Abstract
Objective
This multi-site randomized trial evaluates the quality of life (QOL) benefits of an imagery-based group intervention titled “Envision the Rhythms of Life” (ERL).
Methods
Breast cancer survivors >6 weeks post-treatment were randomized to attend five weekly 4-hour group sessions at a community center with therapist present (live-delivery; LD, n=48); therapist streamed via telemedicine (telemedicine-delivery; TD, n=23); or to a waitlist control group (WL, n=47). Weekly individual phone calls to encourage at-home practice began at session one and continued until the 3-month follow-up. Seven self-report measures of QOL were examined at baseline, 1 and 3 months post-treatment including health-related and breast cancer-specific QOL, fatigue, cognitive function, spirituality, distress, and sleep.
Results
The Bonferroni method was used to correct for multiple comparisons, and alpha was adjusted to 0.01. LMM analyses revealed less fatigue, cognitive dysfunction, and sleep disturbance for LD and TD compared to WL across the follow-up (p’s <0.01). Changes in fatigue, cognitive dysfunction, sleep disturbance, and health-related and breast cancer-related QOL were clinically significant. There were no differences between LD and TD.
Conclusions
Both the live and telemedicine delivered ERL intervention resulted in improvements in multiple QOL domains for breast cancer survivors compared to a waitlist control. Further, there were no significant differences between live- and telemedicine-delivery, suggesting telemedicine delivered ERL intervention may represent an effective and viable option for cancer survivors in remote areas.
Keywords: breast cancer, cancer survivorship, QOL, guided imagery, telemedicine
INTRODUCTION
Growing evidence suggests that psychosocial interventions improve QOL in cancer survivors.[1] Among the efficacious approaches, interventions emphasizing guided imagery have been associated with improved QOL, reduced treatment-related side effects, and improved immune function in cancer survivors[2–4] though not all studies have found this association.[5] A single arm pretest/posttest study using the present imagery-based intervention indicated that 30 post-treatment breast cancer survivors living in rural Alaska experienced increased post-treatment general and breast cancer-specific QOL, improved spiritual well-being, and decreased distress.[6] Thus, a randomized controlled trial is the necessary next step to determine whether such changes can be attributed to the intervention rather than simply the passage of time.
Many cancer survivors face significant barriers to care (e.g., living in remote locations, work schedule, family responsibilities, poor health, psychological distress) that can preclude them from participating in post-treatment care requiring travel to distant medical facilities.[7] Telemedicine is one approach to improving survivor access to care, and has been demonstrated to be an effective and accepted method of providing medical consultations, managing post-treatment symptoms, and delivering psychological counseling or mind-body interventions for cancer survivors.[8–10]
In addition to the traditional telemedicine delivered in-home via telephone or Internet, effective telecare is also being provided to patients at community health centers. For example, oncology patients unable to travel to a major hospital reported reduced pain and depression symptoms following telecare at community-based oncology clinics.[11] Importantly, direct comparison of face-to-face versus videoconference-delivered cognitive intervention for community dwelling older persons revealed no differences in cognitive improvement.[12] Thus, telemedicine delivered at community centers may be a powerful avenue to ensure quality healthcare is available to patients unable to commute to major medical centers. Additionally, group therapy at a community center led by a remote provider (via videoconferencing) can provide patients with important nonspecific social support inherent in group therapy experiences and reduce the time required of providers. However, to the best of our knowledge, no published studies have examined the effects of such an intervention.
Despite its utility, telemedicine is estimated to be used by only 4% of psychosocial cancer care providers.[13] While some suggests this discrepancy is due to a lack of education about telemedicine modalities,[13] only 23% of mental health providers at community-based outpatient clinics indicated that they do not know enough about telemedicine, while 79% believed that more research is needed on the effectiveness of telemedicine.[14] Thus, additional research on the potential effectiveness of telemedicine for delivering psychosocial cancer care is warranted.
The primary aim of the current study was to compare the effects of an intervention entitled “Envision the Rhythms of Life (ERL)” delivered live (LD) or via telemedicine (TD) compared to a waitlist control (WL) on QOL for breast cancer survivors. Specifically, we hypothesized that participants in the LD and TD groups would report improved QOL across the follow-up compared to participants in the WL group. Additionally, though the present study was not designed to test for equivalence, we hypothesized that the LD and TD groups would not significantly differ on any outcome.
METHODS
Participants
Breast cancer survivors, at least 6 weeks after completing their major cancer treatments, were recruited from 2008–2010. Eligibility included confirmed diagnosis of breast cancer, 18 years of age or older, with no major psychiatric illness. Participants were required to be visual and hearing capable, able to read, write and speak English, and demonstrate an orientation to person, place and time. Medical release forms were provided to allow all medical records to be reviewed by the research nurse.
Procedure
Participants were recruited through newspaper ads, cancer support group websites, public presentations, medical referral, posting of flyers, and via TV news and radio coverage. One live delivery (n = 25) and one telemedicine delivery group (n = 23) were conducted in Anchorage, Alaska and one live delivery group (n = 23) was conducted in Seattle, Washington. Therapy groups consisting of up to 25 participants are routinely conducted at these sites, to maximize limited staff and cost effectiveness. Though the authors originally intended for a telemedicine group to also be conducted in Seattle, unforeseen staffing limitations prevented this group from taking place. Seattle was chosen as the secondary location because it was the city in the continental United States for which travel from the therapists’ primary location (in Alaska) was most feasible.
