Abstract
Acute promyelocytic leukemia (APL) comprises approximately 5–10% of childhood acute myeloid leukemia (AML) cases in the US. While variation in this percentage among other populations was noted previously, global patterns of childhood APL have not been thoroughly characterized. In this comprehensive review of childhood APL, we examined its geographic pattern and the potential contribution of environmental factors to observed variation. In 142 studies (spanning >60 countries) identified, variation was apparent—de novo APL represented from 2% (Switzerland) to >50% (Nicaragua) of childhood AML in different geographic regions. Because a limited number of previous studies addressed specific environmental exposures that potentially underlie childhood APL development, we gathered 28 childhood cases of therapy-related APL, which exemplified associations between prior exposures to chemotherapeutic drugs/radiation and APL diagnosis. Future population-based studies examining childhood APL patterns and the potential association with specific environmental exposures and other risk factors are needed.
Keywords: acute promyelocytic leukemia, AML-M3, pediatric leukemia, therapy-related leukemia, environmental exposure, risk factors
INTRODUCTION
Leukemia is the most common type of cancer in children
Leukemia, the most common type of cancer in children [1], accounts for 25–35% of cases of childhood cancer in most populations [1, 2]. Acute myeloid leukemia (AML) and acute lymphocytic leukemia (ALL) comprise the two major subtypes of childhood leukemia, with ALL accounting for 76% of childhood leukemia cases [3, 4]. AML, the second largest subgroup in children but the most common leukemia type among adults [3], represents 15–20% of leukemia cases in children, and is responsible for up to 30% of pediatric leukemia related deaths [4, 5].
De novo acute promyelocytic leukemia (APL), a subtype of AML, represents about 5–10% of childhood AML cases in the United States [6]. Previous studies, in which the majority of cases were reported from clinical trials or treatment protocols, rather than population-based analyses, have suggested that in certain Latin American, European and African populations, APL comprises relatively higher percentages of childhood AML [7]. However, variation in incidence among geographic regions has not been formally explored at a global level.
This review not only provides an overview of childhood APL, but also aims to: 1) examine childhood APL as a proportion of AML in countries around the world in order to gain insight into potential global geographic patterns; 2) analyze whether a previously hypothesized gender predominance in childhood APL cases exists; and, 3) discuss the potential contribution of environmental risk factors to the development of APL, using the example of exposure to previous therapy for primary diseases.
APL is a relatively well-characterized subtype of AML
AML encompasses a heterogeneous group of leukemias characterized by increased proliferation of myeloid cells in the bone marrow [8]. Among the subtypes of AML, APL is of particular interest due to its well-characterized etiology. With targeted treatment involving chemotherapy and all-trans retinoic acid (ATRA), the survival rate of APL in children is relatively high (75–80%) [9]. Additionally, variation in the incidence of APL as a percentage of total childhood AML across certain racial/ethnic groups and geographic regions has been previously observed, and is potentially attributable to certain environmental exposures.
The etiology, molecular mechanisms, and treatment of APL have been comprehensively studied. In 1990, based on the observation that retinoic acid, a vitamin A derivative, is able to induce in vivo differentiation of APL cells into mature granulocytes, a French team of researchers examined the retinoic acid receptor gene (RARα) and discovered that the t(15;17) translocation, characteristic of the majority of APL cases, involved the RARα gene (located on chromosome 17) and the PML locus on chromosome 15, resulting in PML/RARα fusion products [10, 11].
APL classification is based on morphological and cytogenetic information
Under the French-American-British (FAB) classification system, AML is categorized into eight subtypes (AML-M0 to M7) based on morphological features, as well as percentage and maturation of myeloblasts [12]. Under the FAB system, APL is characterized as subtype AML-M3, in which the predominant cells are promyelocytes with heavy granules and Auer rods. Diagnosing the microgranular variant of APL (AML-M3v) can be difficult because its morphological and cytochemical features are often non-specific, leading to misdiagnosis as AML-M4 or AML-M5 [13]. For these reasons, APL diagnosis by morphology alone has its limitations.
Random somatic chromosomal abnormalities resulting in fusion gene rearrangements are common in the malignant cells of patients with AML. About 95% of APL cases are characterized by recurrent chromosomal rearrangements of the RARα gene located on chromosome 17 [14], with the majority involved in a t(15;17)(q24;q21) translocation where the RARα gene fuses to the PML gene on chromosome 15. Eight rare partner genes (in addition to PML) which fuse to RARα have been previously described: NPM1, NUMA1, PLZF, PRKAR1A, FIP1L1, BCOR, STAT5B and a yet unidentified gene. These are represented by cytogenetic abnormalities t(5;17)(q35;q21), t(11;17)(q13;q21), t(11;17)(q23;q21), del(17)(q21;q24)/t(17;17)(q21;q24), t(4;17)(q12;q21), t(X;17)(p11;q12), der(17) and t(3;17)(p25;q21), respectively [15–22].
Recently, the AML committee of the International BFM Study Group published guidelines for the diagnosis and management of AML in children and adolescents with recommendations that cytogenetic and molecular methods should be performed in order to stratify AML subgroups by risk [23]. The advent of cytogenetic analysis has allowed the identification and categorization of recurring chromosomal aberrations associated with some AML subtypes, leading to the World Health Organization (WHO) classification system [24, 25]. Under the WHO classification system [26], AML subtypes are defined using more comprehensive information sources, including genetic, immunophenotypic, biological and clinical features, rather than morphology alone [27]. Under this system, APL (ICD-10 C92.4) falls into a category of myeloid leukemia with recurrent genetic abnormalities [28].
Variation in the geographic distribution of childhood APL may involve genetic and environmental factors
A notable epidemiologic feature of pediatric APL is that observed incidence rates, based on data from hospital-based registries and clinical trials, differ markedly among certain ethnic groups and geographic regions [14, 29]. Previous studies reported a high frequency of APL in certain Latin American, European and African populations, accounting for 17–58% of pediatric AML cases and 22–37% of adult AML cases [7]. APL incidence in studies like these traditionally has been estimated based on its relative frequency among other AML subtypes in large clinical trials because population-based registries did not distinguish APL from other AML subtypes until recently, and as a result, the true incidence rate of APL is nearly unknown [29].
The geographic variation in relative frequency of childhood APL potentially suggests that genetic predisposition towards APL and/or environmental exposures to specific risk factors may be involved [14]. Genetic predisposition may influence susceptibility to breakage at the site involved in chromosomal translocations, such as t(15;17), in APL [7]. In addition, nutritional and environmental factors [30], obesity at diagnosis [31], as well as dietary or metabolic patterns of ingested vitamin A (or its derivatives) [7, 32] have suggested associations with APL. Besides suggested genetic and environmental factors, exposure to chemotherapeutic drugs and other toxins may also contribute to APL development.
Therapy-related childhood APL can occur following treatment for a primary malignancy
Development of therapy-related AML (t-AML) and APL (t-APL) is a potential long-term complication of exposure to high doses of chemotherapy and/or radiation involved in treatment of primary diseases, and leukemia that arises following exposure to chemotherapy is primarily AML [33]. Previous studies have suggested that radiation and chemotherapy with alkylating agents and topoisomerase II inhibitors are potentially implicated in the development of t-APL specifically [34–36]. In recent years, the development of t-AMLs have been a cause of increasing concern due to the increase in the number of individuals surviving primary malignancies [37]. Under the WHO classification of AML (ICD-10 C92.0), t-AMLs following chemotherapy are considered to be distinct diagnostic entities [38], and the system recognizes two types of t-AML based on causative therapy: alkylating agent/radiation–related and a topoisomerase II inhibitor–related types [27]. Similar to therapy-related leukemias with MLL translocations, t-APL following treatment with topoisomerase II inhibitors has distinct breakpoints at chromosomal translocations involving the RARα gene, which appear to be caused by the drug-topoisomerase ‘cleavable complexes’ [39, 40]. Such breakpoint features are a direct link between a causal exposure and leukemia, which hopefully could be extended to de novo or idiopathic APL in the future.
Environmental exposures are implicated in APL and AML development
Other than exposure to radiation and drugs associated with therapy for a primary disease, AML development has also been associated with a variety of different environmental risk factors in both adults and children. In adults, an increased risk of AML has been strongly associated with exposure to ionizing radiation and benzene [41]. Additionally, exposures to other toxic chemicals and occupational hazards have been associated with AML in adults [3]. Due to the relative rarity of childhood AML and APL, fewer epidemiological studies addressing environmental exposure have been conducted [41]. Childhood exposure to petroleum solvents [42], as well as in utero exposure to ionizing radiation [43], and parental smoking [44], are a few among a range of risk factors reported as being potentially associated with development of childhood AML. In a recent case-control study of California children, Heck et al examined associations between air toxics exposures in pregnancy and early life in relation to leukemia in young children, and found that risk of AML was increased with 3rd trimester exposures to chloroform, benzene, and two other traffic-related chemicals (meta/para-xylene and toluene) [45]. There appear to be a limited number of studies that have previously examined childhood exposure and the development of childhood APL specifically.
Relevance of examining the geographic pattern of childhood APL
In this review, we aim to examine regional variation in the global geographic pattern of childhood APL. If the variation exists, it could potentially reflect the involvement of genetic, cultural, and environmental exposure related factors. Therefore, comprehensive characterization of such variation could help with the design of studies to examine the contribution of these factors. Data on childhood APL incidence is lacking for many global regions, however. Ribeiro and Rego have reported previously that a lack of population-based registries in developing countries makes determining the true frequency of APL difficult [29]. Recently, a population-based study of childhood leukemia in Brazil demonstrated that substantial regional differences in the incidence of AML; this finding, which corroborates hospital-based data described previously, warrants further ecological study [46]. Because the vast majority of the published data in APL came from clinical and/or descriptive epidemiologic data, the real incidence rate of APL is still unknown even in well-developed countries.
In the current study, we measured the frequency of childhood APL cases as a percentage of childhood AML, based on data from hospital-, study- or registry-based populations around the world. We sought to examine geographic variation and potential contributions, and to highlight regions of the world where data are not available so further studies including these areas can be conducted to increase the scope of the current understanding of this disease.
MATERIALS AND METHODS
Study Selection and Criteria
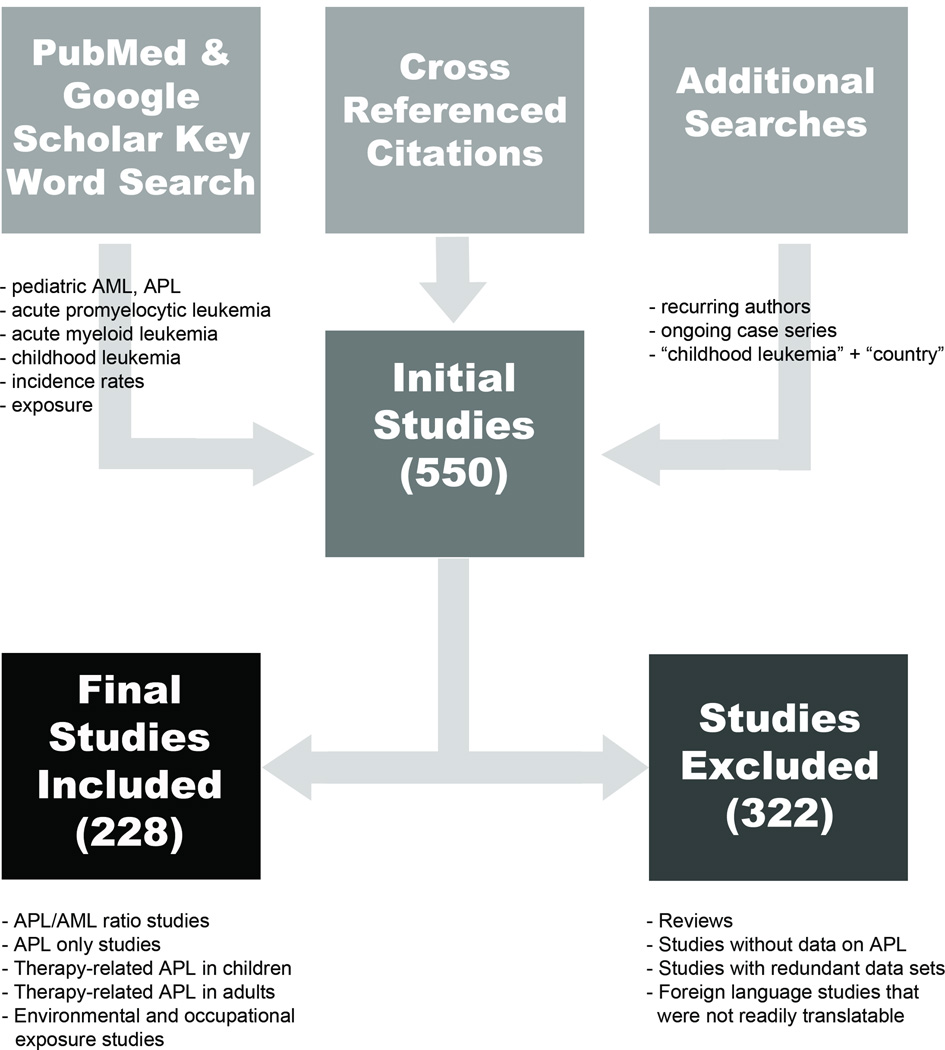
Broad literature searches were conducted from June 2011 to February 2014 using PubMed and Google Scholar databases to locate original, peer-reviewed research and review articles related to childhood AML, APL, t-APL, geographic distributions, potential risk factors. Initially, combinations of relevant key words were used to search for relevant studies, and terms used included: acute myeloid leukemia (leukaemia), pediatric (paediatric) acute promyelocytic leukemia, childhood leukemia, incidence rate, and exposure (Figure 1). Following this initial search, we systematically searched for studies across six continents by using a combination of specific country names with the phrases “acute myeloid leukemia”, “acute promyelocytic leukemia” and/or “childhood leukemia.” Cross-referencing citations of all relevant articles, searching for frequently recurring author names, and searching for ongoing leukemia clinical trials also identified additional studies.
Figure 1. Study selection process.
This flow diagram depicts the logic of the study selection process, the results of which are included in this review. In total, 228 studies were included.
Inclusion criteria
To be included, relevant studies had to contain information regarding a series of leukemia cases in a hospital or registry-based population where the number of cases of both childhood AML and APL were available. If multiple publications reported overlapping data from the same group of subjects, only the study with most recent and complete data was included. Information from the abstracts of relevant studies for which the full text was unobtainable was abstracted when possible. Studies in languages other than English were included if they were readily translatable by coauthors (written in Spanish or Chinese), or if they had a sufficiently informative English abstracts.
Exclusion criteria
Reviews (if all the original studies relevant to APL cited in the review were available to us), studies without APL data, and studies published in languages other than English, Spanish and Chinese that were not readily translatable, were excluded. In additions, studies for which full text publications were unobtainable were excluded if the abstract did not contain sufficient information.
Characteristics of the of Final Studies Included
After examining approximately 550 gathered publications, a total of 228 studies met the criteria for inclusion (Figure 1, Table 1). Of these, 142 studies [2, 5, 7, 24, 30, 41, 47–182] provided information on both childhood de novo APL and AML, and 24 studies [29, 183–205] examined de novo childhood APL only. To examine gender, the de novo childhood APL studies were divided into two groups: AML studies with APL data and APL only studies (Table 1, Supplementary Tables S1a–b). In total, 52 studies provided information about therapy-related APL, with 30 studies examining t-APL [36, 37, 206–233] in children and 26 studies examining t-APL in adults [38, 40, 208, 214, 216, 220, 234–253]. Four studies [208, 214, 216, 220] contained information about both children and adults. In addition, 10 studies [254–263] that discussed environmental and occupational related risk factors for APL in adults were included.
Table 1.
Organization of Studies Included in this Review (N = 228)
| Study Category | Study (N) | Presented In | |
|---|---|---|---|
| Regional Studies: Ratio of APL/AML | 142 | ||
| North America | 13 | Table 2a |  |
| South & Central America | 14 | Table 2b | |
| Europe | 38 | Table 2c | |
| Africa & Middle East | 19 | Table 2d | |
| Asia | 40 | Table 2e | |
| Oceania & Multinational | 18 | Table 2f | |
| APL Studies with Gender Information | |||
| a. APL/AML studies | 15a | Supplementary Table S1a |  |
| b. APL only studies | 24 | Supplementary Table S1b | |
| Therapy-related APL Studies | 52b | ||
| Children | 30 | Table 3, Supplementary Table S2 | Figure 4a–b |
| Adults | 26 | Table 4, Supplementary Table S3 | |
| Environmental & Occupational Exposure Studies | 10 | Table 5 | |
15 studies which contained information about the ratio of APL/AML (Table 2a–f) also contained information about gender.
Data Abstraction and Calculations
From these 142 studies, all relevant data were abstracted regarding the number of cases of AML and APL, the region where the study was conducted, the years during which the data were collected, age at diagnosis, gender, and the method(s) of APL classification when available. However, the criteria for defining childhood leukemia varied widely among these studies (see details in Table 2a–f) e.g. the age of children was defined as ≤ 14, ≤ 18, and ≤ 21 years old.
Table 2.
| a. Distribution of De Novo Childhood APL Cases as a Percentage of AML in North America | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Subregion | Source of Cases |
Inclusion Criteria |
Study (Year) |
Study Series |
Period of Data Collection |
Age | AML | APL | APL/AML |
Methods for APL Classification |
|
| (average %: APL/AML) |
(n) | (n) | (%) | Cytogenetics t(15;17) |
Morphology (FAB) |
|||||||
|
North America |
||||||||||||
|
United States (6.2%) |
N/A | Clinical Trial |
Cohort of survivors analyzed. |
Orgel et. al (2013) [47] |
COG | 2004 – 2009 | 0.3 – 18.6 | 52 | 0 | 0.0 | N/A | x |
| N/A | Case Series |
Absence of prior history of malignant disease or cytotoxic therapy; deceased cases included. |
Martinez-Climent et. al (1995) [48] |
Jul 1981 – Dec 1993 |
< 20 | 115 | 12 | 10.4 | x | x | ||
| California, Michigan, Minnesota, New York, Texas, Utah, Washington DC |
Case Series |
Children diagnosed with ALL or AML who were refractory to primary therapy or experienced relapse and received treatment at participating TACL institutions; deceased cases included. |
Gorman et. al (2010) [49] |
TACLT2005-002 | 1995 – 2004 | 0 – 21 | 99 | 8 | 8.1 | N/A | x | |
| Chicago | Case Series |
Patients admitted consequtively to the University of Chicago Hospitals and Clinics (11), Children's Memorial Hospital (11), University of Illinois (1), Columbus Hospital (1) and Saitama Center (2). |
Kaneko et. al (1982) [50] |
Jun 1977 – Jun 1981 | ≤ 16 | 26 | 3 | 11.5 | x | x | ||
| Tennessee | Clinical Trial |
Patients with secondary leukemia, MDS, excluded. Patients with DS excluded for AML 83, 87 and 97 protocols. |
Ribeiro et. al (2005) [51] |
SJCRHAML 80 |
1980 – 1983 | < 15 | 65 | 1 | 1.5 | x | x | |
| SJCRHAML 83 |
1983 – 1987 | < 15 | 45 | 4 | 8.9 | x | x | |||||
| SJCRHAML 87 |
1987 – 1991 | < 15 | 39 | 4 | 10.3 | x | x | |||||
| SJCRHAML 91 |
1991 – 1996 | < 15 | 62 | 1 | 1.6 | x | x | |||||
| Tennessee | Case- Control |
Treatment for AML at St. Jude Children's Research Hospital. |
Okamoto et. al (2003) [52] |
SJCRH | 1991 – 2000 | N/A | 172 | 2 | 1.2 | x | x | |
| Tennessee | Case Series |
Treatment for AML at St. Jude Children's Research Hospital; children with DS and s-AML excluded. |
Raimondi et. al (1989) [53] |
SJCRH | Apr 1980 – Mar 1987 |
N/A | 121 | 9 | 7.4 | x | x | |
| Tennessee | Case Series |
Consequtively admitted to St. Jude Children's Research Hospital. |
Brodeur et. al (1983) [54] |
SJCRH | Jul 1978 – Dec 1981 |
≤ 19 | 73 | 5 | 6.8 | x | x | |
|
Canada (16.0%) |
Saskatchewan | Case Series |
Children with AL in Regin, Saskatoon clinics of Saskatchewan Cancer Commission; deceased patients included unless lost for follow-up. |
McSheffrey et. al (1975) [55] |
1966 – 1972 | 0 – 16 | 15 | 2 | 13.3 | N/A | x | |
| Toronto | Case Series |
Children with s-AML excluded. |
Abdelhaleem (2007) [56] |
2000 – 2006 | < 18 | 59 | 11 | 18.6 | x | x | ||
|
Mexico (16.4%) |
N/A | Case Series |
N/A | Dorantes-Acosta et. al (2008) [57] |
N/A | 1 – 14 | 17 | 3 | 17.6 | x | x | |
| Mexico City | Case Series |
Residents of Mexico City, newly diagnosed leukemia treated in a hospital in Distrito Federal. |
Perez - Saldivar et. al (2011) [58] |
2006 – 2007 | < 15 | 28 | 3 | 10.7 | N/A | x | ||
| Mexico City | Case Series |
Children at Pediatric Hospital and General Hospital of Mexican Social Security Institute in Mexico City. Only children who are Mexican nationals or whose parents were residents of Mexico City included. |
Mejia- Arangure et. al (2005) [59] |
1996 – 2000 | 0 – 14 | 43 | 9 | 20.9 | N/A | x | ||
| b. Geographic Distribution of De Novo Childhood APL Cases as a Percentage of AML in South & Central America | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Subregion | Source of Cases |
Inclusion Criteria |
Study (Year) |
