Abstract
Background
GTP cyclohydrolase I (GCH1) mutations are the commonest cause of Dopa-responsive dystonia (DRD). Clinical phenotypes can be broad, even within a single family.
Methods
We present clinical, genetic and functional imaging data on a British kindred in which affected subjects display phenotypes ranging from DRD to Parkinson's disease (PD). Twelve family members were studied. Clinical examination, dopamine transporter (DAT) imaging, and molecular genetic analysis of GCH1 and the commonest known familial PD-related genes were performed.
Results
We have identified a novel missense variant, c.5A > G, p.(Glu2Gly), within the GCH1 gene in affected family members displaying a range of phenotypes.
Two affected subjects carrying this variant had abnormal DAT imaging. These two with abnormal DAT imaging had a PD phenotype, while the remaining three subjects with the novel GCH1 variant had normal DAT imaging and a DRD phenotype.
Conclusions
We propose that this GCH1 variant is pathogenic in this family and these findings suggest that similar mechanisms involving abnormal GTP cyclohydolase I may underlie both PD and DRD. GCH1 genetic testing should be considered in patients with PD and a family history of DRD.
Keywords: Parkinson's disease, Dopa responsive dystonia, GCH1, SPECT DAT imaging
Highlights
-
•
We have identified a novel missense variant, c.5A > G, p.(Glu2Gly), within the GCH1 gene in family with Dopa responsive dystonia (DRD) and parkinsonism.
-
•
Those with parkinsonism had abnormal DaTscans, indicating nigrostriatal neurodegeneration.
-
•
These findings suggest that similar mechanisms involving abnormal GTP cyclohydolase I may underlie both Parkinson's disease and Dopa responsive dystonia.
1. Introduction
Dopa-responsive dystonia (DRD) is an autosomal dominant dystonia, now classified as DYT5, with an estimated incidence of between 0.5 and 1 per million [1]. DRD typically manifests as lower limb dystonia in childhood, although the spectrum of symptoms can be broad, even within the same family. Patients typically have an excellent and sustained response to low dose levodopa. Women are more commonly affected with a lower penetrance of mutations in men [2,3].
Mutations in the GTP cyclohydrolase I gene (GCH1) are the most common cause of DRD. GCH1 is located on chromosome 14 (14q22.1-q22.2) and encodes the 32-kDa guanosine 5′-triphosphate cyclohydrolase 1 (GTPCH1) protein. GCH1 contains six exons, and more than 200 different mutations have been identified [1,2].
Single positron emission computerized tomography (SPECT) Dopamine Transporter (DAT) imaging is a demonstration of in vivo striatal dopamine activity. The DAT ligands for SPECT, including [123I]FP-CIT (DaTSCAN) have all shown significantly reduced striatal uptake in PD [4], whilst uptake has usually been normal in DRD [5].
We have studied a family with an inherited movement disorder, with phenotypes ranging from DRD to slowly progressive PD. We report results from clinical, genetic and imaging studies of this kindred.
2. Methods
Twelve members of the kindred were studied. A diagnosis of PD was made according to United Kingdom Parkinson's Disease Society (UKPDS) Brain Bank clinical diagnostic criteria. The Hoehn and Yahr PD rating scale and a Folstein MMSE (Mini Mental State Examination) were performed on each subject. Olfactory function was assessed by use of the University of Pennsylvania Smell Identification Test (UPSIT-40) (Sensonics, Haddon Heights, NJ) and data compared to normative values. Seven subjects (II:1-II:5, III:1 and III:11) underwent dopamine transporter SPECT scanning (DaTSCAN, Amersham Health) and images reviewed by ER, who was blinded to demographic and clinical data.
Molecular genetic screening of the six affected family members was performed by polymerase chain reaction (PCR) and sequencing of the entire coding sequence, and intron-exon boundaries, of the genes SNCA, Parkin, DJ-1, PINK1, LRRK2 and GCHI, using primers and PCR conditions available on request. Multiple ligation-dependent probe amplification (MLPA) was used to detect the presence or absence of copy number variation, using MLPA kits P051 and P052 (MRC Holland), details available on request. Subsequently, six unaffected family members (II:2 and III:4,5,7,9,13) and 150 UK control DNA samples from the PD GEN DNA databank were screened for the novel GCH1 variant.
