Abstract
Complement is a central part of the immune system that has developed as a first defense against non-self cells. Neoplastic transformation is accompanied by an increased capacity of the malignant cells to activate complement. In fact, clinical data demonstrate complement activation in cancer patients. On the basis of the use of protective mechanisms by malignant cells, complement activation has traditionally been considered part of the body's immunosurveillance against cancer. Inhibitory mechanisms of complement activation allow cancer cells to escape from complement-mediated elimination and hamper the clinical efficacy of monoclonal antibody–based cancer immunotherapies. To overcome this limitation, many strategies have been developed with the goal of improving complement-mediated effector mechanisms. However, significant work in recent years has identified new and surprising roles for complement activation within the tumor microenvironment. Recent reports suggest that complement elements can promote tumor growth in the context of chronic inflammation. This chapter reviews the data describing the role of complement activation in cancer immunity, which offers insights that may aid the development of more effective therapeutic approaches to control cancer.
Keywords: Cancer, Tumor microenvironment, Complement activation, Complement inhibitors, Immunotherapy, Monoclonal antibodies, Anaphylatoxins, Angiogenesis, Immunosuppression
A link between cancer and inflammation was first proposed by Rudolf Virchow in the nineteenth century (Grivennikov et al. 2010). Virchow observed that chronic inflammation established an environment that promoted the initiation and growth of malignancy (Balkwill and Mantovani 2001). Since then, a number of epidemiological studies have provided evidence that chronic inflammation predisposes individuals to various types of cancer (Mantovani et al. 2008). Inflammation affects every step of tumorigenesis and fosters multiple hallmarks of cancer (Hanahan and Weinberg 2011), inducing proliferation and survival, promoting angiogenesis and metastasis, evading adaptive immunity, and reducing the response to therapeutic agents. The main features of cancer-related inflammation include infiltration of white blood cells, predominantly tumor-associated macrophages (TAMs), and the presence of pro-inflammatory cytokines and chemokines (Colotta et al. 2009). Many studies have found activation of the complement system in tumors and an elevation of complement activity in the sera of patients with neoplastic diseases. It is interesting that some of the most powerful proinflammatory molecules (e.g., anaphylatoxin C5a) are generated by the activation of the complement system. This chapter reviews the evidence for complement activation within the tumor microenvironment and discusses the implications of its biological actions for cancer progression and anticancer therapy.
11.1 The Complement System and Its Regulation
The complement system has classically been recognized as a central part of the innate immune response, which serves as a first defense against microbes and unwanted host molecules. Physiological functions of complement include defending against pyogenic bacterial infection and disposing of immune complexes and products of inflammatory injury (Walport 2001). However, more recent findings have revealed that complement orchestrates many more immunological and inflammatory processes that contribute substantially to homeostasis (Ricklin et al. 2010). Complement participates in such diverse processes as control of adaptive immunity, enhancement of humoral immunity, removal of apoptotic cells, regulation of the coagulation system, maturation of synapses, angiogenesis, mobilization of hematopoietic stem-progenitor cells, regeneration of tissue, and lipid metabolism. All these activities are mediated by more than 50 circulating or cell surface–bound proteins. These proteins can be zymogens (which become active enzymes upon activation of complement), effectors, receptors, or control proteins that help maintain well-balanced activation and inhibition of the system. There are three well-established mechanisms of complement activation: the classical, lectin, and alternative pathways (Fig. 11.1). The three complement pathways share the common step of activating the central component C3 but differ according to their activation mechanisms of target recognition.
Fig. 11.1.
Cascade of events during the activation of the complement system
11.1.1 The Classical Pathway
The first component of the classical pathway is a complex formed by the hexameric C1q together with C1r and C1s, two serine protease proenzymes (Kojouharova et al. 2010). Activation of this pathway is initiated by the recognition by C1q of the antibody constant regions of μ chains (immunoglobulin [Ig] M) and some γ chains (IgG) bound to target antigens. The classical pathway can also be activated in the absence of antibodies because C1q can recognize endogenous ligands such as dying cells, extracellular matrix proteins, pentraxins, amyloid deposits, prions, and DNA (Nauta et al. 2002 ; Sjoberg et al. 2009 ; McGrath et al. 2006 ; Ying et al. 1993 ; Mitchell et al. 2007 ; Jiang et al. 1992). Binding of C1q activates C1s and C1r. C1s is a serine protease that cleaves C4 into two fragments: C4b, which binds to the cell surface through a thioester bond, and C4a, a soluble small fragment of unknown function that diffuses away. Next, complement C2 binds to C4b and becomes a target for C1s as well. The cleavage of C2 generates two fragments: C2a and C2b, which remains bound to C4b, forming the classical pathway C3 convertase (C4bC2b). In the complex C2b acts as a serine protease that cleaves C3 to C3b and C3a. C3b binds covalently to the cell membrane through its thioester bond and joins to the C3 convertase to form the classical pathway C5 convertase, C4bC2bC3b (Pangburn and Rawal 2002).
11.1.2 The Lectin Pathway
The lectin pathway is analogous to the classical pathway. This pathway is activated by proteins homologous to C1q: mannose-binding lectin (MBL) and H-, L- or M-ficolins (Thiel 2007). These proteins recognize repetitive carbohydrate patterns on pathogens, such as mannose and N-acetyl-glucosamine. After their binding, these proteins form a C1q-like complex with MBL-associated serine protease-2 (MASP-2), cleaving the complement components C4 and C2 to form the C3 convertase C4bC2b, which is common to the classical pathway activation route (Matsushita et al. 2000).
11.1.3 The Alternative Pathway
The alternative pathway is mechanistically distinct from the classical and lectin pathways. It provides an initial line of innate immune defense, being initiated by spontaneous low-level hydrolysis of C3 (Pangburn et al. 1981). The spontaneous hydrolysis of C3, also known as the “tickover” of C3, forms C3(H 2 O). C3(H 2 O) can bind to factor B, which is cleaved by factor D to form the initial alternative pathway C3 convertase, C3(H2 O)Bb (Bexborn et al. 2008). This complex begins to convert C3 into C3b and C3a. In most cases this C3b is rapidly inactivated; however, some C3b can bind to complement-activating surfaces and associate with factor B, which, again, in complex with C3b, can be cleaved by factor D, forming the predominant alternative pathway C3 convertase (C3bBb). The stability of this convertase is enhanced by the binding of properdin (Hourcade 2008). The fragment Bb on the C3 convertase cleaves more C3 and initiates an amplification loop, generating more C3b that can create new alternative C3 convertases and the C5 convertase (C3bBbC3b).
11.1.4 Nonenzymatic Assembly of the Terminal Pathway Components
C5 cleavage by the C5 convertases (C4bC2bC3b or C3bBbC3b) initiates the second phase of complement activation. C5 is cleaved into C5a, the most potent anaphylatoxin in the arsenal, and C5b, which begins the nonenzymatic assembly of the terminal pathway components C5b-9 (membrane attack complex [MAC]), which may lead to lysis in a process known as complement-dependent cytotoxicity (CDC). It is interesting to note that the MAC can also induce other responses in the cell: it can activate immune cells to release inflammatory molecules, increase the resistance of cells to further lytic attack, drive cells to proliferate, and make cells more resistant to apoptosis (Cole and Morgan 2003).
11.1.5 Alternative Routes of Complement Activation
Apart from the three major pathways, there are several bypass routes that have been shown to trigger complement activation at various stages. The lectin pathway can be activated directly by the binding of MBL to natural IgM antibodies bound to ischemic antigens in endothelial cells after ischemia/reperfusion injury (McMullen et al. 2006 ; Zhang et al. 2006). In the absence of C2/C4, but in the presence of alternative pathway components, some antigen-antibody complexes or certain oligosaccharides can lead to C3 activation (Selander et al. 2006). C3 can be cleaved and activated by extrinsic proteases, such as thrombin or kallikrein, pointing to a crosstalk between the complement system and the coagulation cascade (Markiewski et al. 2007). C5 can also be cleaved by thrombin, bypassing C3 (Huber-Lang et al. 2006). Finally, C5 can be cleaved by silica and asbestos fibers through mechanisms involving the generation of free radicals (Governa et al. 2002).
11.1.6 Complement Regulators
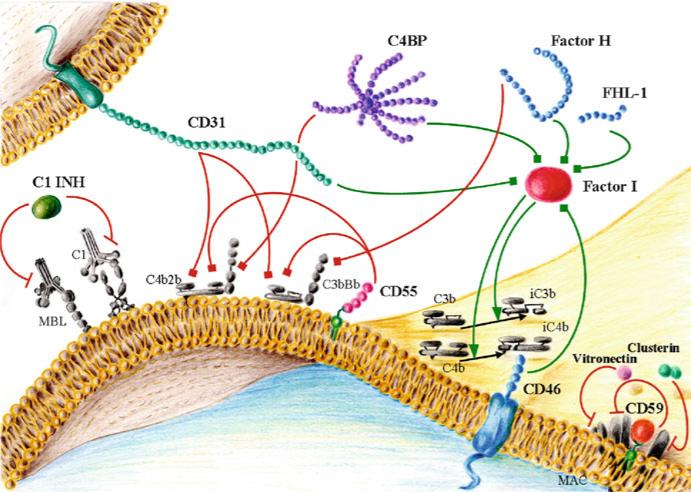
Regulation of complement activation is of critical importance for the homeostasis of the organism. Control proteins regulate complement at three main levels: they can inhibit the protease activity of the proteins involved in the activation cascade, they can facilitate the decay and destruction of convertases, and they can control MAC formation (Fig. 11.2). Many regulators share a varying number of a repeating motifs called short consensus repeats (SCRs), complement control protein repeats, or sushi domains. SCRs are globular domains containing approximately 60 amino acids and have a framework of conserved residues that includes four invariant cysteines, an almost invariant tryptophan, and highly conserved prolines, glycines, and hydro-phobic residues (Kirkitadze and Barlow 2001). These domains are thought to play a role in binding to C3 and C4 and their breakdown products.
Fig. 11.2.
Main complement inhibitors: soluble proteins and membrane-bound complement regulatory proteins. Red lines represent inhibitory activity (when ending in a bar) or accelerated decay activity (when ending in a square). Green lines represent cofactor activity (when ending in a square) or protease activity (when ending in an arrowhead)
Complement regulators have traditionally been grouped into two categories: soluble regulators and membrane-bound regulators. At least six complement regulators can be found in soluble form in plasma: C1 inhibitor, factor I, C4b-binding protein (C4BP), factor H, vitronectin (S protein), and clusterin (SP40,40). C1 inhibitor is a member of the serine family of protease inhibitors that inactivates C1r, C1s, and MASP-2 (Davis et al. 2008). Factor I cleaves and inactivates C4b and C3b (Sim et al. 1993). C4BP is a heterogeneous oligomeric protein that controls the classical complement pathway. After binding to C4b, C4BP inhibits complement by three different mechanisms. It prevents the assembly of the C3 convertase, accelerates the decay of the classical C3 and C5 convertases, and functions as a cofactor in the factor I–mediated inactivation of C4b (Blom et al. 2004). Factor H, with its alternatively spliced variant factor H-like protein 1 (FHL-1), is mostly known as an inhibitor of the alternative pathway. Through its binding to C3b, factor H competes with factor B in the formation of the C3 and C5 convertases, displaces the Bb subunit from the convertases, and acts as a cofactor for factor I in the cleavage of C3b (Jozsi and Zipfel 2008).
Several recent studies have described the association of genetic variations in complement factor H with various diseases. Mutations or polymorphisms that alter the binding of factor H to C3b and polyanions are associated with atypical hemolytic uremic syndrome, whereas mutations that disrupt the plasma activity of factor H, leading to unrestricted activation of the alternative pathway, are associated with membranoproliferative glomerulonephritis type II (de Cordoba and de Jorge 2008). A polymorphism at the factor H locus that causes a Tyr402His amino acid substitution in SCR7 confers a significantly increased risk for age-related macular degeneration (Shaw et al. 2012). Five complement factor H–related proteins encoded by genes closely linked to the factor H locus have been identified. These proteins are involved in complement regulation, but their exact functions are not well-defined (Jozsi and Zipfel 2008). Vitronectin and clusterin inhibit the insertion of the MAC into the membrane (Podack and Muller-Eberhard 1979 ; Jenne and Tschopp 1989). Clusterin can also modulate cell differentiation and regulate the production of major pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-6 (Falgarone and Chiocchia 2009).
Complement activation is also controlled by membrane-bound complement regulatory proteins (mCRPs) such as complement receptor (CR) type 1 (CR1; CD35), membrane cofactor protein (CD46), decay-accelerating factor (CD55), and CD59 (protectin). CR1 is expressed by erythrocytes, neutrophils, eosinophils, monocytes, follicular dendritic cells, glomerular podocytes, B lymphocytes, and some T lymphocytes (Fischer et al. 1986); it functions as a cofactor for the factor I–mediated cleavage of C3b and C4b and accelerates the decay of the classical and alternative convertases (Fearon 1979). CD46 is expressed in most cells (except erythrocytes) and acts as a cofactor of factor I in C3b/C4b cleavage (Liszewski et al. 1991). CD46 also has been implicated in the regulation of T cells (Marie et al. 2002 ; Kemper et al. 2003). CD55 is attached to the membrane by a glycosylphosphatidylinositol (GPI) anchor and is present in all blood elements and most other cell types (Medof et al. 1987). CD55 accelerates the decay of the classical and alternative C3 and C5 convertases (Lublin and Atkinson 1989). CD55 binds to CD97, which is expressed on macrophages, granulocytes, dendritic cells, and activated T and B cells, and simultaneously regulates innate and adaptive immune responses (Abbott et al. 2007). CD59, a GPI-anchored protein, is expressed by all circulating cells, vascular endothelium, epithelium, and most other cell organs (Morgan 1999). CD59 binds C8 during the formation of MAC and inhibits the insertion of C9 into the lipid bilayer (Meri et al. 1990). Alternative roles for CD59, related to its GPI anchor signaling properties, also have been demonstrated in T cells, natural killer cells, and B cells (Kimberley et al. 2007).
