Abstract
Shoulder tendon injuries are common clinical conditions and are a significant source of pain and dysfunction. These conditions are more common in individuals who perform repetitive overhead activities and in individuals who have abnormal scapular kinematics, termed scapular dyskinesis (SD). However, the long term consequences associated with overuse activity in the presence of SD are unknown. Therefore, the objective of this study was to determine the effect of overuse in combination with SD on joint mechanics and properties of the rotator cuff and biceps tendons. A rat model of scapular dyskinesis was used. 90 adult male Sprague-Dawley rats (400–450 grams) were randomized into three groups: nerve transection (SD), sham nerve transection + overuse (OV), or nerve transection + overuse (SD + OV). Rats were sacrificed at 2, 4, and 8 weeks after surgery. Shoulder function and passive joint mechanics were evaluated over time and tendon properties (mechanical, histological, organizational, and compositional) were measured. Results demonstrated that overuse activity and SD are each independently detrimental to tendon properties (e.g., diminished mechanical properties, disorganized collagen). However, tendon damage caused by the addition of overuse may be worse, with more parameters altered, than damage caused by the addition of SD. This study helps define the mechanical mechanisms leading to tendon damage and provides a framework for distinguishing treatment strategies for active patients and those with abnormal scapular mechanics.
Keywords: scapular dyskinesis, animal model, rotator cuff, biceps, overuse injury
Introduction
Shoulder injuries including rotator cuff and biceps tendinitis are widespread and debilitating clinical conditions. These injuries are particularly common in individuals who perform repetitive overhead activities due to their occupation or sport.9 Previous studies have demonstrated that isolated overuse activity, and overuse activity combined with reduced subacromial space, results in the development of rotator cuff tendinopathy.23 Overuse activity can lead to chronic tendon injuries characterized by disorganized collagen fibers, increased cellularity, altered cell shape (for example, more rounded), and decreased mechanical properties, indicative of degenerative tissue.24 These changes may be a result of intrinsic or extrinsic mechanisms, such as excessive repetitive strain or mechanical compression of the rotator cuff under the acromial arch (defined as subacromial impingement), respectively.
Individuals who perform repetitive overuse activities (such as athletes and manual laborers) may also develop abnormal shoulder mechanics. Altered scapulothoracic joint kinematics, caused by muscular inhibition, altered neuromuscular control, or fatigue, is common.2, 12, 29, 30 These changes may contribute to joint injury, such as subacromial impingement. Abnormal scapulothoracic joint kinematics, referred to as scapular dyskinesis, may disrupt glenohumeral joint motion, which presents as increased joint translations due to laxity of the static restraints, and possibly predisposing the dynamic stabilizers, such as the rotator cuff and biceps, to injury. Several studies have demonstrated a strong association between scapular dyskinesis and shoulder injuries, such as shoulder impingement and rotator cuff disease;6, 8, 10, 25, 27 however, additional basic science studies are warranted in order to determine this association and identify cause and effect relationships. Identification of scapular dyskinesis as a risk factor for shoulder injury in an active population may help guide the development of prevention programs.
Using an animal model, we have previously demonstrated that scapular dyskinesis can contribute directly to the initiation and progression of rotator cuff and biceps tendon injuries.19 However, the long term consequences associated with overuse activity in the presence of scapular dyskinesis are unknown. The underlying mechanisms and specific relationship by which scapular dyskinesis affects the rotator cuff and biceps can only be elucidated in an animal model where time from injury and post-operative activity levels can be carefully controlled and evaluated over time.
Therefore, the objective of this study was to determine the effect of overuse in combination with scapular dyskinesis on joint mechanics and properties of the rotator cuff and biceps tendon in a rat model. We hypothesized that scapular dyskinesis in combination with repetitive overuse will alter joint function and diminish tendon properties when compared to scapular dyskinesis alone (H1) and when compared to overuse alone (H2).
Methods
Study Design
A rat model of scapular dyskinesis was used19, in this Institutional Animal Care and Use Committee (IACUC) approved study. Ninety male Sprague-Dawley rats (400–450 g) were randomized into 3 groups: nerve transection (SD, N=30), sham nerve transection + overuse activity (OV, N=30), or nerve transection + overuse activity (SD + OV, N=30). Methods for surgical transection of the accessory and long thoracic nerves were previously published.19 First, a 2 cm vertical incision was made 1 cm posterior to the ear. The cervical nerve, clavo-trapezius, and acromiotrapezius muscles were identified. The clavo-trapezius and acromio-trapezius muscles were separated cranial to the cervical nerve to expose the spinal accessory nerve, located above the omotransversarius muscle. The spinal accessory nerve was transected 2 mm and 5 mm proximal to the acromio-trapezius and removed. The site was irrigated with sterile saline and the overlying skin was closed with staples. Next, the animal‧s forelimb was abducted and internally rotated, and a 3 cm axillary incision was made in the caudal direction along the abdominal fascia, exposing the serratus anterior muscle. Next, the latissimus dorsi muscle was identified, an incision was made along the fascial interface, and a blunt dissection was performed between the latissimus dorsi muscle and serratus anterior to gain proximal exposure to the serratus anterior muscle and long thoracic nerve. The long thoracic nerve was transected 2 mm and 5 mm proximal to the serratus anterior and removed. The site was irrigated with sterile saline, the abdominal fasica was sutured closed with 4-0 Vicryl suture (Ethicon, Inc., Blue Ash, OH), and the overlying skin was then closed with staples. Overuse activity was modeled as stated in our previous publications.18, 24 Initially, rats in overuse groups underwent a 2-week treadmill training period to acclimate them to running at the desired speed and for the desired duration. All groups then underwent unilateral surgical transection (or sham transection) of the spinal accessory and long thoracic nerves, resulting in denervation of the trapezius and serratus anterior muscles, respectively. Pre- and post-operative analgesics (buprenorphine, 0.05 mg/kg) were administered up to 2 days following surgery. Animals returned to unrestricted cage activity for 5 days and were then subjected to overuse activity or continued unrestricted cage activity. Overuse activity consisted of treadmill running at 17 m/min on a 10° decline for 1 hour per day 5 days per week. Rats were sacrificed 2, 4, and 8 weeks following surgery and either frozen (for mechanical testing, N=10) or fixed in formalin (for histology and immunohistochemistry, N=5).
Quantitative Ambulatory Assessment
Forelimb ground reaction forces (medial/lateral, braking, propulsion, and vertical) were quantified using an instrumented walkway, consisting of two 6 degree-of-freedom load/torque cells.20 Rats were acclimated prior to formal recording of ambulatory data. Data was collected one day prior to surgery (baseline), and at days 5, 7, 14, 28, 42, and 56 days post-surgery. All parameters were normalized to animal body-weight.
Passive Joint Mechanics
Passive shoulder joint range of motion (ROM) and stiffness were quantified using a custom device.21 Briefly, the animal was placed under anesthesia and the forearm was secured into a rotating clamp at 90° of elbow flexion and 90° of glenohumeral forward flexion. The scapula was manually stabilized (to isolate glenohumeral motion) and the arm was rotated through the full range of internal and external rotation three times. Range of motion was calculated using the average of the three maximum values for both internal and external rotation. A bilinear fit was applied to calculate joint stiffness in the toe and linear regions for both internal and external rotation. Data was collected one day prior to surgery (baseline), and at days 14, 28, and 56 days post-surgery. All parameters were normalized to baseline values.
