Abstract
Fetal therapy can be defined as any prenatal treatment administered to the mother with the primary indication to improve perinatal or long-term outcomes for the fetus or newborn. This review provides an update of the pharmacological therapies that are solely directed at the fetus with anomalies and outlines a future transcriptomic approach. Fetal anomalies targeted with prenatal pharmacotherapy are a heterogeneous group of structural, endocrine, and metabolic conditions, including congenital cystic adenomatoid malformation (CCAM), congenital adrenal hyperplasia, congenital heart block, fetal tachyarrhythmias, inborn errors of metabolism, fetal thyroid disorders, and polyhydramnios. To date, the majority of pharmacotherapies for fetal anomalies have been evaluated only in retrospective, uncontrolled studies. The way forward will be with an evidence-based approach to prenatal pharmacological interventions.
Keywords: fetal therapy, CCAM, congenital adrenal hyperplasia, fetal arrhythmias, fetal goiter, polyhydramnios, fetal transcriptome
INTRODUCTION
Fetal therapy can be defined as any prenatal treatment administered to the mother with the primary indication to improve perinatal or long-term outcomes for the fetus or newborn. Even with this broad definition, it is apparent that recent advances have occurred primarily in the surgical and minimally invasive modalities. While there is growing interest in the potential applications of gene and stem cell therapy for treatment of fetal anomalies, these currently remain experimental techniques that have not yet transitioned to routine clinical care.
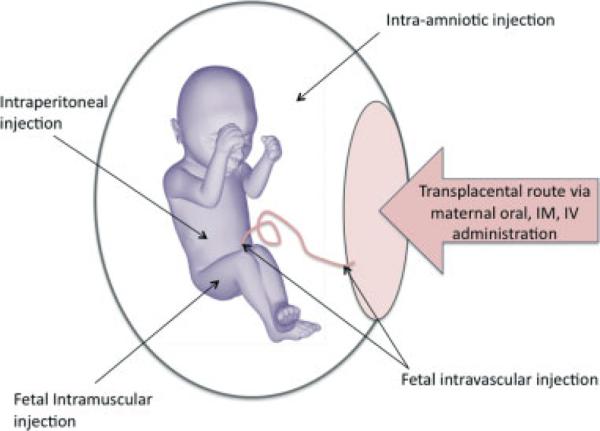
There are several well-established conditions for which medications are administered to the mother for their transplacental action on the fetus. The most common indication for transplacental fetal pharmacotherapy is threatened preterm birth and the use of maternal prenatal corticosteroids to enhance fetal lung maturity. There are also other more invasive routes of administration such as the direct fetal intramuscular or intravascular route (Figure 1). This review will provide an update of the pharmacological fetal therapies that are solely directed at the fetus with anomalies, without concurrent maternal indications for treatment (Table 1). Those fetal conditions that have dedicated reviews elsewhere in this journal issue will not be included in this article. Also excluded are medical disorders that have concurrent maternal indication for treatment, such as preeclampsia, diabetes, and infection, or obstetric disorders such as placental insufficiency and preterm labor/birth, as these are large topics beyond the scope of this review.
Figure 1.
The different routes by which fetal therapeutics may be administered prenatally
Table 1.
Summary of current conditions treated with prenatal pharmacotherapy
| Fetal condition | Prenatal medical therapy | Route of administration |
|---|---|---|
| CCAM | Betamethasone | Maternal intramuscular |
| CAH | Dexamethasone | Maternal oral |
| CHB | Dexamethasone | Maternal oral |
| IVIG | Maternal intravenous | |
| Tachyarrhythmias | Antiarrhythmics (e.g. digoxin, sotalol, flecainide, amiodarone) | Maternal oral, maternal intravenous, fetal intramuscular, fetal intravenous |
| Methylmalonic acidemia | Vitamin B12 | Maternal oral, maternal intramuscular |
| Multiple carboxylase synthetase deficiency | Biotin | Maternal oral |
| 3-Phosphoglycerate-dehydrogenase deficiency | L-serine | Maternal oral |
| Pyridoxine-dependent seizures | Pyridoxine | Maternal oral |
| SLOS | FFP as a source of cholesterol | Fetal intravascular, intraperitoneal transfusion |
| Fetal goitrous hypothyroidism | Levothyroxine | Intra-amniotic, fetal intramuscular |
| Fetal thyrotoxicosis post maternal thyroid ablation | Propylthiouracil | Maternal oral |
| Polyhydramnios, monochorionic monoamniotic (MCMA) twins, Bartter syndrome | NSAIDs (sulindac, indomethacin) | Maternal oral |
CAH, congenital adrenal hyperplasia; CCAM, congenital cystic adenomatous malformation; CHB, congenital heart block; FFP, fresh frozen plasma; IVIG, intravenous immunoglobulin; NSAIDs, nonsteroidal anti-inflammatory drugs; SLOS, Smith-Lemli-Opitz syndrome
MATERNAL STEROID TREATMENT FOR ANOMALIES
Betamethasone for cystic adenomatoid lung malformations
Congenital cystic adenomatoid malformations (CCAMs) of the lung—also known as congenital pulmonary airway malformations—of the lung constitute the majority of prenatally detected thoracic lesions. These hamartomatous pulmonary lesions result from an abnormal proliferation of bronchiolar-like air spaces and lack normal alveoli. The fetal prognosis depends primarily on the size of the lesion and its associated mass effect, including the degree of mediastinal shift, hydrops, and polyhydramnios. The worst outcomes occur in the small proportion of patients with very large CCAMs and hydrops (Crombleholme et al., 2002). Prenatally, CCAMs are classified sonographically as either macro-cystic or microcystic depending on their echotexture. Minimally invasive surgical treatment may be feasible in macrocystic CCAMs, where a dominant cyst can be drained or shunted to relieve the mass effect. Resection via open fetal surgery or during third-trimester ex utero intrapartum treatment are more invasive surgical options for large CCAMS (Grethel et al., 2007).
For large microcystic CCAMs, a novel medical approach is currently being evaluated. Several case series have reported a reduction in CCAM size after maternal administration of betamethasone (Tsao et al., 2003; Peranteau et al., 2007; Curran et al., 2010), although one study had less favorable results (Morris et al., 2009). When the data from these retrospective studies were combined by Curran et al. (2010), the use of prenatal steroids in high-risk microcystic CCAM lesions was associated with resolution of hydrops in 80% and an overall survival to discharge of 87.1%. A US-based, blinded, randomized controlled trial (RCT) is now underway, comparing the treatment of large microcystic CCAMs with maternal corticosteroids to placebo (CCAM steroids trial ClinicalTrials.gov Identifier: NCT00881517). The primary outcome is the incidence of fetal hydrops and the secondary outcomes are 1-month postnatal survival and the relative size of the CCAM. Depending on the outcome of this clinical trial, a single course of betamethasone could represent a new medical therapy for fetuses with microcystic CCAMs that have had historically poor outcomes.
