Abstract
There have been few reports of acute liver failure (ALF, with encephalopathy and coagulopathy) due to infiltration of the liver by malignant cells. We describe a case series of 27 patients with ALF caused by malignancy. We examined a large, multi-center ALF registry (1910 patients; mean age, 47.1±13.9 years) and found only 27 cases (1.4%) of ALF attributed to malignancy. Twenty cases (74%) presented with abdominal pain and 11 with ascites. The malignancies included lymphoma or leukemia (33%), breast cancer, (30%), and colon cancer (7%); 90% of the patients with lymphoma or leukemia had no history of cancer, compared to 25% of patients with breast cancer. Overall, 44% of the patients had evidence of liver masses by imaging. Diagnosis was confirmed by biopsy in 15 (55%) and autopsy for 6 cases. Twenty-four patients (89%) died within 3 weeks of ALF.
Keywords: acute liver failure, malignancy, liver transplantation
Background & Aims
Acute liver failure (ALF) is defined as the presence of encephalopathy and coagulopathy (INR > 1.5) in the absence of pre-existing liver disease, with an illness of <26 weeks duration1. Among the multitude of known etiologies of ALF in adults, APAP overdose (46%), idiosyncratic DILI (11%), viral hepatitis (9%) and autoimmune hepatitis (7%) are most common2. A rarely reported cause of ALF is malignant infiltration of the liver due to either a solid organ tumor or hematological malignancy5–20. Despite the fact that the liver is the most common location for hematogenous spread of solid tumors, clinically severe hepatic dysfunction with coagulopathy and encephalopathy as a result of metastatic tumor involvement is rare. In a large single center study performed at the King’s College in London3, only 18 patients (0.44%) with this condition were identified over an 18-year period (1978–1995).
The Acute Liver Failure Study Group (ALFSG) network has investigated the causes and outcomes of ALF in United States adults since 1998. In this study, we determined the incidence and outcomes of ALF due to malignant infiltration of the liver over a 15-year period.
Methods
Between January 1998 and February 2012, 1910 adult patients with ALF of all etiologies were enrolled in the ALFSG registry from a total of 23 sites. All patients exhibited some degree of encephalopathy and coagulopathy (prothrombin time >15 seconds or international normalized ratio [INR] > 1.5) in the absence of pre-existing liver cirrhosis, with an illness of <26 weeks duration1. Cases of ALF secondary to malignancy were identified in 27 of 1910 patients (1.4%).
Informed consent was obtained from the each patient’s next of kin prior to study enrollment. Study approval was obtained from the local IRB at all 23 sites.
Results
Patient demographics
The patient group was relatively young, with mean age of 47.1. Eighteen were female (Table 1). Common etiologies were leukemia/lymphoma in 11 (41%) and breast cancer in 8 (30%). The remainder were colon cancer (2), and one each signet ring adenocarcinoma, uterus, prostate, thyroid, pancreas and small cell lung cancer. All tumors were metastatic with no liver or biliary primary tumors leading to ALF.
Table 1.
Clinical and laboratory features of patients admitted with malignant infiltration of the liver presenting as ALF.
