Abstract
Background
Maternal vitamin D status in pregnancy is linked to foetal growth and may impact infant growth.
Aim
This study examined the association between maternal vitamin D status and infant anthropometry.
Subjects and methods
Data came from n = 2473 mother–child pairs from the 12-site US Collaborative Perinatal Project (1959–1965). Maternal serum 25-hydroxyvitamin D (25(OH)D) was measured at ≤26 weeks gestation. Multivariate-adjusted linear mixed models were used to relate maternal vitamin D status to infant z-scores for length (LAZ), head circumference (HCZ), weight (WAZ) and BMI (BMIZ), measured at birth and 4, 8 and 12 months.
Results
Infants with maternal 25(OH)D ≥30 nmol/L vs <30 nmol/L had LAZ and HCZ measures 0.13 (95% CI = 0.03–0.23) and 0.20 (95% CI = 0.11–0.28) units higher, respectively, across the first year of life. Similar differences in WAZ and BMIZ at birth were resolved by 12 months of age due to interactions indicating steeper age slopes in infants with maternal 25(OH)D <30 nmol/L.
Conclusion
Low maternal vitamin D status was associated with deficits at birth in infant weight and BMI that were recouped across the first year of life; associations with reduced measures of linear and skeletal growth were sustained from birth to 12 months.
Keywords: 25-hydroxyvitamin D, catch-up growth, infant growth, pregnancy
Introduction
Vitamin D deficiency is common in pregnant women worldwide and is associated with a variety of poor maternal health outcomes (Schroth et al., 2005; Bodnar et al., 2007; Collins-Fulea et al., 2012; Vandevijvere et al., 2012). Research increasingly recognizes that low maternal vitamin D status may also impact child health, including foetal and post-natal growth (Ponsonby et al., 2010; Dror, 2011; Thorne-Lyman & Fawzi, 2012).
With respect to foetal growth, maternal pre-natal vitamin D deficiency has been associated with lower birth weight and an increased risk of babies born small for gestational age (SGA; weight <10th percentile for gestational age) (Leffelaar et al., 2010; Aghajafari et al., 2013; Gernand et al., 2013, 2014). A Cochrane review of pre-natal vitamin D supplementation trials reported a tendency for supplemented women to be less likely to give birth to babies with low birth weight (LBW; <2500 g), although statistical significance was borderline (De-Regil et al., 2012).
The association of maternal vitamin D deficiency in pregnancy with child growth may continue beyond birth. First, negative impacts on bone development during foetal growth may be sustained to affect later stature (Namgung & Tsang, 2000, 2003; Weiler et al., 2005; Mahon et al., 2010; Viljakainen et al., 2010). Additional possible mechanisms originating during the foetal period include extra-skeletal pathways involving foetal programming and gene expression (Hossein-Nezhad & Holick, 2012, 2013). Post-natal growth in infancy may also be affected through pathways mediated by birth size or neonatal vitamin D status, which is correlated with maternal status (Novakovic et al., 2012). Studies of vitamin D status in older children, adolescents and adults suggest associations with pancreatic function, insulin secretion and adiposity, which may also impact weight and body composition (Chiu et al., 2004; Gilbert-Diamond et al., 2010; Lee et al., 2013; Oliveira et al., 2013).
However, the epidemiologic literature relating maternal pre-natal vitamin D status to post-natal anthropometric indicators of child growth is inconclusive and sparse, particularly for the infancy period, which is characterized by high growth velocity. We are only aware of three observational studies and a related follow-up study (Gale et al., 2008; Sayers & Tobias, 2009; Leffelaar et al., 2010; van Eijsden et al., 2013), which differed in the cut points used to define maternal vitamin D deficiency; the anthropometric data collected; and the ages of children at follow-up, with only one study reporting longitudinal data in infancy (Leffelaar et al., 2010). Results across studies were mixed. Three small controlled trials of maternal vitamin D supplementation included anthropometric parameters for infants with mixed results, all within Asian populations with high underlying levels of under-nutrition that have limited generalizability to the US and other populations (Brooke et al., 1981; Kalra et al., 2012; Roth et al., 2013).
Our objective was to examine the association between maternal vitamin D status in pregnancy, indicated by serum 25-hydroxyvitamin D concentration [25(OH)D] measured at ≤ 26 weeks gestation and longitudinal measures of multiple anthropometric indicators of growth across the first year of life among infants in a large US cohort.
Methods
The Collaborative Perinatal Project (CPP) was a birth cohort study that enrolled pregnant women between 1959–1965 at 12 medical centres across the US (n = 55 908) (Niswander, 1972; Hardy, 2003).We performed a secondary analysis of CPP data linked with maternal 25(OH)D concentrations assayed from banked maternal serum samples as part of an existing study on vitamin D and adverse pregnancy outcomes (Bodnar et al., 2014). Pregnancies eligible for 25(OH)D assessment were singleton pregnancies delivered at ≤42 weeks gestation; white, African-American or Puerto Rican maternal race/ ethnicity; no pre-existing diabetes or hypertension; pre-natal care beginning at ≤26 weeks gestation; and availability of a stored serum sample from ≤26 weeks gestation (n = 28 429). Of these eligible women, 3074 were randomly selected for maternal 25(OH)D assessment. If women had multiple available blood samples at ≤26 weeks gestation, a single sample was randomly selected.
For the current analysis, we excluded stillbirths (n = 32), children of mothers with serum unsuitable for 25(OH)D measurement (n = 122), children lacking follow-up anthropometric data beyond birth or children with implausible anthropometric data beyond birth (n = 229) and maternal–child pairs with missing covariates (n = 218). This led to an analytic sample of 2473 singleton, live born infants born to 2438 mothers. Follow-up infant anthropometric measures were collected at 4 months (n = 2330), 8 months (n = 983) and 12 months of age (n = 2125). This study used de-identified data and was exempt from ethical review.
