Abstract
The Ediacaran fossil Corumbella is important because it is hypothesized to be a scyphozoan cnidarian, and thus might be one of the rare examples of bona fide Neoproterozoic animals. Unfortunately, its mode of life, style of skeletonization, and taxonomic affinity have been very controversial. Here, we use X-ray micro-CT, SEM, and taphonomic analysis to compare preservational modes of Corumbella, in order to better understand the symmetry, mode of construction, preservational style, and taxonomy of this group. Results suggest that articulated and disarticulated specimens of Corumbella from the Ediacaran of Brazil, Paraguay, and the United States, although sometimes preserved very differently, represent the same taxon—Corumbella werneri. Corumbellids had a thick but flexible theca and probably lived with their basalmost part anchored in the sediment, much like Conotubus. When considered together, these results suggest that Corumbella was one of the first animals to build a skeleton, employing a lamellar microfabric similar to conulariids.
Introduction
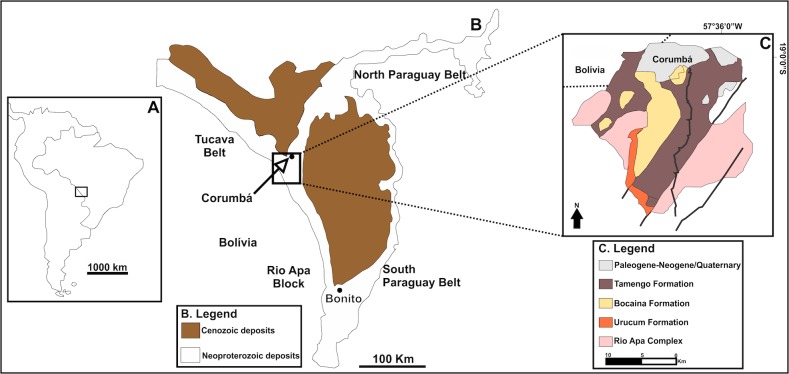
The Corumbá Group of Mato Grosso do Sul, southwest Brazil (Fig. 1), has the most diverse assemblage of Neoproterozoic fossils in South America [1, 2]. These include vase-shaped microfossils, algae (Tyrasotaenia sp.), and metazoans (Corumbella werneri, Cloudina lucianoi) [3–8].
Fig 1. Simplified map of South America (A) with detail of the geological map of the Paraguay Belt (B), and Corumbá Group (C) Modified from Oliveira, 2010, [48].
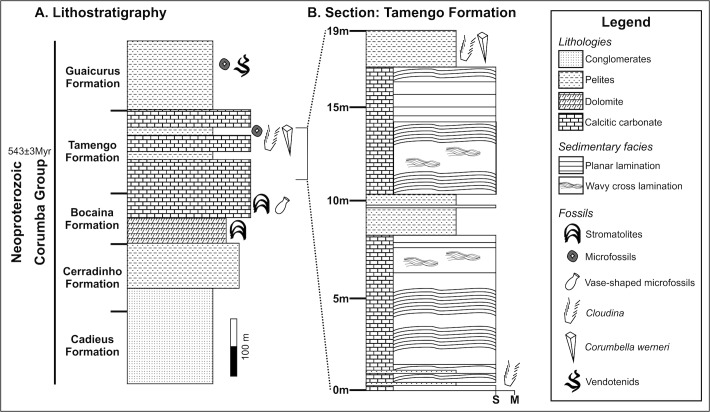
Perhaps the most enigmatic of these taxa is Corumbella werneri, a polyhedral multi-segmented flexible organism. It has been associated with scyphozoan cnidarians or other extinct cnidarian clades, such as the conulariids [4–6, 9]. This fossil is abundant in the limestone mines located in Corumbá and Ladário, Brazil, where it occurs in the Ediacaran Tamengo Formation (Fig. 2). Co-occurrence of C. werneri with the Ediacaran index fossil Cloudina suggests that the Tamengo Formation fossils are part of the Ediacara biota [2, 8, 10–12].
Fig 2. Lithostratigraphic column for Corumbá Group and section for the upper part of Tamengo Formation (Neoproterozoic) at the Saladeiro quarry.
Detail of the position where Corumbella werneri and other fossils occur. M—mud; S—sand. Modified from Warren et al., 2012, [17]; and Morais, 2013, [49].
Although the Tamengo Formation lacks other biomineralized Ediacaran fossils, such as Namacalathus [13, 14] and Namapoikia [15], its shales and marls show a range of preservational style of fossils. Corumbella also occurs in the grainstones and shales of the coeval Itapucumi Group of Paraguay [16, 17], as well as in the sandstones of the Wood Canyon Formation of the USA [9]. In these three deposits, Corumbella occurs as casts, molds, and body-fossils, in bed-parallel or bed-perpendicular orientations, and as single specimens or as clasts within pavements.
This diversity of preservational styles, together with an abundance of new corumbellid specimens, allows reassessment of the ecology, biology, and taxonomy of this organism, and places it among the novel evolutionary changes that occurred during the end of the Ediacaran period, such as the rise of skeletonized animals and carnivores predators [15, 17]. New specimens of Corumbella were collected from the Corumbá and Ladário exposures in Brazil, which were analyzed and compared to previously described specimens from Brazil, Paraguay and the USA, aiming to better understand its taxonomy and paleobiology.
Historical Taxonomy and Morphology
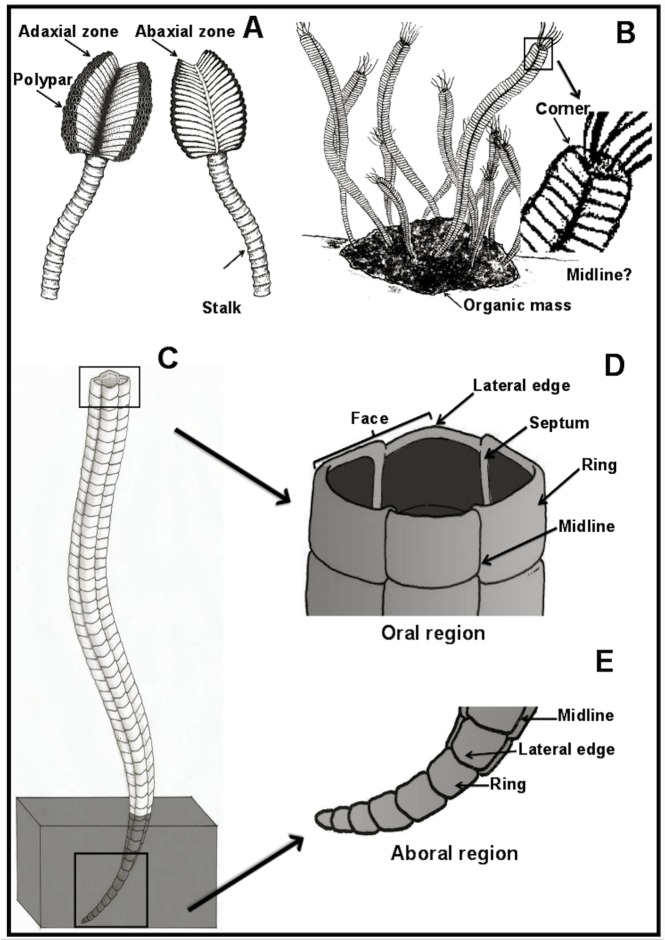
The first taxonomic description and paleoecologic and paleobiogeographic interpretations of Corumbella werneri were based on material from marls and shales of the Saladeiro quarry (Fig. 2), located in Ladário, adjacent to Corumbá. Originally, this material was assigned to a new subclass Corumbellata, and placed among the Cnidaria Scyphozoa [4]. Two distinct body regions were recognized in Corumbella werneri (Fig. 3A). The first is the proximal region, consisting of a curved, elongate, unbranched tubular periderm (or stalk), designated as the “primary polypar”, which is made up of isolated, and hypothesized, chitinous rings, externally and internally reinforced on the sides with four small, short internal sclerosepta. The second is a distal region, consisting of a biseriate arrangement of secondary, contiguous polypars, each formed by a small, distinct chitinous periderm tube, with no clear ring formation or visible sclerosepta. Secondary polypars are merged to each other at the adaxial surface. The set of two secondary polypars has a morphology similar to the primary polypar, differing only in the diameter. The biseriate portion is slightly wider and shorter (8–10 cm) than the uniseriate one. An apical part (here named attachment portion) in the primary polypar was not recognized (though basalmost oriented).