Tendon Sample Preparation
Tendon tissue samples were prepared at the time of mechanical testing. 19 Briefly, the animals were thawed and the scapula and humerus were dissected out with the biceps and supraspinatus tendons intact. The tendons were fine dissected under a stereomicroscope to remove surrounding excess tissue. Cross-sectional area of each tendon was measured using a custom laser device.
Tendon Mechanical Testing
Tendon mechanical testing was performed.18 Briefly, stain lines, for local optical strain measurements, were placed on the biceps and supraspinatus tendons, dividing the insertion and mid-substance regions. The scapula and humerus were embedded using polymethymethacrylate (PMMA) and secured in a custom testing fixture. The proximal end of each tendon was gripped with cyanoacrylate annealed sand paper and secured using custom grips. The specimens were immersed in PBS at 37°C during testing. Uniaxial tensile testing was performed with preconditioning (10 cycles from 0.1 N to 0.5 N), stress relaxation (4% strain for biceps and 5% strain for supraspinatus at a rate of 5 %/sec for 600 sec), and ramp to failure (0.3%/sec). Stress (force divided by cross-sectional area) and strain (determined from stain line displacements that were tracked using custom texture tracking software) were calculated and elastic modulus was determined using a linear regression of the linear region of the stress-strain curve. The viscoelastic parameter, percent relaxation, was calculated through analysis of the stress-relaxation curve and determination of the peak and equilibrium loads.
Tendon Histology
Histologic analysis was performed for the biceps and supraspinatus tendons.19 Briefly, tissues were harvested immediately following sacrifice and processed using standard paraffin procedures. Sagittal sections (7µm) were collected, stained with Hematoxylin–Eosin (H&E), and imaged at the insertion and mid-substance using traditional and polarized light microscopy at 200X and 100X magnifications, respectively. The mid-substance of the biceps was further subdivided into four regions: insertion site (INS), intra-articular space (INTRA), proximal groove (PROX), and distal groove (DIS). 15 Cell density (number of cells/mm2) and cell shape (aspect ratio; 0–1, with 1 being a circle) were quantified in the traditional light images using a bioquantification software system (Bioquant Osteo II; BIOQUANT Image Analysis Corp, Nashville, TN, USA). Polarized light images were analyzed using custom software to evaluate tendon organization.5 The angular deviation (AD) of the collagen orientation for each specimen, a measure of the collagen fiber alignment, was calculated in each tendon location.
Tendon Immunohistochemistry
Tissue specimens used for histology were also used to localize ECM proteins using previous immunohistochemical techniques.14, 19 Staining for collagens type II and III, the proteoglycan decorin, and the inflammatory marker, IL1-β was performed and the proteins were visualized using DAB (Table I). Staining results were independently graded by three blinded investigators, using a scale of 0–3 (0=undetectable, 1=low, 2= medium, 3=high) and the mode of these values was used as the final specimen score.
Table I.
Primary an tibodies used for immunohistochemical staining
| Protein Target |
Anti body |
Hos t |
Type | Enzyme pretreatment |
Diluti on |
Incubation period (h) |
Source |
|---|---|---|---|---|---|---|---|
| Collage n II |
II- 116B 3 |
Mo use |
Monoc lonal |
Hyaluronidase | 1:4 | 16 | DSHB, Iowa City, IA, USA |
| Collage n III |
c780 5 |
Mo use |
Monoc lonal |
Hyaluronidase | 1:500 | 38 | Sigma, St. Louis, MO, USA |
| Decorin | LF- 113 |
Rab bit |
Polycl onal |
Chondroitinas e ABC |
1:300 | 38 | L. Fisher, Bethesda, MD, USA |
| IL1-β | AB18 32 |
Rab bit |
Polycl onal |
Pepsin | 1:250 | 16 | Millipore, Billerica, MA, USA |
Statistical Analysis
For the ambulatory assessment, multiple imputations were conducted using the Markov chain Monte Carlo method for missing data points (~10%). For both ambulatory assessment and passive joint mechanics, significance was assessed using a 2-way ANOVA with repeated measures on time with follow-up t-tests between groups according to our hypotheses (H1: SD vs. SD + OV and H2: OV vs. SD + OV) at each time point. Tissue mechanics and histologic parameters between groups were assessed using a 1-way ANOVA with follow-up t-tests. Immunohistochemistry scores were evaluated using a Kruskal Wallis with follow-up Mann-Whitney tests. Bonferroni corrections were used for multiple comparisons and significance was set at p<0.05/2=0.025, trends at p<0.1/2=0.05.