Exposure
Sera were shipped to the laboratory of Dr Michael Holick at Boston University, which is a vitamin D External Quality Assessment Scheme-proficient and Clinical Laboratory Improvement Amendments certified laboratory. Samples were assayed for total 25-hydroxyvitamin D (25(OH)D) [25(OH)D2 + 25(OH)D3] using liquid-chromatography-tandem mass spectrometry by US National Institute of Standards and Technology standards (Holick et al., 2005). The assay had a lower detection limit of 1 ng/mL and no upper limit. None of the 25(OH)D concentrations fell below the detectable range. The intra-assay coefficient of variation was 8.2% and 5.9% for 25(OH)D2 and 25(OH)D3, respectively. Samples were stored at −20 °C for over 40 years; long-term storage is unlikely to result in significant degradation of 25(OH)D (Zerwekh, 2004; Bodnar et al., 2009). Our analyses tested both a dichotomous categorization of maternal 25(OH)D concentration based on the cut-point for vitamin D deficiency of 25(OH)D <30 nmol/L per IOM guidelines (IOM, 2011), as well as categorization into four groups using other common cut points (<30, 30–49, 50–74 and ≥75 nmol/L) (Holick et al., 2011).
Outcomes
Newborn weight, length and head circumference were measured within 24 hours of birth. Child anthropometric data in the first year of life were collected by trained study staff at research visits scheduled at 4, 8 and 12 months of age. We included measurements taken within a window of ± 1 month of the scheduled visits for cross-sectional analyses and included all available data in longitudinal analyses. At each visit, length was measured with the child in the supine position, with shoes removed, to the nearest 0.5 cm using a standardized measuring board. Weight was measured without clothing or diaper to the nearest 30 g. Head circumference was measured to the nearest 0.5 cm with a flexible steel or non-stretchable tape applied over the supra-orbital ridges and the occiput at the back of the head to provide the maximum circumference. Infant body mass index (BMI) was calculated by dividing weight (kg) by length squared (m2).
Outcome variables were sex-specific length-for-age, head circumference-for-age, weight-for-age and BMI-for-age z-scores (LAZ, HCZ, WAZ and BMIZ, respectively) calculated at each measurement point using the 2006 World Health Organization (WHO) growth standards, recommended for use in US children <24 months of age (Grummer-Strawn et al., 2010). Z-scores >|6| for WAZ and LAZ and >|5| for BMIZ and HCZ were considered implausible and set to missing.
Covariates
Maternal characteristics included parity (primiparous/multiparous); socioeconomic status (a continuous scale as described previously) (Hardy, 2003); maternal pre-pregnancy BMI (pre-pregnancy weight (kg) self-reported at registration divided by measured height squared (m2); marital status (married/not married), self-reported smoking at study registration (yes/no); gestational age at maternal blood draw; season of maternal blood categorized as winter (December–February), spring (March–May), summer (June–August) or fall (September–November); maternal age at enrolment; and race/ethnicity. The CPP study defined race/ethnicity as white, African-American or Puerto Rican. Infant characteristics included sex and feeding mode at hospital discharge, as no follow-up feeding mode data were available. Feeding mode was categorized as breast-fed, formula fed or mixed fed (breastfeeding combined with formula and/or tube feeding).
Statistical analysis
Means and standard deviations and frequencies and percentages were used to describe maternal and infant characteristics. Cross-sectional analyses and t-tests were used to describe the crude relationships between maternal vitamin D status and infant z-scores across the first year of life. Data from the 8 month visit were consistent with observed trends, but are not presented in descriptive tables due to smaller sample sizes at this measurement point (<1000 observations vs >2000 observations for the other time points), although all available anthropometric data were used in multivariate models.
Theory-based casual diagrams were used to identify potential confounders (maternal age, race/ethnicity, socioeconomic status, pre-pregnancy BMI, parity, marital status, smoking at study entry, infant feeding mode, latitude of study site, season of blood sampling, gestational age at blood sampling and study site) for our longitudinal models (Greenland et al., 1999). We fit mixed effects linear regression models to estimate the association between maternal vitamin D status and infant z-scores across the first year of life. We used mixed effects models to allow for unequally spaced repeated outcome measures; account for potential clustering within study sites, mothers and infants; and maximize sample size and power by accommodating missing observations. Initially we ran models iteratively using three different ways of specifying maternal 25(OH)D concentration: as a continuous variable, as a dichotomous variable (>30 nmol/L vs ≤30 nmol/L) and as a categorical variable with four categories (<30, 30–49, 50–74 and ≥75 nmol/L). Because the relationships observed were not linear, we present only the results using the categorical expressions.
We tested whether potential confounders changed the main associations by >10% by removing each covariate from full models among the study population with complete data. Our final models retained infant age, maternal socioeconomic status, maternal race/ethnicity, season of maternal blood draw, infant feeding mode and study site. We also included infant sex and maternal pre-pregnancy BMI as covariates out of convention. The mixed effects models specified study site, mother and child as random effects to account for clustering within these groups. Effect modification of the main effect was examined separately for infant age, infant sex, parity and maternal race/ethnicity in full models with all potential confounders. A cut point of α ≤ 0.10 was used to determine significant interaction terms. For infant age, we tested interactions using linear splines with knots at each scheduled measurement age. The age splines were not statistically significant (by the Wald test), thus effect modification by infant age was evaluated by modelling age as a continuous variable. Age interaction terms were retained in the models for BMI and WAZ. Analyses were conducted using Stata 13.0 software (StataCorp, College Station, TX).
