Abstract
Antifungal prophylaxis with azoles is considered standard in allogeneic hematopoietic stem-cell transplant (allo-HCT). Although sirolimus is being used increasingly for prevention of graft-versus-host disease (GVHD), it is a substrate of CYP3A4, which is inhibited by voriconazole, and concurrent administration can lead to significantly increased exposure to sirolimus. We identified 67 patients with hematologic malignancies who underwent allo-HCT with sirolimus, tacrolimus, and low-dose methotrexate and received concomitant voriconazole prophylaxis from April-2008 to June-2011. All patients underwent a non-myeloablative or reduced-intensity conditioned allo-HCT. Patients received sirolimus and voriconazole concurrently for a median of 113 days. The median daily dose reduction of sirolimus at start of coadministration was 90%. The median serum sirolimus trough-level before and at steady-state of coadministration were 5.8ng/mL (range 0-47.6) and 6.1ng/mL (range 1-14.2) (p=0.45), respectively. One patient with an average sirolimus level of 6 ng/mL developed sirolimus-related thrombotic microangiopathy that resolved after sirolimus discontinuation. No sinusoidal-obstructive syndrome was reported. Seventeen patients (25%) prematurely discontinued voriconazole because of adverse events. Only 2 patients (3%) presented with possible IFI at day100. We demonstrate that sirolimus and voriconazole coadministration with an empiric 90% sirolimus dose-reduction and close monitoring of sirolimus trough levels is safe and well tolerated.
Keywords: Sirolimus, Voriconazole, Tacrolimus, Drug interaction
Introduction
Invasive fungal infections (IFI) remain a significant cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (allo-HCT) (1, 2), and antifungal prophylaxis with azoles is considered standard in allo-H C T. The epidemiology of IFIs has changed in the last two decades (3, 4), and recent data show that 60-70% of IFIs in HCT patients are caused by invasive Aspergillus (IA) (2, 5, 6). Voriconazole has a broad in vitro spectrum against yeast and moulds and is used for fungal prophylaxis after allo-HCT (7-9). Sirolimus, an mTOR inhibitor that was initially developed as an antifungal agent (10), is increasingly used for prevention of graft-versus-host disease (GVHD) (11-14). However, there are significant drug interactions between voriconazole and sirolimus. Voriconazole is a substrate and inhibitor of cytochrome P450 (CYP) 2C19 (CYP2C19), CYP2C9 and CYP3A4 isoenzymes (15), and tacrolimus and sirolimus are both substrates of CYP3A4. Concomitant use with voriconazole can lead to significantly increased exposure to these drugs and contribute to increased adverse events (16, 17).
Data on sirolimus and voriconazole coadministration are scarce (18, 19), and the manufacturer of voriconazole contraindicates coadministration with sirolimus given reports that this can lead to significantly increased systemic exposure to sirolimus (Product Information: Vfend. New York: Pfizer). We now report our results in 67 patients who were treated with both drugs and demonstrate that coadministration of sirolimus and voriconazole with an empiric 90% dose reduction of sirolimus and close monitoring of sirolimus trough levels is safe and well tolerated.
Patients and methods
Patient characteristics
Sixty-seven consecutive patients with hematologic malignancies received voriconazole prophylaxis after a non-myeloablative (NMA) or reduced intensity conditioning (RIC) allo-HCT with the combination of sirolimus/tacrolimus with low-dose methotrexate for GVHD prophylaxis at a single institution (Memorial Sloan Kettering Cancer Center) between April 2008 and June 2011. Patients were included if they received at least one dose of voriconazole and sirolimus concurrently for > 1 day. Sixty-one of the patients in this study were included in our recent report that specifically looked at GVHD outcomes in patients who received tacrolimus, sirolimus and mini-methotrexate as GVHD prophylaxis (14). Written informed consent for treatment was obtained from all patients. Approval for this retrospective review was obtained from the Institutional Review and Privacy Board.