Dexamethasone for CAH
Another fetal condition for which maternally administered steroids have been used is congenital adrenal hyperplasia (CAH). CAH is an autosomal recessive disorder of steroidogenesis, most commonly due to 21-hydroxylase deficiency. This enzyme deficiency in the fetus results in prenatal cortisol deficiency and a subsequent adrenocorticotropic hormone (ACTH)-driven excess production of adrenal androgens. In the more severe classical form, excess of prenatal androgen causes external genital masculinization and ambiguity in newborn females and progressive postnatal virilization in males. The clinical situation in which prenatal diagnosis and treatment are usually offered is in the setting of a previously affected child. The critical time for development of the external genitalia is weeks 7–12, which is before chorionic villus sampling (CVS) can be performed to diagnose fetal sex. Prenatal treatment of CAH to prevent virilization of female fetuses must therefore begin before invasive tests can tell whether the fetus is affected and whether it is male or female.
Fetal sex determination using cell-free fetal DNA in maternal plasma is a noninvasive method of prenatal diagnosis that allows women carrying male fetuses to avoid prenatal dexamethasone treatment (Avent and Chitty, 2006). Clinical experience with noninvasive fetal sex determination has been positive in the UK, with the two English centers performing this test reporting 99.5% accuracy from 7 weeks’ gestation onward (Hill et al., 2010). The Endocrine Society supports the use of noninvasive testing in their clinical guidelines and recommends that it becomes a required component of all prenatal CAH treatment research protocols as soon as a consistently accurate test is available in early gestation (Speiser et al., 2010). However, many centers still rely on traditional invasive methods of diagnosis as noninvasive testing for fetal sex remains unavailable in many countries.
If prenatal treatment is undertaken, maternal oral dexamethasone should commence before 8 weeks’ gestation (Clayton et al., 2002). Doses of 20 μg/kg of pre-pregnancy maternal weight/day up to a maximum of 1.5 mg/day have been shown to normalize androgen precursor levels in affected fetuses (Forest et al., 1989). The need for ongoing dexamethasone treatment is then reassessed after invasive prenatal testing for CYP21 gene mutations by CVS at 10–13 weeks or amniocentesis at 15–17 weeks. If the fetus is unaffected or male, then maternal dexamethasone is discontinued. Prenatal treatment prevents virilization in approximately 85% of female fetuses. Treatment failures are usually attributed to poor compliance, cessation in later pregnancy, or late onset of treatment (Vos and Bruinse, 2010).
While this approach ensures that all affected females are treated during the early critical period, seven out of eight women and their fetuses will be unnecessarily exposed to dexamethasone unless noninvasive fetal sex determination is performed in first trimester. This exposure of unaffected fetuses and mothers is the subject of ongoing concerns, both in the medical and the bioethics communities. In the latest Clinical Practice Guideline of the Endocrine Society, prenatal treatment is still given an ‘experimental’ status and a specific prenatal protocol is not recommended (Speiser et al., 2010). The authors of the guideline explain that they place ‘a higher value on preventing unnecessary prenatal exposure of mother and fetus to dexamethasone and avoiding potential harms associated with this exposure, and a relatively lower value on minimizing the emotional toll of ambiguous genitalia on parents and patients’. The significant maternal risks of long-term dexamethasone therapy include hypertension, abnormal glucose tolerance, infection, and (potentially) osteoporosis or cataracts. Fetal risks include growth restriction, possible disruption of the hypothalamic-pituitary-adrenal (HPA) axis with long-term behavioral changes, and neurodevelopmental changes.
Postnatal genitoplasty and hormone therapy remain alternatives to prenatal steroid use to treat female virilization, but this does not address the postulated psychological effects of androgen exposure in affected females. There are similar concerns about the potential negative metabolic, cognitive, and behavioral effects of long-term prenatal steroid treatment on the growing fetus and the child (Lajic et al., 2008). A large European follow-up study of children prenatally exposed to dexamethasone was reassuring, with no reported differences in psychopathology, behavioral problems, or adaptive functioning, as compared to nonexposed children (Hirvikoski et al., 2008). A US-based comprehensive follow-up study investigating potential long-term adverse side effects of prenatal dexamethasone treatment in children with 21-hydroxylase deficiency and their mothers is ongoing (ClinicalTrials.gov Identifier: NCT00617292). Despite decades of medical experience with prenatal dexamethasone, CAH remains a controversial topic and debate continues online (www.fetaldex.org), as well as in the bioethical and medical media (McCullough et al., 2010a, 2010b; New, 2010). These debates are unlikely to be completely settled by scientific evidence alone, as they are inextricably linked with sociological, ethical, and political factors (Kamenova, 2010).
TREATMENT OF FETAL ARRHYTHMIAS
Congenital heart block
Fetal heart block is defined as a persistent baseline heart rate of <100 beats per minute (bpm). In fetuses with a structurally normal heart and 1 : 1 atrioventricular (AV) conduction, more than half of congenital heart block (CHB) is caused by the transplacental effects of maternal anti-ribonucleoprotein antibodies (anti-SSA/SSB antibodies). These maternal antibodies, which are associated with connective tissue diseases such as systemic lupus erythematosus, cross the placenta, bind to myocardial cells, and cause inflammation and scarring to the AV node. The overall risk of CHB is 1–2% in anti-SSA/SSB positive mothers, but rises to 17% if there is a history of a previously affected pregnancy (Llanos et al., 2009). Poor prognostic factors in those fetuses with CHB but structurally normal hearts include hydrops, a fetal heart rate <55 bpm, myocardial dysfunction, endocardial fibroelastosis, and preterm delivery. Approaches to in utero treatment for high-risk fetuses have concentrated on the use of maternally administered agents to suppress inflammation in the fetal myocardium, with or without concurrent β-agonist therapy.
Retrospective observational studies have reported beneficial results from maternal corticosteroid administration, including 90% survival in dexamethasone-treated cases of third-degree heart block compared with 46% survival in untreated cases (Jaeggi et al., 2004). However, in a review of 15 studies that included 93 cases of CHB, Breur et al. (2004) concluded that there was no evidence that maternal dexamethasone was either safe or effective for the treatment of CHB. There are also persistent concerns regarding the long-term effects of dexamethasone on fetal growth and development. This is confounded by the fact that prematurity and growth restriction are common findings in CHB and are unable to be distinguished from the effects of treatment in non-randomized trials.
The PR Interval and Dexamethasone Evaluation (PRIDE) study was the first attempt to conduct a RCT of maternal dexamethasone treatment for fetal CHB (Friedman et al., 2009). The original study design was a blinded RCT of maternal oral dexamethasone (4 mg/day) compared with placebo. However, due to the obstacles of slow local institutional review board approval systems and physician and patient reluctance to undergo randomization, this proved to be an unfeasible design.
The final form of the PRIDE study was therefore a multicenter open-label nonrandomized study that recruited women with anti-SSA or anti-SSB antibodies and any degree of fetal heart block diagnosed on fetal echocardiogram. Thirty women were treated with dexamethasone and ten were untreated. The study confirmed the high morbidity and mortality associated with CHB, with pacemaker use at 2 years of age in 40–50% of both the treated and untreated groups. There was no reversal of third-degree CHB in any of the 22 cases, either spontaneously or with therapy. The higher mortality seen in the treated group compared with the untreated group (20 vs 0%) is likely to have been influenced by selection bias given the nonrandomized study design. Although there was no discernable benefit of dexamethasone treatment for third-degree CHB, the authors postulated a possible role for dexamethasone in firstand second-degree CHB. The limitations of the study design, however, prevented any firm conclusions regarding treatment benefit and risks in this small group of patients.