Study Series |
Period of Data Collection |
Age | AML | APL | APL/AML |
Methods for APL Classification |
|
| (average %: APL/AML) |
(n) | (n) | (%) | Cytogenetics t(15;17) |
Morphology (FAB) |
|||||||
| South & Central America | ||||||||||||
|
Argentina (18.8%) |
N/A | Case Series |
Children with DS, s-AML excluded. |
Acevedo et. al (1994) [24] |
1990 – 1992 | 0 – 15 | 17 | 1 | 5.9 | x | x | |
| La Plata | Case Series |
Previously untreated patients at Sor Maria Ludovica Hospital. |
Gomez et. al (2001) [60] |
Apr 1994 – May 1999 |
≤ 16 | 41 | 13 | 31.7 | x | x | ||
|
Bolivia (10.5%) |
Case Series |
Leukemias diagnosed in la Unidada de Biologica Celular de las Facultad de Medicina de la UMSA of Bolivia; patients with biphenotypic leukemia excluded. |
Amaru et. al (2012) [180] |
Jan 1999 – May 2012 |
0 – 18 | 172 | 18 | 10.5 | x | x | ||
|
Brazil (15.1%) |
N/A | Case Series |
Age range for criteria was 0–23 months, but 39 children aged 18– 23 months included to account for delay in identification of acute leukemia in areas of Brazil; patients with MDS, DS excluded. |
Emerenciano et. al (2006) [61] |
BCSGIL | Jan 1998 – Jan 2005 |
< 2 | 62 | 5 | 8.1 | x | x |
| Hospital das Clínicas, Universidade Federal de Minas Gerais |
Clinical Trial |
Children with previous history of chemotherapy, treatment with protocol designed for adults, MDS or death before treatment start excluded. |
Viana et. al (2003) [181] |
1986 – 2000 | < 16 | 83 | 18 | 21.7 | N/A | x | ||
| Rio Grande do Sul |
Case Series |
Patients treated at Hospital de Clinicas de Porto Alegre, which treats patients from Brazilian state Rio Grande do Sul; patients with s- AML, MDS, history of chemotherapy or CML excluded. |
Onsten et. al (2006) [62] |
1990 – 2002 | < 20 | 47 | 13 | 27.7 | x | x | ||
| South, Southeast, Northeast, Middle West regions of Brazil |
Case Series |
Childhood leukemia associated with DS, monosomy 8, Fanconi anemia, Bloom syndrome, ataxia telangiectasia, neurofibromatosis, MDS, and children older than 24 months excluded. |
Emerenciano et. al (2013) [63] |
BCSGIAL | Jan 2000 – Jan 2011 |
≤ 2 | 160 | 5 | 3.1 | x | x | |
|
Costa Rica (10.2%) |
N/A | Registrya | Newly diagnosed leukemia; deceased cases included. |
Monge et. al (2002) [2] |
1981 – 1996 | < 15 | 144 | 19 | 13.2 | N/A | x | |
| San Jose | Case Series |
Patients referred to National Children's Hospital (only reference center for pediatric hematology in country); cases of s-AML, MDS excluded. |
Santamaria- Quesada et. al (2009) [64] |
Jan 2006 – May 2007 |
< 14 | 14 | 1 | 7.1 | x | x | ||
|
Cuba (31.3%) |
All Cuban Provinces |
Case Series |
N/A | Hernandez et. al (2000) [7] |
Jan 1993 – Dec 1997 |
< 15 | 83 | 26 | 31.3 | x | x | |
|
Chile (13.9%) |
N/A | Clinical Trial |
Newly diagnosed AML at 11 Chilean hospitals; patients with DS, secondary myeloblastic leukemia and myelosarcoma excluded. |
Quintana et. al (2005) [5] |
PINDA 87 |
Mar 1987 – Nov 1991 |
< 15 | 106 | 12 | 11.3 | x | x |
| PINDA 92 |
Jan 1992 – Jan 1998 |
< 15 | 151 | 25 | 16.6 | x | x | |||||
|
Guatemala (34.4%) |
Results obtained at Unidad Nacional de Oncologia Pedatrica in Guatemala City, Guatemala, referral center for whole country. Patients with previous chemotherapy or assesment of nutritional status more than 48 hours after beginning chemotherapy excluded. MDS excluded from analysis. |
Sala et. al (2008) [65] |
Oct 2004 – Sept 2006 |
1 – 18 | 32 | 11 | 34.4 | N/A | N/A | |||
|
Nicaragua (58.8%) |
N/A | Case Series |
Patients referred to Managua Children's Hospital, only pediatric hematology- oncology service in country. |
Malta Corea et. al (1993) [66] |
1990 – 1992 | 6 – 15.5 | 17 | 10 | 58.8 | x | x | |
|
Venezuela (26.3%) |
Zulia | Case Series |
Patients referred to Instituto Hematologico de Occidente. |
De Salvo et. al (1989) [67] |
1982 – 1987 | < 10 | 19 | 5 | 26.3 | x | x | |
| c. Geographic Distribution of De Novo Childhood APL Cases as a Percentage of AML in Europe | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Subregion | Source of Cases |
Inclusion Criteria |
Study (Year) |
Study Series |
Period of Data Collection |
Age | AML | APL | APL/AML |
Methods for APL Classification |
|
| (average %: APL/AML) |
(n) | (n) | (%) | Cytogenetics t(15;17) |
Morphology (FAB) |
|||||||
| Europe | ||||||||||||
|
Austria (4.4%) |
Vienna | Case Series |
Children with AML or TMD diagnosed in Austria, registered at single institution. |
Strehl et. al (2001) [68] |
1993 – 1998 |
0.01 – 16.4 | 67 | 5 | 7.5 | x | x | |
| Vienna | Case Series |
N/A | Haas et. al (1993) [69] |
1978 – 1989 |
N/A | 71 | 1 | 1.4 | x | x | ||
|
Belarus (18.5%) |
N/A | Registry a |
Treatment at Belarusian Center for Pediatric Oncology, which treats more than 70% of children in Belarus with cancer. |
Lipay et. al (2011) [70] |
2000 – 2009 |
0.8 – 21 | 151 | 28 | 18.5 | N/A | N/A | |
|
Czech Republic (18.5%) |
N/A | Clinical Trial |
Patients with death before treatment, s- AML, major protocol violations, MDS and DS excluded. |
Sramkova et. al (2013) [71] |
AMLBFM 1993 /1998 |
Jun 1993 – Feb 2004 |
0 – 19 | 125 | 11 | 8.8 | x | x |
| AMLBFM 2004 |
Mar 2004 – Dec 2009 |
0 – 18 | 57 | 9 | 15.8 | x | x | |||||
| N/A | Case Series |
All children diagnosed and treated in Czech Pediatric Hematology Working Group centers. |
Burjanivova et. al (2006) [72] |
N/A | 1 – 14 | 13 | 4 | 30.8 | x | N/A | ||
|
Finland (10.5%) |
N/A | Case Series |
Patients diagnosed and treated at Hospital for Children and Adolescents, Helsinki University Central Hospital and Kuopio University Hospital, Finland. |
Huhta et. al (1999) [73] |
N/A | 1–15.6 | 19 | 2b | 10.5 | x | x | |
|
France (11.3%) |
Paris | Case Series |
Treated at Hopital Saint Louis; previously treated patients excluded. |
Leverger et. al (1988) [74] |
Sept 1977 – Dec 1986 |
< 16 | 130 | 11 | 8.5 | x | x | |
| Paris, Lille | Case Series |
Diagnosed at Trousseau Hospital, Claude Huriez Hospital. |
Lapillonne et. al (2006) [75] |
LAME 88/91 LAME 99 (APL) |
Mar 1993 – Jan 2002 |
0.003 – 18.7 | 92 | 13 | 14.1 | x | x | |
|
Germany (4.0%) |
West Germany |
Clinical Trial |
Children treated in 30 West German hospitals without prior malignancy or without prior treatment for more than 14 days; three infants with congenital leukemia, DS excluded. |
Creutzig et. al (1985) [76] |
AMLBFM 78 |
Dec 1978 – Oct 1982 |
< 17 | 151 | 6 | 4.0 | N/A | x |
|
Greece (15.8%) |
N/A | Case Series |
N/A | Manola et. al (2013) [77] |
1998 – 2010 |
≤ 21 | 133c | 21 | 15.8 | x | x | |
|
Hungary (6.5%) |
N/A | Clinical Trial |
N/A | Szegedi et. al (2013) [78] |
HPOG AMLBFM 98 |
2001 – 2011 |
< 18 | 112 | 9 | 8.0 | x | x |
| N/A | Case Series |
Children diagnosed at 10 centers of Leukemia Working Party in Hungary. |
Revesz et. al (1985) [79] |
1971 – 1982 |
< 15 | 123 | 2 | 1.6 | N/A | x | ||
| N/A | Case Series |
Patients in Hungarian Study Group on Childhood Leukemia. |
Keleti et. al (1978) [80] |
1971 – 1975 |
N/A | 41 | 2 | 4.9 | N/A | x | ||
| Budapest | Case Series |
Newly diagnosed AML at Semmelweis University or another pediatric hematological center in Budapest. |
Haltrich et. al (2006) [81] |
1997 – 2003 |
0.58 – 18 | 26d | 3 | 11.5 | x | x | ||
|
Italy (21.9%) |
N/A | Clinical Trial |
Newly diagnosed AML, patients with granulytic sarcoma, MDS, DS, s- AML or pretreatment > 14 days excluded. |
Pession et. al (2005) [82] |
AEIOP LAM-87 |
Jan 1987 – Feb 1993 |
0 – 15 | 151 | 27 | 17.9 | x | x |
| AEIOP LAM-87M |
Feb 1989 – May 1993 |
0 – 15 | 77 | 20 | 26.0 | x | x | |||||
| N/A | Registry a | Newly diagnosed AML admitted and treated at 29 AEIOP institutions. |
Biondi et. al (1994) [83] |
AEIOP | Apr 1989 – Nov 1993 |
< 15 | 314 | 54 | 17.2 | x | x | |
| Catania, Florence, Genoa, Monza, Padua, Rome, Trieste, Turin |
Case Series |
Admitted to treatment at one of 8 Italian centers.e |
Castagnola et. al (2010) [84] |
Jan 1998 – Dec 2005 |
< 15 | 240 | 33 | 13.8 | N/A | x | ||
| Monza | Case Series |
Diagnosed at Clinica Pediatrica Universita di Milano Bicocca. |
Arrigoni et. al (2003) [85] |
Jan 1985 – Dec 2000 |
< 18 | 119 | 32 | 26.9 | x | x | ||
| Monza | Case Series |
Pediatric AML cases observed at Clinica Pediatrica Universita di Milano. |
Cantu- Rajnoldi et. al (1993) [86] |
1970 – 1992 |
N/A | 151 | 46 | 30.5 | x | x | ||
| Piedmont | Registry a | N/A | Maule et. al (2008) [87] |
1980 – 2003 |
< 15 | 121 | 26 | 21.5 | x | x | ||
|
Netherlands (4.5%) |
N/A | Clinical Trial |
Newly diagnosed AML; patients with corticosteroids or chemotherapy longer > 2 weeks before diagnosis, DS, myelosarcoma, MDS excluded. |
Kardos et. al (2005) [88] |
DCOG AML-82 |
Jan 1983 – Jun 1987 |
0 – 15 | 48 | 2 | 4.2 | N/A | x |
| DCOG AML-87 |
Jun 1987 – Oct 1992 |
0 – 15 | 83 | 3 | 3.6 | x | x | |||||
| DCOG AML: 92/94 |
Oct 1992 – Jun 1998 |
0 – 15 | 78 | 2 | 2.6 | x | x | |||||
| N/A | Clinical Trial |
Newly diagnosed AML. |
De Bont et. al (2002) [89] |
DCLSG | 1988 – 1998 |
0–14 | 47 | 3 | 6.4 | x | x | |
| Amsterdam | Case Series |
AML referred to Emma Children's Hospital and the Academic Hospital of the Free University in Amsterdam. |
Slater et. al (1983) [90] |
N/A | 0.08 – 15.25 | 17 | 1 | 5.9 | x | x | ||
|
Poland (10.5%) |
N/A | Clinical Trial |
AML with absence of severe congeinital malformations or comorbitities included. Patients with AML following CML, MDS, s- AML, congenital malformations and severe comorbidities including DS, biphenotypic leukemia, death before treatment and pre- treatment with other protocols or incomplete data excluded. |
Balwierz et. al (2013) [91] |
PPLLSG 83 |
1983 – 1994 |
0.1 – 16.6 | 208 | 23 | 11.1 | N/A | x |
| PPLLSG 94 |
1994 – 1997 |
0.6 – 16.6 | 83 | 9 | 10.8 | x | x | |||||
| PPLLSG 98 |
1998 – 2004 |
0.1 – 17.8 | 195 | 23 | 11.8 | x | x | |||||
| PPLLSGAMLBFM 2004 |
2005 – 2011 |
0.006 – 18.1 | 237 | 20 | 8.4 | x | x | |||||
|
Russia (14.4%) |
N/A | Case Series |
Patients at Russian Children's Clinical Hospital. |
Nasedkina et. al (2003) [92] |
N/A | N/A | 76 | 10 | 13.2 | x | N/A | |
| Moscow | Case Series |
Admitted to the Federal Research Center for Pediatric Hematology, Oncology and Immunology. |
Yatsenko et. al (2013) [93] |
2006 – 2010 |
< 17 | 186 | 29 | 15.6 | x | x | ||
|
Serbia (11.2%) |
N/A | Case Series |
N/A | Krstic et. al (2010) [94] |
N/A | 1 – 15.6 | 19 | 3 | 15.8 | x | N/A | |
| Belgrade | Case Series |
Diagnosed at University Children's Hospital and Mother and Child Healthcare Institute. |
Krstovski et. al (2010) [95] |
Jan 1997 – June 2007 |
N/A | 92 | 6 | 6.5 | x | x | ||
|
Spain (15.3%) |
Barcelona | Case Series |
Patients diagnosed and treated at Hospital Vall d'Hebron and Hospital Sant Joan de Deu in Barcelona. |
Armengol et. al (2010) [96] |
1992 – 2002 |
< 17 | 63f | 8 | 12.7 | x | x | |
| Catalonia | Clinical Trial |
Previously untreated AML included in AML- 88 Trial. |
Ortega et. al (2003) [97] |
Apr 1988 – May 2001 |
< 15 | 79 | 10 | 12.7 | x | x | ||
| Girona, Valencia, Zaragoza |
Registry a | Deceased cases included. |
Marco s- Gragera et. al (2010) [98] |
1993 – 2002 |
< 15 | 63 | 13 | 20.6 | N/A | x | ||
|
Sweden (2.9%) |
Southern | Case Series |
N/A | Andersson et. al (2008) [99] |
1995 – 2004 |
0 – 17 | 34 | 1 | 2.9 | x | N/A | |
|
Switzerland (2.4%) |
N/A | Case Series |
DS AML included. |
Betts et. al (2007) [100] |
SPOG | Sept 1994 – Jan 2005 |
0 – 16 | 82g | 2 | 2.4 | x | x |
|
Ukraine 11.3%) |
N/A | Case Series |
Registered at the Institute of Haematology and Transfusiology AMS Ukraine. |
Andreieva et. al (2010) [101] |
1992 – 2008 |
0.33 – 18 | 116 | 13 | 11.2 | x | x | |
| Kiev | Case Series |
Data from Reference Lab for leukemia diagnostics established by Haematopathologists for Patients with Malignant Diseases of the Blood. Survey covers all cases of childhood leukemia registered during the indiciated period for these regions according to the Ukraine Ministry of Health. |
Gluzman et. al (1999) [102] |
1993 – 1997 |
0 – 17 | 44 | 5 | 11.4 | N/A | x | ||
|
United Kingdom (7.6%) |
N/A | Clinical Trial |
Patients with s- AML, DS, MDS, bilineage leukemia excluded from analysis. |
Gibson et. al (2005) [103] |
MRCAML 10 |
May 1988 – Mar 1995 |
0 – 14 | 303 | 27 | 8.9 | x | x |
| MRCAML 12 |
Apr 1995 – May 2002 |
0 – 14 | 455 | 40 | 8.8 | x | x | |||||
| N/A | Case Series |
Patients referred to the Hospital for Sick Children; all children referred for treatment included, even those that died within hours of admission. |
Phillips et. al (1991) [104] |
1972 – 1987 |
N/A | 152 | 10 | 6.6 | N/A | x | ||
| England, Scotland, Wales |
Registry a | N/A | c | 1980 – 1988 |
< 15 | 471 | 28 | 5.9 | N/A | x | ||
| d. Geographic Distribution of De Novo Childhood APL Cases as a Percentage of AML in Africa and the Middle East | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Subregion | Source of Cases |
Inclusion Criteria |
Study (Year) | Study Series |
Period of Data Collection |
Age | AML | APL | APL /AML |
Methods for APL Classification |
|
| (average %: APL/AML) |
(n) | (n) | (%) | Cytogeneticst (15;17) |
Morphology (FAB) |
|||||||
| Africa | ||||||||||||
|
Egypt (13.2%) |
N/A | Case Series |
Newly diagnosed AML admited to Mansoura University Children's Hospital. |
Al-Tonbary et. al (2009) [106] |
Jan 2004 – Jan 2007 |
1 – 15 | 30 | 6 | 20.0 | x | x | |
| N/A | Registrya | Treated at Children's Cancer Hospital Egypt, which receives patients from all Egyptian governorates. |
Ezzat (Personal Correspondence, 2012) [107] |
Jul 2007 – Dec 2011 |
0 – 18 | 353 | 34 | 9.6 | N/A | N/A | ||
| Cairo | Case Series |
AL at Pediatrics Hospital, Ain Shams University. |
Ismail et. al (2012) [108] |
Nov 2007 – Apr 2011 |
1 – 15 | 30 | 3 | 10.0 | x | x | ||
|
Malawi (6.3%) |
Blantyre | Case Series |
Diagnosed at Queen Elizabeth Central Hospital. |
Mukiibi et. all (2001) [109] |
Jan 1994 – Dec 1998 |
0 – 15 | 16 | 1 | 6.3 | N/A | x | |
|
Nigeria (18.2%) |
Ibadan | Case Series |
Indigenous Nigerian residents of Ibadan and surrounding rainforest. |
Williams et. al (1982) [110] |
Jul 1978 – Dec 1981 |
≤ 14 | 11 | 2 | 18.2 | N/A | x | |
|
South Africa (17.9%) |
Cape Province/ Eastern Cape |
Case Series |
Diagnosed at Red Cross War Memorial Children's Hospital, major referral center for Cape Province, with select patients from neighboring provinces and countries. |
Gilbert et. al (1987) [182] |
Jan 1981 – Dec 1985 |
0.67 – 10.92 | 43 | 9 | 20.9 | x | x | |
| Johannes burg |
Case Series |
Diagnosed at three main teaching hospitals attached to University of the Witwatersrand Medical School. |
Bernstein et al (1984) [30] |
Jan 1978 – Apr 1982 |
0 – 15 | 26 | 5 | 19.2 | x | x | ||
| Johannes burg |
Case Series |
Newly diagnosed, untreated AL referred to Childrens Hematology /Oncology Clinics at Transvaal Memorial Hospital for Children, Joahnnesbug Hospital and Baragwanth Hospital. |
Macdougall et. al (1986) [111] |
Jan 1974 – Dec 1982 |
0 – 15 | 52 | 7 | 13.5 | x | x | ||
|
Sudan (18.2%) |
N/A | Case Series |
Cases diagnosed at University of Khartoum Department of Pathology/National Health lab which serves hospitals in three towns of Khartoum province. |
Ahmed et. al (1982) [112] |
Jan 1970 – Dec 1976 |
2 – 19 | 11 | 2 | 18.2 | N/A | x | |
|
Tunisia (16.5%) |
Case Series |
Consecutive ethnic Tunisian patients. |
Gmidene et. al (2012) [113] |
Jan 2000 – Dec 2007 |
0 – 16 | 97 | 16 | 16.5 | x | x | ||
| Middle East | ||||||||||||
| Iraq | Baghdad | Case Series |
Diagnosed at Pediatric Oncology Unit at Al-Mansour Pediatric Hospital, referral center for childhood cancer in Iraq. |
Testi et. al (2006) [114] |
Jan 2002 – Jan 2003 |
< 15 | 32 | 11 | 34.4 | N/A | x | |
| (34.5%) | Oct 2003 – Aug 2004 |
1 –15 | 26 | 9 | 34.6 | N/A | x | |||||
|
Iran (16.0%) |
Tehran | Case Series |
Consecutive patients referred to Hematology- Oncology and Stem Cell Transplantation Research Center, Tehran University of Medical Sciences. |
Hamidieh et. al (2013) [115] |
May 1991 – Jun 2010 |
< 15 | 133 | 14 | 10.5 | N/A | x | |
| Tehran | Case Series |
Newly diagnosed AML at Hematology and Oncology Clinics of Vali- Asr and Ali- Asghar hospitals, affiliated to Tehran University of Medical Sciences and Iran University of Medical Sciences. |
Memarian et. al (2007) [116] |
N/A | 0.33 – 21 | 14 | 3 | 21.4 | N/A | x | ||
|
Israel (8.1%) |
N/A | Case Series |
AML at Schneider Children's Medical Centre of Israel, Soroka Hospital and Kaplan Hospital; children with DS and non- Fanconi birth defects included. |
Stark et. al (2004) [117] |
Jul 1998 – Jan 2003 |
< 20 | 86 | 7 | 8.1 | x | x | |
|
Oman (12.9%) |
N/A | Case Series |
Treated at Sultan Qaboos University Hospital, national referral center for pediatric leukemia. |
al Lamki et. al (2004) [118] |
Jan 1993 – Jan 2003 |
< 12 | 11 | 1 | 9.1 | x | x | |
| Muscat | Case Series |
Treated at Sultan Qaboos University Hospital, national referral center for pediatric leukemia; patients with s- AML, MDS excluded. |
Udayakumar et. al (2007) [119] |
Nov 2001 – Nov 2006 |
≤ 16 | 18 | 3 | 16.7 | x | x | ||
|
Saudi Arabia (3.4%) |
N/A | Case Series |
Primary treatment at King Faisal Specialist Hospital and Research Center. |
Jenkin et al (2000) [120] |
1983 – 1997 | < 17 | 86 | 1 | 1.2 | x | x | |
| Jeddah | Case Series |
All children with AML diagnosed at King Abdulaziz Medical City. |
Khattab et. al (2008) [121] |
Jan 1986 – Nov 2005 |
0.5 – 14 | 54 | 3 | 5.6 | x | N/A | ||
|
Turkey (8.8%) |
N/A | Case Series |
Diagnosed with AML at Cukurova University Medical School. |
Komur et. al (2010) [122] |
N/A | 1 – 17 | 34 | 3 | 8.8 | x | x | |
| e. Geographic Distribution of De Novo Childhood APL Cases as a Percentage of AML in Asia | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Subregion | Source of Cases |
Inclusion Criteria |
Study (Year) |
Study Series |
Period of Data Collection |
Age | AML | APL | APL/ AML |
Methods for APL Classification |
|
| (average %: APL/AML) |
(n) | (n) | (%) | Cytogenetics t(15;17) |
Morphology (FAB) |
|||||||
| Asia | ||||||||||||
|
China (Mainland) |
N/A | Case Series |
N/A | Wang et. al (2012) [123] |
Apr 2005 – Apr 2010 |
≤ 16 | 179 | 27 | 15.1 | x | x | |
| (19.3%) | N/A | Registrya | N/A | Zhang and Zhu (2012) [124] |
1996 – 2004 | N/A | 141 | 51 | 36.2 | x | x | |
| N/A | Case Series |
Patients with cytotoxic chemotherapy, blastic transformation of CML, secondary malignancy or DS excluded. |
Zhai et. al (2011) [125] |
Aug 1994 – Dec 2008 |
< 18 | 68 | 12 | 17.6 | x | x | ||
| Beijing | Case Series |
Newly diagnosed AML. |
Shang et. al (1999) [126] |
Nov 1992 – Mar 1997 |
N/A | 15 | 2 | 13.3 | N/A | x | ||
| Guang zhou |
Case Series |
AML at the department of Pediatrics Nanfang Hospital. |
Feng et. al (2014) [127] |
Jan 2011 – Oct 2012 |
2 – 16 | 38 | 2 | 5.3 | x | x | ||
| Shanghai | Case Series |
Patients with no- pretreatment included. |
Tang et. al (2003) [128] |
N/A | < 14 | 12 | 2 | 16.7 | x | x | ||
| Suzhou | Case Series |
Patients at Children's Hospital of Soochow Unversity. |
Yan-Fang et. al (2013) [129] |
2000 – 2010 | 1 – 13 | 70 | 10 | 14.3 | x | x | ||
| Wuhan | Case Series |
Newly diagnosed AML. |
Jiang et. al (2014) [130] |
Jan 2009 – Aug 2013 |
0.4 – 13 | 241 | 43 | 17.8 | x | x | ||
| Zhejiang | Case Series |
AML at Children's Hospital of Zheijiang University School of Medicine. |
Xu et. al (2010) [131] |
Jan 1997 – Dec 2005 |
< 16 | 185 | 49 | 26.5 | x | x | ||
| Zheijang | Case Series |
AML at First Affiliated Hospital, Zhejiang University College of Medicine, central hospital with more than 95% of patients residents of the province. |
Cheng et. al (2009) [132] |
Dec 1994 – Nov 2007 |
0 – 19 | 146 | 44 | 30.1 | x | x | ||
|
China (Hong Kong) (9.3%) |
Hong Kong |
Case Series |
Consecutive cases at a regional Hong Kong hospital. |
Chan et. al (2004) [133] |
Dec 1996 – Dec 2003 |
0.67 – 16 | 43 | 4 | 9.3 | x | x | |
|
China (Taiwan) (11.0%) |
N/A | Clinical Trial |
Diagnosed at Chang Gung Memorial Hospital and Mackay Memorial Hospital. |
Liang et. al (2013) [134] |
TPOG | Dec 1995 – Jun 2011 |
0 – 19.7 | 206 | 17 | 8.3 | x | x |
| N/A | Case Series |
Treated at Mackay Memorial Hospital. |
Yeh et. al (2007) [135] |
TPOG | Nov 1995 – July 2004 |
< 15 | 48 | 6 | 12.5 | x | x | |
| N/A | Clinical Trial |
DS, systemic chloroma included; s- AML, MDS excluded. |
Liang et. al (2006) [136] |
TPOG | Jan 1997 – Dec 2002 |
0 – 17 | 243 | 24 | 9.9 | x | x | |
| N/A | Case Series |
Diagnosed at Mackay Memorial Hospital and Chang Gung Children's Hospital. |
Liang et. al (2003) [137] |
TPOG | N/A | ≤ 18 | 91 | 12 | 13.2 | x | x | |
|
India (8.8%) |
Chandigarh | Case Series |
Department of Hematology of Post Graduate Institute of Medical Education and Research (PGIMER) in Northern India. |
Bhatia et. al (2012) [138] |
Apr 2010 – Mar 2012 |
0.6 – 12 | 20 | 3 | 15.0 | x | x | |
| New Delhi |
Case Series |
N/A | Agarwal et. al (2011) [139] |
Jun 2004 – Dec 2008 |
1 – 18 | 80 | 1 | 1.3 | x | x | ||
| South India |
Case Series |
N/A | Mir Mazloumi et. al (2013) [140] |
2009 – 2011 | 1 – 14 | 50 | 5 | 10.0 | x | x | ||
|
Indonesia (5.6%) |
North Sumatra |
Case Series |