Results
Close to one quarter of mothers had concentrations of 25(OH)D <30 nmol/L (Table 1). The mean (± standard deviation) maternal 25(OH)D was 58.9 (23.8) nmol/L. Women with 25(OH)D <30 nmol/L in pregnancy were more often overweight and obese, unmarried, African-American and had a lower socioeconomic index compared to women with 25(OH)D ≥30 nmol/L (Table 1).
Table 1.
Maternal and infant characteristics for the sample of women randomly selected for vitamin D assessment from the eligible cohort, Collaborative Perinatal Project.
| Overall, n (%) (n = 2473)* |
25(OH)D <30 nmol/L, n (%) (n = 589) |
25(OH)D ≥30 nmol/L, n (%) (n = 1884) |
p Value† | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Race/ethnicity | <0.001 | |||
| White | 1150 (46.5) | 153 (26.0) | 997 (52.9) | |
| African-American | 1151 (46.5) | 396 (67.2) | 755 (40.1) | |
| Puerto Rican | 172 (7.0) | 40 (6.8) | 132 (7.0) | |
| Socioeconomic index category‡ | <0.001 | |||
| 1 | 183 (7.4) | 63 (10.7) | 120 (6.4) | |
| 2 | 691 (27.9) | 207 (35.1) | 484 (25.7) | |
| 3 | 803 (32.5) | 206 (35.0) | 597 (31.7) | |
| 4 | 492 (19.9) | 82 (13.9) | 410 (21.8) | |
| 5 | 304 (12.3) | 31 (5.3) | 273 (14.5) | |
| Pre-pregnancy BMI, kg/m2 | 0.009 | |||
| <18.5 | 255 (10.3) | 60 (10.2) | 195 (10.4) | |
| 18.5–24.9 | 1777 (71.9) | 397 (67.4) | 1380 (73.3) | |
| 25–29.9 | 331 (13.4) | 97 (16.5) | 234 (12.4) | |
| ≥30 | 110 (4.5) | 35 (5.9) | 75 (4.0) | |
| Nulliparous (at enrolment) | 825 (33.4) | 188 (31.9) | 637 (33.9) | 0.382 |
| Maternal age, years | 0.056 | |||
| <20 | 605 (24.5) | 165 (28.0) | 440 (23.4) | |
| 20–29 | 1490 (60.3) | 333 (56.5) | 1157 (61.4) | |
| ≥30 | 378 (15.3) | 91 (15.5) | 287 (15.2) | |
| Married | 1987 (80.4) | 430 (73.0) | 1557 (82.6) | <0.001 |
| Smoking at study entry | 1173 (47.5) | 298 (50.7) | 875 (46.5) | 0.076 |
| Gestational age at blood sample¶, weeks | 20.7 [15.9, 23.4] | 20.6 [15.9, 23.1] | 20.7 [15.9, 23.4] | 0.494 |
| Latitude of study site | <0.001 | |||
| ≥41 degrees North (7 sites) | 1459 (59.0) | 272 (46.2) | 1187 (63.0) | |
| 36–40 degrees North (3 sites) | 781 (31.6) | 261 (44.3) | 520 (27.6) | |
| ≤35 degrees North (2 sites) | 233 (9.4) | 56 (9.5) | 177 (9.4) | |
| Season of blood sample§ | <0.001 | |||
| Winter | 569 (23.0) | 194 (32.9) | 375 (19.9) | |
| Spring | 627 (25.4) | 204 (34.6) | 423 (22.5) | |
| Summer | 651 (26.3) | 98 (16.6) | 553 (29.4) | |
| Fall | 626 (25.3) | 93 (15.8) | 533 (28.3) | |
| Infant characteristics | ||||
| Sex | 0.352 | |||
| Female | 1238 (50.1) | 285 (48.4) | 953 (50.6) | |
| Male | 1235 (49.9) | 304 (51.6) | 931 (49.4) | |
| Infant Feeding ModeII | <0.001 | |||
| Breastfed | 155 (6.3) | 22 (3.7) | 133 (7.1) | |
| Mixed fed | 511 (20.7) | 88 (14.9) | 423 (22.5) | |
| Formula fed | 1807 (73.1) | 479 (81.3) | 1328 (70.5) | |
| Anthropometric measures at birth# | ||||
| Weight (kg) | 3.11 (0.48) | 3.0 (0.5) | 3.1 (0.5) | <0.001 |
| BMI (kg/m2) | 12.6 (1.5) | 12.4 (1.3) | 12.6 (1.5) | <0.001 |
| Length (cm) | 49.7 (2.7) | 49.3 (2.7) | 49.8 (2.6) | 0.002 |
| Head circumference (cm) | 33.6 (1.5) | 33.3 (1.5) | 33.6 (1.5) | <0.001 |
n = 2473 infants/pregnancies; n = 2438 mothers, due to 35 women who contributed more than one pregnancy. Data are missing for parity (n = 3) and smoking (n = 3).
Differences tested by the Chi-squared test for categories and the one-sided t-test for continuous variables.
Socioeconomic index categories are lowest (1) to highest (5).
Values are median (IQR); difference tested by Kruskal–Wallis rank test.
Winter=December–February; Spring=March–May; Summer=June–August; Fall=September–November. IIFeeding reported during post-partum hospital stay only (~4 days per infant).
Missing anthropometric data at birth resulted a sample size that varied by measurement from n = 2421 to n = 2473.
Infants with maternal 25(OH)D <30 nmol/L were smaller at birth with respect to all raw anthropometric measures compared to infants with maternal 25(OH)D ≥ 30 nmol/L. Infant feeding mode also differed by maternal vitamin D status; infants with maternal 25(OH)D <30 nmol/L were less likely to be breastfed or mixed fed and were more likely to be formula fed than infants with maternal 25(OH)D ≥ 30 nmol/L.
