Introduction
Highly active antiretroviral therapy has decreased mortality from human immunodeficiency virus type 1 infection (HIV-1) in the United States. People with HIV-1 infection in the United States who are treated with antiretroviral therapy have a near-normal life expectancy, and the number of HIV-infected people over age 50 years has increased.1, 2 Aging with HIV-1 infection, however, is accompanied by premature cardiovascular disease, osteoporosis, cirrhosis, renal disease, and dementia.1, 3 These observations have led some investigators to conclude that HIV-1-infected individuals experience premature or accelerated aging.3
“Successful aging” includes both disease burden and the ability to maintain functional status and independence.4, 5 As HIV-1-infected persons age, assessment of functional status is needed to understand the impact of the concomitant processes of aging, HIV-1 infection, comorbid diseases and lifestyle on successful aging and preservation of independence.6, 7 Prior studies assessing functional status in aging HIV-infected cohorts are limited by retrospective design, high proportions of participants with acquired immunodeficiency syndrome (AIDS) wasting syndrome, reliance on participant self-report, wide age range, or assessment of advanced deficits such as inability to perform basic activities of daily living. Few studies have compared measures of frailty and objective, performance-based assessments of higher functional status among persons with HIV-1 infection.8–17
Several functional assessment tools have been validated in older non-HIV-infected populations. As a global assessment including both subjective and objective components, Fried’s Frailty Phenotype (FFP) is associated with morbidity and mortality in elderly persons18. The presence of an FFP-related syndrome described in cohorts of younger HIV-infected individuals suggests a high risk for loss of functional independence or decline in functional status.14, 16–18 The Short Physical Performance Battery (SPPB) and the 400-m walk are widely used tools predictive of poor clinical outcomes, including mortality, in the elderly, but neither tool has been evaluated in HIV-1-infected persons.19–21
We hypothesized that 1) FFP, SPPB, and 400-m walk would have similar performance characteristics for assessing functional status in aging HIV-1-infected persons, 2) functional status tools would identify similar groups of functionally impaired people, and 3) similar clinical risk factors would predict low functional status on the three instruments. To evaluate these hypotheses, the present study compared the FFP, SPPB, and 400-m walk in patients receiving care for HIV-1 infection.
Methods
Study population
All individuals who received care for HIV-1 infection within twelve months prior to February 2010 in the Infectious Diseases Group Practice clinic at the University of Colorado Hospital were evaluated for potential participation. Individuals meeting the following criteria were eligible to participate: 1) 45 to 65 years of age; 2) able to consent and participate in study procedures; and 3) taking effective combination (two or more) antiretroviral therapy for at least six months with one undetectable plasma HIV-1 RNA (<48 copies/mL) and no plasma HIV-1 RNA >200 copies/mL in the prior six months. Eligible individuals were contacted in person, by telephone, or by letter to determine interest in study participation. Approval was obtained from the Colorado Multiple Institutional Review Board, and informed consent was obtained from all participants.
All participants completed a single study visit that included a medical record review, standardized interview, physical activity questionnaire (2-week Minnesota Leisure Time Physical Activity Questionnaire [MLTPAQ]), and a physical function assessment.
Clinical Assessments
All assessments were completed by two clinicians (K.M.E. and S.D.) at the University of Colorado Hospital. Fried’s frailty phenotype was assessed as previously described.18 Shrinking was defined as unintentional weight loss of ≥ 10 pounds, or decrease of 5% of body weight in the last year (self-reported and verified by records when available). Exhaustion was defined by three to four times per week of feeling “everything I do is an effort” or “sometimes I just cannot get going”. Low activity was defined as self-report of being “limited a lot” in vigorous physical activities from the Short-Form (SF)-36 ®, consistent with prior studies of frailty in HIV cohorts.14, 16 Weakness was assessed by the average of three dominant hand grip measurements using a single Lafayette dynamometer, applying previously defined gender and body mass index (BMI) cutoffs.18 Slowness was defined by 4.5-m walk time: men ≤ 173 cm and women ≤ 159 cm in height requiring ≥ 7 seconds or men >173 cm and women >159 cm requiring ≥ 6 seconds met a criterion.18 One point was given for each abnormality. Three to five points were considered low function, one or two points moderate function, and zero points high function.
The SPPB assessed tandem stand, walking speed, and sit-stand test time, with zero points indicating inability to complete a task and four points indicating performance within the expected range.24 Tandem stand was measured by ability to stand heel-to-toe for ten seconds, walking speed by the faster of two 4-m walks at usual pace, and sit-stand test time by five repetitions of sit-to-stand without use of the arms. A score of less than 9 is highly predictive for subsequent disability and was considered low function, 9–11 points moderate function, and 12 points (no deficits) high function.24, 25
400-m walk time was measured on a set walking course by asking the participant to walk as quickly as possible to complete the distance. Inability to complete the walk was classified as low function. To create three functional categories for comparison, high function was defined as completion in < 5.5 minutes, corresponding to a 3.4 miles/hr walking pace, the equivalent Social Security Administration criterion for disability (peak VO2 <18 mL/kg/min).26 Moderate function was defined as ability to complete the walk but requiring ≥ 5.5 minutes. This resulted in groups approximately the same size as the moderate and high function groups on the FFP and SPPB.
The Veterans Aging Cohort Study (VACS) Index was calculated using the following parameters, as previously described: CD4 count, viral load, age, aspartate aminotransferase, alanine aminotransferase, platelets, hemoglobin, hepatitis C, and estimated glomerular filtration rate.22, 23 Of a possible 164 points, higher values indicate greater mortality risk, and scores of ≤ 34 are associated with the lowest mortality.22, 23 Because all subjects in the current study had plasma HIV-1 RNA viral load < 500 copies/mL, the highest possible score was 150.