11.1.7 Opsonization by C3b and Its Related Fragments
Activation of all three complement pathways results in the conversion of C3 into C3b. The nascent C3b molecule can trigger complement amplification, but it can also be inactivated by proteolysis. Initial inactivation of C3b is mediated by factor I and its cofactors. Factor I catalyzes the proteolysis of two peptide bonds in the α′ polypeptide chain of C3b. The resulting products are the membrane-bound iC3b and a small peptide, C3f, which is released from the molecule. A third cleavage, catalyzed by factor I, generates the inert C3c and C3dg; C3c is released, and C3dg is retained on the cell membrane (Law and Dodds 1997). Although further complement amplification is abolished, recognition of C3b and its fragments by complement receptors on cells promotes phagocytosis. Complement receptors are grouped into three families: the SCR family members CR1 and CR2, the β2 integrin family members CR3 and CR4, and the Ig superfamily member CRIg. CR1 (CD35) is expressed in the majority of peripheral blood cells and recognizes C3b, C4b, iC3b, C3dg, C1q, and mannose-binding protein (Klickstein et al. 1997 ; Ghiran et al. 2000). The main function of CR1 is to capture immune complexes on erythrocytes for transport and clearance by the liver (Taylor et al. 1997). CR1 also promotes the secretion of pro-inflammatory molecules, such as IL-1α, IL-1β, and prostaglandins; it also plays a role in the presentation of antigens to B cells and inhibits both the classical and alternative pathways via its decay-accelerating activity and cofactor activity in C3b/C4b cleavage (Krych-Goldberg and Atkinson 2001). CR2 (CD21) is an evolutionary homolog of CR1 but binds only to iC3b, C3d, and C3dg. On B cells, CR2 forms a coreceptor with CD19 and CD81. Binding of this coreceptor to the B-cell antigen receptor lowers the threshold for B-cell activation (Fearon and Carter 1995). CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are expressed by leukocytes and stimulate phagocytosis when bound to iC3b. In addition, they contribute to leukocyte trafficking, adhesion, migration, and costimulation (van Lookeren et al. 2007). CRIg has recently been identified as a complement receptor. It is expressed on a restricted subset of tissue-resident macrophages and may play an important role in phagocytosis (Helmy et al. 2006 ; He et al. 2008).
11.1.8 Biological Effects Mediated by Anaphylatoxins
During complement activation, soluble active fragments are released from C3, and C5. These bioactive peptides, C3a and C5a, were called anaphylatoxins because they were found to be potent multifunctional pro-inflammatory molecules, acting as chemotaxins and leukocyte activators (Kohl 2001). Anaphylatoxin receptors belong to the superfamily of G-protein-coupled receptors. They share high sequence homology but differ in ligand specificity, signal transduction capacity, and function. The anaphylatoxin receptors are C3aR for C3a and C5aR and C5L2 for C5a. For C5a binding, the first recognized receptor was C5aR-1 (Boulay et al. 1991), also known as CD88. The orphan receptor GPR77 was later identified as a second C5a receptor and was called C5a-like receptor 2 (C5L2) (Ohno et al. 2000). C5aR is a classic G protein-coupled receptor, whereas C5L2 is an enigmatic receptor deficient in G-protein coupling. This fact, together with the fact that the pathway for C5L2 after C5a binding is unknown, has prompted the suggestion that C5L2 is a default receptor that attenuates C5a biological responses by competing with C5aR-1. Nevertheless, this role for C5L2 has been challenged by results that point to its function as a positive modulator for both C5a- and C3a-anaphylatoxin-induced responses (Chen et al. 2007).
C3aR and C5aR are expressed in both myeloid and nonmyeloid cell types. The activities of anaphylatoxins are related to the cell types that express their receptors. They can increase vascular permeability; promote smooth muscle contraction; induce leukocyte recruitment; increase chemotaxis, migration, and phagocytosis in white blood cells; and promote the production and release of other pro-inflamma-tory mediators (e.g., histamine) (Haas and van Strijp 2007). Anaphylatoxins have been implicated in brain development (Benard et al. 2004) and tissue regeneration and fibrosis (Strey et al. 2003). As described below, in recent years C5a activity also has been connected to cancer progression.
11.2 Cancer Immunity
A growing body of evidence supports the proposed capacity of the immune system to recognize malignant cells and regulate tumor growth. Cancer cells acquire several sequential genetic and epigenetic abnormalities that dictate malignant growth and produce changes in cell morphology, generating tumor-associated antigens that distinguish malignant cells from their normal counterparts. Those changes can induce the recognition of malignant cells by immune defense mechanisms mediated by T and B cells, protecting the host against the development of cancers (Pardoll 2003). Tumor cells can also become susceptible to natural killer cells as a result of the decreased expression of self-class I major histocompatability complex (Karre et al. 1986), the expression of stress-induced proteins (Bauer et al. 1999), and the presence of mitosis-associated alterations of the cell membrane (Nolte-'t Hoen et al. 2007). Today, immune surveillance in cancer is supported by both epidemiological data and cancer models. Still, immune surveillance represents only one dimension of the complex relationship between the immune system and cancer (Dunn et al. 2004a).
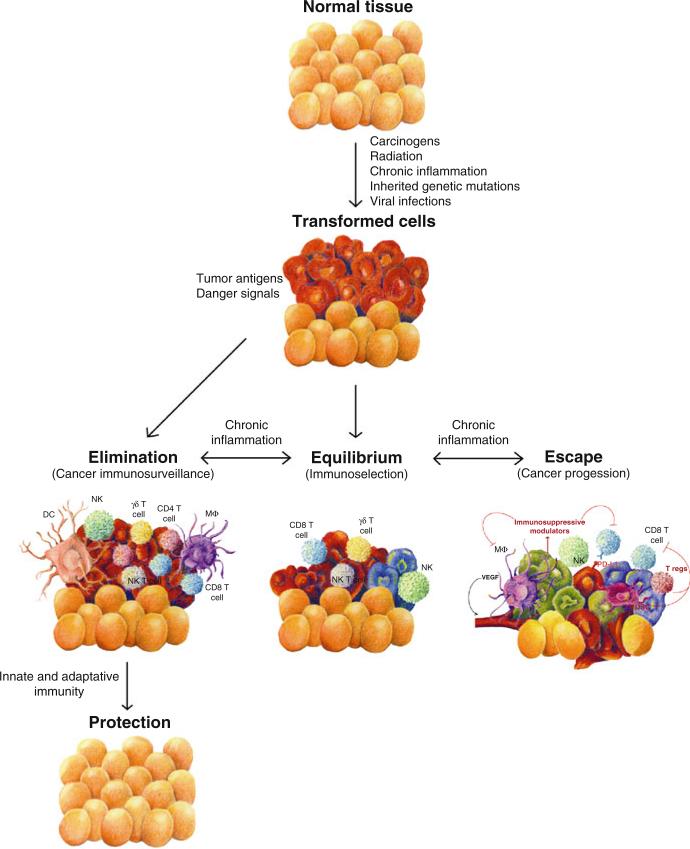
Immune surveillance creates a selective pressure in the tumor microenvironment that can ultimately edit tumor immunogenicity. This idea has prompted the development of the cancer immunoediting hypothesis to explain the dynamic relationship established between cancer and immunity (Dunn et al. 2004b). Cancer immunoediting is a multistep process comprising different phases: recognition, elimination, equilibrium, and escape (Fig. 11.3). Through the process of transformation, normal cells express distinct tumor-specific markers and generate pro-inflammatory “danger” signals that are recognized by the immune system and initiate the process of cancer immunoediting. Once these signals are recognized, cells and molecules of innate and adaptive immunity, which compose the cancer immune surveillance network, can eradicate the nascent tumor cells, protecting the host from tumor formation. This stage is characterized by a lack of clinical evidence of disease; therefore, it is difficult to determine how often tumors are naturally eradicated. In addition, the tumor antigens and the immune mechanisms that underlie this process remain poorly understood (Matsushita et al. 2012). In any case, it is clinically evident that the immune system is unable to get rid of all emerging malignant cells.
Fig. 11.3.
Steps of cancer immunity
When the elimination process is unsuccessful, tumor cells are capable of colonizing sites in the tissue microenvironment and enter the equilibrium phase, in which they may either be maintained chronically or be immunologically induced to change and produce new populations of tumor variants that are less immunogenic or possess mechanisms to control immune activation. In this phase, tumor cells can grow, although the immune system is still capable of controlling tumor progression. The escape phase refers to the final outgrowth of tumors that eventually evolve into a state in which they can effectively evade, suppress, and overcome control by the immune system. Hanahan and Weinberg ( 2011) have included “evading immune destruction” as an emerging hallmark of cancer, in addition to the previously established capabilities acquired during the multistep development of human tumors.
Many immunomodulatory mechanisms operate in tumors. These include the selection of tumor cells that no longer provoke a T cell–mediated immune response as a result of the loss of expression or presentation of tumor antigens (DuPage et al. 2012). At this point, tumors can progress and become clinically detectable. Moreover, at this stage, tumor cells can take advantage of the inflammatory microenvironment associated with the immune response and use it to promote carcinogenesis (Grivennikov et al. 2010). In fact, most solid malignancies trigger an intrinsic inflammatory response that builds up a protumorigenic microenvironment (Mantovani et al. 2008). The heterogeneous role of the immune response in the pathogenesis of cancer is exemplified by the divergent functions of immune cells found within the tumor microenvironment. Immune infiltrates can be located in the center of the tumor, in the invasive margin, or in the adjacent tissue. This component of the tumor microenvironment encompasses a wide variety of immune cells that is extremely diverse from patient to patient; however, most of the cells are macrophages and T cells.
Considerable evidence has been accumulated to support a dual role for TAMs in the regulation of tumor cell proliferation, invasion, and angiogenesis and immune control (Kataki et al. 2002 ; Lewis and Pollard 2006). High TAM content is generally correlated with poor prognosis (Quatromoni and Eruslanov 2012), but, depending on their stage of differentiation and activation, tissue macrophages have the ability to promote or inhibit neoplasia (Montuenga and Pio 2007). T cells can also exert both tumor-suppressive and tumor-promoting effects (Fridman et al. 2012). Tumor-infiltrating T cells can attenuate the metastatic potential of tumor cells and are correlated with better survival in many different tumor types (Galon et al. 2006 ; Laghi et al. 2009). However, many T-cell subsets found in solid tumors are involved in tumor promotion, progression, or metastasis (Roberts et al. 2007 ; Aspord et al. 2007).
In particular, regulatory T cells are considered the most powerful suppressors of antitumor immunity (Zou 2006). Regulatory T cells promote immunosuppression via direct effects on activated T cells or via the secretion of immunosuppressive cytokines such as IL-10 and transforming growth factor (TGF)-β (Thornton and Shevach 1998 ; Hawrylowicz and O'Garra 2005). An increased number of these cells in the tumor microenvironment confers growth and metastatic advantages and predicts a marked reduction in patient survival (Curiel et al. 2004 ; Shimizu et al. 2010). Therefore, the immune microenvironment surrounding the tumor comprises a highly heterogeneous population of immune cells with pro- and antitumor activities.
Whether the immune system limits or promotes tumor growth depends on a delicate balance between opposing forces. As is shown in the next sections, this duality in tumor immunity is also seen in the interrelationship between cancer and complement activation.
11.3 Complement in Immune Surveillance Against Tumors
Once a threatening body is recognized by the complement system, the activating steps initiate an inflammatory reaction, the opsonization of the target cell, and, in some cases, its killing. This conventional role of complement may have an effect on the control of tumor growth. The numerous genetic and epigenetic alterations associated with carcinogenesis dramatically change the morphology and composition of the cell membrane. Altered glycosylation is considered a hallmark of cancer cells (Hakomori 2002 ; Hollingsworth and Swanson 2004 ; Miyagi et al. 2012), and progression of epithelial cells from a normal to malignant phenotype is associated with an aberrant increase in the metabolism of membrane phospholipids (Costello and Franklin 2005 ; Glunde and Serkova 2006 ; Griffin and Kauppinen 2007).
Although there is no irrefutable evidence for the existence of an effective immune surveillance mediated by complement, these changes in the composition of cell membranes may target tumor cells for complement recognition. In fact, several observations support the capacity of complement to recognize malignant cells. In a recent report, lung cancer cell lines were shown to deposit C5 and generate C5a more efficiently than bronchial epithelial cells (Corrales et al. 2012). Moreover, a significant increase in C5a was found in the plasma samples of patients with non-small- cell lung cancer, suggesting that the local generation of C5a within tumors may be followed by its systemic diffusion (Corrales et al. 2012). In primary lung tumors, C3b (but not MAC deposition) can be detected by immunohistochemistry (Niehans et al. 1996). C3c and C4 are elevated in patients with lung cancer (Gminski et al. 1992), and complement levels correlate with tumor size (Nishioka et al. 1976). Several studies of other cancer types also have suggested that the complement system is activated in response to the expression of tumor-associated antigens, with the subsequent deposition of complement components on tumor tissue (Guidi et al. 1988 ; Zurlo et al. 1989 ; Niculescu et al. 1992 ; Baatrup et al. 1994 ; Yamakawa et al. 1994 ; Lucas et al. 1996 ; Gasque et al. 1996 ; Bu et al. 2007). Elevated levels of C3a and soluble C5b-9 are present in the intraperitoneal ascitic fluid of patients with ovarian cancer (Bjorge et al. 2005). The lectin pathway of complement activation has been found to be significantly increased in patients with colorectal cancer when compared to healthy subjects (Ytting et al. 2004), and the MASP-2 concentration in serum has been reported to be an independent prognostic marker for poor survival (Ytting et al. 2005). Higher complement hemolytic activity and C3 levels have been observed in serum samples from children with neuroblastoma (Carli et al. 1979) and elevated complement levels have similarly been reported in patients with carcinomas of the digestive tract (Maness and Orengo 1977) or with brain tumors (Matsutani et al. 1984). In vivo alterations in the activation of the classical pathway have been described in patients with chronic lymphatic leukemia (Fust et al. 1987 ; Schlesinger et al. 1996), with a strong positive correlation between survival and the initial activity of the classical pathway of complement (Varga et al. 1995).
