Abstract
Phenylephrine is often used to treat intra-operative hypotension. Previous studies have shown that the FloTrac cardiac monitor may overestimate cardiac output (CO) changes following phenylephrine administration. A new algorithm (4th generation) has been developed to improve performance in this setting. We performed a prospective observational study to assess the effects of phenylephrine administration on CO values measured by the 3rd and 4th generation FloTrac algorithms. 54 patients were enrolled in this study. We used the Nexfin, a pulse contour method shown to be insensitive to vasopressor administration, as the reference method. Radial arterial pressures were recorded continuously in patients undergoing surgery. Phenylephrine administration times were documented. Arterial pressure recordings were subsequently analyzed offline using three different pulse contour analysis algorithms: FloTrac 3rd generation (G3), FloTrac 4th generation (G4), and Nexfin (nf). One minute of hemodynamic measurements was analyzed immediately before phenylephrine administration and then repeated when the mean arterial pressure peaked. A total of 157 (4.6 ± 3.2 per patient, range 1–15) paired sets of hemodynamic recordings were analyzed. Phenylephrine induced a significant increase in stroke volume (SV) and CO with the FloTrac G3, but not with FloTrac G4 or Nexfin algorithms. Agreement between FloTrac G3 and Nexfin was: 0.23±1.19 l/min and concordance was 51.1%. In contrast, agreement between FloTrac G4 and Nexfin was: 0.19±0.86 l/min and concordance was 87.2%. In conclusion, the pulse contour method of measuring CO, as implemented in FloTrac 4th generation algorithm, has significantly improved its ability to track the changes in CO induced by phenylephrine.
Keywords: arterial pressure, phenylephrine, stroke volume, pulse contour analysis
1 Introduction
Perioperative hemodynamic management guided by minimally invasive or noninvasive cardiac output (CO) monitoring has been recently introduced to clinical practice [1–3]. Uncalibrated CO measurements obtained through pulse contour analysis using the FloTrac/Vigileo system (Edwards Lifesciences, Irvine, CA) has been widely used for perioperative goal-directed fluid management and volume optimization. Patient outcome has been shown to be consequently improved with shorter lengths of hospital stay and/or reduced complication rates [4–8]. However, several studies have suggested that acute changes in arterial blood pressure induced by increased vasomotor tone may alter the ability of this monitor to accurately measure CO [9–11]. Following its initial clinical introduction, a third-generation FloTrac/Vigileo analytical algorithm (FloTrac G3) was developed based on an expanded database that included a larger proportion of hyperdynamic and vasoplegic patients to improve the reliability of the software during a wider range of clinical situations. However, a recent study demonstrated that the FloTrac G3 system still did not accurately track changes in CO following the administration of phenylephrine, a predominantly α1-adrenergic receptor agonist [12].
Recently, a new fourth-generation algorithm (FloTrac G4) has been developed to further improve performance in this setting. In the FloTrac analysis, CO = PR×SD(bp)×χ, where PR is the pulse rate, SD(bp) is the standard deviation of the arterial pressure and χ is the calibration factor that incorporates the assessment of vascular tone based on waveform morphology analysis and patient characteristics [13]. In the FloTrac G3 algorithm, χ is averaged over 1 minute, while PR and SD(bp) are updated every 20 seconds. When vasomotor tone changes suddenly, χ consequently lags in response. In the FloTrac G4 algorithm, a new χ is developed, which is χ = χ1min×χfast, where χ1min is still updated every minute, while χfast is inversely proportional to pressure and is updated every 20 seconds. Since the overall effect of this change is a faster update of the calibration factor, this upgrade allows the FloTrac G4 system to respond faster to changing vascular tone.
This study was designed to compare the effects of phenylephrine administration on changes in CO values measured by the FloTrac G3 and G4 algorithms in a real-world clinical setting. The data were analyzed using the Nexfin algorithm (Edwards BMEYE B.V, Amsterdam, Netherlands) as the reference method. The Nexfin system applies a pulse contour analysis algorithm on the pressure waveform as measured by a volume-clamp method providing noninvasive and continuous hemodynamic and CO monitoring [14,15]. Intraoperative CO measurement using the Nexfin device has been verified as correlating with CO measured by esophageal Doppler, both of which are known to be insensitive to vasopressor administration [16].
2 Materials and methods
After local Institutional Review Board approval, patients scheduled for elective surgical procedures at two university medical centers were considered for enrollment. The inclusion criteria were: male or female, 18 years of age or older; radial arterial cannulation clinically indicated and planned for continuous arterial pressure (AP) monitoring; and planned surgery with a high probability of vasopressor administration to treat intraoperative hemodynamics. Since the CO calculations are based on peripheral arterial pressure waveform morphology, the following exclusion criteria were applied: any contraindication for the placement of the required monitoring radial arterial lines; significant aortic valve regurgitation (e.g., Doppler grade 2+) and intra-aortic balloon pump. Hepatic surgical procedures (transplant, resections, etc.) were excluded because of the potential for sudden mechanical obstruction of venous return or extreme vasoplegia as were patients outside the range of body habitus for which the FloTrac system is well validated (body weight <40 kg; and body mass index greater than 40 kg/m2).