| Demographics | ||||
|---|---|---|---|---|
| Characteristics | Overall | Breast cancer N=8 |
Lymphoma/leukemia n=11 | Other solid tumors n=8 |
| Age (years) | 47.1 ±13.9 | 49.2 ±12.3 | 44.7 ±14.3 | 48.2 ±14.2 |
| Sex, % female | 67% | 100% | 36% | 75% |
| Race % Caucasian |
67% | 63% | 73% | 63% |
| % African-American | 26% | 25% | 18% | 37% |
| % Asian-American | 4% | 12% | - | - |
| % Hispanic | 4% | - | 9% | - |
| Lab features at presentation (Median) | ||||
| AST (IU/L) | 963 (125–7197) | 948 (370–5706) | 2466 (125–7197) | 367 (142–3266) |
| ALT (IU/L) | 673 (46–6904) | 466 (46–2439) | 1161 (112–6904) | 427 (141–1677) |
| Alkaline phosphatase (IU/L) | 393 (110–3301) | 547 (166–902) | 280 (110–2043) | 367 (254–3301) |
| Total bilirubin (mg/dL) | 12.3 (1.3–30.7) | 12.7 (1.3–30.7) | 12.2 (5.1–26.0) | 14.4 (2.7–27.0) |
| Creatinine (mg/dL) | 1.9 (0.5–5.6) | 1.8 (0.5–3.3) | 2.1 (0.6–5.6) | 1.7 (0.7–5.4) |
| INR | 2.1 (1.4–3.9) | 2.2 (1.6–3.9) | 2.0 (1.4 –3.1) | 2.0 (1.6–3.4) |
| WBC (× 103/mm3) | 12.0 (1.8–47.6) | 13.3 (8.5–21.9) | 8.8 (1.8–47.6) | 13.5 (5.8–29.1) |
| Hgb (g/dL) | 9.7 (7.4–16.0) | 10.8 (7.5–14.8) | 8.6 (7.4 –16.0) | 9.5 (7.5–12.5) |
| Plts × 103 uL | 106 (11–303) | 101 (11–259) | 74 (12–141) | 130 (28–303) |
| Clinical status at presentation | ||||
| % Intubated on arrival | 11% (3/27) | 25% (2/8) | 9% (1/11) | 0% (0/8) |
| % Required hemodialysis | 7% (2/27) | - | 9% (1/11) | 12% (1/8) |
| % Infected | 4% (1/27) | 12% (1/8) | - | - |
| % Grade 3–4 Encephalopathy | 22% (6/27) | 25% (2/8) | 27% (3/11) | 13% (1/8) |
| Outcomes | ||||
| % Salvage chemotherapy | 11% (3/27) | 13% (1/8) | 9% (1/11) | 13% (1/8) |
| % OLT | 7% (2/27) | 0% (0/8) | 9% (1/11) | 13% (1/8) |
| % Alive at 3 weeks post-study enrollment | 11% (3/27) | 13% (1/8) | 9% (1/11) | 13% (1/8) |
| Median time to death after admission (days) | 10.5 (3–28) | 7 (3–13) | 12 (6–28) | 14 (8–21) |
All laboratory values refer to those noted on admission.
WBC= White blood cell count; INR = International Normalized Ratio; OLT= Orthotopic liver transplant.
Clinical features
Time between onset of symptoms and admission to the hospital was relatively rapid (median 10 days). Twenty patients presented with abdominal pain and 11 with ascites prior to the onset of jaundice. Only two patients were noted to have splenomegaly and three with hepatomegaly by physical exam.
Malignancies identified
Only 11 patients had a history of prior malignancy. By contrast, presentation with rapid onset liver failure represented a new cancer diagnosis for 16, including 8 of 9 patients diagnosed with lymphoma, 2 patients without a prior history of breast cancer, 2 patients with leukemia, 1 patient each with cancers of the colon, lung (small cell), pancreas, and signet cell type, respectively.
Laboratory values on admission
Laboratory values were not specific for any group of tumors but generally reflected a mixed hepatocellular/cholestatic picture with prolonged INR and low platelet counts (Table 1). Only 12 of 27 (44 %) had evidence of liver infiltration or distinct liver mass(es) noted on imaging (CT, US).
Diagnostic challenges
The diagnosis was obtained by pre-mortem liver biopsy in 16 patients, bone marrow biopsy in 2 patients (both with lymphoma), and in 2 patients only by explant pathology following OLT. One patient with acute leukemia was diagnosed by laboratory results (with evidence of blasts on peripheral smear) as well as hepatomegaly and splenomegaly on imaging. The correct diagnosis was revealed only at autopsy without prior pathology in two of the 27 cases.
Special testing
Immunohistochemistry was performed to obtain hormone receptor status (estrogen receptor, ER, progesterone receptor, PR and Human Epidermal Growth Factor 2, HER2) in 5 of the 8 breast cancer cases. No consistent pattern was observed in this small series-- no correlation between indicators of poor prognosis and the predisposition to develop ALF in this study.
Of the nine lymphoma cases, 5 were diffuse large B-cell lymphomas, the most common type of non-Hodgkin lymphoma in the adult population. Three were T-cell lymphomas. None of the patients had a known history of prior immunosuppression or autoimmune disease.
Clinical Course
Early mortality was nearly universal in this study population, with death occurring within 3 weeks of study enrollment in 23 patients (85%).