After informed consent was obtained, participants were randomized to one of three groups: live delivery group sessions (LD), therapist present via audiovisual technology during group sessions (TD), or waitlist control (WL). Assignment by adaptive randomization (minimization) was balanced by age, gender, stage, chemotherapy, surgery, radiation and hormone use. Participants in LD and TD had five 4-hour weekly group sessions, and received brief (< 10 minute) weekly phone calls to encourage at-home practice that began at the start of treatment and continued for 3 months post-treatment. All self-report questionnaires were completed in the presence of a research assistant, and were collected at baseline and 1 and 3 months post-treatment. The study was approved by the Alaska Regional Hospital institutional review board.
ERL Intervention
LD and TD groups met in a community center, with either the therapist present (LD) or a research assistant present to set up the videoconferencing software (TD). The videoconferencing software enabled the therapist, who was not physically present, and participants to view and interact with one another. Additionally, the therapist was able to control the camera direction, enabling her to interact with small groups and individuals during the interactive portion of the sessions.
The intervention was delivered by the first and second authors, a licensed professional counselor and a family medicine physician, respectively. The format followed a manual developed by the first author, and was identical for both delivery types. The first four sessions were separated into three modules each comprised of 25 minutes of didactic education followed by 25 minutes of interaction with fellow group members (in triads) to discuss and practice the material presented in the didactic portion (Supplement 1). During the fifth session, each participant presented her long-term plan for continuing to practice the activities taught during the group, and participants provided feedback and suggestions to enrich each plan.
The didactic portion of sessions provided education on the mind-body connection and presented research on the impact of mental imagery and sensate experience (e.g., sounds, scent, taste, touch) on physiological processes (e.g., psychoneuroimmunology processes, heart rate variability (HRV), temperature, and circadian rhythms).[15–18] The interactive portion of sessions enabled participants to apply what they just learned and receive feedback from their small group and the therapist, who briefly visited with each triad during the interactive group time. Briefly, throughout the intervention participants identified maladaptive “passive imagery” (e.g., automatic thoughts focused on fear/loss of control); created adaptive “active imagery” (e.g., thoughts focused on empowering, meaning-making themes); and practiced “targeted imagery” (e.g., imagining healthy physiological processes such as HRV, circadian rhythms, and immune function). Participants were instructed to engage all five senses during active and targeted imagery, and to monitor the effects of imagery on their own mind-body health. For example, during the interactive portion of Session 3, participants monitored the effects of targeted imagery on their own HRV using an HRV monitoring device (EmWave PRO; HeartMath LCC; Boulder Creek, CA).
Weekly participants received a 20–30 minute of guided imagery CD related to that week’s topics. During intervention delivery and for 3 months post-treatment, participants were instructed to engage in daily formal (using CDs) and informal (using brief targeted imagery when under stress) practice. Participants received weekly phone calls from their group therapist (approximately 10 minutes) during intervention delivery and for 3 months post-treatment. These phone calls were designed to encouraged participants to engage in practice and trouble-shoot barriers to practice. All sessions were videotaped and 10% were randomly chosen for evaluation of ongoing treatment fidelity. Participants who missed a session were encouraged to attend a one-on-one make-up session with the group therapist, who presented the didactic lessons using the same materials and format as were used in the group session.
Participants in the WL group were offered the ERL intervention delivered with a therapist present after they completed the 3-month follow-up.
Measures
General health-related QOL was measured using the Medical Outcomes Study 36-item short form survey (SF-36).[19] The RAND scoring method was used (0–100) to compute physical component summary (PCS) and mental component summary (MCS) scores, with higher scores representing better QOL.
The 13-item breast cancer-specific subscale of the Functional Assessment of Cancer Therapy-Breast (FACT-B)[20] assessed breast cancer-specific QOL, using a 0–4 Likert scale with higher scores representing better QOL (score range 0–52).
Fatigue was assed using the 13-item FACIT-Fatigue Scale (FACIT-F, version 4), on which participants rate their tiredness during usual daily activities over the past week using a 0–4 Likert scale (score range 0–52), with higher scores indicating less fatigue. A score of < 36 is associated with clinically significant fatigue.[23]
Perceived cognitive function was assessed with the 37-item FACT-Cog (version 2), which uses a 0–4 Likert scale to assesses perceived cognitive impairment (PCI), perceived cognitive abilities (PCA), and impact of perceived cognitive impairments on quality of life (IQL).[24] A total score was calculated by adding the PCI and PCA subscales,[24] with higher scores indicating less perceived impairment (score range 0–116).
Spiritual well-being was measured with the 23-item Functional Assessment of Chronic Illness Therapy Spiritual Well-Being Expanded Scale (FACIT-Sp-Ex; version 4), which uses a 0–4 Likert scale to assess meaning, peace, and faith, with higher scores indicating greater spiritual well-being (score range 0–92).[25]
Psychological distress was assessed using the 18-item Brief Symptom Inventory (BSI-18) Global Severity Index (BSIGSI).[26] BSIGSI scores have been standardized and are represented as T scores in the present paper with higher scores representing worse distress (score range 30–75).
