Abstract
Multiple sclerosis and other demyelinating diseases in the pediatric population have received an increasing level of attention by clinicians and researchers. The low incidence of these diseases in children creates a need for the involvement of multiple clinical centers in research efforts. The Network of Pediatric Multiple Sclerosis Centers was created initially in 2006 to improve the diagnosis and care of children with demyelinating diseases. In 2010, the Network shifted its focus to multicenter research while continuing to advance the care of patients. The Network has obtained support from the National Multiple Sclerosis Society, the Guthy-Jackson Charitable Foundation, and the National Institutes of Health. The Network will continue to serve as a platform for conducting impactful research in pediatric demyelinating diseases of the central nervous system. This article provides a description of the history and development, organization, mission, research priorities, current studies, and future plans of the Network.
Keywords: multiple sclerosis, demyelinating disease, NPMSC, network, multicenter research
Background
The interest in pediatric multiple sclerosis has increased in recent years, as researchers, clinicians, and the general public have become more aware that the disease can affect individuals prior to age 18 years. Furthermore, the frequency of known cases of pediatric multiple sclerosis has increased in recent decades, due either to an increase in actual incidence of the disease or to increased recognition and diagnosis.1 Despite this recent increase, multiple sclerosis in children remains highly uncommon. Diagnosis of multiple sclerosis in children is challenging, hindered in part by numerous potential differential diagnoses in young ages.2 The current understanding of pediatric multiple sclerosis indicates a narrow window of onset with unique cognitive decline,3 and a higher burden of relapses and inflammation relative to adult multiple sclerosis.4 Additionally, the United States pediatric multiple sclerosis population shows greater racial and ethnic diversity compared to adult multiple sclerosis and to pediatric multiple sclerosis outside of the United States.5 These characteristics invite expansion into new research areas that are best pursued through multi-institutional collaborations. In response to the need for large-scale collaborations, efforts have been made, including the initiation of an International Pediatric MS Study Group in 2002 (www.ipmssg.org) and a United States Pediatric Multiple Sclerosis Network in 2006.
In this article, we discuss the efforts of the United States Network of Pediatric Multiple Sclerosis Centers. The mission of the Network is to uncover the key pathogenic mechanisms underlying multiple sclerosis through the study of pediatric patients. The Network holds the potential to advance the understanding of multiple sclerosis disease processes by investigation of the unique features of multiple sclerosis in children and adolescents. Through ongoing studies, the Network is measuring clinical, environmental, and cognitive manifestations of early onset multiple sclerosis and growing the largest collection of well-characterized pediatric multiple sclerosis cases in the world.
Development and Current Structure of the Network
The National Multiple Sclerosis Society founded a nationwide network of pediatric multiple sclerosis centers of excellence in 2006 to establish recognition of pediatric multiple sclerosis and provide comprehensive evaluation and care to children with multiple sclerosis and related demyelinating disorders. At inception the Network was composed of six clinical centers. The clinical center investigators have been meeting regularly since 2006 to develop clinical care systems and explore possible research activities. Initial Network goals to enhance diagnosis and care of children with multiple sclerosis and related disorders and to develop resources for families, healthcare professionals, and the public were readily accomplished. The initial effort to collect data to enable large-scale research initiatives highlighted the specific needs of a multicenter research consortium, consistent protocols, uniform data entry, and appropriate institutional review board approvals. To develop an effective patient registry and facilitate the transition of the centers to a research network the Data Coordinating and Analysis Center at the University of Utah joined the Network in December 2010. The Data Coordinating and Analysis Center presence brought biostatistical, data management, and multi-center pediatric clinical study expertise to existing and future research efforts.
The Network has subsequently made remarkable progress toward becoming a functional, productive research network aimed at conducting high-priority research in pediatric multiple sclerosis. The Network Steering Committee currently conducts monthly conference calls and organizes three in-person meetings per year to develop and implement Network projects. The Network has accomplished several essential steps the past three and a half years including: establishment of specific research priorities to guide Network projects, development of governance policies to formalize Network operations, and initiation of four active research projects.