One patient noted to have spontaneous (or transplant-free) long-term survival was a previously healthy 47-year-old female who initially presented with fever, abdominal pain and distension. She was classified as ALI (Acute Liver Injury), given the lack of encephalopathy on presentation. Laboratory studies on admission revealed ALT 1138, AST 2896, INR 3.5, bilirubin 11.1, and platelets 20,000. Transjugular liver biopsy revealed metastatic breast adenocarcinoma in the liver sinusoids with multiple lobular foci of necrosis (Figure 1), and subsequent mammogram revealed a 4 cm right breast mass. She was HER-2 positive and ER/PR negative. On treatment with carboplatin, 5-FU, and Herceptin she improved clinically, was discharged, and continued on outpatient chemotherapy. Notably, she had remarkable clinical improvement to the point of being able to return to work and no further hospitalizations. Five months later, she was noted to have evidence of local progression in the breast and was switched to paclitaxel and Herceptin. At her 2-year follow-up visit she was alive and well, still working full time.
Figure 1.
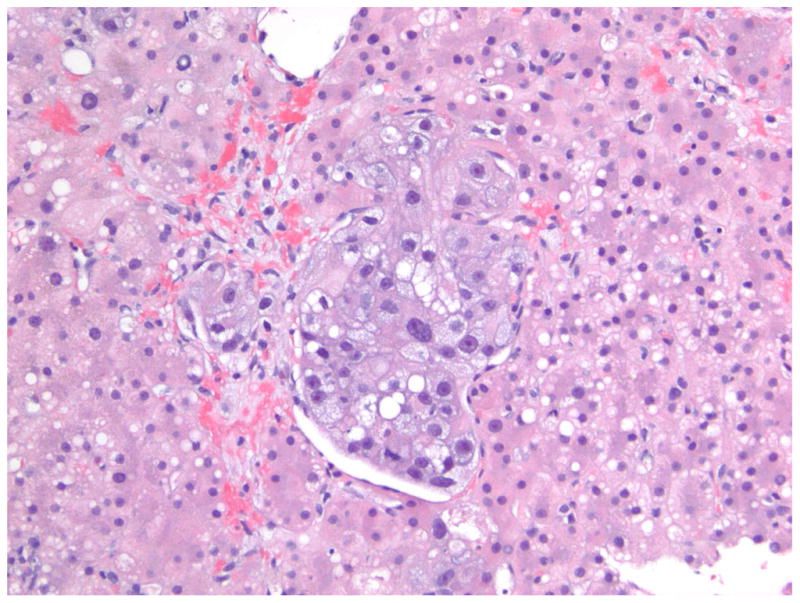
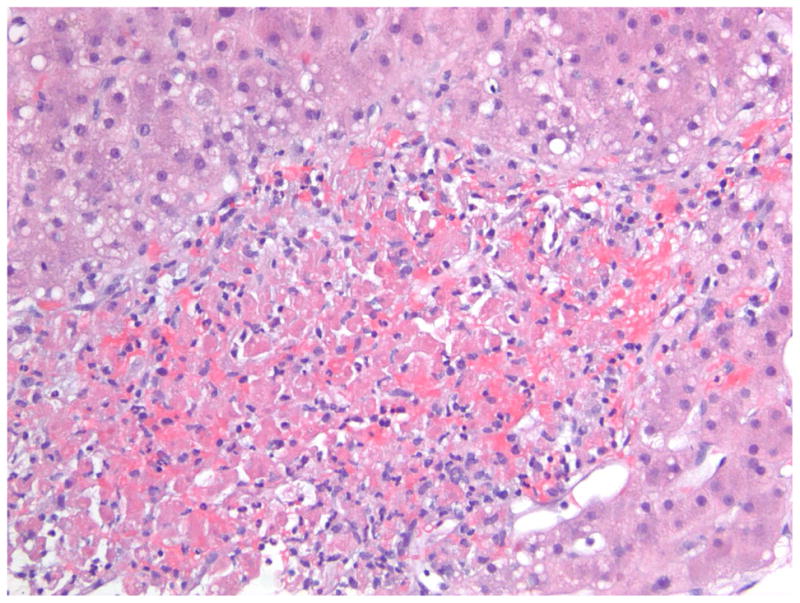
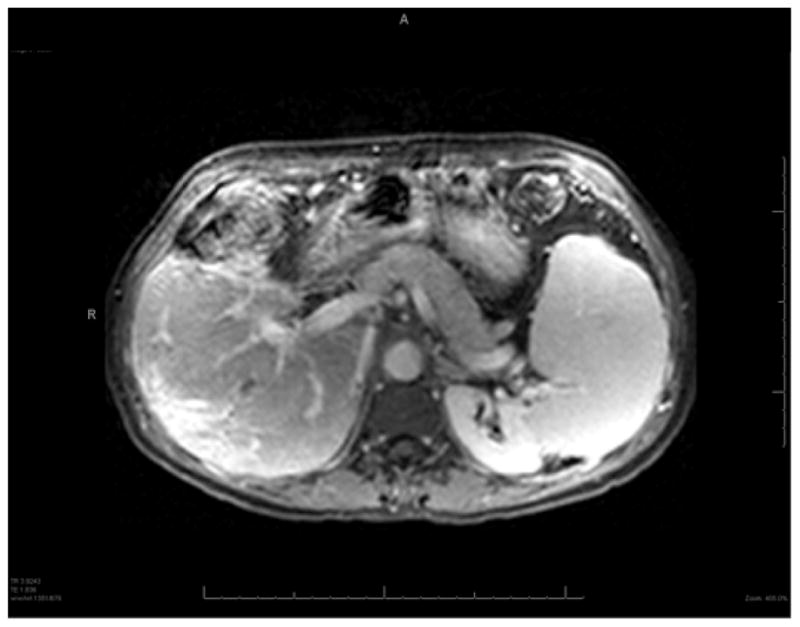
A 47-year old female presented with ALF. A transjugular liver biopsy demonstrated A) In the center of the field, there is a plug of metastatic carcinoma that is filling and obstructing a sinusoid (Hematoxylin and Eosin, 200X). B) At the lower center, there is a zone of acute hemorrhagic necrosis containing necrotic liver cell debris and blood (Hematoxylin and eosin, 200X). The pattern of necrosis suggests an acute interference of incoming blood flow from the portal venule or hepatic artery, presumably embolic in nature from the metastatic breast cancer. (Photomicrographs courtesy of Henry D. Appelman, MD). C) Dynamic contrast enhanced MRI images of the same 47-year-old female with metastatic breast cancer to the liver at month 18 of follow-up following her ALF episode. The liver has a nodular surface contour consistent with “pseudo-cirrhosis” from partially treated malignant infiltration and a small focus of hypointensity indicative of residual tumor. The patient developed esophageal varices during follow-up that required band ligation.