Fig 3. Models for Corumbella werneri.
(A) Reconstruction of Corumbella werneri proposed by Hahn et al. (1982) [4], showing a stalk and polypars (modified by Wilson Soares Jr). (B) Reconstruction of Corumbella werneri as a colonial sessile organism, as interpreted by Babcock et al. (2005) [5] (modified by Abner Santos). Detail of the square symmetry. (C) Reconstruction of Corumbella werneri proposed by Pacheco et al. (2011) [6] and improved in this work (draw by Abner Santos). (D) Detail of the oral region, lateral edges, faces, septa and midline. (E) Aboral region with unisseriated rings on the attachment portion.
In the first description [4], it was also highlighted the internal four-fold radial symmetry of C. werneri, which was implied by the arrangement of sclerosepta in cross-sections of the primary polypar. This character was considered to be similar to the internal arrangement found in the polyps of the recent scyphozoan genus Stephanoscyphistoma Jarms, 1990 (Coronatae; former Stephanoscyphus Allman, 1874), which was used to classify Corumbella as a Scyphozoa (phylum Cnidaria). However, they considered the transition from the proximal uniseriate primary polypar to the distal biseriate secondary polypars of Corumbella werneri to be a distinct character and, on the basis of this hypothesis, the authors erected the family Corumbellidae, order Corumbellida, subclass Corumbellata [4, 10].
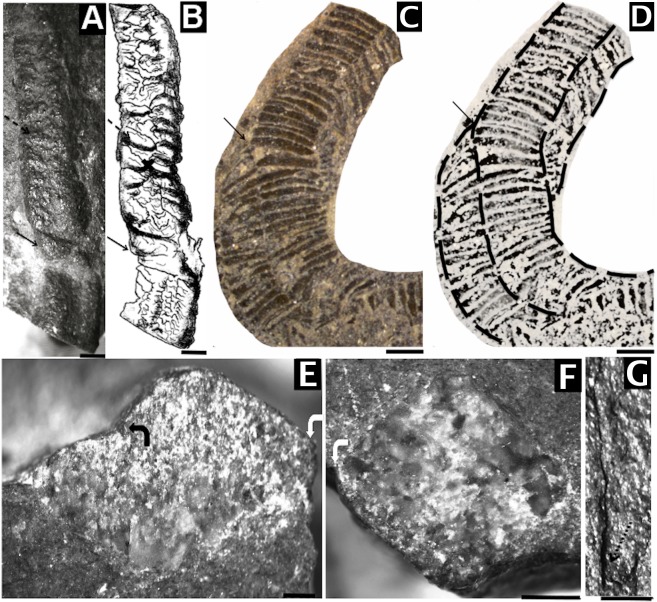
It was described [9] from the Ediacaran portion of the lower member of the Wood Canyon Formation (USA), but not named, “Corumbella n. sp.”, remarking that the two specimens they had found had a single longitudinal groove along the midline and were spirally twisted (Fig. 4A, B). Authors motivate the comparisons between C. werneri and USA specimens [9] by the original description [4], now considered outdated by the more recent studies [5, 6]. They do not consider Corumbella n. sp. conspecific with C. werneri, which in original description [4] lacks a helical twist and has transverse ornamentation, secondary polypars and circular geometry in cross sections (Fig. 4 E, F) [4]. Therefore these specimens were not assigned to Corumbella werneri (Brazil). Corumbella n. sp. was assigned to the Cnidaria [9] because the specimens were similar in symmetry and external ornamentation with conulariids, a group previously allied with Scyphozoa, Cnidaria [18–26].
Fig 4. Corumbella werneri, Wood Canyon (USA) and Tamengo Formation (Brazil). Morphology.
(A) LACMNH 12802 (Wood Canyon Formation). Polyhedral specimen, covered with desert varnish. Observe rings (black dashed arrow) alternately converging on the midline (marked with an “X” in the draw), in continuity with the lateral edges. Detail of the helical twist (black arrow). (B) Draw representing (A) with detail of the rings (black dashed arrow); midline (marked with an “X” in the encounter of two rings in the face) and torsion (black arrow). (C) and (D) GP1E-5808b (Tamengo Formation). Note torsion in the two-dimensional tube (black arrow) (C) and detail of the helical twist represented in (D) (black dashed lines). (E) LACMIP loc. 17130 (Wood Canyon Formation). End section quadrangular pressed torsional in (A). Observe lateral edges (white curved arrow) and septa (black curved arrow). (F) LACMNH 12802. Quadrangular section untwisted end in (A). See lateral edge (white curved arrow) (G) LACMNH loc. 17130. Aboral region. Detail for lateral continuity of the rings (black dotted arrow). Scale: 1 mm.
Then, a new morphological and taxonomic interpretation for C. werneri [5] was presented, in which a tetramerous elongated narrow tube would be secreted from an “apical attachment region” (referred as the apex, though basalmost oriented) (Fig. 3B). The animals were hypothesized to be benthic colonial predators fixed to the substrate by an “organic mass”. The reinterpretation of C. werneri as tubular and its budding reproduction strengthen their similarity with the modern Stephanoscyphistoma, and possibly with the extinct conulariids [5]. However, key morphological features essential for testing this paleoecological and taxonomic interpretation were described but not indicated in the described material [5], including midline thickening, attachment structures, oral region, the “organic mass”, and rectangular sections showing four-fold symmetry.
Other researchers [16] described Corumbella werneri from Itapucumi Group, Paraguay, as long and flattened fragments made up of articulated, apparently organic, narrow, annular elements, possibly corresponding to transverse rod-like skeletal elements that occur in conulariid scyphozoan cnidarians.
Recently, geometric modelling and taphonomic analysis were used to refine the understanding of the original specimens of Corumbella werneri and to carry out a more detailed taxonomic analysis of the group [6]. It was hypothesized that Corumbella would have a uniseriate aboral region that graded to a polyhedral geometry bearing lateral edges (Fig. 3C-E). The theca was constructed of polygonal rings merged into each other at a midline, the central region of the faces, as apothem of a pyramid, the side height of a lateral face—see ref. [6], (Fig. 7, pg. 278); (Fig. 3D).
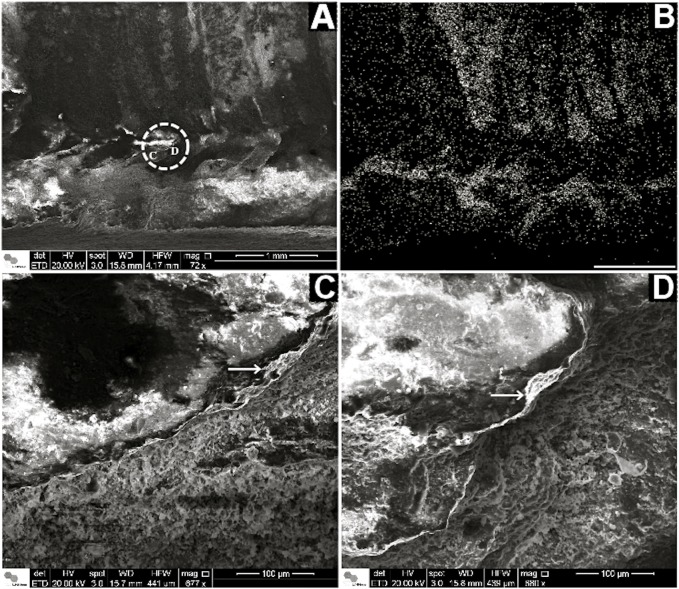
Fig 7. Corumbella werneri: ultrastructure of theca.
(A) SEM of a longitudinal section of a Corumbella theca. Dashed circle marks a detail of a sectioned ring. (B) EDS mapping of (A). Here it is possible to observe higher concentration o calcium in fragments of theca (represented by white dots) in comparison to the rock matrix and molds of fossil without fragments. (C) and (D) are details of the white dashed circle in (A), showing micro layers in Corumbella theca (white arrow). Scale for B: 1mm.
