastocysts. Reprod. Biomed. Online, 3, 25–29 [DOI] [PubMed] [Google Scholar]
- Van Hooser A., Goodrich D.W., Allis C.D., Brinkley B.R., and Mancini M.A. (1998). Histone H3 phosphorylation is required for the initiation, but not maintenance, of mammalian chromosome condensation. J. Cell Sci. 111, 3497–3506 [DOI] [PubMed] [Google Scholar]
- Wei Y., Mizzen C.A., Cook R.G., Gorovsky M.A., and Allis C.D. (1998). Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena. Proc. Natl. Acad. Sci. USA 95, 7480–7484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhusin M.E., Collas P., Marek D., Sullivan E., Stepp P., Pryor J., and Barnes F. (1996). Reducing the amount of cytoplasm available for early embryonic development decreases the quality but not quantity of embryos produced by in vitro fertilization and nuclear transplantation. Theriogenology 46, 243–252 [DOI] [PubMed] [Google Scholar]
- Wilmut I., Beaujean N., de Sousa P.A., Dinnyes A., King T.J., Paterson L.A., Wells D.N., and Young L.E. (2002). Somatic cell nuclear transfer. Nature 419, 583–586 [DOI] [PubMed] [Google Scholar]
- Yamagata K., Iwamoto D., Terashita Y., Li C., Wakayama S., Hayashi-Takanaka Y., Kimura H., Saeki K., and Wakayama T. (2012). Fluorescence cell imaging and manipulation using conventional halogen lamp microscopy. PLoS One 7, e31638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippo A., Serafini R., Rocchigiani M., Pennacchini S., Krepelova A., and Oliviero S. (2009). Histone crosstalk between H3S10ph and H4K16ac generates a histone code that mediates transcription elongation. Cell 138, 1122–1136 [DOI] [PubMed] [Google Scholar]