Comorbid conditions were determined from problem lists, initial clinic intake history, and clinic notes available in the electronic medical record. Current medications were determined by medical record review for prescribed medications and self-report for over-the-counter treatments. Medication count excluded current antiretroviral therapy. To assess the burden of comorbidity, a comorbid count of major diagnoses included: neurological disease (seizure disorder or dementia), stroke or transient ischemic attack, peripheral neuropathy, psychiatric disease (depression, anxiety, bipolar disorder, schizophrenia, or psychiatric disease not otherwise specified), arthritis (osteoarthritis and/or inflammatory arthritis), osteopenia or osteoporosis (prior stress fracture or T-score <−1.0 on bone density scan), diabetes, kidney disease (calculated creatinine clearance <30 mL/min by Cockcroft-Gault), malignancy (excluding non-melanoma skin cancer), solid organ transplant, lung disease (chronic obstructive pulmonary disease, asthma, pulmonary hypertension, or interstitial lung disease), hypertension, cardiovascular disease (ischemic, valvular, peripheral vascular, or congestive heart failure), viral hepatitis (hepatitis B, C, or both), and chronic liver disease of other etiology. Current/prior alcohol use was defined as drinking > 7 drinks per week or self-described abuse history. Self-reported income was dichotomized into low versus other, with less than $12,000 per year considered low. Debilitating pain was defined as responding “moderately”, “quite a bit”, or “extremely” to the SF-36 ® question “During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?”. Obesity was defined as BMI ≥ 30 kg/m2; lipoatrophy was assessed by the question “Have you been told that you have lipoatrophy?”, a positive response was confirmed by clinical evidence of lipoatrophy. To understand the contribution of any physical activity, including both vigorous activities and activities such as gardening or fishing, the MLTPAQ was included, with low reported physical activity was defined as < 500 kilocalories/week.18 Laboratory values were the most recent values available in the medical record. A viral load “blip” was considered an HIV RNA above the limit of detection but < 200 copies/mL.
Statistical Analysis
Data were collected and managed with Research Electronic Data Capture (REDCap) hosted at the University of Colorado.27 Demographic characteristics were summarized with mean and standard deviation (SD) or standard error (SE) for continuous, median and interquartile range for skewed continuous variables, and frequency with percentage for categorical outcomes. Agreement between three-category functional categorization was evaluated by weighted kappa statistic and Bowker’s test for symmetry.28 Relationships of demographics and comorbidities with function were described by odds ratios (OR) and 95% confidence intervals and adjusted for CD4+ lymphocyte count < 350 cells/μL, female gender, and age >50 years. Analyses were performed in SAS v9.2.
Results
Study population
Between February and November 2010, 542 HIV-infected patients from the Infectious Disease Group Practice Clinic fulfilled the inclusion criteria and were asked to participate: 171 either did not respond to correspondence or were not interested in study participation; 2 died prior to obtaining study consent; 369 consented to study participation and 359 completed the study assessment. Participants were 85% male with a median age of 50.8 years and a median CD4+ lymphocyte count of 551 cells/μL (Table 1).
Table 1.
Characteristics of study population.
| Characteristic | Value | N= 359 (%) |
|---|---|---|
| Age | Years (median [IQR])a | 50.8 (47.7–55.7) |
| Gender | Female | 54 (15) |
| Ethnicity | Hispanic or Latino | 65 (18) |
| Race | Caucasian | 265 (74) |
| Employment | Full-time employment | 96 (27) |
| Annual income | < $12,000 | 141 (39) |
| $12,000–25,000 | 102 (28) | |
| $25,000–50,000 | 55 (15) | |
| > $50,000 | 61 (17) | |
| Education | College or post-graduate | 108 (30) |
| Living situation | Live alone | 139 (39) |
| Other | 219 (61) | |
| HIV risk factor | MSMb | 234 (65) |
| Heterosexual | 92 (26) | |
| Intravenous drug use | 76 (21) | |
| Other or unknown | 19 (5) | |
| Current illicit drug use | Intravenous | 1 (<1) |
| Cocaine | 2 (<1) | |
| Marijuana | 81 (23) | |
| Alcohol use or abuse | None or <1 drink/week | 213 (59) |
| Prior heavy use, sober now | 59 (16) | |
| 1–7 drinks per week | 72 (20) | |
| >7 drinks per week | 15 (4) | |
| Tobacco use | Prior smoker | 130 (36) |
| Current smoker | 123 (34) | |
| Current CD4 T-cell count | Cells/μL (median [IQR]a) | 551 (361, 768) |
| HIV-1 viral load | Detectable (≥48 copies/mL) | 17 (5) |
. Interquartile range,
. men who have sex with other men
Comparison of physical function instruments
On FFP, 166 (46%) were high-, 166 (46%) moderate-, and 27 (8%) low-function (Table 2). By SPPB, 223 (62%) were high-, 110 (31%) moderate-, and 26 (7%) low-function. By 400-m walk, 166 (46%) were high-, 182 (51%) moderate-, and 11 (3%) low-function. In pairwise comparisons of the three instruments, agreement ranged from 61–64% (kappa 0.34–0.41). Seven subjects had extreme discordance (low on one assessment but high on the other) between the SPPB and FFP, two between the FFP and the 400-m walk, and none between the SPPB and the 400-m walk. Asymmetry in the classification of discordant subjects was detected for comparison of the FFP to SPPB (p<0.001), FFP to 400-m walk (p=0.03), and SPPB to 400-m walk (p=0.001, Table 2).