An immediate goal of the Network upon incorporating the Data Coordinating and Analysis Center into the Network was to operationalize two funded studies and begin enrolling subjects: the “Pediatric Multiple Sclerosis and other Demyelinating Diseases” database and the “Environmental and Genetic Risk Factors for Pediatric Multiple Sclerosis” study (R01NS071463). All Network clinical centers participate in both studies and both studies continue to accrue subjects at a steady pace.
Along with enrolling in these two studies, the Network has defined a research plan and filled the project pipeline with new projects in the Network’s priority research areas. The Steering Committee established a new concept-to-grant process in early 2011 that has been highly effective in initiating new Network projects. To date, this process has yielded two funded grants, three submitted grants, and three projects actively in the concept and protocol phases. Additionally, six concepts utilizing this process were not moved forward by the Steering Committee and one grant was funded for an external pilot study. The concept development process assures rapid startup of research activities and continuous funding resulting in presentations at national and international meetings and culminating in publications.
The Network has initiated the development of a program to incorporate new clinical sites into the Network. The Steering Committee deemed the external sites participating in the “Environmental and Genetic Risk Factors for Pediatric MS” study that successfully obtained approval for multi-institutional studies and demonstrated the ability to enroll subjects as eligible for inclusion in the Network. Principal Investigators from three of these centers expressed interest in joining the Network and were invited to join Steering Committee meetings and submit concepts as a first step in integrating new sites. These three centers were officially added to the Network in 2012.
The Network has demonstrated devotion to training future generations of researchers. Since the Network was established in 2006, Principal Investigators have mentored several fellows. Many of these fellows have continued on to establish pediatric multiple sclerosis clinics at their institutions and proven to be effective researchers in pediatric multiple sclerosis through funded projects, publications, and presentations contributing to understanding the disease. Publications and presentations produced by the Network have steadily increased since the Network was formed in 2006. To date, over 80 peer-reviewed pediatric multiple sclerosis publications and over 100 presentations have been prepared by the Network Principal Investigators. The full Network has collaborated on 11 publications and has incorporated several manuscripts into their research plan going forward. Network investigators also contributed to the first major textbook on demyelinating disorders of the central nervous system in childhood published in 20116 and have developed a standardized MRI protocol and neuropsychological battery to enhance research efforts. Several manuscripts that will utilize the high-quality aggregate data now available in the core database have been proposed and are being developed by the Network.
Clinical Centers
Network clinical center Principal Investigators all have experience in clinical research, either as lead investigators of multi-institutional studies or as single-site investigators. Each Principal Investigator has a strong publication and funding record with research expertise spanning clinical laboratory basic science research through multi-center clinical trials in both adult and pediatric multiple sclerosis. The clinical centers all have direct access and affiliations with adult multiple sclerosis clinics. Clinical centers were chosen based on their ability to participate in the Network clinical research programs, the number of children seen each year, and the ability to contribute to the science of pediatric multiple sclerosis. The Network clinical centers have diverse expertise that covers many of the important themes in multiple sclerosis research specified by the National Multiple Sclerosis Society:
Immunologic basis of multiple sclerosis
Central nervous system repair and neuroprotection
Biology of glia/myelin/neurons and axons
Genetics and gender differences
Understanding and preventing disease progression
Infectious triggers and risk factors
Cognitive and psychosocial issues
Patient management, care, and rehabilitation
Pathology of multiple sclerosis
Clinical trials
Health care delivery and policy
Measures of disease activity, imaging, surrogates, and biomarkers
Each clinical center has at least one Principal Investigator and one Research Coordinator designated specifically to the Network. The role of the clinical center Principal Investigator is to supervise and oversee their clinical center performance, supervise clinical center research personnel, contribute to development of new concepts and protocols, ensure adherence to study protocols, participate in analysis and publications, and ensure local site institutional review board approval of all studies. The Network Research Coordinators are primarily charged with implementation of Network projects at each clinical center. Responsibilities include recruitment of study patients, completion of case report forms and study files, preparation of institutional review board applications, reporting on study progress, ensuring high-quality data, evaluation of new protocols for feasibility, and assistance with protocol development, among others.
