Abstract
Background
The hypomethylating agents (HMAs) azacitidine and decitabine are most commonly used to treat patients with higher-risk myelodysplastic syndromes (MDS). To the authors' knowledge, the prognosis of patients with low-risk and intermediate-1– risk MDS by the International Prognostic Scoring System (IPSS) after HMA failure has not been explored comprehensively.
Methods
The clinical characteristics and treatment outcome of 438 patients with low-risk and intermediate-1–risk MDS who were treated with HMAs were retrospectively analyzed.
Results
Using the International Working Group response criteria, the overall objective response to HMA was 35% with a median of 6 cycles of HMA administered, and the median response duration was 7 months. Only 7% of patients had disease that transformed into acute myeloid leukemia while receiving therapy. Of the 290 patients who were evaluable at the time of HMA failure, 77% remained in the lower-risk disease categories. On multivariate analysis, baseline neutropenia, intermediate-risk and poor-risk baseline karyotype, and lack of response to HMA were found to be independently associated with a higher risk of disease progression. With a median follow-up of 16 months, the median transformation-free survival and overall survival (OS) after HMA failure were 15 months and 17 months, respectively. On multivariate analysis, only The University of Texas MD Anderson Global Scoring System was found to be independently predictive of outcome, with patients with higher-risk categories having poor transformation-free survival (hazards ratio [HR], 1.5; P=.003) and OS (HR, 1.8; P=.002). The administration of salvage therapy was independently associated with better OS only (HR, 0.8; P=.01).
Conclusions
Outcomes of patients with lower-risk MDS after HMA failure are poor and the treatment of these patients remains an unmet medical need. OS is a reasonable primary endpoint for clinical studies targeting this population.
Keywords: myelodysplastic syndrome, low-risk, hypomethylating agent failure, survival
Introduction
The myelodysplastic syndromes (MDS) are a heterogeneous group of clonal myeloid disorders characterized by ineffective hematopoiesis and an increased risk of transformation to acute myelogenous leukemia (AML).1 Patients with MDS are often divided by the International Prognostic Scoring System (IPSS) into lower-risk (low and intermediate-1) and higher-risk (intermediate-2-and high) subsets.2 The prognosis of patients with lower-risk MDS is heterogeneous, with survival ranging from 14 to 80 months.3 Hypomethylating agents (HMAs; azacitidine and decitabine) are considered the standard of care for patients with higher-risk MDS, although they also are commonly used in patients with lower-risk MDS (and have regulatory approval for such use in the United States), particularly in those with neutropenia and/or thrombocytopenia, or in those for whom other agents (growth factors or lenalidomide) have failed.4-7 We and others have previously reported on the outcome of patients with higher-risk disease after HMA failure. These patients have a poor prognosis, with a median survival of 4 to 6 months.8-10 To our knowledge, the prognosis of patients with low-risk and intermediate-1–risk MDS after HMA failure has not been explored comprehensively,11 despite the limited treatment options available to such patients and implications for the design of future clinical trials.
In the current study, we assessed the outcome of patients with lower-risk MDS after HMA failure and identified prognostic factors for disease progression and survival in those who retained their lower-risk disease designation after HMA failure, and in those who progressed to higher-risk MDS.
Materials and Methods
We reviewed clinical characteristics, treatment details, and outcomes of 438 consecutive patients with IPSS low-risk (145 patients) and intermediate-1-risk (293 patients) disease who were treated with and failed treatment with HMAs at The University of Texas MD Anderson Cancer Center (MDACC; 159 patients) and H. Lee Moffitt Cancer Center (279 patients) between 2000 and 2011. For the purpose of the current study, patients were considered evaluable if karyotype data were available at time of HMA failure. The study was approved by the respective Institutional Review Boards.
