Abstract
Objective
Early childhood caries (ECC) has become a prevalent public health problem among Chinese preschool children. The bacterial microflora is considered to be an important factor in the formation and progress of dental caries. However, high-throughput and large-scale studies of the primary dentition are lacking. The present study aimed to compare oral microbial profiles between children with severe ECC (SECC) and caries-free children.
Methods
Both saliva and supragingival plaque samples were obtained from children with SECC (n = 20) and caries-free children (n = 20) aged 3 to 4 years. The samples were assayed using the Human Oral Microbe Identification Microarray (HOMIM).
Results
A total of 379 bacterial species were detected in both the saliva and supragingival plaque samples from all children. Thirteen (including Streptococcus) and two (Streptococcus and Actinomyces) bacterial species in supragingival plaque and saliva, respectively, showed significant differences in prevalence between the two groups. Of these, the frequency of Streptococcus mutans detection was significantly higher in both saliva (p = 0.026) and plaque (p = 0.006) samples from the SECC group than in those from the caries-free group.
Conclusions
The findings of our study revealed differences in the oral microbiota between the SECC and caries-free groups Several genera, including Streptococcus, Porphyromonas, and Actinomyces, are strongly associated with SECC and can be potential biomarkers of dental caries in the primary dentition.
Introduction
Previous studies on early childhood caries (ECC) have focused on Streptococcus mutans, Actinomyces, and Lactobacillus [1,2,3]. ECC has become a prevalent public health problem among preschool children globally, particularly in China. According to the third national oral health epidemiological survey conducted in China in 2005, the prevalence rate of dental caries among 5-year-olds was 66%, which was significantly higher than the average in other countries [6]. The bacterial microflora is considered to be an important factor in the formation and progress of dental caries. Researchers have explored the bacterial microbiota in dental plaque samples to investigate the etiology of severe ECC (SECC) using methods such as denaturing gradient gel electrophoresis, pyrosequencing analysis, and cultivation [1,2,3].
More than 700 bacterial species or phylotypes exist in the oral cavity, approximately 35% of which have not been cultivated [4]. Conventional microbiological approaches that rely on cultivation for the detection of microorganisms in the oral cavity are not sufficient for such comprehensive and intensive monitoring. These time-consuming techniques require many specialized and complex growth media and yet capture only a small fraction of the oral microbiota [1,5,6]. Therefore, several oral bacterial species remain undetected.
The Human Oral Microbe Identification Microarray (HOMIM), which targets approximately 300 predominant oral bacterial species that include cultivable and not-yet-cultivated phylotypes (HOMIM home page: http://mim.forsyth.org/), has recently been used to determine bacterial profiles and microbial diversity in the oral cavity and compare these between healthy individuals and those with oral diseases such as periodontitis [6,7]. In the present study, we used HOMIM with the aim of comparing the bacterial profiles in saliva and supragingival plaque samples between children with SECC and caries-free children to investigate the etiology of caries in the primary teeth. The data obtained may help in defining differences in the oral microbiota between children with SECC and caries-free children, identify potential biomarkers of ECC in the primary dentition, and further improve our understanding of this complex infectious disease.
Materials and Methods
Ethics Statement
Written informed consent was obtained from the parents of all children included in this study. The study design, protocol, and informed consent forms were approved by the Ethics Committee of Peking University Health Science Center (PKUSSIRB-2013060).
Clinical Methods
A total of 40 Chinese children aged 3 to 4 years (39 to 50 months), including 20 caries-free [decayed, missing, filled surfaces (DMFS) index = 0] children and 20 children with SECC (DMFS ≥ 4 for 3-year-olds, DMFS ≥ 5 for 4-year-olds; Table 1), were included in this study. The definitions and diagnoses of dental caries and SECC were according to the criteria of the World Health Organization [8]. Children with no clinical signs of early caries or white spots were considered to be free of caries. The first molar had not erupted in any of the 40 children, and none of the patients exhibited salivary gland diseases or systemic diseases. No patients had consumed antibiotics within 4 weeks before the study. Samples were obtained only after obtaining informed consent from the children and their parents.
Table 1. Demographic and clinical characteristics of the study population.
| Characteristic | Severe ECC n = 20 | Caries-free n = 20 | P value |
|---|---|---|---|
| Mean age (months) (mean ± SD) | 43.9 ± 3.2 | 44.4 ± 3.7 | 0.62 a |
| No. (%) male | 9 (45) | 10 (50) | 0.759 a |
| Caries status | |||
| DMFS score (mean ± SD) | 11.1 ± 5.6 | 0 | <0.0001 a |
| Low | 4 | 0 | |
| Medium | 10.4 | 0 | |
| High | 24 | 0 |
a Nonparametric Mann–Whitney U test
DMFS: decayed, missing, and filled tooth surfaces
ECC: early childhood caries
All subjects were instructed to refrain from eating or drinking from 2 h before sampling. Stimulated whole saliva samples were collected in 5-mL sterile Eppendorf microcentrifuge tubes. Supragingival pooled plaque samples were obtained from the noncarious enamel surface of each tooth, including the anterior and posterior teeth, and each sample obtained from the same individual was placed in a 1.5-mL microcentrifuge tube containing 1 mL of TE (50 mM Tris-HCl, 1 mM EDTA; pH 7.6). These samples were immediately frozen at −20°C and stored at −80°C until further use.