Results
Ambulatory Data
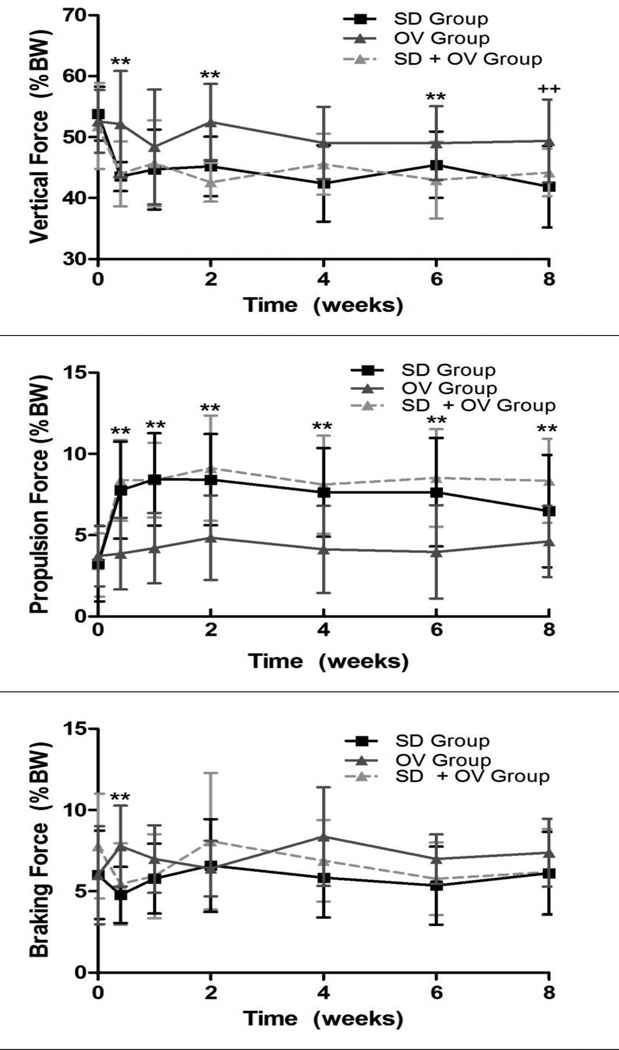
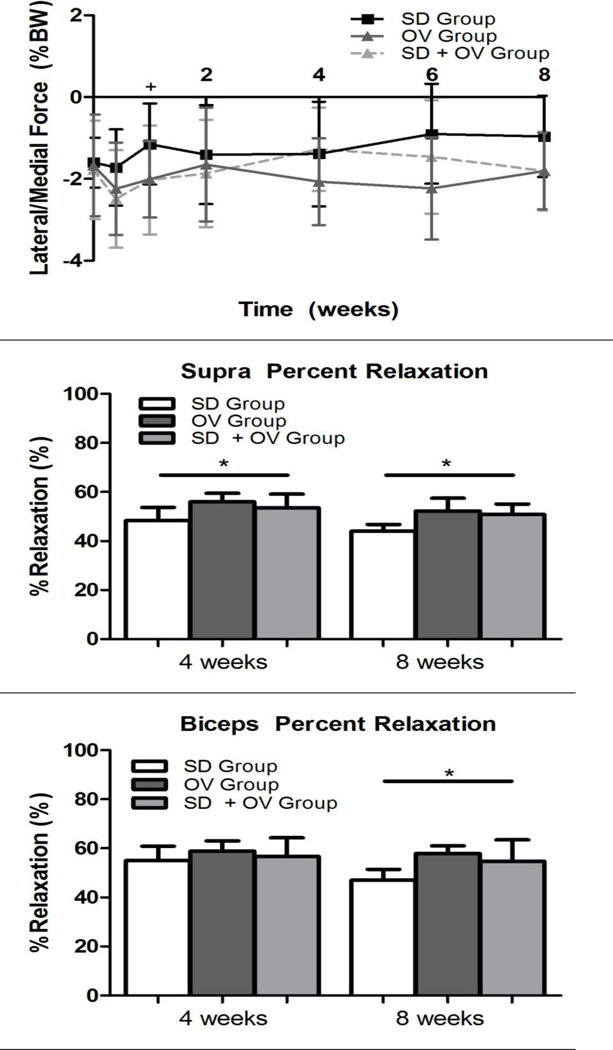
Shoulder function was significantly altered in the SD + OV group (Figure 1). Vertical force was significantly decreased in the SD + OV group compared to OV at 5 days, 2 weeks, and 6 weeks, with a similar trend at 8 weeks (Figure 1A). However, vertical force for the SD and SD + OV groups were not different at any time point. Propulsion force was significantly increased in the SD + OV group compared to OV at all time-points (Figure 1B). However, propulsion force for the SD and SD + OV groups were not different at any time point. Braking force was significantly decreased in the SD + OV group compared to OV at 5 days post-injury (Figure 1C). No other differences in braking force were observed between groups. Lastly, medial-lateral force was significantly more medial 1 week post-injury in the SD group compared to the SD + OV group (Figure 1D). No significant differences in spatio-temporal parameters (step width, stride length, or speed) were observed between groups.
Figure 1.
(A) The SD + OV group had decreased vertical force compared to OV at most time-points (except 1 and 4 weeks following injury). (B) The SD + OV group had increased propulsion force compared to OV at all time-points. (C) The SD + OV group had decreased braking force compared to OV 5 days following injury. (D) No difference in medial/lateral force was observed. Data is shown as mean ± standard deviation (p<0.025 (OV vs. SD + OV**), p<0.05 (OV vs. SD + OV++, SD vs. SD + OV+)).
Passive Joint Mechanics
Passive joint mechanics were altered in the SD + OV group (Table II). External rotation range of motion was significantly decreased in the SD + OV group compared to the SD group at 2 and 8 weeks post-injury and compared to the OV group 2 weeks post-injury. No other significant differences in passive joint mechanics were observed between groups.
Table II.
Passive Joint Mechanics
| Direction | Measurement | Time (wks) | SD | OV | SD + OV |
|---|---|---|---|---|---|
| Internal |
ROM (degrees) |
2 | 7.37±18.2 | 6.52±17.5 | 9.69±6.33 |
| 4 | −0.22±17.3 | 10.7±12.3 | 9.02±14.7 | ||
| 8 | 1.35±16.3 | 1.68±23.8 | 8.64±13.3 | ||
|
Toe Stiffness (N/mm) |
2 | 0.03±0.11 | 0.02±0.09 | 0.10±0.11 | |
| 4 | −0.01±0.11 | 0.04±0.10 | 0.05±0.12 | ||
| 8 | −0.02±0.12 | −0.07±0.17 | 0.04±0.14 | ||
|
Linear Stiffness (N/mm) |
2 | −0.16±0.12 | −0.11±0.18 | −0.06±0.19 | |
| 4 | −0.13±0.11 | −0.02±0.15 | −0.07±0.13 | ||
| 8 | −0.24±0.07 | −0.26±0.14 | −0.20±0.20 | ||
| External |
ROM (degrees) |
2 | 16.0±7.4 | 3.49±14.6 | −7.24±8.38*,** |
| 4 | −2.58±9.5 | 8.75±16.7 | 7.10±19.2 | ||
| 8 | 18.21±10.6 | 8.90±16.6 | 5.92±15.9* | ||
|
Toe Stiffness (N/mm) |
2 | 0.10±0.14 | 0.06±0.10 | 0.06±0.18 | |
| 4 | −0.06±0.19 | 0.03±0.11 | 0.03±0.14 | ||
| 8 | 0.04±0.13 | −0.01±0.14 | 0.06±0.16 | ||
|
Linear Stiffness (N/mm) |
2 | −0.04±0.20 | 0.05±0.16 | 0.06±0.19 | |
| 4 | 0.06±0.16 | 0.06±0.15 | −0.03±0.24 | ||
| 8 | −0.13±0.19 | −0.17±0.20 | −0.17±0.20 |
Results for passive joint mechanics demonstrated decreased external range of motion (ROM) in the SD + OV group compared to the SD group at 2 and 8 weeks and compared to the OV group at 2 weeks. Data is shown as normalized by baseline values (change from baseline) and as mean ± standard deviation (p<0.025 (SD vs. SD + OV*, OV vs. SD + OV**)).
Tendon Mechanics
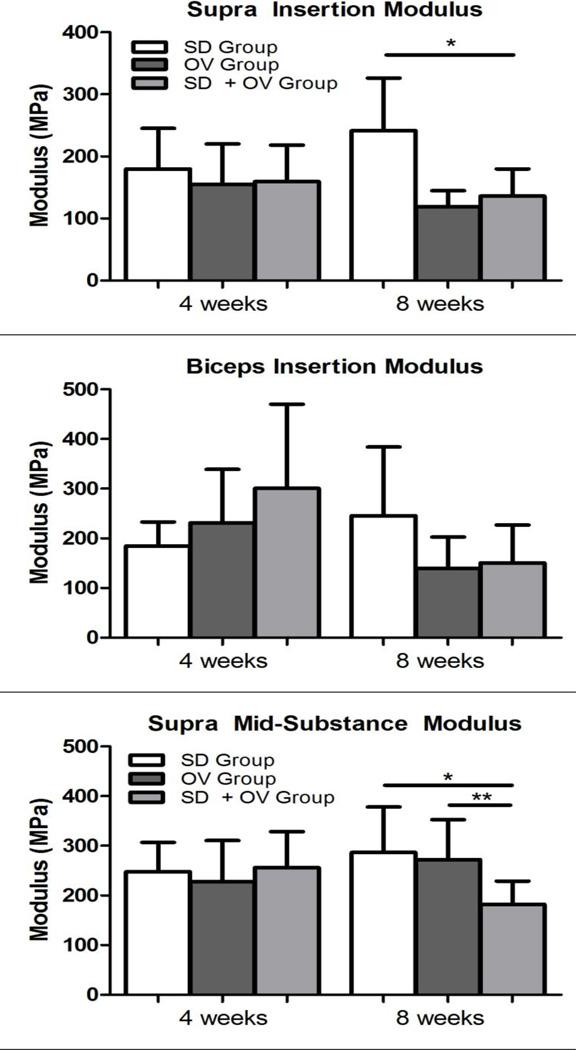
Mechanical parameters were significantly altered in the SD + OV group (Figures 2, 3, and 4). Tendon percent relaxation was significantly increased in the SD + OV group compared to SD for both the supraspinatus (at 4 and 8 weeks post-injury) and the biceps (at 8 weeks post-injury) (Figure 2). Additionally, supraspinatus tendon insertion modulus was significantly decreased in the SD + OV group compared to SD at 8 weeks post-injury (Figure 3A). However, no differences were observed at the biceps tendon insertion at either time-point (Figure 3B). Supraspinatus and biceps tendon mid-substance modulus were significantly decreased in the SD + OV group compared to the SD group and compared to the OV group at 8 weeks post-injury (Figure 4).