Table 2.
Agreement between instruments for assessment of physical function in HIV-1 infected participants.
| FFPa | SPPBb | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| High | Moderate | Low | High | Moderate | Low | |
| 400-m walk | ||||||
| High | 110 | 54 | 2 | 138 | 28 | 0 |
| Moderate | 56 | 105 | 21 | 85 | 77 | 20 |
| Low | 0 | 7 | 4 | 0 | 5 | 6 |
| Agreement | 61% | 62% | ||||
| Kappa | 0.34 (0.26, 0.42) | 0.37 (0.29, 0.45) | ||||
|
| ||||||
| SPPBb | ||||||
| High | 140 | 79 | 4 | |||
| Moderate | 23 | 77 | 10 | |||
| Low | 3 | 10 | 13 | |||
| Agreement | 64% | |||||
| Kappa | 0.41 (0.33, 0.50) | |||||
. Fried’s Frailty Phenotype, scale 0–5 with high function defined as 0 points, moderate function as 1–2 points, and low function as 3–5 points;
. Short Physical Performance Battery, scale 0–12 with high function defined as 12 points, moderate function as 9–11 points, and low function as <9 points. 400-m walk with high function defined as completion in less than 5.5 minutes, moderate function as completion requiring 5.5 minutes or greater, low function as unable to complete. The weighted Kappa strength of agreement can be characterized as: ≤0, poor; 0.01 to 0.20, slight; 0.21 to 0.40, fair; 0.41 to 0.60, moderate; 0.61 to 0.80, substantial; and 0.81 to 1, near perfect
Association of demographic and clinical features with functional status
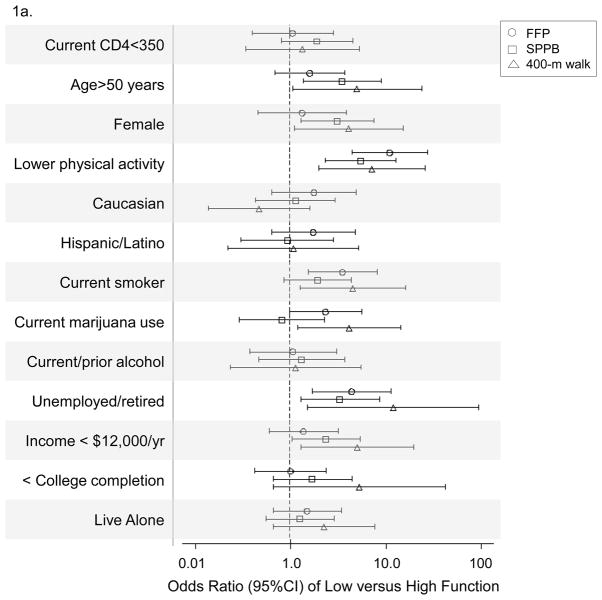
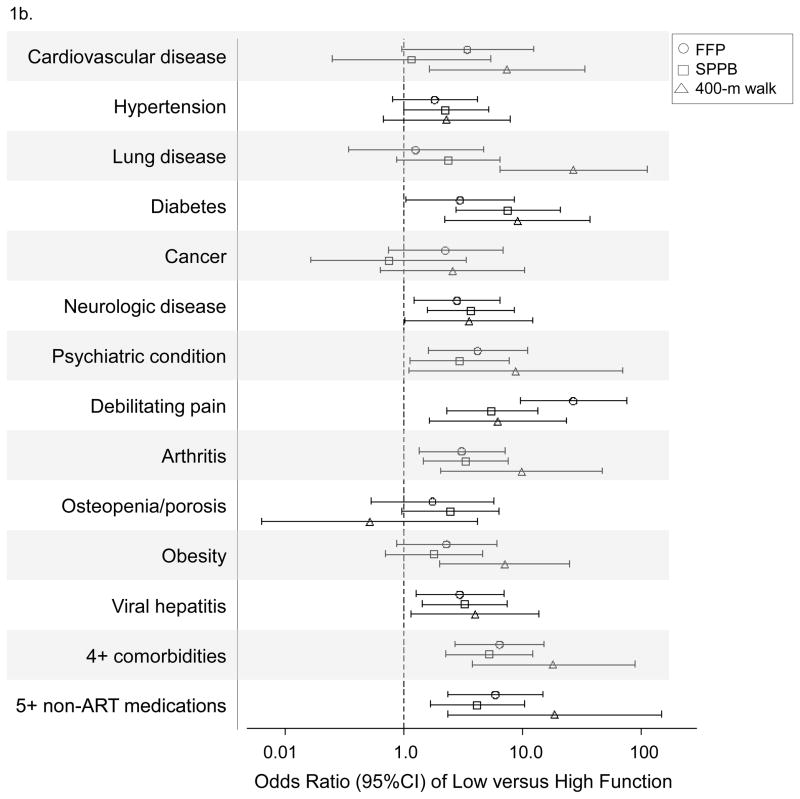
Across all three instruments, lower reported physical activity (OR>5.5; p≤0.005), no current employment (OR>4.2; p<0.02), arthritis (OR>6.5; p<0.02), neurologic disease (OR>2.6; p<0.05), debilitating pain (OR>5.4; p<0.008), psychiatric disease (OR>3.1; p<0.03), four or more comorbidities (OR>3.6; p≤0.005), and five or more non-antiretroviral therapy medications (OR>3.5; p≤0.01) were associated with lower function after adjusting for age, gender, and CD4+ lymphocytes (Figure 1a and b). Compared to the high functioning group, low function persons by FFP had 1.8 (SE 0.3) more comorbid conditions and 2.7 (SE 0.6) more medications (p<0.001); low function persons by SPPB had 1.9 (SE 0.3) more comorbid conditions and 2.5 (SE 0.6) more medications (p<0.001); and low function persons by 400-m walk had 2.5 (SE 0.5) more comorbid conditions and 3.1 (SE 0.8) more medications (p ≤ 0.01).