However, there are still aspects of the morphology of C. werneri that are unknown, such as the nature of inner structures, its aboral region and skeletal micro and ultrastructure. For example, important questions are: Did corumbellids have septa? What was the microfabric or mineralogy of their theca? And did they lay on or attach to the seafloor? In the past, it was difficult to answer these questions because the fossil’s attachment region or internal features are usually obscured by sediment or flattening during compaction, or because specimens previously studied were not well enough preserved to show primary microstructural details. Using microfocus X-ray computed tomography (microCT) in tandem with SEM and ultrastructural analysis of newly collected material, it is possible to fill some of these knowledge gaps and gain insights into the paleobiology, paleoecology, and phylogenetic affinity of these fossils.
Geological Background
The Corumbá Basin outcrops in the southern part of the Paraguay Belt, Brazil (Fig. 1). In this region, the base of the sequence consists of the Cadiueus and Cerradinho formations, which were deposited in an initial continental rift basin, before the widespread of carbonate deposition [2]. Overlying these strata are the Bocaina, Tamengo and Guaicurus formations, which were deposited in a stable marginal basin [27–29]. The Bocaina Formation comprises stromatolitic dolostones and subordinate phosphorites that grade to black limestones, marls and shales of the Tamengo Formation. The Guaicurus Formation, which is the youngest unit of the Corumbá Group, is comprised of thick siltstones and shales overlying the Tamengo Formation (Fig. 2) [28, 32].
The Bocaina, Tamengo, and Guaicurus formations represent the evolution to open shelf sedimentation in a post-rift to drift stage, as indicated by the occurrence of phosphorite at the top of the Bocaina Formation and the presence of the fossil Cloudina in the Tamengo Formation [2, 28], similar to other late marine Ediacaran environments, such as Namibia and Canada [13, 14, 32].
The Corumbella-bearing portion of the Tamengo Formation was dated about 543±3 Ma [30, 31] by U-Pb SHRIMP zircon form an ash bed interbedded in the upper Tamengo Formation (Fig. 2B). This age collaborates the correlation with records of Precambrian-Cambrian boundary in previous age constrains from Siberia and Namibia around 543Ma [33]. Also in Ara Group of Oman, the anomaly negative carbon excursion and abrupt last appearance of Cloudina and Namacalanthus, are dated by U-Pb zircon age in ash interbedded, directly define these event to be at 542±3Ma [34]. This parameters allow confirm that the Corumbella record and the age in Tamengo Formation agree very well with Precambrian-Cambrian boundary showed in previous work in distinct records.
The widespread carbonate deposition of the Tamengo Formation is related to a transgression event also observed in the calcareous grainstones of the Tagatiya Guazu Formation (Itapucumi Group, Paraguay). This unit is thought to be a wave- and tide-dominated depositional environment that represents a shallow tidally-influenced setting on a rimmed carbonate ramp [16], where the association of Cloudina fossils with thrombolites together with remains of Corumbella [17] were found.
The presence of the Cloudina and Corumbella in the Itapucumi Group and Tamengo Formation confirms that these two units are correlated [16]. In Corumbá, Cloudina occurred in a shallow, protected carbonate setting [16], whereas Corumbella lived in a calm terrigenous environment, indicated by its abundance in marls. In Paraguay, these animals probably co-occurred in environments with similar conditions of water temperature, depth, and salinity [17]. The siltstones and very fine-grained sandstones of the lower member of the Wood Canyon Formation in Nevada, USA, also bear Corumbella and Cloudina. Based on chemostratigraphic and biostratigraphic correlation with other radiometrically dated units, it was proposed that the fossil-bearing strata of the Wood Canyon Formation were probably deposited in the latest Ediacaran time, in shallow subtidal nearshore marine environments [9].
Material and Methods
Examined collections
419 samples were analyzed: 401 from Brazil, deposited at the Scientific Collection of the Geoscience Institute of the University of Sao Paulo (IGc/USP) (“GP/1E”), 2 from the Collection of the Laboratory of Paleobiology (IGc/USP), 12 from the Collection of the Department of Paleontology of the National Department of Mineral Production (DNPM), Rio de Janeiro (“DGM”), and 2 from the United States, deposited at the Los Angeles County Natural History Museum (“LACMNH” and “LACMIP”). Regarding the specimens from Paraguay, published data were used [9].
X-ray Microtomography
X-ray imaging can help the visualization of inner structures of fossils because X-rays penetrate opaque minerals and matrices without destructive sectioning of specimens [35]. The contrast between two features of X-ray imaging is based on differences in the attenuation of X-rays, as they pass through materials of different densities and elemental compositions. Computed tomography allows the use of multiple projected images recorded while the sample is rotated around an axis to assess the three-dimensional geometry and internal structures of fossils.
Specimens from the Brazilian and USA collections were examined using a “V|tome|x” (Phoenix X-Ray/General Electric) micro-CT scanner, at 90 kV and 130 μA. Tomographic reconstructions were based on 2000 single projection images equally spaced through 360°. Projection images were captured using a 2000 x 990 pixel coupled scintillator-CCD with 2 s exposure time. The resulting effective voxel size due to geometrical magnification was 12.3 μm3.
Scanning electron microscopy and Energy Dispersive Spectroscopy (SEM/EDS)
The microstructural analyses of fossils were also performed by the means of SEM/EDS at Brazilian Nanotechnology National Laboratory (LNNano). Fossils were examined using a SEM FEI Quanta 650 FE in secondary electrons detection mode, with acceleration voltages of 10 and 20 kV. EDS was performed using an X-Max detector in mapping mode, in order to detect the distribution of calcium in exoskeleton and rock matrix.
Morphological description and systematic analysis of specimens
Morphological and microstructural features are described using the published terminology [4–6, 9, 12, 20, 22]. Whenever possible, we followed the recommendations of other authors [36, 37], viz. (a) the taxonomic study of invertebrate fossils is based on the highest number of samples available, aiming to collect a broad spectrum of morphological and taphonomic data; (b) the study prioritizes the examination of specimens with different types of preservation; (c) comparison is based on morphometric characters of specimens with similar preservation.
New Data on Structure and Morphology
The first reconstructions of C. werneri [4] were based on some specimens that were three-dimensionally preserved. The type-specimens, as well as types, paratypes, and comparable specimens from Paraguay and the USA, were carefully re-examined. In this regard, microCT images and some additional data on SEM/EDS proved to be essential in showing some new aspects of the morphology and taphonomical implications, such as deformation of morphologic features, that could result in wrong taxonomic descriptions. In this sense, it was possible to observe in C. werneri specimens: the morphology and arrangement of the aboral portion or anchor in the substrate, aspects of geometry and construction (Fig. 5), internal exoskeletal, mineralogy, lamellar microfabric, compression and fragmentation (Fig. 6, 7).
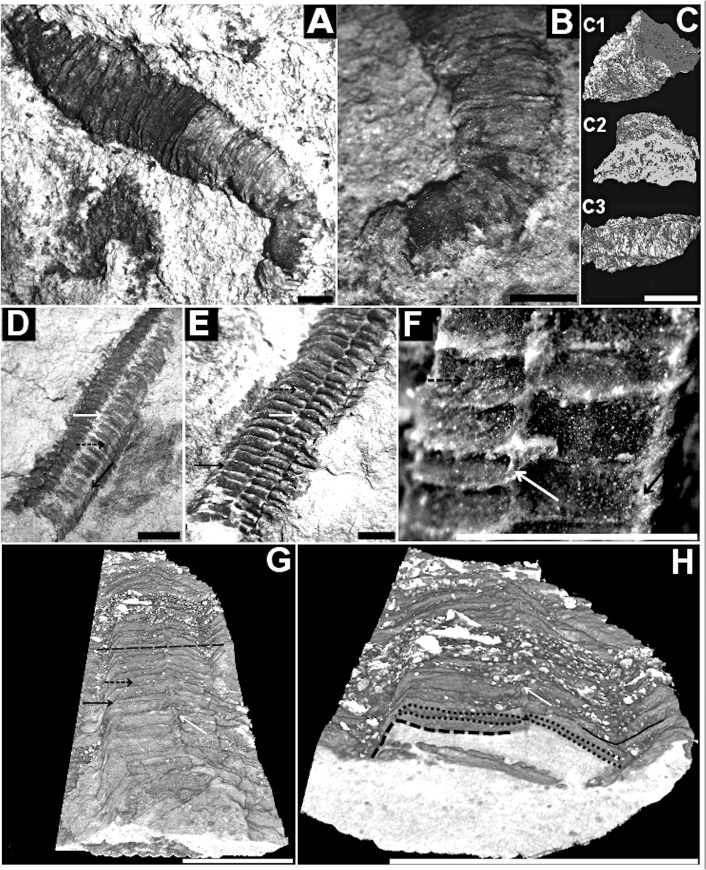
Fig 5. Corumbella werneri: morphology and modes of life.