After arrival in the operating room, the patients were oxygenated, and standard ASA monitors were applied to monitor circulation, oxygenation, ventilation and temperature. Endotracheal intubation was achieved after an intravenous (IV) bolus of propofol (1–2 mg/kg), fentanyl and neuromuscular blockade. General anesthesia was maintained with inhalational and/or intravenous agents at the discretion of the attending anesthesiologist. Ventilation was controlled and an end-tidal CO2 (ETCO2) of 30–40 mm Hg was maintained by adjusting respiratory rate. Positive end-expiratory pressure was set at 5 cm H2O. The radial arterial catheters were placed in either left or right radial arteries for continuous arterial blood pressure monitoring. The FloTrac/Vigileo system was used and radial arterial pressure waveforms were continuously recorded at a digital sampling rate of 100 Hz.
This was an observational study. In response to any clinically significant decrease in blood pressure during surgery, the anesthesiologists, at their discretions, decided when to treat, what vasopressors to administer and what dose the patients should receive. An IV bolus of 50 µg or 100 µg of phenylephrine was usually the starting treatment. If the blood pressure continued to be low, then a fluid bolus or a second IV bolus of 100 µg of phenylephrine was usually administered.
The digital intraoperative arterial pressure recordings were subsequently analyzed offline using three different pulse contour algorithms: FloTrac G3 (FT3rd, the third-generation FloTrac/Vigileo algorithm), FloTrac G4 (FT4th, the fourth-generation FloTrac/Vigileo algorithm), and Nexfin (nf, the COnf algorithm). One minute of hemodynamic measurements were analyzed immediately before any phenylephrine administration and then repeated when the mean arterial pressure peaked.
All data are expressed as mean ± SD. Changes in CO and SV induced by phenylephrine were analyzed using a paired Student's t-test. Bland Altman analysis was used to assess the bias (mean difference) and precision (SD of the bias) between the FloTrac (COFT3rd and COFT4th) and Nexfin (COnf) measurements. Correlations between the FloTrac (COFT3rd and COFT4th) and Nexfin (COnf) systems were determined by linear regression. We also used 4-quadrant concordance plots to analyze the trending abilities of FloTrac systems (COFT3rd and COFT4th) compared to the Nexfin (COnf). Finally, we evaluated whether the CO response to phenylephrine could be predicted by the baseline stroke volume variation (SVV), using a Receiver Operating Characteristic (ROC) analysis. For all statistical analyses, p values < 0.05 were considered statistically significant. Data analysis was conducted using MATLAB software (Mathworks, Nattick, MA).