In unadjusted cross-sectional analyses, all four anthropometric z-score measures (WAZ, BMIZ, LAZ and HCZ) were significantly lower at birth for infants born to mothers with 25(OH)D <30 nmol/L compared with those of infants born to mothers with 25(OH)D ≥ 30 nmol/L (Table 2) and differences remained at 4 and 12 months. However, the differences in WAZ and BMIZ by maternal vitamin D status that were evident at birth were attenuated over time; no significant difference remained by 4 months for BMIZ and no differences for either measure were evident by 12 months of age. These results were generally consistent when anthropometric parameters were analysed as raw measures (data not shown).
Table 2.
Infant z-scores [mean (SD)] by maternal vitamin D status.
| Overall, n (%) (n = 2473)* |
25(OH)D <30 nmol/L, n (%) (n = 589) |
25(OH)D ≥30 nmol/L, n (%) (n = 1884) |
p Value† | |
|---|---|---|---|---|
| Birth | ||||
| Weight-for-age z-score | 0.10 (1.36) | −0.61 (1.11) | −0.37 (1.08) | <0.001 |
| BMI-for-age z-score | −0.43 (1.09) | −0.87 (1.13) | −0.68 (1.14) | <0.001 |
| Length-for-age z-score | −0.73 (1.14) | −0.05 (1.33) | 0.15 (1.37) | 0.003 |
| Head-circumference-for-age z-score 4 months | −0.46 (1.13) | −0.66 (1.11) | −0.40 (1.13) | <0.001 |
| Weight-for-age z-score | 0.02 (1.37) | −0.47 (1.14) | −0.30 (1.07) | 0.001 |
| BMI-for-age z-score | −0.34 (1.09) | −0.53 (1.08) | −0.44 (1.12) | 0.098 |
| Length-for-age z-score | −0.46 (1.11) | −0.12 (1.37) | 0.06 (1.37) | 0.011 |
| Head-circumference-for-age z-score 12 months | −0.11 (1.10) | −0.31 (1.09) | −0.05 (1.10) | <0.001 |
| Weight-for-age z-score | −0.13 (1.20) | 0.34 (1.02) | 0.42 (1.00) | 0.113 |
| BMI-for-age z-score | 0.40 (1.00) | 0.66 (1.12) | 0.64 (1.08) | 0.755 |
| Length-for-age z-score | 0.65 (1.09) | −0.27 (1.21) | −0.09 (1.19) | 0.005 |
| Head-circumference-for-age z-score | 0.23 (1.04) | 0.10 (1.07) | 0.27 (1.03) | 0.002 |
p Values from t-test.
There was variation in sample size by anthropometric measure and follow-up point, overall n ranged from 2421–2473 at birth, 2293–2320 at 4 months and 2102–2118 at 12 months.
There was a significant positive association, with no variation by infant age, between maternal 25(OH)D and infant LAZ and HCZ across the first year of life in multivariate-adjusted linear mixed models adjusted for infant sex, infant age, maternal pre-pregnancy BMI, socioeconomic status, race/ethnicity, season of blood draw and infant feeding (Table 3). In the model with maternal vitamin D dichotomized, infants with maternal 25(OH)D ≥30 nmol/L had LAZ values that were 0.130 (95% CI = 0.028, 0.231) units higher on average than infants with maternal 25(OH)D <30 nmol/L, across the first year of life. When maternal 25(OH)D concentration was further divided into four categories, model coefficients indicated that the observed difference in the model with dichotomized maternal vitamin D status was driven by significant differences between infants with maternal vitamin D status in the upper categories of maternal 25(OH)D concentration (50–74 and ≥75 nmol/L) compared with infants with maternal 25(OH)D <30 nmol/L (Table 3). Multivariate results for HCZ were similar (Table 3).
Table 3.
Association of maternal vitamin D status at ≤ 26 weeks gestation and infant LAZ and HCZ over the first year of life based on multivariate-adjusted* mixed effects linear regression models.
| Length-for-age z-score (n = 2466) | Head circumference-for-age z-score (n = 2465) | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | p Value | β | 95% CI | p Value | |
| Maternal vitamin D status: two categories | ||||||
| <30 nmol/L | ref | ref | ||||
| ≥30 nmol/L | 0.130 | (0.028, 0.231) | 0.012 | 0.195 | (0.107, 0.283) | <0.001 |
| Age, per month | −0.022 | (−0.026, −0.018) | <0.001 | 0.054 | (0.050, 0.057) | <0.001 |
| Maternal vitamin D status: four categories | ||||||
| <30 nmol/L | ref | ref | ||||
| 30–49 nmol/L | 0.091 | (−0.020, 0.202) | 0.107 | 0.173 | (0.077, 0.268) | <0.001 |
| 50–74 nmol/L | 0.174 | (0.052, 0.296) | 0.005 | 0.230 | (0.125, 0.335) | <0.001 |
| ≥75 nmol/L | 0.189 | (0.045, 0.334) | 0.010 | 0.205 | (0.081, 0.330) | 0.001 |
| Age, per month | −0.022 | (−0.026, −0.017) | <0.001 | 0.054 | (0.050, 0.057) | <0.001 |
Adjusted for study site, mother and child specified as random effects and infant age, infant sex, maternal BMI, maternal socioeconomic status, maternal race, season of maternal blood draw and breastfeeding specified as fixed effects.