Of the two OLT recipients, a 36-year-old pregnant female (at 27 weeks) who presented with pruritus and right upper quadrant pain was discovered to have multiple smaller liver masses. Following delivery at 29 weeks, she underwent liver transplantation shortly thereafter, the explant organ revealing metastatic adenocarcinoma to which she succumbed 18 months later.
A previously healthy 57-year-old male who presented with 1 month of malaise and jaundice; a biopsy revealed massive hepatocyte necrosis without fibrosis. He underwent OLT, and the diagnosis of large B-cell lymphoma was made only upon examination of the surgical explant. On chemotherapy with a four -drug regimen with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), and was he was alive and well 5 years after the initial diagnosis.
Discussion
The diagnosis of ALF secondary to malignancy can be challenging and may elude clinicians initially, particularly in patients with no previous cancer history. The two most common etiologies of malignant infiltration of the liver we observed were lymphoma and breast cancer, similar to prior studies and to general trends for common malignancies. Notably, there were no melanomas, endocrine tumors (e.g., APUDomas), or primary hepatic malignancies. There were only two patients with colon cancer.
Common presenting features were jaundice, hepatic encephalopathy and hepatomegaly (noted in 12 of the 27 patients in this study).
Standard laboratory values were not helpful in identifying the presence of malignancy, except in the case of the patient with leukemia. Aminotransferases were generally very elevated (above ~40 times the upper limit of normal) with necrosis of this degree seeming unusual with diffuse infiltration of the liver. Final diagnoses ultimately required tissue diagnosis, absent a prior known tumor; 63% of patients in this study underwent a confirmatory biopsy.
Radiologic studies in this patient group are typically notable for the absence of large mass lesions4, and in some cases reveal only a nodular liver that has been described as “pseudocirrhosis”5, since fulminant presentations appear to be associated commonly with diffuse intra-sinusoidal infiltration. Lymphadenopathy may also be seen on CT. Ultrasound was often nonspecific. CT revealed hepatomegaly or distinct masses in nearly half of our cases. Most, but not all, patients with breast cancer had a previously known diagnosis, and in this setting imaging alone suggested the diagnosis, and only 3 of 8 required liver biopsy. Conversely, in patients with lymphoma, only 1 of 9 patients had a known prior lymphoma diagnosis.