Transplant procedure and supportive care
All patients received a lower-intensity conditioning regimen categorized as RIC or NMA using established consensus criteria (20). GVHD prophylaxis consisted of sirolimus and tacrolimus that were started on day -3, followed by methotrexate 5 mg/m2 on days +1, 3, 6. Doses were adjusted to maintain target serum trough levels of 3-12 ng/mL and 5-10 ng/mL for sirolimus and tacrolimus, respectively. Recipients of MUD (n=26) or MMUD (n=8) grafts were given 2 and 3 doses, respectively of anti-thymocyte globulin (ATG). All patients received supportive care and prophylaxis against opportunistic infections in accordance with standard guidelines. Patients remained on micafungin (150 mg/day) during the cytoreduction and until voriconazole initiation, which typically occurred in the first week after HCT. Voriconazole was administered intravenously at a dose of 6 mg/kg every 12h for 2 doses, then 4 mg/kg every 12h, followed by oral voriconazole 200 mg every 12h until at least day +75 or cessation of intensive immunosuppression. This antifungal prophylaxis is the standard treatment for all recipients of allografts at our center, regardless of baseline risk for IFI. Patients with GVHD or on corticosteroids therapy also received voriconazole until discontinuation of immunosuppression. Patients with a history of intolerance to voriconazole or a prior IFI resistant to voriconazole, received alternative antifungal prophylaxis (micafungin and/or posaconazole) and were excluded from the analysis. Sirolimus and tacrolimus doses were reduced by 90% and 67%, respectively when voriconazole was initiated. Trough sirolimus and tacrolimus levels were drawn daily starting day −1 and then at least weekly once a therapeutic level was achieved. There was no routine drug monitoring of voriconazole.
Data collection
Analyses were performed as of December 31, 2011. Sirolimus and tacrolimus dose before voriconazole initiation, dose adjustment at the time of voriconazole initiation, and the steady-state dose during coadministration were captured from day 0 to day 28 after transplant. Levels that were clearly documented as being obtained inadvertently after administration of the drug (peak levels) were excluded from the analysis. Any concomitant medications that might interact with sirolimus, tacrolimus or voriconazole were recorded. Measurements of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (Alk Phos), serum creatinine, and QT intervals were recorded at baseline and during coadministration of voriconazole and sirolimus. AST, ALT, or Alk Phos levels > 3 times the upper limit of normal or > 3 times baseline if abnormal at baseline were considered clinically significant. Acute renal failure was defined as serum creatinine concentration > 2mg/dL or a 50% increase in serum creatinine if the baseline value was abnormal. Clinical diagnosis of post-transplant thrombotic microangiopathy (TMA) was established according to previously defined international criteria (21). GVHD was diagnosed clinically, confirmed pathologically whenever possible, and classified according to standard criteria (22). Patients who engrafted were evaluable for acute GVHD
IFI risk stratification and diagnosis
Patients with recent neutropenia (<0.5 × 109 neutrophils/L for >10 days), prior history of IFI, prolonged use of steroids (minimum dose of 0.3 mg/kg/day of prednisone or equivalent for >3 weeks), treatment with other T cell immunosuppressants (alemtuzumab, nucleoside analogues) during the past 90 days, were considered as high risk for IFI pre-HSCT (23). The revised definition of IFI was used as proposed by the Cooperative Group of the European Organization for Research and Treatment of Cancer and Mycoses Study Group of the National Institute of Allergy and Infectious Diseases (EORTC/MSG) (24).
Statistical analysis
Descriptive statistics were used to summarize patient characteristics and safety outcomes. A Wilcoxon signed-rank test was used to compare sirolimus and tacrolimus trough levels before and during voriconazole coadministration to account for the paired observations. Similarly, the difference between baseline AST, ALT, Alk Phos and serum creatinine values and peak values during voriconazole coadministration were compared using the signed-rank test and graphically displayed using boxplots. A lowess local regression curve was used to visualize the trend of sirolimus concentrations from the time of transplant to day 28. The incidence of acute GVHD was estimated using the cumulative incidence function, treating relapse and death unrelated to GVHD as competing events. All statistical analyses were done in R (version 2.13.2).
Results
Patient characteristics
Patient characteristics are shown in Table 1. The median age at transplant was 52 years (range: 23 to 69 years). The majority of patients were treated for lymphoid malignancies, with 61% of the patients having non-Hodgkin's lymphoma. Conditioning regimen was NMA in 52 patients (78%) and RIC in 15 patients (22%). All patients except one received peripheral blood stem cells. Twenty-six patients (39%) received a transplant from an HLA-identical sibling donor while the remaining 41 patients received unrelated donor transplantation (10/10 matched in 33 patients, and 9/10 matched in 8 patients). Ninety percent of patients were considered at low risk for IFI pre-HCT.