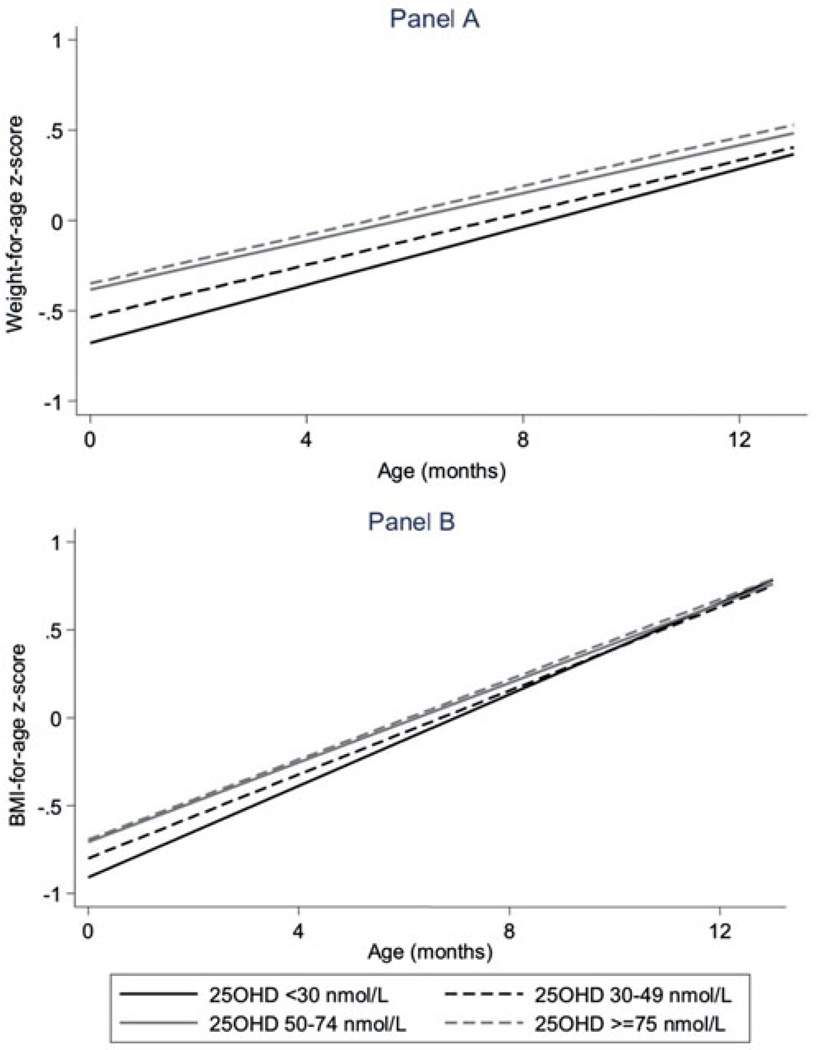
Unlike LAZ and HCZ, the associations of both WAZ and BMIZ with maternal vitamin D status varied by infant age. To provide insight into these interactions, Table 4 includes coefficients for dichotomized maternal vitamin D status with infant age centred at the four ages that align with the measurement points: birth, 4 months, 8 months and 12 months. The steeper age slope across the first year of life in infants with maternal 25(OH)D <30 nmol/L [average gain of 0.080 (0.073, 0.087) z-score units per month] compared with infants with maternal 25(OH)D ≥nmol/L [average gain of 0.065 (0.065, 0.073) z-score units per month, p = 0.007 for interaction] resulted in the differences in WAZ by maternal vitamin D status at birth to diminish over time so that they were no longer statistically significant by the 8- and 12-month follow-up measurements. These differences in WAZ trajectory by maternal vitamin D status were similar, with further division of maternal pre-natal 25(OH)D into four categories, with no significant differences remaining among the groups by the 8-month measurement point (Figure 1(a)). Results for BMIZ were similar to those for WAZ, except that all differences evident at birth had resolved by the 4-month measurement point, regardless of whether maternal vitamin D status was dichotomized (Table 4; p = 0.008 for age interaction) or divided into four categories (Figure 1(b)).
Table 4.
Association of maternal vitamin D status at ≤26 weeks gestation and infant WAZ and BMIZ over the first year of life based on multivariate-adjusted* mixed effects linear regression models, with infant age centred at different points corresponding with ages at measurements.
| Weight-for-age z-score1 (n = 2473) | BMI-for-age z-score1 (n = 2466) | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | p Value | β | 95% CI | p Value | |
| Maternal vitamin D status coefficients | ||||||
| Birth | ||||||
| <30 nmol/L | Ref | Ref | ||||
| ≥30 nmol/L | 0.144 | (0.050, 0.239) | 0.003 | 0.107 | (0.011, 0.202) | 0.028 |
| 4 months | ||||||
| <30 nmol/L | Ref | Ref | ||||
| ≥30 nmol/L | 0.100 | (0.015, 0.186) | 0.022 | 0.050 | (−0.030, 0.129) | 0.218 |
| 8 months | ||||||
| <30 nmol/L | Ref | Ref | ||||
| ≥30 nmol/L | 0.056 | (−0.032, 0.145) | 0.212 | −0.007 | (−0.091, 0.077) | 0.867 |
| 12 months | ||||||
| <30 nmol/L | Ref | Ref | ||||
| ≥30 nmol/L | 0.012 | (−0.089, 0.114) | 0.812 | −0.064 | (−0.171, 0.043) | 0.239 |
| Age coefficients, per month | ||||||
| Age for <30 nmol/L | 0.080 | (0.073, 0.087) | <0.001 | 0.130 | (0.121, 0.139) | <0.001 |
| Age for ≥30 nmol/L | 0.069 | (0.065, 0.073) | <0.001 | 0.116 | (0.110, 0.121) | <0.001 |
Adjusted for study site, mother and child specified as random effects and infant age, infant sex, maternal BMI, maternal socioeconomic status, maternal race, season of maternal blood draw and breastfeeding specified as fixed effects with interaction term for age (continuous) and 25(OH)D (dichotomous at 30 nmol/L). Interaction p<0.01 for weight-for-age and BMI models.
Figure 1.