Intravenous immunoglobulin (IVIG) has also recently attracted interest as an alternative to maternal steroid therapy for management of CHB. In some case reports, it appears to have prevented or ameliorated alloimmune fetal heart block in high-risk pregnancies (Kaaja and Julkunen, 2003; David et al., 2010). There is considerable clinical experience with this medication in pregnancy, as it is an established treatment for fetal alloimmune thrombocytopenia and maternal autoimmune thrombocytopenic purpura. However, two multi-center trials with identical treatment protocols failed to show any benefit. The US-based open-label prospective trial Preventive Intravenous Immune Globulin Therapy for Congenital Heart Block (PITCH) study, and a European multicenter study both failed to show any benefit with a dosage regime of IVIG 400 mg/kg every 3 weeks from weeks 12 to 24 of gestation (Friedman et al., 2010; Pisoni et al., 2010). Twenty women completed the IVIG protocol in the PITCH study before it was terminated. The combined experience of these two trials showed six cases of CHB in 33 women with a history of a prior child with CHB. This is consistent with the recurrence rates in untreated pregnancies (Llanos et al., 2009).
Prevention and prenatal treatment of CHB thus remains an unsolved clinical problem. It may be that higher doses of IVIG, similar to those used in other conditions, such as fetal alloimmune thrombocytopenia, may be required for a transplacental therapeutic effect. At the very least, these two trials show the feasibility of recruiting high-risk women to clinical intervention trials of fetal therapy and contribute more safety data on the use of IVIG in pregnancy.
Tachyarrhythmias
There are a variety of fetal cardiac conduction abnormalities that result in a sustained fetal heart rate > 180 bpm. Antiarrhythmic drugs delivered via the transplacental route is a well-established treatment for these fetal tachyarrythmias (Api and Carvalho, 2008). Sustained fetal tachycardia is associated with increased perinatal morbidity and mortality, particularly in preterm infants. Therefore, prenatal therapy is usually preferred over immediate delivery when intervention is indicated. The current mainstay of assessment is fetal echocardiography. The vast majority of fetal tachyarrhythmias are either AV reentry tachycardias or atrial flutter.
Although the transplacental (maternal) route is generally accepted as the first line of administration in fetal tachyarrhythmias, there is no universal consensus on the choice of drug. This is primarily influenced by the type of arrhythmia, side effects, and prior clinician experience. No prospective controlled trials have been performed to date, but based on the retrospective studies, digoxin is widely accepted as a mainstay of treatment for fetal supraventricular tachycardia (SVT) (Api and Carvalho, 2008; van den Heuvel et al., 2008; Strasburger and Wakai, 2010). Its pharmacokinetics and adverse effects are well described and it can be administered orally or intravenously with good bioavailability to the nonhydropic fetus. Maternal digoxin therapy achieves successful fetal conversion to sinus rhythm in over 60% of nonhydropic fetuses with SVT, but in only approximately 20% of hydropic fetuses (Krapp et al., 2003).
Other second- or third-line transplacental and antiar-rhythmic drugs include sotalol (Oudijk et al., 2000, 2003; Rebelo et al., 2006), flecainide (Frohn-Mulder et al., 1995; D'Souza et al., 2002; Krapp et al., 2003), and amiodarone (Flack et al., 1993; Strasburger et al., 2004).
Direct fetal administration with intramuscular, intraperitoneal or intravenous drugs is usually restricted to refractory cases in which fetal hydrops is present, as transplacental drug transfer may be impaired in this state (Younis and Granat, 1987; Hansmann et al., 1991; Parilla et al., 1996). Fetal intramuscular digoxin is the more favored approach for direct fetal administration, as it avoids the cordocentesis-related complications of intravascular amiodarone therapy (Hansmann et al., 1991). However, direct fetal therapy is not essential in the presence of fetal hydrops, as therapeutic effects can be achieved with combination transplacental drug therapy (Ebenroth et al., 2001; Strasburger et al., 2004; Merriman et al., 2008).
THERAPY FOR FETAL INBORN ERRORS OF METABOLISM
Prenatal replacement therapy for fetuses with an inborn error of metabolism has been reported in only a few conditions in which morbidity is high, and prenatal treatment carries potential benefit for the newborn with minimal maternal risk. The vast majority use the transplacental route to treat the fetus via oral or intramuscular maternal administration.
Case reports of maternal treatment with oral or intramuscular vitamin B12 for prenatal treatment of fetal methylmalonic acidemia (MMA) date as far back as 1975 (Ampola et al., 1975; van der Meer et al., 1990; Soda et al., 1995; Zass et al., 1995; Spada et al., 1999; Huemer et al., 2005; Zhang et al., 2008). MMA can present prenatally as growth restriction or dilated cardiomyopathy (De Bie et al., 2009), or postnatally with recurrent vomiting, failure to thrive, developmental retardation, and hepatomegaly. Recent advances in the prenatal diagnosis of MMA using biochemical and molecular genetic approaches may make the opportunity for prenatal treatment more common (Cavicchi et al., 2006). However, prenatal treatment is not always accompanied by improvements in biochemical markers of disease severity or the prevention of postnatal complications. This highlights the lack of knowledge regarding optimum dosing (Soda et al., 1995) and the influence of fetal treatment on long-term outcomes (Huemer et al., 2005). Monitoring the efficacy of prenatal treatment by measurement of odd-numbered fatty acid accumulation in newborn adipose tissue has been attempted, but its correlation with the prevention of neuropathology is unknown (Zass et al., 1995).
Biotin-responsive multiple carboxylase synthetase deficiency is a rare autosomal recessive inborn error of metabolism that usually presents in the newborn period with severe metabolic acidosis. It is treated with lifelong biotin therapy. Prenatal features include growth restriction and ventriculomegaly (Yokoi et al., 2009). Diagnosis of an affected fetus can be made by analysis of maternal urine or amniotic fluid organic acids, enzymatic activity assay of carboxylases in chorionic villi or amniocytes, or by molecular genetic diagnosis from chorionic villi (Malvagia et al., 2005). Treatment with maternal oral biotin supplementation during pregnancy has been reported to improve the biochemical or clinical features in the newborn in five case reports (Packman et al., 1982; Roth et al., 1982; Suormala et al., 1998; Thuy et al., 1999; Yokoi et al., 2009). However, more severe forms of the disease may only show partial response to the usual 10 mg daily dose (Yokoi et al., 2009).
3-Phosphoglycerate-dehydrogenase deficiency is a treatable disorder of amino acid synthesis that causes congenital microcephaly, severe psychomotor retardation, and intractable seizures. Postnatal oral therapy with l-serine is beneficial for the treatment of seizures, but prevention of congenital microcephaly obviously requires a prenatal approach. A single case report of prenatal maternal administration of l-serine supplementation reported a good outcome with a normal head size at birth and normal psychomotor development at 2 years of age, suggesting that this disorder of amino acid synthesis can be successfully treated prenatally (de Koning et al., 2004).
More recently, another disorder of amino acid synthesis was treated prenatally with apparent success in two families affected by pyridoxine-dependent seizures (Bok et al., 2010). Maternal oral treatment with pyridoxine was given during the second pregnancy in each family and outcomes were compared with the first untreated pregnancy. The authors reported better perinatal and long-term neurodevelopmental outcomes in the prenatally treated pregnancies. Notable outcomes in the prenatally treated children included the absence of intrauterine seizures and higher IQ scores when compared with their untreated siblings.