Table 1. Patient Characteristics.
| Patient Characteristics | N (%) |
|---|---|
| Sample size | 67 |
| Patient median age, y (range) | 52 (23-69) |
| Disease at transplantation | |
| Myelodysplastic syndrome | 1 (1) |
| Acute lymphoid leukemia | 2 (3) |
| Chronic lymphoid leukemia/SLL | 15 (22) |
| Non-Hodgkin lymphoma | 41 (61) |
| Hodgkin lymphoma | 8 (12) |
| Remission status at time of transplant | |
| CR | 34 (51) |
| Active disease | 33 (49) |
| Sex | |
| Male | 43 (64) |
| Female | 24 (36) |
| Donor Type | |
| HLA-identical related | 26 (39) |
| HLA-matched unrelated | 33 (49) |
| HLA-mismatched unrelated | 8 (12) |
| Conditioning regimen | |
| Non myeloablative | 52 (78) |
| Reduced intensity | 15 (22) |
| Source of stem cells | |
| Bone marrow | 1 (1) |
| Mobilized blood | 66 (99) |
| IFI Risk at Transplant | |
| High Risk | 7 (10) |
| Low Risk | 60 (90) |
Study drugs and coadministration parameters
Sirolimus and voriconazole treatment characteristics are summarized in Table 2. All patients received voriconazole for IFI prophylaxis and sirolimus for GVHD prophylaxis. The median day of voriconazole initiation was 8 days after HCT (range: 2-27 days). Patients received voriconazole concurrently with tacrolimus and sirolimus for a median of 113 days (interquartile range (IQR), 13-35; range, 4-1342).
Table 2. Sirolimus Treatment.
| Characteristics | N (%) |
|---|---|
| Indication for voriconazole | |
| IFI Prophylaxis | 61 (91) |
| IFI prophylaxis in setting of GVHD | 6 (9) |
| Day of voriconazole initiation (day, range) | 8 (2-27) |
| Duration of coadministration, days (days, range) | 113 (4-1342) |
| 0-100 days | 43 (64) |
| 100-200 days | 16 (24) |
| 200-300 days | 6 (9) |
| 300-1000 days | 1 (1) |
| >1000 days | 1 (1) |
| Concomitant tacrolimus administration | 67 (100) |
At voriconazole initiation, the daily dose of sirolimus was empirically reduced by a median of 90% (range: 80-92). By the time of the steady state of coadministration, the dose of sirolimus had been reduced by a median of 84% (range: 0-100) from the originally administered dose. Twenty-two patients (33%) required an increase of the sirolimus dose after the initial dose reduction. The median daily dose of sirolimus before voriconazole initiation was 4.6 mg (range: 1-12 mg), with 91% of the patients receiving a sirolimus dose ≥ 4 mg/day. The median sirolimus doses at start of coadministration and at steady state were 0.5 mg (range: 0.1-1.2 mg) and 0.6 mg (range: 0-2 mg), respectively. The median serum sirolimus trough level before voriconazole coadministration was 5.8 ng/mL (range: 0-47.6 ng/mL), which was not different from the level at steady sate of coadministration (6.1 ng/mL, range 1-14.2 ng/mL; p=0.45) (Table 3a).
Table 3a. Sirolimus Coadministration parameters.
| Drug Parameter | Before Coadministration (range) | At start of Coadministration (range) | At steady state of Coadministration (range) |
|---|---|---|---|
| Median daily sirolimus dose, mg | 4.6 (1-12) | 0.5 (0.1-1.2) | 0.6 (0-2) |
| Median daily sirolimus dose reduction, % | 90 (80-92) | 84 (0-100) | |
| Median serum sirolimus trough level, ng/mL | 5.8 (0-47.6) | 6.1 (1-14.2) |
The median daily dose reductions of tacrolimus at initiation and steady state of coadministration were 64% (range: 33-100) and 51% (range: 0-100), respectively (Table 3b). The median daily doses of tacrolimus before, at start and steady state of coadministration were 1.3 mg (range: 0.5-4.7 mg), 0.4 mg (range: 0-2.5 mg) and 0.6 mg (range: 0-5 mg), respectively. The median tacrolimus trough level before voriconazole administration was higher than the level after voriconazole coadministration (10.9 vs. 7.4 ng/mL, p<0.001).