All these observations support the capacity of complement to recognize malignant cells. However, little is known about the tumor-associated antigens that are involved in the recognition of cancer cells by complement and the exact mechanisms that drive this activation. In the TC-1 syngeneic mouse model of cervical cancer, the classical pathway was found to be the main contributor to complement activation (Markiewski et al. 2008). Evidence for the classical pathway of complement activation also has been found in patients with papillary thyroid carcinoma (Lucas et al. 1996), follicular lymphoma, and mucosa-associated lymphoid tissue lymphoma (Bu et al. 2007). In contrast, the alternative complement pathway has been found to be activated in lymphoblastoid cell lines (Budzko et al. 1976 ; Theofilopoulos and Perrin 1976 ; McConnell et al. 1978) and patients with multiple myeloma (Kraut and Sagone 1981). In childhood acute lymphoblastic leukemia, amplification of the alternative pathway after activation of the classical pathway has been suggested (Kalwinsky et al. 1976). On the other hand, the capacity of lung cancer cell lines to produce C5a in the absence of an exogenous source of complement components (i.e., serum), suggests that, apart from the traditional pathways of complement activation, cancer cells may have the capacity to activate complement by an extrinsic activation mechanism (Corrales et al. 2012). The production of anaphylatoxins by cancer cells may be mediated by soluble and membrane-bound pro-teases, such as serine proteases of the coagulation and fibrinolysis systems or cell-bound proteases (Huber-Lang et al. 2002 , 2006 ; Amara et al. 2008). A better analysis of the pathways by which cancer cells activate complement would greatly improve our understanding of the interplay between complement and cancer and may be of value in identifying new diagnostic biomarkers and molecular targets for anticancer therapies. Changes in plasma complement components as part of the host's response to chemotherapy may also be useful as early predictive markers of a response to treatment (Michlmayr et al. 2011).
11.4 Mechanisms for Adaptation and Control of Complement Activation: Implications for Cancer Immunotherapy
There is sufficient basis to propose that neoplastic transformation is accompanied by an increased capacity to activate complement. However, cancer cells exhibit a number of strategies to resist complement attack. Many of these resistance mechanisms also are used by normal cells to avoid accidental activation or bystander effects from a local activation of complement. However, cancer cells develop additional mechanisms to inhibit complement activation (Fig. 11.4). Cancer-associated resistance mechanisms can be divided into two categories: extracellular and intra-cellular (Jurianz et al. 1999). One of the best characterized extracellular mechanisms is the expression of mCRPs. This research area has been extensively reviewed (Gorter and Meri 1999 ; Fishelson et al. 2003 ; Yan et al. 2008 ; Gancz and Fishelson 2009 ; Kolev et al. 2011).
Fig. 11.4.
Mechanisms used by cancer cells to resist complement activation. Red lines represent inhibitory activity and green lines represent activation
With the exception of CR1, most cancers – whatever their tissue origin – express at least two, if not three, mCRPs. In several cancer types, increased levels of CD59 have been found to be associated with resistance to CDC (Brasoveanu et al. 1996 ; Jarvis et al. 1997 ; Chen et al. 2000 ; Coral et al. 2000), increased metastatic potential (Loberg et al. 2005), or poor prognosis (Xu et al. 2005 ; Watson et al. 2006). For example, prostatic tumors and medullary thyroid carcinomas overexpress the regulator CD55 and its receptor CD97 (Loberg et al. 2005 ; Mustafa et al. 2004). A deficiency of CD55 in mice significantly enhances T-cell responses (Liu et al. 2005). In colorectal carcinoma, the expression of CD55 is associated with poor prognosis (Durrant et al. 2003). In contrast, the loss of CD55 has been related to poor prognosis in breast cancer (Madjd et al. 2004). CD46 is perhaps the mCRP with the lowest level of variation between tumors and normal tissue. Nevertheless, CD46 levels are correlated with tumor grade and recurrence in breast tumors (Rushmere et al. 2004 ; Madjd et al. 2005). Cell lines from various cancer types release soluble forms of the mCRPs (Bjorge et al. 2005; Brasoveanu et al. 1997 ; Hindmarsh and Marks 1998 ; Nasu et al. 1998 ; Jurianz et al. 2001 ; Li et al. 2001 ; Morgan et al. 2002 ; Donin et al. 2003), and many of these forms also have been detected in patients with cancer (Niehans et al. 1996 ; Li et al. 2001 ; Morgan et al. 2002 ; Seya et al. 1995 ; Sadallah et al. 1999 ; Gelderman et al. 2002a ; Kawada et al. 2003 ; Hakulinen et al. 2004 ; Kohno et al. 2005). These forms of mCRPs are able to bind to tumor cells and should be considered contributors to the resistance of tumor cells to complement activation.
Soluble complement regulators, including factor H and FHL-1, are also important in the resistance of tumor cells to complement activation and CDC (Bjorge et al. 2005 ; Reiter and Fishelson 1989 ; Ollert et al. 1995 ; Junnikkala et al. 2000 ; Ajona et al. 2004). A clinically approved immunoassay for the detection of bladder cancer in urine is based on the quantification of factor H (Kinders et al. 1998 ; Cheng et al. 2005). H2 glioblastoma cells are able to bind factor H and FHL- 1, promoting the inactivation of C3b (Junnikkala et al. 2000). In the melanoma cell line SK-MEL-93-2, factor H seems to be the dominant factor regulating the activation of complement (Ollert et al. 1995). An anti–factor H antibody enhances the complement-mediated killing of cells obtained from a Burkitt's lymphoma (Corey et al. 1997). Some cancer cells are protected from complement attack by sequestration of factor H to the cell surface through members of the SIBLING family (Fedarko et al. 2000 ; Jain et al. 2002). Factor H and FHL-1 are highly expressed by ovarian carcinomas, and both proteins are abundantly present in ascites from these tumors (Junnikkala et al. 2002). In vitro studies have shown that lung cancer cell lines are more resistant to CDC than are human nasal epithelium primary cell cultures (Varsano et al. 1996 ; Varsano et al. 1998). This resistance may be mediated by the expression and secretion of factor H and FHL-1 to the extracellular milieu (Ajona et al. 2004). Downregulation of factor H reduces the growth of lung cancer cells in vivo (Ajona et al. 2007), and its expression in lung adenocarcinomas may be associated with worse prognosis (Cui et al. 2011).
Non-small-cell lung cancer cell lines also express factor I and C4BP, which efficiently support the cleavage of C3b and C4b in vitro (Okroj et al. 2008). Lung cancer cell lines downregulate the expression of factor H, factor I, CD46, and CD55 under hypoxic conditions and during hypoxia/reoxygenation, implying that, under these conditions, cancer cells reduce their reliance on mechanisms to control complement activation while keeping free from CDC (Okroj et al. 2009). In patients with various nonmetastatic solid tumors, C4BP plasma levels were found to be significantly higher than in control subjects (Battistelli et al. 2005). C4BP is able to bind to SK-OV-3, SW626, and Caov-3 ovarian adenocarcinoma cell lines, and this binding may lead to an increased control of classical pathway activation (Holmberg et al. 2001). Other soluble complement regulatory proteins such as C1 inhibitor (Gasque et al. 1996 ; Bjorge et al. 2005 ; Jurianz et al. 2001 ; Morris et al. 1982 ; Buo et al. 1993) and clusterin (Trougakos and Gonos 2002) may also be involved in the protection of cancer cells from complement activation.
In addition to the expression of mCRP and soluble regulators, there are several alternative mechanisms that can be used by cancer cells to control complement activation. Tumor cells can release proteases that cleave complement components (Ollert et al. 1990) or express them in their cell membrane (Paas et al. 1999 ; Bohana-Kashtan et al. 2005). Tumor cells are able to eliminate the MAC by endocytosis or vesiculation (Morgan 1992 ; Moskovich and Fishelson 2007). Sublytic doses of the MAC can, surprisingly, provide intracellular protection against complement attack. Insertion of the MAC into the cell membrane causes a variety of biological effects, including entrance into the cell cycle, resistance to apoptosis, expression of adhesion molecules, or augmentation of complement resistance (Morgan 1989 ; Liu et al. 2012). The mechanisms responsible for this protection are poorly understood but involve an increase in intracellular concentrations of calcium and the activation of protein kinases (Carney et al. 1990 ; Soane et al. 2001 ; Kraus et al. 2001). The signaling activation triggered by sublytic doses of MAC is discussed in greater detail in the next section.
The effectiveness of complement regulators in protecting tumor tissues from complement injury has led to the idea that inhibiting the function of these regulatory proteins will enhance monoclonal antibody–based immunotherapy. The number of monoclonal antibodies approved for cancer treatment has rapidly increased since rituximab, an anti-CD20 monoclonal antibody used for treatment of malignant lymphoma, was first used for the treatment of lymphomas (Schrama et al. 2006) (Table 11.1). Monoclonal antibodies normally use a combination of mechanisms to direct cytotoxic effects to a tumor cell (Weiner et al. 2010). They target tumor- specific and tumor-associated antigens and block important cancer activities. In addition, many of them are able to activate the immune system and mediate Fc domain–based reactions, such as antibody-dependent cellular cytotoxicity and complement fixation (Kolev et al. 2011). Successful complement activation by these therapeutic antibodies can have multiple effects on the immune response against tumors (i.e., the formation of the MAC, opsonization, and release of proinflammatory anaphylatoxins). However, the above-described protective mechanisms against complement activation hamper the clinical efficacy of cancer therapies based on the use of monoclonal antibodies that can activate complement. For example, rituximab exerts its effects against malignant lymphomas through a variety of mechanisms, including CDC (Di Gaetano et al. 2003 ; Cragg and Glennie 2004 ; Beum et al. 2008). The efficacy of rituximab seems to be limited by the expression of complement regulatory proteins in B-cell lymphoma cell lines (Golay et al. 2000 ; Cardarelli et al. 2002) Therefore, it is logical to assume that the anticancer efficacy of monoclonal antibodies would be enhanced by overcoming the protection exerted by complement regulators.
Table 11.1.
Therapeutic monoclonal antibodies (unconjugated) approved for use in cancer treatment
| Name (trade name) | Isotype | Target | Cancer indication |
|---|---|---|---|
| Rituximab (Rituxan) | Chimeric IgG1 | CD20 | Non-Hodgkin's and follicular lymphoma |
| Trastuzumab (Herceptin) | Humanized IgG1 | HER2/neu | Breast |
| Cetuximab (Erbitux) | Chimeric IgG1 | EGFR | Colorectal and head and neck |
| Bevacizumab (Avastin) | Humanized IgG1 | VEGF | Colorectal, lung, kidney, and brain |
| Alemtuzumab (Campath) | Humanized IgG1 | CD52 | Chronic lymphocytic leukemia |
| Panitumumab (Vectibix) | Human IgG2 | EGFR | Colorectal |
| Ofatumumab (Arzerra) | Human IgG1 | CD20 | Chronic lymphocytic leukemia |
| Ipilimumab (Yervoy) | Human IgG1 | CTLA-4 | Melanoma |
Ig immunoglobulin; EGFR epidermal growth factor receptor; VEGF vascular endothelial growth factor; CTLA-4 cytotoxic T-lymphocyte-associated protein 4
Several strategies to overcome this protection have been tested experimentally in vitro and in animal models (Fishelson et al. 2003 ; Gancz and Fishelson 2009 ; Kolev et al. 2011) (Table 11.2). These strategies include blockade of the activity of the regulators, downregulation of their expression, or their removal from the cell surface (Brasoveanu et al. 1996 ; Ajona et al. 2007 ; Di Gaetano et al. 2001 ; Andoh et al. 2002 ; Blok et al. 2003 ; Nagajothi et al. 2004 ; Terui et al. 2006 ; Shi et al. 2009 ; Gao et al. 2009 ; Hsu et al. 2010 ; Geis et al. 2010 ; Bellone et al. 2012). However, targeting inhibitory molecules to complement regulators in vivo is technically challenging and may have unwelcome consequences for normal cells. To limit the inhibitory effect on the tumor microenvironment, some researchers have proposed strategies such as the use of a biotin-avidin system (Macor et al. 2006) or bispecific monoclonal antibodies that target a tumor antigen and simultaneously block a major complement regulatory protein (Gelderman et al. 2002b , 2004a , 2005). All these strategies are limited by the fact that each tumor may be equipped with specific mechanisms of cell protection, and a concerted action against different protective mechanisms may be needed.
Table 11.2.
Strategies for the improvement of complement-mediated immunotherapy
| Goal | Strategy |
|---|---|
| To overcome the protected capacity of complement regulators | |
| Blockade of the regulatory activity | Neutralizing mono- or bispecific antibodies |
| Downregulation of the regulator expression | RNA interference |
| Antisense oligonucleotides | |
| Pharmacological agents | |
| Cytokines | |
| Removal of the regulatory capacity from the cell membrane | Phosphatidylinositol-specific phospholipase C |
| Desialyzation | |
| Inhibition of MAC removal | Downregulation of mortalin |
| Inhibition of heat shock proteins | |
| To improve complement-mediated effector mechanisms of monoclonal antibodies | |
| Antibody modification | Engineering of the Fc region antibody |
| Bispecific antibodies | Targeting a cancer-associated antigen and a complement regulator |
| Conjugation with complement-activation molecules | Conjugation with CVF |
| Conjugation with C3b | |
| Cocktail of antibodies | Targeting distinct epitopes of the same antigen |
| Fusion proteins including Fc domains | CR2-Fc fusion |
| Immunomodulators | β-glucan |
CVF cobra venom factor; CR2 complement receptor 2
An alternative approach would be to improve the complement-mediated effector mechanisms of monoclonal antibodies through genetic engineering or conjugation. Several strategies have been devised for turning a non-complement-fixing antibody into a complement-fixing antibody to be employed in immunotherapy, including the selection of the Ig subclasses (IgG1 and IgG3) that are most efficient in activating complement and the production of IgG1-containing recombinant variants of Fc that exhibit increased capacity to induce CDC or antibody-dependent cytotoxicity (Macor and Tedesco 2007). Heteroconjugates comprising antitumor antibodies and molecules such as cobra venom factor, C3b, or iC3b have been used (Reiter and Fishelson 1989 ; Gelderman et al. 2002b ; Juhl et al. 1990 , 1995 , 1997 ; Yefenof et al. 1990). Alteration of the glycosylation pattern has been shown to enhance the lytic potential of monoclonal antibodies without affecting their affinity or specificity (Schuster et al. 2005). The Fc region can be engineered to enhance the CDC activity of therapeutic antibodies (Moore et al. 2010), and bispecific antibodies have been engineered to recruit complement effector functions (Holliger et al. 1997). Mixtures of several antibodies have been proposed (Macor et al. 2006 ; Spiridon et al. 2002 ; Kennedy et al. 2003). β-Glucan has been used to induce CR3-dependent cellular cytotoxicity (Gelderman et al. 2004b). In addition, the extracellular domain of the CR2 fused to an IgG Fc domain has been successfully used in syngeneic mouse tumor models (Elvington et al. 2012). The molecular architecture of the antigens selected for immunotherapy and the antibody concentration also seem to be essential for the proper induction of CDC (Ragupathi et al. 2005 ; Livingston et al. 2005 ; Beurskens et al. 2012).