Patients at Subdivision of Pediatric Hematology, School of Medicine, University of North Sumatra /Dr. Pirngadi Hospital Medan. |
Nasution et. al (1991) [141] |
1983 – 1988 | 0 – 15 | 18 | 1 | 5.6 | N/A | x | |
| Japan | N/A | Registrya | N/A | Horibe et. al (2013) [142] |
2006 – 2010 | < 20 | 891 | 70 | 7.9 | N/A | x | |
| (9.0%) | N/A | Clinical Trial |
Newly diagnosed AML, DS included. |
Shimada et al (2012) [143] |
JCACSGAML 99 |
Jan 2000 – Dec 2002 |
0 – 15 | 318 | 32 | 10.1 | x | x |
| N/A | Case Series |
AML from 4 centers.b |
Ohta et. al (2011) [144] |
JPLSG | 1997 – 2007 | N/A | 375 | 42 | 11.2 | N/A | x | |
| N/A | Case Series |
N/A | Miyamura et. al (2004) [145] |
Feb 1999 – May 2002 |
0.33 – 16 | 26c | 3 | 11.5 | x | x | ||
| N/A | Case Series |
Newly diagnosed AML. |
Yamada et. al (2001) [146] |
Jan 1988 – Feb 2000 |
0 – 16 | 159 | 7 | 4.4 | x | x | ||
| N/A | Clinical Trial |
N/A | Iwai et. al (1999) [147] |
CCLSG | N/A | N/A | 94 | 9 | 9.6 | x | x | |
| N/A | Case Series |
Treated at Nagoya University Hospital and affiliates. |
Kondo et. al (1999) 148] |
1985 – 1997 | 0 – 16 | 64 | 3 | 4.7 | N/A | x | ||
| N/A | Case Series |
Treated at Saitama Children's Medical Center. |
Hayashi et. al (1991) [149] |
Apr 1983 – Mar 1990 |
< 15 | 106 | 7 | 6.6 | x | x | ||
| Tokyo | Clinical Trial |
Newly diagnosed AML from 40 participating institutions mainly located in Tokyo and suburbs, covering a third to a fourth of Japanese pediatric population; patients with s- AML, MDS, death before therapy start, undifferentiated and mixed- lineage leukemia excluded. |
Tomizawa et. al (2007) [150] |
TCC SG M91-13, M96-14 |
Aug 1991 – Sept 1998 |
2 – 15 | 216 | 14 | 6.5 | x | x | |
| Tokyo | Case Series |
All new cases of leukemia at University of Tokyo Hospital or affiliated hospitals. |
Bessho (1989)d [151] |
1964 – 1976 | N/A | 36 | 2 | 5.6 | N/A | x | ||
| 1977 – 1989 | N/A | 19 | 4 | 21.1 | N/A | x | ||||||
|
Malaysia (14.4%) |
N/A | Case Series |
AML at University Malaya Medical Centre, tertiary referral center for childhood cancer; patients with s- AML, MDS, or prior chemotherapy excluded; deceased and DS included. |
Chan et. al (2004) [152] |
May 1985 – Dec 1999 |
0 – 15 | 174 | 25 | 14.4 | N/A | x | |
|
Nepal (20.0%) |
Western Nepal |
Case Series |
Patients at the Manipal Teaching hospital in western Nepal. |
Ghartimagar et. al (2012) [153] |
Jan 2000 – June 2011 |
< 15 | 15 | 3 | 20.0 | N/A | x | |
|
Pakistan (24.7%) |
Islamabad | Case Series |
Patients at Pakistan Institue of Medical Sciences; patients already receiving cyotoxic therapy, already diagnosed with AML, CML, myeloproliferative disorders or MDS excluded. |
Asif et. al (2011) [154] |
Jul 2007 – Jul 2009 | 0.17 – 13 | 26 | 8 | 30.8 | N/A | x | |
| Karachi | Case Series |
N/A | Zaki et. al (2002) [155] |
Jan 1987 – Aug 1997 |
< 14 | 23 | 10 | 43.5 | N/A | x | ||
| Karachi | Case Series |
Patients at Aga Khan University Hospital, Karachi. Patients with hematological disorders (MDS, CML, aplastic anaemia), prior chemotherapy/ radiotherapy excluded. Not newly diagnosed cases with or without treatment, relapsed cases excluded. |
Harani et. al (2005) [156] |
Jan 1999 – Dec 2000 |
< 15 | 21 | 0 | 0.0 | N/A | x | ||
|
Singapore (10.6%) |
N/A | Case Series |
Treated at Children's Medical Institute, National University Hospital, deceased included. |
Tan et. al (2007) [157] |
Apr 1988 – Dec 2003 |
0.17 – 15 | 34 | 2 | 5.9 | x | x | |
| N/A | Case Series |
Children treated at the National University of Singapore. |
Quah et. al (1996) [158] |
Jan 1988 – Jan 1994 |
< 12 | 13 | 2 | 15.4 | N/A | x | ||
|
South Korea (7.4%) |
N/A | Case Series |
AML at Samsung Medical Center. |
Sung et. al (2007) [159] |
July 2000 – Apr 2006 |
< 15 | 55 | 5 | 9.1 | x | x | |
| Seoul | Case Series |
Children with AL undergoing allogenic HCT at Asan Medical Center. |
Lee et. al (2009) [160] |
Jan 2000 – Apr 2007 |
0.6 – 15.4 | 35 | 2 | 5.7 | x | N/A | ||
|
Thailand (5.6%) |
Bangkok | Case Series |
Diagnosed with AL at Department of Pediatrics, Faculty of Medicine Rama Thibodi Hospital and Queen Sirikit National Institute of Child Health, Bangkok, Thailand. |
Pakakasama et. al (2008) [161] |
Jan 2004 – Dec 2006 |
0.83 – 13.2 | 20 | 1 | 5.0 | x | x | |
| N/A | Case Series |
Newly diagnosed AML at departments of Pediatrics, Srinakarind Hospital, Faculty of Medicine, Khon Kaen University, Khon Kaen and Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. |
Mukda et. al (2011) [162] |
N/A | 0.17 – 15 | 64 | 4 | 6.3 | N/A | x | ||
| f. Geographic Distribution of De Novo Childhood APL Cases as a Percentage of AML in Oceania and Multinational Studies | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Subregion | Source of Cases |
Inclusion Criteria |
Study Author (Year) |
Study Series |
Period of Data Collection |
Age | AML | APL | APL /AML |
Methods for APL Classification |
|
| (average %: APL/AML) |
(n) | (n) | (%) | Cytogenetics t(15;17) |
Morphology (FAB) |
|||||||
| Oceania | ||||||||||||
|
Australia (17.8%) |
N/A | Clinical Trial |
Previously untreated children with AML; DS and preleukemic myelodysplasia excluded. |
Tiedemann et. al (1993) [163] |
Nov 1984 – Jan 1991 |
0.25 – 16 | 31 | 4 | 12.9 | x | x | |
| Melbourne | Case Series |
Newly diagnosed AML at Royal Children's Hospital, Melbourne; patients too young for cytototoxic therapy or with death before treatment excluded. |
Paton et. al (1982) [164] |
Oct 1974 – Jan 1979 |
1 – 14.67 | 22 | 5 | 22.7 | N/A | x | ||
| Multinational | ||||||||||||
|
North America (7.3%) |
United States & Canada |
Clinical Trial |
CCG 213, children with acute monoblastic leukemia excluded. |
Smith et. al (2005) [165] |
CCG 251 |
Sept 1979 – Oct 1983 |
0 – 21 | 485 | 31 | 6.4 | x | x |
| CCG 213 |
May 1985 – Feb 1989 |
<21 | 532 | 50 | 9.4 | x | x | |||||
| CCG 2891: children with previously untreated AML, acute undifferentiated or biphenotypic leukemia with evidence of myeloid differentiation, MDS or granulocytic sarcoma included. |
CCG 2891 |
Oct 1989 – Apr 1995 |
<21 | 868 | 50 | 5.8 | x | x | ||||
| United States & Canada |
Case- Control |
Newly diagnosed AML, telephone in residence of patient; biological mother of patient had to speak English and be avilable for interview. |
Severson et. al (1993) [41] |
CCG | Jan 1980 – Dec 1984 |
<18 | 187 | 14 | 7.5 | N/A | x | |
|
Central America (14.6%) |
El Salvador, Guatemala, Honduras |
Case Series |
Relapsed AML. Patients from Hospital Nacional de Ninos Benjamin Bloom in San Salvador, Unidad Nacional de Oncologia Pediatrica in Guatemala City, Hospital Escuela in Tegucigalpa and Hospital Rivas in San Pedro Sula; children with induction failure excluded. |
Marjerrison et. al (2014) [166] |
Sept 1997 – Apr 2011 |
<20 | 164 | 24 | 14.6 | N/A | x | |
| Europe | N/A | Clinical Trial |
Patients with myelosarcoma, s-AML, MDS, DS or pre- treatment > 14 days excluded from analysis. |
Creutzig et. al (2005)a [167] |
BFM 83 |
Dec 1982 – Sept 1986 |
0 – 17 | 182 | 5 | 2.7 | x | x |
| BFM 87 |
Dec 1986 – Sept 1992 |
0 – 17 | 307 | 15 | 4.9 | x | x | |||||
| Europe | N/A | Registryb | Patients who underwent autologous HSCT for AML; DS patients excluded. |
Locatelli et. al (2003)a [168] |
Jan 1980 – Dec 1999 |
<16 | 387 | 36 | 9.3 | x | x | |
| Europe | Austria, Czech Republic, Denmark, Finland, Germany, Iceland, Israel, Italy, Netherlands, Norway, Sweden, United Kingdom |
Registryb | DS AML only. |
Forestier et. al (2008)a [169] |
1992 – 2005 | 0 – 12 | 189 | 5 | 2.6 | x | x | |
|
Europe (6.0%) |
Austria, Czech Republic, Germany, Switzerland |
Clinical Trial |
N/A | Creutzig et. al (2010) [170] |
BFM-93,98,04 | Sept 1993 – Dec 2007 |
0 – 18 | 1357 | 81 | 6.0 | x | x |
| Europe | Austria, Denmark, England, Estonia, Finland, France, Iceland, Italy, Netherlands, Poland, Scotland, Slovakia, Slovenia, Spain, Sweden, Switzerland, Wales, West Germany |
Registry | N/A | Gatta et. al (2001)a [171] |
EUROCARE | 1985 – 1989 | <15 | 915 | 40 | 4.4 | x | x |
|
Europe (8.0%) |
Czech Republic, France, Germany, Netherlands |
Clinical Trial |
N/A | Balgobind et. al (2011) [172] |
DCOG, AML- BFM, CPH |
N/A | N/A | 237 | 19 | 8.0 | x | x |
|
Europe (5.3%) |
Denmark, Finland, Iceland, Norway, Sweden (Nordic Region) |
Clinical Trial |
Population based for children < 15; aged 15–18 patients were enrolled by local practice; patients treated with protocols outside of NOPHO- AML, pre- treated with costatic drugs for > 14 days, or with DS, Fanconi anemia, Kostmann syndrome, extramedullary myeloid tumor (without significant bone marrow involvement), t-AML excluded. |
Molgaard- Hansen et. al (2010) [173] |
NOPHO- AML 84,88,93 |
July 1984 – Dec 2003 |
0 – 18 | 525 | 28 | 5.3 | x | x |
|
Europe (6.1%) |
Serbia, Montenegroc |
Case Series |
Newly diagnosed acute leukemia, in previously untreated patients at Mother and Child Health Institute of Serbia, where 60 – 70% of childhood AL cases in region treated. |
Slavkovic et. al (2005) [174] |
Oct 1996 – May 2002 |
0.33 – 17 | 33 | 2 | 6.1 | x | x | |
|
Europe (4.2%) |
Serbia, Montenegro, Slovenia, Croatia, Boznia and Herzegovina, Republic of Macedoniac |
Case Series | N/A | Petkovic et. al (1992) [175] |
N/A | 0.5–15 | 24 | 1 | 4.2 | x | x | |
| Asia | China, Malaysia, India |
Case Series |
N/A | Leow et. al (2011)a [176] |
N/A | 0 – 17 | 150 | 18 | 12.0 | x | x | |
|
Oceania (11.8%) |
Australia, New Zealand |
Clinical Trial |
Previously untreated AML, patients with MDS, s-AML, DS- related leukemic disorders excluded, death before treatment excluded. |
O'Brien et. al (2002) [177] |
ANZCCSG | Dec 1986 – May 1999 |
<18 | 262 | 31 | 11.8 | x | x |
| Multiregional | Australia, Canada,Puerto Rico, Switzerland, United States |
Clinical Trial |
Patients with APL (4), juvenile myelomonocytic leukemia, documented bone marrow failure syndromes, DS or secondary/ treatment related leukemia not eligbile; patients with MDS not eligible unless they presented with karyotypic abnormalities characteristic of de novo AML. |
Cooper et. al (2012)a [178] |
COG | Dec 2003 – Nov 2005 |
>1, ≤21 | 349 | 4 | 1.1 | x | x |
| Multiregional | Australia, Europe, United States |
Case Series |
Patients with secondary leukemia and leukemic cells with abnormal karotype excluded. |
Rowley et. al (1982)a [179] |
N/A | 0 – 19 | 56 | 2 | 3.6 | x | x | |
AL, acute leukemia; ALL, acute lympocytic leukemia; AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; CCG, Children's Cancer Group; COG, Children's Oncology Group; DS, Down syndrome; FAB, French-American-British; MDS, myelodysplastic syndrome; N/A, not available;s-AML, secondary AML; SJCRH, St. Jude Children's Research Hospital; TACL, Therapeautic Advances in Childhood Leukemia Consortium.
National Cancer Registry
AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; BCSGIAL, Brazilian Collaborative Study Group of Infant Acute Leukemias; BCSGIL, Brazilian Cooperative Study Group of Infant Leukemia; CML, chronic myeloid leukemia; DS, Down syndrome; FAB, French-American-British; MDS, myelodysplastic syndrome; N/A, not available; PINDA, National Program for Antineoplastic Drugs for Children; s-AML, secondary AML.
Children's Cancer Subregistry of Belarus, AEIOP National Registry, Childhood Cancer Registry of Piedmont, Zaragoza, Girona and Valencia Registries, National Registry of Childhood Tumors, respectively.
Both cases of APL were classified of FAB M5, but showed t(11;17) cytogenetics characteristic of APL (FAB M3).
Seven patients non M3 t-AML were excluded from original patient total of 140 for purposes of this analysis.
Two non-M3 t-AML were excluded from original patient total of 28 for purposes of this analysis
Centers: G. Gaslini Chidlren's Hospital, Genoa; Bambino Gesu Children's Hospital, Rome; Regina Marghertia-S. Anna Children's Hospital, Turin; Pediatric Clinic, Milano Bicocca University, Monza; Pediatric Hematology Oncology, Padua; Burlo Garofalo Children's Hospital, Trieste; Pediatric Clinic, University of Catania, Catania; A. Meyer Children's Hospital, Florence.
5 secondary AMLs, FAB types M5 (4) and M6 (1) were excluded from original patient total of 68 for purposes of this analysis.
Two non-M3 secondary AMLs were excluded from original patient total of 84 for purposes of this analysis.
AEIOP, Associazione Italiana Ematologia Oncologia Pediatrica; AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; BFM, Berlin-Frankfurt-Münster; CML, chronic myeloid leukemia; DCLSG, Dutch Childhood Leukemia Study Group; DCOG, Dutch Childhood Oncology Group; DS, Down syndrome; EUROCARE, European Cancer Registry; FAB, French-American-British; HPOG, Hungarian Pediatric Oncology-Hematology Group; LAME, Leucémie Aiguë Myéloblastique Enfant; MDS, myelodysplastic syndrome; MRC, UK Medical Research Counci; N/A, not available; PPLLSG, Polish Pediatric Leukemia/Lymphoma Study Group; s-AML, secondary AML; SPOG, Swiss Paediatric Oncology Group; TMD, transient myeloproliferative disorder.
Hospital-based registry at Children's Cancer Hospital Egypt
AL, acute leukemia; AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; DS, Down syndrome; FAB, French-American-British; MDS, myelodysplastic syndrome; N/A, not available; s-AML, secondary AML.
Registry of Hematology and Blood Diseases Hospital of Chinese Academy of Medical Sciences and Peking Union Medical College and the Japan Society of Pediatric Hematology, respectively.
Centers: Department of Pediatrics and Developmental Science, Mie University Graduate School of Medicine; Department of Pediatrics, Osaka University; Center for Clinical Research, National Center for Child Health and Development; Department of Pediatrics, Aichi Medical University.
Two non M3 secondary leukemias excluded for purposes of this analysis.
Authors onducted a slide reclassification study (n=36) and a prospective study (n=19), both shown above.
AL, acute leukemia; AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; CCLSG, Children's Cancer and Leukemia Study Group; CML, chronic myeloid leukemia; DS, Down syndrome; FAB, French-American-British; HCT, hematopoietic stem cell transplantation; JCACSG, Japanese Childhood AML Cooperative Study Group-JPLSG, Japanse Pediatric Leukemia and Lymphoma Study Group; MDS, myelodysplastic syndrome; N/A, not available; s-AML, secondary AML; TCCSG, Tokyo Children's Cancer Study Group; TPOG, Taiwan Pediatric Oncology Group.
Studies not represented in Figure 2 due to patient populations in large non-contiguous geographical regions.
European Blood and Bone Marrow Transplantation Registry and International Berlin-Frankfurt-Munster Registry, respectively.
Studies were conducted in nations formerly known as Serbia & Montenegro and Yugoslavia, respectively.
AL, acute leukemia; AML, acute myeloid leukemia; ANZCCSG, Australian and New Zealand Children's Cancer Study Group; APL, acute promyelocytic leukemia; BFM, Berlin-Frankfurt-Munster; CCG, Children's Cancer Study Group; COG, Children's Oncology Group; CPH, Czech Pediatric Hematology Working Group; DCOG, Dutch Childhood Oncology Group; DS, Down syndrome; FAB, French-American-British; HSCT, hematopoietic stem cell transplantationr; MDS, myelodysplastic syndrome; N/A, not available; NOPHO, Nordic Society of Paediatric Haematology and Oncology; s-AML, secondary AML; t-AML, therapy-related AML.
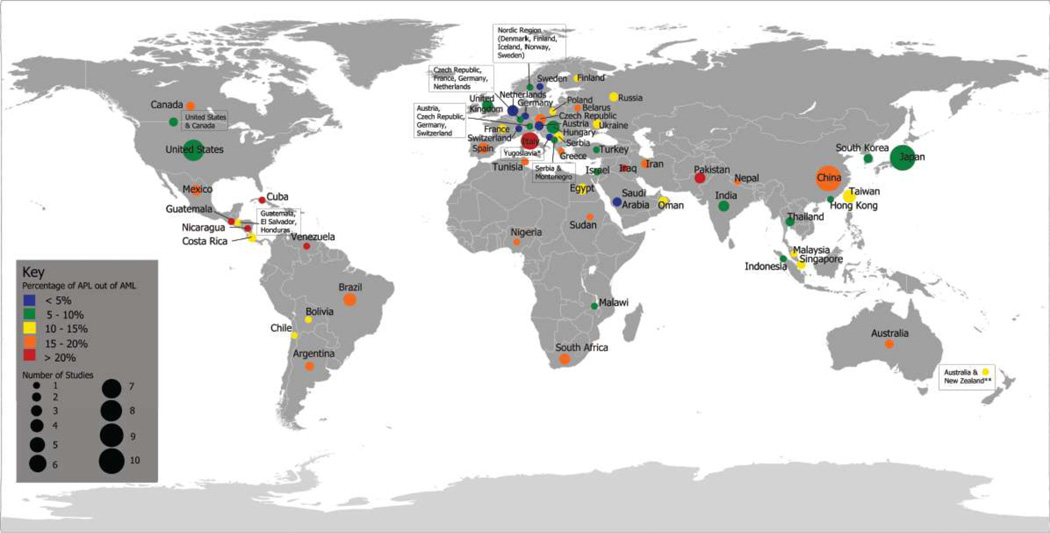
The percentage of childhood AML cases that APL cases comprise was calculated for each study (Table 2a–f). To clearly visualize the data from Table 2a–f, we present data from the 61 countries, or regions spanning multiple countries, in a global map (Figure 2). For each country, childhood APL as a percentage of total AML, averaged across all studies from that country (Table 2a–f), was classified into one of 5 categories: <5%, 5–10%, 10–15%, 15–20%, and >20% (right end point included). These categories are color-coded in Figure 2. The colored dots representing the summary APL statistic for each country are scaled to indicate the number of studies included. Multinational studies that spanned large, non-contiguous geographic areas (noted in Table 2f) were excluded from Figure 2. Averaging across studies to represent entire countries in Figure 2, particularly larger countries like China, Russia and the United States, may mask sub-regional variation in APL prevalence. Therefore, when available, data regarding specific regions or cities was also listed in Table 2a–f. In addition, the source of cases (case series, case-control studies, clinical trials or cancer registries) was noted. For t-APL in children, information regarding the primary malignancy, treatment for primary malignancy, time to APL (latency), karyotype, gender and age at t-APL diagnosis, and outcome were also collected (Supplementary Table S2).
Figure 2. Global map of de novo childhood APL as a percentage of AML.
The percentage of childhood AML cases comprised of APL cases was calculated for each country by averaging all the studies collected for that country. *Study was conducted in region formerly known as Yugoslavia, which includes present day nations of Serbia, Montenegro, Slovenia, Croatia, Bosnia and Herzegovina and the Republic of Macedonia. **One study contained data from patients in both Australia and New Zealand.
Assessment Method for Association Tests
Using the information shown in Table 2, we fit a logistic regression on grouped data to assess the association between the proportion of APL among AML cases with geographical location (continents defined as North America; South/Central America; Europe; Africa; Asia; Oceania), source of cases (case series; clinical trials; case-control studies; cancer registries), period of data collection (median of recruitment time window, in categories following quartile distribution: 1969–87; 1988–95; 1996–2001; 2002–11), eligible age categories (0–2; 0–12; 0–15; 0–19; 0–21), and eligibility of secondary/therapy-related leukemia (no; unknown).
RESULTS & DISCUSSION
Global pattern of childhood APL as a percentage of AML
Geographic area
The 142 studies included in the current review that had relevant data on de novo APL and AML cases represented 61 individual countries or regions spanning multiple countries (Table 2, Figure 2). Information regarding the numbers of childhood AML and APL cases was available for countries in North America (Table 2a), South and Central America (Table 2b), Europe (Table 2c), Africa and the Middle East (Table 2d), Asia (Table 2e), and Oceania (Table 2f). The available data were mostly from Western Europe, South America and Asia. Information regarding numbers of APL cases in Eastern Europe, Southeast Asia, the Middle East and large geographic areas of Africa was more limited. Only six countries in Africa and six countries in the Middle East were represented in the gathered studies.
Table 2 shows that the ratios of de novo APL to total childhood AML cases, calculated for individual studies. Ratios for individual studies were wide ranging, representing from 0% to greater than 50% of AML cases. Figure 2 presents APL as a percentage of total AML, averaged across all studies from that country. In North America, for example, the average ratio of APL to AML is 6.2% in the United States and the rates were around 16% in Canada and Mexico (Table 2a). The lowest proportions of APL (where APL comprised less than 5% of AML cases, colored in blue) were reported in Saudi Arabia and five European countries (Austria, Germany, Netherlands, Sweden, and Switzerland), with the lowest rate of 2.4% in Switzerland (Table 2c). Certain European countries like Italy, the Czech Republic, Belarus and Spain featured relatively higher proportions of APL compared to the rest of Europe. The highest proportions of APL (where APL represented greater than 20% of AML cases, colored in red) were reported in seven countries: Iraq, Pakistan, Italy, Cuba, Nicaragua, Guatemala, and Venezuela, with the highest rate of 58.8% in Nicaragua (Table 2b).
Besides the highest proportions (>20%) of APL in South and Central America referenced above, Brazil and Argentina featured 15–20% of AML cases (Table 2b, Figure 2). APL comprised 10–15% of AML cases in the remaining four studies we gathered from South and Central America, which included patients from Costa Rica, Chile, Bolivia (Table 2b) and a larger multinational region that encompassed Guatemala, Honduras and El Salvador (Table 2f). In the six African countries for which data were available (Table 2d, Figure 2), proportions of APL were varied: APL represented around 15–20% of pediatric AML cases in 4 countries (Nigeria, South Africa, Tunisia, and Sudan), 10–15% of AML cases in Egypt and 5–10% of AML cases in Malawi. Proportions of APL were similarly variable in the Middle East, ranging from as low as 3.4% of AML cases in Saudi Arabia and to as high as 34.5% of AML cases in Iraq (Table 2d, Figure 2). In East Asia, Mainland China featured a relatively higher percentage of APL (19.3%) when compared to the rest of the region (Table 2e, Figure 2), while the highest rate in Asia (~25%) was in Pakistan.