Figure 2.
(A) The supraspinatus tendon demonstrated increased percent relaxation at 4 and 8 weeks post-injury in the SD + OV group compared to SD. (B) The biceps tendon demonstrated increased percent relaxation at 8 weeks post-injury in the SD + OV group compared to SD. Data is shown as mean and standard deviation (p<0.025 (SD vs. SD + OV*)).
Figure 3.
(A) Supraspinatus insertion modulus was decreased at 8 weeks post-injury in the SD + OV group compared to SD. (B) No differences in biceps tendon insertion modulus was observed. Data is shown as mean and standard deviation (p<0.025 (SD vs. SD + OV*)).
Figure 4.
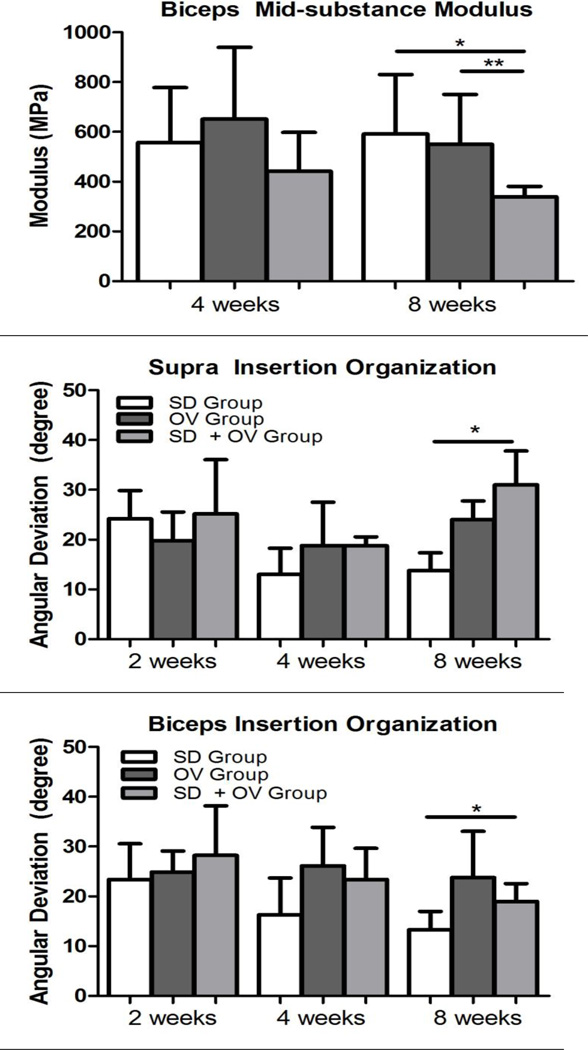
(A) Supraspinatus mid-substance modulus was decreased at 8 weeks post-injury in the SD + OV group compared to SD and compared to OV. (B) Biceps mid-substance modulus was decreased at 8 weeks post-injury in the SD + OV group compared to SD and compared to OV. Data is shown as mean and standard deviation (p<0.025 (SD vs. SD + OV*, OV vs. SD + OV**)).
Tendon Histology
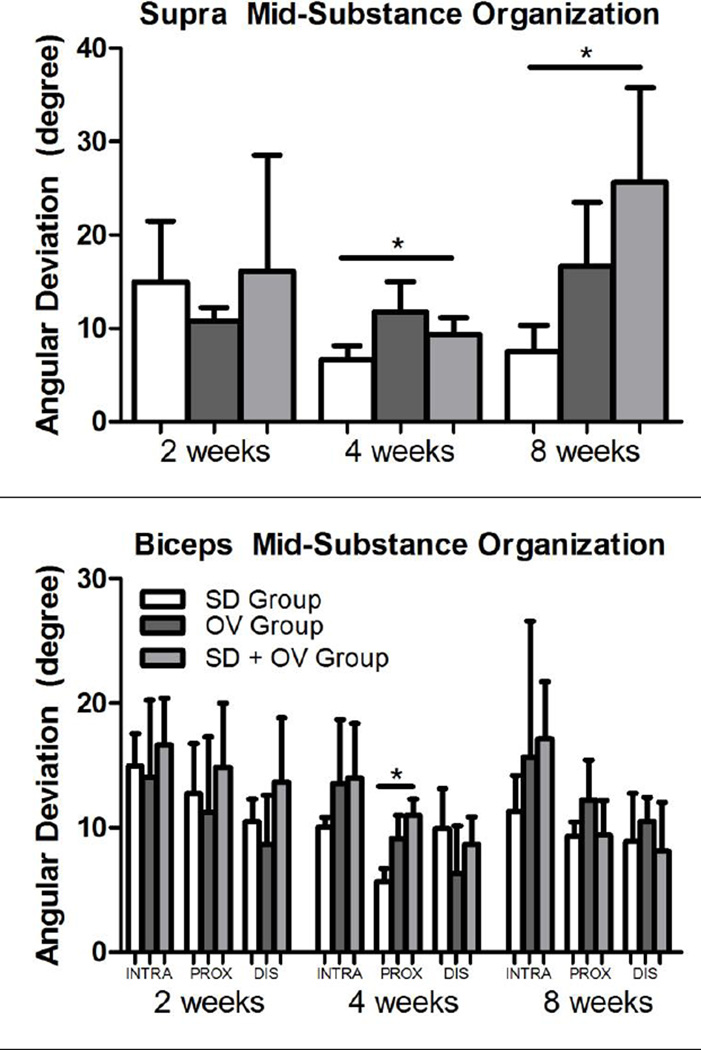
Tendon histology was significantly altered in the SD + OV group (S-Table I and Table II). Cell shape at the biceps distal groove was significantly less rounded in the SD + OV group compared to both the SD and OV groups at 4 weeks post-injury (S-Figure 1). Additionally, cell shape at the supraspinatus mid-substance was significantly more rounded in the SD + OV group compared to the SD group at 8 weeks post-injury (S-Figure 2). No other differences in cell shape were observed. Cell density was significantly increased at the supraspinatus insertion in the SD + OV group compared to the OV group at 8 weeks post-injury (S-Figure 3). No other differences in cell density were observed. Tissue organization was also significantly altered in the SD + OV group (Figure 5). Angular deviation was significantly increased (indicative of greater collagen disorganization) at the supraspinatus and biceps insertion at 8 weeks in the SD + OV group compared to the SD group (Figures 5A and 5B). Additionally, angular deviation was significantly increased at the supraspinatus mid-substance at both 4 and 8 weeks and at the biceps proximal groove at 4 weeks in the SD + OV group compared to SD (Figures 5C and 5D).
Figure 5.