DNA Isolation and Amplification
Bacterial DNA was extracted from saliva and supragingival plaque using the TIANamp Bacteria DNA Kit (Tiangen Qiagen, Hilden, Germany). The lysis procedure was a modification of the conventional procedure [2,3]. All our samples were treated with a cocktail lysis buffer containing mutanolysin, proteinase K, and a lysozyme. The extracts were stored at −20°C until further use. 16S rRNA genes were amplified by polymerase chain reaction (PCR) from the bacterial DNA isolated from the saliva and dental plaque samples using universal 16S rRNA primers. Briefly, two separate PCR assays were performed using either the forward primer 5′-CCA GAGTTT GAT YMT GGC-3′ and reverse primer 5′-GAA GGA GGT GWT CCA RCC GCA-3′ or the forward primer 5′-GAC TAG AGT TTG ATY MTG GC-3′ and reverse primer 5′-GYT ACC TTG TTA CGA CTT-3′ [6]. The results of PCR amplification were examined by electrophoresis in a 1% agarose gel. The two parallel PCRs were combined and the products were purified using the TIAN quick Midi Purification Kit (Tiangen).
Microarray Analysis
For HOMIM 16S rRNA gene microarray analysis, purified DNA samples (two plaque samples from the caries-free group were excluded because of poor quality of DNA) were sent to the MIM Core Facility at the Forsyth Institute (Cambridge, MA, USA, http://mim.forsyth.org). Labeled nucleotide Cy3-dCTP was incorporated during a second nested PCR. Next, hybridization was performed overnight at 55°C. The arrays were then washed at room temperature, spun dry, and stored in a dark container until they were scanned by the Axon 4000B microarray scanner, and crude data were extracted using GenePix Pro software (Molecular Devices, Sunnyvale, CA, USA). Microbial profiles were generated from image files of scanned arrays using a HOMIM online analysis tool (http://bioinformatics.forsyth.org/homim/). Detection of a particular taxon was determined by the presence of a fluorescent spot for that unique probe. The mean intensity for each taxon was calculated from hybridization spots for the same probe. Signals were normalized by comparison of individual signal intensities with the average of signals from universal 16S rRNA probes and categorized into relative intensity values ranging from 0 to 5 (the minimum threshold for signal detection is equivalent to approximately 104 bacterial cells) [9,10,11].
Statistical Analysis