Failure was defined as no response after at least 6 cycles of therapy, loss of response, progression to higher-risk MDS categories, transformation to AML, or discontinuation of therapy due to side effects. Patients who were taken off therapy were analyzed for the reason for HMA discontinuation, as well as for characteristics at the time of HMA treatment and at the time HMA was discontinued. All patients included in the current study were considered to have failed HMA due to no response, lost response, and/or progression to AML. Responses to HMA and subsequent therapies were coded according to the 2006 International Working Group Criteria for response assessment in patients with MDS.12
Patients were categorized for MDS risk at the initiation of HMA therapy and at the time of failure of HMA according to the IPSS,2 the revised IPSS (IPSS-R),13 the MDACC Global Scoring System (MDGSS),14 and the Low-Risk MD Anderson Scoring System (LRMDSS),15 the latter being applicable to patients with low-risk and intermediate-1- risk disease according to the IPSS.
Survival probabilities were calculated using the Kaplan-Meier method, assessed from the time of HMA failure, and compared using the log-rank test. Univariate and multivariate analyses were performed to identify potential prognostic factors associated with progression into higher-risk MDS categories, transformation-free survival (TFS), and overall survival (OS). Multivariate analysis for progression into higher-risk MDS categories used a logistic regression model, and the Cox proportional hazard regression analysis was used for TFS and OS. A P value of <.05 (2-tailed) was considered to be statistically significant.16-18
Results
Baseline Patient Characteristics
Baseline clinical characteristics at the initiation of HMA therapy are summarized in Table 1. Overall, there were no differences noted between the overall population and the 290 evaluable patients who had cytogenetic studies available at the time of HMA failure. Approximately one-third of patients had low-risk disease by the IPSS and two-thirds had intermediate-1–risk disease. The majority of patients had favorable-risk baseline cytogenetics according to the IPSS and were diagnosed with de novo MDS rather than therapy-related disease. Greater than 75% of patients were transfusion-dependent at the time HMA therapy, with serum ferritin measurements of >1000 ng/mL reported in approximately two-thirds of patients. Approximately two-thirds of patients had previously failed to respond to growth factor therapies.
Table 1. Baseline Patient Characteristics.
| No. (%) | ||
|---|---|---|
|
| ||
| Characteristic | Overall (N=438) | Evaluable (N=290) |
| IPSS, low/int-1 | 145 (33)/293 (67) | 91 (32)/193 (68) |
| Age ≥60 y | 356 (81) | 236 (81) |
| Median age (range), y | 69 (20-91) | 68 (20-91) |
| ECOG score 0-1 | 319 (73) | 274 (91) |
| Cytopenia 2-3 | 193 (44) | 126 (43) |
| ANC <1.5 × 109/L | 129 (36) | 79 (34) |
| Hemoglobin <10 g/dL | 237 (54) | 157 (54) |
| Platelets <100 × 109/L | 201 (47) | 124 (43) |
| Median BM blasts (range), % | 3 (0-10) | 3 (0-10) |
| BM blasts ≥5% | 124 (28) | 75 (26) |
| CG-IPSS good | 347 (79) | 230 (80) |
| Therapy-related MDS | 69 (16) | 48 (17) |
| Transfusion-naive | 119 (27) | 67 (23) |
| Ferritin ≥1000 ng/dL | 191 (66) | 145 (70) |
| Prior growth factor therapy | 264 (60) | 184 (63) |
Abbreviations: ANC, absolute neutrophil count; BM, bone marrow; CG, cytogenetic; ECOG, Eastern Cooperative Oncology Group; int, intermediate; IPSS, International Prognostic Scoring System; MDS, myelodysplastic syndrome.
Response to HMA Therapy
The best response to HMA therapy and the reasons for treatment failure are summarized in Table 2. The median duration from diagnosis to HMA therapy was 7 months. The best response to HMA therapy was complete response in 10% of patients, a partial response in 4% of patients, and hematologic improvement in 21% of patients. The overall objective response was 36%. Approximately one-half of the patients (54%) had stable disease while receiving therapy, with no further improvement noted. The median number of HMA cycles administered was 6 (range, 1-64 cycles) and the median response duration was 7 months (range, 1-73 months). The majority of patients discontinued therapy because of loss of response (30%) or primary resistance (45%) as judged by the treating physician. Twenty-six patients (6%) had disease that transformed into AML while receiving HMA therapy, 13 patients (3%) withdrew from therapy because of side effects, and 11 patients (3%) died while receiving therapy. The remaining patients (13%) withdrew from therapy for different reasons (including financial and medical reasons), or were lost to follow-up.