3 Results
Written informed consent was obtained from fifty-four ASA class II–IV patients: 27 males, 27 females, age 62±13 (mean ± SD) years, height 167±9 cm, weight 75±17 kg, and BMI 27±7 kg/m2. All patients were classified as ASA class III or IV. The surgery types included major gastrointestinal surgeries, nephrectomies, cystectomies, hip surgeries, abdominal aortic aneurysm repairs and major gynecological procedures with tumor debunking. Among the 54 patients enrolled, thirty-four received phenylephrine and were included in the final data analysis. Overall, 157 (4.6 ± 3.2 per patient, range 1–15) phenylephrine boluses were administered with the associated hemodynamic recordings analyzed before and after drug administration. The average total dose of phenylephrine was 136 ± 58 µg, ranging from 50 to 400 µg (or 1.82 ± 0.94 µg/kg, ranging from 0.62 to 6.58 µg/kg). There were no unsuccessful or inadequate radial arterial cannulations and the quality of the arterial waveforms was good or excellent in all 34 patients used for the analysis.
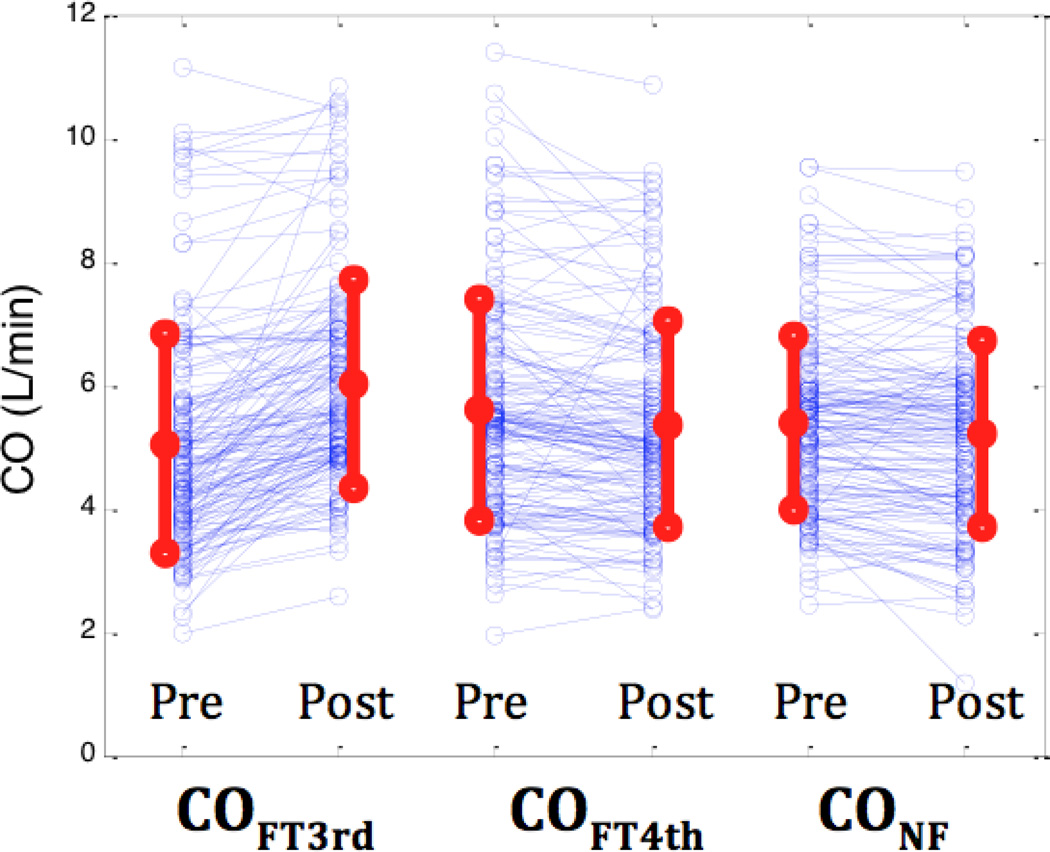
Hemodynamic variables are summarized in Table 1. Phenylephrine administration significantly increased MAP from 61± 9 to 78 ± 15 mmHg (p<0.001) without any significant change in average heart rate. Using the FloTrac G3 algorithm, SV increased from 70 ± 21 to 86 ± 21 ml (p<0.001). In contrast, analysis with the FloTrac G4 and Nexfin algorithms showed no significant differences after phenylephrine administration. Similarly, phenylephrine induced a significant increase in CO with the FloTrac G3 analysis (5.0 ± 1.8 L/min vs 6.0 ± 1.7 L/min, P < 0.001), but not with FloTrac G4 (5.6 ± 1.8 L/min vs 5.4 ± 1.7 L/min, P=0.23) or Nexfin (5.4 ± 1.4 L/min vs. 5.2 ± 1.5 L/min, P=0.31) algorithms (Figure 1).
Table 1.
Hemodynamic effects of phenylephrine administration
| Pre-neo |
Post-neo |
p value | |
|---|---|---|---|
| MAP (mmHg) | 61 ± 9 | 78 ± 15 | <0.001 |
| HR (bpm) | 72 ± 11 | 70 ± 12 | 0.35 |
| SVFT 3rd (ml) | 70 ± 21 | 86 ± 21 | <0.001 |
| SVFT 4th (ml) | 78 ± 21 | 76 ± 20 | 0.40 |
| SVnf (ml) | 75 ± 16 | 73 ± 18 | 0.37 |
| COFT3rd (L/min) | 5.0 ± 1.8 | 6.0 ± 1.7 | <0.001 |
| COFT4th (L/min) | 5.6 ± 1.8 | 5.4 ± 1.7 | 0.23 |
| COnf (L/min) | 5.4 ± 1.4 | 5.2 ± 1.5 | 0.31 |
| SVV (%) | 10 ± 5 | 9 ± 5 | 0.03 |