Assessment of the association
A total of 115 studies were included in the regression while 27 studies were excluded due to missing information for one or several variables under analysis. All variables in the model were significantly associated with the proportion of APL: continent (p < 0.0001); source of cases (p < 0.0001), period of data collection (p < 0.0001); eligible age group (p < 0.0001); and eligibility of secondary/therapy-related leukemias (p < 0.0001). Risk estimates for continent adjusted for other variables in the model indicated that, compared to North America, the proportion of APL among AML was two times higher in South/Central America (odds ratio (OR)=2.01, 95% confidence interval (CI)=1.49–2.71) and Oceania (OR=2.07, 95%CI 1.37–3.12), and approximately 50% higher in Africa (OR=1.52, 95%CI 1.06–2.17). Differences were moderate and non-significant between North America and other geographical locations: Europe (OR=1.12, 95%CI 0.88–1.44), Middle East (OR=1.01, 95% CI 0.68–1.50), and Asia (OR=1.10, 95%CI 0.83–1.46).
We also tested all possible combinations of interaction terms in the model. The interaction was significant (p < 0.05) between continent and data collection period, age, and source of cases. Source of cases interacted with data collection period, age, and inclusion of secondary/therapy-related leukemias. Period of recruitment also interacted with source of cases and age. The main effect of continent only remained significant (p < 0.0001) after adjustment for these interaction terms.
Repeating this analysis after exclusion of studies where eligibility of secondary/therapy-related leukemias was not clearly stated (unknown), a total of 76 studies were included in the regression while 10 studies were excluded due to missing information for one or several variables under analysis. All variables in the model were significantly associated with the proportion of APL: continent (p < 0.0001); source of cases (p < 0.0001), period of data collection (p = 0.0069); and eligible age group (p = 0.0057). Risk estimates for continent adjusted for other variables in the model indicated that, compared to North America, the proportion of APL among AML was more than two times higher in South/Central America (OR=2.43, 95% CI=1.70–3.47) and Oceania (OR=2.28, 95%CI 1.48–3.51. Differences were moderate between North America and other geographical locations: Europe (OR=1.32, 95%CI 0.99–1.77), Africa (OR=1.35, 95% CI 0.81–2.28), Asia (OR=1.40, 95%CI 0.99–1.98), and Middle East (OR=0.73, 95%CI 0.35–1.53).
We also tested all possible combinations of interaction terms in this more restricted model. The interaction was significant (p < 0.05) between continent and period, continent and source of cases, period and source of cases, and period and age. Main effect of continent (p < 0.0001), source of cases (p = 0.0115), and period (p < 0.0001) remained significant after adjustment for these interaction terms.
Data source
A few previous studies using hospital-based data examined the incidence of childhood APL in Northern Italy, Mexico City and El Salvador [59, 86], as well as childhood APL as a proportion of AML [29]. However, these studies examined APL data on a regional scale. In this review we combined information from 142 studies, providing a global view of APL as a proportion of AML. The information abstracted from studies included in this review was based largely on hospital- and study-based populations rather than registries. In total, only 12 studies had data from local or national registries [2, 70, 83, 87, 98, 105, 107, 124, 142, 168, 169, 171]. Out of the remaining 130 studies, 11 hospital-based studies [59, 64–66, 102, 118, 119, 150, 174, 182, 198] had data that were more representative of the population, as the hospitals where patients were treated were either the only referral centers, or the major referral centers, for pediatric leukemia in the region or country. For some countries, information was available from both hospital- and study-based populations as well as registries, allowing us to directly compare calculated ratios of APL to AML. For example, 9 Japanese studies [143–151] were hospital-based and their average proportion of childhood APL among AML was 9.13%, while one recently published study from a Japanese registry [142] reported that childhood APL represented 7.9% of total AML. However for many studies, information regarding regional variation was limited. In some countries for which more than one study was available, we observed that there was often variation between studies regarding the percentage of total AML cases that APL comprised. For example, in Mainland China, APL ranged from 5.3% to 36.2% of AML cases (Table 2e).
Data collection period and APL classification method
The studies included covered a wide range of publication years, with the earliest studies published as early as 1979 and the most recent studies published in 2013 and 2014. In the studies gathered, APL was defined using cellular morphology and/or cytogenetics (based on the presence of the chromosomal translocation t(15;17) or the detection of PML/RARα fusion genes by PCR). Earlier studies largely used morphology combined with the presence of coagulopathy to diagnose APL, while more recent studies, or studies involving the testing of novel therapies, morphology and cytogenetics or PCR were used to confirm the presence of APL. Lack of cytogenetics and/or molecular tests in earlier studies would have potentially contributed to less counted cases due to misdiagnosed variants of APL as other AML subtypes, as described earlier.
Sample size
Ranging from as few as 11 children to more than a thousand children, sample sizes for the included studies were variable. For some countries, few studies were available, or studies that were available had information on a relatively small number of AML cases, which may have artificially raised the calculated ratio of APL to AML cases — the country with the highest ratio of APL to AML (58.8% in Nicaragua) had a smaller study size (n=17). However, a number of studies where the proportion of APL was greater than 20% or less than 5% had relatively larger study sizes, or multiple studies, suggesting that the variation in the geographic distribution of APL observed was not due to study size alone.
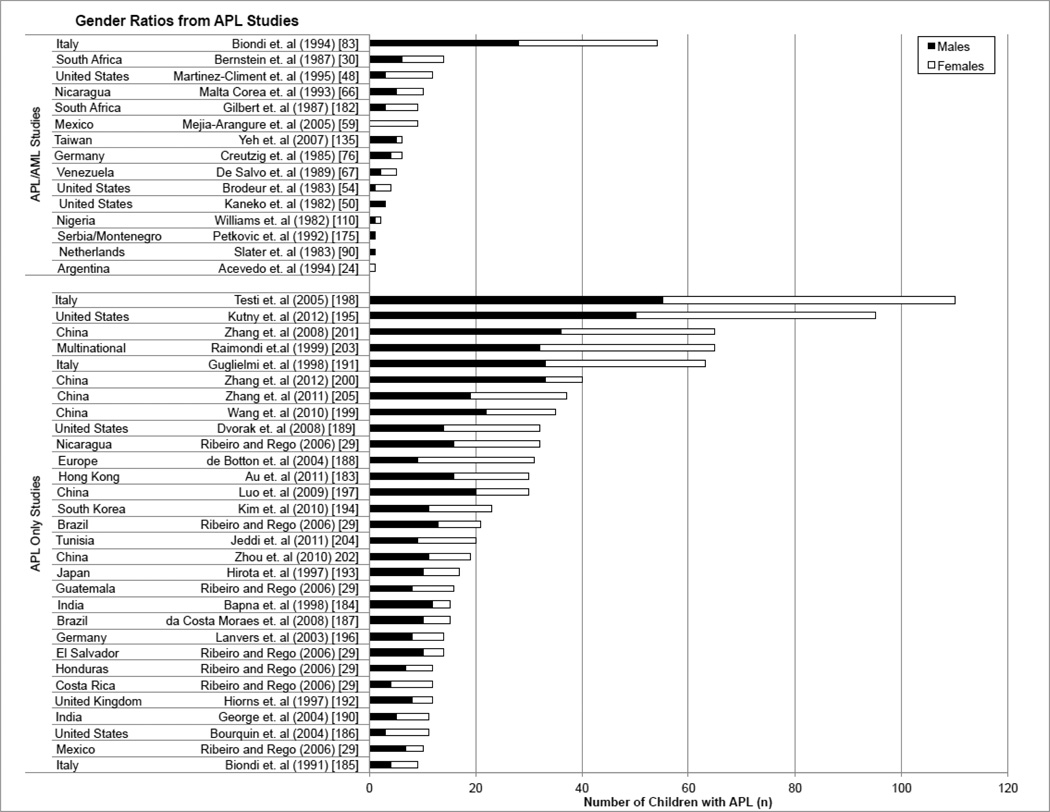
Gender difference in childhood APL varies with study size
Gender ratios of de novo APL studies gathered in this review are presented in Figure 3 and Supplementary Tables S1a–b. We did not find evidence (Figure 3) supporting a predominance of either gender in the APL studies. When study sizes were small, in the de novo cases of APL examined, predominance of male or females was often present, but across the two different groups examined, AML studies with APL data and APL only studies (a and b in Table 1, respectively), as study size increased, gender differences became less apparent (Figure 3, Supplementary Tables S1a–b). For example, in data taken from Yeh et al, the gender ratio (male/female) was 5/1, showing a clear male predominance [135]. Contrastingly, data from Gilbert et al showed a clear female predominance with a gender ratio of 3/6 [182]. In both cases, study sizes were small (n=6 and n=9, respectively). The gender ratio was approximately 50:50 in larger studies, as reflected by studies by Guglielmi et al (n=63, gender ratio: 33/30) and Biondi et al (n=54, gender ratio: 28/26) [83, 191].
Figure 3. Gender ratios for APL in children.
To examine gender, the de novo childhood APL studies were divided into two groups: APL/AML Studies and APL Only Studies. “APL/AML” contained data from studies previously reported in Table 2. “APL Only Studies” contained no information regarding the proportion of APL among AML cases and are represented here for the first time.
Childhood t-APL exemplifies the association between APL and exposure to chemicals
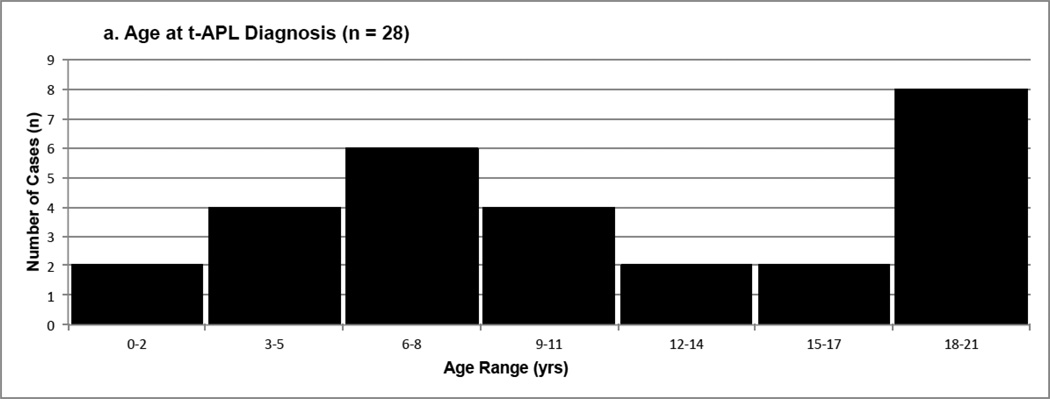
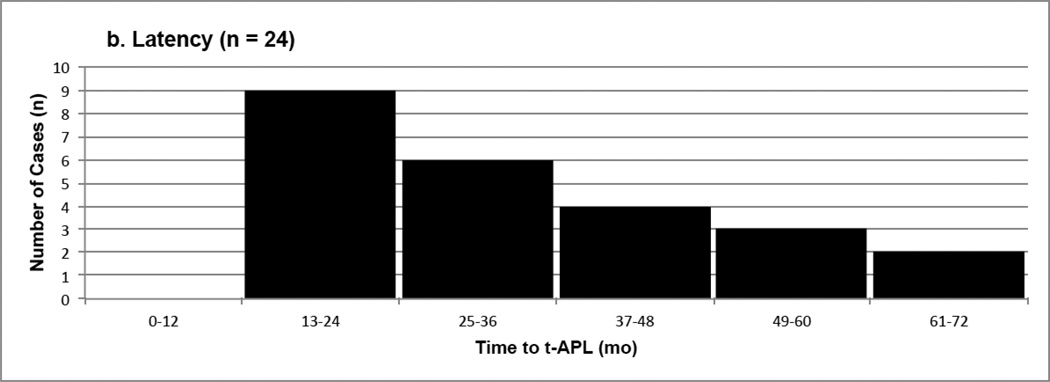
In the current review, 38 pediatric t-APL cases were identified from 30 studies (several studies presented multiple cases of t-APL) in the literature (Table 1, Supplementary Table S2) [36, 37, 206–233]. Ten of these cases were excluded from analysis (Table 3) due to lack of cytogenetic information (the cases were diagnosed by morphology alone) or cytogenetics inconsistent with APL. Characteristics of the final 28 cases are presented in Table 3, and include age at diagnosis of t-APL, gender, latency (time to APL), cytogenetics, primary disease, treatment, and outcome. More detailed information is provided in Supplementary Table S2.
Table 3.
Characteristics of Childhood t-APL Cases (n = 28) a
| Characteristics | Mean | Median (Range) |
No. (%) |
|---|---|---|---|
| Age at t-APL diagnosis (yrs) | 11.4 | 10 (2 – 21) | |
| < 5 | 4 (14) | ||
| 5 – 10 | 12 (43) | ||
| 11 – 15 | 3 (11) | ||
| > 15 | 9 (32) | ||
| Sex | |||
| Male | 12 (43) | ||
| Female | 16 (57) | ||
| Latency (mo) | 35.8 | 32 (18 – 72) | 24 b |
| Cytogenetics | 26 c | ||
| t(15;17) | 18 (69) | ||
| t(15;17) + others | 4 (15) | ||
| t(5;17) | 2 (8) | ||
| t(11;17) | 1 (4) | ||
| Other | 1 (4) | ||
| Primary Disease | |||
| Hematological Diseases | 17 (61) | ||
| Acute Lymphoblastic Leukemia | 1 (4) | ||
| Hemophagocytic Lymphohistiocytosis | 2 (7) | ||
| Hodgkin Lymphoma | 4 (14) | ||
| Langerhans Cell Histiocytosis | 7 (25) | ||
| Non-Hodgkin Lymphoma | 3 (11) | ||
| Solid Tumors | 6 (21) | ||
| Brain astrocytoma | 1 (4) | ||
| Germ cell tumor (choriocarcinoma) | 1 (4) | ||
| Glioblastoma | 1 (4) | ||
| Neuroblastoma | 1 (4) | ||
| Rhabdomyosarcoma | 1 (4) | ||
| Wilms' Tumor | 1 (4) | ||
| Other Conditions | 5 (18) | ||
| Liver Transplant | 2 (7) | ||
| Multiple Sclerosis | 1 (4) | ||
| Psoriasis | 2 (7) | ||
| Treatment for Primary Disease d | |||
| Radiation | 9 (32) | ||
| Topoisomerase II inhibitors/anthracyclines e | 27 (96) | ||
| Alkylating Agents f | 14 (50) | ||
| Vinca Alkaloids g | 12 (43) | ||
| Anti-metabolites h | 8 (29) | ||
| Steroids i | 8 (29) | ||
| Other j | 9 (32) | ||
| Outcome | 19 l | ||
| Complete Remission | 16 (84) | ||
| Death | 3 (16) | ||
Ten additional cases of childhood t-APL were excluded due to cytogenetics inconsistent with APL or diagnosis by morphology alone.
Latency information was not available for one case; three additional cases were excluded because the definition of latency used was not comparable to the rest of the cases.
Cytogenetic information was not available for two cases. APL was confirmed by detection of PML-RARA using RT-PCR.
Multiple types of drugs were involved in treatments for primary disease in many case studies. Drugs were counted in the table each time they were used.
Topoisomerase II inhibitors/anthracyclines: actinomycin-D, doxorubicin/adriamycin, etoposide, mitoxantrone, teniposide, unspecified topoisomerase II inhibitors/anthracyclines.
Alklayting agents: carboplatin, cisplatin, cyclophoshamide, dacarbazine, ifosfamide.
Vinca alkaloids: vinblastine, vincristine/oncovin, vindesine.
Antimetabolites: 6-mercaptopurine, azathioprine, cytarabine/cytosine arabinoside, methotrexate.
Steroids: methylprednisone, prednisolone, prednisone, dexamethasone.
Other: bleomycin, cyclosporine, tacrolimus, L-asparaginase.
information on outcome was unavailable for 9 cases.
Characteristics and primary diseases of childhood t-APL cases
The age at t-APL diagnosis ranged from 2–21 years, with a median age of 10 years and a mean age of 11.4 years (Table 3, Figure 4a). In contrast to the de novo APL findings, in the 28 cases of childhood t-APL included in this review, there was a female predominance of 57% (16 girls versus 12 boys) though the case studies collected provide a limited view which may not be representative of the entire population. Primary diseases included Langerhans cell histiocytosis (LCH), multiple sclerosis (MS), Hodgkin lymphoma (HL), psoriasis, non-Hodgkin lymphoma (NHL), and assorted solid tumors. Among the cases included, the most common primary diseases were LCH, HL, and a variety of solid tumors, which accounted for 25%, 14% and 21% of the included primary diseases, respectively. Excluding leukemia, other hematological disorders—LCH, HL and NHL—comprised 61% of the total primary diseases prior to therapy-related APL.
Figure 4. a–b Distribution of data from therapy-related APL studies in Children.
Figure 4a depicts age (in years) at t-APL diagnosis for n=28 cases. Figure 4b depicts time to APL (latency) for n=24 cases.
Treatment for primary disease
Depending on primary malignancy, treatment for primary disease was variable. Even within cases with the same primary malignancy, there were differences in treatments for individual cases. It has previously been established that exposure to radiation and chemotherapy drugs including mitotic inhibitors and alkylating agents involved in the treatment for a variety of primary malignancies or conditions are associated with the development of t-APL and AML in children [34, 36]. Consistent with this result, in the cases included in this review, the most frequently used drugs to treat primary malignancies prior to secondary APL were topoisomerase II inhibitors (i.e. etoposide, mitoxantrone) and anthracyclines, alkylating agents (i.e. cyclophosphamide, dicarbazine) and vinca alkaloids (i.e. vinblastine, vincristine). In many cases combinations of drugs and radiotherapy were employed. In two cases, secondary APL developed following radiation alone.
Latency definition and distribution
In the cases included, definitions of latency (time to APL) were varied. In this review, latency was defined as either the time from diagnosis of primary disease to development of t-APL or the time from beginning of treatment for primary disease to development of t-APL, depending on the information available from the original studies. For the purposes of this study, we assumed that the time between diagnosis of primary disease and beginning of treatment was negligible allowing us to compare latencies across cases.
A median of 32 months (Table 3, Figure 4b) and a mean of 35.8 months were calculated from 24 cases listed in Supplementary Table S2 (latency information was not available for one case, and three cases were excluded from calculations in Table 3 and Figure 4b due to definitions of latency inconsistent with our definition). Latency times that we recorded were highly varied — development of APL occurred as quickly as 18 months following interruption of primary therapy in one case study or after more than 6 years (72 months) in another (Figure 4b). Information on APL latency (time to APL) indicates that in the majority of cases, t-APL developed within roughly 1–3 years after treatment for the primary illness.
Cytogenetics and prognosis
For cases where cytogenetic information was available (n=26), the majority (84%) of cases had characteristic t(15;17) cytogenetics. Two cases (8%) showed more rare t(5;17) cytogenetics and one case featured a t(11;17) translocation. Of the 28 cases, there were 19 cases with available information regarding prognosis: complete remission following treatment for APL (n=16) and death (n=3). Because the APL case studies included span several decades, it is important to take into account improved outcomes as therapy and supportive care has changed.
While a previous study by Ogami et al, provided a review of 15 cases of pediatric t-APL [228], the current study provides an updated and expanded view of t-APL, examining 28 cases of t-APL in children and adolescents up to 21 years old. While the case studies described here provide some insight into childhood t-APL, there is a lack of comprehensive data regarding the proportion of children that develop APL after treatment for a primary disease.
Comparison of t-APL in children and adults
Twenty-six studies that included information on t-APL in adults, representing a total of 260 cases (age > 21), were identified from the literature (Supplementary Table S3) and are summarized in Table 4 [38, 40, 208, 214, 216, 220, 234–253]. Of the 24 studies that included information about gender, 98 were men and 150 were women. In adult t-APL cases, there was a similar female predominance to childhood t-APL (60% vs 59%, respectively). Among these adults, the most common primary diseases prior to development of APL were breast cancer (94 cases), multiple sclerosis (51 cases) and prostate cancer (21 cases). Similarly, an earlier review examined 326 adult cases of t-APL from the literature and reported the most common conditions prior to therapy-related APL were breast cancer, hematological malignancies, multiple sclerosis and genitourinary malignancies [264]. Hematological malignancies and multiple sclerosis were also among the most frequent diseases prior to childhood t-APL, as discussed in the previous section (Table 3).
Table 4.
Characteristics of Adult t-APL Cases (n = 260)
| Characteristics | Range | No. (%) |
|---|---|---|
| Age Range (yrs) | 21 – 81 | |
| Sex | 248 a | |
| Male | 98 (39) | |
| Female | 150 (60) | |
| Latency (mo) | 1 – 276 | |
| Primary Disease | ||
| Hematological Diseases | 39 (15) | |
| AML/MDS | 3 (1.1) | |
| Diffuse large B-cell lymphoma | 5 (1.9) | |
| Follicular NHL | 2 (0.8) | |
| Folllicular lymphoma | 3 (1.1) | |
| HL | 6 (2.3) | |
| Mucosa-associated lymphoid tissue NHL | 1 (0.4) | |
| Multiple myeloma | 1 (0.4) | |
| NHL | 11(4.2) | |
| Primary-CNS NHL | 1 (0.4) | |
| SLL/CLL | 4 (1.5) | |
| T-cell NHL | 1 (0.4) | |
| Mantle cell lymphoma | 1 (0.4) | |
| Autoimmune Disorders | 4 (1.5) | |
| Chron's disease | 1 (0.4) | |
| Membranous glumerulonephropathy | 1 (0.4) | |
| Rhuematoid arthritis | 2 (0.8) | |
| CNS Disorders | 54 (21) | |
| Hydatiform mole | 1 (0.4) | |
| Lewis-Sumner Syndrome | 1 (0.4) | |
| Multiple sclerosis | 51 (19.6) | |
| Polyradiculoneuritis | 1 (0.4) | |
| Solid Tumors | 165 (63) | |
| Adenocarcinoma | 1 (0.4) | |
| Astrocytoma | 1 (0.4) | |
| Bladder carcinoma | 2 (0.8) | |
| Breast carcinoma | 94 (36.2) | |
| Cervical cancer | 2 (0.8) | |
| Choriocarcinoma | 1 (0.4) | |
| Colon cancer | 6 (2.3) | |
| Corpus-Uteri carcinoma | 2 (0.8) | |
| Endometrium carcinoma | 2 (0.8) | |
| Gestational trophoblastic disease | 1 (0.4) | |
| Head and neck squamous cell carcinoma | 2 (0.8) | |
| Histiocytoma | 1 (0.4) | |
| Laryngeal carcinoma | 2 (0.8) | |
| Lung carcinoma | 3 (1.1) | |
| Malignant fibrous histiocytoma | 2 (0.8) | |
| Mixed germ cell tumor | 1 (0.4) | |
| Neuroectodermal tumor | 1 (0.4) | |
| Neuroepethlioma | 1 (0.4) | |
| Ovarian carcinoma | 4 (1.5) | |
| Pancreatic cancer | 1 (0.4) | |
| Primary location unknown carcinoma | 1 (0.4) | |
| Prostate carcinoma | 21 (8.1) | |
| Seminoma | 2 (0.8) | |
| Squamous cell carcinoma | 2 (0.8) | |
| Stomach cancer | 1 (0.4) | |
| Testicular cancer | 4 (1.5) | |
| Thyroid carcinoma | 3 (1.1) | |
| Tongue carcinoma | 1 (0.4) | |
| Other Conditions | 2 (0.8) | |
| Arthritis | 1 (0.4) | |
| Cardiac transplant | 1 (0.4) | |
| Treatment for Primary Diseaseb | ||
| Radiation | 153 (59) | |
| Topoisomerase I inhibitors c | 1 (0.4) | |
| Topoisomerase II inhibitors/anthracyclines d | 186 (72) | |
| Alkylating agents e | 146 (56) | |
| Vinca alkaloids f | 46 (18) | |
| Antimetabolites g | 101 (39) | |
| Taxanes h | 7 (3) | |
| Steroids i | 17 (7) | |
| Monoclonal Antibodies j | 6 (2) | |
| Hormone Therapy k | 10 (4) | |
| Unspecified Chemotherapy | 1 (0.4) | |
| Other l | 29 (11) | |
Gender information not available for 12 cases.
Multiple types of drugs were involved in treatments for primary disease in many case studies. Drugs were counted in the table each time they were used.
Topoisomerase I inhibitors: irinotecan.