(A) Supraspinatus insertion angular deviation was increased at 8 weeks post-injury in the SD + OV group compared to SD. (B) Biceps insertion angular deviation was increased at 8 weeks post-injury in the SD + OV group compared to SD. (C) Supraspinatus mid-substance angular deviation was increased at 4 and 8 weeks post-injury in the SD + OV group compared to SD. (D) Biceps proximal groove (PROX) angular deviation was increased at 4 weeks in the SD + OV group compared to SD. Data is shown as mean and standard deviation (p<0.025 (SD vs. SD + OV*)).
Tendon Immunohistochemistry
Tendon protein composition was also significantly altered in the SD + OV group (Table III and Table IV). Collagen II was significantly increased at the proximal groove of the biceps tendon at 4 weeks in the SD + OV group compared to the SD group (Table III). Collagen III was significantly decreased at the biceps insertion at 2 weeks and supraspinatus insertion and mid-substance at 4 weeks in the SD + OV group compared to the SD group. Additionally, collagen III was decreased at the proximal groove of the biceps tendon at 8 weeks in the SD + OV group compared to the OV group, with a similar trend compared to the SD group. Decorin was significantly decreased at the supraspinatus mid-substance at 8 weeks in the SD + OV group compared to the SD group (Table IV). However, decorin was significantly increased at the biceps tendon insertion, with a trend at the intra-articular space, at 4 weeks in the SD + OV group compared to the SD group. No differences in IL1-β were observed.
Table III.
Tendon Immunohistochemistry (Col II and Col III)
| Tendo n |
Gro up |
Regi on |
Col II | Col III | ||||
|---|---|---|---|---|---|---|---|---|
| 2 weeks | 4 weeks | 8 weeks | 2 weeks | 4 weeks |
8 weeks | |||
| Biceps | SD | INS | 2 (1–3) | 0(0–0) | 0(0–1) | 1.5 (1–2) | 1(1–2) | 2(2–2) |
| OV | 2 (1.5–2) | 1(1–2) | 1.5(0.75– 2) |
0 (0–0.5) | 2(1–2) | 2(2–2.25) | ||
| SD + OV |
1 (1–1) | 1(0–1) | 0(0–0.25) | 0 (0–0.5)* | 1(1–1) | 2(1–2) | ||
| SD | INT RA |
2 (2–3) | 0(0–0) | 1(0–1) | 2 (2–2) | 1(1–1) | 2(1–2) | |
| OV | 1 (0.5– 1.5) |
1(1–1.5) | 1(0.75– 1.25) |
0 (0–0.5) | 1(1–2) | 3(2.75–3) | ||
| SD + OV |
1 (0.75– 1) |
1(1–2) | 0(0–1) | 0 (0–0) | 1(1–1) | 2(1–2) | ||
| SD | PRO X |
1 (1–3) | 0(0–0) | 1(1–2) | 1(1–1.25) | 1(1–2) | 2(2–2) | |
| OV | 2 (1–2) | 1(1–2) | 1(0.75– 1.5) |
1(0.5–1) | 0(0–1) | 2(2–2.25) | ||
| SD + OV |
0.5 (0–1) | 1(1–1)* | 0(0–1) | 0(0–0.5) | 1(1–1) | 1(1–1)+,** | ||
| SD | DIS | 1 (1–2) | 1(0–1) | 1(0–1) | 0(0–0) | 1(1–2) | 1(0.75– 1.25) |
|
| OV | 1 (1–1) | 1(1–1) | 0(0–0.5) | 0(0–0.5) | 0(0–1) | 1.5(0.75– 2.25) |
||
| SD + OV |
1 (0.5– 1.5) |
1.5(0.75– 2) |
1(1–1) | 0(0–0.5) | 1(0–1) | 1(1–1) | ||
| Supra | SD | INS | 1 (1–2) | 1(1–2) | 2(1–3) | 0(0–0) | 2(1–2) | 2(2–2) |
| OV | 2 (1.5– 2.5) |
2(1–3) | 1(0.5–1) | 0.5(0–1.25) | 0(0– 0.25) |
3(2–3) | ||
| SD + OV |
1 (1–1.5) | 0(0–1) | 1(0–2) | 0(0–0) | 0(0–1)* | 2(2–2) | ||
| SD | MID | 1 (1–1) | 1(0–1) | 1(1–2) | 1(0.75– 1.25) |
1(1–2) | 2(2–2) | |
| OV | 1 (1–1) | 1(1–1) | 1(0.5–1.5) | 0.5(0–1) | 0(0–1) | 1(1–1.5) | ||
| SD + OV |
1 (1–1) | 0(0–0) | 0(0–0.5) | 0.5(0.25– 0.75) |
0(0–0)* | 2(1–2) | ||
At 4 weeks, collagen II staining was increased at the biceps proximal groove (PROX) in the SD + OV group compared to SD. Collagen III staining was decreased at the supraspinatus insertion (INS) and mid-substance (MID) in the SD + OV group compared to SD. At 8 weeks, collagen III staining was decreased at the biceps proximal groove in the SD + OV group compared to OV, with a similar trend compared to SD. Data is shown as median and interquartile range (p<0.025, (SD vs. SD + OV*), (OV vs. SD + OV**), p<0.05 (SD vs. SD+OV+)).
Table IV.
Tendon Immunohistochemistry (Decorin and IL1-β)
| Tendo n |
Gro up |
Regi on |
Decorin | IL1-β | ||||
|---|---|---|---|---|---|---|---|---|
| 2 weeks | 4 weeks | 8 weeks |
2 weeks | 4 weeks | 8 weeks | |||
| Biceps | SD | INS | 1(0.75– 1.25) |
1(1–1) | 2(2–3) | 2(2–2) | 1(1–2) | 2(2–2) |
| OV | 1(0.75–1) | 3(2–3) | 1.5(1– 2) |
1(1–1.25) | 1(1–1.5) | 1.5(1– 2.25) |
||
| SD + OV |
1(0.5–1.5) | 2(2–2)* | 1(1–2) | 1(1–1.25) | 1(1–1) | 2(1–3) | ||
| SD | INTR A |
1(0.5–1.5) | 1(1–2) | 1(1–2) | 2(1–3) | 1(1–2) | 2(2–2) | |
| OV | 1(1–1) | 3(2–3) | 1.5(1– 2) |
1(1–1) | 1.5(1– 2.25) |
2(1.75– 2.25) |
||
| SD + OV |
1(1–1.5) | 2(2–3)+ | 2(1–3) | 1(1–1.25) | 2(1–2) | 2(1.5–2.5) | ||
| SD | PRO X |
1(0.5–1) | 1(1–1) | 1(1–1) | 1(1–1.5) | 1(1–2) | 2(2–2) | |
| OV | 1(1–1) | 2.5(1.75– 3) |
2(1.75– 2) |
1(1–1.5) | 1(1–1.5) | 1(1–1) | ||
| SD + OV |
1(1–1) | 2.5(1.75– 3) |
1(1– 1.5) |
1.5(1–2) | 2(1–2) | 1(1–2) | ||
| SD | DIS | 1(0.5–1) | 1(1–2) | 2(2–2) | 1.5(1– 2.25) |
1(1–2) | 2.5(2–3) | |
| OV | 1(1–1.5) | 3(1.5–3) | 2(2– 2.5) |
1(1–1) | 1(1–1.5) | 1.5(1– 2.25) |
||
| SD + OV |
0(0–0.5) | 3(2.5–3) | 2(2–2) | 2(1.5– 2.5) |
1(1–1.5) | 2(2–2.25) | ||
| Supra | SD | INS | 1(1–1) | 3(2–3) | 2(2–2) | 2(1–2) | 2(2–2) | 3(3–3) |
| OV | 1(1–1) | 2.5(1.75– 3) |
3(2.5– 3) |
3(2–3) | 2(1.75–2) | 3(2.5–3) | ||
| SD + OV |
1.5(1.25– 1.75) |
2(2–3) | 1(1–2) | 1(1–2) | 2(2–2) | 2(2–3) | ||
| SD | MID | 1(1–1) | 3(2.75–3) | 3(3–3) | 1(1–1) | 1.5(1– 2.25) |
3(3–3) | |
| OV | 1(1–1) | 2(1.5–2) | 2(2– 2.5) |
1(1–1.5) | 2(1–2) | 2(1.5–2.5) | ||
| SD + OV |
1(1–1) | 2(1.75– 2.25) |
1.5(1– 2)* |
2(2–2.5) | 1(1–1) | 2(1–2) | ||
At 4 weeks, decorin staining was increased at the biceps insertion, with a similar trend at the intra-articular space (INTRA), in the SD + OV group compared to SD. At 8 weeks, decorin staining was decreased at the supraspinatus mid-substance in the SD + OV group compared to SD. No differences in IL1-β were observed. Data is shown as median and interquartile range (p<0.025, (SD vs. SD + OV*), (OV vs. SD + OV**), p<0.05 (SD vs. SD+OV+)).