Data are presented as mean ± SD. MAP: mean arterial blood pressure; HR: heart rate; bpm: beats per minute; SVFT3rd: stroke volume by FloTrac/Vigileo 3rd generation algorithm; SVFT4th: stroke volume by FloTrac/Vigileo 4th generation algorithm; SVnf: stroke volume by Nexfin; COFT3rd: cardiac output by FloTrac/Vigileo 3rd generation; COFT4th: cardiac output by FloTrac/Vigileo 4th generation; COnf: cardiac output by Nexfin; SVV: stroke volume variation; neo: phenylephrine.
Figure 1.
Cardiac output pre and post phenylephrine administration for 3rd generation FloTrac, 4th generation FloTrac and Nexfin algorithms from left to right, respectively. Red dots and lines indicate mean±SD.
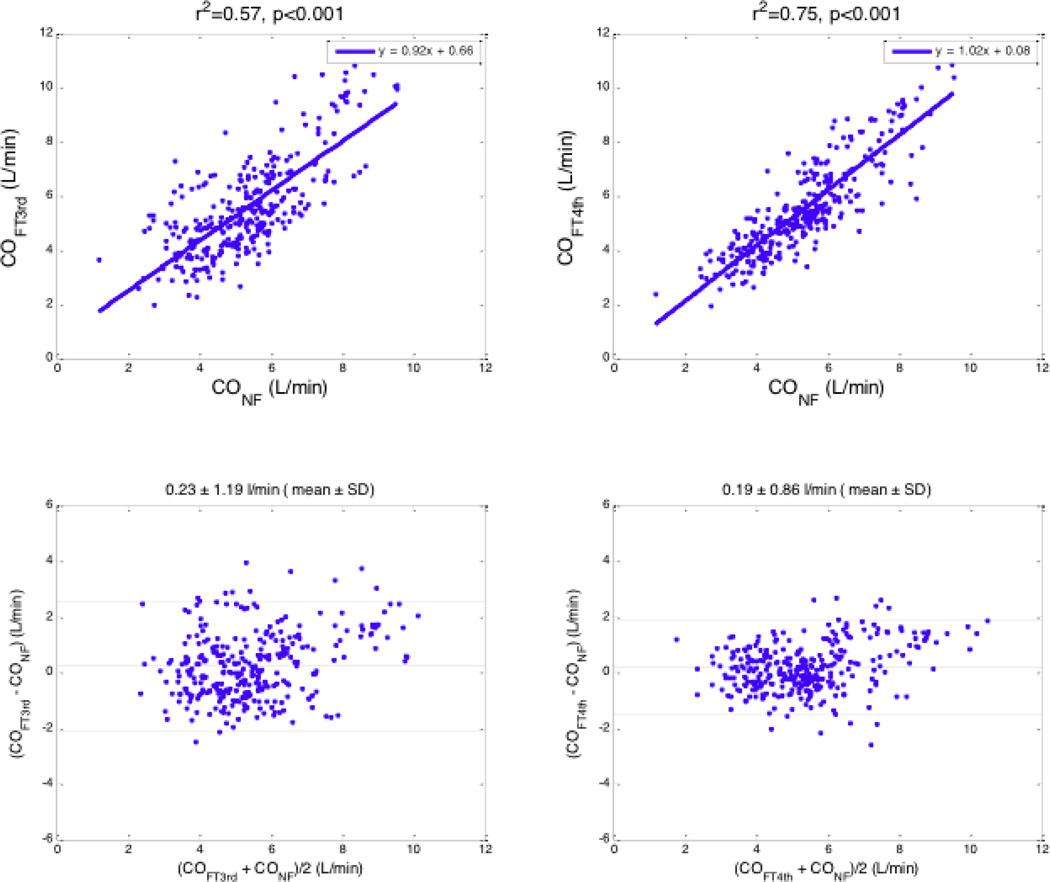
For the 314 total pairs of CO measurements (FloTrac vs. Nexfin), there was a significant relationship between the COFT3rd and COnf (r2 = 0.57; P < 0.001), but the relationship was stronger between the COFT4th and COnf (r2 = 0.75; P < 0.001). Similarly, the difference between paired measurements of COFT3rd and COnf was 0.23 ± 1.19 L/min and the percentage error was 45.9%, while the difference between paired measurements of COFT4th and COnf was 0.19 ± 0.86 L/min (mean ± SD), and the percentage error (1.96 SD/mean) was 31.8%. (Figure 2, Table 2).
Figure 2.
Cardiac outputs measured by FloTrac and Nexfin algorithms before and after an intravenous bolus of phenylephrine. Top row: regression analysis; Bottom row: Bland–Altman analysis. Left column: 3rd generation FloTrac and Nexfin; Right column: 4th generation FloTrac and Nexfin.
Table 2.
Bland-Altman comparison of CO measured by FloTrac vs. Nexfin algorithms
| FloTrac 4G | FloTrac 3G | |
|---|---|---|
| Pre and Post-Phenylephrine data combined (N=314) | ||
| Bias (L/min) | 0.19 | 0.23 |
| Precision (L/min) | 0.86 | 1.19 |
| 2SD/mean (%) | 31.8% | 45.9% |
| Pre-Phenylephrine only (N=157) | ||
| Bias (L/min) | 0.22 | −0.33 |
| Precision (L/min) | 0.88 | 0.93 |
| 2SD/mean (%) | 31.7% | 35.3% |
| Post-Phenylephrine only (N=157) | ||
| Bias (L/min) | 0.16 | 0.80 |
| Precision (L/min) | 0.84 | 1.15 |
| 2SD/mean (%) | 31.9% | 43.8% |
Comparison of cardiac outputs (CO) measured by FloTrac vs. Nexfin based on Bland–Altman analysis. FloTrac 4G, fourth generation algorithm; FloTrac 3G, third generation algorithm; SD, standard deviation.
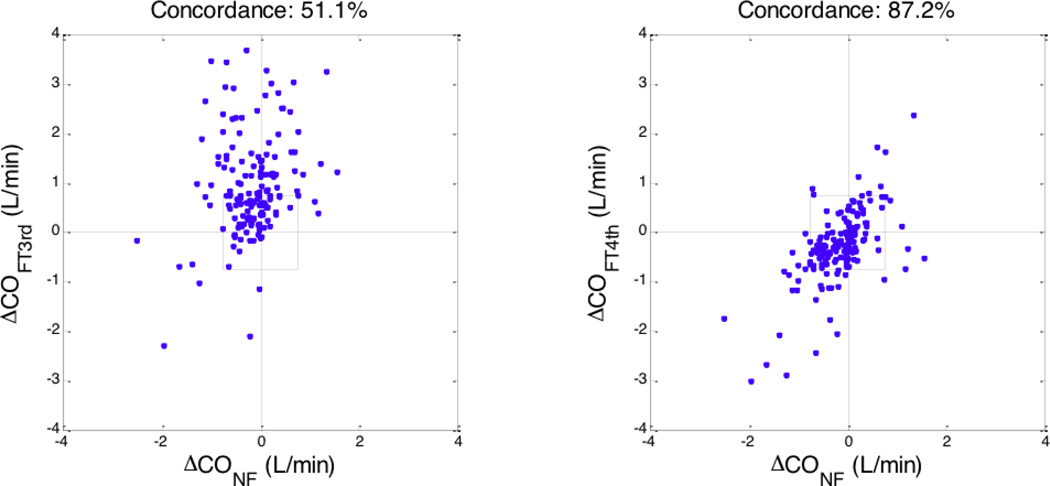
We also used the 4-quadrant concordance analysis described by Critchley et al to evaluate the trending ability of the FloTrac algorithms as compared to the Nexfin [17]. The concordance between CO changes was calculated using an exclusion zone of 0.75 L/min. A 51.1% concordance was observed between changes in COFT3rd and COnf while an 87.2% concordance was found between changes in COFT4th and COnf (post-phenylephrine minus pre-phenylephrine) (Figure 3).
Figure 3.