Sleep disturbances were assessed using 9-item the Pittsburgh Sleep Quality Index (PSQI), which assesses quality of sleep and sleep disturbances over a 1-month period.[27] A total score is derived with a score of 5 or greater associated with being a “poor” sleeper (score range 1–21).[27]
Demographic factors were included in the baseline questionnaires, and medical data were extracted from patients’ medical records. Tracking data were kept regarding class attendance, completion of questionnaires, and attrition.
Data Management and Analysis
Data were scored and analyzed using SAS (version 9.2; SAS, Cary, NC). Descriptive statistics were computed. The PROC MIXED procedure in SAS was used to conduct linear multilevel modeling (LMM) analyses [28] to estimate the effects of group, time, and the group*time effects on each of the eight primary QOL outcomes (SF-36 (PCS, MCS), FACIT scales (FACT-B, FACIT-F, FACT-Cog, FACIT-Sp-Ex), BSIGSI, and PSQI) covarying for the respective QOL baseline measure, and covariates determined a priori (age, months since diagnosis, stage, chemotherapy, surgery, radiation, hormone therapy). LMM efficiently handles unbalanced designs and missing data without excluding participants or imputing values.[29] The Bonferroni method was used to correct for the eight primary QOL outcome measures, taking into account the average correlation between outcome variables (mean r = 0.27), and alpha was adjusted to 0.011.[30] The t test was used for all post hoc group comparisons. Additionally, exploratory analyses were conducted using χ2 tests to examine group differences in the proportion of participants reporting clinically significant sleep disturbances (PSQI ≥ 5) [27] and fatigue (FACIT-F < 36)[23] at each time point. A priori power analyses determined that with 45 patients per each group in this study and a 15% dropout rate (i.e., with 38 evaluable participants per group), we would be able to declare as statistically significant differences between two groups that are at least 0.65 standard deviations assuming a two-sided significance level of 0.05 and 80% power.
Preliminary analyses revealed no differences between cities (Anchorage vs. Seattle) in demographic or medical characteristics. Further, with the exception of baseline FACIT-Sp-Ex, there were no baseline (demographic, medical, or psychosocial) or follow-up differences between the Anchorage and Seattle LD groups (p’s > 0.3). There was a trend for LD participants in Anchorage to report higher FACIT-Sp-Ex than those in Seattle (p = 0.07). Thus, the LD groups were combined for all analyses, and city was entered as a covariate in analyses examining FACIT-Sp-Ex.
RESULTS
Sample Characteristics
Among the 121 participants consented to study, 118 (97.5%) were randomized and provided baseline data (LD=48, TD=23, WL=47), 104 (LD=41, TD=19, WL=44) completed the intervention and 1-month follow-up, and 102 (LD=40, TD=19, WL=43) completed the 3-month follow-up. Reasons for attrition can be seen in the CONSORT diagram (Figure 1). There were no significant group differences in loss to follow-up. The demographic, medical, and baseline psychosocial variables did not differ between participants who did and did not drop out of the study by T2 or T3 follow-ups. There were no group differences in demographic or medical characteristics (Table 1) or in baseline psychosocial measures (Table 2). Additionally, the average class size did not differ between LD and TD.
Figure 1.
Consort Diagram
Table 1.
Demographic and Medical Characteristics by Study Group
| Characteristic | Live Delivery n = 48 |
Telemedicine Delivery n = 23 |
Waitlist Control n = 47 |
|---|---|---|---|
| Mean Age (SD) | 55.44 (8.08) | 55.57 (9.88) | 55.28 (7.90) |
| Ethnicity N (%) | |||
| White | 40 (83.33) | 20 (86.96) | 43 (91.49) |
| African American | 0 (0) | 0 (0) | 1 (2.23) |
| Hispanic/Latino American | 0 (0) | 1 (4.35) | 0 (0) |
| Indian/Alaska Native | 8 (16.67) | 2 (8.70) | 3 (6.38) |
| Marriage Status N (%) | |||
| Married/Cohabitating | 30 (62.50) | 17 (73.91) | 26 (55.32) |
| Divorced/Separated | 13 (27.08) | 4 (17.39) | 13 (27.66) |
| Never Married | 5 (10.42) | 2 (8.70) | 5 (10.64) |
| Education N (%) | |||
| High School Diploma | 2 (4.17) | 1 (4.35) | 2 (4.26) |
| Some College | 11 (22.92) | 4 (17.39) | 11 (23.40) |
| College Degree | 23 (47.92) | 11 (47.83) | 16 (34.04) |
| Graduate Degree | 11 (22.92) | 7 (30.43) | 18 (38.30) |
| City N (%) | |||
| Anchorage | 25 (52.08) | 23 (100) | 24 (51.06) |
| Seattle | 23 (47.92) | 0 (0) | 23 (48.94) |
| Months Since Diagnosis (SD) | 50.48 (41.72) | 62.65 (61.60) | 45.53 (36.68) |
| Stage of Disease N (%) | |||
| 0 | 6 (13.33) | 4 (19.05) | 3 (7.32) |
| I | 16 (35.56) | 6 (28.57) | 16 (39.02) |
| II | 15 (33.33) | 4 (19.05) | 15 (36.59) |
| III | 7 (15.56) | 6 (28.57) | 5 (12.20) |
| IV | 1 (2.22) | 1 (4.76) | 2 (4.88) |
| Surgery N (%) | |||
| Lumpectomy | 15 (31.91) | 12 (52.17) | 19 (42.22) |
| Mastectomy Only | 26 (55.32) | 11 (47.83) | 23 (51.11) |
| Mastectomy with Reconstruction | 6 (12.77) | 0 (0) | 3 (6.67) |
| Chemotherapy N (%) | |||
| Yes | 31 (64.58) | 13 (56.52) | 33 (70.21) |
| Radiation N (%) | |||
| Yes | 34 (70.83) | 17 (73.91) | 36 (76.60) |
| Hormone Therapy N (%) | |||
| Yes | 31 (64.58) | 13 (59.09) | 27 (57.45) |
Table 2.