Table 2. Response to HMA Therapy and Reasons for Failure.
| No. (%) | |
|---|---|
| Best response | |
| Complete response | 42 (10) |
| Partial response | 19 (4) |
| Hematologic improvement | 92 (21) |
| Stable disease | 238 (54) |
| Progressive disease | 36 (8) |
| Died while receiving therapy | 11 (3) |
| Reason for stopping therapy | |
| Loss of response | 133 (30) |
| Primary resistance | 195 (45) |
| Transformation into AML | 26 (6) |
| Side effects | 13 (3) |
| Other | 71 (16) |
Abbreviation: AML, acute myeloid leukemia; HMA, hypomethylating agent.
It is interesting to note that there was no difference in patient characteristics noted between patients treated with azacitidine and those treated with decitabine, nor was any difference observed with regard to response rates between the 2 HMAs administered.
Patient Characteristics at the Time of HMA Failure
Of the 290 patients who were evaluable at the time of HMA failure, the majority (54% to 77%) continued to have lower-risk disease as assessed by the IPSS, the IPSS-R, or the MDGSS. Rates of evolution to higher-risk disease were 23% (high: 8%; intermediate-2: 15%) using the IPSS, 30% (high: 20%; very high: 10%) using the IPSS-R, and 46% (poor: 17%; intermediate-2: 29%) using the MDGSS (Table 3). The MDGSS identified a larger group of patients with higher-risk disease, some of whom were classified as having lower-risk disease by the IPSS and IPSS-R.
Table 3. Staging at the Time of HMA Failure and Outcome After HMA Failurea.
| TFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| % | % | |||||||
|
|
|
|||||||
| Scoring System | No. (%) | Median, Months | 1-Year | 3-Year | Median, Months | 1-Year | 3-Year | |
| IPSS | Low | 69 (24) | 34 | 82 | 58 | 33 | 90 | 62 |
| Int-1 | 155 (53) | 21 | 65 | 37 | 26 | 77 | 38 | |
| Int-2 | 43 (15) | 5 | 17 | 8 | 7 | 37 | 10 | |
| High | 23 (8) | 2 | 15 | 0 | 8 | 39 | 0 | |
| IPSS-R | Very low | 31 (11) | 33 | 76 | 58 | 51 | 82 | 62 |
| Low | 87 (30) | 31 | 76 | 42 | 31 | 79 | 47 | |
| Int | 84 (29) | 21 | 55 | 36 | 22 | 68 | 38 | |
| High | 58 (20) | 5 | 23 | 10 | 9 | 38 | 10 | |
| MDGSS | Very high | 29 (10) | 4 | 22 | 10 | 7 | 36 | 10 |
| Low | 51 (18) | 30 | 79 | 45 | 36 | 77 | 60 | |
| Int-1 | 104 (36) | 29 | 75 | 40 | 29 | 75 | 51 | |
| Int-2 | 85 (29) | 10 | 58 | 18 | 12 | 60 | 21 | |
| Poor | 50 (17) | 5 | 30 | 10 | 8 | 32 | 10 | |
Abbreviations: HMA, hypomethylating agent; Int, intermediate; IPSS, International Prognostic Scoring System; IPSS-R, revised International Prognostic Scoring System; MDGSS, The University of Texas MD Anderson Global Scoring System; OS, overall survival; TFS, transformation-free survival.
This table summarizes TFS and OS (median and at 1-year and 3-year) after HMA failure according to the IPSS, IPSS-R, and MGGSS at the time of HMA failure.
On multivariate analyses, factors associated with disease progression from lower-risk to higher-risk MDS categories among the 290 evaluable patients included baseline neutropenia, intermediate-risk and poor-risk baseline karyotype (according to the IPSS classification), and lack of response to HMAs (Table 4).