Figure 1.
Figure 1a. Adjusted odds of low versus high functional categorization by each assessment tool according to demographics
Figure 1b. Adjusted odds of low versus high functional categorization by each assessment tool according to comorbid diagnoses
Odds ratios with 95% confidence intervals are shown for each comparison. FFP, Fried’s Frailty Phenotype (circles); SPPB, Short Physical Performance Battery (squares); 400-m walk (triangles); ART, antiretroviral therapy.
Compared to high-functioning individuals, low-functioning individuals identified by FFP also had higher odds of smoking, diabetes, and hepatitis (OR>2.9; p<0.05). After adjusting for age, gender, and CD4+ lymphocytes, diabetes was no longer significant (p>0.05); falls, smoking, and psychiatric disease remained highly significant (OR>4.0; p<0.01). Low-functioning individuals identified by SPPB had significantly higher odds of older age, female gender, low income, diabetes, and hepatitis (OR>2.3; p<0.05). After adjusting for age, gender, and CD4+ lymphocytes, income was not significant (p>0.05); only diabetes remained highly significant (OR>9.1; p<0.01). Low-functioning individuals identified by the 400-m walk had higher odds of smoking, marijuana use, older age, female gender, low income, cardiovascular and lung disease, diabetes, obesity, and hepatitis (OR>3.9; p<0.01). After adjusting, hepatitis was not significant (p>0.05); lung disease, diabetes, arthritis, and obesity remained highly significant (OR>7.0; p<0.01). Similar trends were observed for comparison of low- to moderate- and moderate- to high-functioning groups (not shown).
HIV-related characteristics were not significantly different (p>0.05) between low- and high-functioning persons on the FFP, SPPB, or 400-m walk, with the exception that current CD4 <200 cells/μL was more likely among low- (11%) than high- (2%) functioning persons on the FFP (Table 3; p=0.04). By the SPPB, low-functioning persons were less likely to be men reporting sex with men (p=0.02) and more likely to report blood transfusion or “other” risk factor of HIV transmission (p=0.04). VACS Index scores were calculated on each subject and ranged from zero to 78 (mean 18.2, SE 0.7). Differences in VACS Index scores between high- and low-functioning persons were observed for the SPPB (12.4, SE 2.8 points; p=0.001), FFP (8.6, SE 2.8 points; p=0.03) and 400-m walk (11.9, SE 4.2 points; p=0.08, Table 3).
Table 3.
Comparison of HIV-related characteristics between high and low functioning groups by each assessment tool.
| Characteristic | Functional Status Group by Assessment Tool
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fried’s Frailty Phenotype | P value | Short Physical Performance Battery | P value | 400-m walk | P value | ||||
| High N=167 (%) |
Low N=27 (%) |
High N=223 (%) |
Low N=26 (%) |
High N=166 (%) |
Low N=11 (%) |
||||
| Years since HIV diagnosis (mean, standard error) | 14.5 (0.6) | 15.5 (1.5) | 0.54 | 14.2 (0.5) | 16.2 (1.6) | 0.25 | 14.4 (0.6) | 17.9 (2.3) | 0.17 |
| History of AIDS | 105 (63) | 21 (78) | 0.19 | 143 (64) | 18 (69) | 0.67 | 97 (58) | 7 (64) | 0.77 |
| Prior opportunistic infection | 60 (36) | 10 (37) | 1.0 | 74 (33) | 7 (27) | 0.67 | 55 (33) | 2 (18) | 0.35 |
| Lipoatrophy | 32 (19) | 5 (19) | 1.0 | 48 (22) | 7 (27) | 0.62 | 32 (19) | 2 (18) | 1.0 |
| Risk for HIV acquisition | |||||||||
| MSMa | 113 (68) | 19 (70) | 0.83 | 148 (66) | 11 (42) | 0.02 | 118 (71) | 5 (45) | 0.09 |
| Intravenous drug use | 21 (13) | 3 (11) | 1.0 | 35 (16) | 4 (15) | 1.0 | 22 (13) | 2 (18) | 0.65 |
| Heterosexual | 45 (27) | 4 (15) | 0.24 | 56 (25) | 9 (35) | 0.35 | 41 (25) | 3 (27) | 1.0 |
| Other | 6 (4) | 2 (7) | 0.60 | 9 (4) | 4 (15) | 0.04 | 7 (4) | 2 (18) | 0.1 |
| CD4+ T-cells/μL (geometric mean, 95% CIb) | 556 (514–601) | 459 (344–613) | 0.20 | 516 (477–559) | 448 (345–580) | 0.29 | 517 (469–570) | 542 (376–782) | 0.79 |
| Current CD4+ lymphocyte count <200 cells/μL | 3 (2) | 3 (11) | 0.04 | 15 (7) | 3 (12) | 0.41 | 11 (7) | 0 (0) | 0.63 |
| Nadir CD4+ T-cells/μL (geometric mean, 95% CIb) | 88 (69–112) | 58 (34–99) | 0.15 | 91 (74–111) | 75 (44–128) | 0.50 | 99 (78–124) | 112 (51–249) | 0.73 |
| Viral load “blip” | 23 (14) | 4 (15) | 1.0 | 33 (15) | 3 (12) | 0.78 | 20 (12) | 0 (0) | 0.37 |