(A) GP1E-4216: Corumbella werneri and (B) attachment region evidenced by (C) 3D-rendered microCT. (C1) shows a transverse-lateral section of (B). In (C1), it is possible to observe the conical morphology of the final attachment region, obliterated by the rock matrix in (B) (black arrow). (C2) is a detail of the attachment region in transversal section. (C3) is a lateral view of the final attachment region. (D) GP1E-4109: external mold of (E), GP1E-4210: internal mold with prismatic geometry and almost square in cross section. Note lateral edge (black arrow), midline (white arrow) and the alternate disposition of rings (black dashed arrow) across midline, on the face. (F) Zoom of specimen (E) to observe the “u” alternate disposition of rings across midline (white arrow), rings (black dashed arrow) and the continuity of rings on the lateral edges (black arrow). (G) and (H) MicroCT of (E). (G) Transversal section (black dashed line) showing a folded polyhedral tube in (H). Details of the rings (black dotted line), lateral edges (black dashed line) and open folded lateral edge (black line). Scale: 1mm. (A), (B) and (E): reprinted from Pacheco et al. (2011) [6] under a CC BY license, with permission from Luis Alcalá, original copyright 2011.
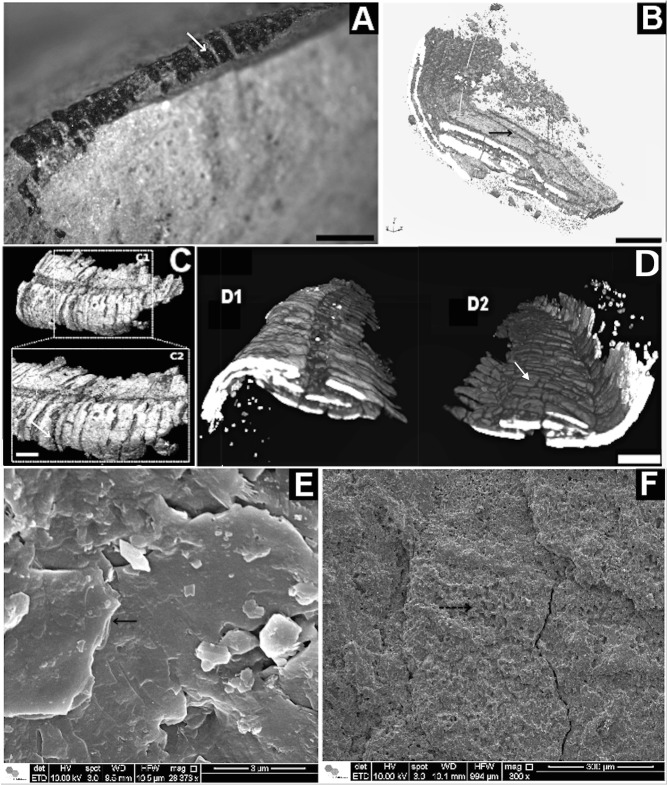
Fig 6. Corumbella werneri: ultrastructure of theca.
(A) GP1E-574a: three-dimensional specimen with theca. (B) 3D-rendered microCT of Corumbella theca,flipped by 180° compared to (A): interior view and detail for lamellae microfabric and plates (black arrow). (C) 3D-rendered microCT of Corumbella theca (A) without flipping in C1 and C2, with details of rings (white arrow). (D) compression and fragmentation along theca. D1 shows a transversal section in the fossil structure. Flipped by 180° of it produces D2, with details of small breakages (white arrow). (E) Details of lamellar plates by SEM (black arrow) and (F) pores in plates (black dashed arrow). Scale: 1mm.
Structural and morphological attributes were considered here in order to compare specimens from South and North America.
Morphology
The bipartite region of C. werneri body (Fig. 3A) may have originally been attributed to fragments of three-dimensional or two-dimensional uniseriate tubes. These tubes were interpreted as part of a primary polyp or stalk (DGM-5601-I, Fig. 8C), and the biseriate region was interpreted as polypars (DGM-5601-I, 5606-DGM-I, Fig. 8C, F). In previously analyzed samples, these parts were often arranged separately. In this paper, they are interpreted as fragments, sometimes from the same individual. Based on this study, it appears that Corumbella werneri is a polyhedral tube (Fig. 5D, E) with oral and aboral regions, and that the aboral region has a conical attachment (Figs. 3C-E and 5A-C) see ref. [6], pg. 277.
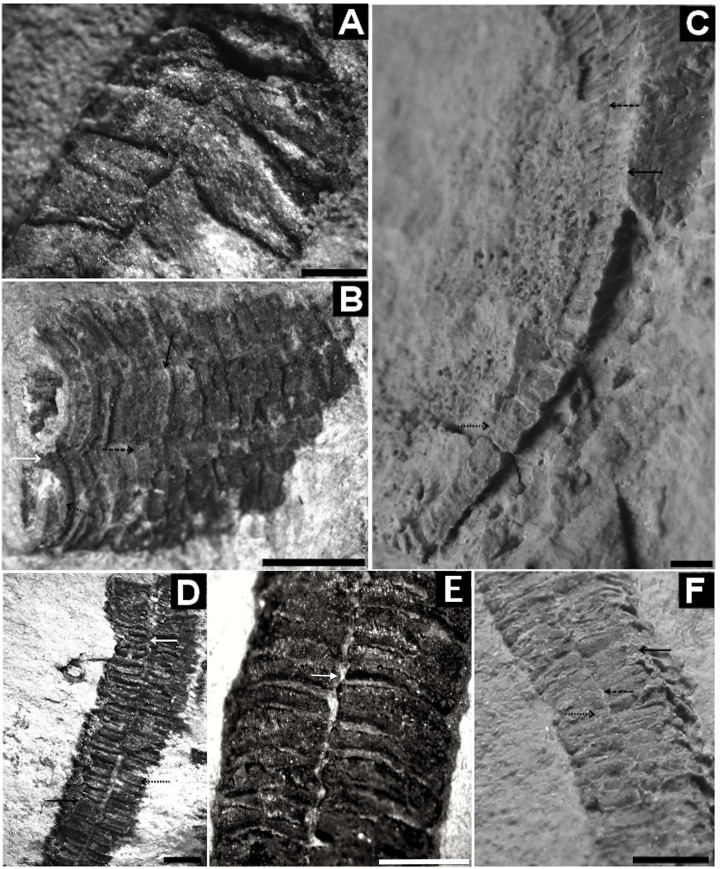
Fig 8. Corumbella werneri: morphology and modes of life.
(A) GP1E-4089: oral region. (B) GP1E-4077: Inflated tube. Detail of a face with septum (white arrow), midline (black dashed arrow), rings (black dotted arrow) and lateral edge (black arrow) relatively compressed. (C) DGM-5601-I: specimen used in the original description by Hahn et al. (1982). Internal mold, recurved. It is observed the aboral region, uniseriate, without evidente midline in the (incomplete) attachment region. These rings (black dotted arrow) grade to an approximately polyhedral portion, with midline (on the face) (black dashed arrow) and lateral edge (black arrow). Notice a break in the longitudinal mid-distal portion of the fossil. (D) GP1E-4204: tube with longitudinal breakage, where it is evident the septum (white arrow), lateral edge (black arrow) and rings (black dotted arrow). (E) GP1E-3093: internal portion of folded tube, thick exoskeleton, where it is possible to see the septum (white arrow) formed by the inner part of the alternate rings (black dashed arrow). (F) DGM-5606-I: internal mold, three-dimensional, with midline (black dashed arrow), rings (black dotted arrow) and fragments of theca on the lateral edge (black arrow). Scale: 1mm. (A), (B), (C) and (D): reprinted from Pacheco et al. (2011) [6] under a CC BY license, with permission from Luis Alcalá, original copyright 2011.