Unadjusted Group Means
| Live Delivery n = 48 |
Telemedicine Delivery n = 23 |
Waitlist Control n = 47 |
Group Effect | Time Effect | Group* Time Effect | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| M | SD | M | SD | M | SD | p-value | p-value | p-value | |
| SF-36 PCS | 0.154 | 0.529 | 0.111 | ||||||
| Baseline | 47.20 | 8.60 | 46.54 | 8.48 | 45.24 | 10.23 | |||
| 1 Month | 48.81 | 9.84 | 48.64 | 9.05 | 43.49 | 11.34 | |||
| 3 Months | 50.54 | 8.49 | 46.95 | 8.04 | 45.44 | 10.24 | |||
| Group LSM, SE* | 48.32 | 0.91 | 49.93 | 1.36 | 46.81 | 0.91 | |||
|
| |||||||||
| SF-36 MCS | 0.020 | 0.612 | 0.661 | ||||||
| Baseline | 42.45 | 10.50 | 43.45 | 8.03 | 42.41 | 10.04 | |||
| 1 Month | 48.51 | 8.72 | 49.25 | 7.97 | 46.50 | 10.40 | |||
| 3 Months | 49.80 | 8.04 | 50.84 | 7.58 | 43.29 | 12.75 | |||
| Group LSM, SE* | 48.77 | 1.24 | 49.40 | 1.86 | 44.30 | 1.25 | |||
|
| |||||||||
| FACT-B | 0.076 | 0.003 | 0.208 | ||||||
| Baseline | 22.63 | 5.98 | 22.09 | 4.03 | 20.32 | 6.06 | |||
| 1 Month | 25.32 | 5.97 | 24.84 | 5.29 | 22.32 | 6.08 | |||
| 3 Months | 26.18 | 5.83 | 27.21 | 4.22 | 22.72 | 5.20 | |||
| Group LSM, SE* | 24.66 | 0.57 | 26.03 | 0.85 | 23.66 | 0.58 | |||
|
| |||||||||
| FACIT-F | 0.002 | 0.084 | 0.321 | ||||||
| Baseline | 29.75 | 10.08 | 29.78 | 10.19 | 29.21 | 11.01 | |||
| 1 Month | 38.44 | 12.02 | 36.74 | 10.68 | 31.05 | 12.15 | |||
| 3 Months | 39.78 | 8.05 | 41.53 | 12.36 | 31.51 | 11.23 | |||
| Group LSM, SE* | 38.16 | 1.34 | 39.62 | 2.00 | 32.26 | 1.34 | |||
|
| |||||||||
| FACT-Cog | 0.001 | 0.154 | 0.687 | ||||||
| Baseline | 47.48 | 20.01 | 50.30 | 21.13 | 50.66 | 18.54 | |||
| 1 Month | 64.20 | 19.46 | 65.42 | 16.69 | 51.41 | 18.93 | |||
| 3 Months | 64.85 | 21.15 | 68.79 | 15.22 | 54.23 | 18.86 | |||
| Group LSM, SE* | 63.75 | 2.18 | 67.69 | 3.24 | 54.48 | 2.17 | |||
|
| |||||||||
| FACIT-Sp-Ex§ | 0.049 | 0.657 | 0.462 | ||||||
| Baseline | 67.68 | 13.70 | 68.87 | 14.81 | 67.64 | 14.22 | |||
| 1 Month | 75.73 | 14.44 | 74.42 | 11.49 | 68.64 | 14.44 | |||
| 3 Months | 74.10 | 14.21 | 75.74 | 12.24 | 69.54 | 16.12 | |||
| Group LSM, SE* | 74.60 | 1.36 | 76.08 | 2.24 | 70.61 | 1.37 | |||
|
| |||||||||
| BSI-GSI | 0.051 | 0.120 | 0.032 | ||||||
| Baseline | 53.98 | 7.75 | 51.77 | 7.81 | 55.51 | 7.26 | |||
| 1 Month | 48.88 | 8.31 | 49.32 | 8.58 | 52.20 | 8.44 | |||
| 3 Months | 46.80 | 7.82 | 49.26 | 7.34 | 53.02 | 8.95 | |||
| Group LSM, SE* | 48.24 | 1.02 | 47.81 | 1.59 | 51.51 | 1.03 | |||
|
| |||||||||
| PSQI | <0.001 | 0.346 | 0.303 | ||||||
| Baseline | 8.79 | 4.11 | 8.30 | 3.74 | 9.96 | 4.74 | |||
| 1 Month | 6.12 | 3.74 | 5.95 | 3.47 | 9.18 | 4.61 | |||
| 3 Months | 6.70 | 3.83 | 5.53 | 2.46 | 9.74 | 4.32 | |||
| Group LSM, SE* | 7.09 | 0.36 | 6.04 | 0.54 | 8.74 | 0.37 | |||
Higher SF-36 and FACIT subscales indicate better QOL. Higher BSIGSI and PSQI indicate more distress and sleep disturbance, respectively. Group, time, and group*time p-values are based on linear multilevel models (LMM) covarying for age, time since diagnoses, stage, and medical treatments (chemotherapy, surgery, radiation, hormone therapy), and baseline level of the outcome variable.