| VACSc Index Score (mean, standard error) | 15.1 (0.9) | 23.8 (3.8) | 0.03 | 15.8 (0.9) | 28.2 (3.4) | 0.001 | 15.8 (1.0) | 27.6 (6.0) | 0.08 |
| Current use of: | |||||||||
| NRTId | 161 (97) | 25 (93) | 0.25 | 217 (97) | 26 (100) | 0.63 | 162 (98) | 11 (100) | 1.0 |
| NNRTIe | 72 (43) | 13 (48) | 0.68 | 99 (44) | 12 (46) | 1.0 | 76 (46) | 7 (64) | 0.35 |
| Protease inhibitor | 82 (49) | 17 (63) | 0.22 | 115 (52) | 11 (42) | 0.41 | 83 (50) | 3 (27) | 0.21 |
| Integrase inhibitor | 37 (22) | 4 (15) | 0.46 | 48 (22) | 5 (19) | 0.81 | 33 (20) | 2 (18) | 1.0 |
| Entry inhibitor | 2 (1) | 0 (0) | 1.0 | 1 (<1) | 0 (0) | 1.0 | 0 (0) | 0 (0) | 1.0 |
| Any (current/prior) use of: | |||||||||
| NRTId | 165 (99) | 25 (93) | 0.05 | 221 (99) | 25 (96) | 0.28 | 163 (98) | 10 (91) | 0.23 |
| NNRTIe | 118 (71) | 21 (78) | 0.50 | 156 (70) | 18 (69) | 1.0 | 119 (72) | 9 (82) | 0.52 |
| Protease inhibitor | 117 (70) | 23 (85) | 0.16 | 163 (73) | 20 (77) | 0.82 | 119 (72) | 7 (64) | 0.73 |
| Integrase inhibitor | 37(22) | 6 (22) | 1.0 | 48 (22) | 8 (31) | 0.32 | 34 (20) | 1 (9) | 0.47 |
| Entry inhibitor | 8 (5) | 2 (7) | 0.63 | 7 (3) | 1 (4) | 1.0 | 4 (2) | 0 (0) | 1.0 |
. Men whom have sex with other men,
. confidence interval,
. Veterans Aging Cohort Study,
. nucleoside reverse transcriptase inhibitor,
. non-nucleoside reverse transcriptase inhibitor
In evaluation of individual exam components across the instruments, SPPB sit-stand score <4 had consistently high sensitivity and specificity for predicting low function on FFP (82% sensitivity, 91% specificity) and 400-m walk (86% sensitivity, 86% specificity). Sit-stand time was the only test on which no low-function individuals (by FFP, SPPB, or 400-m walk) achieved the highest level of performance. Low activity from FFP was sensitive and specific for predicting low-functioning groups on SPPB (80% sensitivity, 91% specificity) and 400-m walk (80% sensitivity, 83% specificity; Table 4).
Table 4.
Comparison of instrument summary scores and individual components between high, moderate, and low functioning groups.
| Exam Component | Fried’s Frailty Phenotype | Short Physical Performance Battery | 400-m walk | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
High N=167 |
Moderate N=165 |
Low N=27 |
High N=223 |
Moderate N=110 |
Low N=26 |
High N=166 |
Moderate N=182 |
Low N=11 |
|
| FFPa Score, mean (SDb) | -- | 1.4 (0.5) | 3.2 (0.5) | 0.5 (0.8) | 1.3 (0.9) | 2.3 (1.3) | 0.5 (0.7) | 1.2 (1.0) | 2.4 (1.3) |
| Weakness (%) | -- | 11 | 70 | 4 | 15 | 27 | 3 | 15 | 36 |
| Weight loss (%) | -- | 14 | 41 | 8 | 11 | 23 | 7 | 12 | 18 |
| Fatigue (%) | -- | 52 | 85 | 21 | 45 | 58 | 19 | 40 | 55 |
| Low activity (%) | -- | 61 | 100 | 20 | 58 | 80 | 17 | 50 | 91 |
| Slow walk (%) | -- | 1 | 22 | 0 | 0 | 31 | 0 | 2 | 36 |
|
| |||||||||
| SPPBc Score, mean (SDb) | 11.7 (0.7) | 10.9 (1.5) | 8.5 (2.9) | -- | 10.3 (0.8) | 6.5 (1.7) | 11.8 (0.6) | 10.7 (1.7) | 7.8 (3.3) |
| 4 m walk | |||||||||
| 0 points (%) | 0 | 0 | 4 | -- | 0 | 4 | 0 | 0 | 9 |
| 1 point (%) | 0 | 1 | 0 | -- | 0 | 4 | 0 | 0 | 9 |
| 2 points (%) | 0 | 1 | 11 | -- | 0 | 19 | 0 | 2 | 9 |
| 3 points (%) | 2 | 8 | 22 | -- | 12 | 38 | 1 | 11 | 18 |
| 4 points (%) | 98 | 90 | 63 | -- | 88 | 35 | 99 | 87 | 55 |
| Sit-stand test | |||||||||
| 0 points (%) | <1 | 2 | 19 | -- | 0 | 38 | 0 | 3 | 37 |
| 1 point (%) | <1 | 7 | 22 | -- | 9 | 35 | <1 | 9 | 9 |
| 2 points (%) | 3 | 10 | 22 | -- | 21 | 19 | 2 | 13 | 18 |
| 3 points (%) | 10 | 24 | 19 | -- | 53 | 8 | 12 | 20 | 27 |
| 4 points (%) | 86 | 57 | 18 | -- | 17 | 0 | 86 | 55 | 9 |
| Tandem stand | |||||||||
| 0 points (%) | 0 | 0 | 7 | -- | 0 | 8 | 0 | 1 | 9 |
| 1 point (%) | 0 | 2 | 4 | -- | 1 | 15 | <1 | 2 | 0 |
| 2 points (%) | 2 | 2 | 19 | -- | 5 | 27 | <1 | 5 | 18 |
| 3 points (%) | 2 | 13 | 4 | -- | 20 | 15 | 2 | 12 | 9 |
| 4 points (%) | 96 | 83 | 63 | -- | 74 | 35 | 97 | 80 | 64 |
|
| |||||||||
| Completed 400-m walkd (%) | 100 | 96 | 85 | 100 | 95 | 77 | -- | -- | -- |
| 400-m walk time in m/sec (SDb) | 1.6 (0.2) | 1.4 (0.3) | 1.2 (0.3) | 1.6 (0.2) | 1.4 (0.2) | 1.0 (0.3) | 1.7 (0.1) | 1.3 (0.2) | -- |
. Fried’s Frailty Phenotype, scale 0–5 with 5 indicating the most frailty. By definition, all high functioning persons by FFP had no deficits in any of the FFP components.