Association of maternal vitamin D status at ≤26 weeks gestation with weight-for-age (a) and BMI-for-age (b) z-scores over the first year of life, Collaborative Perinatal Project, 1959–1965. Maternal vitamin D status was expressed using four categories. Mixed effects linear regression model were adjusted for study site, mother and child specified as random effects; and 25(OH)D, infant age, infant sex, maternal BMI, maternal socioeconomic status, maternal race, season of maternal blood draw and breastfeeding specified as fixed effects. Interaction terms for infant age (continuous) and 25(OH)D (categorical) were significant for both WAZ (p = 0.03) and BMIZ (p = 0.04). For WAZ, there were no significant differences by maternal vitamin D status by the 8-month measurement point. For BMIZ, there were no significant differences by maternal vitamin D status by the 4-month measurement point.
Sensitivity analyses excluding pre-term infants (gestational age <37 weeks) and infants who died during the first year of life resulted in comparable findings (data not shown).
Discussion
In this multi-site US cohort, we found associations between low maternal pre-natal vitamin D status and reduced LAZ and HCZ measures at birth that persisted across infancy. In contrast, initial associations with reduced WAZ and BMIZ at birth were attenuated across infancy so that no differences by maternal vitamin D status remained by 12 months of age. Results were consistent with different specifications of maternal vitamin D status.
These findings suggest that deficits at birth in infant anthropometric indicators linked to skeletal growth (length and head circumference) in infants with low maternal vitamin D status may be difficult to recoup. The observed sustained association of maternal vitamin D status with measures of infant skeletal growth may reflect the correlation between maternal and infant vitamin D status at birth and possibly beyond; the direct effects of vitamin D deficiency on infant bone mineral homeostasis; and/or the cumulative nature of infant bone growth in contrast to the fluctuating nature of weight and body composition. While low maternal vitamin D status was also associated with lower weight and body mass index at birth, catch-up growth pattern in infants with low maternal vitamin D status resulted in the elimination of initial deficits in weight and BMI. However, given that accelerated weight gain in infants has been positively linked to later obesity risk in other studies (Ong et al., 2000; Monteiro & Victora, 2005; Ong & Loos, 2006; Druet & Ong, 2008), further research is needed to determine whether catch-up growth patterns such as we observed might mediate latent effects of low maternal vitamin D status on obesity risk in later childhood.
Our study is primarily comparable to a large cohort study of Dutch singleton term deliveries, the only other study we are aware of with more than one follow-up measurement point in infancy. Similar to our study, low maternal vitamin D status in the Dutch cohort was associated with lower birth weight, as well as an increased risk for babies born SGA (Leffelaar et al., 2010). However, the Dutch infants of mothers with low vitamin D status experienced complete catch-up growth in both weight and length by 12 months of age (Leffelaar et al., 2010), rather than catch-up restricted to weight and BMI as observed in our study. In a follow-up study of the same study population, no differences were apparent in height and leg-length measured between 5–6 years of age (van Eijsden et al., 2013). Possible reasons for the differences observed between our study and the Dutch cohort include differences in the timing of maternal pre-natal 25(OH)D measurement (median of 13 weeks gestation in the Dutch study compared to a median of 20.7 weeks in our sample) and differences in the way outcome measures were collected (paediatric visit measures in the Dutch study, which are subject to more measurement error than the research measures collected in our study).
In contrast to our findings, a study of over 400 births from the UK found that maternal third-trimester vitamin D status was not associated with any anthropometric measure at birth, 9 months or 9 years of age, although maternal 25(OH)D was sampled at a median of 32.6 weeks gestation compared to a median of 20.7 weeks in our sample (Javaid et al., 2006; Gale et al., 2008). In a different UK cohort that primarily focused on measures of bone mass and content, estimated maternal third-trimester ultraviolet radiation exposure was positively associated with offspring birth weight and with height and weight at follow-up at a mean of 9.9 years of age, but infant growth trajectories were not examined (Sayers & Tobias, 2009; Sayers et al., 2009). Moreover, these researchers later reported poor correlation of estimated ultraviolet exposure with maternal 25(OH)D concentration (Lawlor et al., 2013).
We considered how the time period of the CPP study may have impacted our findings. First, the inherent differences in this historical dataset may reduce the generalizability of our findings to present day infant populations. For example, almost all of the z-score measures in the CPP population were negative at birth, indicating that the CPP population was smaller on average than the WHO reference population. These size differences at birth likely reflect other differences related to the age of the data; the CPP data are drawn from births between 1959–1965 and the study sample had high rates of maternal smoking during pregnancy (47.5%) and pre-pregnancy maternal underweight (10.3% with BMI ≤ 18.5). It should be noted that, while the CPP data share many similarities with the population of primarily formula-fed US children used in the older US Centers for Disease Control and Prevention growth references, we believe the 2006 WHO growth standards currently recommended for use in this age group in US populations (Grummer-Strawn et al., 2010) are the correct choice for use in our analyses. Moreover, the WHO references were constructed from a diverse breastfed population to produce a universal standard of healthy normative growth (de Onis et al., 2004), making them the appropriate reference for any population.
The CPP study was not originally designed to answer the research question addressed in this paper, thus our study had limitations. Ideally, our exposure data would have included maternal serum pre-natal 25(OH)D measured in each trimester of pregnancy to determine whether findings differed by timing of measurement and foetal exposure to low maternal vitamin D status. Additionally, assessment of infant vitamin D status across the first year of life would have been useful for verifying an inter-generational association. Ideally, outcome data would have included direct measures of body composition and long-term follow-up data to clarify the contribution of changes in fat vs lean body mass over time to the observed differences in BMI and to determine whether early growth patterns are predictive of size and body composition beyond infancy. Lastly, unmeasured confounding may be a concern and measurement error in variables on which we had data may have led to bias. For example, the infant feeding data collected for CPP were limited to feeding mode at hospital discharge and did not include measures of exclusivity or duration of breastfeeding. In addition, the manner in which the race/ethnicity data were collected for the CPP study only allowed for comparisons among three discrete categories (white, African-American or Puerto Rican) that do not distinguish “race” from “ethnicity” and did not allow participants to be categorised in multiple ways to reflect our current nuanced understanding of these labels.