11.5 Complement Activation Can Promote Carcinogenesis
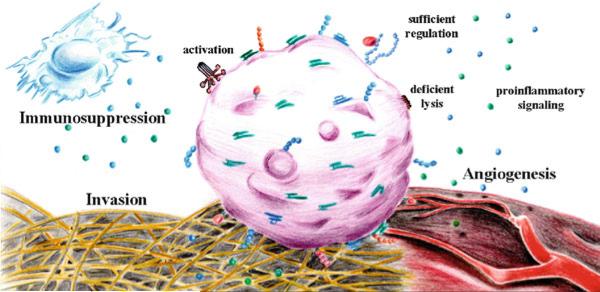
In the cancer setting, researchers have traditionally focused on the role of complement in the tagging and elimination of tumor cells. However, recent work has challenged this conventional view. The fact that mice deficient in C3 or C5aR show decreased tumor growth when compared to wild-type mice suggests that complement proteins may perversely promote malignancy (Corrales et al. 2012 ; Markiewski et al. 2008 ; Nunez-Cruz et al. 2012). In line with this hypothesis, several studies have demonstrated a role for activated components of the complement system in the various stages of carcinogenesis. Complement can assist the escape of tumor cells from immunosurveillance, promote angiogenesis, activate mitogenic signaling pathways, sustain cellular proliferation and insensitivity to apoptosis, and participate in tumor cell invasion and migration (Rutkowski et al. 2010a) (Fig. 11.5).
Fig. 11.5.
Potential tumor-promoting roles of complement proteins in the tumor microenvironment
11.5.1 Complement and Immunosuppression
Activation of specific T cells against tumor-associated antigens has been demonstrated in cancer patients and mouse models (Boon et al. 1997 ; Peterson et al. 2003). However, multiple evasion mechanisms in tumor and stromal cells reduce immune function, causing a miscarriage of tumor rejection by effector immune cells (Umansky and Sevko 2013). Tumor-derived immunosuppressive mechanisms can be summarized as the downregulation (or loss) of major histocompatibility complex class I molecules, tumor-associated antigens, or danger signals and as the secretion of immunosuppressive factors such as vascular endothelial growth factor (VEGF), TGF-β, IL-10, reactive oxygen species, and prostaglandins (Kim et al. 2006). Immunosuppression is orchestrated by cells of lymphoid and myeloid origin that are recruited and activated in the tumor microenvironment. These immunosuppressive cells include regulatory T cells, TAMs, regulatory/tolerogenic dendritic cells, and myeloid-derived suppressor cells (MDSCs) (Zou 2005). Recent studies have linked complement activation to the induction of a suppressive immune response. Differentiation of regulatory T cells is correlated with the C5a concentration within the tumor (Gunn et al. 2012). It also has been proposed that the generation of inducible regulatory T cells can be mediated by co-engagement of CD3 and the complement regulator CD46 in the presence of IL-2 (Kemper et al. 2003). Nevertheless, the effect of this complement receptor (highly expressed on lymphocytes) on the generation of inducible regulatory T cells within tumors has not yet been demonstrated. On the other hand, several studies have emphasized the pivotal role of MDSCs in tumor immunosuppression (Gabrilovich and Nagaraj 2009 ; Ostrand-Rosenberg and Sinha 2009). Like their mature counterparts (monocytes and neutrophils), MDSCs also respond to C5a anaphylatoxin. C5a released as a result of complement activation on tumor cells is connected to the recruitment and activation of MDSCs into tumors (Markiewski et al. 2008). Among the various mechanisms used by MDSCs to inhibit T-cell function, the production and release of reactive oxygen and nitrogen species seem to be critical for their suppressive capabilities. C5a may play a key role as a chemoattractant for a subpopulation of MDSCs that is morphologically related to neutrophils (polymorphonuclear MDSCs) and as an activator of the production of reactive oxygen and nitrogen species in the monocyte-like subpopulation (Markiewski et al. 2008). The role of C5a in the immunosuppressive function of MDSCs was confirmed ex vivo when isolated MDSCs from C5aR-deficient mice were unable to inhibit T-cell proliferation (Markiewski et al. 2008). Moreover, in a lung cancer mouse model, the blockade of C5a signaling downregulated the expression of key immunosuppressive molecules within the tumors. These molecules included ARG1, IL-10, IL-6, CTLA4, LAG3, and PDL1 (Corrales et al. 2012). All these studies suggest that C5a can suppress the T-cell-mediated antitumor response by promoting an immunosuppressive microenvironment and recruiting regulatory T cells and MDSCs into the tumor.
11.5.2 Complement and Angiogenesis
Angiogenesis, the creation of new vessels from preexisting ones, is a key mechanism of carcinogenesis that is directly related to the aggressiveness of the tumor (Carmeliet 2003). Complement-activated factors have been related, either directly or indirectly, to neovascularization in several diseases. Because of the heterogeneity of the studies and diseases examined, there is some controversy about the pro- or anti-angiogenic role of the complement system in neovascularization.
An anti-angiogenic effect of C3 and C5 was observed in a model of retinopathy of prematurity, in which C5a stimulated macrophages toward an angiogenesis-inhibitory phenotype and induced the secretion of the anti-angiogenic soluble VEGF receptor-1 (Langer et al. 2010). This anti-angiogenic factor also was upregulated in monocytes by complement activation products in an antibody-independent model of spontaneous miscarriage and intrauterine growth restriction (Girardi et al. 2006). The soluble receptor was able to sequester circulating VEGF and placental growth factor, altering the balance of angiogenic factors in pregnancy (Girardi et al. 2006).
In contrast, a role for complement in the activation of angiogenesis has been demonstrated in age-related macular degeneration, a disease caused by choroidal neovascularization. Both C3a and C5a are present in the lipoproteinaceous deposits, also called drusen, that appear between the choroid and the retinal pigmented epithelium in patients with age-related macular degeneration and in animals with laser- induced choroidal neovascularization (Nozaki et al. 2006). Both anaphylatoxins seem to be involved in the induction of VEGF expression in retinal pigmented epithelium cells, thereby promoting the generation of new vessels. In addition, MAC and C3 are deposited in the eyes of animals with laser-induced choroidal neovascularization, concomitant with an increase in the expression of the angiogenic factors VEGF, TGF-β2, and basic fibroblast growth factor (Bora et al. 2005). In a mouse model of epithelial ovarian cancer, a genetic C3 deficiency impaired tumor vascularization by altering the function of endothelial cells (Nunez-Cruz et al. 2012). However, in end point tumor specimens in the murine TC-1 cervical cancer model, C5aR blockade did not impair tumor angiogenesis (Markiewski et al. 2008). These results suggest that complement activation may be important in the promotion of angiogenesis only during the early steps of tumor formation. In vitro studies using endothelial cells support this conclusion. C5a stimulates chemotaxis and the formation of tube-like structures in gelled Matrigel in both human umbilical endothelial cells (Corrales et al. 2012 ; Schraufstatter et al. 2002) and human microvascular endothelial cells (Nunez-Cruz et al. 2012). Like the response to TNF-α and lipopolysaccharide, the endothelial cell response to C5a involves the activation of genes that participate in endothelial adhesion, migration, and angiogenesis (Albrecht et al. 2004).
11.5.3 Complement and Tumor Cell Signaling
Various complement factors have been linked to the activation of signaling pathways in tumor cells. Deposition of C5b-9 has been demonstrated in various human malignancies (Vlaicu et al. 2013). However, as mentioned earlier, tumor cells acquire resistance to complement attack, leading to the deposition of sublytic doses of the MAC on the cell membrane. Whereas a lytic dose of MAC is detrimental to cells because it induces an influx of Ca 2+ , mitochondrial damage, and adenosine triphosphate depletion (Kim et al. 1987), sublytic doses of the MAC play a role in cell activation, proliferation, differentiation and the inhibition of apoptosis (Tegla et al. 2011). These effects may be a result of the regulation of cell cycle genes activated by the phosphoinositide 3-kinase/Akt and extracellular signal-regulated kinase (ERK) 1 pathways (Vlaicu et al. 2013). Sublytic MACs activate several prooncogenic pathways such as the mitogen-activated protein kinase family of proteins, ERKs, p38 mitogen-activated protein kinases, and Jun N-terminal kinases (Kraus et al. 2001); the phosphatidylinositol 3-kinase pathway (Niculescu et al. 1999); Ras (Niculescu et al. 1997); p70 S6 kinase; and the Janus kinase/signal transducers and activators of transcription pathway (Niculescu et al. 1999). C5b-9 also inhibits apoptosis by inducing the phosphorylation of Bad and blocking the activation of FLIP, caspase-8, and Bid (Tegla et al. 2011). Several genes are regulated by sublytic doses of complement in oligodendrocytes (Badea et al. 1998). Among these genes, designated response genes to complement (RGCs), is RGC-32, which was shown to bind and increase the kinase activity of CDC2/cyclin B1 and thus regulate the cell cycle (Badea et al. 2002). It is likely that RGC-32 is involved in cell proliferation in vivo because it is overexpressed in malignant tumors of the human colon, kidney, stomach, and ovary (Fosbrink et al. 2005). Finally, some complement-activated factors have been linked to the production of growth factors and cytokines that support neoplastic transformation. Signaling via C3aR and C5aR has been shown to be necessary for the survival of liver cells after partial hepatectomy through the induction of the cytokines IL-6 and TNF-α, both of which are necessary for liver regeneration and (in the case of IL-6) hepatoprotection (Markiewski et al. 2009). Moreover, in several animal models of central nervous system pathology, C5a has been shown to mediate neuroprotection and exert an antiapoptotic effect (Mukherjee and Pasinetti 2001 ; Mukherjee et al. 2008). Overall, it is becoming more evident that complement factors can trigger oncogenic pathways, establishing the basis for the use of complement inhibitors in the treatment of cancer.
11.5.4 Complement and Tumor Cell Invasion and Migration
Metastasis is estimated to be the responsible for ~90 % of cancer deaths. This multistep process involves genetic and molecular changes in tumor and stromal cells, leading to local invasion, intravasation into the tumor vasculature, transit within the blood, extravasation into secondary sites, and, finally, the formation of metastases (Hanahan and Weinberg 2011).
Complement proteins can participate in some of the processes that orchestrate invasion and metastasis. One of the key events in this process is the generation of mesenchymal derivatives from epithelial phenotypes, also called the epithelial-tomesenchymal transition (EMT). Activation of an EMT program during tumorigenesis often requires signaling between cancer and stromal cells such as fibroblasts, myofibroblasts, granulocytes, macrophages, mesenchymal stem cells, and lymphocytes. These cells create a “reactive” stroma that seems to result in the release of EMT-inducing signals (Chaffer and Weinberg 2011). Complement activation by tumor cells releases anaphylatoxins that can recruit stromal cells to the tumor. C3a and C5a have been shown to promote the chemotaxis of human bone marrow– derived mesenchymal stem cells and robustly activate ERK1/2 and Akt (Schraufstatter et al. 2009). In the development of tubulointerstitial injury, C3a release seems to induce the EMT, at least partially in response to a decrease in the expression of E-cadherin (Tang et al. 2009). It is well documented that the expression of E-cadherin antagonizes invasion and metastasis, whereas a decrease in its expression has the opposite effect (Hanahan and Weinberg 2011).
Complement proteins can also promote the degradation of the extracellular matrix (Rutkowski et al. 2010b). Although C1s can directly degrade collagens and gelatin in human cartilage, complement activation can also induce the release and activation of proteases such as matrix metalloproteinase (MMP)-2 and -9 (Bandyopadhyay and Rohrer 2012). In particular, C5a signaling through C5aR promotes the release of MMP-9 by macrophages (Gonzalez et al. 2011). Proteases can, conversely, inactivate complement proteins, protecting tumor cells from complement attack. Overexpression of MMP-1 in a murine melanoma cell line protected these cells from the damaging effects of complement and promoted the formation of lung metastasis in vivo (Rozanov et al. 2006). In melanoma cells, the overexpression of procathepsin-L, another protease with anticomplement capacity, similarly increased the tumorigenicity of the cells and switched their phenotype from nonmetastatic to highly metastatic (Frade et al. 1998). Therefore, the context-dependent interaction between matrix proteases and complement activation illustrates once again the duality in the relationship between cancer and the complement system.
11.6 Concluding Remarks
An anticancer function for complement is well illustrated by its contribution to the clinical efficacy of monoclonal antibodies for the treatment of neoplasias. However, the biological functions of the complement system are much more diverse than a simple elimination of target cells. In fact, complement recognition of cancer cells may be an element of immunosurveillance, with complement taking part in the elimination of tumors and at the same time serving as a force for immunoselection. This idea is entirely consistent with our growing recognition of the homeostatic function of the complement system. Furthermore, in the context of chronic inflammation, complement elements can promote tumor growth. Recent reports of the role of complement activation in the pathogenesis of cancer stress this duality. The expression of immune modulators in the tumor microenvironment dictates the balance between antitumor and tumor-promoting complement activities. It is clinically evident that when complement fails to protect an organism from growing tumors, this balance is tilted toward protumor inflammation. However, to date, we still have only fragmentary knowledge concerning the interplay between complement activation and tumor cells. We need to both identify those tumor-associated antigens that are able to stimulate complement and better understand the intricate mechanisms of activation and resistance. These studies will permit the development of new therapeutic strategies for cancer that are aimed at modulating this interaction and enhancing immunologically based cancer therapies. Additional in vivo models are needed to validate the strategy of using complement inhibitors to treat cancer.
Acknowledgments
Work in our laboratories is funded by the UTE project CIMA, Instituto de Salud Carlos III: Red Temática de Investigación Cooperativa en Cáncer (RD12/0036/0040), Ministerio de Economía y Competitividad (PI1100618), and National Institutes of Health grant AI068730. L.C. is supported by a postdoctoral fellowship from Fundación Ramón Areces. We thank Dr. Deborah McClellan for editorial assistance.