Three-dimensional modeling of Corumbella specimens from the Wood Canyon Formation indicates that their structure and anatomy are strikingly similar to the Tamengo Formation specimens. They have an elongated tubular polyhedral (Fig. 4A) appearance and are formed by four faces and four lateral edges, showing an almost quadrangular cross-section (Fig. 4F). The rings are inconspicuous, but it is possible to see they are polygonal, and merge with each other across midline (Fig. 4A, B). There are lateral edges (Fig. 4E, F) and faces with no clear demarcation of midlines, but with internal septa (Fig. 4E). These specimens were stretched, and fossilized in sandstone, and the fragments of the theca are partly obscured by desert varnish. Unlike the Tamengo Formation’s specimens, the Wood Canyon Formation’s specimens have a thinner theca without longitudinal striations, and there is no evidence of the oral region in these specimens. One specimen has an aboral region (Fig. 4G), but not a clear attachment portion.
Structure and ultrastructure
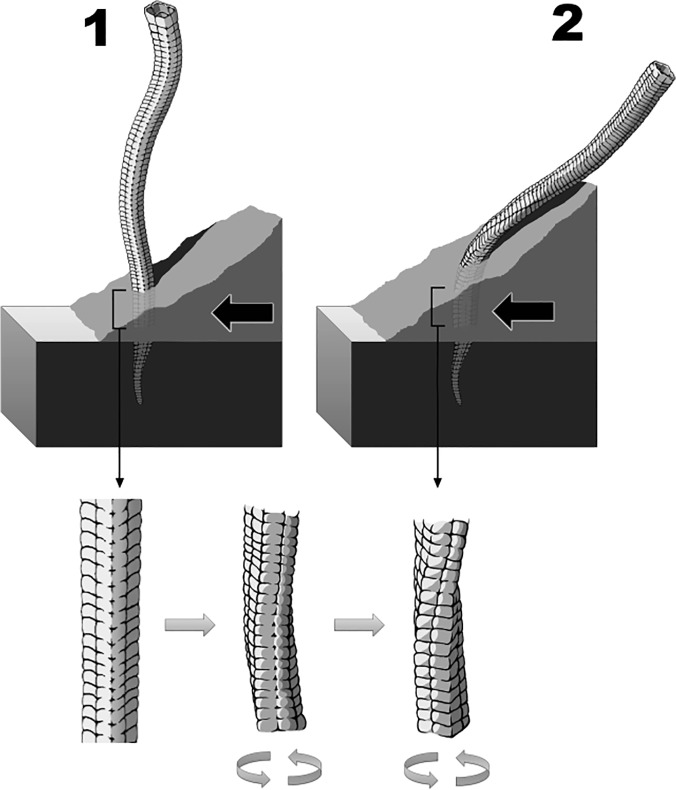
Unlike completely biomineralized integuments of other metazoans, biomineralized Ediacara fossils often have weak or unusually flexible integuments (e.g. Cloudina). Thecae of C. werneri are similar—they show twisting and stretching in response to deformation, which suggests they had some plasticity. For example, both specimens from the USA and Brazil show torsions along the tube (Fig. 4A-D). In Brazilian specimens, the basalmost part ends directly in the substrate (Fig. 5A-C), just as described for Conotubus [38], suggesting that adults might be benthic. Corumbella werneri from the USA also has an aboral region, recognized as a basalmost ending attachment portion. In this sense, the presence of helical twisted regions suggests that these torsions result from mechanical processes acting on the pyramidal structure of the tube. When twisting a polyhedron, this usually happens due to its asymmetric geometry and/or due to mechanic torques (Fig. 9). In the case of Corumbella, helical twisting could have happened if the animal was partially buried when it was subjected to an external twisting force (such as a current or turbid flow that might also bury the organism). Hence, the response of tube to external forces depends not only on the rigidity of the theca, but also on their internal filling. Wood Canyon specimens are commonly filed with sediments coarser than the surrounding matrix, which causes a differential mechanical response to external torques, such as those produced on the burial process. Torsions can result from this transfer of forces from the matrix sediments to the structure, which can be more pronounced depending on the grain size of the sediment.
Fig 9. Simplified model for the mechanical origin of the torsion observed on the Corumbella specimen from USA (LACMNH 12802), which is dependent of the physical conditions of the animal and/or of the external forces.
Subfigures 2 to 1 correspond to different stages: black arrows show a reconstitution from helical twisting happening when a Corumbella tube becomes partially buried (stage 2) until a possibility of Corumbella life mode (stage 1). Gray arrows point details of twisting until stage 2. Gray circular arrows show possibilities of twisting degrees in tubes.
Since the structure of the tube was not entirely rigid, but fairly flexible in some twisted specimens, only a restricted area of the animal's body was twisted. If the skeleton was completely hard (e.g. strongly mineralized), it could not be twisted. A simplified version of this scenario is presented (Fig. 9) in order to explain the observed deformation and twisting of some Corumbella specimens.
Although the theca of Corumbella was flexible, its tube-like body and its segments were not elastic, which is supported by the evidence that its structure was susceptible to breakage. For example, in the Tamengo Formation, Corumbella fossils are highly fragmented and fractured (Fig. 6C, D), including specimens that show evidence of syndepositional and/or pre-burial fragmentation and cracking. For instance, some fragments were broken prior to deposition, or are oriented inconsistently with the vertical compaction processes attributable to burial compaction. Similarly, abundant intricate specimens are sometimes concentrated in a single horizon, forming a pavement of Corumbella remains.
Considering these characteristics, thecae of C. werneri (Corumbá Group) may have been as flexible but not as functionally elastic as the periderm of modern coronates [39] and of some cnidarian fossils, like Byronia [40] or Olivooides [41] for example.
The coronate periderm is composed of thin lamellar layers of chitin [42] in a smooth microtexture arrangement [39, 43], functionally resulting in a soft tube that can be compressed and return to its original shape without damage or injury [39]. Morphologically, thecae of C. werneri also consist of a lamellar microfabric but made of polygonal plates (Figs. 6B, E and 7D, E); see ref. [17], like the Ordovician scyphozoan Sphenothallus that have integuments quite similar to some chitino-mineralized thecae of conulariids [17, 44, 45], and linguliids [46]. However, the theca of Corumbella is morphologically unique, because its polygonal plates sometimes have pores and papillae (Fig. 6F); see ref. [17].
The EDS mapping detected a higher concentration of calcium in remains of Corumbella thecae in comparison to the rock matrix (Fig. 7B). It suggests a biomineralized theca. But we have not ruled out the possibility that the calcium concentrations may be the result of diagenetic mineralization of organic tissue, rather than original biomineralization.
Nevertheless, skeletogenesis is the process of synthesizing skeletons and not necessarily involves biomineralization (which is the process that organisms precipitate solid materials to the formation of skeletal structures). Many animals synthesize completely organic skeletons, like insects, for example. Because our main issue is to investigate elements related to skeletogenesys process (including biomineralization as well), we also consider other elements related to cnidarian biomineralization, such as phosphate for example. However, we have not detected yet phosphate and other important elements related to this process besides calcium. We even had tried the chemical test for phosphate (1 dg of ammonium molybdate in reaction with 1:1 HNO3) [17] and it was not detected.
The ultrastructure of Corumbella also lacks the standard parabolic design characteristic of chitin-protein complexes, common to the former chitinous integuments exclusive from the Cambrian, such as the scyphozoan Byronia robusta [40, 47]. Moreover, unlike Corumbella and other current cnidarians, Byronia integuments are thinner, exclusively organic, and almost always preserved as carbonaceous films that are flattened but not fragmented [40, 47].
In summary, the plastic deformation of C. werneri suggests that its theca was flexible, organic, or even weakly mineralized. Furthermore, we hypothesize that the lamellar nature, thickness (and mineralization?) of its integument (Figs. 6B, E and 8E) may have contributed to its vulnerability to fragmentation (Fig. 6C, D). These characteristics suggest that the theca may have been originally thicker and less elastic than the periderms of modern coronates and Byronia, and that it was originally flexible but inelastic.