Trending ability of FloTrac/Vigileo algorithms vs. Nexfin based on 4-quadrant concordance analysis. Change in cardiac output (post phenylephrine minus pre phenylephrine); left: 3rd generation FloTrac vs. Nexfin; right: 4th generation FloTrac vs. Nexfin. The exclusion zone for the concordance calculation was 0.75 L/min.
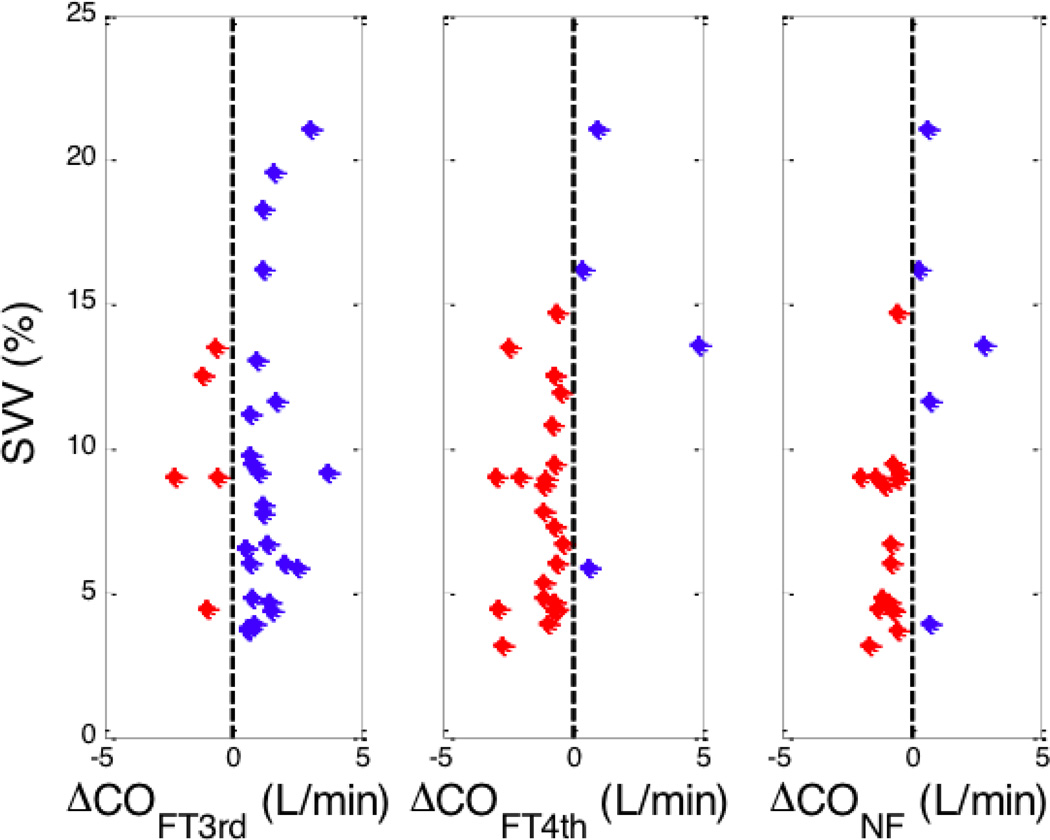
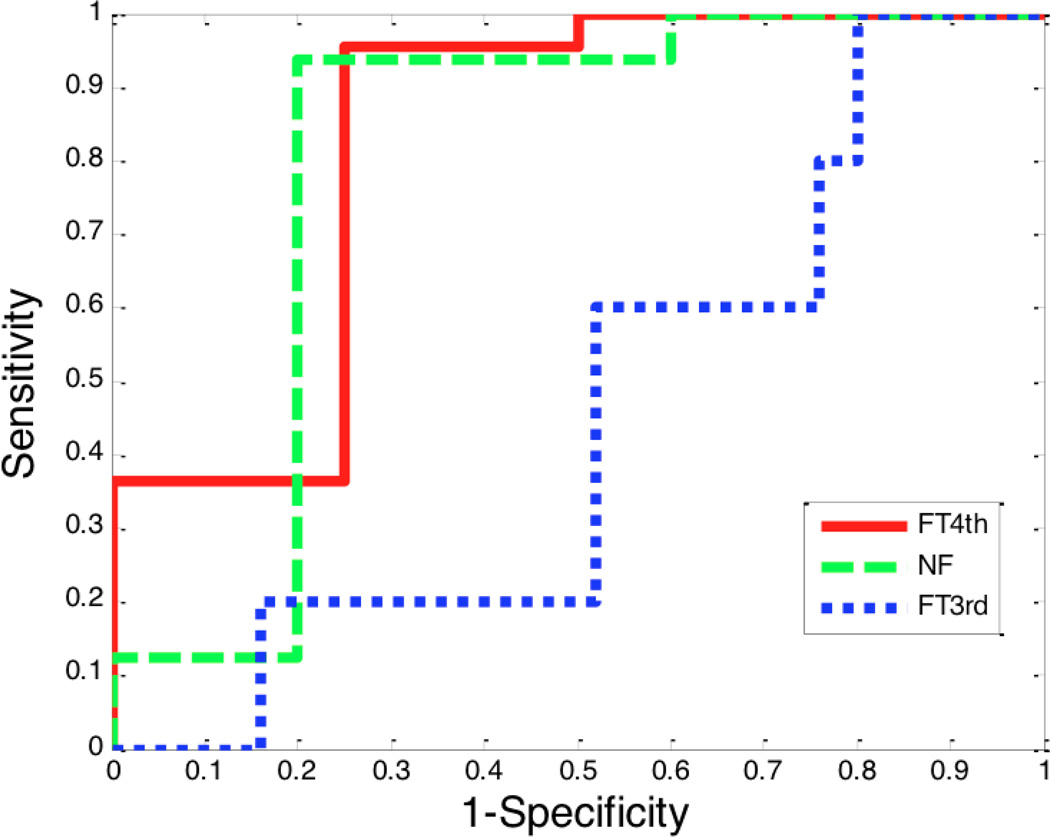
Lastly, we evaluated whether the CO response to phenylephrine could be predicted by the baseline stroke volume variation (SVV) [18]. It is known that for SVV to predict fluid responsiveness, the patients should be mechanically ventilated with a tidal volume of at least 8 ml/kg (ideal body weight), should have a closed chest and no sustained arrhythmia. Among the 34 patients, twenty-two of them were either open chest operations and/or the tidal volume was less than 8 ml/kg and were therefore excluded from this analysis. Therefore, 12 patients who received a total of 40 phenylephrine boluses were analyzed. As cardiac output varies from beat to beat, we considered only those cardiac outputs whose change induced by phenylephrine was greater than 10%. Figure 4 plots the baseline SVV against the CO change induced by phenylephrine. Data with high SVV is limited, but when SVV is high, the CO tends to increase. When SVV was low (<15%), the CO decreased with the FloTrac G4 (36/38 measurements) and Nexfin (35/38 measurements) algorithms but not with the FloTrac G3 (5/36 measurements) algorithm. We also used receiver operator characteristic (ROC) curves to compare the ability of the baseline SVV to predict the CO response to phenylephrine (Figure 5). The areas under the ROC curves for FloTrac G4, Nexfin, and FloTrac G3 algorithms are 0.83, 0.80, and 0.45, respectively. The best SVV threshold value based on Youden index method [19] and its corresponding sensitivity (with 95% confidence interval in parentheses) and specificity (with 95% confidence interval in parentheses) are 13.5%, 1.0 (0.57–1.0), and 0.2 (0.09–0.39) for the FloTrac G3 algorithm, 13.5%, 0.95 (0.78–0.99), and 0.75 (0.30–0.95) for the FloTrac G4 and 9.5%, 0.94 (0.72–0.99), and 0.80 (0.38–0.96) for the Nexfin.
Figure 4.
Stroke volume variation vs. change in cardiac output following administration of phenylephrine. SVV – strove volume variation, FT3rd - 3rd generation FloTrac algorithm, FT4th – 4th generation FloTrac algorithm, NF Nexfin algorithm
Figure 5.
Receiver operating characteristic (ROC) curve for baseline SVV as predictor of CO response to phenylephrine. FT3rd: 3rd generation FloTrac algorithm, FT4th: 4th generation FloTrac algorithm, NF: Nexfin algorithm
4 Discussion