Discussion
Overhead athletes and manual laborers commonly develop scapular dyskinesis and continue performing overuse activity, potentially placing their shoulders at increased risk of injury. However, the long term consequences of continuing overuse activity in the presence of abnormal scapulothoracic joint kinematics have not been fully elucidated. Using a variety of measurements, results from this study demonstrate that overuse activity in the presence of scapular dyskinesis is detrimental to shoulder properties and that overuse activity alone has a greater effect (more parameters altered) on shoulder properties than scapular dyskinesis alone (Table V). These results suggest that overuse activity in the presence of scapular dyskinesis likely contributes to shoulder injury.
Table V.
Results Summary
| Scapular Dyskinesis + Overuse vs. | ||
|---|---|---|
| Parameter | Scapular Dyskinesis | Overuse |
| Ground Reaction Force | ||
| Vertical force | NS | Day 5, 14, 48 |
| ↑Propulsion force | NS | All time points |
| ↓Braking force | NS | Day 5 |
| Medial/lateral force | NS | NS |
| Passive Joint Mechanics | ||
| ↑Range of Motion | Day 14, 64 (External) | Day 14 (External) |
| Toe Stiffness | NS | NS |
| Linear Stiffness | NS | NS |
| Tendon Mechanics | ||
| ↑Percent Relaxation | All time points (S) and at day 56 (B) | NS |
| ↓Insertion Modulus | Day 56 (S) | NS |
| ↓Mid Modulus | Day 56 (S+B) | Day 64 (S+B) |
| Tendon Histology | ||
| ↓Aspect Ratio | Day 28 (B) | Day 28 (B) |
| ↑Aspect Ratio | Day 56 (S) | NS |
| ↑Cell Density | NS | Day 56 (S) |
| ↓Insertion Organization | Day 56 (S+B) | NS |
| ↓Mid Organization | All time points (S) and at day 28 (B) | NS |
| Tendon Immunohistochemistry | ||
| ↑Col II | Day 28 (B) | NS |
| ↓Col III | Day 28 (S) | Day 56 (B) |
| ↑Decorin | Day 28 (B) | NS |
| ↓Decorin | Day 56 (S) | NS |
| ↑IL1-β | NS | NS |
Comparison of all parameters between groups (SD=scapular dyskinesis, NS=no significance, S=supraspinatus, B=biceps)
Results of this study demonstrated that overuse activity does not alter joint function (opposite H1) while scapular dyskinesis significantly alters shoulder function (consistent with H2). No differences were observed with the addition of overuse (SD vs. SD + OV) while vertical and braking force decreased and propulsion force increased with scapular dyskinesis (OV vs. SD + OV). The altered loading profile placed on the shoulder joint in the presence of scapular dyskinesis may relate to functional deficits and associated pain and could have a significant effect on tendon properties. The changes due to scapular dyskinesis are similar to those previously described.19 Alternatively, overuse activity did not diminish joint function. Therefore, we can conclude that the mechanical mechanism by which overuse activity alters tendon properties and by which scapular dyskinesis alters tendon properties are distinct.
Passive joint mechanics were significantly altered with the addition of overuse (SD vs. SD + OV). The addition of overuse activity in the SD + OV group resulted in decreased external rotation range of motion compared to SD alone (at two time points) and compared to OV alone (at one time point). Passive motion is often used as a clinical measure of shoulder range of motion and stiffness. Several factors can contribute to shoulder stiffness including tightening of the capsuloligamentous structures and/or surrounding musculature, arthritis, adhesion formation, and inflammation. In this study, we observed decreased external rotation range of motion, indicative of tightening of the anterior structures. The changes observed in this study could be related to increased reliance on the surrounding muscles (such as the subscapularis, pectoralis major, teres major, or the latissimus dorsi) in response to eccentric overuse activity and leading to subsequent tightening.16, 31 Alternatively, the addition of scapular dyskinesis in the presence of overuse (OV vs. SD + OV) had less of an effect on passive joint mechanics, except at 2 weeks post-injury. The initial insult of scapular dyskinesis may have had a significant effect on the secondary joint stabilizers, such as the joint capsule and surrounding musculature, leading to decreased range of motion. However, as both groups continued repetitive overuse activity, these changes were no longer apparent, suggesting that the effect of overuse may mask any underlying deficits due to scapular dyskinesis.
Consistent with our first hypothesis, overuse activity in the presence of scapular dyskinesis led to compromised tendon properties when compared to scapular dyskinesis alone (SD vs. SD + OV). Tendon percent relaxation was increased for both the supraspinatus and biceps tendons with the addition of overuse, consistent with previous findings for injured tendon.3 Additionally, supraspinatus insertion and mid-substance modulus and biceps mid-substance modulus were significantly diminished. Results for tendon organization support the mechanical changes observed. The supraspinatus and biceps insertion and mid-substance regions were more disorganized with the addition of overuse activity. In healthy tendon, collagen fibers typically align with the axis of loading. Alterations in loading (e.g. shear or compressive) could lead to a more random orientation of collagen fibers and diminish the mechanical integrity of the tendon as observed in this study (decreased viscoelastic and elastic properties). Additionally, the changes in collagen alignment may be related to microtrauma caused by mechanical compression and shear of the tendons due to repetitive excursion of the tendons under the acromion during overuse activity. Histologic changes in the tendon also supported the mechanical changes observed. A more rounded cell shape was observed in the supraspinatus mid-substance, consistent with a tendinopathic condition and likely indicative of altered cellular function (e.g., maintenance, repair, and remodeling of tendon matrix). Alternatively, cell shape was less rounded in the biceps tendon distal groove, which may indicate increased eccentric stretching and tensile loading in this region, which does not pass under the acromial arch, with overuse.