. Standard deviation.
. Short Physical Performance Battery, scale 0–12, with 0 points indicating that a task could not be completed and a total score of 0 indicating the lowest function. By definition, all high functioning persons by SPPB scored 4 points in each category.
. By definition, all low functioning persons on the 400-m walk were unable to complete the walk.
Discussion
Although declining functional capacity in elderly persons is well described, few studies have investigated the effect of aging on impairments in both objective and subjective functional status in people with HIV-1 infection.13, 15–17, 29, 30 The FFP, SPPB and the 400-m walk tests are commonly used to assess frailty and disability in older persons and they correlate with morbidity and mortality.18, 31, 32 The comparative performance of these instruments in HIV-1-infected persons, who are at risk for functional impairment at younger ages, had not been investigated previously.1, 13, 14, 30, 33 Our study provides the first prospective comparison of these three instruments for assessing functional status in persons receiving care for HIV-1 infection.
The entry criteria for the study resulted in the recruitment of middle-aged HIV-1-infected persons who had evidence of successful antiretroviral therapy with suppression of plasma HIV-1 RNA below limits of detection. In this study population, all three instruments revealed high levels of impairment. Greater than 50% of participants were unable to maintain a walking speed faster than 3.4 miles per hour in the 400-m walk test, thereby meeting a criterion for Social Security Administration disability26, and an additional 3% of individuals were unable to walk a distance of 400-m at any pace. Over 7% of the study population met criteria for frailty. These frequencies of functional impairment are surprising given the relatively young age range of the cohort. Indeed, the frequency of functional impairment in the study population was similar to that described in other studies of non-HIV-infected persons who were 20–30 years older than our participants.14, 34–38 Although the cross-sectional design of our study did not allow for examination of trends over time, the frequency of moderate to severe functional impairment among middle-aged HIV-1-infected persons is concerning, and raises questions about future resource needs and expertise to care for aging, functionally impaired HIV-1-infected persons.
Despite the finding that all three instruments detected similar frequencies of functional impairment in the study population, there was only modest agreement across instruments for the degree of impairment. These discordances can be explained, in part, by the characteristics of the assessments: the FFP is a global assessment including subjective components that may overlap with depressive symptoms, the SPPB focuses on lower extremity function, and the 400-m walk requires more endurance and incorporates the broad impact of multiple disease processes (Table 5).18, 19, 32, 34, 39, 40
Table 5.
Summary of functional status assessments
| Highly significant predictorsa (p<0.01) | Strengths | Weaknesses | |
|---|---|---|---|
| FFPb | Psychiatric disease Tobacco use More comorbidities and medications Low physical activity Debilitating pain |
Existing data in HIV-infected adults [14–17] Global assessment of function [18] |
Overlap with symptoms of depression [37] Subjective criteria Requires tool to measure grip strength |
| SPPBc | Diabetes More comorbidities and medications Low physical activity Debilitating pain |
Objective and standardized Data on sit-stand time in HIV-infected adults [13] |
Ceiling effect in higher functioning persons [37] Focus on lower extremity function [21] |
| 400-m walk | Obesity Diabetes Arthritis Lung disease More comorbidities and medications Debilitating pain |
Objective and standardized Continuous variable Incorporates impact of multiple diseases [38] No ceiling or floor effect [37] |
Requires more space and time than SPPB |
. Highly significant predictors (p<0.01) after adjusting for age > 50 years, female gender, and CD4+ lymphocyte count < 350 cells/μL
. Fried’s Frailty Phenotype;
. Short Physical Performance Battery
Several aspects of this study limit generalizability to other clinical settings. First, by recruiting patients on suppressive antiretroviral therapy and in a defined “pre-geriatric” age range, we sought to limit the effect of the confounding processes of ongoing viremia and aging but may have selected for a cohort with better treatment adherence, less likely to be using illicit substances, and more engaged in medical care. Second, a number of eligible patients chose not to participate in our study for unknown reasons, which could have affected study outcomes. Persons who chose not to participate may have had greater impairments; conversely, time constraints from full-time employment in higher functional persons may have prohibited study involvement. The predominately male and Caucasian study population, although reflective of the underlying clinic population, may not be reflective of functional status findings in more diverse settings. As demonstrated by the large confidence intervals, select comorbidities and low functional status occurred relatively infrequently. Variability and reliability between observers was not tested. Lastly, because the cross-sectional design of the study captured function only at a single time point, we were not able to assess temporal changes in functional status. Longitudinal studies are needed to define the rate of functional status change, particularly among patients who have moderately impaired function at relatively young ages, and to assess the potential efficacy of interventions to slow or reverse impairment.