Group least squared means and standard errors are associated with the group effect (collapsed across time) in the LMMs described above.
The LMM predicting FACT-Sp-Ex also covarys for city.
Abbreviations: SF-36 = Medical Outcomes Study 36-item short form survey; PCS = physical component summary; MCS = mental component summary; FACT-B = Functional Assessment of Cancer Therapy-Breast; FACIT-F = Functional Assessment of Chronic Illness Therapy Fatigue Scale; FACT-Cog = Functional Assessment of Cancer Therapy-Cognitive Function Scale FACIT-Sp-Ex = Functional Assessment of Chronic Illness Therapy Spiritual Well-Being Expanded Scale; BSI-GSI = Brief Symptom Inventory-Global Severity Index; PSQI = Pittsburgh Sleep Quality Index.
Adherence
Five of the 48 participants randomized to LD withdrew from the study prior to attending any classes; two participants attended some classes before dropping out due to deaths in the family. Thirty-five of the remaining 41 LD participants attended all sessions as scheduled, 6 missed one session, but attended a make-up session, and 1 missed the final session and did not perform a make-up. One of the 23 participants randomized to TD withdrew without attending any classes; three attended some classes before dropping out due to personal reasons. Eighteen of the remaining 19 TD participants attended all sessions as scheduled and one participant missed one session, but received a make-up session.
QOL Outcomes for Live-Delivery vs. Telemedicine-Delivery vs. Waitlist
Unadjusted group means and standard deviations at baseline, 1- and 3-month follow-up and adjusted group means collapsed across time can be seen in Table 2. Using a Bonferroni correction for multiple QOL comparisons (alpha = 0.011), there was an effect of group on FACIT-F, FACT-Cog, and PSQI (p’s ≤ 0.002). A priori pairwise comparisons indicated individuals in LD and TD reported higher FACIT-F and FACT-Cog and lower PSQI scores compared to individuals in the WL group (p’s < 0.01). Using the adjusted alpha, there was no group effect on PCS, MCS, FACT-B, FACIT-Sp-Ex, or BSIGSI, though means were in the expected direction. Exploratory pairwise comparisons following up on group effects that reached p ≤ 0.05 revealed that women in LD and TD reported higher MCS and FACIT-Sp-Ex and lower BSIGSI scores compared to women in the WL group (p’s < 0.05). Additionally, pairwise comparisons revealed no differences between LD and TD groups on any outcome measure.
There was an effect of time on FACT-B (p = 0.003), with scores increasing over time. There was no effect of time on any other outcome.
Though there were no group*time effects that reached the adjusted alpha level of 0.011, there was a group*time effect on BSIGSI scores at the p < 0.05 level (p = 0.032). Pairwise comparisons of groups at each time point revealed that neither TD or LD differed from WL at the 1-month follow-up (p’s > 0.3), both LD (p = 0.011) and TD (p = 0.004) reported lower BSIGSI than WL at the 3-month follow-up, and TD and LD did not differ from one another at either time point (p’s > 0.7). No other group*time effects reached significance.