The Chi-squared test was performed to compare the frequency of each probe between the SECC and caries-free groups. The nonparametric Mann–Whitney U test was performed to compare the abundance of species between the two groups. Adjustment for multiple comparisons was performed using the false-discovery rate (Benjamini and Hochberg, 1995). A p-value of ≤0.05 was considered statistically significant. Statistical analyses were performed using the SPSS 19.0 software.
Results
The severe ECC and caries-free (control) groups were matched for age, gender, and race (Table 1). In total, 379 bacterial species were detected in both the saliva and supragingival plaque samples from all children. All collected samples were successfully analyzed.
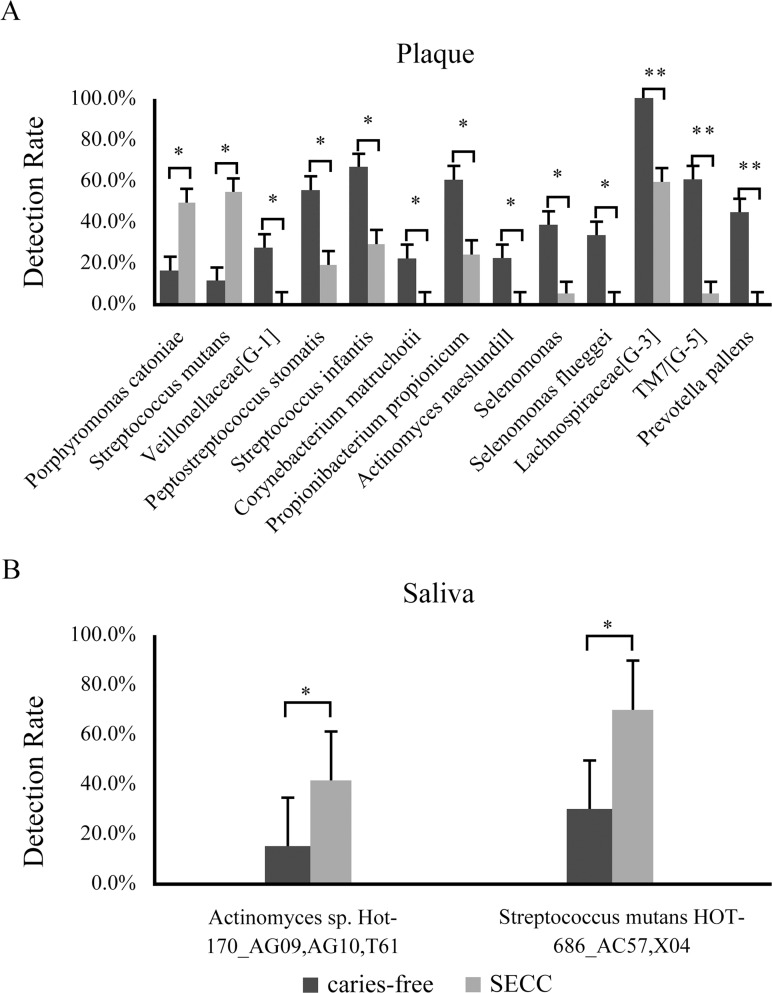
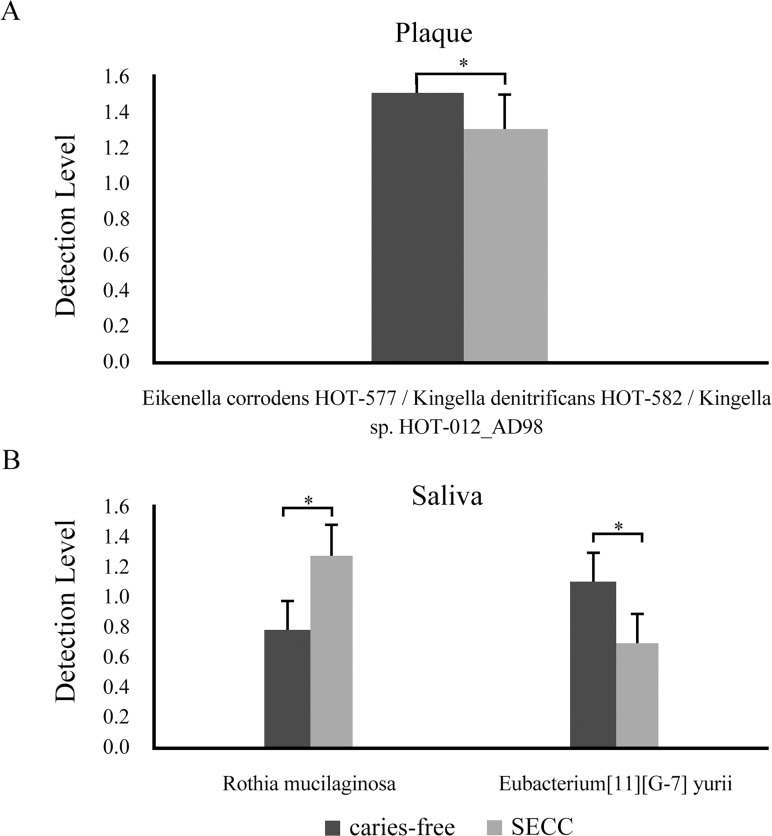
Thirteen bacterial species showed significant differences in prevalence in supragingival plaque between the SECC and caries-free groups (Fig 1A), while two bacterial species showed significant differences in prevalence in saliva (Fig 1B). With regard to abundance, one bacterial species, Eikenella corrodens HOT-577/Kingella denitrificans HOT-582/Kingella sp. HOT-012_AD98, in supragingival plaque (p = 0.014, Mann–Whitney U test; Fig 2A) and two bacterial species, Rothia mucilaginosa (p = 0.046, Mann–Whitney U test) and Eubacterium (p = 0.021, Mann–Whitney U test), in saliva (Fig 2B) showed significant differences between the SECC and caries-free groups. Of these species, S. mutans was detected significantly more frequently in the SECC group than in the caries-free group in both saliva (p = 0.026, Chi-squared test) and plaque (p = 0.006, Chi-squared test). In addition, Porphyromonas catoniae and Actinomyces in plaque (p = 0.043, Chi-squared test) and saliva (p = 0.041, Chi-squared test), respectively, were more prevalent in the SECC group than in the caries-free group. R. mucilaginosa was more abundant in the saliva of children with SECC than in that of caries-free children.
Fig 1. Bacterial species showing significant differences in prevalence between the severe ECC group and caries-free group.
Significant differences in prevalence are observed for 13 bacterial species in supragingival plaque (A) and two bacterial species in saliva (B). *p ≤ 0.005, **p ≤ 0.05 according to the Chi-square test.
Fig 2. Bacterial species showing significant differences in abundance between the severe ECC group and caries-free group.
One and three bacterial species showed significant differences in abundance in supragingival plaque (A) and saliva (B), respectively. *p<0.05 according to the Mann–Whitney Rank Sum Test.
Each of the 17 species was confirmed to be somehow relevant to the development of ECC or dental caries (Table 2). Table 3 summarizes the bacterial counts for each species.