A single case report of prenatal cholesterol replacement for Smith–Lemli–Opitz syndrome (SLOS) is remarkable for utilizing direct fetal therapy via intraperitoneal and intravascular infusions of fresh frozen plasma (FFP)(Irons et al., 1999). Clinical manifestations of SLOS include prenatal and postnatal growth restriction, microcephaly, distinctive facial appearance, intellectual disability, and ambiguous genitalia. In this case report, three infusions of FFP were given between 34 and 37 weeks’ gestation. This resulted in increased levels of cholesterol in fetal blood and an increased fetal mean erythrocyte cellular volume, suggesting that the cholesterol had been incorporated in the erythrocyte cell membranes. Prenatal growth velocity also improved, with an increase from the 7th to the 15th percentiles during treatment.
While there is insufficient evidence to formally assess the effectiveness of any of these replacement therapies, the lack of reported toxicities and their biological rationales make them interesting—albeit highly experimental—considerations for prenatal treatment of rare metabolic diseases with associated anomalies and congenital morbidities.
TREATMENT FOR FETAL THYROID DISORDERS
Intra-amniotic therapy for fetal goitrous hypothyroidism
Intra-amniotic instillation of medication has been well described in fetal goitrous hypothyroidism, a condition in which transplacental thyroxine supplementation is not possible due to placental impermeability and maternal side effects. (Abuhamad et al., 1995; Agrawal et al., 2002; Hashimoto et al., 2006; Miyata et al., 2007; Hanono et al., 2009; Ribault et al., 2009). Potential causes of fetal goiter include primary dyshormonogenesis (Börgel et al., 2005) and side effects of maternal antithyroid medication (Miyata et al., 2007). In severe cases of this condition, the mass effect of a large fetal goiter can lead to polyhydramnios, head hyperextension, and airway obstruction at birth.
Diagnosis is usually made by sonography with or without fetal blood or amniotic fluid sampling (Thorpe-Beeston et al., 1992). Intra-amniotic replacement therapy for hypothyroidism is primarily aimed at reducing the size of the goiter and the complications associated with the mass effect. Intra-amniotic installation of thyroxine allows for fetal oral ingestion of the hormone, but also carries risks associated with repeated amniocentesis, including premature rupture of membranes, preterm birth, and sepsis. Case reports describe regimes of levothyroxine (150–600 μg) injected into the amniotic cavity every 7–10 days. Given that the aim is reduction of goiter size, assessment of a therapeutic response is usually managed noninvasively by sonographic examination. There have been no trials to assess this therapy. It is currently unknown if long-term developmental outcomes are altered by initiating hormone replacement therapy in the prenatal period, as compared with postnatal replacement only. Successful fetal intramuscular injection has been reported in a case of failed intra-amniotic therapy, where initial treatment failure was attributed to severe esophageal obstruction impeding swallowing (Corral et al., 2010).
Antithyroid treatment for fetal thyrotoxicosis
Fetal thyrotoxicosis, most commonly caused by maternal Graves’ disease, can lead to serious complications such as growth restriction and fetal hydrops. Traditional maternal therapy for Graves’ disease with thioamides counteracts the effect of the thyroid-stimulating immunoglobulins (TSIs) and prevents both maternal and fetal complications of hyperthyroidism. However, a woman who has been successfully treated for Graves’ disease with thyroid ablation or surgery can still transfer thyroid-stimulating antibodies to the fetus. In the absence of maternal antithyroid medication, these TSIs can cause fetal hyperthyroidism with persistent fetal tachycardia, goiter, growth restriction, or hydrops (Heckel et al., 1997; Srisupundit et al., 2008; Ting et al., 1999).
The diagnosis of fetal thyrotoxicosis can be established by performing fetal thyroid function tests on umbilical cord blood or based on the high levels of maternal TSI antibodies and a medical history of Graves’ disease. There are many case series of successful intrauterine treatment for fetal hyperthyroidism using standard doses of antithyroid drugs (150 mg/day of propylthiouracil) via maternal oral administration (Wallace et al., 1995; Watson and Fiegen, 1995; Heckel et al., 1997; Bowman et al., 1998; Duncombe and Dickinson, 2001; Srisupundit et al., 2008). Maternal hypothyroidism is a potential complication of this approach, but maternal thyroid hormone replacement therapy is possible, as this does not cross the placenta to any significant degree.
NSAIDs TO REDUCE AFV
Excessive amniotic fluid volume (AFV), defined as an AF index >97.5th percentile or >24 cm (Moise, 1997), can be associated with either maternal or fetal diseases, although the majority of pregnancies affected by mild polyhydramnios are normal. The risk of a major fetal anomaly, including aneuploidy, increases with the extent of polyhydramnios. Up to 28% of fetuses with ‘idiopathic’ polyhydramnios have significant abnormalities detected in the first year of life (Dorleijn et al., 2009). Detailed prenatal sonographic studies can detect structural abnormalities and screen for fetal anemia. In the absence of a specific fetal condition amenable to targeted interventions, treatment of polyhydramnios is usually only indicated in severe cases for the relief of maternal symptoms or to reduce the risk of preterm birth.
The treatment of polyhydramnios has surgical and medical approaches. Directly draining off the excess amniotic fluid via amniocentesis provides rapid relief, but may be only a temporary solution, as the excessive fluid often recurs. Amnioreduction also carries the risks of preterm labor, abruption, preterm rupture of membranes, fetal distress, and sepsis, which accumulate with serial procedures.
In the 1990s, indomethacin was investigated in multiple studies for the medical treatment of polyhydramnios (as reviewed in Moise, 1997). Indomethacin is a nonsteroidal anti-inflammatory drug (NSAID) that reduces AFV by reducing fetal urine output (Kirshon et al., 1988, 1991), increasing fetal breathing movements (Hallak et al., 1992), and reducing pulmonary fluid production (Stevenson and Lumbers, 1992). Since then, indomethacin has largely fallen out of favor for the treatment of polyhydramnios due to subsequently recognized fetal side effects of in utero constriction of the ductus arteriosus and postnatal renal insufficiency. Long-term maternal use of indomethacin during pregnancy, particularly after 32 weeks, is also associated with other serious perinatal complications such as fetal hydrops, persistent fetal circulation, necrotizing enterocolitis, and ileal perforation (Moise, 1997).
A rare condition in which a specific preference for prenatal indomethacin treatment may exist is fetal Bartter syndrome, an inherited renal tubular disorder in which fetal polyuria is associated with elevated levels of prostaglandin E (Konrad et al., 1999; Dane et al., 2010). In most clinical situations, however, the severe antiprostaglandin effects of indomethacin are not desired. Sulindac appears to be a less fetotoxic alternative NSAID if medical treatment of polyhydramnios is indicated. This drug has less constrictive effects on the ductus arteriosus (Rasanen and Jouppila, 1995) and reduces AFV with fewer fetal side effects (Carlan et al., 1992).
Sulindac has been used to reduce AFV in both singleton (Jayagopal et al., 2007) and twin pregnancies. Of special interest is its use in monochorionic monoamniotic twin pregnancies to prevent fetal death due to cord complications (Peek et al., 1997; Pasquini et al., 2006). In these uncontrolled case series, the use of prophylactic sulindac from 20 weeks’ gestation in monochorionic monoamniotic twins, in combination with ultrasound surveillance and delivery at 32 weeks, was associated with stabilization of fetal lie and a higher rate of peri-natal survival compared with historical outcomes.