Data Coordinating and Analysis Center
The Data Coordinating and Analysis Center is located in the Department of Pediatrics in the Division of Critical Care at the University of Utah. The Center houses multiple research networks for pediatric emergency medicine, critical care, hydrocephalus, and demyelinating disorders. The Center serves as the project management, data management, and biostatistical core for the Network. Resources available include study design and management, database development, statistical analysis, manuscript preparation, meeting coordination and hosting, among others. In addition, the Data Coordinating and Analysis Center is the prime awardee for the Network infrastructure grant and subcontracts to Network clinical centers for financial matters. The Center team currently consists of two Principal Investigators (biostatistics and multiple sclerosis expertise), a program director, a project manager, a clinical data manager, statisticians, and other auxiliary personnel.
Steering Committee
The Steering Committee is the executive entity for the Network and consists of 1–2 investigators from each of the clinical centers and the Data Coordinating and Analysis Center. Each center is allotted one vote when voting is required, such as for concepts, protocols, or grants, or when new policies are implemented.
A Steering Committee Chair is nominated and voted upon by the Steering Committee. The Chair leads in-person Steering Committee meetings and oversees Network development and research priorities. The Chair also represents the Network at national/international meetings. An Associate Chair is also nominated and voted upon by the Steering Committee. This individual assists the Chair with Network development, research priorities, and strategic direction of the Network. The term of both the Chair and Associate Chair positions is 2 years.
There are currently five Network subcommittees that report to the Steering Committee. The first is the strategic development subcommittee, comprised of senior investigators and charged with discussing and providing recommendations regarding the prioritization of Network activities and use of Network resources. The second is the Feasibility and Budget Subcommittee. This subcommittee helps to ensure that new projects plan for adequate support of additional resources beyond those provided by the Network infrastructure. The third committee engages new investigators and the final two subcommittees are the MRI repository and Bio-repository subcommittees. Their charge is to explore options for imaging and biological sample storage for use in Network research projects. This involves evaluating both what is needed to set up such repositories and the work that would be required at the clinical centers and the Data Coordinating and Analysis Center in order to maintain and contribute to these resources. Subcommittees have no power to take action on behalf of the Network, but rather report to the Steering Committee in an advisory capacity.
Project Development
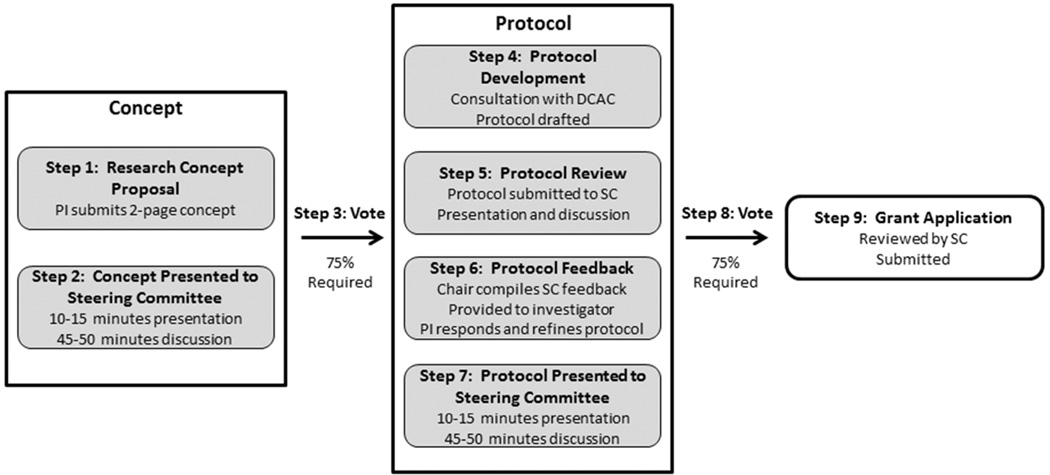
The Network has a process for investigators to bring research ideas to the Network and develop these into protocols and grant applications for extramural funding. This allows for full Network collaboration on projects, beginning at or close to their inception. It also provides extra layers of review and feedback prior to decisive reviews by funding agencies. The concept to grant process is presented in Figure 1.
Figure 1.
Concept-to-grant process in the US Network of Pediatric Multiple Sclerosis Centers. PI=principal investigator; DCAC=data coordinating and analysis center; SC=steering committee.