Contributor Information
Ruben Pio, Oncology Division (CIMA), and Department of Biochemistry and Genetics (School of Science), University of Navarra, Pamplona, Spain rpio@unav.es.
Leticia Corrales, Department of Pathology, University of Chicago, Chicago, IL, USA lcorrales@bsd.uchicago.edu.
John D. Lambris, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA, USA
References
- Abbott RJ, Spendlove I, Roversi P, et al. Structural and functional characterization of a novel T cell receptor co-regulatory protein complex, CD97-CD55. J Biol Chem. 2007;282(30):22023–22032. doi: 10.1074/jbc.M702588200. [DOI] [PubMed] [Google Scholar]
- Ajona D, Castano Z, Garayoa M, et al. Expression of complement factor H by lung cancer cells: effects on the activation of the alternative pathway of complement. Cancer Res. 2004;64(17):6310–6318. doi: 10.1158/0008-5472.CAN-03-2328. [DOI] [PubMed] [Google Scholar]
- Ajona D, Hsu YF, Corrales L, et al. Down-regulation of human complement factor H sensitizes non-small cell lung cancer cells to complement attack and reduces in vivo tumor growth. J Immunol. 2007;178(9):5991–5998. doi: 10.4049/jimmunol.178.9.5991. [DOI] [PubMed] [Google Scholar]
- Albrecht EA, Chinnaiyan AM, Varambally S, et al. C5a-Induced gene expression in human umbilical vein endothelial cells. Am J Pathol. 2004;164(3):849–859. doi: 10.1016/S0002-9440(10)63173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara U, Rittirsch D, Flierl M, et al. Interaction between the coagulation and complement system. Adv Exp Med Biol. 2008;632:71–79. doi: 10.1007/978-0-387-78952-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh A, Shimada M, Araki Y, et al. Sodium butyrate enhances complement-mediated cell injury via down-regulation of decay-accelerating factor expression in colonic cancer cells. Cancer Immunol Immunother. 2002;50(12):663–672. doi: 10.1007/s00262-001-0239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspord C, Pedroza-Gonzalez A, Gallegos M, et al. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204(5):1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baatrup G, Qvist N, Junker A, et al. Activity and activation of the complement system in patients being operated on for cancer of the colon. Eur J Surg. 1994;160(9):503–510. [PubMed] [Google Scholar]
- Badea TC, Niculescu FI, Soane L, et al. Molecular cloning and characterization of RGC-32, a novel gene induced by complement activation in oligodendrocytes. J Biol Chem. 1998;273(41):26977–26981. doi: 10.1074/jbc.273.41.26977. [DOI] [PubMed] [Google Scholar]
- Badea T, Niculescu F, Soane L, et al. RGC-32 increases p34CDC2 kinase activity and entry of aortic smooth muscle cells into S-phase. J Biol Chem. 2002;277(1):502–508. doi: 10.1074/jbc.M109354200. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay M, Rohrer B. Matrix metalloproteinase activity creates pro-angiogenic environment in primary human retinal pigment epithelial cells exposed to complement. Invest Ophthalmol Vis Sci. 2012;53(4):1953–1961. doi: 10.1167/iovs.11-8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistelli S, Vittoria A, Cappelli R, et al. Protein S in cancer patients with non-metastatic solid tumours. Eur J Surg Oncol. 2005;31(7):798–802. doi: 10.1016/j.ejso.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285(5428):727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- Bellone S, Roque D, Cocco E, et al. Down regulation of membrane complement inhibitors CD55 and CD59 by siRNA sensitises uterine serous carcinoma overexpressing Her2/neu to complement and antibody-dependent cell cytotoxicity in vitro: implications for Trastuzumab-based immunotherapy. Br J Cancer. 2012;106(9):1543–1550. doi: 10.1038/bjc.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard M, Gonzalez BJ, Schouft MT, et al. Characterization of C3a and C5a receptors in rat cerebellar granule neurons during maturation. Neuroprotective effect of C5a against apoptotic cell death. J Biol Chem. 2004;279(42):43487–43496. doi: 10.1074/jbc.M404124200. [DOI] [PubMed] [Google Scholar]
- Beum PV, Lindorfer MA, Beurskens F, et al. Complement activation on B lymphocytes opsonized with rituximab or ofatumumab produces substantial changes in membrane structure preceding cell lysis. J Immunol. 2008;181(1):822–832. doi: 10.4049/jimmunol.181.1.822. [DOI] [PubMed] [Google Scholar]
- Beurskens FJ, Lindorfer MA, Farooqui M, et al. Exhaustion of cytotoxic effector systems may limit monoclonal antibody-based immunotherapy in cancer patients. J Immunol. 2012;188(7):3532–3541. doi: 10.4049/jimmunol.1103693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexborn F, Andersson PO, Chen H, et al. The tick-over theory revisited: formation and regulation of the soluble alternative complement C3 convertase (C3(H2O)Bb). Mol Immunol. 2008;45(8):2370–2379. doi: 10.1016/j.molimm.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorge L, Hakulinen J, Vintermyr OK, et al. Ascitic complement system in ovarian cancer. Br J Cancer. 2005;92(5):895–905. doi: 10.1038/sj.bjc.6602334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blok VT, Gelderman KA, Tijsma OH, et al. Cytokines affect resistance of human renal tumour cells to complement-mediated injury. Scand J Immunol. 2003;57(6):591–599. doi: 10.1046/j.1365-3083.2003.01265.x. [DOI] [PubMed] [Google Scholar]
- Blom AM, Villoutreix BO, Dahlback B. Complement inhibitor C4b-binding protein-friend or foe in the innate immune system? Mol Immunol. 2004;40(18):1333–1346. doi: 10.1016/j.molimm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Bohana-Kashtan O, Pinna LA, Fishelson Z. Extracellular phosphorylation of C9 by protein kinase CK2 regulates complement-mediated lysis. Eur J Immunol. 2005;35(6):1939–1948. doi: 10.1002/eji.200425716. [DOI] [PubMed] [Google Scholar]
- Boon T, Coulie PG, Van den Eynde B. Tumor antigens recognized by T cells. Immunol Today. 1997;18(6):267–268. doi: 10.1016/s0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- Bora PS, Sohn JH, Cruz JM, et al. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J Immunol. 2005;174(1):491–497. doi: 10.4049/jimmunol.174.1.491. [DOI] [PubMed] [Google Scholar]
- Boulay F, Mery L, Tardif M, et al. Expression cloning of a receptor for C5a anaphylatoxin on differentiated HL-60 cells. Biochemistry. 1991;30(12):2993–2999. doi: 10.1021/bi00226a002. [DOI] [PubMed] [Google Scholar]
- Brasoveanu LI, Altomonte M, Fonsatti E, et al. Levels of cell membrane CD59 regulate the extent of complement-mediated lysis of human melanoma cells. Lab Invest. 1996;74(1):33–42. [PubMed] [Google Scholar]
- Brasoveanu LI, Fonsatti E, Visintin A, et al. Melanoma cells constitutively release an anchor-positive soluble form of protectin (sCD59) that retains functional activities in homologous complement-mediated cytotoxicity. J Clin Invest. 1997;100(5):1248–1255. doi: 10.1172/JCI119638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu X, Zheng Z, Wang C, et al. Significance of C4d deposition in the follicular lymphoma and MALT lymphoma and their relationship with follicular dendritic cells. Pathol Res Pract. 2007;203(3):163–167. doi: 10.1016/j.prp.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Budzko DB, Lachmann PJ, McConnell I. Activation of the alternative complement pathway by lymphoblastoid cell lines derived from patients with Burkitt's lymphoma and infectious mononucleosis. Cell Immunol. 1976;22(1):98–109. doi: 10.1016/0008-8749(76)90011-3. [DOI] [PubMed] [Google Scholar]
- Buo L, Karlsrud TS, Dyrhaug G, et al. Differential diagnosis of human ascites: inhibitors of the contact system and total proteins. Scand J Gastroenterol. 1993;28(9):777–782. doi: 10.3109/00365529309104008. [DOI] [PubMed] [Google Scholar]
- Cardarelli PM, Quinn M, Buckman D, et al. Binding to CD20 by anti-B1 antibody or F(ab') (2) is sufficient for induction of apoptosis in B-cell lines. Cancer Immunol Immunother. 2002;51(1):15–24. doi: 10.1007/s00262-001-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli M, Bucolo C, Pannunzio MT, et al. Fluctuation of serum complement levels in children with neuroblastoma. Cancer. 1979;43(6):2399–2404. doi: 10.1002/1097-0142(197906)43:6<2399::aid-cncr2820430634>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Carney DF, Lang TJ, Shin ML. Multiple signal messengers generated by terminal complement complexes and their role in terminal complement complex elimination. J Immunol. 1990;145(2):623–629. [PubMed] [Google Scholar]
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- Chen S, Caragine T, Cheung NK, et al. CD59 expressed on a tumor cell surface modulates decay-accelerating factor expression and enhances tumor growth in a rat model of human neuroblastoma. Cancer Res. 2000;60(11):3013–3018. [PubMed] [Google Scholar]
- Chen NJ, Mirtsos C, Suh D, et al. C5L2 Is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature. 2007;446(7132):203–207. doi: 10.1038/nature05559. [DOI] [PubMed] [Google Scholar]
- Cheng ZZ, Corey MJ, Parepalo M, et al. Complement factor H as a marker for detection of bladder cancer. Clin Chem. 2005;51(5):856–863. doi: 10.1373/clinchem.2004.042192. [DOI] [PubMed] [Google Scholar]
- Cole DS, Morgan BP. Beyond lysis: how complement influences cell fate. Clin Sci (Lond) 2003;104(5):455–466. doi: 10.1042/CS20020362. [DOI] [PubMed] [Google Scholar]
- Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- Coral S, Fonsatti E, Sigalotti L, et al. Overexpression of protectin (CD59) down-modulates the susceptibility of human melanoma cells to homologous complement. J Cell Physiol. 2000;185(3):317–323. doi: 10.1002/1097-4652(200012)185:3<317::AID-JCP1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Corey MJ, Kinders RJ, Brown LG, et al. A very sensitive coupled luminescent assay for cytotoxicity and complement-mediated lysis. J Immunol Methods. 1997;207(1):43–51. doi: 10.1016/s0022-1759(97)00098-7. [DOI] [PubMed] [Google Scholar]
- Corrales L, Ajona D, Rafail S, et al. Anaphylatoxin c5a creates a favorable microenvironment for lung cancer progression. J Immunol. 2012;189(9):4674–4683. doi: 10.4049/jimmunol.1201654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello LC, Franklin RB. ‘Why do tumour cells glycolyse?’: from glycolysis through citrate to lipogenesis. Mol Cell Biochem. 2005;280(1–2):1–8. doi: 10.1007/s11010-005-8841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg MS, Glennie MJ. Antibody specificity controls in vivo effector mechanisms of anti-CD20 reagents. Blood. 2004;103(7):2738–2743. doi: 10.1182/blood-2003-06-2031. [DOI] [PubMed] [Google Scholar]
- Cui T, Chen Y, Knosel T, et al. Human complement factor H is a novel diagnostic marker for lung adenocarcinoma. Int J Oncol. 2011;39(1):161–168. doi: 10.3892/ijo.2011.1010. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Davis AE, 3rd, Mejia P, Lu F. Biological activities of C1 inhibitor. Mol Immunol. 2008;45(16):4057–4063. doi: 10.1016/j.molimm.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cordoba SR, de Jorge EG. Translational mini-review series on complement factor H: genetics and disease associations of human complement factor H. Clin Exp Immunol. 2008;151(1):1–13. doi: 10.1111/j.1365-2249.2007.03552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gaetano N, Xiao Y, Erba E, et al. Synergism between fludarabine and rituximab revealed in a follicular lymphoma cell line resistant to the cytotoxic activity of either drug alone. Br J Haematol. 2001;114(4):800–809. doi: 10.1046/j.1365-2141.2001.03014.x. [DOI] [PubMed] [Google Scholar]
- Di Gaetano N, Cittera E, Nota R, et al. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2003;171(3):1581–1587. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- Donin N, Jurianz K, Ziporen L, et al. Complement resistance of human carcinoma cells depends on membrane regulatory proteins, protein kinases and sialic acid. Clin Exp Immunol. 2003;131(2):254–263. doi: 10.1046/j.1365-2249.2003.02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004a;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004b;21(2):137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- DuPage M, Mazumdar C, Schmidt LM, et al. Expression of tumour-specific antigens underlies cancer immunoediting. Nature. 2012;482(7385):405–409. doi: 10.1038/nature10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant LG, Chapman MA, Buckley DJ, et al. Enhanced expression of the complement regulatory protein CD55 predicts a poor prognosis in colorectal cancer patients. Cancer Immunol Immunother. 2003;52(10):638–642. doi: 10.1007/s00262-003-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvington M, Huang Y, Morgan BP, et al. A targeted complement-dependent strategy to improve the outcome of mAb therapy, and characterization in a murine model of metastatic cancer. Blood. 2012;119(25):6043–6051. doi: 10.1182/blood-2011-10-383232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falgarone G, Chiocchia G. Chapter 8: clusterin: a multifacet protein at the crossroad of inflammation and autoimmunity. Adv Cancer Res. 2009;104:139–170. doi: 10.1016/S0065-230X(09)04008-1. [DOI] [PubMed] [Google Scholar]
- Fearon DT. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979;76(11):5867–5871. doi: 10.1073/pnas.76.11.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon DT, Carter RH. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol. 1995;13:127–149. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- Fedarko NS, Fohr B, Robey PG, et al. Factor H binding to bone sialoprotein and osteopontin enables tumor cell evasion of complement-mediated attack. J Biol Chem. 2000;275(22):16666–16672. doi: 10.1074/jbc.M001123200. [DOI] [PubMed] [Google Scholar]
- Fischer E, Appay MD, Cook J, et al. Characterization of the human glomerular C3 receptor as the C3b/C4b complement type one (CR1) receptor. J Immunol. 1986;136(4):1373–1377. [PubMed] [Google Scholar]