THE FUTURE OF MEDICAL THERAPY
To date, pharmacotherapy has largely been based on adaptations of postnatal approaches for similar conditions. In the future, however, it is conceivable that a ‘personalized medicine’ approach can and will be developed for each fetus with anomalies. For example, Slonim et al. (2009) performed gene expression analysis on cell-free mRNA isolated from the second-trimester amniotic fluid of euploid fetuses and fetuses with trisomy 21. A list of statistically significant differentially regulated genes for the fetuses with trisomy 21 was developed and uploaded into a freely available database known as the Connectivity Map (www.broadinstitute.org/CMAP). The Connectivity Map suggests specific bioactive, US Food and Drug Administration (FDA)-approved small molecules that can mimic or negate the fetal gene expression signature. In this report, oxidative stress was the major functional abnormality for the fetuses with trisomy 21. The Connectivity Map suggested a number of different antioxidants as treatment. Although this study only addressed fetuses with Down syndrome, it could easily be generalized to treat any fetuses with an isolated anomaly or a syndrome with a consistent phenotype.
CONCLUSIONS
Fetal anomalies that are targeted with prenatal pharmacotherapy comprise a heterogeneous group of structural, endocrine, and metabolic conditions. It is notable that very few fetal therapies included in this review have undergone prospective studies. The experience in invasive fetal therapy shows that it is feasible to randomize surgical interventions for high-risk fetal conditions. However, pharmacological therapies for fetal conditions appear to have been less rigorously evaluated to date, perhaps because management of these disorders is less centralized than for novel surgical procedures. Some long-standing therapies, while lacking quality clinical trial evidence, have enough accumulated clinical experience to comfortably guide treatment, such as digoxin for fetal SVT. Similarly, treatment of fetal thyroid conditions relies on known biological mechanisms and accumulated data from case series. Extremely rare conditions, such as the inborn errors of metabolism, are unlikely to ever generate enough evidence to establish the efficacy of prenatal intervention.
Noninvasive determination of fetal sex in first trimester may allow targeted treatment of female fetuses with suspected CAH and remove some of the concerns regarding unnecessary dexamethasone exposure to unaffected fetuses and mothers. However, the continuing debate over prenatal treatment of CAH shows that controversies persist about the ethics of treating pregnant women and their fetuses in the context of inadequate clinical data.
The field of medical therapy for fetal anomalies is beginning to adopt an evidence-based approach to pharmacological interventions. The appearance of prospective trials of medical fetal therapies in recent years such as the CCAM steroid trial, the PITCH, and PRIDE studies contrasts with the experience from prior years with retrospective case series and isolated case reports. This change is welcome, as prospective trials of prenatal fetal therapy provide much more than evidence to guide treatment: they also necessitate a commitment to multicenter collaboration and ethical scrutiny that can only benefit the field as a whole.
REFERENCES
- Abuhamad AZ, Fisher DA, Warsof SL, et al. Antenatal diagnosis and treatment of fetal goitrous hypothyroidism: case report and review of the literature. Ultrasound Obstet Gynecol. 1995;6:368–371. doi: 10.1046/j.1469-0705.1995.06050368.x. [DOI] [PubMed] [Google Scholar]
- Agrawal P, Ogilvy-Stuart A, Lees C. Intrauterine diagnosis and management of congenital goitrous hypothyroidism. Ultrasound Obstet Gynecol. 2002;5:501–505. doi: 10.1046/j.1469-0705.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- Ampola MG, Mahoney MJ, Nakamura E, Tanaka K. Prenatal therapy of a patient with vitamin-B12-responsive methylmalonic acidemia. N Engl J Med. 1975;293:313–317. doi: 10.1056/NEJM197508142930701. [DOI] [PubMed] [Google Scholar]
- Api O, Carvalho JS. Fetal dysrhythmias. Best Pract Res Clin Obstet Gynaecol. 2008;22:31–48. doi: 10.1016/j.bpobgyn.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Avent ND, Chitty LS. Non-invasive diagnosis of fetal sex; utilisation of free fetal DNA in maternal plasma and ultrasound. Prenat Diagn. 2006;26:598–603. doi: 10.1002/pd.1493. [DOI] [PubMed] [Google Scholar]
- Bok LA, Been JV, Struys EA, Jakobs C, Rijper EA, Willemsen MA. Antenatal treatment in two Dutch families with pyridoxine-dependent seizures. Eur J Pediatr. 2010;169:297–303. doi: 10.1007/s00431-009-1020-2. [DOI] [PubMed] [Google Scholar]
- Börgel K, Pohlenz J, Holzgreve W, Bramswig J. Intrauterine therapy of goitrous hypothyroidism in a boy with a new compound heterozygous mutation (Y453D and C800R) in the thyroid peroxidase gene. A long-term follow-up. Am J Obstet Gynecol. 2005;193:857–858. doi: 10.1016/j.ajog.2005.01.060. [DOI] [PubMed] [Google Scholar]
- Bowman ML, Bergmann M, Smith JF. Intrapartum labetalol for the treatment of maternal and fetal thyrotoxicosis. Thyroid. 1998;8:795–796. doi: 10.1089/thy.1998.8.795. [DOI] [PubMed] [Google Scholar]
- Breur JM, Visser GH, Kruize AA, Stoutenbeek P, Meijboom EJ. Treatment of fetal heart block with maternal steroid therapy: case report and review of the literature. Ultrasound Obstet Gynecol. 2004;24:467–472. doi: 10.1002/uog.1713. [DOI] [PubMed] [Google Scholar]
- Carlan SJ, O'Brien WF, O'Leary TD, Mastrogiannis D. Randomized comparative trial of indomethacin and sulindac for the treatment of refractory preterm labor. Obstet Gynecol. 1992;79:223–228. [PubMed] [Google Scholar]
- Cavicchi C, Donati MA, Funghini S, et al. Genetic and biochemical approach to early prenatal diagnosis in a family with mut methylmalonic aciduria. Clin Genet. 2006;69:72–76. doi: 10.1111/j.1399-0004.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- Clayton PE, Miller WL, Oberfield SE, Ritzen EM, Sippell WG, Speiser PW. Consensus statement on 21-hydroxylase deficiency from the European Society for Paediatric Endocrinology and the Lawson Wilkins Pediatric Endocrine Society. Horm Res. 2002;58:188–195. doi: 10.1159/000065490. [DOI] [PubMed] [Google Scholar]