Table 2. Bacterial species showing significant differences between the severe early childhood caries (SECC) and caries-free groups.
| Significant differences in prevalence in plaque | P value | References | Opinions |
|---|---|---|---|
| Actinomyces naeslundii | 0.041 a | Beker et al.2002 [ 14 , 15 ] | Associated with SECC |
| Corynebacterium matruchotii | 0.041 a | Tanner et al.2011 [ 1 , 19 ] | Associated with oral health |
| Lachnospiraceae [G-3] | 0.003 a | Gross et al.2010 [ 19 ] | Associated with SECC |
| Porphyromonas catoniae | 0.043 a | Vercher et al.2014 [ 17 ] | Caries-related |
| Prevotella pallens | 0.001 a | Tanner et al.2011 [ 1 ] | Associated with SECC |
| Propionibacterium propionicum | 0.047 a | Gross et al.2010 [ 19 ] | Associated with SECC |
| Selenomonas spp. | 0.016 a | Xu et al.2012 [ 20 ] | Associated with oral health |
| Selenomonas flueggei | 0.007 a | Luo et al.2012 [ 12 ] | Caries-related |
| Streptococcus infantis | 0.05 a | Gross et al.2010 [ 19 ] | Associated with oral health |
| Streptococcus mutans | 0.006 a | Li et al.2007 [ 2 , 3 ] | Caries Pathogens |
| TM7 [G-5] | 0.002 a | Vercher et al.2014 [ 17 ] | Caries-related |
| Peptostreptococcus stomatis | 0.042 a | Colombo et al.2009 [ 9 ] | Associated with oral periodontitis |
| Veillonellaceae [G-1] | 0.017 a | Tanner et al.2012 [ 25 ] | Associated with white-spot lesions |
| Significant differences in abundance in plaque | P value | References | Opinions |
| Kingella denitrificans | 0.014 b | Torlakovic et al.2012 [ 7 ] | Associated with oral health |
| Significant differences in prevalence in saliva | P value | References | Opinions |
| Actinomyces | 0.041 a | Beker et al.2002 [ 14 , 15 ] | Associated with SECC |
| Streptococcus mutans | 0.026 a | Li et al.2007 [ 2 , 3 ] | Caries Pathogen |
| Significant differences in abundance in saliva | P value | References | Opinions |
| Eubacterium/ Peptostreptococcaceae | 0.021 b | Gross et al.2010 [ 19 ] | Associated with oral health |
| Rothia mucilaginosa | 0.046 b | Colombo et al.2009 [ 9 ] | Associated with peritonitis |
The table shows 17 bacterial species that showed significant differences between the caries-free and SECC groups The studies showed that each species was somehow related to early childhood caries or dental caries.
a Chi-squared test
b Nonparametric Mann–Whitney U test
Table 3. Human Oral Microbe Identification Microarray (HOMIM) score distribution for the 17 bacterial species that showed significant differences between the severe early childhood caries (SECC) and caries-free groups.
| HOMIM scores | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial species | Severe ECC n = 20 | Caries-free n = 18/ 20 | ||||||||
| ≤1 | 2 | 3 | 4 | 5 | ≤1 | 2 | 3 | 4 | 5 | |
| Actinomyces naeslundii II | 20 | 0 | 0 | 0 | 0 | 17 | 1 | 0 | 0 | 0 |
| Corynebacterium matruchotii | 20 | 0 | 0 | 0 | 0 | 18 | 0 | 0 | 0 | 0 |
| Lachnospiraceae [G-3] | 12 | 8 | 0 | 0 | 0 | 2 | 14 | 2 | 0 | 0 |
| Porphyromonas catoniae | 14 | 6 | 0 | 0 | 0 | 17 | 1 | 0 | 0 | 0 |
| Prevotella pallens | 20 | 0 | 0 | 0 | 0 | 15 | 3 | 0 | 0 | 0 |
| Propionibacterium propionicum | 19 | 1 | 0 | 0 | 0 | 14 | 4 | 0 | 0 | 0 |
| Selenomonas | 20 | 0 | 0 | 0 | 0 | 12 | 6 | 0 | 0 | 0 |
| Selenomonas flueggei | 20 | 0 | 0 | 0 | 0 | 17 | 1 | 0 | 0 | 0 |
| Streptococcus infantis | 18 | 2 | 0 | 0 | 0 | 17 | 1 | 0 | 0 | 0 |
| Streptococcus mutans (in plaque) | 12 | 3 | 3 | 1 | 1 | 17 | 1 | 0 | 0 | 0 |
| TM7[G-5] | 19 | 1 | 0 | 0 | 0 | 12 | 6 | 0 | 0 | 0 |
| Peptostreptococcus stomatis | 18 | 2 | 0 | 0 | 0 | 11 | 7 | 0 | 0 | 0 |
| Veillonellaceae [G-1] | 20 | 0 | 0 | 0 | 0 | 16 | 2 | 0 | 0 | 0 |
| Kingella denitrificans | 9 | 11 | 0 | 0 | 0 | 9 | 8 | 0 | 1 | 0 |
| Actinomyces | 17 | 3 | 0 | 0 | 0 | 20 | 0 | 0 | 0 | 0 |
| Streptococcus mutans (in saliva) | 13 | 2 | 5 | 0 | 0 | 18 | 2 | 0 | 0 | 0 |
| Eubacterium/ Peptostreptococcaceae | 16 | 4 | 0 | 0 | 0 | 13 | 7 | 0 | 0 | 0 |
| Rothia mucilaginosa | 11 | 7 | 2 | 0 | 0 | 15 | 5 | 0 | 0 | 0 |
The table shows the bacterial counts obtained by HOMIM for each species that are significantly different between the SECC and caries-free groups (ranging from 0 to 5, the minimum threshold for signal detection is equivalent to approximately 104 bacterial cells).