Table 3b. Tacrolimus Coadministration parameters.
| Drug Parameter | Before Coadministration (range) | At start of Coadministration (range) | At steady state of Coadministration (range) |
|---|---|---|---|
| Median daily tacrolimus dose, mg | 1.3 (0.5-4.7) | 0.5 (0-2.5) | 0.6 (0-5) |
| Median daily tacrolimus dose reduction, % | 64 (33-100) | 51 (0-100) | |
| Median serum tacrolimus trough level, ng/mL | 10.9 (0-30) | 7.4 (2-24.2) |
Safety and breakthrough invasive fungal infection
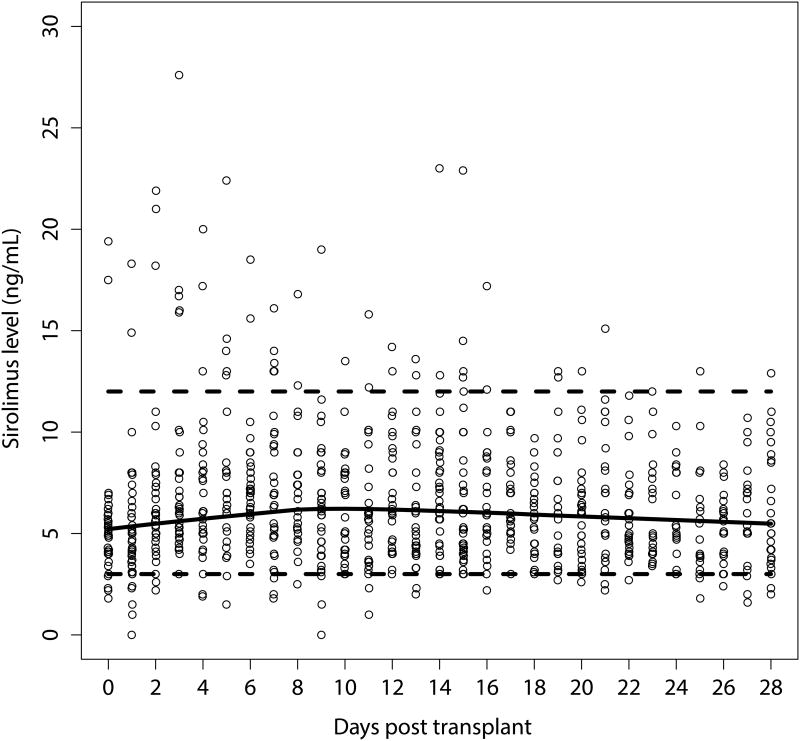
The majority of the patients tolerated sirolimus-voriconazole coadministration well and did not experience sirolimus-related adverse events. A total of 838 sirolimus blood levels were drawn over the study period (median 12/patient; range, 9-21). The sirolimus blood levels were relatively stable within the therapeutic range (3 – 12 ng/mL): 40 measurements (4.8%) from 24 patients were sub-therapeutic and 52 measurements (6.2%) from 17 patients were above the therapeutic target, occurring early in the course of prophylaxis (Figure 1). Clinically significant changes in the median serum creatinine levels and LFTs, when baseline values were compared with peak values during voriconazole coadministration (Figure 2), were noted in a minority of patients. The median serum creatinine was 0.8 mg/dL (range 0.3 – 2.3) at baseline, and increased to 1.3 mg/dL (range 0.7 – 4.2) at peak values. Seven patients (10%) developed renal toxicity attributable to tacrolimus, 4 of them requiring modification of the immunosuppressant treatment (tacrolimus to MMF in 3 and to MMF plus steroids in 1, who could not receive full dose methotrexate). Three of the 7 patients with tacrolimus-related renal toxicity had at least one serum level of tacrolimus above 10 ng/mL, but none had a serum level above 15 ng/mL during coadministration. One patient with an average sirolimus level of 6 ng/mL developed sirolimus-related TMA that resolved after changing the immunosuppression regimen (mycophenolate mofetil [MMF] instead of sirolimus). An additional 4 patients had at least a doubling of their baseline creatinine with a peak serum creatinine that ranged from 1.5 to 2.0 mg/dL at peak level. These patients were able to continue on their planned prophylaxis. Overall, 14 patients (21%) developed clinically significant hepatotoxicity while on voriconazole prophylaxis. No cases of SOS were observed.