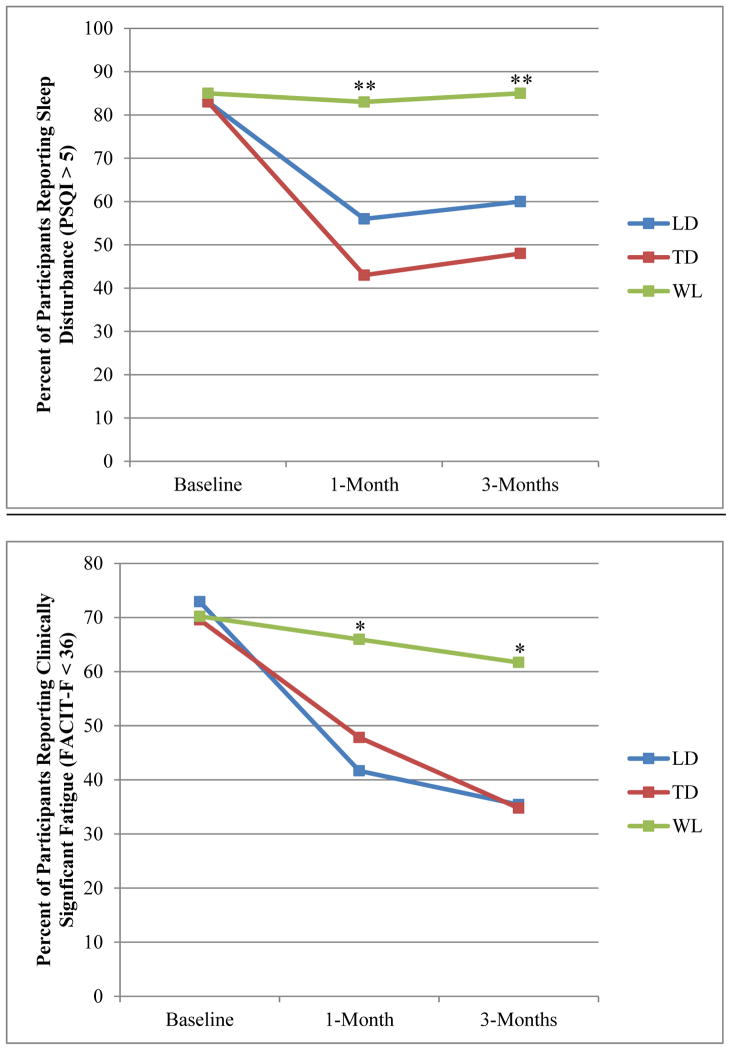
Exploratory χ2 analyses indicated no group differences in the percent of participants reporting clinically significant sleep disturbances (PSQI ≥ 5) at baseline (p = 0.77). However, significantly fewer individuals in the LD and TD reported clinically significant sleep disturbance compared to the WL at the 1- and 3-month follow-up (p’s < 0.01; Figure 2a). Similarly, groups did not differ in the percent of participants reporting clinically significant fatigue (FACIT-F < 36) at baseline, but fewer individuals in the LD and TD reported clinically significant fatigue compared to the WL at 1- and 3-month follow-up (p’s < 0.05; Figure 2b).
Figure 2.
Percent of participants reporting clinically significant sleep disturbance and fatigue
PSQI = Pittsburgh Sleep Quality Index; FACIT-F = Functional Assessment of Chronic Illness Therapy Fatigue Scale; **WL differed from TD and LD p < 0.01; *WL differed from TD and LD p < 0.05.
DISCUSSION
Our results are consistent with other studies that have shown beneficial effects of psychosocial interventions for improving QOL in cancer survivors.[1–3] Though SF-36 scores in the present study are slightly higher than those reported by oncology patients receiving telecare for pain and depression in rural community centers, pre- to post-treatment change in SF-36 scores was similar.[11] Additionally, the current sample reported baseline PSQI scores similar to women with breast cancer enrolled in MBSR, but showed greater improvement in PSQI scores following the intervention.[31] Further, a review of exercise interventions for breast cancer survivors indicated baseline FACIT-F scores almost 10 points higher than the present study, yet end of intervention FACIT-F scores in the present study are on par with those of exercise interventions.[32]
To our knowledge, the present study represents the first randomized trial of a telemedicine mind-body intervention delivered to a group of cancer survivors. Though the study was not powered to test equivalence, participating in the telemedicine-delivered intervention did not result in different outcomes compared to the intervention delivered in person. Further, using a conservative Bonferroni adjustment, the either form of the intervention resulted in significantly better cognitive function, and less fatigue, and sleep disturbance compared to individuals in WL group. Though the intervention and control groups did not significantly differ on health-related or breast cancer-specific QOL, distress, or spiritual well-being using the adjusted alpha, means were in the expected directions.
The improvements in cognitive function, fatigue, sleep disturbance, and mental health-related and breast cancer-related QOL were considered clinically significant. The intervention groups, but not the waitlist group, reported a ≥ 10 point improvement in cognitive function and fatigue from baseline to 3-month follow-up.[33, 34] Additionally, there were clinically significant improvements in sleep quality and fatigue in both interventions groups and no changes in the WL group.[23, 27] Further, though group differences in mental health-related and breast cancer-specific QOL did not reach statistical significance, means suggest that individuals in the intervention groups, but not in the waitlist group, experienced a clinically significant improvement in mental health-related QOL (≥ 5 point increase in MCS) and in breast cancer-related QOL (≥ 3 point increase in FACT-B subscale) at the 3-month follow-up.[35, 36] Thus, the ERL intervention results in clinically significant improvements in many facets of QOL.