At the genus level, the most abundant bacterial genera detected in both the saliva and plaque samples from the SECC group were Streptococcus, Actinomyces, Campylobacter, Capnocytophaga, Fusobacterium, Gemella, Haemophilus, and Neisseria. Interestingly, these species were also the most abundant in the caries-free group (Table 4) as well as the most commonly detected genera in both groups (Table 5).We calculated the Simpson index to compare the difference in alpha-diversity between the SECC and caries-free groups, and no significant differences for both the saliva (p = 0.829, Mann–Whitney U test) and plaque (p = 0.101, Mann–Whitney U test) samples. Saliva exhibited a greater diversity in the overall microbial population compared with plaque in the caries-free group (p = 0.005, Mann–Whitney U test), but not in the SECC group (p = 0.33, Mann–Whitney U test).
Table 4. The most abundant species in the severe early childhood caries (SECC) and caries-free groups.
| Bacterial species | Mean score | SD |
|---|---|---|
| Most abundant species in saliva in the SECC group | ||
| Streptococcus oralis | 4.1 | 0.6 |
| Haemophilus parainfluenzae | 3.7 | 1.0 |
| Neisseria | 2.7 | 1.3 |
| Fusobacterium | 2.6 | 0.6 |
| Cardiobacterium hominis | 2.5 | 0.5 |
| Most abundant species in plaque in the SECC group | ||
| Fusobacterium | 3.3 | 0.9 |
| Haemophilus parainfluenzae | 3.3 | 1.2 |
| Capnocytophaga granulosa | 3.3 | 1.1 |
| Streptococcus oralis | 3.3 | 1.0 |
| Campylobacter gracilis | 2.9 | 0.9 |
| Cardiobacterium hominis | 2.7 | 0.7 |
| Most abundant species in saliva in the caries-free group | ||
| Streptococcus oralis | 4.4 | 0.5 |
| Haemophilus parainfluenzae | 3.9 | 0.6 |
| Neisseria | 3.0 | 0.8 |
| Fusobacterium | 2.8 | 0.7 |
| Cardiobacterium hominis | 2.6 | 0.5 |
| Most abundant species in plaque in the caries-free group | ||
| Fusobacterium | 3.3 | 0.7 |
| Capnocytophaga granulosa | 3.2 | 1.0 |
| Streptococcus oralis/Streptococcus sp. | 3.0 | 0.9 |
| Campylobacter gracilis | 2.9 | 0.9 |
| Cardiobacterium hominis | 2.8 | 0.8 |
| Haemophilus parainfluenzae | 2.8 | 1.0 |
| Fusobacterium periodonticum | 2.6 | 1.0 |
The table shows the most abundant bacterial species in the SECC group and the caries-free group. Abundance values are represented as the mean intensity value (from the Human Oral Microbe Identification Microarray-ranked signal scale of 0–5) for each group.
“Mean” indicates the mean abundance of species in the group
SD = standard deviation
Table 5. Species with the highest prevalances in the severe early childhood caries (SECC) and caries-free groups.