This is the first study to evaluate the tracking abilities of the new FloTrac 4th generation cardiac output algorithm (COFT4th) after phenylephrine administration and to compare CO measurements among FloTrac algorithms (COFT3rd and COFT4th) and the Nexfin (Conf) system. The results show that in contrast to the 3rd generation FloTrac/Vigileo algorithm, the CO measurements with the 4th generation FloTrac/Vigileo system and Nexfin are not significantly affected by acute changes in vasomotor tone. The concordance between CO measurements with the FloTrac G3 and Nexfin was only fair (51.1%) while the concordance between CO measurements with the FloTrac G4 and Nexfin was >87%.
There is increasing interest in perioperative goal-directed fluid optimization and hemodynamic management in critical ill patients during high-risk surgeries [1]. Several technologies are now available for minimally invasive or noninvasive hemodynamic monitoring including pulse contour analysis systems like the FloTrac/Vigileo and Nexfin systems along with other transesophageal and bioimpedance technologies [20]. To calculate CO, the FloTrac/Vigileo software uses an algorithm based on the relationship between arterial pulse pressure and stroke volume while considering vessel compliance and peripheral resistance. Although the third-generation software was based on an expanded dataset to improve the reliability of monitoring hemodynamic variables in rapidly changing clinical scenarios, previous studies showed that it was still affected by acute changes in vasomotor tone. The observed phenylephrine induced CO increase measured by this FloTrac/Vigileo system was opposite to CO changes measured by esophageal Doppler systems [12]. Similar observations have been reported with norepinephrine and other acute hemodynamic interventions in studies comparing COFT3rd with thermodilution and other CO measurement techniques [9–11].
Nexfin determines a beat-to-beat SV by dividing the area under the systolic blood pressure curve by the aortic input impedance (Zin) similar to the method described by Wesseling et al and Westerhof et al [21,22]. In calculating CO, the Nexfin COnf algorithm first transforms the radial artery waveform into a brachial waveform morphology with a specific filter [23]. The area under the systolic waveform is input to the model, which, in combination with Zin, directly yields SV. CO is calculated by multiplying beat-to-beat SV with the instantaneous heart rate (HR). When compared with the pulmonary artery catheter (PAC) the Nexfin system can reliably track preload-induced changes in CO in stable patients after cardiac surgery in the presence of moderate vasopressor and inotropic therapy [23].