Systematic Paleontology
The systematic review proposed here takes into account the approaches established for Brazilian specimens of Corumbella werneri [4–6, 12], which are applied to better constrain the systematic affinities of the incompletely described corumbellids from the lower member of the Wood Canyon Formation [9]. In order to facilitate this study and a comparison between Brazilian and USA specimens, we summarized data on the Brazilian specimens in a synoptic treatment, presented in Table 1.
Table 1. Revision of morphological terminology and taxonomic affinities of Corumbella werneri.
| Description | Hahn et al. (1982) | Zaine (1991) | Babcock et al. (2005) | This study |
|---|---|---|---|---|
| Body Organization | Growth polarity | Growth polarity not evident | Growth polarity | Growth polarity |
| Bipartite organization: composed of primary and secondary polyps | No bipartite organization | Description of oral region and observation of an apical attachment region | Observation of oral-aboral organization | |
| No secondary polyps | No secondary polyps | |||
| Articulation and cross continuity of rings | ||||
| Geometry | Uni- and biseriate cylindrical parts, circular in section | One to four longitudinal series of hollow flat, nearly cylindrical compartments | Elongate tube, square in cross-section | Elongated polyhedral tube (pyramidal), approximately quadrangular in cross-section |
| Symmetry and internal thickening | Sclerosepta only in primary polyp (tetraseptation) | Not observed | Carinae (attributed to the midline) | Presence of septa |
| Carinae not observed | ||||
| Tetramery | Tetramery | Tetramery | ||
| External structures | No external structures (e.g. midlines and corners) corresponding to internal septations | Absent | Midline and corner | Midline, lateral edges and faces |
| Covering | Isolated rings in the proximal part | Isolated chitinous rings | Isolated rings | Rings are alternate across midline and continued at the lateral edges |
| No rings in polypar | Thick carapace, sometimes with longitudinal striations | |||
| Chitinous periderm | Possible chitinous periderm | Composition of carapace probably organic (chitinous?) or weakly mineralized | ||
| Attachment structures | Not observed | Not observed | Apical region attached to an organic mass | Apical region attached to the substrate |
| Paleoecological considerations | Colonial | Reproductive modes not evident | Reproduction by budding | Inferred gregarious and/or colonial habit |
| Taxonomy | Subclass Corumbellata, Order Corumbellida, Family Corumbellidae | Vendozoa, Vendobiont | Cnidaria, Scyphozoa, Conulata | Metazoa, Cnidaria, Scyphozoa, |
| Phylogenetic relationships | Cnidaria: Stephanoscyphus | Giant protists related to an extinct order (or subclass) of rhizopods | Cnidaria: Modern Stephanoscyphus and fossil conulariid | Cnidaria: fossil conulariid |
| Pennatulacea, Charniidae |
Kingdom METAZOA Linnaeus 1758
Phylum CNIDARIA Verrill 1865
Class SCYPHOZOA Goette 1887
Family CORUMBELLIDAE Hahn, Hahn, Leonardos, Pflug e Walde 1982
Genus Corumbella Hahn, Hahn, Leonardos, Pflug and Walde 1982
Type species. Corumbella werneri Hahn, Hahn, Leonardos, Pflug and Walde 1982, by monotypy.
Emended diagnosis. Polyhedral, elongated tube, with internal septa formed by junction of alternate rings at midlines.
Corumbella werneri Hahn, Hahn, Leonardos, Pflug and Walde 1982
Corumbella werneri Hahn et al., 1982: p. 4–9, Tables 1–3, Figs. 3–5, 9, 11.
Corumbella n. sp. Hagadorn and Waggoner 2000: Fig. 5 p. 356.
Emended diagnosis. Elongated polyhedral pyramidal exoskeleton (theca), thick, diameter along tube slightly variable; cross section circular at basal part, otherwise quadratic distalwards; external midline groove formed by junction of polygonal rings at apothem, continuous along the polyhedral tube; internal septa located internally to midline, when present; rings continuous over lateral edges, absence of carinae.
Description. Sample DGM-5601-I (Fig. 8C): three-dimensional internal mold, slightly compressed; tube "J"-shaped, 34 mm in height; aboral region of tube polyhedral, elongated, 9 mm in height; midline and lateral edges present. Sample GP/1E 4210a (Fig. 5E, F): internal mold of part of a three-dimensional polyhedral tube, quadrangular geometry, lateral edges visible; rings continuous at lateral edges; midline demarcated by merging of polygonal rings, in "U" conformation, with fragments of theca, alternately on face apothem (Fig. 5F); maximum length 9 mm, width 2 mm, maximum width of ring 1 mm, maximum length of ring 0.3mm. Sample DGM-5606-I (Fig. 8F): internal mold of part of a three-dimensional polyhedral compressed tube; lateral edges; rings continuous at lateral edges; midline demarcated by merging of rings alternately in face apothem, maximum length 19 mm, width 2.4 mm. Sample GP/1E 3093 (Fig. 8E) internal view of fragment compressed theca, thick; rings continuous at lateral edges; septa formed by merging of rings internally; thickness 0.05–0.2 mm, maximum length 9 mm. Sample GP/1E 4089 (Fig. 8A): oral region compressed at lateral edges, rings and lateral edges present; oral opening characterized by well marked hole. Sample GP/1E 4077 (Fig. 8B): tube inflated with three-dimensional rings, midline and septum present, lateral edges relatively compressed. Sample GP/1E 4204 (Fig. 8D): part of a three-dimensional polyhedral tube broken at the face and lateral edges; presence of polygonal rings; lateral edges and septa at midline visible in some parts of tube, absence of carinae. Sample LACMNH 12802 (Fig. 4A): three-dimensional polyhedral tube, presence of septa at midline and lateral edges; rings alternately converging at midline, apothem of face, twisting; specimen square in cross section; carinae absent.
Type series. DGM-5601I, DGM-5604I, DGM-5605I, DGM-5606I, DGM-5608I, DGM-5609I, DGM-5610I, DGM-5611I, DGM-5612I, DGM-5613I (Hahn et al., 1982, p. 4–9,Tables 1–3, Figs. 3–5, 9, 11), LACMNH 12802, LACMNH loc. 17130, LACMIP loc. 17130 (Hagadorn and Waggoner, 2000, p. 356, Fig. 5).
Examined Material. DNPM-RJ (DGM), 10 specimens; GSA/IGc-USP (GP/1E), 6 specimens; NHMLA (USA) (LACMNH, LACMIP), 2 specimens.
Discussion. All specimens studied here have similar morphology, and are summarized under the diagnosis of the genus Corumbella. The specimens from USA, which were previously assigned to “Corumbella n. sp.”, show longitudinal grooves at the midline and the alternated rings at the center of the polygonal faces. In the USA fossils, the midline is also indicated by the presence of its respective internal thickening (septum), which is visible in cross section. Septa are not always visible in all specimens, even in the Brazilian ones, a pattern that may be attributed to decay prior to burial. Comparisons between USA and Brazilian specimens showed that they can be considered the same species, Corumbella werneri, as evidenced by the presence of an external midline, formed by the alternation of polygonal rings at the apothem (Figs. 4A and 5F, H). This midline is continuous throughout the polyhedral tube, showing an unbroken continuity of the rings at the lateral edges (Figs. 4A and 5F-H). In considering these specimens, it is possible that the tube was occasionally expanded. Such expansion might have occurred since the flexible integument was susceptible to sediment infilling (maybe during life as in the case of some modern coronates) [50], or movement of the tube (Fig. 9). Hence, there is not a diagnostic and unique characteristic that justifies the assignment of a new species to the Corumbella from USA [9]; after comparisons based on [4]. Re-examination of these three-dimensional molds showed spaces between the molds and the rock matrix (Fig. 4A, G), which indicates the previous existence of a relatively thick theca—one that would be similar in thickness to the skeletal integument of the specimens from the Tamengo Formation of Brazil and the Tagatiya Guazu Formation of Paraguay. The quadrangular cross-section along the length of the tube (Figs. 4A, F and 5D, H) reinforces our interpretation that the skeletal structure of Corumbella was more resistant than many fossils that are always found flattened or compressed but never fragments or with breaks (such as Byronia).
Conclusions and Future Directions
Our data not only confirms previously published observations [5, 9], but also add new information about morphology (e.g. attachment portion, oral region, rings disposition and midline) and ultrastructure (e.g. lamellae microfabric) that contributes an important complement to the interpretation and a new description of C. werneri.