All subjects gave informed written consent to take part in the study. The study had appropriate ethical approval from South Birmingham LREC and Sandwell and West Birmingham LREC.
3. Results
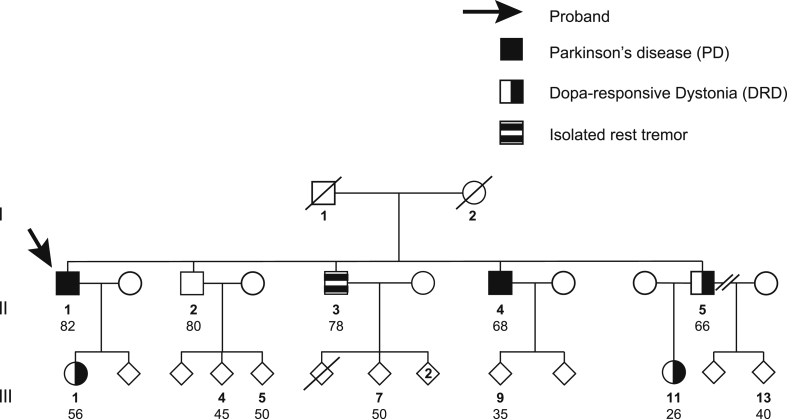
Twelve individuals in the pedigree (Fig. 1, those annoted with an age) were examined in detail. We noted features consistent with slowly progressive PD in individuals II:1 and II:4 (Supplementary video), with additonal levodopa-induced dyskinesias and dementia in subject II:1. Disease onset was at 58 and 50 years in II:1 and II:4 respectively. Subject II:3 had an isolated rest tremor (asymmetrical, right upper limb), with an older age of onset of 75 years, had not progressed over six years and did not meet diagnostic criteria for PD. Subjects II:5, III:1 and III:11 had features consistent with DRD, with median age of disease onset 17 years and median disease duration of 22 years. The remaining members of the family were unaffected. All the studied subjects are Caucasian and clinical details of affected individuals are summarized in Table 1 (more clinical details in Supplementary data).
Fig. 1.
Pedigree of study family. Numbers refer to the age (in years) at clinical evaluation and blood sampling of the twelve family members studied.
Table 1.
Summary of the clinical, functional neuro-imaging and molecular genetic data on subjects from the study family. PD denotes Parkinson's disease; DRD Dopa responsive dystonia; N/A not done or not appropriate; MMSE mini mental state examination score; UPSIT University of Pennsylvania Smell Identification Test; DaTSCAN dopamine transporter imaging; Mod. moderate. Age and sex corrected percentile values for UPSIT scores are presented in brackets.
| Subject & gender | Age at eval. | Age at sympt. onset | Clinical diagnosis | L-dopa response | H&Y stage | MMSE | UPSIT score (percentile) | DaT scan | GCH1 mut. p.E2G |
|---|---|---|---|---|---|---|---|---|---|
| II:1 M | 82 | 58 | PD | Y | 3 | 27 | 21 (40th) Severe microsmia |
A | Y |
| II:2 M | 80 | N/A | Unaffected | N/A | N/A | 27 | 31 (71st) Mild microsmia |
N | N |
| II:3 M | 78 | 75 | Isolated rest tremor | No Rx | 1 | 30 | 21 (23rd) Severe microsmia |
1 A 2 N |
N |
| II:4 M | 68 | 50 | PD | Y | 2 | 29 | 28 (31st) Mod. microsmia |
1 A 2 A |
Y |
| II:5 M | 66 | 44 | DRD | Y | N/A | 28 | 26 (24th) Mod. microsmia |
N | Y |
| III:1 F | 56 | 17 | DRD | Y | N/A | 30 | 35 (38th) Normosomia |
N | Y |
| III:11 F | 26 | 6 | DRD | Y | N/A | 29 | 37 (40th) Normosmia |
N | Y |
Supplementary video related to this article can be found at http://dx.doi.org/10.1016/j.parkreldis.2015.01.004.