Table 4. Univariate and Multivariate Analysis for Disease Progression Into Higher-Risk Categories.
| Univariate | Multivariate | |||
|---|---|---|---|---|
|
| ||||
| Parameter | No. (%) | P | OR | P |
| ANC <1.5 × 109/L | 79 (27) | .03 | 3.2 | .02 |
| BM blasts ≥5% | 75 (26) | .05 | NA | NS |
| CG-IPSS int/poor | 60 (21) | .01 | 6 | .01 |
| IPSS: int-1 | 193 (67) | .004 | NA | NS |
| IPSS-R: higher risk | 93 (32) | .002 | NA | NS |
| MDGSS: higher risk | 63 (22) | .001 | NA | NS |
| LRMDSS: high risk | 81 (28) | .009 | NA | NS |
| Lack of response to HMA | 176 (61) | <.001 | 1.8 | .02 |
Abbreviations: ANC, absolute neutrophil count; BM, bone marrow; CG, cytogenetic; HMA, hypomethylating agent; int, intermediate; IPSS, International Prognostic Scoring System; IPSS-R, revised International Prognostic Scoring System; LRMDSS, Low-Risk MD Anderson Scoring System; MDGSS, The University of Texas MD Anderson Global Scoring System; NA, not applicable; NS, not significant; OR, odds ratio.
Outcome After HMA Failure
A total of 89 patients (31%) were still alive at a median follow-up of 16 months (range, 1-80 months) from HMA failure. A total of 204 patients (70%) subsequently experienced a transformation to AML (93 patients) or died (111 patients). The median TFS and OS after HMA failure was 15 months (range, 1-80 months) and 17 months (range, 1-80 months), respectively (Fig. 1A). There was no difference in outcome noted between the 290 evaluable patients and the entire cohort of 438 patients (median survival, 15 months [range, 1-80 months] overall) (Fig. 1B). Furthermore, there was no difference noted in terms of leukemia-free and OS after HMA failure between the 2 types of HMAs administered.
Figure 1.
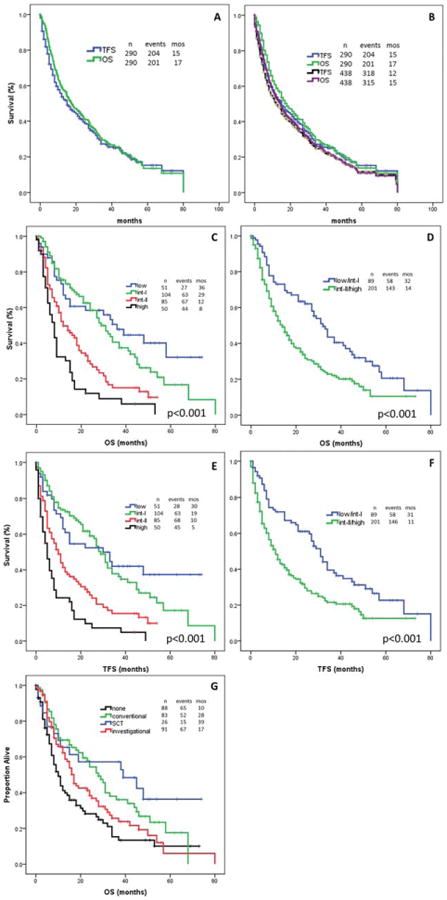
Outcome is shown. (A) Overall survival (OS) and transformation-free survival (TFS) are shown for the 290 evaluable patients. (B) OS and TFS are shown for the entire patient population (438 patients). (C) OS is shown by The University of Texas MD Anderson Cancer Center Global Scoring System (MDGSS) (4 categories). (D) OS is shown by the MDGSS (2 categories). (E) TFS is shown by the MDGSS (4 categories). (F) TFS is shown by the MDGSS (2 categories). (G) OS is shown by salvage therapy received. int-I indicates intermediate-1 risk; int-II, intermediate-2 risk; SCT, stem cell transplantation.