Topoisomerase II inhibitors/anthracyclines: actinomycin-D, daunorubicin, doxorubicin/adriamycin, ellipticine, epirubicin, etoposide, hydroxydanunorubicin, idarubicin, mitoxantrone, teniposide, unspecified topoisomerase II inhibitors.
Alklayting agents: busulfan, carboplatin, chlorambucil, cisplatin, cyclophoshamide, dacarbazine, ifosfamide, lomustin, mechlorethamine, melphalan, mitomycin, oxaliplatin, prednimustine, thiotepa, procarbazine, unspecified alkylating agents.
Vinca alkaloids: vinblastine, vincristine/oncovin, vindesine, vinorelbine.
Antimetabolites: 5-FU fluorouracil, 6-mercaptopurine, azathioprine, capacitabine, cytarabine/cytosine arabinoside, fludarabine, methotrexate, thioguanine, unspecified antimetabolite chemotherapy.
Taxanes: docetaxel, paclitaxel, unspecified taxanesantimetabolite chemotherapy.
Steroids: methylprednisone, prednisolone, prednisone, unspecified steroids.
Monoclonal antibodies: herceptin, rituximab.
Hormone therapy: anastrazole, leuprolide, medroxyprogesterone acetate, tamoxifen, unspecified hormone therapy.
Other: bleomycin, immunospuression, interferon, interferon-beta, TNF-alpha inhibitor.
AML, acute myeloid leukemia; CNS, central nervous system; HL, Hodgkin lymphoma; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; SLL/CLL, small lymphocytic lymphoma/chronic lymphocytic leukemia.
Similar to children, some of the most common treatments prior to development of t-APL in adults were treatment with topoisomerase II inhibitors/anthracyclines, alkylating agents, and radiation therapy. Radiation was involved in therapy for 153 of the cases (Table 4). Time to APL diagnosis varied among studies, ranging from as short a period of time one month to as long as 276 months.
Environmental and occupational exposure related factors associated with APL
One of the goals of our study was to examine the potential contribution of exposure-related risk factors to geographic variations in childhood APL. However, few studies have specifically examined childhood APL and exposure.
Increased risk of APL
In one of two cases examined in a single study, a t(15;17) translocation was determined to have arisen prenatally, over 10 years before clinical manifestation of childhood APL [265]. Though confirmation in additional patients is needed, it is probable that prenatal and postnatal exposures may play a role in the origination and development of childhood APL. In utero exposure to ionizing radiation [43], 3rd trimester air toxics exposures to chloroform, benzene, and two other traffic-related chemicals (meta/para-xylene and toluene) [45], and parental smoking [44], as well as childhood exposure to petroleum solvents [42] are potentially assosicated with development of childhood AML. Increased risk of childhood ALL has been associated with accelerated fetal growth [266], home exposure to herbicides (chlorthal, and possibly alachlor) [267] and paint [42], paternal ever smoking, particularly preconception [268]. Elevated ALL risks associated with use of paints in the home (ever) and indoor insecticides (pre-birth) were found to be limited to subjects carrying specific haplotypes of CYP2C8 and ABCB1, respectively [269]. Exposure to benzene, a well-known risk factor for adult AML, may also be associated with both childhood ALL and AML [270]. Larger studies are needed to determine the risk of these prenatal and postnatal exposures on development of childhood APL and the contribution of genetic susceptibility.
In adults, an increased risk of APL has been previously associated with various environmental, occupational and life-style related risk factors. For this review, ten studies (6 case-control studies and 4 case series studies) suggesting an association between certain risk factors and APL in adults were identified and are summarized in Table 5 [254–263]. Occupational and industrial exposures associated with an increased risk of APL included general construction (OR=2.28, 95% CI=1.03–50.05), metal work (OR=14.00, 95% CI=1.72–113.77) [254] and shoe making (OR= 6.3, 95% CI=1.2–31.1) [255]. The study authors suggest that the relationship between APL and shoe making is potentially related to exposure to benzene, which is present in glues used for shoe making [255].
Table 5.
Occupational, Environmental and Life-style Related Factors Associated with an Increased Risk of APL in Adults
| a. Case-Control | Subgroup | OR (95% CI) | APL (n) | Control (n) | Reference |
|---|---|---|---|---|---|
| Occupational & Industrial | |||||
| Benzene | 1.95 (0.98–3.88) | 18 | 20 | Wong et. al (2010) [254] | |
| Metal work | 14.00 (1.72–113.77) | 7 | 1 | Wong et. al (2010) [254] | |
| Textile and other fabric manufacturing | 2.5 (1.10–5.66) | 15 | 14 | Wong et. al (2010) [254] | |
| General construction | 2.28 (1.03–5.05) | 15 | 15 | Wong et. al (2010) [254] | |
| Metals | 2.28 (1.03–5.05) | 10 | 8 | Wong et. al (2010) [254] | |
| Shoe making | 6.3 (1.3–31.1) | 2 | 17 | Mele et. al (1995) [255] | |
| Environmental & Lifestyle | |||||
| Smoking | ever smoking | 0.32 (0.1–1) a | 4 | 168 | Bjork et. al (2001) [256] |
| non-smoker | 14 | 166 | |||
| ever smoking | 0.57 (0.32–1) | 23 | 943 | Moorman et. al (2002) [257] | |
| current smoking | 0.47 (0.23–0.96) | 11 | 461 | ||
| past smoking | 0.72 (0.35–1.49) | 12 | 472 | ||
| 0.56 (0.30–1.03) | 53 | 618 | Sandler et. al (1993) [258] | ||
| Alcohol consumption | 0.96 (0.68–1.69) | 44 | 83 | Wong et. al (2009) [259] | |
| BMI | underweight | 0.81 (0.35–1.85) | 10 | 26 | Wong et. al (2009) b [259] |
| desirable | 1.00 (reference) | 65 | 149 | ||
| overweight | 1.55 (0.92–2.63) | 30 | 60 | ||
| obese | 2.15 (0.89–5.20) | 11 | 13 | ||
| Home/workplace renovation | 2.02 (1.06–3.85) | 20 | 21 | Wong et. al (2009) [259] | |
| 2.01 (1.01–4.00) | N/A | N/A | Wong et. al (2010) [254] | ||
| b. Case Series | Subgroup | OR (95% CI) | APL | AML | References |
| Occupational & Industrial | (n) | (n) | |||
| Benzene | 4 | 9 | Travis et. al (1994) [260] | ||
| 1 | 20 | Yin et. al (1989) [263] | |||
| Electricians | 4.4 (2.1–9.1) c | 22 | 11 | Pulsoni et. al (1998) [261] | |
| Environmental & Lifestye | BMI | BMI | |||
| BMI | 25th %tile BMI | 22.8 | 21.3 | Estey et. al (1997) d [262] | |
| 50th %tile BMI | 26 | 24.2 | |||
| 75th %tile BMI | 28.9 | 27.6 | |||
Odds ratio for ever smokers versus life-long non-smokers
APL had a positive trend of OR by increasing BMI (p-trend = 0.03)
Risk of APL versus other AML expressed as an OR.
Wilcoxon Mann–Whitney test for hypothesis of no difference between distribution of BMI in APL and other AML patients, p=0.0003
AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; BMI, body mass index; CI, confidence interval; N/A, not available; OR, odds ratio.
Benzene exposure and APL
Additional previous studies have supported an association between benzene exposure and development of APL. In a case-control study by Richardson et al examining occupational risk factors for acute leukemia, the authors reported that exposed cases, (which included workers exposed to benzene), had more AML-M3 subtypes than non-exposed cases (7.8% versus 5.2%) [271]. One case of APL was also reported in benzene-exposed workers in a study by Yin et al in China [263]. In addition, in a cohort study of benzene-exposed Chinese factory workers, APL was the most common form of AML diagnosed, representing 4 out of a total of 9 AML cases [260]. In a study by Wong et al (2010) of 722 newly diagnosed AML cases and 1,444 individually gender- and aged-matched patient controls in Shanghai, a borderline significant risk (OR= 1.95, 95% CI=0.98–3.88) of almost two-fold was found between benzene exposure and APL development (Table 5). This study, as well as an additional study by the same author (Wong et al, 2009) found that home and workplace renovation was associated with an increased risk for APL (OR= 2.01, 95% CI=1.01–4.00) and OR=2.02, 95% CI=1.06–3.85, respectively) in adults in Shanghai [254, 259]. These studies named paints, adhesives, glues, solvents, preservatives, dusts, treated fabrics and building materials as potential exposures present in these environments, specifically noting the high benzene levels associated with Chinese commercial painting and elevated levels of formaldehyde and benzene (known human leukemogens) as well as toluene, xylene and other volatile organic chemicals in newly renovated homes in China [254, 259].
Body mass index, smoking and APL
With regards to life style and environment, increased body mass index (BMI) was reportedly associated with APL, with ORs increasing with increasing BMI (ptrend = 0.03, Table 5), in adults in Shanghai [259]. In a study by Estey et al, increasing BMI in adults was associated with diagnosis of APL [262]. Similarly, in children, obesity at diagnosis has previously reported to be associated with APL. In a study of fifty children by Feusner et al, 13 of them (26%) were obese, compared to an expected overall incidence of 11% in a healthy pediatric population [31]. In the studies we identified that examined associations between smoking and specific AML subtypes, an association between smoking and development of APL was not found [256, 257].
CONCLUSIONS
This study provides the first comprehensive overview of global variation in the proportion of childhood APL among AML cases. In the 142 studies gathered, we found the lowest percent of APL among AML in Switzerland (2.4%) and the highest in Nicaragua (58.8%). Compared to North America, the assessed APL risk among AML cases was more than two times higher in South/Central America (OR=2.43, 95% CI=1.70–3.47). A goal of this study was to examine whether known or potential environmental exposures and lifestyle-related factors may contribute to this apparent global variation. Due to the limited number of studies directly addressing childhood APL development and exposure, we examined 28 childhood cases of therapy-related APL, which exemplified associations between prior exposures to chemotherapeutic drugs and APL development.
FUTURE DIRECTIONS
More studies examining both the incidence of APL, in countries and regions for which information is lacking, and the association of APL with specific risk factors are needed. In this review, we have examined the distribution of APL mostly in hospital- and study-based populations; further studies examining the distribution of APL using population-based data like national and/or regional cancer registries are needed. Since APL is a relatively rare subtype of acute childhood leukemia, studying exposure from individual studies is difficult. The Childhood Leukemia International Consortium (CLIC) may be better positioned to study APL [272].
Supplementary Material
PRACTICE POINTS.
Geographic patterns may potentially reflect specific genetic and environmental factors involved in development of APL.
In light of increasing globalization, where patients have lived previously may have increasing relevance to their risk of APL.
A predominance of either gender in de novo childhood APL was unclear. Although in the t-APL cases gathered a female predominance was observed, the number of cases was too few to draw conclusions about a potential gender difference.
Development of t-APL in children is potentially associated with exposure to certain chemotherapy drugs/radiation given for treatment for a primary disease.
RESEARCH AGENDA
Geographic distribution of childhood APL using population-based data from registries
Contribution of specific environmental factors to childhood APL risk
Association between obesity and APL in children
ACKNOWLEDGEMENT
This study was supported in part by the National Institute of Environmental Health Sciences, NIEHS, and National Cancer Institute, NCI, (grant R01ES009137), NIEHS (grants P42ES04705 and P01ES018172), and the US Environmental Protection Agency, US EPA (grant RD83451101). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS, NCI and US EPA. Aaida Samad, an undergraduate, and a summer intern of STEER program (2011) at UC Berkeley was partially funded by grants P01ES018172 and RD83451101 and the Council for Education and Research on Toxics (CERT). This work would not have been possible without the early support and contributions of Professor Patricia Buffler (1938–2013). Thank you to Dr. Cliona McHale for her scientific insight and assistance with editing. We are grateful to Dr. Sameera Ezzat for providing her unpublished data regarding childhood APL in Egypt.
ABBREVIATIONS LIST
- ALL
Acute Lymphocytic Leukemia
- AML
Acute Myeloid Leukemia
- APL
Acute Promyelocytic Leukemia
- ATRA
All-trans Retinoic Acid
- BMI
Body Mass Index
- CI
Confidence Interval
- CLIC
The Childhood Leukemia International Consortium
- FAB
French-American-British
- HL
Hodgkin Lymphoma
- ICD-10
10th revision of the International Statistical Classification of Diseases and Related Health Problems
- LCH
Langerhans Cell Histiocytosis
- MS
Multiple Sclerosis
- NHL
Non-Hodgkin Lymphoma
- OR
Odds Ratio
- t-AML
Therapy-related AML
- t-APL
Therapy-related APL
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
Dr. Smith has received consulting and expert testimony fees from lawyers representing both plaintiffs and defendants in cases involving claims related to exposure to chemicals and leukemia. The remaining authors declare that there are no conflicts of interest.
REFERENCES
- 1.Kaatsch P. Epidemiology of childhood cancer. Cancer Treatment Reviews. 2010;36:277–285. doi: 10.1016/j.ctrv.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Monge P, Wesseling C, Rodríguez AC, Cantor KP, Weiderpass E, Reutfors J, et al. Childhood leukaemia in Costa Rica, 1981–96. Paediatric and Perinatal Epidemiology. 2002;16:210–218. doi: 10.1046/j.1365-3016.2002.00422.x. [DOI] [PubMed] [Google Scholar]
- 3.Deschler B, Lubbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107:2099–2107. doi: 10.1002/cncr.22233. [DOI] [PubMed] [Google Scholar]
- 4.Margolin JF. Molecular diagnosis and risk-adjusted therapy in pediatric hematologic malignancies: a primer for pediatricians. Eur J Pediatr. 2011;170:419–425. doi: 10.1007/s00431-011-1424-7. [DOI] [PubMed] [Google Scholar]
- 5.Quintana J, Advis P, Becker A, Beresi V, Campbell M, Vines EF, et al. Acute myelogenous leukemia in Chile PINDA protocols 87 and 92 results. Leukemia. 2005;19:2143–2146. doi: 10.1038/sj.leu.2403959. [DOI] [PubMed] [Google Scholar]
- 6.Gregory J, Feusner J. Acute Promyelocytic Leukemia in Childhood. Curr Oncol Rep. 2009;11:439–445. doi: 10.1007/s11912-009-0060-0. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez P, Milanes MT, Svarch E, Martinez G, Ballester JM. High relative proportion of acute promyelocytic leukemia in children: experience of a multicenter study in Cuba. Leuk Res. 2000;24:739–740. doi: 10.1016/s0145-2126(00)00012-6. [DOI] [PubMed] [Google Scholar]
- 8.Lowenberg B, Downing JR, Burnett A. Acute Myeloid Leukemia. New England Journal of Medicine. 1999;341:1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 9.St. Jude Children’s Research hospital, editor. Leukemias / Lymphomas: Acute Promyelocytic Leukemia (APL) Disease Information. Memphis: St. Jude Children's Research Hospital; 2013. Available from: http://www.stjude.org/stjude/v/index.jsp?vgnextoid=9c0c061585f70110VgnVCM1000001e0215cRCRD. [Google Scholar]
- 10.de The H, Chomienne C, Lanotte M, Degos L, Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature. 1990;347:558–561. doi: 10.1038/347558a0. [DOI] [PubMed] [Google Scholar]
- 11.Longo L, Pandolfi PP, Biondi A, Rambaldi A, Mencarelli A, Lo Coco F, et al. Rearrangements and aberrant expression of the retinoic acid receptor alpha gene in acute promyelocytic leukemias. J Exp Med. 1990;172:1571–1575. doi: 10.1084/jem.172.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bain BJ. The Nature of Leukaemia, Cytology, Cytochemistry and the FAB Classification of Acute Leukaemia. Leukaemia Diagnosis: Wiley-Blackwell. 2010:1–63. [Google Scholar]
- 13.Nagendra S, Meyerson H, Skallerud G, Rosenthal N. Leukemias resembling acute promyelocytic leukemia, microgranular variant. Am J Clin Pathol. 2002;117:651–657. doi: 10.1309/KD1G-NUR1-J75P-HQ28. [DOI] [PubMed] [Google Scholar]
- 14.Rizzari C, Biondi A. Tailoring treatment strategy for acute promyelocytic leukemia in low-income countries. Pediatr Blood Cancer. 2009;53:303–305. doi: 10.1002/pbc.22087. [DOI] [PubMed] [Google Scholar]
- 15.Huret J. t(17;17)(q21;q24) PRKAR1A/RARA; del(17)(q21q24) PRKAR1A/RARA. Atlas Genet Cytogenet Oncol Haematol. 2011;16:49–50. [Google Scholar]
- 16.Mistry AR, Pedersen EW, Solomon E, Grimwade D. The molecular pathogenesis of acute promyelocytic leukaemia: implications for the clinical management of the disease. Blood Rev. 2003;17:71–97. doi: 10.1016/s0268-960x(02)00075-9. [DOI] [PubMed] [Google Scholar]
- 17.Redner RL. Variations on a theme: the alternate translocations in APL. Leukemia. 2002;16:1927–1932. doi: 10.1038/sj.leu.2402720. [DOI] [PubMed] [Google Scholar]
- 18.Redner RL, Contis LC, Craig F, Evans C, Sherer ME, Shekhter-Levin S. A novel t(3;17)(p25;q21) variant translocation of acute promyelocytic leukemia with rearrangement of the RARA locus. Leukemia. 2006;20:376–379. doi: 10.1038/sj.leu.2404062. [DOI] [PubMed] [Google Scholar]
- 19.Strehl S, Konig M, Boztug H, Cooper BW, Suzukawa K, Zhang SJ, et al. All-trans retinoic acid and arsenic trioxide resistance of acute promyelocytic leukemia with the variant STAT5B-RARA fusion gene. Leukemia. 2013;27:1606–1610. doi: 10.1038/leu.2012.371. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto Y, Tsuzuki S, Tsuzuki M, Handa K, Inaguma Y, Emi N. BCOR as a novel fusion partner of retinoic acid receptor alpha in a t(X;17)(p11;q12) variant of acute promyelocytic leukemia. Blood. 2010;116:4274–4283. doi: 10.1182/blood-2010-01-264432. [DOI] [PubMed] [Google Scholar]
- 21.Kondo T, Mori A, Darmanin S, Hashino S, Tanaka J, Asaka M. The seventh pathogenic fusion gene FIP1L1-RARA was isolated from a t(4;17)-positive acute promyelocytic leukemia. Haematologica. 2008;93:1414–1416. doi: 10.3324/haematol.12854. [DOI] [PubMed] [Google Scholar]
- 22.Catalano A, Dawson MA, Somana K, Opat S, Schwarer A, Campbell LJ, et al. The PRKAR1A gene is fused to RARA in a new variant acute promyelocytic leukemia. Blood. 2007;110:4073–4076. doi: 10.1182/blood-2007-06-095554. [DOI] [PubMed] [Google Scholar]
- 23.Creutzig U, van den Heuvel-Eibrink MM, Gibson B, Dworzak MN, Adachi S, de Bont E, et al. Diagnosis and management of acute myeloid leukemia in children and adolescents: recommendations from an international expert panel. Blood. 2012;120:3187–3205. doi: 10.1182/blood-2012-03-362608. [DOI] [PubMed] [Google Scholar]
- 24.Acevedo S, Slavutsky I, Andreoli G, Larripa I. Cytogenetic Study of 50 De-Novo Cases of ANLL from Argentina. Haematologica. 1994;79:40–45. [PubMed] [Google Scholar]
- 25.Swerdlow S, Campo E, Harris NL, Jaffe ES, Pileri SA, STein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue World Health Organization. 2008
- 26.Imbach P. Acute Myeloid Leukemia. In: Imbach P, Kühne T, Arceci RJJ, editors. Pediatric Oncology. Berlin Heidelberg: Springer; 2011. pp. 21–33. [Google Scholar]
- 27.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 28.Mantadakis E, Samonis G, Kalmanti M. A comprehensive review of acute promyelocytic leukemia in children. Acta Haematol. 2008;119:73–82. doi: 10.1159/000117712. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro RC, Rego E. Management of APl in developing countries: epidemiology, challenges and opportunities for international collaboration. Hematology Am Soc Hematol Educ Program. 2006;162 doi: 10.1182/asheducation-2006.1.162. [DOI] [PubMed] [Google Scholar]
- 30.Bernstein R, Macdougall LG, Pinto MR. Chromosome Patterns in 26 South African Children with Acute Nonlymphocytic Leukemia (ANLL) Cancer Genet Cytogenet. 1984;11:199–214. doi: 10.1016/0165-4608(84)90114-6. [DOI] [PubMed] [Google Scholar]
- 31.Feusner J, Kim H, Gregory J, Alonzo T, Woods W, Weinstein H, et al. Obesity in Newly Diagnosed Childhood Acute Promyelocytic Leukemia. ASH Annual Meeting Abstracts. 2006;108 4494-. [Google Scholar]
- 32.Biondi A, Testi AM, Gibson BES. Acute Promyelocytic Leukaemia. 2010:83–108. [Google Scholar]
- 33.Pyatt DW, Aylward LL, Hays SM. Is Age an Independent Risk Factor for Chemically Induced Acute Myelogenous Leukemia in Children? Journal of Toxicology and Environmental Health, Part B. 2007;10:379–400. doi: 10.1080/15287390600975061. [DOI] [PubMed] [Google Scholar]
- 34.Sandoval C, Pui C-H, Bowman LC, Heaton D, Hurwitz CA, Raimondi SC, et al. Secondary acute myeloid leukmeia in children perviously treated with alkylating agents, interclating topoisomeraise II inhibitors and irradiation. Journal of Clinical Oncology. 1993;11:1039–1045. doi: 10.1200/JCO.1993.11.6.1039. [DOI] [PubMed] [Google Scholar]
- 35.Jantunen E, Heinonen K, Mahlamäki E, Penttilä K, Kuittinen T, Lehtonen P, et al. Secondary acute promyelocytic leukemia: An increasingly common entity. Leuk Lymphoma. 2007;48:190–191. doi: 10.1080/10428190600961736. [DOI] [PubMed] [Google Scholar]
- 36.Detourmignies L, Castaigne S, Stoppa AM, Harousseau JL, Sadoun A, Janvier M, et al. Therapy-related acute promyelocytic leukemia: a report on 16 cases. J Clin Oncol. 1992;10:1430–1435. doi: 10.1200/JCO.1992.10.9.1430. [DOI] [PubMed] [Google Scholar]