| Bacterial species | Prevalence | Mean score |
|---|---|---|
| Most frequent species in saliva in the SECC group | ||
| Granulicatella adiacens/Granulicatella elegans | 96% | 1.6 |
| Campylobacter concisus/Campylobacter rectus | 95% | 2.3 |
| Streptococcus australis | 95% | 3.3 |
| Streptococcus mitis/Streptococcus sp. | 95% | 2.4 |
| Veillonella dispar/Veillonella parvula | 95% | 2.0 |
| Most frequent species in plaque in the SECC group | ||
| Actinomyces naeslundii | 95% | 2.3 |
| Campylobacter gracilis | 95% | 2.9 |
| Capnocytophaga granulosa | 95% | 3.3 |
| Campylobacter showae | 95% | 2.6 |
| Gemella haemolysans | 95% | 3.3 |
| Haemophilus parainfluenzae | 95% | 3.3 |
| Leptotrichia hofstadii/Leptotrichia sp. | 95% | 2.2 |
| Lachnoanaerobaculum saburreum | 95% | 2.1 |
| Streptococcus anginosus/Streptococcus gordonii | 95% | 2.8 |
| Streptococcus constellatus/Streptococcus intermedius | 95% | 2.7 |
| Streptococcus cristatus | 95% | 2.7 |
| Veillonella dispar/Veillonella parvula | 95% | 1.9 |
| Most frequent species in saliva in the caries-free group | ||
| Abiotrophia defectiva | 100% | 2.3 |
| Cardiobacterium hominis | 100% | 2.6 |
| Fusobacterium | 100% | 2.8 |
| Gemella haemolysans/Gemella sanguinis | 100% | 4.3 |
| Rothia dentocariosa/Rothia mucilaginosa | 100% | 2.0 |
| Streptococcus australis | 100% | 3.5 |
| Campylobacter concisus/Campylobacter rectus | 100% | 2.7 |
| Haemophilus parainfluenzae | 100% | 3.9 |
| Neisseria | 100% | 3.0 |
| Streptococcus constellatus/Streptococcus intermedius | 100% | 3.2 |
| Streptococcus infantis | 100% | 2.5 |
| Streptococcus mitis | 100% | 2.5 |
| Streptococcus oralis | 100% | 4.4 |
| Streptococcus salivarius/Streptococcus vestibularis | 100% | 4.1 |
| Most frequent species in plaque in the caries-free group | ||
| Campylobacter gracilis | 100% | 2.9 |
| Campylobacter showae | 100% | 2.6 |
| Capnocytophaga granulosa | 100% | 2.8 |
| Cardiobacterium hominis | 100% | 2.8 |
| Fusobacterium | 100% | 3.3 |
| Gemella haemolysans | 100% | 3.2 |
| Haemophilus parainfluenzae | 100% | 2.8 |
| Neisseria | 100% | 2.3 |
| Streptococcus oralis | 100% | 3.0 |
The table shows the most frequently detected bacterial species in the SECC group and the caries-free group.
“Prevalence” is expressed as the percentage of all samples that showed a positive signal for the designated Human Oral Microbe Identification Microarray probe.
“Mean score” indicates the mean abundance of species in the group.
Discussion
Dental caries is one of the most prevalent and cost-ineffective oral infectious diseases worldwide. The prevalence of caries in the primary dentition underlies the importance of its prediction and prevention. Furthermore, ECC has become a prevalent public health problem among preschool children worldwide. However, little is known about the microbial community in ECC. The detection of specific bacteria associated with ECC can facilitate the prevention and treatment of dental caries in young children.
Although several previous studies have focused on the oral microbiota of children with and without dental caries, our research is the first, as per our knowledge, to use HOMIM to determine the bacterial profiles in both saliva and supragingival plaque to investigate the etiology of SECC in the primary dentition. The data generated will help in defining the microbial diversity in saliva and plaque, identify potential biomarkers of SECC, and provide new insights into the disease as will be discussed below.
Our study confirmed differences in microbial profiles between caries-free children and children with SECC and identified several bacterial species that could play a destructive role in this disease. S. mutans was found to be strongly associated with ECC in both saliva and supragingival plaque, confirming the findings of previous reports concerning dental caries and ECC [1,2]. Other species associated with SECC included P. catoniae, Actinomyces, and R. mucilaginosa. The presence of these species in healthy children can represent a marker of ECC risk, facilitating the detection of this disease in healthy individuals.
HOMIM was also conducted in a similar study by Luo et al. [12], who collected saliva from 50 children aged 6–8 years old to determine differences in bacterial profiles between children with and without caries. They concluded that Bacteroidetes, Capnocytophaga sputigena, Tannerella, Campylobacter showae, Selenomonas, and Parvimonas were significantly more common in the active caries group (DMFS>8) than in the caries-free group; however, our saliva samples showed insignificant differences (p > 0.05) for these species between two groups.
S. mutans is strongly associated with SECC in numerous investigations using different methods [13]. As opposed to the finding of Luo et al., who detected S. mutans in only one sample from the active caries group [12], S. mutans was detected in 14 saliva and 11 plaque samples from the SECC group in our study, which was a significantly higher prevalence than that in the caries-free group.
Actinomyces was detected significantly more frequently in the saliva samples from the SECC group, whereas there were no differences between the two groups in Luo et al.’s study [12]. With most evidence linking the species to root surface caries, Actinomyces has also been suspected to play a role in ECC. Beker’s and Jiang’s teams both observed high mean levels of Actinomyces in the earliest stage of childhood caries [14,15]; it was speculated that Actinomyces may play a major role in the initial formation stage of caries.
Differences in the age groups of patients included in Luo et al.’s study may be the reason for the differences in results between our study and theirs, because the microbial composition of the deciduous dentition is not the same as that of the mixed dentition. Moreover, the bacterial lysis method employed in our study may have yielded a better representation of gram-positive and gram-negative organisms. Considering that gram-positive bacteria such as Streptococcus are difficult to lyse, particularly in plaque, we modified the lysis procedure. All our samples were treated with a cocktail lysis buffer containing mutanolysin, proteinase K, and a lysozyme, which led to high quality and increased concentration of the total bacterial genomic DNA and a wide 16S rRNA gene diversity [2].