Conclusion
Our study demonstrated that middle-aged persons with suppressed HIV-1 infection on antiretroviral therapy frequently have functional impairments similar to HIV-uninfected persons 20–30 years older.14, 34–37 Similar frequencies of impairment were detected by all three functional assessment instruments. Although each instrument was associated with unique demographic or clinical risk factors for impairment, across all three instruments, the strongest associations were seen with low physical activity, high comorbid disease and medication burden, psychiatric disease, arthritis, and debilitating pain. Consistent with geriatric syndromes, the findings emphasize that the accumulation of disease and medication burden account for impairments in functional capacity. Furthermore, the findings underscore the importance of psychological well-being in maintaining functional independence with aging. Although the direction of these relationships cannot be established through this cross-sectional study, our findings suggest that regular physical activity could have important implications in maintenance of functional independence among persons aging with HIV-infection through prevention of modifiable diseases, avoidance of additional medication burden, reduction in arthritis symptoms, and improvement in psychological well-being.
Acknowledgments
We gratefully acknowledge the study participants and Janet Tate for her assistance in use of the VACS index.
This work was supported by the National Center for Research Resources at the National Institutes of Health (Colorado CTSA 5UL1RR025780 and T32 AI007447-1); the Hartford Foundation Center of Excellence; and GlaxoSmithKline HIV Collaborative Investigator Research Award. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
Conflicts of Interest: None
References
- 1.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008 Aug 15;47(4):542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison KM, Song R, Zhang X. Life expectancy after HIV diagnosis based on national HIV surveillance data from 25 states, United States. Journal of acquired immune deficiency syndromes. 2010 Jan 1;53(1):124–130. doi: 10.1097/QAI.0b013e3181b563e7. [DOI] [PubMed] [Google Scholar]
- 3.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Topics in HIV medicine : a publication of the International AIDS Society, USA. 2009 Sep-Oct;17(4):118–123. [PubMed] [Google Scholar]
- 4.Rowe JW, Kahn RL. Successful aging. The Gerontologist. 1997 Aug;37(4):433–440. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- 5.Hawton A, Green C, Dickens AP, et al. The impact of social isolation on the health status and health-related quality of life of older people. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2011 Feb;20(1):57–67. doi: 10.1007/s11136-010-9717-2. [DOI] [PubMed] [Google Scholar]
- 6.High KP, Bradley S, Loeb M, Palmer R, Quagliarello V, Yoshikawa T. A new paradigm for clinical investigation of infectious syndromes in older adults: assessment of functional status as a risk factor and outcome measure. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005 Jan 1;40(1):114–122. doi: 10.1086/426082. [DOI] [PubMed] [Google Scholar]
- 7.Justice AC. HIV and aging: time for a new paradigm. Current HIV/AIDS reports. 2010 May;7(2):69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- 8.Oursler KK, Goulet JL, Crystal S, et al. Association of age and comorbidity with physical function in HIV-infected and uninfected patients: results from the Veterans Aging Cohort Study. AIDS patient care and STDs. 2011 Jan;25(1):13–20. doi: 10.1089/apc.2010.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oursler KK, Goulet JL, Leaf DA, et al. Association of comorbidity with physical disability in older HIV-infected adults. AIDS patient care and STDs. 2006 Nov;20(11):782–791. doi: 10.1089/apc.2006.20.782. [DOI] [PubMed] [Google Scholar]
- 10.Stanton DL, Wu AW, Moore RD, et al. Functional status of persons with HIV infection in an ambulatory setting. Journal of acquired immune deficiency syndromes. 1994 Oct;7(10):1050–1056. [PubMed] [Google Scholar]
- 11.O’Dell MW, Hubert HB, Lubeck DP, O’Driscoll P. Pre-AIDS physical disability: data from the AIDS Time-Oriented Health Outcome Study. Archives of physical medicine and rehabilitation. 1998 Oct;79(10):1200–1205. doi: 10.1016/s0003-9993(98)90262-3. [DOI] [PubMed] [Google Scholar]
- 12.Rusch M, Nixon S, Schilder A, Braitstein P, Chan K, Hogg RS. Impairments, activity limitations and participation restrictions: prevalence and associations among persons living with HIV/AIDS in British Columbia. Health and quality of life outcomes. 2004;2:46. doi: 10.1186/1477-7525-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richert L, Dehail P, Mercie P, et al. High frequency of poor locomotor performance in HIV-infected patients. AIDS. 2011 Mar 27;25(6):797–805. doi: 10.1097/QAD.0b013e3283455dff. [DOI] [PubMed] [Google Scholar]