There are some limitations to the current study. The overall sample size was relatively small, and unforeseen staffing limitations resulted in the telemedicine group being smaller than expected. Thus, it is possible that the present study was underpowered to detect differences between telemedicine compared to live and waitlist groups. However, post-hoc power analyses suggested that a sample size of >600 would be required to detect statistically significant differences between the two intervention groups on all outcomes aside from the PSQI. On the PSQI, a sample size of 424 would be required to detect significant intervention group differences, with the means in favor of the TD group.[37] Nonetheless, the relatively small sample size, particularly for the TD group, necessitates caution in interpreting these results and calls for validation of these findings in a larger study. Adherence to home practice was not documented, limiting our ability to examine a “dose effect” of the intervention. Future studies could document this by providing patients with practice logs or devices (such as MP3 players) equipped to document use of audio files, eliminating the bias inherent in self-reported practice. The lack of an active control group with which to compare the ERL program (versus just usual care) limits the ability to know that the effects are directly attributable to the specific content of the program versus non-specific effects such as social support or attention. Additionally, the present study did not specifically assess social support, making it difficult to examine or control for change in social support in the present analyses. In light of this, a subsequent trial should include an attention control group or other active program for comparison. Future research is needed to test the long-term benefits of participating in an imagery-based group intervention, after contact with therapists has concluded. Additionally, though the present study provides support for the use of telemedicine delivered at community centers in areas that may not have access to mental healthcare providers, future research is needed to examine home- or internet-based telemedicine interventions for survivors unable to travel even to community centers. Further, though the time commitment (five 4-hour weekly sessions) of the present intervention is similar to MBSR (eight 2.5-hour weekly sessions plus a full-day retreat), future research altering the modules to fit within the schedule of a work-week is warranted.[38] As is the challenge of all mind-body interventions,[39] determining optimal length and essential components of interventions is paramount to the dissemination of evidence-based treatment. There was low minority representation in the study and future research will examine the efficacy of the ERL program for minority groups. Finally, the present findings reflect subjective QOL outcomes, and do not include corroborating objective, biological measures of QOL.
The ERL program represents a mind-body program that comprehensively addresses many facets of QOL relevant to breast cancer survivors, including general health- and cancer-related QOL, spiritual well-being, cognitive function, fatigue, sleep disturbance, and distress. Further, our results suggest that telemedicine is an effective and viable method to deliver a group intervention aimed at improving QOL in breast cancer survivors.
Supplementary Material
Acknowledgments
This work was supported by a Small Business Innovation Research grant from NIH/NCI #2 R44 CA117597-02A2; and by a cancer prevention fellowship for Chelsea Ratcliff supported by the NCI grant R25T CA057730, Shine Chang, Ph.D., Principal Investigator.
We would like to acknowledge the Alaska Regional Hospital in for their unwavering support and the use of their facilities, the Washington State Army of Women for their help in recruitment, and the late Dr. Candace Pert for her unique contribution in designing the neuropeptides animation utilized in the study.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Andersen BL, Thornton LM, Shapiro CL, et al. Biobehavioral, Immune, and Health Benefits following Recurrence for Psychological Intervention Participants. Clinical Cancer Research. 2010 doi: 10.1158/1078-0432.CCR-10-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roffe L, Schmidt K, Ernst E. A systematic review of guided imagery as an adjuvant cancer therapy. Psychooncology. 2005;14(8):607–17. doi: 10.1002/pon.889. [DOI] [PubMed] [Google Scholar]
- 3.Eremin O, Walker MB, Simpson E, et al. Immuno-modulatory effects of relaxation training and guided imagery in women with locally advanced breast cancer undergoing multimodality therapy: a randomised controlled trial. Breast. 2009;18(1):17–25. doi: 10.1016/j.breast.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Lengacher CA, Bennett MP, Gonzalez L, et al. Immune responses to guided imagery during breast cancer treatment. Biol Res Nurs. 2008;9(3):205–14. doi: 10.1177/1099800407309374. [DOI] [PubMed] [Google Scholar]
- 5.Nunes DF, Rodriguez AL, da Silva Hoffmann F, et al. Relaxation and guided imagery program in patients with breast cancer undergoing radiotherapy is not associated with neuroimmunomodulatory effects. J Psychosom Res. 2007;63(6):647–55. doi: 10.1016/j.jpsychores.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Freeman L, Cohen L, Stewart M, et al. Imagery intervention for recovering breast cancer patients: clinical trial of safety and efficacy. J Soc Integr Oncol. 2008;6(2):67–75. [PubMed] [Google Scholar]