Lactobacillus has also been consistently associated with caries and is believed to be an important secondary pathogen in dental caries [1,2,3]. Surprisingly, we rarely detected this genus in both the SECC and caries-free groups in our study, consistent with the findings of Luo et al. This interesting phenomenon needs further investigation.
A novel finding of the current study was the association of P. catoniae with SECC, which suggested this bacteria to be a potential risk factor for the disease. Known as periodontal pathogens, P. catoniae organisms are saccharolytic, gram-negative, anaerobic rods belonging to early colonizers among obligate anaerobes [16]. A recent study showed that children with a poor caries status possessed a significantly higher level of Porphyromonas in their saliva [17]. However, Crielaard et al. found a positive association between the signal of the probe targeting P. catoniae in caries-free children, and the abundance of this species correlated negatively with the DMFS score [26]. Fabris et al. discovered that Porphyromonas was predominant in the root canals of primary teeth with necrotic pulp and was associated with primary endodontic infections and acute periradicular abscesses [18]. However, further studies of P. catoniae and its correlation with ECC are required for clearer conclusions.
Another interesting finding in the present study pertained to health-associated species. Some species were more prevalent in the caries-free group and were present in larger quantities and proportions; these included Actinomyces naeslundii II, Corynebacterium matruchotii, Eubacterium, Lachnospiraceae, Selenomonas, S. infantis, Veillonellaceae, and Propionibacterium propionicum. These health-associated species help in maintaining the balance of the oral microbiota. Tanner et al. reported a higher prevalence of C. matruchotii and Actinomyces Cluster 1 in the caries-free group than in the SECC group [1]. Gross et al. reported that the levels of S. infantis, C. matruchotii, Eubacterium IR009, and Lachnospiraceae sp. C1 showed a significant decrease with caries progression [19]. Xu et al. found Selenomonas in supragingival plaque samples from caries-free children [20].
In our study, saliva exhibited a greater diversity in the overall microbial population compared with plaque in the caries-free group. The opposite was true for the SECC group, although the difference was not statistically significant (p = 0.33). Furthermore, there were significant differences between saliva and plaque in the prevalence and abundance of species. Considerable differences in bacterial composition and diversity between individual sites and surfaces in the oral cavity have been demonstrated [21]. Previous checkerboard hybridization and 16S rDNA sequencing studies have confirmed that the salivary flora is more similar to tongue flora than to dental plaque flora [22]. Some studies have found an association between microbiota and disease in plaque samples, but not in saliva samples, from patients with both gingivitis and dental caries [23,24].
Major limitations of the current study was the lack of identification of the underlying mechanism and lack of adequate follow-up for both groups; therefore, the development of fresh caries in high-risk children was not monitored and the microbiota in pre- and post-treatment samples from children with SECC was not compared.
A confirmatory study and a follow-up of 6–12 months would enable the determination of more specific associations between some of these species and SECC. Such a study will also lead to an increased understanding of the role of these bacteria in the initiation and progression of ECC and facilitate the prevention and treatment of dental caries in young children.
Supporting Information
Signals were normalized by comparison of individual signal intensities with the average of signals from universal 16S rRNA probes and categorized into relative intensity values ranging from 0 to 5.
(XLS)
(TIF)
(XLS)
Acknowledgments
We would like to thank the native English speaking scientists of Elixigen Company for editing our manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants 81200762 and 81070815 from National Natural Science Foundation of China, by the funding from Peking University School of Stomatology (PKUSS20130210), and by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tanner AC, Kent RL Jr., Holgerson PL, Hughes CV, Loo CY, Kanasi E, et al. Microbiota of severe early childhood caries before and after therapy. J Dent Res. 2011;90: 1298–1305. 10.1177/0022034511421201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Y, Ge Y, Saxena D, Caufield PW. Genetic profiling of the oral microbiota associated with severe early-childhood caries. J Clin Microbiol. 2007;45: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Y, Wang W. Predicting caries in permanent teeth from caries in primary teeth: an eight-year cohort study. J Dent Res. 2002;81: 561–566. [DOI] [PubMed] [Google Scholar]
- 4. Dewhirst FE, Tuste Chen, Jacques Izard, Paster BJ, Tanner AC, Yu WH, et al. The human oral microbiome. Journal of Bacteriology. 2010;192: 5002–5017. 10.1128/JB.00542-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, Sahasrabudhe A, Dewhirst FE. Bacterial diversity in human subgingival plaque. Journal of Bacteriology. 2001;183: 3770–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xin BC, Luo AH, Qin J, Paster BJ, Xu YL, Li YL, et al. Microbial diversity in the oral cavity of healthy Chinese Han children. Oral Diseases. 2013;19: 401–405. 10.1111/odi.12018 [DOI] [PubMed] [Google Scholar]