An investigator with a research concept drafts an initial written concept proposal and submits to the Chair to have the project assigned to an in-person meeting for review. The investigator submits a 2-page concept summary to the Steering Committee. The concept is then presented to the Steering Committee for review. The paper and presentation should address the importance of the topic, the proposed study methods, the population to be studied, approximate sample size requirements, and funding options. The purpose of the presentation is to encourage scientific dialogue, to develop a broad understanding of the proposal among Network members, and to review and consider the scientific merit.
The presentation is followed by a discussion and a vote by secret ballot to determine whether or not the concept should be endorsed for further development into a Network protocol. One vote per center is allowed. A 75% majority vote is required for concept endorsement. The majority required will ensure that there is not only recognition of scientific merit, but wide enthusiasm for developing the concept into a research protocol for implementation in the Network. Concepts that receive 50–74% approval may be revised and resubmitted for discussion and reconsideration at a future meeting at the discretion of the investigator. Concepts receiving less than 50% approval will not be reconsidered.
Once the research concept is approved, the investigator identifies a group of co-investigators or collaborators, and with the help of this group, the concept is developed into a research protocol. The protocol can take the format of a standard protocol, as might be submitted to an IRB, or of a grant application. Irrespective of the format, the protocol/grant provides details about the proposed study so that the Network may assess scientific merit and feasibility. The protocol includes a preliminary budget and budget narrative. After the protocol is preliminarily developed by the study group, investigators are required to meet (preferably in person) with the Data Coordinating and Analysis Center to further revise the protocol.
The protocol is submitted to the Network for a scientific review at least four weeks prior to a Steering Committee meeting. Network members conduct a detailed review of the protocol. The protocol is presented and discussed at the Steering Committee meeting. The purpose of this review and discussion is to provide constructive feedback to the investigator and the study group. After receiving the reviews, the investigator will make appropriate revisions to the protocol. The investigator must submit the revised protocol at least 2 weeks prior to the next Steering Committee meeting, highlighting where substantive changes were made, particularly in regard to feedback from the previous meeting.
The investigator also submits point-by-point responses to the major comments from the previous meeting, as one would do in resubmission of a manuscript. It is the responsibility of the Chair to assess the revised protocol and identify any remaining issues. The Steering Committee then reviews the protocol and related documents before the vote on the protocol.
The investigator presents the revised protocol at a Steering Committee meeting. The investigator summarizes the goals, aims, methods and other aspects of the protocol in detail. Following the presentation and subsequent discussion, a vote is held by secret ballot. Protocols receiving 75% support are approved for further development of a grant application. Protocols receiving 50%–75% approval may be revised and resubmitted at the following Steering Committee meeting. Protocols receiving less than 50% approval are not considered further for Network implementation.
If Network approval is granted and the appropriate funding is successfully obtained, then the project is implemented by the Network. The original proposing investigator becomes the study Principal Investigator and takes responsibility for all aspects of the research. With the Data Coordinating and Analysis Center, they prepare the protocol for institutional review board submission at all centers. The study Principal Investigator leads training efforts and communicates with the Data Coordinating and Analysis Center on data collection, management, and analysis issues.
Goals and Priorities
Strategic planning for an infrastructure to support research in pediatric demyelinating diseases requires consensus on specific research topic areas that have the highest priority. The Network has developed research priorities that function to guide the research going forward. The Network refined and approved four research priorities:
Determine etiology and predictors of acquired pediatric demyelinating diseases
Define causes and consequences of cognitive dysfunction in pediatric multiple sclerosis
Identify determinants of disability accrual in pediatric multiple sclerosis
Identify distinct injury and repair mechanisms in younger patients to better treat multiple sclerosis
Priority 1-Determine etiology and predictors of acquired pediatric demyelinating diseases
The causes of pediatric demyelinating diseases remain unknown and likely differ among disease types. Studies in adults have provided evidence of both genetic and environmental risk factors for development of multiple sclerosis and other demyelinating diseases, but environmental risk factors remain elusive. Research in children has been limited due, in part, to the rarity of these diseases in the pediatric population. Investigating possible causes of a disorder in a younger population has the potential advantage of fewer confounding factors, and the potential to more reliably identify risk factors, as these exposures have occurred within a shorter time frame.7 In addition, observing patients at an age closer to true disease onset implies that there have been fewer peripheral exposures and more readily identified immune responses. Identification of risk factors associated with demyelinating diseases will lead to more effective preventative and therapeutic strategies.8
Priority 2-Define causes and consequences of cognitive dysfunction in pediatric multiple sclerosis
Cognitive impairment is a troubling and frequently disabling problem that affects approximately half of all adults with multiple sclerosis.9 In pediatric multiple sclerosis, cognitive problems remain poorly understood.3 In children, who typically have an increased relapse rate compared to adults, the effects of cognitive impairment during the critical school-age development years may be substantial and have devastating consequences on the development of a successful adult life. Characterizing cognitive problems and their course in the pediatric multiple sclerosis population will help inform educators, educational system administrators, and clinicians in the design of interventions that could improve the lives of these children and their achievements as young adults. In addition, as cognitive dysfunction is more readily detected in pediatric multiple sclerosis, it may inform on causative disease processes.