Figure 1.
Scatter plot of all sirolimus concentrations, with a lowess local regression curve (red). The suggested therapeutic range of 3-12 ng/mL and the median concentration is outlined for reference.
Figure 2.
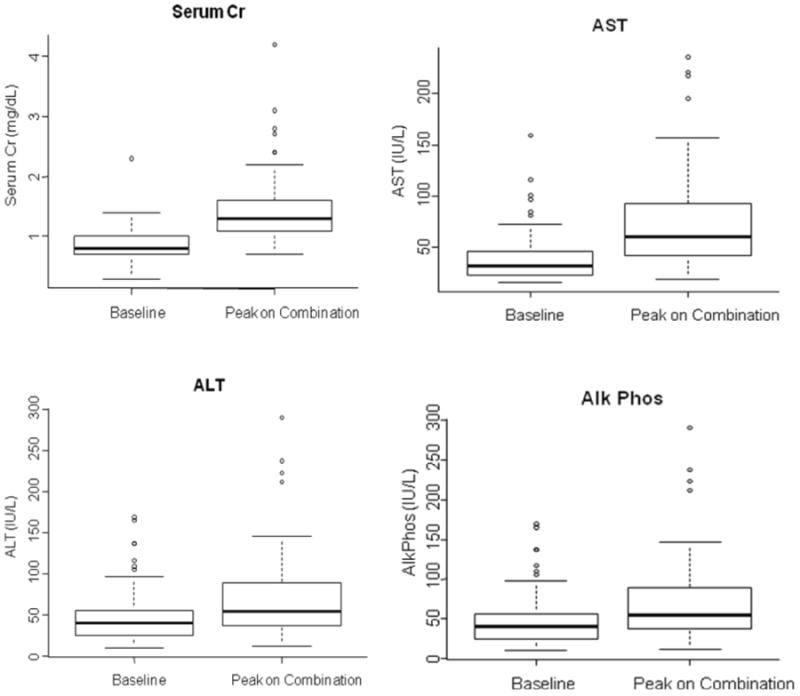
Boxplots that illustrate the difference in serum creatinine (2.A), ALT (2.B), AST (2.C) and Alk Phos (AP) (2.D) at baseline and during voriconazole coadministration. The squares represent the interquartile range (IQR). Capped whiskers represent the upper and lower adjacent values.
Seventeen patients (25%) prematurely discontinued voriconazole after a median of 28 days (range: 8-51 days) because of adverse events: ten (15%) had elevated LFTs (12% clinically significant), two (3%) QT-interval prolongation, two (3%) visual disturbances, one (1.5%) rash and two (3%) other adverse events.
One patient with low risk for IFI pre-HCT and one with high risk presented with possible IFI at day 100. The first patient was being treated with steroids for GVHD and was diagnosed with IFI while on micafungin due to intolerance to azoles.
Graft-versus-host disease
The cumulative incidence (95% CI) of grade 2-4 acute GVHD at day 100 was 0.134 (0.066, 0.228), with one patient developing grade 3 (skin) and no patients having grade 4. Among the 9 patients with acute GVHD prior to day 100, 4 received systemic steroids and 3 were treated with budesonide for grade 2 gastrointestinal GVHD. The other 2 were treated with topical steroids for skin only GVHD.
Discussion
Despite a label contraindication to sirolimus and voriconazole coadministration and limited data, this combination is increasingly used by clinicians. We now report our experience in 67 patients who received sirolimus and voriconazole concomitantly after allo-HCT, and show that this combination can be safely used with appropriate adjustments and monitoring. Furthermore, it should be noted that all the patients also received concurrent tacrolimus. A median empirical reduction of sirolimus by 90% and tacrolimus by 64%, and close monitoring of drug concentrations resulted in trough sirolimus and tacrolimus levels similar to those obtained before voriconazole coadministration. The exposure to concomitant sirolimus and voriconazole was prolonged, with a median of 113 days, and was overall well tolerated. None of the 17 patients with an elevated sirolimus trough level (> 12 ng/mL) had sirolimus-related adverse events. TMA was observed in a single patient, who had therapeutic sirolimus levels at the time, and in whom sirolimus discontinuation led to resolution without further sequelae. No patient developed SOS or sirolimus-related renal toxicity. Seven patients with tacrolimus levels < 15 ng/mL experienced renal insufficiency possibly attributable to tacrolimus.