- 7. Torlakovic L, Klepac-Ceraj V, Ogaard B, Cotton SL, Paster BJ and Olsen I. Microbial community succession on developing lesions on human enamel. J Oral Microbiol 4 10.3402/jom.v4i0.16125 Epub 2012, Mar 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American Academy on Pediatric Dentistry. American Academy of Pediatrics Policy on early childhood caries (ECC): Classifications, consequences, and preventive strategies. Pediatr Dent. 2008;30: 40–43. [PubMed] [Google Scholar]
- 9. Colombo AP, Boches SK, Cotton SL, Goodson JM, Kent R, Haffajee AD, et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis and periodontal health using the Human Oral Microbe Identification Microarray. J Periodontol. 2009;80: 1421–1432. 10.1902/jop.2009.090185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shaddox LM, Huang H, Lin T, Hou W, Harrison PL, Aukhil I, et al. Microbiological characterization in children with aggressive periodontitis. J Dent Res. 2012;91: 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rudney JD, Chen R, Lenton P, Li J, Li Y, Jones RS, et al. A Reproducible Oral Microcosm Biofilm Model for Testing Dental Materials. Journal of applied Microbiology. 2012;113: 1540–1553. 10.1111/j.1365-2672.2012.05439.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luo AH, Yang DQ, Xin BC, Paster BJ, Qin J. Microbial profiles in saliva from children with and without caries in mixed dentition. Oral Diseases. 2012;18: 595–601. 10.1111/j.1601-0825.2012.01915.x [DOI] [PubMed] [Google Scholar]
- 13. Tanner AC, Mathney JMJ, Kent RL Jr, Chalmers NI, Hughes CV, Loo CY, et al. Cultivable anaerobic microbiota of severe early childhood caries. Journal of clinical microbiology. 2011;49: 1464–1474. 10.1128/JCM.02427-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, et al. Molecular analysis of bacterial species associated with childhood caries. Journal of clinical microbiology. 2002;40: 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang W, Ling Z, Lin X, Chen Y, Zhang J, Xu J, et al. Pyrosequencing analysis of oral microbiota shifting in various caries states in childhood. Microbial Ecology. 2014;67: 962–969. 10.1007/s00248-014-0372-y [DOI] [PubMed] [Google Scholar]
- 16. Kononen E. Development of oral bacterial flora in young children. Ann Med. 2000;32: 107–112. [DOI] [PubMed] [Google Scholar]
- 17.Vercher SG, Rubio RC, Montiel JM, Almerich JM. Relationship of children’s salivary microbiota with their caries status: a pyrosequencing study. Clinical Oral Investigations. 2014: 10.1007/s00784-014-1200-y [DOI] [PubMed]
- 18. Fabris AS, Nakano V, Avila MJ. Bacteriological analysis of necrotic pulp and fistulae in primary teeth. J Appl Oral Sci. 2014;22: 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gross EL, Leys EJ, Gasparovich SR, Firestone ND, Schwartzbaum JA, Janies DA, et al. Bacterial 16S sequence analysis of severe caries in young permanent teeth. Journal of clinical microbiology. 2010;48: 4121–4128. 10.1128/JCM.01232-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu H, Hao WJ, Zhou Q, Wang WH, Xia ZK, Liu C, et al. Plaque bacterial microbiome diversity in children younger than 30 months with or without caries prior to eruption of second primary molars. Plos One. 2014;9: e89269 10.1371/journal.pone.0089269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simon-Soro A, Tomas I, Cabrera-Rubio R, Catalan MD, Nyvad B, Mira A. Microbial geography of the oral cavity. J Dent Res. 2013;92: 616–621. 10.1177/0022034513488119 [DOI] [PubMed] [Google Scholar]
- 22. Mager DL, Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Distribution of selected bacterial species on intraoral surfaces. J Clin Periodontol. 2003;30: 644–654. [DOI] [PubMed] [Google Scholar]
- 23. Huang S, Yang F, Zeng X, Chen J, Li R, Wen T, et al. Preliminary characterization of the oral microbiota of Chinese adults with and without gingivitis. BMC Oral Health. 2011;11: 33 10.1186/1472-6831-11-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ling Z, Kong J, Jia P, Wei C, Wang Y, Pan Z, et al. Analysis of oral microbiota in children with dental caries by PCR-DGGE and barcoded pyrosequencing. Microbial ecology. 2010;60: 677–690. 10.1007/s00248-010-9712-8 [DOI] [PubMed] [Google Scholar]
- 25. Tanner AC, Sonis AL, Holgerson PL, Starr JR, Nunez Y, Kressirer CA, et al. White-spot lesions and gingivitis microbiotas in orthodontic patients. J Dent Res. 2012;91: 853–858. 10.1177/0022034512455031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crielaard W, Zaura E, Schuller AA, Huse SM, Montijn RC, Keijser BJ. Exploring the oral microbiota of children at various developmental stages of their dentition in the relation to their oral health. BMC Med Genomics. 2011;4: 22 10.1186/1755-8794-4-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Signals were normalized by comparison of individual signal intensities with the average of signals from universal 16S rRNA probes and categorized into relative intensity values ranging from 0 to 5.
(XLS)
(TIF)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.