This study is consistent with the idea that Corumbella is a scyphozoan cnidarian and that specimens from Brazil, Paraguay, and the USA are part of a similar taxonomic and benthic assemblage. The occurrence of specimens with the tip of Corumbella’s tube-like skeleton embedded in the sediment suggests that their mode of life was possibly similar to that of helicoplacoids, in which the animal was attached to or rooted in the substrate. Corumbella also had a relatively thick and sturdy theca that showed complex growth and flexure.
The taphonomic aspects considered here are only those essential to better perform morphological studies (e.g. distortion, compression) refusing bias in taxonomic descriptions. As the flexible exoskeleton of Corumbella may be susceptible to molding processes and/or sediments filling, we performed some insights into the importance of some deformations (e.g. twisting) to the interpretation of how some taphonomic classes, such as three-dimensional, can be preserved and incur in important specimens for taxonomic descriptions. Here we only offer a model that justifies the twists observed in Corumbella are not intrinsic to the organism (morphological) but an artifact of deformation related to external forces on its polyhedral structure during burial (see Fig. 9).
In this sense, some causes of the preservational style of its theca still remain enigmatic. The distribution of Corumbella remains among the marls and shales of Tamengo Formation lacks satisfactory paleoenvironmental explanation and taphonomic models. Detailed taphonomic studies (involving the compilation of a deep sedimentological database) are still crucial to reveal fundamental aspects of fossilization process in Corumbella specimens.
In addition, further research about the possible mineralized nature (phosphatic or calcitic) of specimens may shed light on the corumbellids’s role in the development of metazoan biomineralization.
Acknowledgments
This study was supported by research grants from FAPESP (Proc. 2009/02312-4) and NAP-Astrobio (PRP-USP). ACM had support from FAPESP (Proc. 2011/50242-5), CNPq (562143/2010-6, 458555/2013-4), and CAPES. The authors would like to acknowledge the Astrobiology Laboratory (AstroLab, IAG-USP) for proving research facilities to carry out modelling of taphonomic processes. The authors would like to thank LNNano/CNPEM for technical support and for providing infrastructure, in particular for allowing the use of Scanning Microscope FEI Quanta 650 FEG under the Quanta-15068 and 14 861 proposals. Part of the microCT experiments was performed on the ID19 beamline at the European Synchrotron Radiation Facility (ESRF), Grenoble, France. We are grateful to Local Contact at ESRF for providing assistance in using beamline ID19. Parts of this research were also carried out at the light source Doris III at DESY, a member of the Helmholtz Association (HGF). We would like to thank the Local Contact for assistance in using beamline W2. We also would like to thank Fabio Ramos Dias de Andrade (IGc/USP) for translating the original description of Corumbella werneri, Adriana Furtado Fonseca for reviewing the manuscript, Rodrigo da Rocha Machado (Museu de Ciências da Terra, DNPM, Brazil) for allowing the study of the type series; Mary Stecheson (Collection of the Natural History Museum of Los Angeles, USA) for loaning the material to be analyzed. The paper was substantially improved by the drawings of the paleoartists Abner Santos, Pedro Busana (UFSCar-Sorocaba, Brazil) and Wilson Soares Junior (USP-Sao Paulo, Brazil). Two scientists from the NP-BioMar and NAP-Astrobio (both from University of São Paulo) contributed greatly to this study.
Funding Statement
This study was supported by research grants from FAPESP (Proc. 2009/02312-4) and NAP-Astrobio (PRP-USP). ACM had support from FAPESP (Proc. 2011/50242-5), CNPq (562143/2010-6, 458555/2013-4), and CAPES. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Glaessner MF (1984) The Dawn of Animal Life. Cambridge: Cambridge University Press, 244 pp. [Google Scholar]
- 2. Gaucher C, Boggiani PC, Sprechmann P, Sial AN, Fairchild TR (2003) Integrated correlation of the Vendian to Cambrian Arroyo del Soldado and Corumbá Group (Uruguay and Brazil): palaeogeographic, palaeoclimatic and palaeobiologic implications. Precambrian Research 120: 241–278. [Google Scholar]
- 3. Fairchild TR, Barbour AP, Haralyi NLE (1978) Microfossils in the “Eopaleozoic” Jacadigo Group at Urucum, Mato Grosso, Southwest Brazil. Boletim do Instituto Geológico 9: 74–79. 10.1088/0953-8984/24/34/346001 [DOI] [PubMed] [Google Scholar]
- 4. Hahn G, Hahn R, Leonardos OH, Pflug HD, Walde DHG (1982) Kfrperlich erhaltene Scyphozoen-Reste aus dem Jungprekambrium Brasiliens. Geologica et Paleontologica 16: 1–18. [Google Scholar]
- 5. Babcock LE, Grunow AM, Sadowski GR, Leslie SA (2005) Corumbella, na Ediacaran-grade organism from the Late Neoproterozoic of Brazil. Palaeogeography, Palaeoclimatology, Palaeoecology 220: 7–18. [Google Scholar]
- 6. Pacheco MLAF, Leme JM, Machado AF (2011) Taphonomic analysis and geometric modelling for the reconstitution of the Ediacaran metazoan Corumbella werneri Hahn et al. 1982 (Tamengo Formation, Corumbá Basin, Brazil). Journal of Taphonomy 9(4): 269–283. [Google Scholar]
- 7. Fairchild TR, Sanchez EAM, Pacheco MLAF, Leme JM (2012) Evolution of Precambrian life in the Brazilian geological record. International Journal of Astrobiology 20: 1–15. [Google Scholar]
- 8. Kerber BB, Rosa ALZ, Gabas SG, Leme JM, Pacheco MLAF (2013) O registro fossilífero de metazoários ediacaranos na América do Sul e duas implicações nos estudos sobre origem e complexificação da vida animal. Geologia USP Serie Cientifica 13: 51–64. [Google Scholar]
- 9. Hagadorn JW, Waggoner B (2000) Ediacaran fossils from the southwestern Great Basin, United States. Journal of Paleontology 74: 349–359. [Google Scholar]
- 10. Walde DHG, Leonardos OH, Hahn G, Hahn R, Pflug H (1982) The first Precambrian megafossil from South América, Corumbella werneri . Anais da Academia Brasileira de Ciências 54(2): 461. [Google Scholar]
- 11. Hahn G, Pflug HD (1985) Die Cloudinidae n. fam., Kalk-Rfhren aus dem Vendium und Unter-Kambrium. Senckenbergiana Lethaea 65: 413–431. [Google Scholar]
- 12.Zaine MF (1991) Analise dos fósseis de parte da Faixa Paraguai (MS, MT) e seu contexto temporal e paleoambiental. Programa de Pós-graduação em Geologia Sedimentar, Universidade de São Paulo, Tese de Doutorado, 218 pp.