Coadministration of sirolimus and voriconazole was initially contraindicated, based on a study among healthy volunteers showing that oral voriconazole causes an 11-fold increase in the area under the curve (AUC) and a 7-fold increase in the sirolimus Cmax. After some case reports of concomitant use of sirolimus and voriconazole in renal transplant recipients (25, 26), two small series reported safe coadministration in HCT patients (18, 19). In the study by Marty et al. (19), 11 HCT recipients were safely treated with voriconazole after an empiric initial 90% sirolimus dose reduction along with careful monitoring of sirolimus trough levels. Another study of 23 patients, that included 16 HCT recipients, also reported that sirolimus and voriconazole could be safely coadministered and suggested a dose-reduction strategy based on the baseline sirolimus dose and concentration (18). The results of our study are consistent with and further expand the results of these prior reports.
A number of reports have addressed interactions between tacrolimus and voriconazole, and dose reduction and frequent monitoring of tacrolimus blood concentrations to avoid toxicity are also recommended (27-30). All our patients received tacrolimus concomitantly to voriconazole and sirolimus. Tacrolimus and voriconazole coadministration was similarly well tolerated with a median 64% tacrolimus dose reduction at the start of coadministration. None of the seven patients that developed renal toxicity possibly attributed to tacrolimus had tacrolimus levels above 15 ng/mL. The renal toxicity resolved in 6 out of the 7 patients after tacrolimus discontinuation.
Hepatic dysfunction in allo-HCT recipients is common and may result from various confounding conditions including toxicity from the preparative regimen and other medications, infection and GVHD of the liver. A recent meta-analysis of randomized trials reported that 20% of 881 patients receiving voriconazole experienced an elevation of liver enzymes, and 12% required the cessation of voriconazole (31). We have previously reported that, in a cohort of 200 patients receiving voriconazole after allo-HCT, 25% experienced clinically significant elevations in liver enzymes and 17% of patients required voriconazole therapy discontinuation (32). Consistent with our previous report, in this cohort 12% of the patients developed clinically significant reversible hepatic toxicity, with 15% of the patients requiring cessation of voriconazole. The higher incidence of clinically significant elevation in liver enzymes in the previous study can be explained in part by the use of myeloablative conditioning in 70% of patients in that cohort.
In addition to its retrospective nature, the main limitation of the present study is the fact that we did not routinely measure voriconazole levels. Voriconazole has a nonlinear pharmacokinetic profile with wide inter and intra-individual variability, and inconsistent absorption and genetic polymorphisms in the isoenzyme CYP2C19, which primarily metabolizes voriconazole (33, 34). Variations in voriconazole concentrations can affect the CYP3A4 inhibition and result in sirolimus and tacrolimus concentration variations. Additionally, the therapeutic window of voriconazole is narrow (35, 36).
Finally, given the fact that most patients were at low risk for IFI, we cannot draw definitive conclusions regarding the efficacy of the antifungal prophylaxis in our study. As noted above, sirolimus itself has been shown to have antifungal activity. The lower risk of IFI in our study was also observed post transplant and was associated with a low incidence of GVHD and a small number of patients requiring systemic steroids. The overall risk of IFI in recipients of allografts at our institution, including patients at higher risk with acute leukemia and aplastic anemia, has historically been 5% (G. Papanicolaou, unpublished). Therefore the 2 cases of breakthrough IFI would certainly fall within that range.
This report represents the largest experience to date on the coadministration of sirolimus and voriconazole and supports its feasibility and safety. The empirical dose-reduction strategy and close serum drug monitoring ensured that sirolimus blood concentrations remain within the narrow therapeutic range, reducing toxicity and maximizing efficacy.
Acknowledgments
We gratefully acknowledge the expert care provided to our patients by the fellows, physician assistants, nurse practitioners and nurses of Memorial Sloan Kettering Cancer Center.
Footnotes
Financial disclosure: I. Ceberio received research support from Pfizer Spain. M-A Perales has served on advisory boards for Astellas and Merck.