The following is the supplementary video related to this article:
Video of subject II:4. The subject gave informed, written consent to be videoed for publication.
The findings from DaTSCAN images on six affected subjects (II:1, II:3-II:5, III:1 and III:11) and one unaffected subject (II:2) are summarized in Table 1 (Supplementary data). Subjects II:3 and II:4 had a second DaTSCAN performed 18 months after the first scan due to atypical disease progression (ie only very slowly progressing which would be unexpected in idiopathic PD).
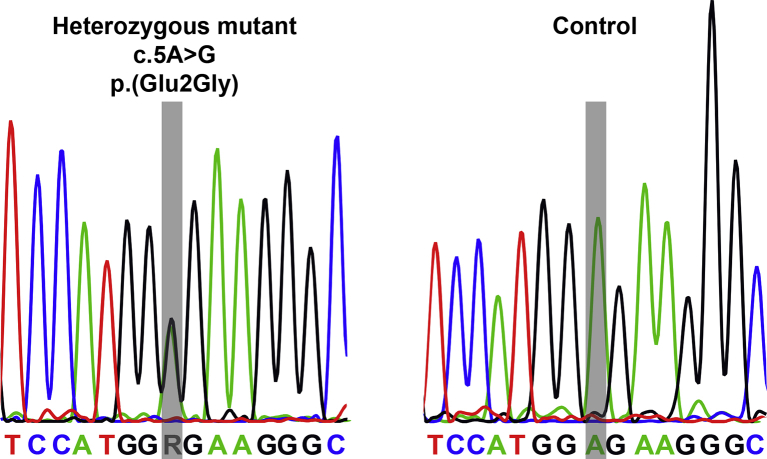
No novel or previously documented mutations were identified in the coding sequences of SNCA, Parkin, PINK1, DJ-1 and LRRK2. However, a novel heterozygous substitution was identified in the first exon of GCH1 affecting the fifth nucleotide of the open reading frame (c.5A > G) (Supplementary data). This change is predicted to replace a glutamic acid residue with glycine (p.(Glu2Gly)). This mutation (GenBank accession NM_000161.2, NP_000152.1.) is not listed as a known mutation or polymorphism in standard databases (dbSNP138 (http://www.ncbi.nlm.nih.gov/SNP/); 1000 genomes (release October 2013, http://browser.1000genomes.org.); Exome Variant Server (HLBI-EPS6500, http://evs.gs.washington.edu/EVS/database accessed Sept, 2014). The novel GCH1 variant was identified in five of the affected members of the family, but not in subject II:3 or unaffected subjects II:2 and III:4,5,7,9,13, nor in 300 UK control chromosomes. MLPA did not reveal any copy number variation in SNCA, Parkin, PINK1, DJ-1 and LRRK2 or GCH1.
4. Discussion
We have identified a novel heterozygous missense variant within GCH1 (c.5A > G) in five family members affected by PD or DRD. Several arguments support the contention that this variant is pathogenic. The variant cosegregates with disease state in the family; it is absent from 300 UK control chromosomes tested here, and from all the large public databases; furthermore, it is almost completely conserved among species (Supplementary Fig. 2), and it is predicted to replace one of the larger hydrophilic amino acids with a small hydrophobic glycine within the N-terminal region of the protein. It is well known that in-silico prediction tools possess limited accuracy [6]. This mutation is predicted as pathogenic by SIFT and SNP&GO, but not by PolyPhen-2 and Mutation Taster. The clinical symptoms and signs observed in subjects II:1 and II:4 met diagnostic criteria for PD. In both cases the disease was more slowly progressive than is usual in idiopathic PD. Subject II:3 had an isolated rest tremor and subjects II:5, III:1 and III:11 were diagnosed with DRD. The symptoms in subjects III:1 and III:11 started at a young age and in the case of subject III:11 were more severe than those seen in subject II:5, illustrating the extreme phenotypic heterogeneity in this kindred and possibly reflecting the increased penetrance of GCH1 mutations in females.