We assessed survival according to different scoring systems applied at the time of HMA failure (Table 3). As expected, patients with higher-risk disease at time of HMA failure had a worse outcome. The median OS ranged from <1 year for patients with higher-risk disease to approximately 3 years in patients with lower-risk disease after HMA failure (Fig. 1C). Using the MDGSS at the time of HMA failure, we were able to divide our population into 2 groups. The first group had lower-risk disease at the time of the initiation of treatment with HMAs and higher-risk disease at the time of HMA failure (approximately two-thirds of patients), with a median survival of 1 year. The second group had lower-risk disease at the time of HMA initiation and at HMA failure, with a median survival of approximately 3 years (Fig. 1D). Similarly, the median TFS ranged from 7 to 12 months in patients with lower-risk disease to <3 years in patients with higher-risk disease at the time of HMA failure (Fig. 1E). In addition, we identified 2 groups of patients with a high risk and low risk of transformation, with a median TFS of 11 months and 31 months, respectively (Fig. 1F).
Outcome by Post-HMA Therapy
After HMA failure, 200 patients received salvage therapy. Salvage therapy included investigational agents, a cytarabine-based regimen, and allogeneic stem cell transplantation (ASCT). Ninety-one patients (31%) received investigational agents, 26 patients (9%) underwent ASCT, and 83 patients (29%) received conventional or noninvestigational therapies, mainly with cytarabine-based regimens or additional HMAs; 90 patients (31%) elected not to receive any further treatment beyond supportive care. Of the 91 patients receiving cytarabine-based therapy, 16 (18%) responded. Of the 91 patients receiving investigational therapy, 15 (16%) responded. Eighteen of the 26 patients who underwent ASCT achieved a complete remission. The administration of salvage therapy was associated with better survival. The median survival from HMA failure was 10 months, 28 months, 17 months, and 39 months, respectively, for patients not receiving further therapy and for those treated with conventional agents, investigational agents, and ASCT (P = .001) (Fig. 1G). Because patients not pursuing salvage therapy may have had advanced disease and may not have been candidates for further therapy, we performed a landmark analysis at 1 month and 2 months after HMA failure. The landmark analysis confirmed the superior outcome in patients receiving salvage therapy after HMA failure.
Prognostic Factors for Survival After HMA Failure
Using univariate and multivariate analysis, we assessed factors associated with survival at the time of HMA failure. On univariate analysis, thrombocytopenia; bone marrow blasts >5%; intermediate-risk and poor-risk cytogenetics; higher-risk disease categories by the IPSS, IPSS-R, MDGSS, and LRMDSS; lack of response to HMA therapy; and lack of salvage treatment after HMA failure were associated with worse outcome (Table 5). Previous therapy with erythropoietin-stimulating agents (ESAs) did not appear to affect outcome after HMA failure on the univariate analysis and therefore it was not included in the multivariate analyses for leukemia-free survival and OS. On multivariate analyses, only the MDGSS was found to be independently predictive of outcome, with patients with higher-risk MDS found to have poor TFS (hazards ratio [HR], 1.5; P=.003) and OS (HR, 1.8; P=.002). The administration of salvage therapy rather than only supportive care was found to be independently associated with better OS only (HR, 0.8; P=.01) but not with a decrease in the rates of disease progression.
Table 5. Univariate and Multivariate Analysis for Survival After HMA Failure.
| TFS | OS | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| UVA | MVA | UVA | MVA | |||
|
| ||||||
| Parameter | P | HR | P | P | HR | P |
| Platelets <100 × 109/L | <.001 | NA | NS | <.001 | NA | NS |
| BM blasts >5% | <.001 | NA | NS | <.001 | NA | NS |
| CG-IPSS: int/poor | <.001 | NA | NS | <.001 | NA | NS |
| IPSS: higher risk | <.001 | NA | NS | <.001 | NA | NS |
| IPSS-R higher risk | <.001 | NA | NS | <.001 | NA | NS |
| MDGSS: higher risk | <.001 | 1.5 | .003 | <.001 | 1.8 | .002 |
| LRMDSS: higher risk | <.001 | NA | NS | <.001 | NA | NS |
| Lack of response to HMA | <.001 | NA | NS | .002 | NA | NS |
| Salvage therapy | NS | NA | NA | .001 | 0.8 | .01 |
Abbreviation: BM, bone marrow; CG, cytogenetic; HMA, hypomethylating agent; HR, hazards ratio; int, intermediate; IPSS, International Prognostic Scoring System; IPSS-R, revised International Prognostic Scoring System; LRMDSS, Low-Risk MD Anderson Scoring System; MDGSS, The University of Texas MD Anderson Global Scoring System; MVA, multivariate analysis; NA, not applicable; NS, not significant; OS, overall survival; TFS, transformation-free survival; UVA, univariate analysis.