References
- 1.Mikulska M, Raiola AM, Bruno B, Furfaro E, Van Lint MT, Bregante S, et al. Risk factors for invasive aspergillosis and related mortality in recipients of allogeneic SCT from alternative donors: an analysis of 306 patients. Bone Marrow Transplant. 2009;44(6):361–70. doi: 10.1038/bmt.2009.39. [DOI] [PubMed] [Google Scholar]
- 2.Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48(3):265–73. doi: 10.1086/595846. [DOI] [PubMed] [Google Scholar]
- 3.Lass-Florl C, Alastruey-Izquierdo A, Cuenca-Estrella M, Perkhofer S, Rodriguez-Tudela JL. In vitro activities of various antifungal drugs against Aspergillus terreus: Global assessment using the methodology of the European committee on antimicrobial susceptibility testing. Antimicrob Agents Chemother. 2009;53(2):794–5. doi: 10.1128/AAC.00335-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Ami R, Lewis RE, Kontoyiannis DP. Invasive mould infections in the setting of hematopoietic cell transplantation: current trends and new challenges. Curr Opin Infect Dis. 2009;22(4):376–84. doi: 10.1097/QCO.0b013e32832db9f3. [DOI] [PubMed] [Google Scholar]
- 5.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50(8):1091–100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 6.Kurosawa M, Yonezumi M, Hashino S, Tanaka J, Nishio M, Kaneda M, et al. Epidemiology and treatment outcome of invasive fungal infections in patients with hematological malignancies. Int J Hematol. 2012;96(6):748–57. doi: 10.1007/s12185-012-1210-y. [DOI] [PubMed] [Google Scholar]
- 7.Cecil JA, Wenzel RP. Voriconazole: a broad-spectrum triazole for the treatment of invasive fungal infections. Expert Rev Hematol. 2009;2(3):237–54. doi: 10.1586/ehm.09.13. [DOI] [PubMed] [Google Scholar]
- 8.Perfect JR, Marr KA, Walsh TJ, Greenberg RN, DuPont B, de la Torre-Cisneros J, et al. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin Infect Dis. 2003;36(9):1122–31. doi: 10.1086/374557. [DOI] [PubMed] [Google Scholar]
- 9.Troke P, Aguirrebengoa K, Arteaga C, Ellis D, Heath CH, Lutsar I, et al. Treatment of scedosporiosis with voriconazole: clinical experience with 107 patients. Antimicrob Agents Chemother. 2008;52(5):1743–50. doi: 10.1128/AAC.01388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35(3 Suppl):7S–14S. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 11.Alyea EP, Li S, Kim HT, Cutler C, Ho V, Soiffer RJ, et al. Sirolimus, tacrolimus, and low-dose methotrexate as graft-versus-host disease prophylaxis in related and unrelated donor reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2008;14(8):920–6. doi: 10.1016/j.bbmt.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho VT, Aldridge J, Kim HT, Cutler C, Koreth J, Armand P, et al. Comparison of Tacrolimus and Sirolimus (Tac/Sir) versus Tacrolimus, Sirolimus, and mini-methotrexate (Tac/Sir/MTX) as acute graft-versus-host disease prophylaxis after reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(7):844–50. doi: 10.1016/j.bbmt.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Simon JA, Martino R, Parody R, Cabrero M, Lopez-Corral L, Valcarcel D, et al. The combination of sirolimus plus tacrolimus improves outcome after reduced-intensity conditioning, unrelated donor hematopoietic stem cell transplantation compared with cyclosporine plus mycofenolate. Haematologica. 2013;98(4):526–32. doi: 10.3324/haematol.2012.065599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceberio I, Devlin SM, Sauter C, Barker JN, Castro-Malaspina H, Giralt S, et al. Sirolimus, tacrolimus and low-dose methotrexate based graft-versus-host disease prophylaxis after non-ablative or reduced intensity conditioning in related and unrelated donor allogeneic hematopoietic cell transplant. Leuk Lymphoma. 2014:1–8. doi: 10.3109/10428194.2014.930851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruggemann RJ, Alffenaar JW, Blijlevens NM, Billaud EM, Kosterink JG, Verweij PE, et al. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis. 2009;48(10):1441–58. doi: 10.1086/598327. [DOI] [PubMed] [Google Scholar]
- 16.Saad AH, DePestel DD, Carver PL. Factors influencing the magnitude and clinical significance of drug interactions between azole antifungals and select immunosuppressants. Pharmacotherapy. 2006;26(12):1730–44. doi: 10.1592/phco.26.12.1730. [DOI] [PubMed] [Google Scholar]