- 37.Kudo K, Yoshida H, Kiyoi H, Numata S, Horibe K, Naoe T. Etoposide-related acute promyelocytic leukemia. Leukemia. 1998;12:1171–1175. doi: 10.1038/sj.leu.2401089. [DOI] [PubMed] [Google Scholar]
- 38.Au W, Ma S, Chung L, Chim C, Kwong Y. Two cases of therapy-related acute promyelocytic leukemia (t-APL) after mantle cell lymphoma and gestational trophoblastic disease. Annals of Hematology. 2002;81:659–661. doi: 10.1007/s00277-002-0552-6. [DOI] [PubMed] [Google Scholar]
- 39.Joannides M, Mays AN, Mistry AR, Hasan SK, Reiter A, Wiemels JL, et al. Molecular pathogenesis of secondary acute promyelocytic leukemia. Mediterr J Hematol Infect Dis. 2011;3:e2011045. doi: 10.4084/MJHID.2011.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mistry AR, Felix CA, Whitmarsh RJ, Mason A, Reiter A, Cassinat B, et al. DNA topoisomerase II in therapy-related acute promyelocytic leukemia. N Engl J Med. 2005;352:1529–1538. doi: 10.1056/NEJMoa042715. [DOI] [PubMed] [Google Scholar]
- 41.Severson RK, Buckley JD, Woods WG, Benjamin D, Robison LL. Cigarette smoking and alcohol consumption by parents of children with acute myeloid leukemia — an analysis within morphological subgroups-a report from the Children's Cancer Group. Cancer Epidemiology Biomarkers & Prevention. 1993;2:433–439. [PubMed] [Google Scholar]
- 42.Scelo G, Metayer C, Zhang L, Wiemels JL, Aldrich MC, Selvin S, et al. Household exposure to paint and petroleum solvents, chromosomal translocations, and the risk of childhood leukemia. Environmental Health Perspectives. 2009;117:133–139. doi: 10.1289/ehp.11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandler DP, Ross JA. Epidemiology of acute leukemia in children and adults. Seminars in Oncology. 1997;24:3–16. [PubMed] [Google Scholar]
- 44.Chang JS, Selvin S, Metayer C, Crouse V, Golembesky A, Buffler PA. Parental Smoking and the Risk of Childhood Leukemia. American Journal of Epidemiology. 2006;163:1091–1100. doi: 10.1093/aje/kwj143. [DOI] [PubMed] [Google Scholar]
- 45.Heck JE, Park AS, Qiu J, Cockburn M, Ritz B. Risk of leukemia in relation to exposure to ambient air toxics in pregnancy and early childhood. Int J Hyg Environ Health. 2014;217:662–668. doi: 10.1016/j.ijheh.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Souza Reis R, Sr, de Camargo B, de Oliveira Santos M, de Oliveira JM, Azevedo Silva F, Pombo-de-Oliveira MS. Childhood leukemia incidence in Brazil according to different geographical regions. Pediatr Blood Cancer. 2011;56:58–64. doi: 10.1002/pbc.22736. [DOI] [PubMed] [Google Scholar]
- 47.Orgel E, Zung L, Ji L, Finklestein J, Feusner J, Freyer DR. Early cardiac outcomes following contemporary treatment for childhood acute myeloid leukemia: a North American perspective. Pediatr Blood Cancer. 2013;60:1528–1533. doi: 10.1002/pbc.24498. [DOI] [PubMed] [Google Scholar]
- 48.Martinez-Climent JA, Lane NJ, Rubin CM, Morgan E, Johnstone HS, Mick R, et al. Clinical and prognostic significance of chromosomal abnormalities in childhood acute myeloid leukemia de novo. Leukemia. 1995;9:95–101. [PubMed] [Google Scholar]
- 49.Gorman MF, Ji L, Ko RH, Barnette P, Bostrom B, Hutchinson R, et al. Outcome for children treated for relapsed or refractory acute myelogenous leukemia (rAML): a Therapeutic Advances in Childhood Leukemia (TACL) Consortium study. Pediatr Blood Cancer. 2010;55:421–429. doi: 10.1002/pbc.22612. [DOI] [PubMed] [Google Scholar]
- 50.Kaneko Y, Rowley JD, Maurer HS, Variakojis D, Moohr JW. Chromosome pattern in childhood acute nonlymphocytic leukemia (ANLL) Blood. 1982;60:389–399. [PubMed] [Google Scholar]
- 51.Ribeiro RC, Razzouk BI, Pounds S, Hijiya N, Pui CH, Rubnitz JE. Successive clinical trials for childhood acute myeloid leukemia at St Jude Children's Research Hospital, from 1980 to 2000. Leukemia. 2005;19:2125–2129. doi: 10.1038/sj.leu.2403872. [DOI] [PubMed] [Google Scholar]
- 52.Okamoto Y, Ribeiro RC, Srivastava DK, Shenep JL, Pui CH, Razzouk BI. Viridans streptococcal sepsis: clinical features and complications in childhood acute myeloid leukemia. J Pediatr Hematol Oncol. 2003;25:696–703. doi: 10.1097/00043426-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Raimondi SC, Kalwinsky DK, Hayashi Y, Behm FG, Mirro J, Jr, Williams DL. Cytogenetics of childhood acute nonlymphocytic leukemia. Cancer Genet Cytogenet. 1989;40:13–27. doi: 10.1016/0165-4608(89)90141-6. [DOI] [PubMed] [Google Scholar]
- 54.Brodeur GM, Williams DL, Kalwinsky DK, Williams KJ, Dahl GV. Cytogenetic features of acute nonlymphoblastic leukemia in 73 children and adolescents. Cancer Genet Cytogenet. 1983;8:93–105. doi: 10.1016/0165-4608(83)90041-9. [DOI] [PubMed] [Google Scholar]
- 55.McSheffrey JB, Naidoo A, Hirte WE. Acute leukemia in children: experience in Saskatchewan in 1966–72. Can Med Assoc J. 1975;113:295–298. [PMC free article] [PubMed] [Google Scholar]
- 56.Abdelhaleem M. Frequent but nonrandom expression of lymphoid markers on de novo childhood acute myeloid leukemia. Exp Mol Pathol. 2007;83:259–263. doi: 10.1016/j.yexmp.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Dorantes-Acosta E, Chavez-Gonzalez A, Santos JI, Medina-Sanson A, Mayani H. Defective in vitro growth of primitive hematopoietic cells from pediatric patients with acute myeloid leukemia. Pediatr Blood Cancer. 2008;51:741–746. doi: 10.1002/pbc.21706. [DOI] [PubMed] [Google Scholar]
- 58.Perez-Saldivar ML, Fajardo-Gutierrez A, Bernaldez-Rios R, Martinez-Avalos A, Medina-Sanson A, Espinosa-Hernandez L, et al. Childhood acute leukemias are frequent in Mexico City: descriptive epidemiology. BMC Cancer. 2011;11:355. doi: 10.1186/1471-2407-11-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mejia-Arangure JM, Bonilla M, Lorenzana R, Juarez-Ocana S, de Reyes G, Perez-Saldivar ML, et al. Incidence of leukemias in children from El Salvador and Mexico City between 1996 and 2000: population-based data. BMC Cancer. 2005;5:33. doi: 10.1186/1471-2407-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gomez SM, Schuttenberg V, Armendariz H, Alba L, Martinez M, Fynn A, et al. Childhood acute leukemia: a single institution experience in La Plata, Argentina. Med Pediatr Oncol. 2001;36:383–385. doi: 10.1002/mpo.1090. [DOI] [PubMed] [Google Scholar]
- 61.Emerenciano M, Agudelo Arias DP, Coser VM, de Brito GD, Macedo Silva ML, Pombode-Oliveira MS. Molecular cytogenetic findings of acute leukemia included in the Brazilian Collaborative Study Group of Infant acute leukemia. Pediatr Blood Cancer. 2006;47:549–554. doi: 10.1002/pbc.20654. [DOI] [PubMed] [Google Scholar]
- 62.Onsten T, Girardi FM, Coelho GM, Lima Frey MC, Paskulin G. Cytogenetic and morphological findings in 166 patients with de novo acute myeloid leukemia in southern Brazil. Cancer Genet Cytogenet. 2006;170:167–170. doi: 10.1016/j.cancergencyto.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 63.Emerenciano M, Meyer C, Mansur MB, Marschalek R, Pombo-de-Oliveira MS. The distribution of MLL breakpoints correlates with outcome in infant acute leukaemia. Br J Haematol. 2013;161:224–236. doi: 10.1111/bjh.12250. [DOI] [PubMed] [Google Scholar]
- 64.Santamaria-Quesada C, Vargas M, Venegas P, Calvo M, Obando C, Valverde B, et al. Molecular and epidemiologic findings of childhood acute leukemia in Costa Rica. J Pediatr Hematol Oncol. 2009;31:131–135. doi: 10.1097/MPH.0b013e31818c919e. [DOI] [PubMed] [Google Scholar]
- 65.Sala A, Rossi E, Antillon F. Nutritional status at diagnosis in children and adolescents with cancer in the Asociacion de Hemato-Oncologia Pediatrica de Centro America (AHOPCA) countries: preliminary results from Guatemala. Pediatr Blood Cancer. 2008;50:499–501. doi: 10.1002/pbc.21399. discussion 17. [DOI] [PubMed] [Google Scholar]
- 66.Malta Corea A, Pacheco Espinoza C, Cantu Rajnoldi A, Conter V, Lietti G, Masera G, et al. Childhood acute promyelocytic leukemia in Nicaragua. Ann Oncol. 1993;4:892–894. doi: 10.1093/oxfordjournals.annonc.a058400. [DOI] [PubMed] [Google Scholar]
- 67.De Salvo L, Weir Medina J, Gomez Sanchez O, de Baena ES, de Ramos BU, Guevara J, Luengo, Vera J, de Vizcaino MA, Sanchez H, de Leon E. Acute Promyelocytic Leukemia in the West of Venezuela. Sangre. 1989;34:329–331. [PubMed] [Google Scholar]
- 68.Strehl S, Konig M, Mann G, Haas OA. Multiplex reverse transcriptase-polymerase chain reaction screening in childhood acute myeloblastic leukemia. Blood. 2001;97:805–808. doi: 10.1182/blood.v97.3.805. [DOI] [PubMed] [Google Scholar]
- 69.Haas OA, Kronberger M, Mayerhofer L. Cytogenetic Abnormalities Associated with Childhood Acute Myeloblastic Leukemia. In: Ludwig W-D, Thiel E, editors. Recent Advances in Cell Biology of Acute Leukemia. Berlin Heidelberg: Springer; 1993. pp. 103–112. [DOI] [PubMed] [Google Scholar]
- 70.Lipay NV, Zmitrovich AI, Aleinikova OV. Epidemiology of venous thromboembolism in children with malignant diseases: A single-center study of the Belarusian Center for Pediatric Oncology and Hematology. Thromb Res. 2011;128:130–134. doi: 10.1016/j.thromres.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 71.Sramkova L, Sterba J, Hrstkova H, Mihal V, Blazek B, Timr P, et al. Development of treatment and clinical results in childhood acute myeloid leukemia in the Czech Republic. memo - Magazine of European Medical Oncology. 2013;6:41–45. [Google Scholar]
- 72.Burjanivova T, Madzo J, Muzikova K, Meyer C, Schneider B, Votava F, et al. Prenatal origin of childhood AML occurs less frequently than in childhood ALL. BMC Cancer. 2006;6:100. doi: 10.1186/1471-2407-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huhta T, Vettenranta K, Heinonen K, Kanerva J, Larramendy ML, Mahlamaki E, et al. Comparative genomic hybridization and conventional cytogenetic analyses in childhood acute myeloid leukemia. Leuk Lymphoma. 1999;35:311–315. doi: 10.3109/10428199909145735. [DOI] [PubMed] [Google Scholar]
- 74.Leverger G, Bernheim A, Daniel MT, Flandrin G, Schaison G, Berger R. Cytogenetic study of 130 Childhood Acute Nonlymphocytic Leukemias. Medical and Pediatric Oncology. 1988;16:227–232. doi: 10.1002/mpo.2950160402. [DOI] [PubMed] [Google Scholar]
- 75.Lapillonne H, Renneville A, Auvrignon A, Flamant C, Blaise A, Perot C, et al. High WT1 expression after induction therapy predicts high risk of relapse and death in pediatric acute myeloid leukemia. J Clin Oncol. 2006;24:1507–1515. doi: 10.1200/JCO.2005.03.5303. [DOI] [PubMed] [Google Scholar]
- 76.Creutzig U, Ritter J, Riehm H, Langermann HJ, Henze G, Kabisch H, et al. Improved Treatment Results in Childhood Acute Myelogenous Leukemia - A Report of the German Cooperative Study AML-BFM-78. Blood. 1985;65:298–304. [PubMed] [Google Scholar]
- 77.Manola KN, Panitsas F, Polychronopoulou S, Daraki A, Karakosta M, Stavropoulou C, et al. Cytogenetic abnormalities and monosomal karyotypes in children and adolescents with acute myeloid leukemia: correlations with clinical characteristics and outcome. Cancer Genet. 2013;206:63–72. doi: 10.1016/j.cancergen.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 78.Szegedi I, Jakab Z, Masat P, Kiss C. Development of treatment and clinical results in childhood acute myeloid leukemias in Hungary. memo - Magazine of European Medical Oncology. 2013;6:69–72. [Google Scholar]
- 79.Revesz T, Kardos G, Koos R, Vargha M, Kabos S, Szollar J, et al. Acute myeloid leukemia in childhood: 12 years experience of treatment in Hungary. Haematologia (Budap) 1985;18:13–21. [PubMed] [Google Scholar]
- 80.Keleti J, Revesz T, Schuler D. Morphological diagnosis in childhood leukaemia. Br J Haematol. 1978;40:501–502. doi: 10.1111/j.1365-2141.1978.tb05820.x. [DOI] [PubMed] [Google Scholar]
- 81.Haltrich I, Kost-Alimova M, Kovacs G, Klein G, Fekete G, Imreh S. Multipoint interphase FISH analysis of chromosome 3 abnormalities in 28 childhood AML patients. Eur J Haematol. 2006;76:124–133. doi: 10.1111/j.1600-0609.2005.00576.x. [DOI] [PubMed] [Google Scholar]
- 82.Pession A, Rondelli R, Basso G, Rizzari C, Testi AM, Fagioli F, et al. Treatment and long-term results in children with acute myeloid leukaemia treated according to the AIEOP AML protocols. Leukemia. 2005;19:2043–2053. doi: 10.1038/sj.leu.2403869. [DOI] [PubMed] [Google Scholar]
- 83.Biondi A, Rovelli A, Cantu Rajnoldi A, Fenu S, Basso G, Luciano A, et al. Acute Promyelocytic Leukemia in Children- Experience of the Italian Pediatric Hematology and Oncology Group (AIEOP) Leukemia. 1994;8:S66–S70. [PubMed] [Google Scholar]
- 84.Castagnola E, Rossi MR, Cesaro S, Livadiotti S, Giacchino M, Zanazzo G, et al. Incidence of bacteremias and invasive mycoses in children with acute non-lymphoblastic leukemia: results from a multi-center Italian study. Pediatr Blood Cancer. 2010;55:1103–1107. doi: 10.1002/pbc.22750. [DOI] [PubMed] [Google Scholar]
- 85.Arrigoni P, Beretta C, Silvestri D, Rossi V, Rizzari C, Valsecchi MG, et al. FLT3 internal tandem duplication in childhood acute myeloid leukaemia: association with hyperleucocytosis in acute promyelocytic leukaemia. Br J Haematol. 2003;120:89–92. doi: 10.1046/j.1365-2141.2003.04032.x. [DOI] [PubMed] [Google Scholar]
- 86.Cantu-Rajnoldi A, Biondi A, Jankovic M, Masera G, Rovelli A, Uderzo C, et al. Diagnosis and Incidence of APL in Childhood. Blood. 1993;81:2209–2211. [PubMed] [Google Scholar]
- 87.Maule MM, Dama E, Mosso ML, Magnani C, Pastore G, Merletti F. High incidence of acute promyelocytic leukemia in children in northwest Italy, 1980–2003: a report from the Childhood Cancer Registry of Piedmont. Leukemia. 2008;22:439–441. doi: 10.1038/sj.leu.2404916. [DOI] [PubMed] [Google Scholar]
- 88.Kardos G, Zwaan CM, Kaspers GJ, de-Graaf SS, de Bont ES, Postma A, et al. Treatment strategy and results in children treated on three Dutch Childhood Oncology Group acute myeloid leukemia trials. Leukemia. 2005;19:2063–2071. doi: 10.1038/sj.leu.2403873. [DOI] [PubMed] [Google Scholar]
- 89.de Bont E, Fidler V, Meeuwsen T, Scherpen F, Hahlen K, Kamps WA. Vascular endothelial growth factor secretion is an independent prognostic factor for relapse-free survival in pediatric acute myeloid leukemia patients. Clinical Cancer Research. 2002;8:2856–2861. [PubMed] [Google Scholar]
- 90.Slater RM, Behrendt H, De Waal FC. Chromosome studies on acute nonlymphocytic leukaemia in children. Pediatr Res. 1983;17:398–405. doi: 10.1203/00006450-198305000-00016. [DOI] [PubMed] [Google Scholar]
- 91.Balwierz W, Pawinska-Wasikowska K, Klekawka T, Czogala M, Matysiak M, Fic-Sikorska B, et al. Development of treatment and clinical results in childhood acute myeloid leukemia in Poland. Memo. 2013;6:54–62. doi: 10.1007/s12254-012-0061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nasedkina TV, Zharinov VS, Isaeva EA, Mityaeva ON, Yurasov RN, Surzhikov SA, et al. Clinical screening of gene rearrangements in childhood leukemia by using a multiplex polymerase chain reaction-microarray approach. Clinical Cancer Research. 2003;9:5620–5629. [PubMed] [Google Scholar]
- 93.Yatsenko Y, Kalennik O, Maschan M, Kalinina I, Maschan A, Nasedkina T. NPM1, FLT3, and c-KIT Mutations in Pediatric Acute Myeloid Leukemia in Russian Population. J Pediatr Hematol Oncol. 2013;35:e100–e108. doi: 10.1097/MPH.0b013e318286d261. [DOI] [PubMed] [Google Scholar]
- 94.Krstic AD, Micic D, Lakic N, Guc-Scekic M, Janic D. Molecular diagnosis of childhood acute leukemia: Serbian experience. Pediatr Blood Cancer. 2010;55:394–395. doi: 10.1002/pbc.22571. [DOI] [PubMed] [Google Scholar]
- 95.Krstovski N, Tosic N, Janic D, Dokmanovic L, Kuzmanovic M, Spasovski V, et al. Incidence of FLT3 and nucleophosmin gene mutations in childhood acute myeloid leukemia: Serbian experience and the review of the literature. Med Oncol. 2010;27:640–645. doi: 10.1007/s12032-009-9261-5. [DOI] [PubMed] [Google Scholar]
- 96.Armengol G, Canellas A, Alvarez Y, Bastida P, Toledo JS, Perez-Iribarne Mdel M, et al. Genetic changes including gene copy number alterations and their relation to prognosis in childhood acute myeloid leukemia. Leuk Lymphoma. 2010;51:114–124. doi: 10.3109/10428190903350397. [DOI] [PubMed] [Google Scholar]
- 97.Ortega JJ, Diaz de Heredia C, Olive T, Bastida P, Llort A, Armadans L, et al. Allogeneic and autologous bone marrow transplantation after consolidation therapy in high-risk acute myeloid leukemia in children. Towards a risk-oriented therapy. Haematologica. 2003;88:290–299. [PubMed] [Google Scholar]
- 98.Marcos-Gragera R, Cervantes-Amat M, Vicente ML, de Sanjose S, Guallar E, Godoy C, et al. Population-based incidence of childhood leukaemias and lymphomas in Spain (1993–2002) Eur J Cancer Prev. 2010;19:247–255. doi: 10.1097/CEJ.0b013e328339e2f3. [DOI] [PubMed] [Google Scholar]
- 99.Andersson A, Paulsson K, Lilljebjorn H, Lassen C, Strombeck B, Heldrup J, et al. FLT3 mutations in a 10 year consecutive series of 177 childhood acute leukemias and their impact on global gene expression patterns. Genes Chromosomes Cancer. 2008;47:64–70. doi: 10.1002/gcc.20508. [DOI] [PubMed] [Google Scholar]
- 100.Betts DR, Ammann RA, Hirt A, Hengartner H, Beck-Popovic M, Kuhne T, et al. The prognostic significance of cytogenetic aberrations in childhood acute myeloid leukaemia. A study of the Swiss Paediatric Oncology Group (SPOG) Eur J Haematol. 2007;78:468–476. doi: 10.1111/j.1600-0609.2007.00854.x. [DOI] [PubMed] [Google Scholar]
- 101.Andreieva SV, Drozdova VD, Kavardakova NV. Phenomenon of the evolution of clonal chromosomal abnormalities in childhood acute myeloid leukemia. Tsitol Genet. 2010;44:41–52. [PubMed] [Google Scholar]
- 102.Gluzman DF, Abramenko IV, Sklyarenko LM, Nadgornaya VA, Zavelevich MP, Bilous NI, et al. Acute leukemias in children from the city of Kiev and Kiev region after the Chernobyl NPP catastrophe. Pediatr Hematol Oncol. 1999;16:355–360. doi: 10.1080/088800199277191. [DOI] [PubMed] [Google Scholar]
- 103.Gibson BE, Wheatley K, Hann IM, Stevens RF, Webb D, Hills RK, et al. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia. 2005;19:2130–2138. doi: 10.1038/sj.leu.2403924. [DOI] [PubMed] [Google Scholar]
- 104.Phillips M, Richards S, Chessells J. Acute myeloid leukaemia in childhood: the costs and benefits of intensive treatment. Br J Haematol. 1991;77:473–477. doi: 10.1111/j.1365-2141.1991.tb08612.x. [DOI] [PubMed] [Google Scholar]
- 105.Stiller CA, Eatock EM. Survival from acute non-lymphocytic leukaemia, 1971–88: a population based study. Arch Dis Child. 1994;70:219–223. doi: 10.1136/adc.70.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Al-Tonbary Y, Mansour AK, Ghazy H, Elghannam DM, Abd-Elghaffar HA. Prognostic significance of foetal-like tyrosine kinase 3 mutation in Egyptian children with acute leukaemia. Int J Lab Hematol. 2009;31:320–326. doi: 10.1111/j.1751-553X.2008.01039.x. [DOI] [PubMed] [Google Scholar]
- 107.Ezzat S. In: Report of cases presenting to CCHE 57357 in the period between 07/07/2007 until 31/12/2011(Hospital Based Cancer Registry Data) Zhang L, editor. Berkeley: 2012. [Google Scholar]
- 108.Ismail EA, Mahmoud HM, Tawfik LM, Habashy DM, Adly AA, El-Sherif NH, et al. BIRC6/Apollon gene expression in childhood acute leukemia: impact on therapeutic response and prognosis. Eur J Haematol. 2012;88:118–127. doi: 10.1111/j.1600-0609.2011.01734.x. [DOI] [PubMed] [Google Scholar]
- 109.Mukiibi JM, Nyirenda CM, Adewuyi JO, Mzula ELB, Magombo ED, Mbvundula EM. Leukemia at the Queen Elizabeth Central Hospital in Blantyre, Malawi. East African Medical Journal. 2001;78:349–354. doi: 10.4314/eamj.v78i7.9006. [DOI] [PubMed] [Google Scholar]
- 110.Williams CKO, Folami AO, Laditan AAO, Ukaejiofo EO. Childhood Acute Leukemia in a Tropical Population. British Journal of Cancer. 1982;46:89–94. doi: 10.1038/bjc.1982.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Macdougall LG, Jankowitz P, Cohn R, Bernstein R. Acute childhood leukemia in Johannesburg. Ethnic differences in incidence, cell type, and survival. Am J Pediatr Hematol Oncol. 1986;8:43–51. doi: 10.1097/00043426-198608010-00009. [DOI] [PubMed] [Google Scholar]
- 112.Ahmed MA, Kordofani AA, Hidaytalla A, Omer A. Leukaemia in the Democratic Republic of Sudan. East Afr Med J. 1982;59:533–538. [PubMed] [Google Scholar]
- 113.Gmidene A, Sennana H, Wahchi I, Youssef YB, Jeddi R, Elloumi M, et al. Cytogenetic profile of a large cohort of Tunisian de novo acute myeloid leukemia. Hematology. 2012;17:9–14. doi: 10.1179/102453312X13221316477417. [DOI] [PubMed] [Google Scholar]
- 114.Testi AM, Al-Hadad SA, Al-Jadiry MFF, Moleti ML, Mandelli F, Foa R. Impact of international collaboration on the prognosis of childhood acute promyelocytic leukemia in Iraq. Haematologica-the Hematology Journal. 2006;91:509–512. [PubMed] [Google Scholar]
- 115.Hamidieh AA, Alimoghaddam K, Jahani M, Bahar B, Mousavi SA, Iravani M, et al. Non-TBI hematopoietic stem cell transplantation in pediatric AML patients: a single-center experience. J Pediatr Hematol Oncol. 2013;35:e239–e245. doi: 10.1097/MPH.0b013e31827080fc. [DOI] [PubMed] [Google Scholar]
- 116.Memarian A, Jeddi Tehrani M, Vossough P, Sharifian RA, Rabbani H, Shokri F. Expression profile of Wnt molecules in leukemic cells from Iranian patients with acute myeloblastic leukemia. Iran J Immunol. 2007;4:145–154. [PubMed] [Google Scholar]