Discussion
To the best of our knowledge, the current study is the first to present the outcome of a large series of patients with low-risk and intermediate-1–risk MDS according to the IPSS who were treated with HMAs and whose disease failed to respond to an HMA or progressed after an initial clinical response. The median OS of 16 months for these patients confirmed their general poor outcome and the urgent need for improved salvage therapies. This is in keeping with a preliminary previous report from Prebet et al, who reported a median survival of 17 months after HMA failure in 59 patients with lower-risk disease.11
The results of our multivariate models demonstrated that simple clinical and biologic characteristics, as well as the initial response to HMA, can predict progression into higher-risk disease categories (23%), and that the MDGSS, applied at the time of HMA therapy failure, can predict independently of any other parameters TFS and OS, thus dividing patients into 2 categories of those with low-risk and high-risk disease with approximate median survivals of 3 years and 1 year, respectively. It is interesting to note that the outcome of patients who remained in lower-risk categories was similar to that of those with resistance to ESAs, in whom the median survival after ESA failure was reported to be 40 months.19 Determining which patients with lower-risk disease are in need of HMA therapy is warranted. A randomized trial assessing the impact of HMA in newly diagnosed patients with lower-risk MDS is currently ongoing.
The MDGSS predicted survival in this large cohort of patients with lower-risk disease, a finding that is similar to our previous report of patients with high-risk disease after decitabine failure.8 This score includes poor performance, older age, thrombocytopenia, anemia, increased bone marrow blasts, leukocytosis, chromosome 7 or complex (≥3) abnormalities, and prior transfusions. It allows risk assessment of any patient, regardless of prior therapy, at different time points in the course of the disease.14 In the report by Prebet et al, age, bone marrow blast count, and cytogenetics (all of which were included in our model) were found to have prognostic value as well.9 In addition, and similar to the recent report published by Prebet et al regarding the outcome of patients with lower-risk MDS after HMA failure,11 the initial response to HMA was not found to have an impact on survival after failure.
The results of the current analysis are important for patient and physician decision-making, as well as to establish individual patient expectations and to assess the benefit of newer therapies. At the time of HMA failure, based on the MDGSS, patients can be divided into low-risk and high-risk groups with median survivals of 3 years and 1 year, respectively. This simple risk model could thus be used to advise patients of their prognosis and treatment options, and to evaluate the benefit of newer therapies after failure of HMA therapy. That being said and regardless of disease categorization at the time of HMA failure, these patients continue to have a poor prognosis and newer therapies are indicated. OS is a reasonable primary endpoint for clinical studies targeting this population.
A variety of salvage regimens were administered to patients in the current cohort. The outcome after any type of treatment appeared better than supportive care as confirmed in our multivariate model, as well by performing a landmark analysis at different time points after HMA failure. ASCT remained the option with the best outcome, with long-term survival noted in a substantial percentage of patients even if some patients underwent transplantation with progressive disease.20 This is in keeping with the study by Prebet et al, in which the median survival of patients who underwent ASCT was 19 months, which is significantly superior to that of other treatments.9
In addition, we also observed an improved outcome with investigational and standard treatments involving cytarabine-based and HMA-based regimens. These findings are in keeping with previous results of patients with high-risk disease after decitabine failure, in whom we reported response rates between 15% to 30% with the use of investigational agents and standard approaches.8,21 However, these data should be interpreted with caution due to the inherent confounding factors of such an analysis such as performance status, accelerated disease, physician bias, etc. Dedicated studies for each type of treatment will be necessary to refine the response rates and prognostic factors associated with each group of patients.