- Corral E, Reascos M, Preiss Y, Rompel SM, Sepulveda W. Treatment of fetal goitrous hypothyroidism: value of direct intramuscular L-thyroxine therapy. Prenat Diagn. 2010;30:899–901. doi: 10.1002/pd.2560. [DOI] [PubMed] [Google Scholar]
- Crombleholme TM, Coleman B, Hedrick H, et al. Cystic adenomatoid malformation volume ratio predicts outcome in prenatally diagnosed cystic adenomatoid malformation of the lung. J Pediatr Surg. 2002;37:331–338. doi: 10.1053/jpsu.2002.30832. [DOI] [PubMed] [Google Scholar]
- Curran PF, Jelin EB, Rand L, et al. Prenatal steroids for microcystic congenital cystic adenomatoid malformations. J Pediatr Surg. 2010;45:145–150. doi: 10.1016/j.jpedsurg.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Dane B, Dane C, Aksoy F. Antenatal Bartter syndrome: analysis of two cases with placental findings. Fetal Pediatr Pathol. 2010;29:121–126. doi: 10.3109/15513811003777276. [DOI] [PubMed] [Google Scholar]
- David AL, Ataullah I, Yates R, Sullivan I, Charles P, Williams D. Congenital fetal heart block: a potential therapeutic role for intravenous immunoglobulin. Obstet Gynecol. 2010;116(Suppl. 2):43–47. doi: 10.1097/AOG.0b013e3181e75a4a. [DOI] [PubMed] [Google Scholar]
- De Bie I, Nizard SD, Mitchell GA. Fetal dilated cardiomyopathy: an unsuspected presentation of methylmalonic aciduria and hyperhomocystinuria, cblC type. Prenat Diagn. 2009;3:266–270. doi: 10.1002/pd.2218. [DOI] [PubMed] [Google Scholar]
- de Koning TJ, Klomp LW, van Oppen AC, et al. Prenatal and early postnatal treatment in 3-phosphoglycerate-dehydrogenase deficiency. Lancet. 2004;364:2221–2222. doi: 10.1016/S0140-6736(04)17596-X. [DOI] [PubMed] [Google Scholar]
- Dorleijn DM, Cohen-Overbeek TE, Groenendaal F, Bruinse HW, Stoutenbeek P. Idiopathic polyhydramnios and postnatal findings. J Matern Fetal Neonatal Med. 2009;22:315–320. doi: 10.1080/14767050802531870. [DOI] [PubMed] [Google Scholar]
- D'Souza D, MacKenzie WE, Martin WL. Transplacental flecainide therapy in the treatment of fetal supraventricular tachycardia. J Obstet Gynaecol. 2002;22:320–322. doi: 10.1080/01443610252971249. [DOI] [PubMed] [Google Scholar]
- Duncombe GJ, Dickinson JE. Fetal thyrotoxicosis after maternal thyroidectomy. Aust N Z J Obstet Gynaecol. 2001;41:224–227. doi: 10.1111/j.1479-828x.2001.tb01215.x. [DOI] [PubMed] [Google Scholar]
- Ebenroth ES, Cordes TM, Darragh RK. Second-line treatment of fetal supraventricular tachycardia using flecainide acetate. Pediatr Cardiol. 2001;22:483–487. doi: 10.1007/s002460010279. [DOI] [PubMed] [Google Scholar]
- Flack NJ, Zosmer N, Bennett PR. Amiodarone given by three routes to terminate fetal atrial flutter associated with severe hydrops. Obstet Gynecol. 1993;82(4 Pt 2 Suppl):714–716. [PubMed] [Google Scholar]
- Forest MG, Betuel H, David M. Prenatal treatment in congenital adrenal hyperplasia due to 21-hydroxylase deficiency: up-date 88 of the French multicentric study. Endocr Res. 1989;15:277–301. doi: 10.1080/07435808909039101. [DOI] [PubMed] [Google Scholar]
- Friedman DM, Kim MY, Copel JA, Llanos C, Davis C, Buyon JP. Prospective evaluation of fetuses with autoimmune-associated congenital heart block followed in the PR Interval and Dexamethasone Evaluation (PRIDE) Study. Am J Cardiol. 2009;103:1102–1106. doi: 10.1016/j.amjcard.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DM, Llanos C, Izmirly PM, et al. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: results of a multicenter, prospective, open-label clinical trial. Arthritis Rheum. 2010;62:1138–1146. doi: 10.1002/art.27308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohn-Mulder IM, Stewart PA, Witsenburg M, Den Hollander NS, Wladimiroff JW, Hess J. The efficacy of flecainide versus digoxin in the management of fetal supraventricular tachycardia. Prenat Diagn. 1995;15:1297–1302. doi: 10.1002/pd.1970151309. [DOI] [PubMed] [Google Scholar]
- Grethel EJ, Wagner AJ, Clifton MS, et al. Fetal intervention for mass lesions and hydrops improves outcome: a 15-year experience. J Pediatr Surg. 2007;42:117–123. doi: 10.1016/j.jpedsurg.2006.09.060. [DOI] [PubMed] [Google Scholar]
- Hallak M, Moise K, Jr, Lira N, Dorman KF, Smith EO, Cotton DB. The effect of tocolytic agents (indomethacin and terbutaline) on fetal breathing and body movements: a prospective, randomized, double-blind, placebo-controlled clinical trial. Am J Obstet Gynecol. 1992;167:1059–1063. doi: 10.1016/s0002-9378(12)80038-x. [DOI] [PubMed] [Google Scholar]
- Hanono A, Shah B, David R, et al. Antenatal treatment of fetal goiter: a therapeutic challenge. J Matern Fetal Neonatal Med. 2009;22:76–80. doi: 10.1080/14767050802448299. [DOI] [PubMed] [Google Scholar]
- Hansmann M, Gembruch U, Bald R, Manz M, Redel DA. Fetal tachyarrhythmias: transplacental and direct treatment of the fetus-a report of 60 cases. Ultrasound Obstet Gynecol. 1991;1:162–168. doi: 10.1046/j.1469-0705.1991.01030162.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Hashimoto K, Suehara N. Successful in utero treatment of fetal goitrous hypothyroidism: case report and review of the literature. Fetal Diagn Ther. 2006;21:360–365. doi: 10.1159/000092466. [DOI] [PubMed] [Google Scholar]
- Heckel S, Favre R, Schlienger JL, Soskin P. Diagnosis and successful in utero treatment of a fetal goitrous hyperthyroidism caused by maternal Graves disease. A case study. Fetal Diagn Ther. 1997;12:54–58. doi: 10.1159/000264428. [DOI] [PubMed] [Google Scholar]
- Hill M, Finning K, Martin P, et al. Non-invasive prenatal determination of fetal sex: translating research into clinical practice. Clin Genet. 2010 doi: 10.1111/j.1399-0004.2010.01533.x. [Epub ahead of print]. DOI: 10.1111/j.1399-0004.2010.01533.x. [DOI] [PubMed] [Google Scholar]
- Hirvikoski T, Nordenström A, Lindholm T, Lindblad F, Ritzen EM, Lajic S. Long-term follow-up of prenatally treated children at risk for congenital adrenal hyperplasia: does dexamethasone cause behavioural problems? Eur J Endocrinol. 2008;159:309–316. doi: 10.1530/EJE-08-0280. [DOI] [PubMed] [Google Scholar]