- 117.Stark B, Jeison M, Gabay LG, Mardoukh J, Luria D, Bar-Am I, et al. Classical and molecular cytogenetic abnormalities and outcome of childhood acute myeloid leukaemia: report from a referral centre in Israel. Br J Haematol. 2004;126:320–337. doi: 10.1111/j.1365-2141.2004.05038.x. [DOI] [PubMed] [Google Scholar]
- 118.al Lamki Z, Wali YA, Shah WM, Zachariah M. Relapsed acute leukemia in children: Oman experience. Pediatr Hematol Oncol. 2004;21:167–173. doi: 10.1080/08880010490273127. [DOI] [PubMed] [Google Scholar]
- 119.Udayakumar AM, Pathare AV, Al-Kindi S, Khan H, Rehmen JU, Zia F, et al. Cytogenetic, morphological, and immunophenotypic patterns in Omani patients with de novo acute myeloid leukemia. Cancer Genet Cytogenet. 2007;177:89–94. doi: 10.1016/j.cancergencyto.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 120.Jenkin RD, Al-Shabanah M, Al-Nasser A, El-Solh H, Aur R, Al Sudairy R, et al. Extramedullary myeloid tumors in children: the limited value of local treatment. J Pediatr Hematol Oncol. 2000;22:34–40. doi: 10.1097/00043426-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 121.Khattab TM, Atra AA, Elimam NA, Kassar A, Zayed A, Baothman A. Improved outcome of children with acute myeloid leukemia treated on 2 consecutive protocols. Saudi Med J. 2008;29:776–777. [PubMed] [Google Scholar]
- 122.Komur M, Erbey F, Bayram I, Tanyeli A. Incidence and Prognostic Importance of Molecular Genetic Defects in Children with Acute Myeloblastic Leukemia. Asian Pacific Journal of Cancer Prevention. 2010;11:1393–1395. [PubMed] [Google Scholar]
- 123.Wang YQ, Zhou JF, Ruan M, Yi XL, An WB, Yang WY, et al. Diagnostic value of fluorescence in situ hybridization for children with acute myeloid leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012;20:1099–1102. [PubMed] [Google Scholar]
- 124.Zhang L, Zhu X. Epidemiology, diagnosis and treatment of acute promyelocytic leukemia in children: the experience in china. Mediterr J Hematol Infect Dis. 2012;4:e2012012. doi: 10.4084/MJHID.2012.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhai XW, Cheng FW, Lee V, Leung WK, Ng MH, Tsang KS, et al. Improved survival outcome of childhood acute myeloid leukemia with intensified chemotherapy in Chinese children. Pediatr Hematol Oncol. 2011;28:269–278. doi: 10.3109/08880018.2010.533249. [DOI] [PubMed] [Google Scholar]
- 126.Shang X, Yin H, Lu A, Zhang L. Application of recombinant human granulocyte colony stimulating factor in children with acute myeloid leukemia. Chin Med J (Engl) 1999;112:620–622. [PubMed] [Google Scholar]
- 127.Feng X, Ruan Y, He Y, Zhang Y, Wu X, Liu H, et al. Prophylactic First-Line Antibiotics Reduce Infectious Fever and Shorten Hospital Stay during Chemotherapy-Induced Agranulocytosis in Childhood Acute Myeloid Leukemia. Acta Haematol. 2014;132:112–117. doi: 10.1159/000356626. [DOI] [PubMed] [Google Scholar]
- 128.Tang J, Xue H, Pan C, Chen J, Gu L, Zhao H. A homoharringtonine-based regimen for childhood acute myelogenous leukemia. Med Pediatr Oncol. 2003;41:70–72. doi: 10.1002/mpo.10264. [DOI] [PubMed] [Google Scholar]
- 129.Yan-Fang T, Jian N, Jun L, Na W, Pei-Fang X, Wen-Li Z, et al. The promoter of miR-663 is hypermethylated in Chinese pediatric acute myeloid leukemia (AML) BMC Med Genet. 2013;14:74. doi: 10.1186/1471-2350-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jiang M, Li XQ, Hu D, Qiu YN, Zhang ZQ, Zhang BY, et al. Clinical and biological characteristics of childhood acute myeloid leukemia with EVI1 gene positive expression. Zhongguo Dang Dai Er Ke Za Zhi. 2014;16:129–134. [PubMed] [Google Scholar]
- 131.Xu XJ, Tang YM, Song H, Yang SL, Shi SW, Wei J. Long-term outcome of childhood acute myeloid leukemia in a developing country: experience from a children's hospital in China. Leuk Lymphoma. 2010;51:2262–2269. doi: 10.3109/10428194.2010.518653. [DOI] [PubMed] [Google Scholar]
- 132.Cheng Y, Wang Y, Wang H, Chen Z, Lou J, Xu H, et al. Cytogenetic profile of de novo acute myeloid leukemia: a study based on 1432 patients in a single institution of China. Leukemia. 2009;23:1801–1806. doi: 10.1038/leu.2009.107. [DOI] [PubMed] [Google Scholar]
- 133.Chan NP, Wong WS, Ng MH, Tsang KS, Lau TT, Leung Y, et al. Childhood acute myeloid leukemia with CBFbeta-MYH11 rearrangement: study of incidence, morphology, cytogenetics, and clinical outcomes of Chinese in Hong Kong. Am J Hematol. 2004;76:300–303. doi: 10.1002/ajh.20081. [DOI] [PubMed] [Google Scholar]
- 134.Liang DC, Liu HC, Yang CP, Jaing TH, Hung IJ, Yeh TC, et al. Cooperating gene mutations in childhood acute myeloid leukemia with special reference on mutations of ASXL1, TET2, IDH1, IDH2, and DNMT3A. Blood. 2013;121:2988–2995. doi: 10.1182/blood-2012-06-436782. [DOI] [PubMed] [Google Scholar]
- 135.Yeh TC, Liu HC, Wang LY, Chen SH, Lin WY, Liang DC. The development of a novel protocol for the treatment of de novo childhood acute myeloid leukemia in a single institution in Taiwan. J Pediatr Hematol Oncol. 2007;29:826–831. doi: 10.1097/MPH.0b013e31815a05aa. [DOI] [PubMed] [Google Scholar]
- 136.Liang DC, Chan TT, Lin KH, Lin DT, Lu MY, Chen SH, et al. Improved treatment results for childhood acute myeloid leukemia in Taiwan. Leukemia. 2006;20:136–141. doi: 10.1038/sj.leu.2403979. [DOI] [PubMed] [Google Scholar]
- 137.Liang DC, Shih LY, Hung IJ, Yang CP, Chen SH, Jaing TH, et al. FLT3-TKD mutation in childhood acute myeloid leukemia. Leukemia. 2003;17:883–886. doi: 10.1038/sj.leu.2402928. [DOI] [PubMed] [Google Scholar]
- 138.Bhatia P, Binota J, Varma N, Marwaha R, Malhotra P, Varma S. Incidence of Common Fusion Transcripts in Adult and Pediatric Acute Myeloid Leukemia (AML) Cases: Experience of a Tertiary Care Research institute. Mediterr J Hematol Infect Dis. 2012;4:e2012042. doi: 10.4084/MJHID.2012.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Agarwal R, Vishnubhatla S, Gupta R, Bakhshi S. Diagnostic and follow-up eosinophilia is not predictive of outcome in childhood acute myeloid leukemia. J Pediatr Hematol Oncol. 2011;33:e51–e53. doi: 10.1097/MPH.0b013e3181f46a91. [DOI] [PubMed] [Google Scholar]
- 140.Mir Mazloumi SH, Appaji L, Madhumathi DS, Prasannakumari G-banding and fluorescence in situ hybridization in childhood acute myeloid leukemia from South India. Arch Iran Med. 2013;16:459–462. [PubMed] [Google Scholar]
- 141.Nasution F, Arifin Z, Sutjipto A. Acute non lymphoblastic leukemia in the Department of Child Health School of Medicine, University of North Sumatera/Dr. Pirngadi Hospital Medan (1983–1988), a preliminary study. Paediatr Indones. 1991;31:268–272. [PubMed] [Google Scholar]
- 142.Horibe K, Saito AM, Takimoto T, Tsuchida M, Manabe A, Shima M, et al. Incidence and survival rates of hematological malignancies in Japanese children and adolescents (2006–2010): based on registry data from the Japanese Society of Pediatric Hematology. Int J Hematol. 2013;98:74–88. doi: 10.1007/s12185-013-1364-2. [DOI] [PubMed] [Google Scholar]
- 143.Shimada A, Taki T, Koga D, Tabuchi K, Tawa A, Hanada R, et al. High WT1 mRNA expression after induction chemotherapy and FLT3-ITD have prognostic impact in pediatric acute myeloid leukemia: a study of the Japanese Childhood AML Cooperative Study Group. Int J Hematol. 2012;96:469–476. doi: 10.1007/s12185-012-1163-1. [DOI] [PubMed] [Google Scholar]
- 144.Ohta H, Iwamoto S, Kiyokawa N, Tsurusawa M, Deguchi T, Takase K, et al. Flow cytometric analysis of de novo acute myeloid leukemia in childhood: report from the Japanese Pediatric Leukemia/Lymphoma Study Group. Int J Hematol. 2011;93:135–137. doi: 10.1007/s12185-010-0754-y. [DOI] [PubMed] [Google Scholar]
- 145.Miyamura T, Sakata N, Okamura T, Yasui M, Inoue M, Yagi K, et al. Clinical significance of minimal residual disease in childhood acute myeloid leukemia. Int J Hematol. 2004;79:243–249. doi: 10.1532/ijh97.03113. [DOI] [PubMed] [Google Scholar]
- 146.Yamada S, Hongo T, Okada S, Watanabe C, Fujii Y, Ohzeki T. Clinical relevance of in vitro chemoresistance in childhood acute myeloid leukemia. Leukemia. 2001;15:1892–1897. doi: 10.1038/sj.leu.2402305. [DOI] [PubMed] [Google Scholar]
- 147.Iwai T, Yokota S, Nakao M, Okamoto T, Taniwaki M, Onodera N, et al. Internal tandem duplication of the FLT3 gene and clinical evaluation in childhood acute myeloid leukemia. The Children's Cancer and Leukemia Study Group, Japan. Leukemia. 1999;13:38–43. doi: 10.1038/sj.leu.2401241. [DOI] [PubMed] [Google Scholar]
- 148.Kondo M, Horibe K, Takahashi Y, Matsumoto K, Fukuda M, Inaba J, et al. Prognostic value of internal tandem duplication of the FLT3 gene in childhood acute myelogenous leukemia. Med Pediatr Oncol. 1999;33:525–529. doi: 10.1002/(sici)1096-911x(199912)33:6<525::aid-mpo1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 149.Hayashi Y, Hanada R, Yamamoto K. Chromosome abnormalities and prognosis in childhood acute leukemia. Acta Paediatrica Japonica. 1991;33:497–506. doi: 10.1111/j.1442-200x.1991.tb02579.x. [DOI] [PubMed] [Google Scholar]
- 150.Tomizawa D, Tabuchi K, Kinoshita A, Hanada R, Kigasawa H, Tsukimoto I, et al. Repetitive cycles of high-dose cytarabine are effective for childhood acute myeloid leukemia: long-term outcome of the children with AML treated on two consecutive trials of Tokyo Children's Cancer Study Group. Pediatr Blood Cancer. 2007;49:127–132. doi: 10.1002/pbc.20944. [DOI] [PubMed] [Google Scholar]
- 151.Bessho F. Acute Non lymphocytic Leukemia Is Not a Major Type of Childhood Leukemia in Japan. Eur J Cancer Clin Oncol. 1989;25:729–732. doi: 10.1016/0277-5379(89)90210-1. [DOI] [PubMed] [Google Scholar]
- 152.Chan LL, Abdel-Latif ME, Ariffin WA, Ariffin H, Lin HP. Treating childhood acute myeloid leukaemia with the AML-BFM-83 protocol: experience in a developing country. Br J Haematol. 2004;126:799–805. doi: 10.1111/j.1365-2141.2004.05129.x. [DOI] [PubMed] [Google Scholar]
- 153.Ghartimagar D, Ghosh A, Narasimhan R, Talwar OP. Patterns of hematological and non-hematological malignancies in bone marrow in a tertiary care hospital in Nepal--11 years study. Nepal Med Coll J. 2012;14:187–192. [PubMed] [Google Scholar]
- 154.Asif N, Hassan K, Yasmeen N. Acute Myeloblastic Leukemia in Children. International Journal of Pathology. 2011;9:67–70. [Google Scholar]
- 155.Zaki S, Burney IA, Khurshid M. Acute Myeloid Leukemia in Children in Pakistan- an Audit. Journal of Pakistan Medical Association. 2002;247 [PubMed] [Google Scholar]
- 156.Harani MS, Adil SN, Shaikh MU, Kakepoto GN, Khurshid M. Frequency of fab subtypes in acute myeloid leukemia patients at Aga Khan University Hospital Karachi. J Ayub Med Coll Abbottabad. 2005;17:26–29. [PubMed] [Google Scholar]
- 157.Tan RM, Quah TC, Aung L, Liang S, Kirk RC, Yeoh AE. Improved outcome in childhood acute myeloid leukemia in Singapore with the MRC AML 10 protocol. Pediatr Blood Cancer. 2007;48:262–267. doi: 10.1002/pbc.20834. [DOI] [PubMed] [Google Scholar]
- 158.Quah TC, Sun L, Chew FT, Yeoh A, Lee BW. Survival of childhood leukemia in Singapore. Med Pediatr Oncol. 1996;26:318–324. doi: 10.1002/(SICI)1096-911X(199605)26:5<318::AID-MPO3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 159.Sung KW, Choi J, Hwang YK, Lee SJ, Kim HJ, Lee SH, et al. Overexpression of Apollon, an antiapoptotic protein, is associated with poor prognosis in childhood de novo acute myeloid leukemia. Clin Cancer Res. 2007;13:5109–5114. doi: 10.1158/1078-0432.CCR-07-0693. [DOI] [PubMed] [Google Scholar]
- 160.Lee JH, Yoon HS, Song JS, Choi ES, Moon HN, Seo JJ, et al. Unrelated hematopoietic stem cell transplantation for children with acute leukemia: experience at a single institution. J Korean Med Sci. 2009;24:904–909. doi: 10.3346/jkms.2009.24.5.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pakakasama S, Kajanachumpol S, Kanjanapongkul S, Sirachainan N, Meekaewkunchorn A, Ningsanond V, et al. Simple multiplex RT-PCR for identifying common fusion transcripts in childhood acute leukemia. Int J Lab Hematol. 2008;30:286–291. doi: 10.1111/j.1751-553X.2007.00954.x. [DOI] [PubMed] [Google Scholar]
- 162.Mukda E, Pintaraks K, Sawangpanich R, Wiangnon S, Pakakasama S. FLT3 and NPM1 gene mutations in childhood acute myeloblastic leukemia. Asian Pac J Cancer Prev. 2011;12:1827–1831. [PubMed] [Google Scholar]
- 163.Tiedemann K, Waters KD, Tauro GP, Tucker D, Ekert H. Results of intensive therapy in childhood acute myeloid leukemia, incorporating high-dose melphalan and autologous bone marrow transplantation in first complete remission. Blood. 1993;82:3730–3738. [PubMed] [Google Scholar]
- 164.Paton CM, Ekert H, Waters KD, Matthews RN, Toogood IR. Treatment of acute myeloid leukaemia in children. Aust N Z J Med. 1982;12:143–146. doi: 10.1111/j.1445-5994.1982.tb02446.x. [DOI] [PubMed] [Google Scholar]
- 165.Smith FO, Alonzo TA, Gerbing RB, Woods WG, Arceci RJ. Long-term results of children with acute myeloid leukemia: a report of three consecutive Phase III trials by the Children's Cancer Group: CCG 251, CCG 213 and CCG 2891. Leukemia. 2005;19:2054–2062. doi: 10.1038/sj.leu.2403925. [DOI] [PubMed] [Google Scholar]
- 166.Marjerrison S, Antillon F, Bonilla M, Fu L, Martinez R, Valverde P, et al. Outcome of children treated for relapsed acute myeloid leukemia in Central America. Pediatr Blood Cancer. 2014;61:1222–1226. doi: 10.1002/pbc.24942. [DOI] [PubMed] [Google Scholar]
- 167.Creutzig U, Zimmermann M, Ritter J, Reinhardt D, Hermann J, Henze G, et al. Treatment strategies and long-term results in paediatric patients treated in four consecutive AML-BFM trials. Leukemia. 2005;19:2030–2042. doi: 10.1038/sj.leu.2403920. [DOI] [PubMed] [Google Scholar]
- 168.Locatelli F, Labopin M, Ortega J, Meloni G, Dini G, Messina C, et al. Factors influencing outcome and incidence of long-term complications in children who underwent autologous stem cell transplantation for acute myeloid leukemia in first complete remission. Blood. 2003;101:1611–1619. doi: 10.1182/blood-2002-03-0764. [DOI] [PubMed] [Google Scholar]
- 169.Forestier E, Izraeli S, Beverloo B, Haas O, Pession A, Michalova K, et al. Cytogenetic features of acute lymphoblastic and myeloid leukemias in pediatric patients with Down syndrome: an iBFM-SG study. Blood. 2008;111:1575–1583. doi: 10.1182/blood-2007-09-114231. [DOI] [PubMed] [Google Scholar]
- 170.Creutzig U, Zimmermann M, Dworzak M, Urban C, Henze G, Kremens B, et al. Favourable outcome of patients with childhood acute promyelocytic leukaemia after treatment with reduced cumulative anthracycline doses. Br J Haematol. 2010;149:399–409. doi: 10.1111/j.1365-2141.2010.08107.x. [DOI] [PubMed] [Google Scholar]
- 171.Gatta G, Luksch R, Coleman MP, Corazziari I. Survival from acute non-lymphocytic leukaemia (ANLL) and chronic myeloid leukaemia (CML) in European children since 1978: a population-based study. Eur J Cancer. 2001;37:695–702. doi: 10.1016/s0959-8049(01)00045-4. [DOI] [PubMed] [Google Scholar]
- 172.Balgobind BV, Hollink IH, Arentsen-Peters ST, Zimmermann M, Harbott J, Beverloo HB, et al. Integrative analysis of type-I and type-II aberrations underscores the genetic heterogeneity of pediatric acute myeloid leukemia. Haematologica. 2011;96:1478–1487. doi: 10.3324/haematol.2010.038976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Molgaard-Hansen L, Mottonen M, Glosli H, Jonmundsson GK, Abrahamsson J, Hasle H. Early and treatment-related deaths in childhood acute myeloid leukaemia in the Nordic countries: 1984–2003. Br J Haematol. 2010;151:447–459. doi: 10.1111/j.1365-2141.2010.08389.x. [DOI] [PubMed] [Google Scholar]
- 174.Slavkovic B, Guc-Scekic M, Bunjevacki G, Djuricic S, Krstic A, Micic D, et al. Acute Leukemia of Childhood- A Single Institution's Experience. Archives of Biological Sciences. 2005;57:11–17. [Google Scholar]
- 175.Petkovic I, Konja J, Nakic M. Cytogenetic Analysis in Children with Acute Nonlymphocytic Leukemia. Cancer Genet Cytogenet. 1992;58:155–159. doi: 10.1016/0165-4608(92)90103-f. [DOI] [PubMed] [Google Scholar]
- 176.Leow S, Kham SK, Ariffin H, Quah TC, Yeoh AE. FLT3 mutation and expression did not adversely affect clinical outcome of childhood acute leukaemia: a study of 531 Southeast Asian children by the Ma-Spore study group. Hematol Oncol. 2011;29:211–219. doi: 10.1002/hon.987. [DOI] [PubMed] [Google Scholar]
- 177.O'Brien TA, Russell SJ, Vowels MR, Oswald CM, Tiedemann K, Shaw PJ, et al. Results of consecutive trials for children newly diagnosed with acute myeloid leukemia from the Australian and New Zealand Children's Cancer Study Group. Blood. 2002;100:2708–2716. doi: 10.1182/blood.V100.8.2708. [DOI] [PubMed] [Google Scholar]
- 178.Cooper TM, Franklin J, Gerbing RB, Alonzo TA, Hurwitz C, Raimondi SC, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: a report from the Children's Oncology Group. Cancer. 2012;118:761–769. doi: 10.1002/cncr.26190. [DOI] [PubMed] [Google Scholar]
- 179.Rowley JD, Alimena G, Garson OM, Hagemeijer A, Mitelman F, Prigogina EL. A Collaborative Study of the Relationship of the Morphological Type of Acute Nonlymphocytic Leukemia with Patient Age and Karyotype. Blood. 1982;59:1013–1022. [PubMed] [Google Scholar]
- 180.Amaru R, Torres G, Penaloza R, Miguez H, Velarde J, Huarachi N, et al. Epidemiologia de las Leucemias en Bolivia: 1473 Casos Enero 1999 a Mayo de 2012. Revista Medica La Paz. 2012;18:9–19. [Google Scholar]
- 181.Viana MB, Cunha KC, Ramos G, Murao M. Acute myeloid leukemia in childhood: 15-year experience in a single institution. J Pediatr (Rio J) 2003;79:489–496. [PubMed] [Google Scholar]
- 182.Gilbert RD, Karabus CD, Mills AE. Acute promyelocytic leukemia. A childhood cluster. Cancer. 1987;59:933–935. doi: 10.1002/1097-0142(19870301)59:5<933::aid-cncr2820590513>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 183.Au WY, Kumana CR, Lee HK, Lin SY, Liu H, Yeung DY, et al. Oral arsenic trioxide-based maintenance regimens for first complete remission of acute promyelocytic leukemia: a 10-year follow-up study. Blood. 2011;118:6535–6543. doi: 10.1182/blood-2011-05-354530. [DOI] [PubMed] [Google Scholar]
- 184.Bapna A, Nair R, Tapan KS, Nair CN, Kadam P, Gladstone B, et al. All-trans-retinoic acid (ATRA): pediatric acute promyelocytic leukemia. Pediatr Hematol Oncol. 1998;15:243–248. doi: 10.3109/08880019809028791. [DOI] [PubMed] [Google Scholar]
- 185.Biondi A, Rambaldi A, Alcalay M, Pandolfi PP, Lo Coco F, Diverio D, et al. RAR-alpha gene rearrangements as a genetic marker for diagnosis and monitoring in acute promyelocytic leukemia. Blood. 1991;77:1418–1422. [PubMed] [Google Scholar]
- 186.Bourquin JP, Thornley I, Neuberg D, Brennan L, Kung A, Clark J, et al. Favorable outcome of allogeneic hematopoietic stem cell transplantation for relapsed or refractory acute promyelocytic leukemia in childhood. Bone Marrow Transplant. 2004;34:795–798. doi: 10.1038/sj.bmt.1704676. [DOI] [PubMed] [Google Scholar]
- 187.da Costa Moraes C, Trompieri N, Calvalcante FF. Pediatric acute promyelocytic leukemia: all-transretinoic acid therapy in a Brazilian pediatric hospital. J Pediatr Hematol Oncol. 2008;30:387–390. doi: 10.1097/MPH.0b013e3181662493. [DOI] [PubMed] [Google Scholar]
- 188.de Botton S, Coiteux V, Chevret S, Rayon C, Vilmer E, Sanz M, et al. Outcome of childhood acute promyelocytic leukemia with all-trans-retinoic acid and chemotherapy. J Clin Oncol. 2004;22:1404–1412. doi: 10.1200/JCO.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 189.Dvorak CC, Agarwal R, Dahl GV, Gregory JJ, Feusner JH. Hematopoietic stem cell transplant for pediatric acute promyelocytic leukemia. Biol Blood Marrow Transplant. 2008;14:824–830. doi: 10.1016/j.bbmt.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.George B, Mathews V, Poonkuzhali B, Shaji RV, Srivastava A, Chandy M. Treatment of children with newly diagnosed acute promyelocytic leukemia with arsenic trioxide: a single center experience. Leukemia. 2004;18:1587–1590. doi: 10.1038/sj.leu.2403480. [DOI] [PubMed] [Google Scholar]
- 191.Guglielmi C, Martelli MP, Diverio D, Fenu S, Vegna ML, Cantu-Rajnoldi A, et al. Immunophenotype of adult and childhood acute promyelocytic leukaemia: correlation with morphology, type of PML gene breakpoint and clinical outcome. A cooperative Italian study on 196 cases. Br J Haematol. 1998;102:1035–1041. doi: 10.1046/j.1365-2141.1998.00871.x. [DOI] [PubMed] [Google Scholar]
- 192.Hiorns L, Swansbury G, Mehta J, Min T, Dainton M, Treleaven J, et al. Additional chromosome abnormalities confer worse prognosis in acute promyelocytic leukaemia. J Haematol. 1997;96:314–321. doi: 10.1046/j.1365-2141.1997.d01-2037.x. [DOI] [PubMed] [Google Scholar]
- 193.Hirota T, Fujimoto T, Katano N, Tsurasawa M, Eguchi H, Nakadate N, et al. Treatment results of intermittent and cyclic regimen with ATRA and chemotherapy in childhood acute promyelocytic leukemia. Children's Cancer and Leukemia Study Group. Rinsho Ketsueki. 1997;38:1177–1182. [PubMed] [Google Scholar]
- 194.Kim MH, Choi CS, Lee JW, Jang PS, Chung NG, Cho B, et al. Outcome of childhood acute promyelocytic leukemia treated using a modified AIDA protocol. Korean J Hematol. 2010;45:236–241. doi: 10.5045/kjh.2010.45.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kutny MA, Moser BK, Laumann K, Feusner JH, Gamis A, Gregory J, et al. FLT3 mutation status is a predictor of early death in pediatric acute promyelocytic leukemia: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2012;59:662–667. doi: 10.1002/pbc.24122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lanvers C, Reinhardt D, Dubbers A, Wagner-Bohn A, Creutzig U, Ritter J, et al. Pharmacology of all-trans-retinoic acid in children with acute promyelocytic leukemia. Med Pediatr Oncol. 2003;40:293–301. doi: 10.1002/mpo.10257. [DOI] [PubMed] [Google Scholar]