In order to further elucidate the mechanical changes observed with overuse, protein expression was evaluated. Overuse activity resulted in increased collagen II in the biceps tendon, decreased collagen III in both the supraspinatus and biceps tendons, and decreased and increased decorin in the supraspinatus and biceps. The increased presence of collagen II in tendon may be related to changes in the mechanical loading environment observed by the tendon (such as increased compressive or shear loading), leading to abnormal collagen metabolism and resulting in the subsequent mechanical changes in the tendon at later time points. The reduction in collagen III with overuse is surprising, as the presence of collagen III in tendon has been associated with tendon microtrauma and decreased mechanical strength.1, 4 However, in the supraspinatus and biceps, these findings were only observed early (at 4 weeks and 2 weeks, respectively) and were not present later (at 8 weeks). This alteration in collagen III is indicative of abnormal collagen metabolism. The ratio of collagen I to collagen III deposition is important in tendon and the initial decrease in collagen III may be a compensatory strategy to maintain proper coordination and synthesis of the collagen matrix. The increased decorin observed in the biceps tendon at 4 weeks may be related to the tendon’s initial response to changes in the mechanical loading environment and is consistent with the increased proteoglycan content observed with tendinopathy.7 Additionally, some in vitro studies have suggested that decorin inhibits collagen fibrillogenesis17, 28, which is important for proper tendon function and force transmission. Therefore, the increase in decorin which occurred at 4 weeks post-injury may alter collagen fibrillogenesis, which could explain the mechanical deficits at 8 weeks. The decreased decorin observed in the supraspinatus tendon at 8 weeks is consistent with mechanical changes (increased percent relaxation and decreased modulus) observed at the same time point and with previous studies that have demonstrated downregulation of decorin following tendon injury.1, 26
Consistent with our second hypothesis, scapular dyskinesis did further diminish tendon properties (OV vs. SD + OV). Supraspinatus and biceps mid-substance modulus were both diminished with the addition of scapular dyskinesis. However, the tendon changes due to scapular dyskinesis were not as prominent (only one mechanical parameter) as changes due to overuse (several mechanical parameters). Additionally, no differences in organization were observed with the addition of scapular dyskinesis. However, a few changes in histologic parameters (cell shape and cell density) were observed, with less rounded cell shape in the biceps distal groove and increased cell density at the supraspinatus insertion. Changes in protein were also observed with the addition of scapular dyskinesis. Decreased Collagen III was observed in the biceps tendon (OV vs. SD + OV), as similarly observed due to overuse. However, consistent with our mechanical and histological findings, immunohistochemical changes due to the addition of scapular dyskinesis were not as prominent as changes due to overuse alone. These findings indicate that overuse activity alone may have a greater effect on tendon properties than scapular dyskinesis alone. Future studies will implement additional biologic assays to quantify mRNA and protein expression in order to further characterize the biologic mechanisms involved in this tendon injury.
Results from this study demonstrate that overuse activity and abnormal scapular mechanics can each independently lead to tendon mechanical damage. However, the effect of overuse activity seems to be more substantial, with more parameters altered, then the effect of abnormal scapular mechanics. It is clear that the loading profile in each of these scenarios is different, as evidenced by differences in kinetic variables, which may explain why the effect on tendon properties is different. Scapular dyskinesis significantly altered joint mechanics while overuse activity did not. However, overuse activity resulted in significantly more structural and biological adaptations (such as decreased range of motion, diminished tendon properties, and altered matrix synthesis) than scapular dyskinesis alone. Overuse activity in the presence of scapular dyskinesis may be a more significant injury due to the extreme physiological demands placed on the joint. Clinically, asymmetric scapular motion is often present in healthy subjects (absent of symptoms),11, 13 suggesting that while scapular dyskinesis may be a risk factor, not everyone will develop significant shoulder injuries. Taken together, results from this study suggest that the risk for shoulder injury in patients with scapular dyskinesis may be higher in an active population.
This study has several limitations. First, we acknowledge that the use of an animal model does not exactly replicate the human condition. However, the rat shoulder has been well-established as an appropriate model for rotator cuff disease and the rat rotator cuff model has been cited over 200 times.22 Additionally, this model has been shown to reproducibly develop tendinopathy following repetitive overuse activity.24 Second, acute transection of the spinal accessory and long thoracic nerves to induce scapular dyskinesis does not exactly mimic the clinical scenario. However, this model successfully and consistently creates scapular dyskinesis. The cause and effect relationships addressed in this clinical scenario can only be studied in an animal model where time from injury and activity level can be controlled and tissues rigorously evaluated. Despite these limitations, results clearly and consistently demonstrate that overuse activity and scapular dyskinesis are detrimental to shoulder tendon properties.
Identification of overuse activity as a risk factor for shoulder injury in individuals with scapular dyskinesis will help inform and guide clinicians in developing prevention and treatment programs. Specifically, activity levels of athletes and laborers could be monitored in order to prevent permanent shoulder injury. Additionally, early detection and intervention programs can be developed, such as preventative neuromuscular training and scapular movement screens, which could assist in prevention of further detrimental changes due to scapular dyskinesis.
Supplementary Material
Tendon histology was quantified for cell shape for regions of the biceps. A representative image for the biceps distal groove is displayed. Cell shape was significantly less rounded in the SD + OV group (C) compared to SD (A) and compared to OV (B) at 4 weeks post-injury. Note: For publication, filters (auto-tone, auto-contrast, and auto-color) were individually applied to images.
Tendon histology was quantified for cell shape for regions of the supraspinatus. A representative image for the supraspinatus mid-substance is displayed. Cell shape was significantly more rounded in the SD + OV group (B) compared to SD (A) at 8 weeks post-injury. Note: For publication, filters (auto-tone, auto-contrast, and auto-color) were individually applied to images.
Tendon histology was quantified for cell density for regions of the supraspinatus and biceps. A representative image for the supraspinatus insertion is displayed. Cell density was significantly increased in the SD + OV group (B) compared to SD (A) at 8 weeks post-injury. Note: For publication, filters (auto-tone, auto-contrast, and auto-color) were individually applied to images.
Acknowledgements
The authors acknowledge Adam Pardes, Julianne Huegel, and Pankti Bhatt for their assistance with histology. The study was funded by NIH/NIAMS (R01AR056658) and the Penn Center for Musculoskeletal Disorders (P30AR050950).