- 13. Grotzinger JP, Watters WA, Knoll AH (2000) Calcified metazoans in thrombolite-stromatolite reefs of the terminal Proterozoic Nama Group, Namibia. Paleobiology 26(3): 334–359. [Google Scholar]
- 14. Hofmann HJ, Mountjoy EW (2001) Namacalathus-Cloudina assemblage in Neoproterozoic Miette Group (Byng Formation), British Columbia: Canada’s oldest shelly fossils. Geology 29(12): 1091–1094. [Google Scholar]
- 15. Wood R.A (2011) Paleoecology of the earliest skeletal metazoan communities: Implications for early biomineralization. Earth Science Reviews 106(1–2): 184–190. [Google Scholar]
- 16. Warren LV, Fairchild TR, Gaucher C, Boggiani PC, Poiré DG, Anelli LE, et al. (2011) Corumbella and in situ Cloudina in association with thrombolites in the Ediacaran Itapucumi Group, Paraguay. Terra Nova 23: 382–389. [Google Scholar]
- 17. Warren LV, Pacheco MLAF, Fairchild TR, Simões MG, Riccomini C, Boggiani PC, et al. (2012) The dawn of animal skeletogenesis: ultrastructural analysis of the Ediacaran metazoan Corumbella werneri . Geology 40(8): 691–694. 10.1002/dc.21598 [DOI] [PubMed] [Google Scholar]
- 18. Van Iten H (1991) Evolutionary affinities of conulariids In: Simonetta A.M. & Morris S.C. (Eds.) The early evolution of Metazoa and the significance of problematic fossil taxa, Cambridge University Press, p. 145–155. [Google Scholar]
- 19. Van Iten H (1992. a) Morphology and phylogenetic significance of the corners and midlines of the conulariid test. Paleontology 35: 335–358. [Google Scholar]
- 20. Van Iten H (1992. b) Microstructure and growth of the conulariid test: implications for conulariid affinities. Paleontology 35: 359–372. [Google Scholar]
- 21. Van Iten H, Fitzke JA, Cox RS (1996) Problematical fossil cnidarians from the Upper Ordovician of the North-Central USA. Paleontology 39: 1037–1064. [Google Scholar]
- 22. Van Iten H, Leme JM, Simoes MG (2006) Additional observations on the gross morphology and microstructure of Baccaconularia Hughes, Gunderson et Weedon, 2000, a Cambrian (Furongian) conulariid from the north-central USA. Palaeoworld 15: 294–306. [Google Scholar]
- 23. Marques AC, Collins AG (2004) Cladistic analysis of Medusozoa and cnidarian evolution. Invertebrate Biology 123: 23–42. [Google Scholar]
- 24. Leme JM, Simões M, Marques A, Van Iten H (2008. a) Cladistic analysis of the suborder Conulariina Miller and Gurley 1896 (Cnidaria, Scyphozoa, Vendian/Triassic). Paleontology 51: 649–662. [Google Scholar]
- 25. Leme JM, Simões M, Rodrigues S, Van Iten H, Marques A (2008. b). Major developments in conulariid (Cnidaria) research: problems of interpretation and future perspectives. Ameghiniana 45: 407–420. [Google Scholar]
- 26. Leme JM, Simões M, Van Iten H (2010). Phylogenetic Systematics and evolution of conulariids Saarbrücken: Lap Lambert. Academic Publishing Gmb H & Co., 49 p. [Google Scholar]
- 27. Almeida FFM (1984) Província Tocantins. Setor sudoeste In: Almeida de F.F.M. & Hasuy Y. (coord.). O Pré-Cambriano do Brasil. São Paulo: Edgard Blücher, p. 265–281. [Google Scholar]
- 28.Boggiani PC (1998) Análise Estratigráfica da Bacia Corumbá (Neoproterozóico)—Mato Grosso do Sul. Tese de Doutorado, Instituto de Geociências, Universidade de São Paulo, 181 pp.
- 29. Alvarenga CJS, Boggiani PC, Babinski M, Dardenne MA, Figueiredo MF, Santos RV, et al. (2009) The Amazonian Paleocontinent In: Gaucher C.; Sial A.N.; Halverson G.P. & Frimmel H.E. (Ed.). Neoproterozoic-Cambrian tectonics, global change and evolution: a focus on southwest Gondwana. Amsterdam: Elsevier, 498 pp. [Google Scholar]
- 30. Babinski M, Boggiani PC, Fanning M, Simon CM, Sial AN (2008) U-Pb shrimp geochronology and isotope chemostratigraphy (C, O, Sr) of the Tamengo Formation, southern Paraguay belt, Brazil. In: South American Symposium on Isotope Geology, 6, San Carlos de Bariloche. Proceedings 1: 160. [Google Scholar]
- 31. Babinski M, Trindade RIF, Alvarenga CJS, Boggiani PC, Liu D, Santos RV, et al. (2006) Cronology of Neoproterozoic ice ages in Central Brazil. In: South American Symposium on Isotope Geology, 5, Punta del Este. Short Papers 1: 223–226. [Google Scholar]
- 32. Boggiani PC, Gaucher C, Sial AN, Babinsky M, Simon CM, Riccomini C, et al. (2010) Chemostratigraphy of the Tamengo Formation (Corumbá Group, Brazil): a contribution to the calibration of the Ediacaran carbon-isotope curve. Precambrian Reseach 182: 382–401. [Google Scholar]
- 33. Grotzinger JP, Bowring SA, Saylor BZ, Kaufman AJ (1995) Biostratigraphic and geochronologic constraints on early animal evolution. Science 270: 598–604. [Google Scholar]
- 34. Amthor JE, Grotzinger JP, Schröeder S, Bowring SA, Ramezani J, Martin MW, et al. (2003) Extinction of Cloudina and Namacalathus at the Precambrian-Cambrian boundary in Oman. Geological Society of America.31(5): 431–434. [Google Scholar]
- 35. Tafforeau P, Boistel R, Boller E, Bravin A, Brunet M, Chaimanee Y, et al. (2006) Applications of X-ray synchrotron microtomography for non-destructive 3D studies of paleontological specimens. Applied Physics A 83(2): 195–202. [Google Scholar]
- 36. Lucas S (2001) Taphotaxon. Lethaia 34: 30. [Google Scholar]
- 37. Rodrigues S, Simões M, Leme JM (2003) Tafonomia Comparada dos Conulatae (Cnidaria), Formação Ponta Grossa (Devoniano), Bacia do Paraná, Estado do Paraná. Revista Brasileira de Geociências 33(4): 165–186. [Google Scholar]
- 38. Cai Y, Schiffbauer JD, Hua H, Xiao S (2011) Morphology and paleoecology of the late Ediacaran tubular fossil Conotubus hemiannulatus from the Gaojiashan Lagerstätte of southern Shaanxi Province, South China. Precambrian Research 191: 46–57. [Google Scholar]
- 39. Chapman DM, Werner B (1972) Structure of a solitary and a colonial species of Stephanoscyphus (Scyphozoa, Coronatae) with observations on periderm repair Helgoländer wiss. Meeresunters 23: 393–421. [Google Scholar]
- 40. Mierzejewska G, Mierzejewski P (1979) Traces of bacterial activity on the Ordovician polychaete jaws. Acta Medica Polona 20: 35–36. [PubMed] [Google Scholar]
- 41. Dong XP, Cunningham JA, Bengston S, Thomas CW, Liu J, StampanoniI M, et al. (2013) Embryos, polyps and medusae of the early cambrian scyphozoan Olivooides . Proceedings of Royal Society B280: 20130071 10.1098/rspb.2013.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chapman DM (1966) Evolution of the Scyphistoma In: Rees W.J. (Ed.). The Cnidaria and their Evolution—Symposium of the Zoological Society of London. Londres: Academic Press, 16, p. 51–75. [Google Scholar]
- 43. Jarms G, Morandini AC, da Silveira FL (2002) Polyps of the families Atorellidae and Nausithoidae (Scyphozoa: Coronatae) new to the Brazilian fauna. Biota Neotropica 2(1): 1–11. [Google Scholar]
- 44. Van Iten H, Leme JM, Rodrigues SC, Simões MG (2005. a) Reinterpretation of a conulariid-like fossil from the Vendian of Russia. Palaeontology 48: 619–622. [Google Scholar]
- 45. Van Iten H, Vyhlasova Z, Zhu MY, Yi Q (2005. b) Widespread occurrence of microscopic pores in conulariids. Journal of Paleontology 79: 400–407. [Google Scholar]
- 46. Zabini C, Schiffbauer JD, Xiao S, Kowalewski M (2012) Biomineralization, taphonomy, and diagenesis of Paleozoic lingulide brachiopod shells preserved in silicified mudstone concretions. Palaeogeography, Palaeoclimatology, Palaeoecology 326–328: 118–127. [Google Scholar]
- 47. Mierzejewski P (1986) Ultrastructure, taxonomy and affinities of some Ordovician and Silurian organic microfossils. Palaeontologia Polonica 47: 129–220. [Google Scholar]
- 48.Oliveira RS (2010) Depósitos de rampa carbonática neoproterozóica do Grupo Corumbá, região de Corumbá, Mato Grosso. Dissertação de Mestrado, Instituto de Geociências, Universidade Federal do Pará, 105 pp.
- 49.Morais LPC (2013) Paleobiologia da Formação Bocaina (Grupo Corumbá), Ediacarano, Mato Grosso do Sul. Dissertação de Mestrado, Instituto de Geociências, Universidade de São Paulo, 122 pp.
- 50. Holst S, Jarms G (2006) Responses of solitary and colonial coronate polyps (Cnidaria, Scyphozoa, Coronatae) to sedimentation and burial. J.E.M.B.E. 329: 230–238. [Google Scholar]