Despite these issues, the CPP dataset was well suited to answering our research question. There are few datasets, either current or historical, that provide such a rich array of linked maternal and infant data including maternal circulating 25(OH)D in pregnancy, longitudinal infant anthropometry and measures of potential confounders such as maternal pre-pregnancy BMI. Additionally, our inclusion of different categorizations of maternal 25(OH)D concentration contributes to the collective understanding of whether research findings may vary when using different cut-points for categorization of vitamin D status.
Conclusion
This work highlights the inter-generational impact of maternal vitamin D status on infant size and growth and indicates that initial deficits in infant weight and body mass index that result from constrained foetal growth related to low maternal vitamin D status may be recouped, while associations with markers of linear or skeletal growth may be sustained. Future research steps should include analyses to determine the extent to which observed associations of maternal vitamin D status and child growth are mediated entirely by foetal growth, reflect direct growth effects in both the foetal and post-natal period or are the result of related effects via other pathways such as foetal programming of pancreatic function. Additionally, research is needed to determine whether catch-up growth patterns among infants of deficient mothers are related to subsequent risk for growth-related health outcomes such as obesity.
Acknowledgements
The authors thank Nandita Perumal for assistance with calculating z-scores and writing analysis code.
Funding support came from NIH/NICHD grant R01 HD056999.
Dr Gernand’s research is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under BIRCWH award number K12HD055882, ‘Career Development Program in Women’s Health Research at Penn State’. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Declaration of interest
The authors report no conflicts of interest.
References
- Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169. doi: 10.1136/bmj.f1169. [DOI] [PubMed] [Google Scholar]
- Bodnar LM, Catov JM, Wisner KL, Klebanoff MA. Racial and seasonal differences in 25-hydroxyvitamin D detected in maternal sera frozen for over 40 years. Br J Nutr. 2009;101:278–284. doi: 10.1017/S0007114508981460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar LM, Klebanoff MA, Gernand AD, Platt RW, Parks WT, Catov JM, Simhan HN. Maternal vitamin D status and spontaneous preterm birth by placental histology in the US Collaborative Perinatal Project. Am J Epidemiol. 2014;179:168–176. doi: 10.1093/aje/kwt237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137:447–452. doi: 10.1093/jn/137.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke OG, Butters F, Wood C. Intrauterine vitamin D nutrition and postnatal growth in Asian infants. Br Med J (Clin Res Ed) 1981;283:1024. doi: 10.1136/bmj.283.6298.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- Collins-Fulea C, Klima K, Wegienka GR. Prevalence of low vitamin D levels in an urban midwestern obstetric practice. J Midwifery Womens Health. 2012;57:439–444. doi: 10.1111/j.1542-2011.2012.00167.x. [DOI] [PubMed] [Google Scholar]
- de Onis M, Garza C, Victora CG, Onyango AW, Frongillo EA, Martines J. The WHO Multicentre Growth Reference Study: planning, study design, and methodology. Food Nutr Bull. 2004;25:S15–S26. doi: 10.1177/15648265040251S103. [DOI] [PubMed] [Google Scholar]
- De-Regil LM, Palacios C, Ansary A, Kulier R, Pena-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2012;2:CD008873. doi: 10.1002/14651858.CD008873.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror DK. Vitamin D status during pregnancy: maternal, fetal, and postnatal outcomes. Curr Opin Obstet Gynecol. 2011;23:422–426. doi: 10.1097/GCO.0b013e32834cb791. [DOI] [PubMed] [Google Scholar]
- Druet C, Ong KK. Early childhood predictors of adult body composition. Best Pract Res Clin Endocrinol Metab. 2008;22:489–502. doi: 10.1016/j.beem.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C Princess Anne Hospital Study Group. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62:68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernand AD, Simhan HN, Klebanoff MA, Bodnar LM. Maternal serum 25-hydroxyvitamin D and measures of newborn and placental weight in a U.S. multicenter cohort study. J Clin Endocrinol Metab. 2013;98:398–404. doi: 10.1210/jc.2012-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernand AD, Simhan HN, Caritis S, Bodnar LM. Maternal vitamin D status and small-for-gestational-age offspring in women at high risk for preeclampsia. Obstet Gynecol. 2014;123:40–48. doi: 10.1097/AOG.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert-Diamond D, Baylin A, Mora-Plazas M, Marin C, Arsenault JE, Hughes MD, Willett WC, Villamor E. Vitamin D deficiency and anthropometric indicators of adiposity in school-age children: a prospective study. Am J Clin Nutr. 2010;92:1446–1451. doi: 10.3945/ajcn.2010.29746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- Grummer-Strawn LM, Reinold C, Krebs NF. Use of World Health Organization and CDC growth charts for children aged 0–59 months in the United States. MMWR Recomm Rep. 2010;59:1–15. [PubMed] [Google Scholar]
- Hardy JB. The Collaborative Perinatal Project: lessons and legacy. Ann Epidemiol. 2003;13:303–311. doi: 10.1016/s1047-2797(02)00479-9. [DOI] [PubMed] [Google Scholar]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- Hossein-Nezhad A, Holick MF. Optimize dietary intake of vitamin D: an epigenetic perspective. Curr Opin Clin Nutr Metab Care. 2012;15:567–579. doi: 10.1097/MCO.0b013e3283594978. [DOI] [PubMed] [Google Scholar]