- Fishelson Z, Donin N, Zell S, et al. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol. 2003;40(2–4):109–123. doi: 10.1016/s0161-5890(03)00112-3. [DOI] [PubMed] [Google Scholar]
- Fosbrink M, Cudrici C, Niculescu F, et al. Overexpression of RGC-32 in colon cancer and other tumors. Exp Mol Pathol. 2005;78(2):116–122. doi: 10.1016/j.yexmp.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Frade R, Rodrigues-Lima F, Huang S, et al. Procathepsin-L, a proteinase that cleaves human C3 (the third component of complement), confers high tumorigenic and metastatic properties to human melanoma cells. Cancer Res. 1998;58(13):2733–2736. [PubMed] [Google Scholar]
- Fridman WH, Pages F, Sautes-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- Fust G, Miszlay Z, Czink E, et al. C1 and C4 abnormalities in chronic lymphocytic leukaemia and their significance. Immunol Lett. 1987;14(3):255–259. doi: 10.1016/0165-2478(87)90110-6. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Gancz D, Fishelson Z. Cancer resistance to complement-dependent cytotoxicity (CDC): problem-oriented research and development. Mol Immunol. 2009;46(14):2794–2800. doi: 10.1016/j.molimm.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Gao LJ, Guo SY, Cai YQ, et al. Cooperation of decay-accelerating factor and membrane cofactor protein in regulating survival of human cervical cancer cells. BMC Cancer. 2009;9:384. doi: 10.1186/1471-2407-9-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasque P, Thomas A, Fontaine M, et al. Complement activation on human neuroblastoma cell lines in vitro: route of activation and expression of functional complement regulatory proteins. J Neuroimmunol. 1996;66(1–2):29–40. doi: 10.1016/0165-5728(96)00015-x. [DOI] [PubMed] [Google Scholar]
- Geis N, Zell S, Rutz R, et al. Inhibition of membrane complement inhibitor expression (CD46, CD55, CD59) by siRNA sensitizes tumor cells to complement attack in vitro. Curr Cancer Drug Targets. 2010;10(8):922–931. doi: 10.2174/156800910793357952. [DOI] [PubMed] [Google Scholar]
- Gelderman KA, Blok VT, Fleuren GJ, et al. The inhibitory effect of CD46, CD55, and CD59 on complement activation after immunotherapeutic treatment of cervical carcinoma cells with monoclonal antibodies or bispecific monoclonal antibodies. Lab Invest. 2002a;82(4):483–493. doi: 10.1038/labinvest.3780441. [DOI] [PubMed] [Google Scholar]
- Gelderman KA, Kuppen PJ, Bruin W, et al. Enhancement of the complement activating capacity of 17-1A mAb to overcome the effect of membrane-bound complement regulatory proteins on colorectal carcinoma. Eur J Immunol. 2002b;32(1):128–135. doi: 10.1002/1521-4141(200201)32:1<128::AID-IMMU128>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Gelderman KA, Kuppen PJ, Okada N, et al. Tumor-specific inhibition of membrane-bound complement regulatory protein crry with bispecific monoclonal antibodies prevents tumor outgrowth in a rat colorectal cancer lung metastases model. Cancer Res. 2004a;64(12):4366–4372. doi: 10.1158/0008-5472.CAN-03-2131. [DOI] [PubMed] [Google Scholar]
- Gelderman KA, Tomlinson S, Ross GD, et al. Complement function in mAb-mediated cancer immunotherapy. Trends Immunol. 2004b;25(3):158–164. doi: 10.1016/j.it.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Gelderman KA, Lam S, Gorter A. Inhibiting complement regulators in cancer immuno-therapy with bispecific mAbs. Expert Opin Biol Ther. 2005;5(12):1593–1601. doi: 10.1517/14712598.5.12.1593. [DOI] [PubMed] [Google Scholar]
- Ghiran I, Barbashov SF, Klickstein LB, et al. Complement receptor 1/CD35 is a receptor for mannan-binding lectin. J Exp Med. 2000;192(12):1797–1808. doi: 10.1084/jem.192.12.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi G, Yarilin D, Thurman JM, et al. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203(9):2165–2175. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glunde K, Serkova NJ. Therapeutic targets and biomarkers identified in cancer choline phospholipid metabolism. Pharmacogenomics. 2006;7(7):1109–1123. doi: 10.2217/14622416.7.7.1109. [DOI] [PubMed] [Google Scholar]
- Gminski J, Mykala-Ciesla J, Machalski M, et al. Immunoglobulins and complement components levels in patients with lung cancer. Rom J Intern Med. 1992;30(1):39–44. [PubMed] [Google Scholar]
- Golay J, Zaffaroni L, Vaccari T, et al. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood. 2000;95(12):3900–3908. [PubMed] [Google Scholar]
- Gonzalez JM, Franzke CW, Yang F, et al. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am J Pathol. 2011;179(2):838–849. doi: 10.1016/j.ajpath.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter A, Meri S. Immune evasion of tumor cells using membrane-bound complement regulatory proteins. Immunol Today. 1999;20(12):576–582. doi: 10.1016/s0167-5699(99)01537-6. [DOI] [PubMed] [Google Scholar]
- Governa M, Fenoglio I, Amati M, et al. Cleavage of the fifth component of human complement and release of a split product with C5a-like activity by crystalline silica through free radical generation and kallikrein activation. Toxicol Appl Pharmacol. 2002;179(3):129–136. doi: 10.1006/taap.2002.9351. [DOI] [PubMed] [Google Scholar]
- Griffin JL, Kauppinen RA. Tumour metabolomics in animal models of human cancer. J Proteome Res. 2007;6(2):498–505. doi: 10.1021/pr060464h. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi L, Baroni R, Bartoloni C, et al. Immune complexes in solid tumours precipitable by 3.5% Polyethylene glycol: analysis of some nonspecific components. Diagn Clin Immunol. 1988;5(6):284–288. [PubMed] [Google Scholar]
- Gunn L, Ding C, Liu M, et al. Opposing roles for complement component C5a in tumor progression and the tumor microenvironment. J Immunol. 2012;189(6):2985–2994. doi: 10.4049/jimmunol.1200846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas PJ, van Strijp J. Anaphylatoxins: their role in bacterial infection and inflammation. Immunol Res. 2007;37(3):161–175. doi: 10.1007/BF02697367. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci U S A. 2002;99(16):10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakulinen J, Junnikkala S, Sorsa T, et al. Complement inhibitor membrane cofactor protein (MCP; CD46) is constitutively shed from cancer cell membranes in vesicles and converted by a metalloproteinase to a functionally active soluble form. Eur J Immunol. 2004;34(9):2620–2629. doi: 10.1002/eji.200424969. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5(4):271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- He JQ, Wiesmann C, van Lookeren CM. A role of macrophage complement receptor CRIg in immune clearance and inflammation. Mol Immunol. 2008;45(16):4041–4047. doi: 10.1016/j.molimm.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Helmy KY, Katschke KJ, Jr, Gorgani NN, et al. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124(5):915–927. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Hindmarsh EJ, Marks RM. Decay-accelerating factor is a component of subendothelial extracellular matrix in vitro, and is augmented by activation of endothelial protein kinase C. Eur J Immunol. 1998;28(3):1052–1062. doi: 10.1002/(SICI)1521-4141(199803)28:03<1052::AID-IMMU1052>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Holliger P, Wing M, Pound JD, et al. Retargeting serum immunoglobulin with bispecific diabodies. Nat Biotechnol. 1997;15(7):632–636. doi: 10.1038/nbt0797-632. [DOI] [PubMed] [Google Scholar]
- Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4(1):45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- Holmberg MT, Blom AM, Meri S. Regulation of complement classical pathway by association of C4b-binding protein to the surfaces of SK-OV-3 and caov-3 ovarian adenocarcinoma cells. J Immunol. 2001;167(2):935–939. doi: 10.4049/jimmunol.167.2.935. [DOI] [PubMed] [Google Scholar]
- Hourcade DE. Properdin and complement activation: a fresh perspective. Curr Drug Targets. 2008;9(2):158–164. doi: 10.2174/138945008783502458. [DOI] [PubMed] [Google Scholar]
- Hsu YF, Ajona D, Corrales L, et al. Complement activation mediates cetuximab inhibition of non-small cell lung cancer tumor growth in vivo. Mol Cancer. 2010;9:139. doi: 10.1186/1476-4598-9-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber-Lang M, Younkin EM, Sarma JV, et al. Generation of C5a by phagocytic cells. Am J Pathol. 2002;161(5):1849–1859. doi: 10.1016/S0002-9440(10)64461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber-Lang M, Sarma JV, Zetoune FS, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12(6):682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- Jain A, Karadag A, Fohr B, et al. Three SIBLINGs (small integrin-binding ligand, N-linked glycoproteins) enhance factor H's cofactor activity enabling MCP-like cellular evasion of complement- mediated attack. J Biol Chem. 2002;277(16):13700–13708. doi: 10.1074/jbc.M110757200. [DOI] [PubMed] [Google Scholar]
- Jarvis GA, Li J, Hakulinen J, et al. Expression and function of the complement membrane attack complex inhibitor protectin (CD59) in human prostate cancer. Int J Cancer. 1997;71(6):1049–1055. doi: 10.1002/(sici)1097-0215(19970611)71:6<1049::aid-ijc22>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Jenne DE, Tschopp J. Molecular structure and functional characterization of a human complement cytolysis inhibitor found in blood and seminal plasma: identity to sulfated glycoprotein 2, a constituent of rat testis fluid. Proc Natl Acad Sci U S A. 1989;86(18):7123–7127. doi: 10.1073/pnas.86.18.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Cooper B, Robey FA, et al. DNA binds and activates complement via residues 14–26 of the human C1q a chain. J Biol Chem. 1992;267(35):25597–25601. [PubMed] [Google Scholar]
- Jozsi M, Zipfel PF. Factor H family proteins and human diseases. Trends Immunol. 2008;29(8):380–387. doi: 10.1016/j.it.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Juhl H, Petrella EC, Cheung NK, et al. Complement killing of human Neuroblastoma cells: a cytotoxic monoclonal antibody and its F(ab')2-cobra venom factor conjugate are equally cytotoxic. Mol Immunol. 1990;27(10):957–964. doi: 10.1016/0161-5890(90)90118-j. [DOI] [PubMed] [Google Scholar]
- Juhl H, Sievers M, Baltzer K, et al. A monoclonal antibody-cobra venom factor conjugate increases the tumor-specific uptake of a 99mTc-labeled anti-carcinoembryonic antigen antibody by a two-step approach. Cancer Res. 1995;55(23 Suppl):5749s–5755s. [PubMed] [Google Scholar]
- Juhl H, Petrella EC, Cheung NK, et al. Additive cytotoxicity of different monoclonal antibody- cobra venom factor conjugates for human Neuroblastoma cells. Immunobiology. 1997;197(5):444–459. doi: 10.1016/s0171-2985(97)80078-2. [DOI] [PubMed] [Google Scholar]
- Junnikkala S, Jokiranta TS, Friese MA, et al. Exceptional resistance of human H2 glioblastoma cells to complement-mediated killing by expression and utilization of factor H and factor H-like protein 1. J Immunol. 2000;164(11):6075–6081. doi: 10.4049/jimmunol.164.11.6075. [DOI] [PubMed] [Google Scholar]
- Junnikkala S, Hakulinen J, Jarva H, et al. Secretion of soluble complement inhibitors factor H and factor H-like protein (FHL-1) by ovarian tumour cells. Br J Cancer. 2002;87(10):1119–1127. doi: 10.1038/sj.bjc.6600614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurianz K, Ziegler S, Garcia-Schuler H, et al. Complement resistance of tumor cells: basal and induced mechanisms. Mol Immunol. 1999;36(13–14):929–939. doi: 10.1016/s0161-5890(99)00115-7. [DOI] [PubMed] [Google Scholar]
- Jurianz K, Ziegler S, Donin N, et al. K562 Erythroleukemic cells are equipped with multiple mechanisms of resistance to lysis by complement. Int J Cancer. 2001;93(6):848–854. doi: 10.1002/ijc.1406. [DOI] [PubMed] [Google Scholar]
- Kalwinsky DK, Urmson JR, Stitzel AE, et al. Activation of the alternative pathway of complement in childhood acute lymphoblastic leukemia. J Lab Clin Med. 1976;88(5):745–756. [PubMed] [Google Scholar]
- Karre K, Ljunggren HG, Piontek G, et al. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Kataki A, Scheid P, Piet M, et al. Tumor infiltrating lymphocytes and macrophages have a potential dual role in lung cancer by supporting both host-defense and tumor progression. J Lab Clin Med. 2002;140(5):320–328. doi: 10.1067/mlc.2002.128317. [DOI] [PubMed] [Google Scholar]
- Kawada M, Mizuno M, Nasu J, et al. Release of decay-accelerating factor into stools of patients with colorectal cancer by means of cleavage at the site of glycosylphosphatidylinositol anchor. J Lab Clin Med. 2003;142(5):306–312. doi: 10.1016/S0022-2143(03)00137-9. [DOI] [PubMed] [Google Scholar]
- Kemper C, Chan AC, Green JM, et al. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421(6921):388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- Kennedy AD, Solga MD, Schuman TA, et al. An anti-C3b(i) mAb enhances complement activation, C3b(i) deposition, and killing of CD20+ cells by rituximab. Blood. 2003;101(3):1071–1079. doi: 10.1182/blood-2002-03-0876. [DOI] [PubMed] [Google Scholar]
- Kim SH, Carney DF, Hammer CH, et al. Nucleated cell killing by complement: effects of C5b-9 channel size and extracellular Ca2+ on the lytic process. J Immunol. 1987;138(5):1530–1536. [PubMed] [Google Scholar]
- Kim R, Emi M, Tanabe K, et al. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66(11):5527–5536. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- Kimberley FC, Sivasankar B, Paul Morgan B. Alternative roles for CD59. Mol Immunol. 2007;44(1–3):73–81. doi: 10.1016/j.molimm.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Kinders R, Jones T, Root R, et al. Complement factor H or a related protein is a marker for transitional cell cancer of the bladder. Clin Cancer Res. 1998;4(10):2511–2520. [PubMed] [Google Scholar]
- Kirkitadze MD, Barlow PN. Structure and flexibility of the multiple domain proteins that regulate complement activation. Immunol Rev. 2001;180:146–161. doi: 10.1034/j.1600-065x.2001.1800113.x. [DOI] [PubMed] [Google Scholar]