References
- 1.Berglund M, Reno C, Hart DA, Wiig M. Patterns of mRNA Expression for Matrix Molecules and Growth Factors in Flexor Tendon Injury: Differences in the Regulation Between Tendon and Tendon Sheath. The Journal of Hand Surgery. 2006;31:1279–1287. doi: 10.1016/j.jhsa.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Clarsen B, Bahr R, Andersson S, Kristensen R, Myklebust G. Risk factors for overuse shoulder injuries among male professional handball players. Br J Sports Med. 2014;48:579. doi: 10.1136/bjsports-2014-093702. [DOI] [PubMed] [Google Scholar]
- 3.Dourte LM, Perry SM, Getz CL, Soslowsky LJ. Tendon properties remain altered in a chronic rat rotator cuff model. Clin Orthop Relat Res. 2010;468:1485–1492. doi: 10.1007/s11999-009-1206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eriksen HA, Pajala A, Leppilahti J, Risteli J. Increased content of type III collagen at the rupture site of human Achilles tendon. Journal of Orthopaedic Research. 2002;20:1352–1357. doi: 10.1016/S0736-0266(02)00064-5. [DOI] [PubMed] [Google Scholar]
- 5.Gimbel JA, Van Kleunen JP, Mehta S, Perry SM, Williams GR, Soslowsky LJ. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech. 2004;37:739–749. doi: 10.1016/j.jbiomech.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Green RA, Taylor NF, Watson L, Ardern C. Altered scapula position in elite young cricketers with shoulder problems. J Sci Med Sport. 2013;16:22–27. doi: 10.1016/j.jsams.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Joseph M, Maresh CM, McCarthy MB, Kraemer WJ, Ledgard F, Arciero CL, Anderson JM, Nindl BC, Mazzocca AD. Histological and molecular analysis of the biceps tendon long head post-tenotomy. Journal of Orthopaedic Research. 2009;27:1379–1385. doi: 10.1002/jor.20868. [DOI] [PubMed] [Google Scholar]
- 8.Kibler WB, McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11:142–151. doi: 10.5435/00124635-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Lo YP, Hsu YC, Chan KM. Epidemiology of shoulder impingement in upper arm sports events. Br J Sports Med. 1990;24:173–177. doi: 10.1136/bjsm.24.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludewig PM, Reynolds JF. The association of scapular kinematics and glenohumeral joint pathologies. J Orthop Sports Phys Ther. 2009;39:90–104. doi: 10.2519/jospt.2009.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morais NV, Pascoal AG. Scapular positioning assessment: is side-to-side comparison clinically acceptable? Man Ther. 2013;18:46–53. doi: 10.1016/j.math.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Myers JB, Laudner KG, Pasquale MR, Bradley JP, Lephart SM. Scapular position and orientation in throwing athletes. Am J Sports Med. 2005;33:263–271. doi: 10.1177/0363546504268138. [DOI] [PubMed] [Google Scholar]
- 13.Oyama S, Myers JB, Wassinger CA, Daniel Ricci R, Lephart SM. Asymmetric resting scapular posture in healthy overhead athletes. J Athl Train. 2008;43:565–570. doi: 10.4085/1062-6050-43.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peltz CD, Hsu JE, Zgonis MH, Trasolini NA, Glaser DL, Soslowsky LJ. Intra-articular changes precede extra-articular changes in the biceps tendon after rotator cuff tears in a rat model. J Shoulder Elbow Surg. 2012;21:873–881. doi: 10.1016/j.jse.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peltz CD, Perry SM, Getz CL, Soslowsky LJ. Mechanical properties of the long-head of the biceps tendon are altered in the presence of rotator cuff tears in a rat model. J Orthop Res. 2009;27:416–420. doi: 10.1002/jor.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proske U, Morgan DL. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001;537:333–345. doi: 10.1111/j.1469-7793.2001.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reese SP, Underwood CJ, Weiss JA. Effects of decorin proteoglycan on fibrillogenesis, ultrastructure, and mechanics of type I collagen gels. Matrix Biol. 2013;32:414–423. doi: 10.1016/j.matbio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reuther KE, Thomas SJ, Tucker JJ, Sarver JJ, Gray CF, Rooney SI, Glaser DL, Soslowsky LJ. Disruption of the anterior-posterior rotator cuff force balance alters joint function and leads to joint damage in a rat model. J Orthop Res. 2014;32:638–644. doi: 10.1002/jor.22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reuther KE, Thomas SJ, Tucker JJ, Yannascoli SM, Caro AC, Vafa RP, Liu SS, Gordon JA, Bhatt PR, Kuntz AF, Soslowsky LJ. Scapular Dyskinesis is Deterimental to Shoulder Tendon Properties and Joint Mechanics in a Rat Model. Journal of Orthopaedic Research. 2014 doi: 10.1002/jor.22693. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarver JJ, Dishowitz MI, Kim SY, Soslowsky LJ. Transient decreases in forelimb gait and ground reaction forces following rotator cuff injury and repair in a rat model. J Biomech. 2010;43:778–782. doi: 10.1016/j.jbiomech.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarver JJ, Peltz CD, Dourte L, Reddy S, Williams GR, Soslowsky LJ. After rotator cuff repair, stiffness--but not the loss in range of motion--increased transiently for immobilized shoulders in a rat model. J Shoulder Elbow Surg. 2008;17:108S–113S. doi: 10.1016/j.jse.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 23.Soslowsky LJ, Thomopoulos S, Esmail A, Flanagan CL, Iannotti JP, Williamson JD, 3rd, Carpenter JE. Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors. Ann Biomed Eng. 2002;30:1057–1063. doi: 10.1114/1.1509765. [DOI] [PubMed] [Google Scholar]
- 24.Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, Carpenter JE. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. Neer Award 1999. [PubMed] [Google Scholar]
- 25.Struyf F, Nijs J, Baeyens JP, Mottram S, Meeusen R. Scapular positioning and movement in unimpaired shoulders, shoulder impingement syndrome, and glenohumeral instability. Scand J Med Sci Sports. 2011;21:352–358. doi: 10.1111/j.1600-0838.2010.01274.x. [DOI] [PubMed] [Google Scholar]
- 26.Thomopoulos S, Hattersley G, Rosen V, Mertens M, Galatz L, Williams GR, Soslowsky LJ. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J Orthop Res. 2002;20:454–463. doi: 10.1016/S0736-0266(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 27.Timmons MK, Thigpen CA, Seitz AL, Karduna AR, Arnold BL, Michener LA. Scapular kinematics and subacromial-impingement syndrome: a meta-analysis. J Sport Rehabil. 21:354–370. doi: 10.1123/jsr.21.4.354. [DOI] [PubMed] [Google Scholar]
- 28.Vogel KG, Paulsson M, Heinegard D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984;223:587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wadsworth DJ, Bullock-Saxton JE. Recruitment patterns of the scapular rotator muscles in freestyle swimmers with subacromial impingement. Int J Sports Med. 1997;18:618–624. doi: 10.1055/s-2007-972692. [DOI] [PubMed] [Google Scholar]
- 30.Weldon EJ, 3rd, Richardson AB. Upper extremity overuse injuries in swimming. A discussion of swimmer’s shoulder. Clin Sports Med. 2001;20:423–438. doi: 10.1016/s0278-5919(05)70260-x. [DOI] [PubMed] [Google Scholar]
- 31.Whitehead NP, Weerakkody NS, Gregory JE, Morgan DL, Proske U. Changes in passive tension of muscle in humans and animals after eccentric exercise. J Physiol. 2001;533:593–604. doi: 10.1111/j.1469-7793.2001.0593a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tendon histology was quantified for cell shape for regions of the biceps. A representative image for the biceps distal groove is displayed. Cell shape was significantly less rounded in the SD + OV group (C) compared to SD (A) and compared to OV (B) at 4 weeks post-injury. Note: For publication, filters (auto-tone, auto-contrast, and auto-color) were individually applied to images.
Tendon histology was quantified for cell shape for regions of the supraspinatus. A representative image for the supraspinatus mid-substance is displayed. Cell shape was significantly more rounded in the SD + OV group (B) compared to SD (A) at 8 weeks post-injury. Note: For publication, filters (auto-tone, auto-contrast, and auto-color) were individually applied to images.
Tendon histology was quantified for cell density for regions of the supraspinatus and biceps. A representative image for the supraspinatus insertion is displayed. Cell density was significantly increased in the SD + OV group (B) compared to SD (A) at 8 weeks post-injury. Note: For publication, filters (auto-tone, auto-contrast, and auto-color) were individually applied to images.