Liver biopsy changed the initial diagnosis from indeterminate to cancer in 10 of 27 patients in this series, highlighting the importance of early tissue sampling. It seems likely that patients presenting with rapid onset of liver failure with high aminotransferases represent examples of sinusoidal obstruction with micro-infarcts.3, 19.
In most patients with ALF secondary to malignant infiltration of the liver, the prognosis is dismal. Metastatic malignancy has long been considered an absolute contraindication to liver transplantation. However, it is of interest that two transplanted patients in our series were unrecognized prior to transplantation and did indeed have prolonged survival, one still alive after more than 5 years. Had the diagnosis of malignancy been established beforehand, it is probable that both of these individuals would have been excluded as transplant candidates.
Conclusions
Clinicians must have a high index of suspicion when approaching a case of ALF of indeterminate etiology. Hepatomegaly may be one of the only clues. Imaging is often non-diagnostic. Early liver biopsy in indeterminate cases must be considered as it can indicate the direction of future care: avoidance of needless transplantation and enabling more appropriate end-of-life care, or more aggressive steps in some circumstances. There may be a role for OLT in very select patients with ALF secondary to malignancy, particularly those with tumor types known to be sensitive to chemotherapy and when long-term survival is probable.
Acknowledgments
The authors would like to thank Dr. Henry D. Appelman, (Professor, Dept of Pathology, University of Michigan Health System) for the preparation of photomicrographs for this article.
Grant Support: The ALFSG was supported by NIDDK U01-DK-58369 to UT Southwestern Medical Center at Dallas.
Abbreviations
- ALF
Acute Liver Failure
- ALFSG
Acute Liver Failure Study Group
Footnotes
Disclosures: None
Writing Assistance: None
Author Contributions: See above.
ALFSG Sites 2010–2012: UT Southwestern Medical Center; University of Washington; University of California-San Francisco; Northwestern University; University of California-Los Angeles; University of Michigan; Yale University; University of Alabama Birmingham; Massachusetts General Hospital; Medical University of South Carolina; University of Pennsylvania; Virginia Commonwealth University; California Pacific Medical Center
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nicole E. Rich, Email: Nicole.rich@phhs.org, The University of Texas Southwestern Medical Center, Dallas, Texas. (Acquisition of data, analysis and interpretation of data; drafting of the manuscript)
Corron Sanders, Email: Corron.sanders@UTSouthwestern.edu, The University of Texas Southwestern Medical Center, Dallas, Texas. (Acquisition of data, analysis and interpretation of data; statistical analysis)
Randall S. Hughes, Email: Randall.Hughes@UTSouthwestern.edu, The University of Texas Southwestern Medical Center, Dallas, Texas. (Critical revision of the manuscript for important intellectual content)
Robert J. Fontana, Email: rfontana@med.umich.edu, The University of Michigan Medical Center, Ann Arbor, Michigan. (Acquisition of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content)
R. Todd Stravitz, Email: rstravit@vcu.edu, Virginia Commonwealth University, Richmond, Virginia. (Acquisition of data)
Oren Fix, Email: Oren.fix@ucsf.edu, University of California, San Francisco, San Francisco, California. (Acquisition of data)
Steven H. Han, Email: sbhan@mednet.ucla.edu, University of California, Los Angeles, Los Angeles, California. (Acquisition of data)
Willscott E. Naugler, Email: nauglers@ohsu.edu, Oregon Health and Science University, Portland, Oregon (Acquisition of data)
Atif Zaman, Email: zamana@ohsu.edu, Oregon Health and Science University, Portland, Oregon. (Acquisition of data)
William M. Lee, Email: William.lee@UTSouthwestern.edu, The University of Texas Southwestern Medical Center, Dallas, Texas. (Study concept and design, drafting of the manuscript, critical revision of the manuscript, study supervision)
References