DaTSCAN data supported the clinical diagnoses in most cases. The clinical phenotypes of DRD and PD and correspondingly normal and abnormal scans were found in subjects carrying the same GCH1 mutation. DAT imaging is typically normal in DRD. Indeed DAT imaging has been proposed as a diagnostic tool to help differentiate between DRD and early onset PD [5]. There have been case reports of individuals with adult onset dystonia-parkinsonism [7] or PD without any dystonia [8] carrying a GCH1 mutation and an abnormal DaTSCAN.
Recently, Mencacci and colleagues reported 4 unrelated individuals with adult onset parkinsonism, presumed pathogenic GCH1 mutations and abnormal DaTSCANs [9].
These cases, and the two in our family (subjects III:1 and III:4), raise the question as to whether PD, in patients with presumed pathogenic GCH1 mutations, is in fact a rare phenotype of DRD. Certainly the phenotype of DRD is suggested to be broad, as illustrated by a recently reported family with classical DRD, adult onset PD and an MSA-like phenotype associated with a GCH1 two exon deletion [3]. As recently speculated by Mencacci and colleagues [9], chronic dopamine deficiency resulting from GCH1 deficiency could directly predispose to nigral cell death (rather than classical lewy body associated neurodegeneration as typically seen in PD). Although earlier studies screening for a GCH1 mutations in PD cohorts did not identify a significant role for genetic variation in GCH1, recent studies have implicated GCH1 as a risk locus for PD [9,10].
An alternative, but less plausible, explanation in our family is the coincident occurrence of two separate movement disorders: a GCH1 mutation causing DRD in some individuals an additional mutation (ie an unidentified mutation in an as yet unidentified PD gene) causing PD coincidentally in others. In those with a GCH1 mutation and PD but no dystonia the lack of DRD phenotype could then be explained by incomplete penetrance of the GCH1 mutation.
Olfactory dysfunction is found in 70–100% of PD patients. As DRD is a result of dopamine deficiency without neuronal cell death, we would predict that UPSIT-40 data in DRD should be normal. In our study, the mean UPSIT-40 score for affected subjects with the GCH1 mutation was 29.4, compared to a previous study of olfaction in PD, in which the mean score in 18 subjects with idiopathic PD was 17.1, with a score of 27.6 in 27 age-matched controls [11]. The small sample sizes which result from separating our individuals with the GCH1 mutation limit any conclusions but the 2 individuals with PD had a mean score of 24.5 versus the 3 with DRD who had a mean score 32.7.
The DaTSCAN data for subject III:3, who had an isolated rest tremor, is difficult to explain. His first scan was abnormal, but the second scan performed 18 months later was normal. One explanation is that he suffered a vascular embolic event occurring around the time of the first scan. An alternative explanation is that there was a technical problem with one of the scans. There is also a category of subjects with parkinsonism who have normal DaTSCANs, so called SWEDDs (Scans Without Evidence of Dopaminergic Deficit), with 11–15% of subjects with PD being found to have normal nigrostriatal uptake of presynaptic ligands [12]. The novel GCH1 mutation is not carried by subject III:3 and the most likely explanation for his symptoms is that he has isolated rest tremor due to an unrelated mechanism.
In further studies we will screen for GCH1 variants in subjects with familial PD, and in subjects with parkinsonism who have normal DAT scans, as well as pursuing further studies into the functional effects of the novel variant described here. We anticipate that future post-mortem histological data on affected individuals, particularly those with the PD phenotype will shed more light on disease mechanisms in this interesting family.
Author roles
The study was designed by AJL, with support and advice by DJN, VB, and KEM. AJL and TDL carried out the clinical assessments and the LRRK2 sequencing work. SO, MQ, EJS, and VB carried out the further genetic analyses (sequencing and MLPA). EBR assessed the DaT Scans. AJL and TDL drafted the manuscript. All the co-Authors contributed to revising the manuscript for intellectual content and approved the final version for publication.
Conflict of interest
None of the authors has any conflict of interest to disclose.
Acknowledgments
Funding agencies: The Parkinson's Disease Society of the UK, Sandwell and West Birmingham Hospital NHS Trust and the Midland Neuroscience Teaching and Research Fund. Stichting ParkinsonFonds, The Netherlands (research grant to VB).
