Abstract
Osteosarcomas are highly malignant bone tumours. Its appearance in craniofacial bones is a rare entity and accounts for only 1% of all head and neck malignancies. We present an uncommon case report of a 42 year old male patient with osteosarcoma of left maxilla, which was successfully excised under general anesthesia. The patient reported to us with a history of pain, swelling, pus discharge and tooth extraction, which led to the differential diagnosis of suppurative osteomyelitis, dentoalveolar abscess, benign odontogenic tumour, an infected cyst etc. Histopathological examination of incisional biopsy was reported as chondromyxoid fibroma which is a rare benign tumour. However the excisional biopsy specimen was reported as osteosarcoma of maxilla. The clinical presentation, diagnostic challenges and its therapeutic approach are addressed. This case serves to emphasize the need to recognize osteosarcoma when it presents in unexpected locations, especially because of its rarity.
Keywords: Osteosarcoma, Maxilla
Introduction
Osteosarcomas are highly malignant and rare bone tumours. The long bones, particularly in the knee region are the most commonly affected area. The incidence of craniofacial osteosarcoma as mentioned in the published literature vary and is as follows, 7% [1], 6% [5], 8% [7], 6–13% [3, 9]. Craniofacial osteosarcomas account for only 1% of all head and neck malignancies [1]. In general, osteosarcoma of the jaws tend to occur later in life than osteosarcoma of the long bones [2]. Presenting signs and symptoms of craniofacial osteosarcomas include regional swelling, pain and paresthesia, change in tooth position, loose teeth or change in fit of dental prosthesis. Many of these signs and symptoms are nonspecific and they can be produced by a number of different developmental, infectious, benign neoplastic lesions or malignancies. Thus an osteosarcoma of maxillofacial region often goes undiagnosed for a significant period of time [3, 4]. The diagnosis of these tumours is sometimes difficult also due to their rarity [5]. Radiographically they may present with an expansion of bone, an incidental radiographic finding of a radiopacity, or a widened periodontal ligament (PDL) space (garrington sign) [4]. Biopsy of the lesion may be diagnostic with histopathological features consistent with osteosarcoma. However, described variants including osteoblastic and chondroblastic osteosarcomas as well as heterogenous appearances may make histopathological diagnosis difficult [5]. The mainstay of treatment of head and neck osteosarcoma is surgery [3, 5, 6]. Over the last decade, chemotherapy has been consistently added to the therapeutic armamentarium. However, the role of induction or adjuvant chemotherapy remains uncertain and no prospective studies have examined these strategies [5, 6]. Adjuvant postoperative radiotherapy is indicated for those with close or positive margins [6].
The main purpose of this case report is to highlight its unusual presentation as a maxillary expansile swelling bulging into the maxillary sinus, as well as its benign histopathological appearance on incisional biopsy which lead to an initial diagnosis of chondromyxoid fibroma.
Case Report
Male patient aged 45, a farmer by occupation, reported to Department of Oral and Maxillofacial Surgery with the chief complaint of swelling in his upper left back teeth region since two months and numbness in the same region since 15 days. Patient gave history of undergoing extraction of upper left second molar tooth due to increased mobility two months ago, following which he developed swelling in the same region. Patient also complained of foul odor, heaviness of head and pain which was dull aching, intermittent in nature and radiating to left temporal region. Patient had temporary relief from pain following consumption of pain killers. History of pus discharge on and off was present in relation to the swelling. However there was no history of fever/loss of appetite/loss of weight/disturbed sleep/epistaxis/nasal obstruction/nasal discharge/change in taste/change in voice/change in vision.
There was no relevant medical history. Patient did not have any deleterious habits such as smoking/pan chewing/alcohol consumption. Also, no significant finding was noted on general physical examination. On extraoral examination, there was no gross facial asymmetry observed. Mouth opening was adequate. TMJ and lateral jaw movements were satisfactory. On extraoral palpation no significant finding was noted. Intraorally on inspection, a solitary lobulated swelling was present approximately measuring 2 × 2 cm, extending from upper left first molar to maxillary tuberosity region on the same side. The upper left second and third molar were missing. There was both expansion of the buccal and palatal cortices with blanching of the overlying mucosa. However there was no obliteration of the vestibular sulcus/sinus opening/ulceration (refer Fig. 1). On palpation, inspectory findings were confirmed. Swelling was firm and non tender. There was no pulsation, bruit, fluctuation or crepitus. Adjacent teeth were firm without any evidence of dental caries and non tender on percussion. The remaining dentition was periodontally sound, oral hygiene was fair; although with significant halitosis.