- 17.Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clinical pharmacokinetics. 2000;38(1):41–57. doi: 10.2165/00003088-200038010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Surowiec D, DePestel DD, Carver PL. Concurrent administration of sirolimus and voriconazole: a pilot study assessing safety and approaches to appropriate management. Pharmacotherapy. 2008;28(6):719–29. doi: 10.1592/phco.28.6.719. [DOI] [PubMed] [Google Scholar]
- 19.Marty FM, Lowry CM, Cutler CS, Campbell BJ, Fiumara K, Baden LR, et al. Voriconazole and sirolimus coadministration after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(5):552–9. doi: 10.1016/j.bbmt.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 20.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–33. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho VT, Cutler C, Carter S, Martin P, Adams R, Horowitz M, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(8):571–5. doi: 10.1016/j.bbmt.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97(4):855–64. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 23.Cornely OA, Bohme A, Reichert D, Reuter S, Maschmeyer G, Maertens J, et al. Risk factors for breakthrough invasive fungal infection during secondary prophylaxis. J Antimicrob Chemother. 2008;61(4):939–46. doi: 10.1093/jac/dkn027. [DOI] [PubMed] [Google Scholar]
- 24.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadaba B, Campanero MA, Quetglas EG, Azanza JR. Clinical relevance of sirolimus drug interactions in transplant patients. Transplant Proc. 2004;36(10):3226–8. doi: 10.1016/j.transproceed.2004.10.056. [DOI] [PubMed] [Google Scholar]
- 26.Mathis AS, Shah NK, Friedman GS. Combined use of sirolimus and voriconazole in renal transplantation: a report of two cases. Transplant Proc. 2004;36(9):2708–9. doi: 10.1016/j.transproceed.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 27.Kuypers DR, Claes K, Evenepoel P, Maes B, Vandecasteele S, Vanrenterghem Y, et al. Drug interaction between itraconazole and sirolimus in a primary renal allograft recipient. Transplantation. 2005;79(6):737. doi: 10.1097/01.tp.0000147462.86886.f3. [DOI] [PubMed] [Google Scholar]
- 28.Pai MP, Allen S. Voriconazole inhibition of tacrolimus metabolism. Clin Infect Dis. 2003;36(8):1089–91. doi: 10.1086/374252. [DOI] [PubMed] [Google Scholar]
- 29.Mori T, Kato J, Yamane A, Sakurai M, Kohashi S, Kikuchi T, et al. Drug interaction between voriconazole and tacrolimus and its association with the bioavailability of oral voriconazole in recipients of allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2012;95(5):564–9. doi: 10.1007/s12185-012-1057-2. [DOI] [PubMed] [Google Scholar]
- 30.Trifilio SM, Scheetz MH, Pi J, Mehta J. Tacrolimus use in adult allogeneic stem cell transplant recipients receiving voriconazole: preemptive dose modification and therapeutic drug monitoring. Bone Marrow Transplant. 2010;45(8):1352–6. doi: 10.1038/bmt.2009.345. [DOI] [PubMed] [Google Scholar]
- 31.Wang JL, Chang CH, Young-Xu Y, Chan KA. Systematic review and meta-analysis of the tolerability and hepatotoxicity of antifungals in empirical and definitive therapy for invasive fungal infection. Antimicrob Agents Chemother. 2010;54(6):2409–19. doi: 10.1128/AAC.01657-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amigues I, Cohen N, Chung D, Seo SK, Plescia C, Jakubowski A, et al. Hepatic safety of voriconazole after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16(1):46–52. doi: 10.1016/j.bbmt.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andes D, Pascual A, Marchetti O. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother. 2009;53(1):24–34. doi: 10.1128/AAC.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scholz I, Oberwittler H, Riedel KD, Burhenne J, Weiss J, Haefeli WE, et al. Pharmacokinetics, metabolism and bioavailability of the triazole antifungal agent voriconazole in relation to CYP2C19 genotype. British journal of clinical pharmacology. 2009;68(6):906–15. doi: 10.1111/j.1365-2125.2009.03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolton MJ, Ray JE, Chen SC, Ng K, Pont LG, McLachlan AJ. Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring. Antimicrob Agents Chemother. 2012;56(9):4793–9. doi: 10.1128/AAC.00626-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46(2):201–11. doi: 10.1086/524669. [DOI] [PubMed] [Google Scholar]