- Klickstein LB, Barbashov SF, Liu T, et al. Complement receptor type 1 (CR1, CD35) is a receptor for C1q. Immunity. 1997;7(3):345–355. doi: 10.1016/s1074-7613(00)80356-8. [DOI] [PubMed] [Google Scholar]
- Kohl J. Anaphylatoxins and infectious and non-infectious inflammatory diseases. Mol Immunol. 2001;38(2–3):175–187. doi: 10.1016/s0161-5890(01)00041-4. [DOI] [PubMed] [Google Scholar]
- Kohno H, Mizuno M, Nasu J, et al. Stool decay-accelerating factor as a marker for monitoring the disease activity during leukocyte apheresis therapy in patients with refractory ulcerative colitis. J Gastroenterol Hepatol. 2005;20(1):73–78. doi: 10.1111/j.1440-1746.2004.03545.x. [DOI] [PubMed] [Google Scholar]
- Kojouharova M, Reid K, Gadjeva M. New insights into the molecular mechanisms of classical complement activation. Mol Immunol. 2010;47(13):2154–2160. doi: 10.1016/j.molimm.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Kolev M, Towner L, Donev R. Complement in cancer and cancer immunotherapy. Arch Immunol Ther Exp (Warsz) 2011;59(6):407–419. doi: 10.1007/s00005-011-0146-x. [DOI] [PubMed] [Google Scholar]
- Kraus S, Seger R, Fishelson Z. Involvement of the ERK mitogen-activated protein kinase in cell resistance to complement-mediated lysis. Clin Exp Immunol. 2001;123(3):366–374. doi: 10.1046/j.1365-2249.2001.01477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraut EH, Sagone AL., Jr Alternative pathway of complement in multiple myeloma. Am J Hematol. 1981;11(4):335–345. doi: 10.1002/ajh.2830110402. [DOI] [PubMed] [Google Scholar]
- Krych-Goldberg M, Atkinson JP. Structure-function relationships of complement receptor type 1. Immunol Rev. 2001;180:112–122. doi: 10.1034/j.1600-065x.2001.1800110.x. [DOI] [PubMed] [Google Scholar]
- Laghi L, Bianchi P, Miranda E, et al. CD3+ Cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10(9):877–884. doi: 10.1016/S1470-2045(09)70186-X. [DOI] [PubMed] [Google Scholar]
- Langer HF, Chung KJ, Orlova VV, et al. Complement-mediated inhibition of neovascularization reveals a point of convergence between innate immunity and angiogenesis. Blood. 2010;116(22):4395–4403. doi: 10.1182/blood-2010-01-261503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law SK, Dodds AW. The internal thioester and the covalent binding properties of the complement proteins C3 and C4. Protein Sci. 1997;6(2):263–274. doi: 10.1002/pro.5560060201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66(2):605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- Li L, Spendlove I, Morgan J, et al. CD55 is over-expressed in the tumour environment. Br J Cancer. 2001;84(1):80–86. doi: 10.1054/bjoc.2000.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszewski MK, Post TW, Atkinson JP. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- Liu J, Miwa T, Hilliard B, et al. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J Exp Med. 2005;201(4):567–577. doi: 10.1084/jem.20040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Li W, Li Z, et al. Sublytic complement protects prostate cancer cells from tumour necrosis factor-alpha-induced cell death. Clin Exp Immunol. 2012;169(2):100–108. doi: 10.1111/j.1365-2249.2012.04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston PO, Hood C, Krug LM, et al. Selection of GM2, fucosyl GM1, globo H and polysialic acid as targets on small cell lung cancers for antibody mediated immunotherapy. Cancer Immunol Immunother. 2005;54(10):1018–1025. doi: 10.1007/s00262-005-0663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loberg RD, Wojno KJ, Day LL, et al. Analysis of membrane-bound complement regulatory proteins in prostate cancer. Urology. 2005;66(6):1321–1326. doi: 10.1016/j.urology.2005.06.094. [DOI] [PubMed] [Google Scholar]
- Lublin DM, Atkinson JP. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- Lucas SD, Karlsson-Parra A, Nilsson B, et al. Tumor-specific deposition of immunoglobulin G and complement in papillary thyroid carcinoma. Hum Pathol. 1996;27(12):1329–1335. doi: 10.1016/s0046-8177(96)90346-9. [DOI] [PubMed] [Google Scholar]
- Macor P, Tedesco F. Complement as effector system in cancer immunotherapy. Immunol Lett. 2007;111(1):6–13. doi: 10.1016/j.imlet.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Macor P, Mezzanzanica D, Cossetti C, et al. Complement activated by chimeric anti-folate receptor antibodies is an efficient effector system to control ovarian carcinoma. Cancer Res. 2006;66(7):3876–3883. doi: 10.1158/0008-5472.CAN-05-3434. [DOI] [PubMed] [Google Scholar]
- Madjd Z, Durrant LG, Bradley R, et al. Loss of CD55 is associated with aggressive breast tumors. Clin Cancer Res. 2004;10(8):2797–2803. doi: 10.1158/1078-0432.ccr-1073-03. [DOI] [PubMed] [Google Scholar]
- Madjd Z, Durrant LG, Pinder SE, et al. Do poor-prognosis breast tumours express membrane cofactor proteins (CD46)? Cancer Immunol Immunother. 2005;54(2):149–156. doi: 10.1007/s00262-004-0590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness PF, Orengo A. Serum complement levels in patients with digestive tract carcinomas and other neoplastic diseases. Oncology. 1977;34(2):87–89. doi: 10.1159/000225191. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Marie JC, Astier AL, Rivailler P, et al. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nat Immunol. 2002;3(7):659–666. doi: 10.1038/ni810. [DOI] [PubMed] [Google Scholar]
- Markiewski MM, Nilsson B, Ekdahl KN, et al. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28(4):184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Markiewski MM, DeAngelis RA, Benencia F, et al. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9(11):1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewski MM, DeAngelis RA, Strey CW, et al. The regulation of liver cell survival by complement. J Immunol. 2009;182(9):5412–5418. doi: 10.4049/jimmunol.0804179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M, Thiel S, Jensenius JC, et al. Proteolytic activities of two types of mannose-binding lectin-associated serine protease. J Immunol. 2000;165(5):2637–2642. doi: 10.4049/jimmunol.165.5.2637. [DOI] [PubMed] [Google Scholar]
- Matsushita H, Vesely MD, Koboldt DC, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482(7385):400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsutani M, Suzuki T, Hori T, et al. Cellular immunity and complement levels in hosts with brain tumours. Neurosurg Rev. 1984;7(1):29–35. doi: 10.1007/BF01743288. [DOI] [PubMed] [Google Scholar]
- McConnell I, Klein G, Lint TF, et al. Activation of the alternative complement pathway by human B cell lymphoma lines is associated with Epstein-Barr virus transformation of the cells. Eur J Immunol. 1978;8(7):453–458. doi: 10.1002/eji.1830080702. [DOI] [PubMed] [Google Scholar]
- McGrath FD, Brouwer MC, Arlaud GJ, et al. Evidence that complement protein C1q interacts with C-reactive protein through its globular head region. J Immunol. 2006;176(5):2950–2957. doi: 10.4049/jimmunol.176.5.2950. [DOI] [PubMed] [Google Scholar]
- McMullen ME, Hart ML, Walsh MC, et al. Mannose-binding lectin binds IgM to activate the lectin complement pathway in vitro and in vivo. Immunobiology. 2006;211(10):759–766. doi: 10.1016/j.imbio.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Medof ME, Walter EI, Rutgers JL, et al. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med. 1987;165(3):848–864. doi: 10.1084/jem.165.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meri S, Morgan BP, Davies A, et al. Human protectin (CD59), an 18,000-20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990;71(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Michlmayr A, Bachleitner-Hofmann T, Baumann S, et al. Modulation of plasma complement by the initial dose of Epirubicin/docetaxel therapy in breast cancer and its predictive value. Br J Cancer. 2011;103(8):1201–1208. doi: 10.1038/sj.bjc.6605909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DA, Kirby L, Paulin SM, et al. Prion protein activates and fixes complement directly via the classical pathway: implications for the mechanism of scrapie agent propagation in lymphoid tissue. Mol Immunol. 2007;44(11):2997–3004. doi: 10.1016/j.molimm.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Miyagi T, Takahashi K, Moriya S, et al. Altered expression of sialidases in human cancer. Adv Exp Med Biol. 2012;749:257–267. doi: 10.1007/978-1-4614-3381-1_17. [DOI] [PubMed] [Google Scholar]
- Montuenga LM, Pio R. Tumour-associated macrophages in nonsmall cell lung cancer: the role of interleukin-10. Eur Respir J. 2007;30(4):608–610. doi: 10.1183/09031936.00091707. [DOI] [PubMed] [Google Scholar]
- Moore GL, Chen H, Karki S, et al. Engineered Fc variant antibodies with enhanced ability to recruit complement and mediate effector functions. MAbs. 2010;2(2):181–189. doi: 10.4161/mabs.2.2.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan BP. Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J. 1989;264(1):1–14. doi: 10.1042/bj2640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan BP. Effects of the membrane attack complex of complement on nucleated cells. Curr Top Microbiol Immunol. 1992;178:115–140. doi: 10.1007/978-3-642-77014-2_8. [DOI] [PubMed] [Google Scholar]
- Morgan BP. Regulation of the complement membrane attack pathway. Crit Rev Immunol. 1999;19(3):173–198. [PubMed] [Google Scholar]
- Morgan J, Spendlove I, Durrant LG. The role of CD55 in protecting the tumour environment from complement attack. Tissue Antigens. 2002;60(3):213–223. doi: 10.1034/j.1399-0039.2002.600303.x. [DOI] [PubMed] [Google Scholar]
- Morris KM, Aden DP, Knowles BB, et al. Complement biosynthesis by the human hepatoma-derived cell line HepG2. J Clin Invest. 1982;70(4):906–913. doi: 10.1172/JCI110687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovich O, Fishelson Z. Live cell imaging of outward and inward vesiculation induced by the complement c5b-9 complex. J Biol Chem. 2007;282(41):29977–29986. doi: 10.1074/jbc.M703742200. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Pasinetti GM. Complement anaphylatoxin C5a neuroprotects through mitogen-activated protein kinase-dependent inhibition of caspase 3. J Neurochem. 2001;77(1):43–49. doi: 10.1046/j.1471-4159.2001.00167.x. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Thomas S, Pasinetti GM. Complement anaphylatoxin C5a neuroprotects through regulation of glutamate receptor subunit 2 in vitro and in vivo. J Neuroinflammation. 2008;5:5. doi: 10.1186/1742-2094-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa T, Klonisch T, Hombach-Klonisch S, et al. Expression of CD97 and CD55 in human medullary thyroid carcinomas. Int J Oncol. 2004;24(2):285–294. [PubMed] [Google Scholar]
- Nagajothi N, Matsui WH, Mukhina GL, et al. Enhanced cytotoxicity of rituximab following genetic and biochemical disruption of glycosylphosphatidylinositol anchored proteins. Leuk Lymphoma. 2004;45(4):795–799. doi: 10.1080/10428190310001625700. [DOI] [PubMed] [Google Scholar]
- Nasu J, Mizuno M, Uesu T, et al. Cytokine-stimulated release of decay-accelerating factor (DAF;CD55) from HT-29 human intestinal epithelial cells. Clin Exp Immunol. 1998;113(3):379–385. doi: 10.1046/j.1365-2249.1998.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta AJ, Trouw LA, Daha MR, et al. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur J Immunol. 2002;32(6):1726–1736. doi: 10.1002/1521-4141(200206)32:6<1726::AID-IMMU1726>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Niculescu F, Rus HG, Retegan M, et al. Persistent complement activation on tumor cells in breast cancer. Am J Pathol. 1992;140(5):1039–1043. [PMC free article] [PubMed] [Google Scholar]
- Niculescu F, Rus H, van Biesen T, et al. Activation of Ras and mitogen-activated protein kinase pathway by terminal complement complexes is G protein dependent. J Immunol. 1997;158(9):4405–4412. [PubMed] [Google Scholar]
- Niculescu F, Badea T, Rus H. Sublytic C5b-9 induces proliferation of human aortic smooth muscle cells: role of mitogen activated protein kinase and phosphatidylinositol 3-kinase. Atherosclerosis. 1999;142(1):47–56. doi: 10.1016/s0021-9150(98)00185-3. [DOI] [PubMed] [Google Scholar]
- Niehans GA, Cherwitz DL, Staley NA, et al. Human carcinomas variably express the complement inhibitory proteins CD46 (membrane cofactor protein), CD55 (decay-accelerating factor), and CD59 (protectin). Am J Pathol. 1996;149(1):129–142. [PMC free article] [PubMed] [Google Scholar]
- Nishioka K, Kawamura K, Hirayama T, et al. The complement system in tumor immunity: significance of elevated levels of complement in tumor bearing hosts. Ann N Y Acad Sci. 1976;276:303–315. doi: 10.1111/j.1749-6632.1976.tb41656.x. [DOI] [PubMed] [Google Scholar]
- Nolte-'t Hoen EN, Almeida CR, Cohen NR, et al. Increased surveillance of cells in mitosis by human NK cells suggests a novel strategy for limiting tumor growth and viral replication. Blood. 2007;109(2):670–673. doi: 10.1182/blood-2006-07-036509. [DOI] [PubMed] [Google Scholar]
- Nozaki M, Raisler BJ, Sakurai E, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A. 2006;103(7):2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-Cruz S, Gimotty PA, Guerra MW, et al. Genetic and pharmacologic inhibition of complement impairs endothelial cell function and ablates ovarian cancer neovascularization. Neoplasia. 2012;14(11):994–1004. doi: 10.1593/neo.121262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Hirata T, Enomoto M, et al. A putative chemoattractant receptor, C5L2, is expressed in granulocyte and immature dendritic cells, but not in mature dendritic cells. Mol Immunol. 2000;37(8):407–412. doi: 10.1016/s0161-5890(00)00067-5. [DOI] [PubMed] [Google Scholar]