To address the observed limits of the FloTrac/Vigileo system, a new FloTrac G4 algorithm has been developed. The present study showed that the phenylephrine induced increase in stroke volume (SV) and CO calculated by the FloTrac G3 algorithm were not observed with either the FloTrac G4 or Nexfin systems. With respect to CO, the agreement between the FloTrac G4 and Nexfin systems was comparable to that observed with the FloTrac G3 but the concordance was greatly improved. The observed phenylephrine induced changes in SV and CO were consistent with previously published studies [12,16].
Phenylephrine has two potentially conflicting actions through which it could alter cardiac output. The first is an increase in ventricular afterload due to arterial constriction with consequent decreases in stroke volume and cardiac output. The second is an increase in venous return due to venous constriction with consequent decreased venous capacitance, raising central venous pressure, stroke volume and thus cardiac output. Cannesson et al in a recent animal study demonstrated that the effect of phenylephrine on cardiac output depended on the position of the heart on the Frank-Starling curve [18]. They also demonstrated that baseline SVV or pulse pressure variation (PPV) can predict the response of cardiac output to phenylephrine. Maas et al similarly showed, in a recent clinical study, that norepinephrine can induce either an increase or decrease in CO and the direction of change can be predicted by the baseline stroke volume variation [24]. Similar results are shown in this study when the FloTrac G4 and Nexfin algorithms are used for the analysis, while the response of cardiac output changes analyzed by the FloTrac G3 algorithm is not predictive (ROCAUC= 0.45).
There are limitations to this study. We did not compare the calculated CO measurements with the gold standard reference pulmonary artery catheter thermodilution methodology. This is because our main goal was to assess the response to acute changes induced by phenylephrine. Bolus thermodilution cannot assess these beat-to-beat changes so we chose to use the Nexfin as a reference as it was previously reported to be a reliable CO monitoring method in this clinical setting [14–16,25]. Further studies are still required to assess the accuracy of the Nexfin and FloTrac G4 devices in more challenging situations such as extremes of blood pressure or CO. In addition, only 34 consented patients received phenylephrine. The percentage of 63% receiving phenylephrine is typical in our clinical setting, however, this decreased the population studied from 54 to 34, further studies in a larger population would increase the statistical power of our observations. Similarly, in order to use only the most robust data for the ROC analysis, it was done with a relatively small sample size. Further studies are also needed to confirm these initial results. Nevertheless, the existing data consistently showed the advantage of the FloTrac G4 and Nexfin algorithms when compared to the FloTrac G3 algorithm.
In conclusion, the pulse contour method of measuring CO, as implemented in the FloTrac 4th generation algorithm, has significantly improved its ability to accurately track the changes in CO induced by phenylephrine.
Acknowledgments
The authors thank Zhongping Jian, PhD of Edwards Lifesciences for assisting with the data analysis and members of the Department of Anesthesiology and Perioperative Care, University of California, Irvine for assisting with part of the data collection.
Funding/Support:
This work was supported by the University of California Davis Health System Department of Anesthesiology and Pain Medicine, and NIH grant UL1 TR000002. This study was supported by a grant from Edwards Lifesciences, a grant from Jiangsu Province’s by Key Provincial Talents Program, China (Fuhai Ji), by Jiangsu province’s six major peak talents program, China (Fuhai Ji) and by Suzhou science and No.SYS201111 (Fuhai Ji) from Technology Bureau’s program, China.
References
- 1.Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112:1392–1402. doi: 10.1213/ANE.0b013e3181eeaae5. [DOI] [PubMed] [Google Scholar]
- 2.Rhodes A, Cecconi M, Hamilton M, Poloniecki J, Woods J, Boyd O, Bennett D, Grounds RM. Goal-directed therapy in high-risk surgical patients: a 15-year follow-up study. Intensive Care Med. 2010;36:1327–1332. doi: 10.1007/s00134-010-1869-6. [DOI] [PubMed] [Google Scholar]
- 3.Dalfino L, Giglio MT, Puntillo F, Marucci M, Brienza N. Haemodynamic goal-directed therapy and postoperative infections: earlier is better. A systematic review and meta-analysis. Crit Care. 2011 Jun 24;15(3):R154. doi: 10.1186/cc10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benes J, Chytra I, Altmann P, Hluchy M, Kasal E, Svitak R, Pradl R, Stepan M. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care. 2010;14:R118. doi: 10.1186/cc9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramsingh DS, Sanghvi C, Gamboa J, Cannesson M, Applegate RL., 2nd Outcome impact of goal directed fluid therapy during high risk abdominal surgery in low to moderate risk patients: a randomized controlled trial. J Clin Monit Comput. 2013 Jun;27(3):249–257. doi: 10.1007/s10877-012-9422-5. [DOI] [PubMed] [Google Scholar]