Priority 3-Identify determinants of disability accrual in pediatric multiple sclerosis
While disability accrual due to multiple sclerosis can be slower in the pediatric population, early-onset multiple sclerosis leads to a longer period of disease exposure in which cumulative effects can be substantial. Accumulation of both physical and cognitive disabilities limits the later capabilities of pediatric multiple sclerosis patients. Limitations in early adulthood leave these individuals particularly vulnerable in multiple facets, including personal development, education, employment, and access to health insurance. Studies comparing pediatric-onset multiple sclerosis to adult-onset multiple sclerosis patients can provide new insights into the effects of age in modifying disability accrual, and may identify new therapeutic targets.
Priority 4-Identify distinct injury and repair mechanisms in younger patients to better treat multiple sclerosis
Pediatric multiple sclerosis patients differ from their adult counterparts in timing and characterization of injury to the central nervous system and in recovery from those injuries. The parameters of both destructive and regenerative mechanisms must be thoroughly investigated in children, as specific processes that exist in childhood may help develop new therapeutic strategies for multiple sclerosis in general. Studies in children may provide new insights into neuroimmunological mechanisms of early disease, and modifying factors. The outcomes that are used in adult multiple sclerosis research are often applied to studies in children, yet many of these measures have not been validated in children. Some therapeutics strategies may be best first tested in the pediatric multiple sclerosis population rather than the common first application in adult multiple sclerosis, based on the concept that repair mechanism may be more robust earlier in life.
Current Studies of the NPMSC
Pediatric Multiple Sclerosis and Other Demyelinating Diseases Database
This study, funded by the National Multiple Sclerosis Society includes patients with suspected onset of demyelinating disease of the central nervous system prior to age 18. The purposes of the database are to describe the number and characteristics of patients with suspected early onset of demyelinating disease and to support hypothesis generation and study design development for clinical trials and observational studies to be carried out by the Network. Information is collected and entered into a web-based electronic data capture system. Categories of data elements collected are enrollment, registration (demographics, family and medical history, vaccinations), history of disease (medications, blood tests, other biological samples, MRIs), clinic visits (diagnosis, Neurostatus expanded disability status scale, growth), events/relapses (symptoms, localization, disease onset), and neuropsychological testing. Important features of the registry include (a) uniformity in data collection and submission, (b) careful monitoring of the data and queries for data discrepancies, and (c) a process for access to individual center data for Network investigators, among others.