- Okroj M, Hsu YF, Ajona D, et al. Non-small cell lung cancer cells produce a functional set of complement factor I and its soluble cofactors. Mol Immunol. 2008;45(1):169–179. doi: 10.1016/j.molimm.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Okroj M, Corrales L, Stokowska A, et al. Hypoxia increases susceptibility of non-small cell lung cancer cells to complement attack. Cancer Immunol Immunother. 2009;58(11):1771–1780. doi: 10.1007/s00262-009-0685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollert MW, Frade R, Fiandino A, et al. C3-Cleaving membrane proteinase. A new complement regulatory protein of human melanoma cells. J Immunol. 1990;144(10):3862–3867. [PubMed] [Google Scholar]
- Ollert MW, David K, Bredehorst R, et al. Classical complement pathway activation on nucleated cells. Role of factor H in the control of deposited C3b. J Immunol. 1995;155(10):4955–4962. [PubMed] [Google Scholar]
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182(8):4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paas Y, Bohana-Kashtan O, Fishelson Z. Phosphorylation of the complement component, C9, by an ecto-protein kinase of human leukemic cells. Immunopharmacology. 1999;42(1–3):175–185. doi: 10.1016/s0162-3109(99)00027-2. [DOI] [PubMed] [Google Scholar]
- Pangburn MK, Rawal N. Structure and function of complement C5 convertase enzymes. Biochem Soc Trans. 2002;30(Pt 6):1006–1010. doi: 10.1042/bst0301006. [DOI] [PubMed] [Google Scholar]
- Pangburn MK, Schreiber RD, Muller-Eberhard HJ. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J Exp Med. 1981;154(3):856–867. doi: 10.1084/jem.154.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- Peterson AC, Harlin H, Gajewski TF. Immunization with melan-a peptide-pulsed peripheral blood mononuclear cells plus recombinant human interleukin-12 induces clinical activity and T-cell responses in advanced melanoma. J Clin Oncol. 2003;21(12):2342–2348. doi: 10.1200/JCO.2003.12.144. [DOI] [PubMed] [Google Scholar]
- Podack ER, Muller-Eberhard HJ. Isolation of human S-protein, an inhibitor of the membrane attack complex of complement. J Biol Chem. 1979;254(19):9808–9814. [PubMed] [Google Scholar]
- Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res. 2012;4(4):376–389. [PMC free article] [PubMed] [Google Scholar]
- Ragupathi G, Liu NX, Musselli C, et al. Antibodies against tumor cell glycolipids and proteins, but not mucins, mediate complement-dependent cytotoxicity. J Immunol. 2005;174(9):5706–5712. doi: 10.4049/jimmunol.174.9.5706. [DOI] [PubMed] [Google Scholar]
- Reiter Y, Fishelson Z. Targeting of complement to tumor cells by heteroconjugates composed of antibodies and of the complement component C3b. J Immunol. 1989;142(8):2771–2777. [PubMed] [Google Scholar]
- Ricklin D, Hajishengallis G, Yang K, et al. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SJ, Ng BY, Filler RB, et al. Characterizing tumor-promoting T cells in chemically induced cutaneous carcinogenesis. Proc Natl Acad Sci U S A. 2007;104(16):6770–6775. doi: 10.1073/pnas.0604982104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanov DV, Savinov AY, Golubkov VS, et al. Interference with the complement system by tumor cell membrane type-1 matrix metalloproteinase plays a significant role in promoting metastasis in mice. Cancer Res. 2006;66(12):6258–6263. doi: 10.1158/0008-5472.CAN-06-0539. [DOI] [PubMed] [Google Scholar]
- Rushmere NK, Knowlden JM, Gee JM, et al. Analysis of the level of mRNA expression of the membrane regulators of complement, CD59, CD55 and CD46, in breast cancer. Int J Cancer. 2004;108(6):930–936. doi: 10.1002/ijc.11606. [DOI] [PubMed] [Google Scholar]
- Rutkowski MJ, Sughrue ME, Kane AJ, et al. Cancer and the complement cascade. Mol Cancer Res. 2010a;8(11):1453–1465. doi: 10.1158/1541-7786.MCR-10-0225. [DOI] [PubMed] [Google Scholar]
- Rutkowski MJ, Sughrue ME, Kane AJ, et al. The complement cascade as a mediator of tissue growth and regeneration. Inflamm Res. 2010b;59(11):897–905. doi: 10.1007/s00011-010-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadallah S, Lach E, Schwarz S, et al. Soluble complement receptor 1 is increased in patients with leukemia and after administration of granulocyte colony-stimulating factor. J Leukoc Biol. 1999;65(1):94–101. doi: 10.1002/jlb.65.1.94. [DOI] [PubMed] [Google Scholar]
- Schlesinger M, Broman I, Lugassy G. The complement system is defective in chronic lymphatic leukemia patients and in their healthy relatives. Leukemia. 1996;10(9):1509–1513. [PubMed] [Google Scholar]
- Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5(2):147–159. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- Schraufstatter IU, Trieu K, Sikora L, et al. Complement c3a and c5a induce different signal transduction cascades in endothelial cells. J Immunol. 2002;169(4):2102–2110. doi: 10.4049/jimmunol.169.4.2102. [DOI] [PubMed] [Google Scholar]
- Schraufstatter IU, Discipio RG, Zhao M, et al. C3a and C5a are chemotactic factors for human mesenchymal stem cells, which cause prolonged ERK1/2 phosphorylation. J Immunol. 2009;182(6):3827–3836. doi: 10.4049/jimmunol.0803055. [DOI] [PubMed] [Google Scholar]
- Schuster M, Umana P, Ferrara C, et al. Improved effector functions of a therapeutic monoclonal Lewis Y-specific antibody by glycoform engineering. Cancer Res. 2005;65(17):7934–7941. doi: 10.1158/0008-5472.CAN-04-4212. [DOI] [PubMed] [Google Scholar]
- Selander B, Martensson U, Weintraub A, et al. Mannan-binding lectin activates C3 and the alternative complement pathway without involvement of C2. J Clin Invest. 2006;116(5):1425–1434. doi: 10.1172/JCI25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seya T, Hara T, Iwata K, et al. Purification and functional properties of soluble forms of membrane cofactor protein (CD46) of complement: identification of forms increased in cancer patients’ sera. Int Immunol. 1995;7(5):727–736. doi: 10.1093/intimm/7.5.727. [DOI] [PubMed] [Google Scholar]
- Shaw PX, Zhang L, Zhang M, et al. Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids. Proc Natl Acad Sci U S A. 2012;109(34):13757–13762. doi: 10.1073/pnas.1121309109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi XX, Zhang B, Zang JL, et al. CD59 silencing via retrovirus-mediated RNA interference enhanced complement-mediated cell damage in ovary cancer. Cell Mol Immunol. 2009;6(1):61–66. doi: 10.1038/cmi.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Nakata M, Hirami Y, et al. Tumor-infiltrating Foxp3+ regulatory T cells are correlated with cyclooxygenase-2 expression and are associated with recurrence in resected non-small cell lung cancer. J Thorac Oncol. 2010;5(5):585–590. doi: 10.1097/JTO.0b013e3181d60fd7. [DOI] [PubMed] [Google Scholar]
- Sim RB, Day AJ, Moffatt BE, et al. Complement factor I and cofactors in control of complement system convertase enzymes. Methods Enzymol. 1993;223:13–35. doi: 10.1016/0076-6879(93)23035-l. [DOI] [PubMed] [Google Scholar]
- Sjoberg AP, Manderson GA, Morgelin M, et al. Short leucine-rich glycoproteins of the extra-cellular matrix display diverse patterns of complement interaction and activation. Mol Immunol. 2009;46(5):830–839. doi: 10.1016/j.molimm.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soane L, Cho HJ, Niculescu F, et al. C5b-9 Terminal complement complex protects oligodendrocytes from death by regulating Bad through phosphatidylinositol 3-kinase/Akt pathway. J Immunol. 2001;167(4):2305–2311. doi: 10.4049/jimmunol.167.4.2305. [DOI] [PubMed] [Google Scholar]
- Spiridon CI, Ghetie MA, Uhr J, et al. Targeting multiple Her-2 epitopes with monoclonal antibodies results in improved antigrowth activity of a human breast cancer cell line in vitro and in vivo. Clin Cancer Res. 2002;8(6):1720–1730. [PubMed] [Google Scholar]
- Strey CW, Markiewski M, Mastellos D, et al. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J Exp Med. 2003;198(6):913–923. doi: 10.1084/jem.20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Lu B, Hatch E, et al. C3a Mediates epithelial-to-mesenchymal transition in protein-uric nephropathy. J Am Soc Nephrol. 2009;20(3):593–603. doi: 10.1681/ASN.2008040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RP, Ferguson PJ, Martin EN, et al. Immune complexes bound to the primate erythrocyte complement receptor (CR1) via anti-CR1 mAbs are cleared simultaneously with loss of CR1 in a concerted reaction in a rhesus monkey model. Clin Immunol Immunopathol. 1997;82(1):49–59. doi: 10.1006/clin.1996.4286. [DOI] [PubMed] [Google Scholar]
- Tegla CA, Cudrici C, Patel S, et al. Membrane attack by complement: the assembly and biology of terminal complement complexes. Immunol Res. 2011;51(1):45–60. doi: 10.1007/s12026-011-8239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terui Y, Sakurai T, Mishima Y, et al. Blockade of bulky lymphoma-associated CD55 expression by RNA interference overcomes resistance to complement-dependent cytotoxicity with rituximab. Cancer Sci. 2006;97(1):72–79. doi: 10.1111/j.1349-7006.2006.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos AN, Perrin LH. Binding of components of the properdin system to cultured human lymphoblastoid cells and B lymphocytes. J Exp Med. 1976;143(2):271–289. doi: 10.1084/jem.143.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel S. Complement activating soluble pattern recognition molecules with collagen-like regions, mannan-binding lectin, ficolins and associated proteins. Mol Immunol. 2007;44(16):3875–3888. doi: 10.1016/j.molimm.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Thornton AM, Shevach EM. CD4+CD25+ Immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188(2):287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trougakos IP, Gonos ES. Clusterin/apolipoprotein J in human aging and cancer. Int J Biochem Cell Biol. 2002;34(11):1430–1448. doi: 10.1016/s1357-2725(02)00041-9. [DOI] [PubMed] [Google Scholar]
- Umansky V, Sevko A. Tumor microenvironment and myeloid-derived suppressor cells. Cancer Microenviron. 2013;6(2):169–177. doi: 10.1007/s12307-012-0126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lookeren CM, Wiesmann C, Brown EJ. Macrophage complement receptors and pathogen clearance. Cell Microbiol. 2007;9(9):2095–2102. doi: 10.1111/j.1462-5822.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- Varga L, Czink E, Miszlai Z, et al. Low activity of the classical complement pathway predicts short survival of patients with chronic lymphocytic leukaemia. Clin Exp Immunol. 1995;99(1):112–116. doi: 10.1111/j.1365-2249.1995.tb03480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varsano S, Frolkis I, Rashkovsky L, et al. Protection of human nasal respiratory epithelium from complement-mediated lysis by cell-membrane regulators of complement activation. Am J Respir Cell Mol Biol. 1996;15(6):731–737. doi: 10.1165/ajrcmb.15.6.8969267. [DOI] [PubMed] [Google Scholar]
- Varsano S, Rashkovsky L, Shapiro H, et al. Human lung cancer cell lines express cell membrane complement Inhibitory proteins and are extremely resistant to complement-mediated lysis; a comparison with normal human respiratory epithelium in vitro, and an insight into mechanism(s) of resistance. Clin Exp Immunol. 1998;113(2):173–182. doi: 10.1046/j.1365-2249.1998.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaicu SI, Tegla CA, Cudrici CD, et al. Role of C5b-9 complement complex and response gene to complement-32 (RGC-32) in cancer. Immunol Res. 2013;56(1):109–121. doi: 10.1007/s12026-012-8381-8. [DOI] [PubMed] [Google Scholar]
- Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344(14):1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- Watson NF, Durrant LG, Madjd Z, et al. Expression of the membrane complement regulatory protein CD59 (protectin) is associated with reduced survival in colorectal cancer patients. Cancer Immunol Immunother. 2006;55(8):973–980. doi: 10.1007/s00262-005-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10(5):317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Jung M, Burkhardt M, et al. Increased CD59 protein expression predicts a PSA relapse in patients after radical prostatectomy. Prostate. 2005;62(3):224–232. doi: 10.1002/pros.20134. [DOI] [PubMed] [Google Scholar]
- Yamakawa M, Yamada K, Tsuge T, et al. Protection of thyroid cancer cells by complement-regulatory factors. Cancer. 1994;73(11):2808–2817. doi: 10.1002/1097-0142(19940601)73:11<2808::aid-cncr2820731125>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Yan J, Allendorf DJ, Li B, et al. The role of membrane complement regulatory proteins in cancer immunotherapy. Adv Exp Med Biol. 2008;632:159–174. [PubMed] [Google Scholar]
- Yefenof E, Zehavi-Feferman R, Guy R. Control of primary and secondary antibody responses by cytotoxic T lymphocytes specific for a soluble antigen. Eur J Immunol. 1990;20(8):1849–1853. doi: 10.1002/eji.1830200833. [DOI] [PubMed] [Google Scholar]
- Ying SC, Gewurz AT, Jiang H, et al. Human serum amyloid P component oligomers bind and activate the classical complement pathway via residues 14–26 and 76–92 of the a chain collagen- like region of C1q. J Immunol. 1993;150(1):169–176. [PubMed] [Google Scholar]
- Ytting H, Jensenius JC, Christensen IJ, et al. Increased activity of the mannan-binding lectin complement activation pathway in patients with colorectal cancer. Scand J Gastroenterol. 2004;39(7):674–679. doi: 10.1080/00365520410005603. [DOI] [PubMed] [Google Scholar]
- Ytting H, Christensen IJ, Thiel S, et al. Serum mannan-binding lectin-associated serine protease 2 levels in colorectal cancer: relation to recurrence and mortality. Clin Cancer Res. 2005;11(4):1441–1446. doi: 10.1158/1078-0432.CCR-04-1272. [DOI] [PubMed] [Google Scholar]
- Zhang M, Takahashi K, Alicot EM, et al. Activation of the lectin pathway by natural IgM in a model of ischemia/reperfusion injury. J Immunol. 2006;177(7):4727–4734. doi: 10.4049/jimmunol.177.7.4727. [DOI] [PubMed] [Google Scholar]
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- Zurlo JJ, Schechter GP, Fries LF. Complement abnormalities in multiple myeloma. Am J Med. 1989;87(4):411–420. doi: 10.1016/s0002-9343(89)80824-1. [DOI] [PubMed] [Google Scholar]