- 7.Hendren S, Chin N, Fisher S, et al. Patients’ barriers to receipt of cancer care, and factors associated with needing more assistance from a patient navigator. J Natl Med Assoc. 2011;103(8):701–10. doi: 10.1016/s0027-9684(15)30409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pruthi S, Stange KJ, Malagrino GD, Jr, Chawla KS, LaRusso NF, Kaur JS. Successful implementation of a telemedicine-based counseling program for high-risk patients with breast cancer. Mayo Clin Proc. 2013;88(1):68–73. doi: 10.1016/j.mayocp.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Sherman DW, Haber J, Hoskins CN, et al. The effects of psychoeducation and telephone counseling on the adjustment of women with early-stage breast cancer. Appl Nurs Res. 2012;25(1):3–16. doi: 10.1016/j.apnr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Zernicke KA, Campbell TS, Speca M, Kelley MR, Flowers S, Carlson LE. A Randomized Wait-List Controlled Trial of Feasibility and Efficacy of an Online Mindfulness-Based Cancer Recovery Program: The eTherapy for Cancer Applying Mindfulness Trial. Psychosomatic Medicine. 2014;76(4):257–267. doi: 10.1097/PSY.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 11.Kroenke K, Theobald D, Wu J, et al. Effect of telecare management on pain and depression in patients with cancer: a randomized trial. JAMA. 2010;304(2):163–71. doi: 10.1001/jama.2010.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poon P, Hui E, Dai D, Kwok T, Woo J. Cognitive intervention for community- dwelling older persons with memory problems: telemedicine versus face-to-face treatment. Int J Geriatr Psychiatry. 2005;20(3):285–6. doi: 10.1002/gps.1282. [DOI] [PubMed] [Google Scholar]
- 13.Schnur JB, Montgomery GH. E-counseling in psychosocial cancer care: a survey of practice, attitudes, and training among providers. Telemed J E Health. 2012;18(4):305–8. doi: 10.1089/tmj.2011.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jameson JP, Farmer MS, Head KJ, Fortney J, Teal CR. VA community mental health service providers’ utilization of and attitudes toward telemental health care: the gatekeeper’s perspective. J Rural Health. 2011;27(4):425–32. doi: 10.1111/j.1748-0361.2011.00364.x. [DOI] [PubMed] [Google Scholar]
- 15.Freeman LW, Lawlis GF. Mosby’s complementary & alternative medicine. Mosby; 2004. [Google Scholar]
- 16.Núñez MaJ, Mañá P, Liñares D, et al. Music, immunity and cancer. Life Sciences. 2002;71(9):1047–1057. doi: 10.1016/s0024-3205(02)01796-4. [DOI] [PubMed] [Google Scholar]
- 17.Manyande A, Berg S, Gettins D, et al. Preoperative rehearsal of active coping imagery influences subjective and hormonal responses to abdominal surgery. Psychosom Med. 1995;57(2):177–82. doi: 10.1097/00006842-199503000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Kiecolt-Glaser JK, Graham JE, Malarkey WB, Porter K, Lemeshow S, Glaser R. Olfactory influences on mood and autonomic, endocrine, and immune function. Psychoneuroendocrinology. 2008;33(3):328–39. doi: 10.1016/j.psyneuen.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ware J, Sherbourne C. The MOS 36-item shortform health survey. Medical Care. 1992:30. [PubMed] [Google Scholar]
- 20.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 21.Hwang SS, V, Chang T, Kasimis BS. A comparison of three fatigue measures in veterans with cancer. Cancer Invest. 2003;21(3):363–73. doi: 10.1081/cnv-120018227. [DOI] [PubMed] [Google Scholar]
- 22.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander S, Minton O, Stone PC. Evaluation of screening instruments for cancer- related fatigue syndrome in breast cancer survivors. Journal of Clinical Oncology. 2009;27(8):1197–1201. doi: 10.1200/JCO.2008.19.1668. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs SR, Jacobsen PB, Booth-Jones M, Wagner LI, Anasetti C. Evaluation of the functional assessment of cancer therapy cognitive scale with hematopoietic stem cell transplant patients. J Pain Symptom Manage. 2007;33(1):13–23. doi: 10.1016/j.jpainsymman.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Peterman AH, Min GFD, Brady MJ, Cella D. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy—Spiritual Well-being Scale (FACIT-Sp) Annals of Behavioral Medicine. 2002;24(1):49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 26.Derogatis LR. BSI, Brief Symptom Inventory: administration, scoring & procedures manual. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- 27.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 28.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, SO . SAS for Mixed Models. 2. Cary, NC: SAS Institute Inc; 2006. [Google Scholar]
- 29.Gibbons RD, Hedeker D, Waternaux C, Davis JM. Random regression models: a comprehensive approach to the analysis of longitudinal psychiatric data. Psychopharmacology Bulletin. 1988;24:438–443. [PubMed] [Google Scholar]
- 30.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Statistics in Medicine. 1997;16(22):2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 31.Lengacher CA, Reich RR, Paterson CL, et al. The effects of mindfulness-based stress reduction on objective and subjective sleep parameters in women with breast cancer: a randomized controlled trial. Psychooncology. 2014 doi: 10.1002/pon.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Battaglini CL, Mills RC, Phillips BL, et al. Twenty-five years of research on the effects of exercise training in breast cancer survivors: A systematic review of the literature. World J Clin Oncol. 2014;5(2):177–90. doi: 10.5306/wjco.v5.i2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547–61. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 34.Chan A, Foo YL, Guo MSH, et al. Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: Cognitive function (FACT-Cog) in breast cancer patients. Journal of Clinical Oncology. 2013;31(suppl):abstr 6589. doi: 10.1016/j.jclinepi.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Sloan JA, Frost MH, Berzon R, et al. The clinical significance of quality of life assessments in oncology: a summary for clinicians. Supportive Care in Cancer. 2006;14(10):988–998. doi: 10.1007/s00520-006-0085-y. [DOI] [PubMed] [Google Scholar]
- 36.Eton DT, Cella D, Yost KJ, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004;57(9):898–910. doi: 10.1016/j.jclinepi.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 38.Kabat-Zinn J. Mindfulness-based interventions in context: past, present, and future. Clinical Psychology: Science and Practice. 2003;10(2):144–156. [Google Scholar]
- 39.Carmody J, Baer RA. How long does a mindfulness-based stress reduction program need to be? A review of class contact hours and effect sizes for psychological distress. J Clin Psychol. 2009;65(6):627–38. doi: 10.1002/jclp.20555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.