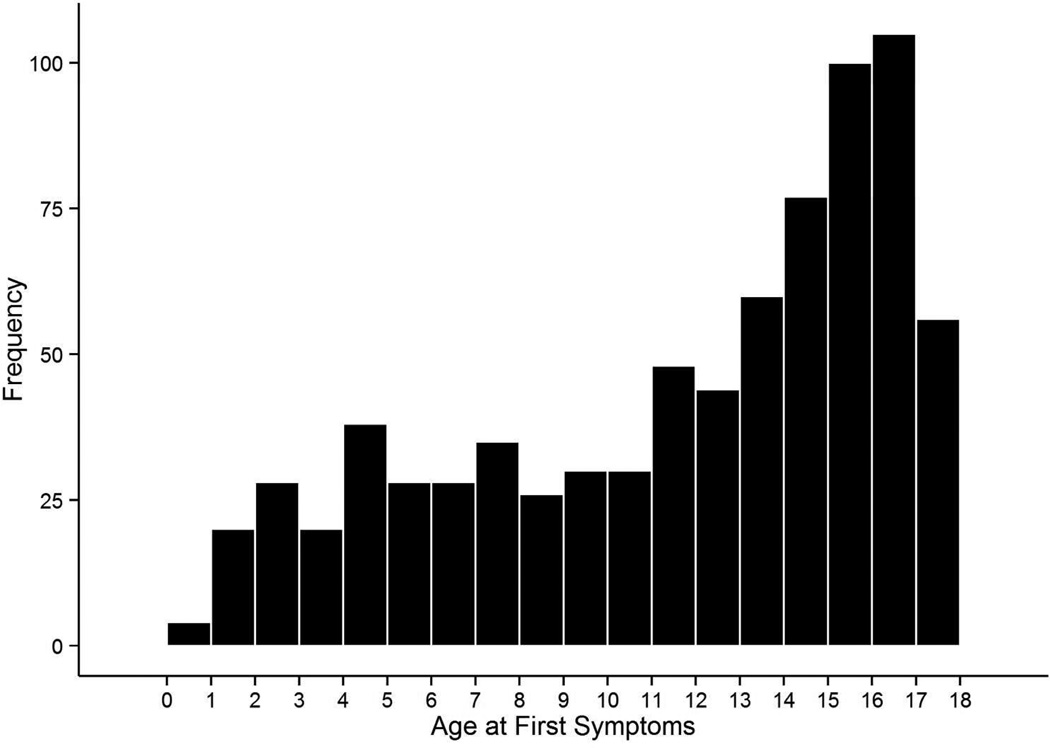
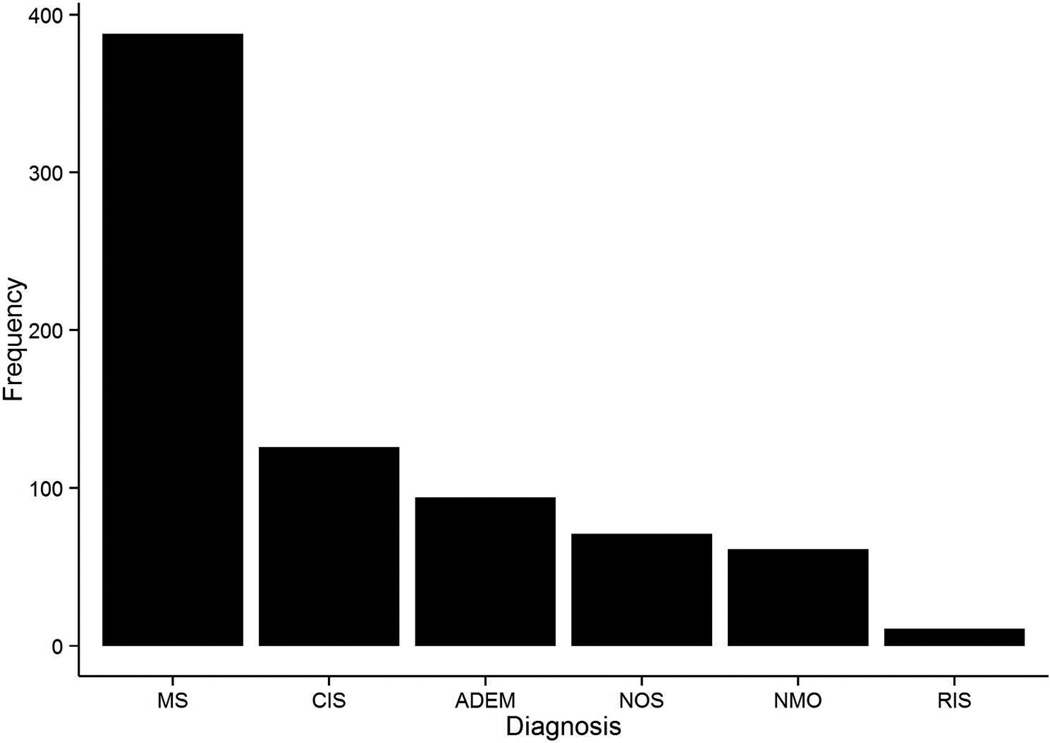
Enrollment of subjects into the database began in May 2011. As of December 31, 2013, a total of 979 patients had been enrolled, and 751 of these had a diagnosis of demyelinating disease. Enrollment is predominantly female (62.5%). The distribution of age at onset is shown in Figure 2. The breakdown of most recent diagnosis is shown in Figure 3, with multiple sclerosis as the most common diagnosis (N=388), followed by clinically isolated syndrome (N=126), acute disseminated encephalomyelitis (N=94), demyelinating disease not otherwise specified (N=71), neuromyelitis optica (N=61), and radiologically isolated syndrome (N=11). Detailed information was collected at a total of 2,617 clinic visits and histories include approximately 1,758 demyelinating attacks. Data from the database have been used to show feasibility and provide preliminary analyses for Network grants that have been submitted. Currently, data are being compiled into analysis datasets for Network manuscripts that are in progress.