- 6.Cecconi M, Fasano N, Langiano N, Divella M, Costa MG, Rhodes A, Della Rocca G. Goal-directed haemodynamic therapy during elective total hip arthroplasty under regional anaesthesia. Crit Care. 2011;15(3):R132. doi: 10.1186/cc10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer J, Boldt J, Mengistu AM, Röhm KD, Suttner S. Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: a randomized, controlled trial. Crit Care. 2010;14:R18. doi: 10.1186/cc8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutierrez MC, Moore PG, Liu H. Goal Directed Therapy in Intraoperative Fluid and Hemodynamic Management. The Journal of Biomedical Research. 2013;27(5):357–365. doi: 10.7555/JBR.27.20120128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eleftheriadis S, Galatoudis Z, Didilis V, Bougioukas I, Schön J, Heinze H, Berger KU, Heringlake M. Variations in arterial blood pressure are associated with parallel changes in FloTrac/Vigileo-derived cardiac output measurements: a prospective comparison study. Crit Care. 2009;13:R179. doi: 10.1186/cc8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorsomradee S, Cromheecke S, De Hert SG. Uncalibrated arterial pulse contour analysis versus continuous thermodilution technique: effects of alterations in arterial waveform. J Cardiothorac Vasc Anesth. 2007;21:636–643. doi: 10.1053/j.jvca.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Monnet X, Anguel N, Naudin B, Jabot J, Richard C, Teboul JL. Arterial pressure-based cardiac output in septic patients: different accuracy of pulse contour and uncalibrated pressure waveform devices. Crit Care. 2010;14:R109. doi: 10.1186/cc9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng L, Tran NP, Alexander BS, Laning K, Chen G, Kain ZN, Cannesson M. The impact of phenylephrine, ephedrine, and increased preload on third-generation Vigileo-FloTrac and esophageal doppler cardiac output measurements. Anesth Analg. 2011;113:751–757. doi: 10.1213/ANE.0b013e31822649fb. [DOI] [PubMed] [Google Scholar]
- 13.Pratt B, Roteliuk L, Hatib F, Frazier J, Wallen RD. Calculating arterial pressure based cardiac output using a novel measurement and analysis method. Biomed Instrum Technol. 2007;41:403–411. doi: 10.2345/0899-8205(2007)41[403:CAPCOU]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Bogert LW, Wesseling KH, Schraa O, Van Lieshout EJ, de Mol BA, van Goudoever J, Westerhof BE, van Lieshout JJ. Pulse contour cardiac output derived from non-invasive arterial pressure in cardiovascular disease. Anaesthesia. 2010;65:1119–1125. doi: 10.1111/j.1365-2044.2010.06511.x. [DOI] [PubMed] [Google Scholar]
- 15.Broch O, Renner J, Gruenewald M, Meybohm P, Schöttler J, Caliebe A, Steinfath M, Malbrain M, Bein B. A comparison of the Nexfin® and transcardiopulmonary thermodilution to estimate cardiac output during coronary artery surgery. Anaesthesia. 2012;67(4):377–383. doi: 10.1111/j.1365-2044.2011.07018.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen G, Meng L, Alexander B, Tran NP, Kain ZN, Cannesson M. Comparison of noninvasive cardiac output measurements using the Nexfin monitoring device and the esophageal Doppler. J Clin Anesth. 2012;24:275–283. doi: 10.1016/j.jclinane.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Critchley LA, Lee A, Ho AM. A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg. 2010;111:1180–1192. doi: 10.1213/ANE.0b013e3181f08a5b. [DOI] [PubMed] [Google Scholar]
- 18.Cannesson M, Jian Z, Chen G, Vu TQ, Hatib F. Effects of phenylephrine on cardiac output and venous return depend on the position of the heart on the Frank-Starling relationship. J Appl Physiol. 2012;113:281–289. doi: 10.1152/japplphysiol.00126.2012. [DOI] [PubMed] [Google Scholar]
- 19.Yin J, Tian L. Joint confidence region estimation for area under ROC curve and Youden index. Stat Med. 2014;33:985–1000. doi: 10.1002/sim.5992. [DOI] [PubMed] [Google Scholar]
- 20.Peyton PJ, Chong SW. Minimally invasive measurement of cardiac output during surgery and critical care: a meta-analysis of accuracy and precision. Anesthesiology. 2010;113:1220–1235. doi: 10.1097/ALN.0b013e3181ee3130. [DOI] [PubMed] [Google Scholar]
- 21.Wesseling KH, Smith NT, Nichols WW, Weber H, De Wit B, Beneken JE. Beat-to-beat cardiac output from the arterial pressure pulse contour. In: Feldman SA, Leigh JM, Spierdijk J, editors. Measurement in Anaesthesia. Leiden, The Netherlands: Leiden University Press; 1974. pp. 148–164. [Google Scholar]
- 22.Westerhof N, Lankhaar JW, Westerhof BE. The arterial Windkessel. Med Biol Eng Comput. 2009;47:131–141. doi: 10.1007/s11517-008-0359-2. [DOI] [PubMed] [Google Scholar]
- 23.Bubenek-Turconi SI, Craciun M, Miclea I, Perel A. Noninvasive continuous cardiac output by the Nexfin before and after preload-modifying maneuvers: a comparison with intermittent thermodilution cardiac output. Anesth Analg. 2013 Aug;117(2):366–372. doi: 10.1213/ANE.0b013e31829562c3. [DOI] [PubMed] [Google Scholar]
- 24.Maas JJ, Pinsky MR, de Wilde RB, de Jonge E, Jansen JR. Cardiac output response to norepinephrine in postoperative cardiac surgery patients: interpretation with venous return and cardiac function curves. Crit Care Med. 2013 Jan;41(1):143–150. doi: 10.1097/CCM.0b013e318265ea64. [DOI] [PubMed] [Google Scholar]
- 25.Bubenek-Turconi SI, Craciun M, Miclea I, Perel A. Noninvasive continuous cardiac output by the Nexfin before and after preload-modifying maneuvers: a comparison with intermittent thermodilution cardiac output. Anesth Analg. 2013 Aug;117(2):366–372. doi: 10.1213/ANE.0b013e31829562c3. [DOI] [PubMed] [Google Scholar]