The results of the current study define the poor outcome in patients with lower-risk MDS after HMA failure (median survival, 17 months). The treatment of these patients remains an unmet medical need. We believe the results presented herein will help in the design of future clinical trials in this population, and indicate that OS is a reasonable primary endpoint for such studies.
Acknowledgments
Funding Support: Supported in part by the following grants: the MDS Clinical Research Consortium sponsored by the Aplastic Anemia and MDS International Foundation (made possible by funding from the Edward P. Evans Foundation); The University of Texas MD Anderson Cancer Center Support Grant CA016672; the Fundacion Ramon Areces (Madrid, Spain); and generous philanthropic contributions to The University of Texas MD Anderson Cancer Center Moon Shots Program.
Footnotes
Conflict of Interest Disclosures: Dr. Jabbour has received a grant from GlaxoSmithKline for work performed outside of the current study. Dr. Padron has received a grant from the Edward P. Evans Foundation for work performed as part of the current study. Dr. Lancet has acted as a paid consultant for and received research support from Celgene for work performed outside of the current study. Dr. Steensma has acted as a paid consultant for Celgene and Incyte. He also owns stock equity in ARIAD Pharmaceuticals and has been a paid member of the Data Safety Monitoring Board for Amgen and Novartis for work performed outside of the current study. Dr. Sekeres has acted as a paid member of the advisory boards for Celgene, Amgen, and Boehringer Ingelheim for work performed outside of the current study. Dr. Gore has received a grant and personal fees from Celgene for work performed outside of the current study. Dr. List has acted as a paid consultant for Celgene for work performed outside of the current study.
References
- 1.Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361:1872–1885. doi: 10.1056/NEJMra0902908. [DOI] [PubMed] [Google Scholar]
- 2.Bejar R, Levine R, Ebert BL, et al. Unraveling the molecular pathophysiology of myelodysplastic syndromes. J Clin Oncol. 2011;29:504–515. doi: 10.1200/JCO.2010.31.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 4.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 5.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. International Vidaza High-Risk MDS Survival Study Group. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–57. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 8.Jabbour E, Garcia-Manero G, Batty N, et al. Outcome of patients with myelodysplastic syndrome after failure of decitabine therapy. Cancer. 2010;116:3830–3834. doi: 10.1002/cncr.25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prebet T, Gore SD, Esterni B, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol. 2011;29:3322–3327. doi: 10.1200/JCO.2011.35.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duong VH, Lin K, Reljic T, et al. Poor outcome of patients with myelodysplastic syndrome after azacitidine treatment failure. Clin Lymphoma Myeloma Leuk. 2013;13:711–715. doi: 10.1016/j.clml.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Prebet T, Thepot S, Gore SD, Dreyfus F, Fenaux P, Vey N. Outcome of patients with low-risk myelodysplasia after azacitidine treatment failure. Haematologica. 2013;98:e18–e19. doi: 10.3324/haematol.2012.071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantarjian H, O'Brien S, Ravandi F, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113:1351–1361. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Manero G, Shan J, Faderl S, et al. A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia. 2008;22:538–543. doi: 10.1038/sj.leu.2405070. [DOI] [PubMed] [Google Scholar]
- 16.Agresti A. Categorical Data Analysis. 2nd. Hoboken, NJ: John Wiley & Sons Inc; 1990. [Google Scholar]
- 17.Kaplan EL, Maier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 19.Kelaidi C, Park S, Sapena R, et al. Long-term outcome of anemic lower-risk myelodysplastic syndromes without 5q deletion refractory to or relapsing after erythropoiesis-stimulating agents. Leukemia. 2013;27:1283–1290. doi: 10.1038/leu.2013.16. [DOI] [PubMed] [Google Scholar]
- 20.Litzow MR, Tarima S, Perez WS, et al. Allogeneic transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood. 2010;115:1850–1857. doi: 10.1182/blood-2009-10-249128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borthakur G, Ahdab SE, Ravandi F, et al. Activity of decitabine in patients with myelodysplastic syndrome previously treated with azacitidine. Leuk Lymphoma. 2008;49:690–695. doi: 10.1080/10428190701882146. [DOI] [PMC free article] [PubMed] [Google Scholar]