- Hossein-Nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc. 2013;88:720–755. doi: 10.1016/j.mayocp.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOM. Dietary Reference Intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, et al. Princess Anne Hospital Study Group. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- Kalra P, Das V, Agarwal A, Kumar M, Ramesh V, Bhatia E, Gupta S, Singh S, Saxena P, Bhatia V. Effect of vitamin D supplementation during pregnancy on neonatal mineral homeostasis and anthropometry of the newborn and infant. Br J Nutr. 2012;108:1052–1058. doi: 10.1017/S0007114511006246. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Wills AK, Fraser A, Sayers A, Fraser WD, Tobias JH. Association of maternal vitamin D status during pregnancy with bone-mineral content in offspring: a prospective cohort study. Lancet. 2013;381:2176–2183. doi: 10.1016/S0140-6736(12)62203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HA, Kim YJ, Lee H, Gwak HS, Park EA, Cho SJ, Oh SY, et al. Association of vitamin D concentrations with adiposity indices among preadolescent children in Korea. J Pediatr Endocrinol Metab. 2013;26:849–854. doi: 10.1515/jpem-2012-0416. [DOI] [PubMed] [Google Scholar]
- Leffelaar ER, Vrijkotte TG, Van Eijsden M. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: results of the multi-ethnic Amsterdam Born Children and their Development cohort. Br J Nutr. 2010;104:108–117. doi: 10.1017/S000711451000022X. [DOI] [PubMed] [Google Scholar]
- Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N, Swaminathan R, et al. SWS Group. Low maternal vitamin D status and fetal bone development: cohort study. J Bone Miner Res. 2010;25:14–19. doi: 10.1359/jbmr.090701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro P, Victora C. Rapid growth in infancy and childhood and obesity in later life—a systematic review. Obes Rev. 2005;6:143–154. doi: 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- Namgung R, Tsang RC. Factors affecting newborn bone mineral content: in utero effects on newborn bone mineralization. Proc Nutr Soc. 2000;59:55–63. doi: 10.1017/s0029665100000070. [DOI] [PubMed] [Google Scholar]
- Namgung R, Tsang RC. Bone in the pregnant mother and newborn at birth. Clin Chim Acta. 2003;333:1–11. doi: 10.1016/s0009-8981(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Niswander K. The Collaborative Perinatal Study of the National Institute of Neurological Diseases and Stroke: The women and their pregnancies. Philadelphia, PA: WB Saunders; 1972. [Google Scholar]
- Novakovic B, Galati JC, Chen A, Morley R, Craig JM, Saffery R. Maternal vitamin D predominates over genetic factors in determining neonatal circulating vitamin D concentrations. Am J Clin Nutr. 2012;96:188–195. doi: 10.3945/ajcn.112.035683. [DOI] [PubMed] [Google Scholar]
- Oliveira RM, Novaes JF, Azeredo LM, Candido AP, Leite IC. Association of vitamin D insufficiency with adiposity and metabolic disorders in Brazilian adolescents. Public Health Nutr. 2013;17:787–794. doi: 10.1017/S1368980013001225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967–671. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KK, Loos R. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95:904–908. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- Ponsonby AL, Lucas RM, Lewis S, Halliday J. Vitamin D status during pregnancy and aspects of offspring health. Nutrients. 2010;2:389–407. doi: 10.3390/nu2030389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DE, Perumal N, Al Mahmud A, Baqui AH. Maternal vitamin D3 supplementation during the third trimester of pregnancy: Effects on infant growth in a longitudinal follow-up study in Bangladesh. J Pediatr. 2013;163:1605–1611. doi: 10.1016/j.jpeds.2013.07.030. [DOI] [PubMed] [Google Scholar]
- Sayers A, Tilling K, Boucher BJ, Noonan K, Tobias JH. Predicting ambient ultraviolet from routine meteorological data; its potential use as an instrumental variable for vitamin D status in pregnancy in a longitudinal birth cohort in the UK. Int J Epidemiol. 2009;38:1681–1688. doi: 10.1093/ije/dyp237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers A, Tobias JH. Estimated maternal ultraviolet B exposure levels in pregnancy influence skeletal development of the child. J Clin Endocrinol Metab. 2009;94:765–771. doi: 10.1210/jc.2008-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroth RJ, Lavelle CL, Moffatt ME. Review of vitamin D deficiency during pregnancy: who is affected? Int J Circumpolar Health. 2005;64:112–120. doi: 10.3402/ijch.v64i2.17964. [DOI] [PubMed] [Google Scholar]
- Thorne-Lyman A, Fawzi WW. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2012;26:75–90. doi: 10.1111/j.1365-3016.2012.01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eijsden M, Snijder MB, Brouwer I, Vrijkotte TG. Maternal early-pregnancy vitamin D status in relation to linear growth at the age of 5–6 years: results of the ABCD cohort. Eur J Clin Nutr. 2013;67:972–977. doi: 10.1038/ejcn.2013.106. [DOI] [PubMed] [Google Scholar]
- Vandevijvere S, Amsalkhir S, Van Oyen H, Moreno-Reyes R. High prevalence of vitamin D deficiency in pregnant women: a national cross-sectional survey. PLoS One. 2012;7:e43868. doi: 10.1371/journal.pone.0043868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljakainen HT, Saarnio E, Hytinantti T, Miettinen M, Surcel H, Makitie O, Andersson S, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95:1749–1757. doi: 10.1210/jc.2009-1391. [DOI] [PubMed] [Google Scholar]
- Weiler H, Fitzpatrick-Wong S, Veitch R, Kovacs H, Schellenberg J, McCloy U, Yuen CK. Vitamin D deficiency and whole-body and femur bone mass relative to weight in healthy newborns. CMAJ. 2005;172:757–761. doi: 10.1503/cmaj.1040508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerwekh JE. The measurement of vitamin D: analytical aspects. Ann Clin Biochem. 2004;41:272–281. doi: 10.1258/0004563041201464. [DOI] [PubMed] [Google Scholar]