- 1.Polson J, Lee WM. AASLD position paper: The management of acute liver failure. Hepatology. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 2.Ostapowicz G, Fontana RJ, Schiødt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 3.Rowbotham D, Wendon J, Williams R. Acute liver failure secondary to hepatic infiltration: a single centre experience of 18 cases. Gut. 1998;42:576–580. doi: 10.1136/gut.42.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allison KH, Fligner CL, Parks WT. Radiographically occult, diffuse intrasinusoidal hepatic metastases from primary breast carcinomas: a clinicopathologic study of 3 autopsy cases. Arch Pathol Lab Med. 2004;128:1418–1423. doi: 10.5858/2004-128-1418-RODIHM. [DOI] [PubMed] [Google Scholar]
- 5.Sass DA, Clark K, Grzybicki Diffuse desmoplastic metastatic breast cancer simulating cirrhosis with severe portal hypertension: a case of “pseudocirrhosis”. Dig Dis Sci. 2007;52:749–752. doi: 10.1007/s10620-006-9332-9. [DOI] [PubMed] [Google Scholar]
- 6.Esfahani K, Gold P, Wakil S, et al. Acute liver failure because of chronic lymphocytic leukemia: case report and review of the literature. Curr Oncol. 2011;18:39–42. doi: 10.3747/co.v18i1.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson DR, Faust TW, Stone MJ, et al. Hepatic failure as the presenting manifestation of malignant lymphoma. Clin Lymphoma. 2001;2:123–128. doi: 10.3816/clm.2001.n.018. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh P, Fox IJ, Rader AM, et al. Fulminant hepatic failure as the initial manifestation of non-Hodgkins lymphoma. Am J Gastroenterol. 1995;90:2207–2209. [PubMed] [Google Scholar]
- 9.Lettieri CJ, Berg BW. Clinical features of Non-Hodgkins lymphoma presenting with acute liver failure: a report of five cases and review of published experience. Am J Gastroenterol. 2003;98:1641–1646. doi: 10.1111/j.1572-0241.2003.07536.x. [DOI] [PubMed] [Google Scholar]
- 10.Goswami R, Babich M, Farah K. Occult breast malignancy masquerading as acute hepatic failure. Gastroenterology & Hepatology. 2011;7:62–64. [PMC free article] [PubMed] [Google Scholar]
- 11.Nazario HE, Lepe R, Trotter JF. Metastatic breast cancer presenting as acute hepatic failure. Gastroenterology & Hepatology. 2011;7:65–66. [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal K, Jones DE, Burt AD, et al. Metastatic breast carcinoma presenting as acute liver failure and portal hypertension. Gastroenterol Hepatol. 2002;97:750–751. doi: 10.1111/j.1572-0241.2002.05559.x. [DOI] [PubMed] [Google Scholar]
- 13.Montero JL, Muntane J, de las Heras S, et al. Acute liver failure caused by diffuse hepatic melanoma infiltration. Journal of Hepatology. 2002;37:540–541. doi: 10.1016/s0168-8278(02)00219-2. [DOI] [PubMed] [Google Scholar]
- 14.Te HS, Schiano TD, Kahaleh M, et al. Fulminant hepatic failure secondary to malignant melanoma: case report and review of the literature. Am J Gastroenterol. 1999;94:262–266. doi: 10.1111/j.1572-0241.1999.00811.x. [DOI] [PubMed] [Google Scholar]
- 15.Lowenthal A, Tur-Kaspa R, Brower RG, et al. Acute liver failure complicating ductal breast carcinoma: two cases and literature review. Scand J Gastroenterol. 2003;38:1095–1096. doi: 10.1080/00365520310005370. [DOI] [PubMed] [Google Scholar]
- 16.Sawabe M, Kato Y, Ichirou O, et al. Diffuse intrasinusoidal metastasis of gastric carcinoma to the liver leading to fulminant hepatic failure. Cancer. 1990;65:169–173. doi: 10.1002/1097-0142(19900101)65:1<169::aid-cncr2820650132>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Kaira K, Takise A, Watanabe R, et al. Fulminant hepatic failure resulting from small-cell lung cancer and dramatic response of chemotherapy. World J Gastroenterol. 2006;12:2466–2468. doi: 10.3748/wjg.v12.i15.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagedorn S, Kelly W. Small cell carcinoma presenting as fulminant hepatic failure. Chest. 2011;140 Meeting abstracts 1A. [Google Scholar]
- 19.Ihara N, et al. Diffuse intrasinusoidal liver metastasis of small cell lung cancer causing fulminant hepatic failure: CT findings –a case report. Radiation Medicine. 2001;19:275–277. [PubMed] [Google Scholar]
- 20.Saikia UN, Sharma N, Duseja A, et al. Anaplastic large cell lymphoma presenting as acute liver failure: A report of two cases with review of literature. Ann Hepatology. 2010;9:457–461. [PubMed] [Google Scholar]