- 14.Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. The journals of gerontology. Series A, Biological sciences and medical sciences. 2007 Nov;62(11):1279–1286. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 15.Desquilbet L, Jacobson LP, Fried LP, et al. A frailty-related phenotype before HAART initiation as an independent risk factor for AIDS or death after HAART among HIV-infected men. The journals of gerontology. Series A, Biological sciences and medical sciences. 2011 Sep;66(9):1030–1038. doi: 10.1093/gerona/glr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onen NF, Agbebi A, Shacham E, Stamm KE, Onen AR, Overton ET. Frailty among HIV-infected persons in an urban outpatient care setting. The Journal of infection. 2009 Nov;59(5):346–352. doi: 10.1016/j.jinf.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Terzian AS, Holman S, Nathwani N, et al. Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART. Journal of women’s health. 2009 Dec;18(12):1965–1974. doi: 10.1089/jwh.2008.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology. Series A, Biological sciences and medical sciences. 2001 Mar;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 19.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA : the journal of the American Medical Association. 2011 Jan 5;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vestergaard S, Patel KV, Bandinelli S, Ferrucci L, Guralnik JM. Characteristics of 400-meter walk test performance and subsequent mortality in older adults. Rejuvenation research. 2009 Jun;12(3):177–184. doi: 10.1089/rej.2009.0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolland Y, Lauwers-Cances V, Cesari M, Vellas B, Pahor M, Grandjean H. Physical performance measures as predictors of mortality in a cohort of community-dwelling older French women. European journal of epidemiology. 2006;21(2):113–122. doi: 10.1007/s10654-005-5458-x. [DOI] [PubMed] [Google Scholar]
- 22.Tate JJAC. Change in a prognostic index for survival in HIV infection after on year on cART by level of adherence. Presented at: 48th Annual IDSA Meeting; 2010; Vancouver. [Google Scholar]
- 23.Justice AC, McGinnis KA, Skanderson M, et al. Towards a combined prognostic index for survival in HIV infection: the role of ‘non-HIV’ biomarkers. HIV medicine. 2010 Feb;11(2):143–151. doi: 10.1111/j.1468-1293.2009.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of gerontology. 1994 Mar;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 25.Chiarantini D, Volpato S, Sioulis F, et al. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J Card Fail. 2010 May;16(5):390–395. doi: 10.1016/j.cardfail.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Alexander NB, Dengel DR, Olson RJ, Krajewski KM. Oxygen-uptake (VO2) kinetics and functional mobility performance in impaired older adults. The journals of gerontology. Series A, Biological sciences and medical sciences. 2003 Aug;58(8):734–739. doi: 10.1093/gerona/58.8.m734. [DOI] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–174. [PubMed] [Google Scholar]
- 29.Oursler KK, Katzel LI, Smith BA, Scott WB, Russ DW, Sorkin JD. Prediction of cardiorespiratory fitness in older men infected with the human immunodeficiency virus: clinical factors and value of the six-minute walk distance. Journal of the American Geriatrics Society. 2009 Nov;57(11):2055–2061. doi: 10.1111/j.1532-5415.2009.02495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oursler KK, Sorkin JD, Smith BA, Katzel LI. Reduced aerobic capacity and physical functioning in older HIV-infected men. AIDS research and human retroviruses. 2006 Nov;22(11):1113–1121. doi: 10.1089/aid.2006.22.1113. [DOI] [PubMed] [Google Scholar]
- 31.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA : the journal of the American Medical Association. 2006 May 3;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 32.Vasunilashorn S, Coppin AK, Patel KV, et al. Use of the Short Physical Performance Battery Score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. The journals of gerontology. Series A, Biological sciences and medical sciences. 2009 Feb;64(2):223–229. doi: 10.1093/gerona/gln022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onen NF, Overton ET. A review of premature frailty in HIV-infected persons; another manifestation of HIV-related accelerated aging. Current aging science. 2011 Feb;4(1):33–41. [PubMed] [Google Scholar]
- 34.Cesari M, Onder G, Zamboni V, et al. Physical function and self-rated health status as predictors of mortality: results from longitudinal analysis in the ilSIRENTE study. BMC geriatrics. 2008;8:34. doi: 10.1186/1471-2318-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel KV, Coppin AK, Manini TM, et al. Midlife physical activity and mobility in older age: The InCHIANTI study. American journal of preventive medicine. 2006 Sep;31(3):217–224. doi: 10.1016/j.amepre.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Syddall H, Roberts HC, Evandrou M, Cooper C, Bergman H, Aihie Sayer A. Prevalence and correlates of frailty among community-dwelling older men and women: findings from the Hertfordshire Cohort Study. Age and ageing. 2010 Mar;39(2):197–203. doi: 10.1093/ageing/afp204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Espinoza SE, Jung I, Hazuda H. Lower frailty incidence in older Mexican Americans than in older European Americans: the San Antonio Longitudinal Study of Aging. Journal of the American Geriatrics Society. 2010 Nov;58(11):2142–2148. doi: 10.1111/j.1532-5415.2010.03153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morie M, Reid KF, Miciek R, et al. Habitual physical activity levels are associated with performance in measures of physical function and mobility in older men. Journal of the American Geriatrics Society. 2010 Sep;58(9):1727–1733. doi: 10.1111/j.1532-5415.2010.03012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drey M, Pfeifer K, Sieber CC, Bauer JM. The Fried frailty criteria as inclusion criteria for a randomized controlled trial: personal experience and literature review. Gerontology. 2011;57(1):11–18. doi: 10.1159/000313433. [DOI] [PubMed] [Google Scholar]
- 40.Sayers SP, Guralnik JM, Newman AB, Brach JS, Fielding RA. Concordance and discordance between two measures of lower extremity function: 400 meter self-paced walk and SPPB. Aging clinical and experimental research. 2006 Apr;18(2):100–106. doi: 10.1007/BF03327424. [DOI] [PubMed] [Google Scholar]