We thank the participating family for all their cooperation with this work. A subset of DNA samples from subjects with PD, and controls, was supplied from the PD GEN DNA databank (Prof C.E. Clarke, Prof K.E. Morrison & Prof K. Wheatley, funded by the Medical Research Council of the UK). We gratefully acknowledge our funding sources The Parkinson's Disease Society of the UK, Sandwell and West Birmingham Hospital NHS Trust and the Midland Neuroscience Teaching and Research Fund, and the Stichting ParkinsonFonds, The Netherlands (research grant to VB).
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplementary Fig. 1.
Electropherograms from subjects who are wild-type and heterozygote for the novel GCH1 mutation c.5A > G. Bars indicate position of nucleotide variation.
Supplementary Fig. 2.

Alignment of the GCH1 protein homologs. Arrow indicates position of the mutated amino acid.
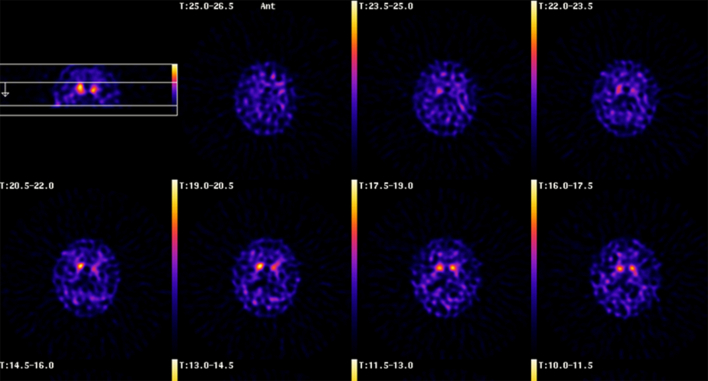
Supplementary Fig. 3.
DaTSCAN of subject II:1. An abnormal scan, which shows bilateral reduced striatal uptake of [123I]FP-CIT, worse on the left side, as demonstrated by the arrow. Images 1–7 represent transverse sections through the brain, where 1 is the most superior and 7 the most inferior section. A normal DaTSCAN shows symmetrical bilateral striatal uptake which confers a comma-shaped configuration. There should be only minor background activity. The head of comma represents caudate activity whilst tail is due to uptake within putamen. In this example there is loss of uptake within each putamen. Caudate activity especially that on the left is also diminished. Note increased background activity. An MRI excluded a possible structural cause for these findings.
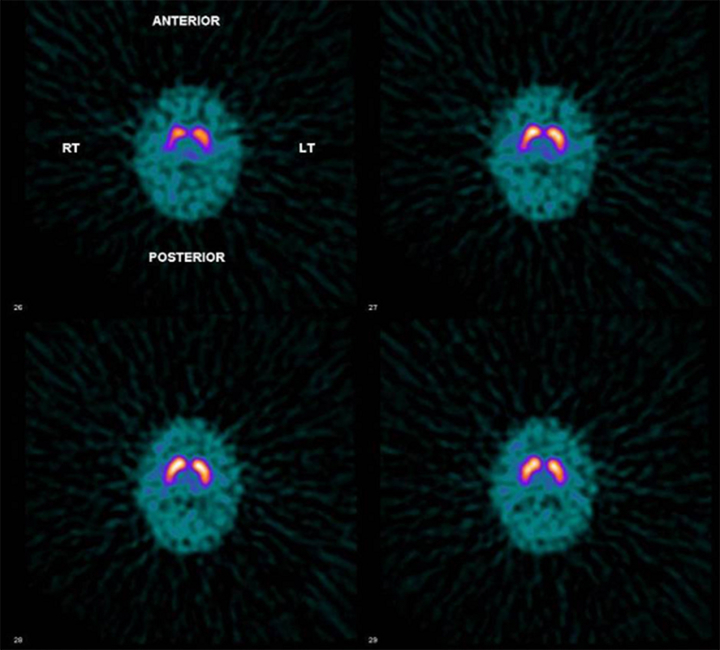
Supplementary Fig. 4.