- Huemer M, Simma B, Fowler B, Suormala T, Bodamer OA, Sass JO. Prenatal and postnatal treatment in cobalamin C defect. J Pediatr. 2005;147:469–472. doi: 10.1016/j.jpeds.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Irons MB, Nores J, Stewart TL, et al. Antenatal therapy of Smith-Lemli Opitz syndrome. Fetal Diagn Ther. 1999;14:133–137. doi: 10.1159/000020906. [DOI] [PubMed] [Google Scholar]
- Jaeggi ET, Fouron JC, Silverman ED, Ryan G, Smallhorn J, Hornberger LK. Transplacental fetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease. Circulation. 2004;110:1542–1548. doi: 10.1161/01.CIR.0000142046.58632.3A. [DOI] [PubMed] [Google Scholar]
- Jayagopal N, Sinha D, Bhatti NR. Use of sulindac in polyhydramnios. J Obstet Gynaecol. 2007;8:850–850. doi: 10.1080/01443610701748187. [DOI] [PubMed] [Google Scholar]
- Kaaja R, Julkunen H. Prevention of recurrence of congenital heart block with intravenous immunoglobulin and corticosteroid therapy: comment on the editorial by Buyon, et al. Arthritis Rheum. 2003;48:280–281. doi: 10.1002/art.10716. author reply 281–282. [DOI] [PubMed] [Google Scholar]
- Kamenova K. Politics and persuasion in medical controversies. Am J Bioeth. 2010;10:68–69. doi: 10.1080/15265161.2010.499585. [DOI] [PubMed] [Google Scholar]
- Kirshon B, Moise KJ, Jr, Wasserstrum N, Ou CN, Huhta JC. Influence of short-term indomethacin therapy on fetal urine output. Obstet Gynecol. 1988;72:51–53. [PubMed] [Google Scholar]
- Kirshon B, Moise KJ, Jr, Mari G, Willis R. Long-term indomethacin therapy decreases fetal urine output and results in oligohydramnios. Am J Perinatol. 1991;8:86–88. doi: 10.1055/s-2007-999349. [DOI] [PubMed] [Google Scholar]
- Konrad M, Leonhardt A, Hensen P, Seyberth HW, Kockerling A. Prenatal and postnatal management of hyperprostaglandin E syndrome after genetic diagnosis from amniocytes. Pediatrics. 1999;103:678–683. doi: 10.1542/peds.103.3.678. [DOI] [PubMed] [Google Scholar]
- Krapp M, Kohl T, Simpson JM, Sharland GK, Katalinic A, Gembruch U. Review of diagnosis, treatment, and outcome of fetal atrial flutter compared with supraventricular tachycardia. Heart. 2003;89:913–917. doi: 10.1136/heart.89.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajic S, Nordenstrom A, Hirvikoski T. Long-term outcome of prenatal treatment of congenital adrenal hyperplasia. Endocr Dev. 2008;13:82–98. doi: 10.1159/000134827. [DOI] [PubMed] [Google Scholar]
- Llanos C, Izmirly PM, Katholi M, et al. Recurrence rates of cardiac manifestations associated with neonatal lupus and maternal/fetal risk factors. Arthritis Rheum. 2009;60:3091–3097. doi: 10.1002/art.24768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvagia S, Morrone A, Pasquini E, et al. First prenatal molecular diagnosis in a family with holocarboxylase synthetase deficiency. Prenat Diagn. 2005;25:1117–1119. doi: 10.1002/pd.1291. [DOI] [PubMed] [Google Scholar]
- McCullough LB, Chervenak FA, Brent RL, Hippen B. A case study in unethical transgressive bioethics: letter of concern from bioethicists about the prenatal administration of dexamethasone. Am J Bioeth. 2010a;10:35–45. doi: 10.1080/15265161.2010.499745. [DOI] [PubMed] [Google Scholar]
- McCullough LB, Chervenak FA, Brent RL, Hippen B. The intellectual and moral integrity of bioethics: response to commentaries on “a case study in unethical transgressive bioethics: ‘letter of concern from bioethicists’ about the prenatal administration of dexamethasone”. Am J Bioeth. 2010b;10:W3–W5. doi: 10.1080/15265161.2010.505143. [DOI] [PubMed] [Google Scholar]
- Merriman JB, Gonzalez JM, Rychik J, Ural SH. Can digoxin and sotalol therapy for fetal supraventricular tachycardia and hydrops be successful? A case report. J Reprod Med. 2008;53:357–359. [PubMed] [Google Scholar]
- Miyata I, Abe-Gotyo N, Tajima A, et al. Successful intrauterine therapy for fetal goitrous hypothyroidism during late gestation. Endocr J. 2007;54:813–817. doi: 10.1507/endocrj.k07-047. [DOI] [PubMed] [Google Scholar]
- Moise KJ., Jr Polyhydramnios. Clin Obstet Gynecol. 1997;40:266–279. doi: 10.1097/00003081-199706000-00004. [DOI] [PubMed] [Google Scholar]
- Morris LM, Lim FY, Livingston JC, Polzin WJ, Crombleholme TM. High-risk fetal congenital pulmonary airway malformations have a variable response to steroids. J Pediatr Surg. 2009;44:60–65. doi: 10.1016/j.jpedsurg.2008.10.012. [DOI] [PubMed] [Google Scholar]
- New M. Description and defense of prenatal diagnosis and treatment with low-dose dexamethasone for congenital adrenal hyperplasia. Am J Bioeth. 2010;10:48–51. doi: 10.1080/15265161.2010.507652. [DOI] [PubMed] [Google Scholar]
- Oudijk MA, Michon MM, Kleinman CS, et al. Sotalol in the treatment of fetal dysrhythmias. Circulation. 2000;101:2721–2726. doi: 10.1161/01.cir.101.23.2721. [DOI] [PubMed] [Google Scholar]
- Oudijk MA, Ruskamp JM, Ververs FF, et al. Treatment of fetal tachycardia with sotalol: transplacental pharmacokinetics and pharmacodynamics. J Am Coll Cardiol. 2003;42:765–770. doi: 10.1016/s0735-1097(03)00779-4. [DOI] [PubMed] [Google Scholar]
- Packman S, Cowan MJ, Golbus MS, et al. Prenatal treatment of biotin responsive multiple carboxylase deficiency. Lancet. 1982;1:1435–1438. doi: 10.1016/s0140-6736(82)92452-7. [DOI] [PubMed] [Google Scholar]
- Parilla BV, Strasburger JF, Socol ML. Fetal supraventricular tachycardia complicated by hydrops fetalis: a role for direct fetal intramuscular therapy. Am J Perinatol. 1996;13:483–486. doi: 10.1055/s-2007-994432. [DOI] [PubMed] [Google Scholar]
- Pasquini L, Wimalasundera RC, Fichera A, Barigye O, Chappell L, Fisk NM. High perinatal survival in monoamniotic twins managed by prophylactic sulindac, intensive ultrasound surveillance, and Cesarean delivery at 32 weeks’ gestation. Ultrasound Obstet Gynecol. 2006;28:681–687. doi: 10.1002/uog.3811. [DOI] [PubMed] [Google Scholar]
- Peek MJ, McCarthy A, Kyle P, Sepulveda W, Fisk NM. Medical amnioreduction with sulindac to reduce cord complications in monoamniotic twins. Am J Obstet Gynecol. 1997;176:334–336. doi: 10.1016/s0002-9378(97)70494-0. [DOI] [PubMed] [Google Scholar]