Figure 2.
Distribution of age at first symptoms for patients with suspected demyelinating disease enrolled in the Pediatric Multiple Sclerosis and other Demyelinating Diseases database.
Figure 3.
Breakdown of most recent diagnosis for demyelinating disease patients in the Pediatric Multiple Sclerosis and other Demyelinating Diseases database. MS=multiple sclerosis (n=388); CIS=clinically isolated syndrome (n=126); ADEM=acute disseminated encephalomyelitis (n=94); NOS=demyelinating disease not otherwise specified (n=71); NMO=neuromyelitis optica (n=61); RIS=radiologically isolated syndrome (n=11)
Environmental and Genetic Risk Factors for Pediatric Multiple Sclerosis
The primary objective of this Network study, funded by the National Institute of Neurological Disorders and Stroke (R01NS071463), is to determine if risk factors identified for adult multiple sclerosis such as HLA-DRB1 1501/1503, Epstein-Barr virus, 25(OH) vitamin D insufficiency, and exposure to cigarette smoke are also risk factors for pediatric multiple sclerosis, and if there are interactions between them. This objective will be addressed by enrolling 640 early pediatric-onset multiple sclerosis cases and 1280 matched controls. Several additional environmental factors are also being collected through a questionnaire which can be filled out by families on-line. The study also involves a separate validated diet survey. Fourteen sites are now enrolling in the study. As of June 15, 2014, over 240 cases and over 400 controls have been enrolled.
Microbiomes in Pediatric Multiple Sclerosis
The overarching goal of this study (National Multiple Sclerosis Society, RG 4861A13) is to advance the understanding of how commensal bacteria are associated with multiple sclerosis. The longer term objectives of this research are to understand whether the microbiomes modify the course of multiple sclerosis and to develop preventative and therapeutic strategies. The ongoing study of risk factors in pediatric multiple sclerosis will be leveraged to collecting nasal, oropharyngeal, and stool samples in 70 cases and 70 matched controls as a pilot study of microbiota. Examining children with the disease overcomes the confounders introduced from the study of an adult patient population that over the years has had multiple exposures irrelevant to disease onset and that have altered their microbiota. The study is currently being initiated at seven centers and 3 cases have been enrolled.
NMO SPECTRUM
This study was awarded funding by the Guthy-Jackson Charitable Foundation. This study will compare the clinical and radiological presentation and course of pediatric-onset neuromyelitis optica spectrum disorders to other demyelinating disorders using the core database. These data will lead to an improved understanding of the presenting features of neuromyelitis optica in children and enhanced diagnosis and treatment of this disorder. A final objective of the study is to summarize current collection of biological samples in pediatric neuromyelitis optica patients. Cases identified as potentially meeting criteria for neuromyelitis optica, relapsing-remitting multiple sclerosis, or acute disseminated encephalomyelitis have been reviewed by an expert panel and classified for analysis. The analysis compares features between the three groups, as well as examines the features of other patients identified as possible neuromyelitis optica cases.