DaTSCAN of subject III:1. A normal scan, showing normal striatal uptake of [123I]FP-CIT bilaterally. Images 1–7 represent transverse sections through the brain, where 1 is the most superior and 7 the most inferior section. In normal DaT image striatum shown as comma shaped region with caudate and putamen appearing as high intensity against a low background. With increasing age background may become slightly more apparent. Compensating for any patient rotation, activity should be evenly distributed within each striatum with symmetrical configuration of comma.
References
- 1.Zirn B., Steinberger D., Troidi C., Brockmann K., von der Hagen M., Feiner C. Frequency of GCH1 deletions in dopa-responsive dystonia. J Neurol Neurosurg Psychiatr. 2008;79(2):183–186. doi: 10.1136/jnnp.2007.128413. [DOI] [PubMed] [Google Scholar]
- 2.Bernal-Pacheco O., Oyama G., Briton A., Singleton A.B., Fernandez H.H., Rodriguez R.L. A novel DYT-5 mutation with phenotypic variability within a Colombian family. Tremor Other Hyperkinet Mov (N Y) 2013;3 doi: 10.7916/D86W98SW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceravolo R., Nicoletti V., Garavaglia B., Reale C., Kiferle L., Bonuccelli U. Expanding the clinical phenotype of DYT5 mutations: is multiple system atrophy a possible one? Neurology. 2013;81(3):301–302. doi: 10.1212/WNL.0b013e31829bfd7c. [DOI] [PubMed] [Google Scholar]
- 4.Ba F., Martin W.R.W. Dopamine transporter imaging as a diagnostic tool for parkinsonism and related disorders in clinical practice. Parkinsonism Relat Disord. 2015;21(2):87–94. doi: 10.1016/j.parkreldis.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Brajkovic L.D., Svetel M.V., Kostic V.S., Sobic-Saranovic D.P., Pavlovic S.V., Artiko V.M. Dopamine transporter imaging (123)I-FP-CIT (DaTSCAN) SPET in differential diagnosis of dopa-responsive dystonia and young-onset Parkinson's disease. Hell J Nucl Med. 2012;15(2):134–138. [PubMed] [Google Scholar]
- 6.Thusberg J., Olatubosun A., Vihinen M. Performance of mutation pathogenicity prediction methods on missense variants. Hum Mutat. 2011;32:358–368. doi: 10.1002/humu.21445. [DOI] [PubMed] [Google Scholar]
- 7.Hjermind L.E., Johannsen L.G., Blau N., Wevers R.A., Lucking C.B., Hertz J.M. Dopa-responsive dystonia and early-onset Parkinson's disease in patient with GTP cyclohydrolase I deficiency? Mov Disord. 2006;21:679–682. doi: 10.1002/mds.20773. [DOI] [PubMed] [Google Scholar]
- 8.Eggers C., Volk A.E., Kahraman D., Fink G.R., Leube B., Schmidt M. Are dopa-responsive dystonia and Parkinson's disease related disorders? A case report. Parkinsonism Relat Disord. 2012;18(5):666–668. doi: 10.1016/j.parkreldis.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Mencacci N.E., Isaias I.U., Reich M.M., Ganos C., Plagnol V., Polke J.M. Parkinson's disease in GTP cyclohydrolase 1 mutation carriers. Brain. 2014;137(9):2480–2492. doi: 10.1093/brain/awu179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nalls N.A., Pankratz N., Lill C.M., Do C.B., Hernandez D.G., Saad M. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nat Genet. 2014;46:989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katzenschlager R., Zijlmans J., Evans A., Watt H., Lees A.J. Olfactory function distinguishes vascular parkinsonism from Parkinson's disease. J Neurol Neurosurg Psychiatr. 2004;75(12):1749–1752. doi: 10.1136/jnnp.2003.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider S.A., Edwards M.J., Mir P., Cordivari C., Hooker J., Dickson J. Patients with adult-onset dystonic tremor resembling parkinsonian tremor have scans without evidence of dopaminergic deficit (SWEDDs) Mov Disord. 2007;22(15):2210–2215. doi: 10.1002/mds.21685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video of subject II:4. The subject gave informed, written consent to be videoed for publication.