- Peranteau WH, Wilson RD, Liechty KW, et al. Effect of maternal betamethasone administration on prenatal congenital cystic adenomatoid malformation growth and fetal survival. Fetal Diagn Ther. 2007;22:365–371. doi: 10.1159/000103298. [DOI] [PubMed] [Google Scholar]
- Pisoni CN, Brucato A, Ruffatti A, et al. Failure of intravenous immunoglobulin to prevent congenital heart block: findings of a multicenter, prospective, observational study. Arthritis Rheum. 2010;62:1147–1152. doi: 10.1002/art.27350. [DOI] [PubMed] [Google Scholar]
- Rasanen J, Jouppila P. Fetal cardiac function and ductus arteriosus during indomethacin and sulindac therapy for threatened preterm labor: a randomized study. Am J Obstet Gynecol. 1995;173:20–25. doi: 10.1016/0002-9378(95)90163-9. [DOI] [PubMed] [Google Scholar]
- Rebelo M, Macedo AJ, Nogueira G, Trigo C, Kaku S. Sotalol in the treatment of fetal tachyarrhythmia. Rev Port Cardiol. 2006;25:477–481. [PubMed] [Google Scholar]
- Ribault V, Castanet M, Bertrand AM, et al. Experience with intraamniotic thyroxine treatment in nonimmune fetal goitrous hypothyroidism in 12 cases. J Clin Endocrinol Metab. 2009;94:3731–3739. doi: 10.1210/jc.2008-2681. [DOI] [PubMed] [Google Scholar]
- Roth KS, Yang W, Allan L, Saunders M, Gravel RA, Dakshinamurti K. Prenatal administration of biotin in biotin responsive multiple carboxylase deficiency. Pediatr Res. 1982;16:126–129. doi: 10.1203/00006450-198202000-00010. [DOI] [PubMed] [Google Scholar]
- Slonim DK, Koide K, Johnson KL, et al. Functional genomic analysis of amniotic fluid cell-free mRNA suggests that oxidative stress is significant in Down syndrome fetuses. Proc Natl Acad Sci U S A. 2009;106:9425–9429. doi: 10.1073/pnas.0903909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soda H, Ohura T, Yoshida I, et al. Prenatal diagnosis and therapy for a patient with vitamin B12-responsive methylmalonic acidaemia. J Inherit Metab Dis. 1995;18:295–298. doi: 10.1007/BF00710418. [DOI] [PubMed] [Google Scholar]
- Spada M, Fowler B, Bonetti G. Fetal therapy in combined methylmalonic aciduria and homocystinuria. J Inherit Metab Dis. 1999;22(S1):91–91. [Google Scholar]
- Speiser PW, Azziz R, Baskin LS, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4133–4160. doi: 10.1210/jc.2009-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisupundit K, Sirichotiyakul S, Tongprasert F, Luewan S, Tongsong T. Fetal therapy in fetal thyrotoxicosis: a case report. Fetal Diagn Ther. 2008;23:114–116. doi: 10.1159/000111589. [DOI] [PubMed] [Google Scholar]
- Stevenson KM, Lumbers ER. Effects of indomethacin on fetal renal function, renal and umbilicoplacental blood flow and lung liquid production. J Dev Physiol. 1992;17:257–264. [PubMed] [Google Scholar]
- Strasburger JF, Wakai RT. Fetal cardiac arrhythmia detection and in utero therapy. Nat Rev Cardiol. 2010;7:277–290. doi: 10.1038/nrcardio.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasburger JF, Cuneo BF, Michon MM, et al. Amiodarone therapy for drug-refractory fetal tachycardia. Circulation. 2004;109:375–379. doi: 10.1161/01.CIR.0000109494.05317.58. [DOI] [PubMed] [Google Scholar]
- Suormala T, Fowler B, Jakobs C, et al. Late-onset holocarboxylase synthetase-deficiency: pre- and post-natal diagnosis and evaluation of effectiveness of antenatal biotin therapy. Eur J Pediatr. 1998;157:570–575. doi: 10.1007/s004310050881. [DOI] [PubMed] [Google Scholar]
- Thorpe-Beeston JG, Nicolaides KH, McGregor AM. Fetal thyroid function. Thyroid. 1992;2:207–217. doi: 10.1089/thy.1992.2.207. [DOI] [PubMed] [Google Scholar]
- Thuy LP, Belmont J, Nyhan WL. Prenatal diagnosis and treatment of holocarboxylase synthetase deficiency. Prenat Diagn. 1999;19:108–112. doi: 10.1002/(sici)1097-0223(199902)19:2<108::aid-pd476>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Ting MK, Hsu BR, Huang YY, Lin JD, Chen TC. Recurrent fetal thyrotoxicosis in a woman with Graves’ disease: case report. Changgeng Yi Xue Za Zhi. 1999;22:492–497. [PubMed] [Google Scholar]
- Tsao K, Hawgood S, Vu L. Resolution of hydrops fetalis in congenital cystic adenomatoid malformation after prenatal steroid therapy. J Pediatr Surg. 38:508–510. doi: 10.1053/jpsu.2003.50089. [DOI] [PubMed] [Google Scholar]
- van den Heuvel F, Bink-Boelkens MT, du Marchie Sarvaas GJ, Berger RM. Drug management of fetal tachyarrhythmias: are we ready for a systematic and evidence-based approach? Pacing Clin Electrophysiol. 2008;31(Suppl. 1):S54–S57. doi: 10.1111/j.1540-8159.2008.00958.x. [DOI] [PubMed] [Google Scholar]
- van der Meer SB, Spaapen LJ, Fowler B, Jakobs C, Kleijer WJ, Wendel U. Prenatal treatment of a patient with vitamin B12-responsive methylmalonic acidemia. J Pediatr. 1990;117:923–926. doi: 10.1016/s0022-3476(05)80138-6. [DOI] [PubMed] [Google Scholar]
- Vos AA, Bruinse HW. Congenital adrenal hyperplasia: do the benefits of prenatal treatment defeat the risks? Obstet Gynecol Surv. 2010;65:196–205. doi: 10.1097/OGX.0b013e3181d61046. [DOI] [PubMed] [Google Scholar]
- Wallace C, Couch R, Ginsberg J. Fetal thyrotoxicosis: a case report and recommendations for prediction, diagnosis, and treatment. Thyroid. 1995;5:125–128. doi: 10.1089/thy.1995.5.125. [DOI] [PubMed] [Google Scholar]
- Watson WJ, Fiegen MM. Fetal thyrotoxicosis associated with nonimmune hydrops. Am J Obstet Gynecol. 1995;172:1039–1040. doi: 10.1016/0002-9378(95)90040-3. [DOI] [PubMed] [Google Scholar]
- Yokoi K, Ito T, Maeda Y, et al. A case of holocarboxylase synthetase deficiency with insufficient response to prenatal biotin therapy. Brain Dev. 2009;31:775–778. doi: 10.1016/j.braindev.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Younis JS, Granat M. Insufficient transplacental digoxin transfer in severe hydrops fetalis. Am J Obstet Gynecol. 1987;157:1268–1269. doi: 10.1016/s0002-9378(87)80309-5. [DOI] [PubMed] [Google Scholar]
- Zass R, Leupold D, Fernandez MA, Wendel U. Evaluation of prenatal treatment in newborns with cobalamin-responsive methylmalonic acidaemia. J Inherit Metab Dis. 1995;18:100–101. doi: 10.1007/BF00711393. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yang YL, Hasegawa Y, et al. Prenatal diagnosis of methylmalonic aciduria by analysis of organic acids and total homocysteine in amniotic fluid. Chin Med J (Engl) 2008;121:216–219. [PubMed] [Google Scholar]