- 197.Luo XQ, Ke ZY, Huang LB, Guan XQ, Zhang YC, Zhang XL. Improved outcome for Chinese children with acute promyelocytic leukemia: a comparison of two protocols. Pediatr Blood Cancer. 2009;53:325–328. doi: 10.1002/pbc.22042. [DOI] [PubMed] [Google Scholar]
- 198.Testi AM, Biondi A, Lo Coco F, Moleti ML, Giona F, Vignetti M, et al. GIMEMA-AIEOPAIDA protocol for the treatment of newly diagnosed acute promyelocytic leukemia (APL) in children. Blood. 2005;106:447–453. doi: 10.1182/blood-2004-05-1971. [DOI] [PubMed] [Google Scholar]
- 199.Wang H, Hao L, Wang X, Li J, Wu Q, Bian S. Retrospective study of arsenic trioxide for childhood acute promyelocytic leukemia in China: a single-center experience. Int J Hematol. 2010;91:820–825. doi: 10.1007/s12185-010-0575-z. [DOI] [PubMed] [Google Scholar]
- 200.Zhang L, Cao Z, Zou Y, Ruan M, Li Q, Wang J, et al. Quantification of PML/RARa transcript after induction predicts outcome in children with acute promyelocytic leukemia. Int J Hematol. 2012;95:500–508. doi: 10.1007/s12185-012-1034-9. [DOI] [PubMed] [Google Scholar]
- 201.Zhang L, Zhao H, Zhu X, Chen Y, Zou Y, Chen X. Retrospective analysis of 65 Chinese children with acute promyelocytic leukemia: a single center experience. Pediatr Blood Cancer. 2008;51:210–215. doi: 10.1002/pbc.21510. [DOI] [PubMed] [Google Scholar]
- 202.Zhou J, Zhang Y, Li J, Li X, Hou J, Zhao Y, et al. Single-agent arsenic trioxide in the treatment of children with newly diagnosed acute promyelocytic leukemia. Blood. 2010;115:1697–1702. doi: 10.1182/blood-2009-07-230805. [DOI] [PubMed] [Google Scholar]
- 203.Raimondi SC, Chang MN, Ravindranath Y, Behm FG, Gresik MV, Steuber CP, et al. Chromosomal abnormalities in 478 children with acute myeloid leukemia: Clinical characteristics and treatment outcome in a cooperative pediatric oncology group Study - POG 8821. Blood. 1999;94:3707–3716. [PubMed] [Google Scholar]
- 204.Jeddi R, Ghedira H, Ben Abdennebi Y, Kacem K, Ben Amor R, Aissaoui L, et al. ATRA and anthracycline-based chemotherapy in the treatment of childhood acute promyelocytic leukemia (APL): A 10-year experience in Tunisia. Med Oncol. 2011;28:1618–1623. doi: 10.1007/s12032-010-9642-9. [DOI] [PubMed] [Google Scholar]
- 205.Zhang L, Zhu X, Zou Y, Chen Y, Chen X. Effect of arsenic trioxide on the treatment of children with newly diagnosed acute promyelocytic leukemia in China. Int J Hematol. 2011;93:199–205. doi: 10.1007/s12185-011-0768-0. [DOI] [PubMed] [Google Scholar]
- 206.Pui CH, Behm FG, Raimondi SC, Dodge RK, George SL, Rivera GK, et al. Secondary acute myeloid leukemia in children treated for acute lymphoid leukemia. N Engl J Med. 1989;321:136–142. doi: 10.1056/NEJM198907203210302. [DOI] [PubMed] [Google Scholar]
- 207.Pui CH, Ribeiro RC, Hancock ML, Rivera GK, Evans WE, Raimondi SC, et al. Acute myeloid leukemia in children treated with epipodophyllotoxins for acute lymphoblastic leukemia. N Engl J Med. 1991;325:1682–1687. doi: 10.1056/NEJM199112123252402. [DOI] [PubMed] [Google Scholar]
- 208.Beaumont M, Sanz M, Carli PM, Maloisel F, Thomas X, Detourmignies L, et al. Therapy-related acute promyelocytic leukemia. J Clin Oncol. 2003;21:2123–2137. doi: 10.1200/JCO.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 209.Smith MA, Rubinstein L, Anderson JR, Arthur D, Catalano PJ, Freidlin B, et al. Secondary leukemia or myelodysplastic syndrome after treatment with epipodophyllotoxins. J Clin Oncol. 1999;17:569–577. doi: 10.1200/JCO.1999.17.2.569. [DOI] [PubMed] [Google Scholar]
- 210.Attili VSSD, Hemant, Sundereshan TS, Bapsy PP, Sahoo TP, Anupama G. Therapy related acute promyelocytic leukemia. Indian Journal of Medical and Paediatric Oncology. 2006;27:32–34. [Google Scholar]
- 211.Rudd E, Goransdotter Ericson K, Zheng C, Uysal Z, Ozkan A, Gurgey A, et al. Spectrum and clinical implications of syntaxin 11 gene mutations in familial haemophagocytic lymphohistiocytosis: association with disease-free remissions and haematopoietic malignancies. J Med Genet. 2006;43:e14. doi: 10.1136/jmg.2005.035253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sathiyamoorthy S, Shad A, Ozdemirli M. Acute promyelocytic leukemia following chemotherapy for EBV-associated hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2011;56:850–852. doi: 10.1002/pbc.22718. [DOI] [PubMed] [Google Scholar]
- 213.Elezovic I, Colovic M, Tomin D, Boskovic D. Pregnancy after treatment of secondary acute promyelocytic leukemia following Hodgkin's disease: a case report. Med Oncol. 2000;17:222–224. doi: 10.1007/BF02780532. [DOI] [PubMed] [Google Scholar]
- 214.Hasan SK, Ottone T, Schlenk RF, Xiao Y, Wiemels JL, Mitra ME, et al. Analysis of t(15;17) chromosomal breakpoint sequences in therapy-related versus de novo acute promyelocytic leukemia: association of DNA breaks with specific DNA motifs at PML and RARA loci. Genes Chromosomes Cancer. 2010;49:726–732. doi: 10.1002/gcc.20783. [DOI] [PubMed] [Google Scholar]
- 215.Antonijevic N, Suvajdzic N, Terzic T, Jakovljevic B, Jankovic G, Elezovic I, et al. Favourable prognostic factors in therapy related acute myeloid leukaemia. Srp Arh Celok Lek. 2011;139:347–352. doi: 10.2298/sarh1106347a. [DOI] [PubMed] [Google Scholar]
- 216.Ottone T, Cicconi L, Hasan SK, Lavorgna S, Divona M, Voso MT, et al. Comparative molecular analysis of therapy-related and de novo acute promyelocytic leukemia. Leuk Res. 2012;36:474–478. doi: 10.1016/j.leukres.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 217.Sugita K, Furukawa T, Tsuchida M, Okawa Y, Nakazawa S, Akatsuka J, et al. High frequency of etoposide (VP-16)-related secondary leukemia in children with non-Hodgkin's lymphoma. Am J Pediatr Hematol Oncol. 1993;15:99–104. doi: 10.1097/00043426-199302000-00013. [DOI] [PubMed] [Google Scholar]
- 218.Dallorso S, Sessarego M, Garre ML, Haupt R, Pasino M, Sansone R. Secondary acute promyelocytic leukemia with t(8;21) and t(9;22) at onset and loss of the Philadelphia chromosome at relapse. Cancer Genet Cytogenet. 1990;47:41–46. doi: 10.1016/0165-4608(90)90260-h. [DOI] [PubMed] [Google Scholar]
- 219.Dufour C, Lanciotti M, Micalizzi C, Valetto A, Haupt R. Non-identical twin sisters concordant for Langerhans cell histiocytosis and discordant for secondary acute promyelocytic leukemia. Med Pediatr Oncol. 2001;37:70–72. doi: 10.1002/mpo.1169. [DOI] [PubMed] [Google Scholar]
- 220.Ellis R, Boggild M. Therapy-related acute leukaemia with Mitoxantrone: what is the risk and can we minimise it? Mult Scler. 2009;15:505–508. doi: 10.1177/1352458508100967. [DOI] [PubMed] [Google Scholar]
- 221.Haupt R, Fears TR, Heise A, Gadner H, Loiacono G, De Terlizzi M, et al. Risk of secondary leukemia after treatment with etoposide (VP-16) for Langerhans' cell histiocytosis in Italian and Austrian-German populations. Int J Cancer. 1997;71:9–13. doi: 10.1002/(sici)1097-0215(19970328)71:1<9::aid-ijc3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 222.Horibe K, Matsushita T, Numata S, Miyajima Y, Katayama I, Kitabayashi T, et al. Acute promyelocytic leukemia with t(15;17) abnormality after chemotherapy containing etoposide for Langerhans cell histiocytosis. Cancer. 1993;72:3723–3726. doi: 10.1002/1097-0142(19931215)72:12<3723::aid-cncr2820721226>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 223.Ledda A, Caocci G, Spinicci G, Cocco E, Mamusa E, La Nasa G. Two new cases of acute promyelocytic leukemia following mitoxantrone treatment in patients with multiple sclerosis. Leukemia. 2006;20:2217–2218. doi: 10.1038/sj.leu.2404443. [DOI] [PubMed] [Google Scholar]
- 224.Li YS, Zhao YL, Jiang QP, Yang CL. Specific chromosome changes and nonoccupational exposure to potentially carcinogenic agents in acute leukemia in China. Leuk Res. 1989;13:367–376. doi: 10.1016/0145-2126(89)90076-3. [DOI] [PubMed] [Google Scholar]
- 225.Lopes LF, de Camargo B. Secondary acute promyelocytic leukemia after treatment with etoposide for Langerhans cell histiocytosis (LCH) Med Pediatr Oncol. 1999;32:315. doi: 10.1002/(sici)1096-911x(199904)32:4<315::aid-mpo17>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 226.Lopez-Andrew JA, Ferris J, Verdeguer A, Esquembre C, Senent ML, Castel V. Secondary acute promyelocytic leukemia in a child treated with epipodophyllotoxins. Am J Pediatr Hematol Oncol. 1994;16:384–386. [PubMed] [Google Scholar]
- 227.Matsuzaki A, Inamitsu T, Watanabe T, Ohga S, Ishii E, Nagotoshi Y, et al. Acute promyelocytic leukaemia in a patient treated with etoposide for Langerhans cell histiocytosis. Br J Haematol. 1994;86:887–889. doi: 10.1111/j.1365-2141.1994.tb04851.x. [DOI] [PubMed] [Google Scholar]
- 228.Ogami A, Morimoto A, Hibi S, Todo S, Sugimoto T, Mori K, et al. Secondary acute promyelocytic leukemia following chemotherapy for non-Hodgkin's lymphoma in a child. Journal of Pediatric Hematology Oncology. 2004;26:427–430. doi: 10.1097/00043426-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 229.Pai MR, Advani SH, Gopal R, Nair CN, Saikia T, Kamat DM. Acute leukaemia following malignant ependymoma: a case report. J Surg Oncol. 1985;29:1–4. doi: 10.1002/jso.2930290102. [DOI] [PubMed] [Google Scholar]
- 230.Rubin CM, Arthur DC, Woods WG, Lange BJ, Nowell PC, Rowley JD, et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia in children: correlation between chromosomal abnormalities and prior therapy. Blood. 1991;78:2982–2988. [PubMed] [Google Scholar]
- 231.Sato T, Kobayashi R, Iguchi A, Nakajima M, Koizumi S, Furukawa H, et al. Acute promyelocytic leukemia after living donor partial orthotopic liver transplantation in two Japanese girls. Leuk Lymphoma. 2005;46:1057–1060. doi: 10.1080/10428190500097706. [DOI] [PubMed] [Google Scholar]
- 232.Schiavetti A, Varrasso G, Maurizi P, Castello MA. Two secondary leukemias among 15 children given oral etoposide. Med Pediatr Oncol. 2001;37:148–149. doi: 10.1002/mpo.1187. [DOI] [PubMed] [Google Scholar]
- 233.Xue Y, Lu D, Guo Y, Lin B. Specific chromosomal translocations and therapy-related leukemia induced by bimolane therapy for psoriasis. Leuk Res. 1992;16:1113–1123. doi: 10.1016/0145-2126(92)90050-h. [DOI] [PubMed] [Google Scholar]
- 234.Bhavnani M, Azzawi SA, Yin JA, Lucas GS. Therapy-related acute promyelocytic leukaemia. Br J Haematol. 1994;86:231–232. doi: 10.1111/j.1365-2141.1994.tb03288.x. [DOI] [PubMed] [Google Scholar]
- 235.Chen Z, Mostafavi HS, Shevrin DH, Morgan R, Vye MV, Stone JF, et al. A case of therapy-related extramedullary acute promyelocytic leukemia. Cancer Genet Cytogenet. 1996;86:29–30. doi: 10.1016/0165-4608(95)00163-8. [DOI] [PubMed] [Google Scholar]
- 236.Colovic N, Suvajdzic N, Kraguljac Kurtovic N, Djordjevic V, Dencic Fekete M, Drulovic J, et al. Therapy-related acute leukemia in two patients with multiple sclerosis treated with Mitoxantrone. Biomed Pharmacother. 2012;66:173–174. doi: 10.1016/j.biopha.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 237.Dayyani F, Kantarjian H, O'Brien S, Pierce S, Jones D, Faderl S, et al. Outcome of therapy-related acute promyelocytic leukemia with or without arsenic trioxide as a component of frontline therapy. Cancer. 2011;117:110–115. doi: 10.1002/cncr.25585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Duffield AS, Aoki J, Levis M, Cowan K, Gocke CD, Burns KH, et al. Clinical and pathologic features of secondary acute promyelocytic leukemia. Am J Clin Pathol. 2012;137:395–402. doi: 10.1309/AJCPE0MV0YTWLUUE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Elliott MA, Letendre L, Tefferi A, Hogan WJ, Hook C, Kaufmann SH, et al. Therapy-related acute promyelocytic leukemia: observations relating to APL pathogenesis and therapy. Eur J Haematol. 2012;88:237–243. doi: 10.1111/j.1600-0609.2011.01727.x. [DOI] [PubMed] [Google Scholar]
- 240.Ghalie RG, Mauch E, Edan G, Hartung HP, Gonsette RE, Eisenmann S, et al. A study of therapy-related acute leukaemia after mitoxantrone therapy for multiple sclerosis. Mult Scler. 2002;8:441–445. doi: 10.1191/1352458502ms836oa. [DOI] [PubMed] [Google Scholar]
- 241.Gillis S, Sofer O, Zelig O, Dann EJ, Lotan H, Ben Yehuda D, et al. Acute promyelocytic leukaemia with t(15;17) following treatment of Hodgkin's disease--a report of 4 cases. Ann Oncol. 1995;6:777–779. doi: 10.1093/oxfordjournals.annonc.a059315. [DOI] [PubMed] [Google Scholar]
- 242.Hasan SK, Mays AN, Ottone T, Ledda A, La Nasa G, Cattaneo C, et al. Molecular analysis of t(15;17) genomic breakpoints in secondary acute promyelocytic leukemia arising after treatment of multiple sclerosis. Blood. 2008;112:3383–3390. doi: 10.1182/blood-2007-10-115600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hoffmann L, Moller P, Pedersen-Bjergaard J, Waage A, Pedersen M, Hirsch FR. Therapy-related acute promyelocytic leukemia with t(15;17) (q22;q12) following chemotherapy with drugs targeting DNA topoisomerase II. A report of two cases and a review of the literature. Ann Oncol. 1995;6:781–788. doi: 10.1093/oxfordjournals.annonc.a059316. [DOI] [PubMed] [Google Scholar]
- 244.Linassier C, Barin C, Calais G, Letortorec S, Bremond JL, Delain M, et al. Early secondary acute myelogenous leukemia in breast cancer patients after treatment with mitoxantrone, cyclophosphamide, fluorouracil and radiation therapy. Ann Oncol. 2000;11:1289–1294. doi: 10.1023/a:1008375016038. [DOI] [PubMed] [Google Scholar]
- 245.Malhotra P, Varma N, Arora N, Das R, Nath A, Patel FD, et al. Treatment of therapy related acute promyelocytic leukemia with the combination of all trans retinoic acid and arsenic trioxide without chemotherapy: a series of three patients. Leuk Lymphoma. 2010;51:933–936. doi: 10.3109/10428191003697484. [DOI] [PubMed] [Google Scholar]
- 246.Martin J, Majumdar G. Platinum compounds and therapy related acute promyelocytic leukemia. Hematol J. 2002;3:321–323. doi: 10.1038/sj.thj.6200198. [DOI] [PubMed] [Google Scholar]
- 247.Mays AN, Osheroff N, Xiao Y, Wiemels JL, Felix CA, Byl JA, et al. Evidence for direct involvement of epirubicin in the formation of chromosomal translocations in t(15;17) therapy-related acute promyelocytic leukemia. Blood. 2010;115:326–330. doi: 10.1182/blood-2009-07-235051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Molero T, Lemes A, de la Iglesia S, Gomez Casares MT, del Mar Perera M, Jimenez S. Acute promyelocytic leukemia developing after radiotherapy for prostate cancer in a patient with chronic lymphocytic leukemia. Cancer Genet Cytogenet. 2001;131:141–143. doi: 10.1016/s0165-4608(01)00503-9. [DOI] [PubMed] [Google Scholar]
- 249.Ono M, Watanabe T, Shimizu C, Hiramoto N, Goto Y, Yonemori K, et al. Therapy-related acute promyelocytic leukemia caused by hormonal therapy and radiation in a patient with recurrent breast cancer. Jpn J Clin Oncol. 2008;38:567–570. doi: 10.1093/jjco/hyn057. [DOI] [PubMed] [Google Scholar]
- 250.Pascual AM, Tellez N, Bosca I, Mallada J, Belenguer A, Abellan I, et al. Revision of the risk of secondary leukaemia after mitoxantrone in multiple sclerosis populations is required. Mult Scler. 2009;15:1303–1310. doi: 10.1177/1352458509107015. [DOI] [PubMed] [Google Scholar]
- 251.Ramkumar B, Chadha MK, Barcos M, Sait SN, Heyman MR, Baer MR. Acute promyelocytic leukemia after mitoxantrone therapy for multiple sclerosis. Cancer Genet Cytogenet. 2008;182:126–129. doi: 10.1016/j.cancergencyto.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 252.Sinha S, Aish L, Oo TH. Morphologic heterogeneity of acute promyelocytic leukemia: therapy-related acute promyelocytic leukemia presenting with FAB-M2 morphology. Am J Hematol. 2006;81:475–476. doi: 10.1002/ajh.20577. [DOI] [PubMed] [Google Scholar]
- 253.Yin CC, Glassman AB, Lin P, Valbuena JR, Jones D, Luthra R, et al. Morphologic, cytogenetic, and molecular abnormalities in therapy-related acute promyelocytic leukemia. Am J Clin Pathol. 2005;123:840–848. doi: 10.1309/TJFF-K819-RPCL-FKJ0. [DOI] [PubMed] [Google Scholar]
- 254.Wong O, Harris F, Armstrong TW, Hua F. A hospital-based case-control study of acute myeloid leukemia in Shanghai: analysis of environmental and occupational risk factors by subtypes of the WHO classification. Chem Biol Interact. 2010;184:112–128. doi: 10.1016/j.cbi.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 255.Mele A, Stazi MA, Pulsoni A, Visani G, Monarca B, Castelli G, et al. Epidemiology of acute promyelocytic leukemia. Haematologica. 1995;80:405–408. [PubMed] [Google Scholar]
- 256.Bjork J, Albin M, Mauritzson N, Stromberg U, Johansson B, Hagmar L. Smoking and acute myeloid leukemia: associations with morphology and karyotypic patterns and evaluation of dose-response relations. Leuk Res. 2001;25:865–872. doi: 10.1016/s0145-2126(01)00048-0. [DOI] [PubMed] [Google Scholar]
- 257.Moorman AV, Roman E, Cartwright RA, Morgan GJ. Smoking and the risk of acute myeloid leukaemia in cytogenetic subgroups. Br J Cancer. 2002;86:60–62. doi: 10.1038/sj.bjc.6600010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Sandler DP, Shore DL, Anderson JR, Davey FR, Arthur D, Mayer RJ, et al. Cigarette smoking and risk of acute leukemia: associations with morphology and cytogenetic abnormalities in bone marrow. J Natl Cancer Inst. 1993;85:1994–2003. doi: 10.1093/jnci/85.24.1994. [DOI] [PubMed] [Google Scholar]
- 259.Wong O, Harris F, Yiying W, Hua F. A hospital-based case-control study of acute myeloid leukemia in Shanghai: analysis of personal characteristics, lifestyle and environmental risk factors by subtypes of the WHO classification. Regul Toxicol Pharmacol. 2009;55:340–352. doi: 10.1016/j.yrtph.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 260.Travis LB, Li CY, Zhang ZN, Li DG, Yin SN, Chow WH, et al. Hematopoietic malignancies and related disorders among benzene-exposed workers in China. Leuk Lymphoma. 1994;14:91–102. doi: 10.3109/10428199409049654. [DOI] [PubMed] [Google Scholar]
- 261.Pulsoni A, Stazi A, Cotichini R, Allione B, Cerri R, Di Bona E, et al. Acute promyelocytic leukaemia: epidemiology and risk factors. A report of the GIMEMA Italian archive of adult acute leukaemia. GIMEMA Cooperative Group. Eur J Haematol. 1998;61:327–332. doi: 10.1111/j.1600-0609.1998.tb01096.x. [DOI] [PubMed] [Google Scholar]
- 262.Estey E, Thall P, Kantarjian H, Pierce S, Kornblau S, Keating M. Association between increased body mass index and a diagnosis of acute promyelocytic leukemia in patients with acute myeloid leukemia. Leukemia. 1997;11:1661–1664. doi: 10.1038/sj.leu.2400783. [DOI] [PubMed] [Google Scholar]
- 263.Yin SN, Li GL, Tain FD, Fu ZI, Jin C, Chen YJ, et al. A retrospective cohort study of leukemia and other cancers in benzene workers. Environ Health Perspect. 1989;82:207–213. doi: 10.1289/ehp.8982207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Rashidi A, Fisher SI. Therapy-related acute promyelocytic leukemia: a systematic review. Med Oncol. 2013;30:625. doi: 10.1007/s12032-013-0625-5. [DOI] [PubMed] [Google Scholar]
- 265.McHale CM, Wiemels JL, Zhang L, Ma X, Buffler PA, Feusner J, et al. Prenatal origin of childhood acute myeloid leukemias harboring chromosomal rearrangements t(15;17) and inv(16) Blood. 2003;101:4640–4641. doi: 10.1182/blood-2003-01-0313. [DOI] [PubMed] [Google Scholar]
- 266.Milne E, Greenop KR, Metayer C, Schuz J, Petridou E, Pombo-de-Oliveira MS, et al. Fetal growth and childhood acute lymphoblastic leukemia: findings from the childhood leukemia international consortium. Int J Cancer. 2013;133:2968–2979. doi: 10.1002/ijc.28314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Metayer C, Colt JS, Buffler PA, Reed HD, Selvin S, Crouse V, et al. Exposure to herbicides in house dust and risk of childhood acute lymphoblastic leukemia. J Expo Sci Environ Epidemiol. 2013;23:363–370. doi: 10.1038/jes.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Liu R, Zhang L, McHale CM, Hammond SK. Paternal smoking and risk of childhood acute lymphoblastic leukemia: systematic review and meta-analysis. J Oncol. 2011;2011:854584. doi: 10.1155/2011/854584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Chokkalingam AP, Metayer C, Scelo GA, Chang JS, Urayama KY, Aldrich MC, et al. Variation in xenobiotic transport and metabolism genes, household chemical exposures, and risk of childhood acute lymphoblastic leukemia. Cancer Causes Control. 2012;23:1367–1375. doi: 10.1007/s10552-012-9947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Smith MT. Advances in understanding benzene health effects and susceptibility. Annu Rev Public Health. 2010;31:133–148. doi: 10.1146/annurev.publhealth.012809.103646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Richardson S, Zittoun R, Bastuji-Garin S, Lasserre V, Guihenneuc C, Cadiou M, et al. Occupational risk factors for acute leukaemia: a case-control study. Int J Epidemiol. 1992;21:1063–1073. doi: 10.1093/ije/21.6.1063. [DOI] [PubMed] [Google Scholar]
- 272.Metayer C, Milne E, Clavel J, Infante-Rivard C, Petridou E, Taylor M, et al. The Childhood Leukemia International Consortium. Cancer Epidemiol. 2013;37:336–347. doi: 10.1016/j.canep.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.