Summary and Conclusions
The United States Network of Pediatric Multiple Sclerosis Centers is now established as an independent entity with research support from the National Multiple Sclerosis Society, National Institutes of Health, and the Guthy-Jackson Charitable Foundation. The Network has developed and defined the structure and functions to facilitate multicenter research. The Network has established four central research priorities. Ongoing studies include the core Pediatric Multiple Sclerosis and Other Demyelinating Diseases database, Environmental and Genetic Risk Factors, Microbiomes and NMO SPECTRUM projects. These projects should help define the nature of pediatric demyelinating diseases and begin to define the pathogenesis of these diseases. Multiple additional concepts and proposals are in development. Future objectives are to 1) foster a productive pipeline of funded research addressing the established priorities, 2) develop pathways for development of new investigators, and 3) expansion of the Network to facilitate its research, clinical care, and educational missions.
Acknowledgements
Participating centers (with collaborators) are listed below in alphabetical order: Children's Hospital of Alabama (J. Ness, Y. Harris), Boston Children’s Hospital (M. Gorman, L. Benson), Loma Linda University Medical Center (G. Aaen), Massachusetts General Hospital for Children (T. Chitnis), Mayo Clinic (J. Tillema, J. M. Rodriguez), State University of New York at Stony Brook (L. Krupp, A. Belman), Texas Children’s Hospital (T. Lotze), University of Buffalo (B. Weinstock-Guttman, O. Farooq), University of California at San Francisco (E. Waubant, J. Graves), University of Utah Data Coordinating and Analysis Center (T. C. Casper, J. Rose, S. Roalstad, T. Hunt, C. Olsen, T. Simmons, W. Weber, B. Brown, E. Roan).
Funding (Financial Disclosure)
This work was funded by the National Multiple Sclerosis Society (HC 0165). Current projects are funded by the National Institutes of Health (R01NS071463), the National Multiple Sclerosis Society (RG 4861A13), and the Guthy-Jackson Charitable Foundation (Eureka Grant).
Footnotes
Author Contribution (Roles)
TCC, SR and JR wrote the first draft of the article. All authors contributed to the development of the network and reviewed and edited the article.
Declaration of Conflicting Interests
Nothing to declare
Ethical Approval
The Pediatric Multiple Sclerosis and Other Demyelinating Diseases database, as well as all other studies mentioned in this article, were reviewed and approved by each participating center’s institutional review board. Informed consent was obtained as required by each center’s review board.
References
- 1.Chitnis T, Krupp L, Yeh A, et al. Pediatric multiple sclerosis. Neurol Clin. 2011;29(2):481–505. doi: 10.1016/j.ncl.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Waubant E, Chabas D. Pediatric multiple sclerosis. Curr Treat Options Neurol. 2009;11(3):203–210. doi: 10.1007/s11940-009-0024-6. [DOI] [PubMed] [Google Scholar]
- 3.Julian L, Serafin D, Charvet L, et al. Cognitive impairment occurs in children and adolescents with multiple sclerosis: results from a united states network. J Child Neurol. 2013;28(1):102–107. doi: 10.1177/0883073812464816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waubant E, Chabas D, Okuda DT, et al. Difference in disease burden and activity in pediatric patients on brain magnetic resonance imaging at time of multiple sclerosis onset vs adults. Archives of neurology. 2009;66(8):967–971. doi: 10.1001/archneurol.2009.135. [DOI] [PubMed] [Google Scholar]
- 5.Chitnis T, Glanz B, Jaffin S, Healy B. Demographics of pediatric-onset multiple sclerosis in an MS center population from the Northeastern United States. Multiple Sclerosis. 2009;15(5):627–631. doi: 10.1177/1352458508101933. [DOI] [PubMed] [Google Scholar]
- 6.Chabas D, Waubant E, editors. Demyelinating Disorders of the Central Nervous System in Childhood. New York: Cambridge University Press; 2011. [Google Scholar]
- 7.Vargas-Lowy D, Chitnis T. Pathogenesis of Pediatric Multiple Sclerosis. J Child Neurol. 2012;27(11):1394–1407. doi: 10.1177/0883073812456084. [DOI] [PubMed] [Google Scholar]
- 8.Munger KL, Chitnis T, Frazier AL, et al. Dietary intake of vitamin D during adolescence and risk of multiple sclerosis. J Neurol. 2011;258(3):479–485. doi: 10.1007/s00415-010-5783-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao S, Leo G, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis: frequency, patterns, and predictions. Neurology. 1991;41:385–691. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]