Fig. 1.
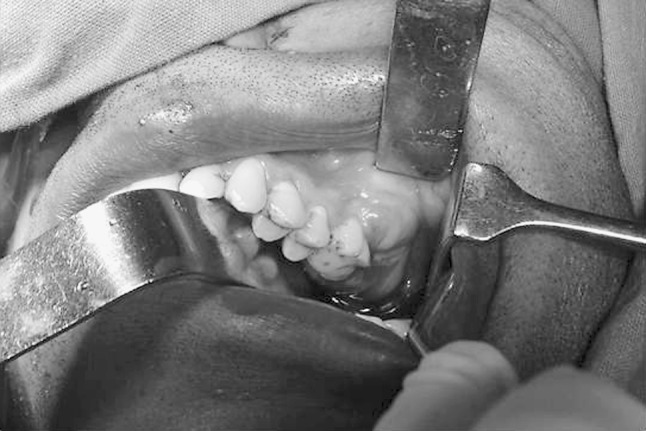
Solitary lobulated swelling extending from upper left first molar to maxillary tuberosity region with missing upper left second and third molar
Orthopantamograph (OPG) revealed radiolucency of extraction socket in relation to upper left second molar and vertically impacted third molar with periapical radiolucency and widening of PDL space (refer Fig. 2). Paranasal sinus (PNS) radiograph showed haziness of the left maxillary sinus. Based on clinical examination and basic radiologic investigation, a provisional diagnosis of an infected cyst was made. Differential diagnosis included suppurative osteomyelitis, infected cyst, benign odontogenic tumour and non odontogenic bone tumour. An incisional biopsy was performed under local anesthesia and sent for histopathological examination (HPE) which was reported as chondromyxoid fibroma of left maxilla.
Fig. 2.
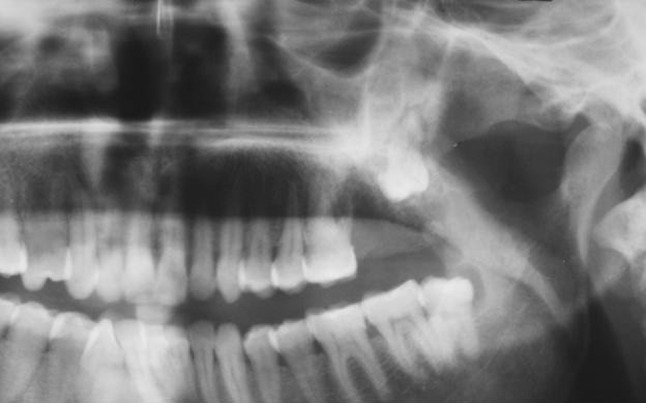
OPG revealing garrington’s sign in relation to upper left impacted third molar
Computed tomography (CT) with IV contrast, axial and coronal cuts revealed poorly defined areas of sclerosis with few lytic areas seen involving the maxillary sinus and areas of left half of maxilla causing reduction in size of left maxillary sinus. The involved bone showed areas of rarefaction and disruption of normal architecture. Frontal sinus, anterior, middle and posterior group of ethmoid air cells and sphenoid sinus were normal (refer Fig. 3).
Fig. 3.
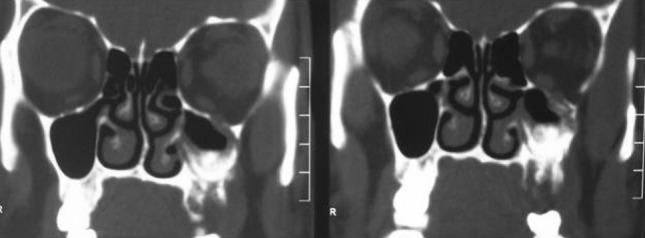
CT coronal cuts presenting poorly defined areas of sclerosis involving left maxillary sinus
Based on the investigations and incisional biopsy report, wide local excision of the tumour was planned followed by reconstruction with buccal fat pad/temporalis muscle flap under GA and prosthetic rehabilitation at a later date.
Right nasal endotracheal intubation was performed. A modified Weber Ferguson incision was used to expose the tumour. The second premolar on left side was extracted, osteotomy cuts were made on the buccal and palatal aspect along the premolar socket and extended superiorly and posteriorly to separate the posterior part of maxilla. Cuts were completed using osteotomes and the pterygoid plates separated. The tumour was seen bulging into the maxillary sinus, however it was not attached to any of the walls of the sinus except the posterolateral wall (refer Fig. 4). The entire tumour was removed in toto (refer Fig. 5) and defect closed with buccal fat pad. Primary mucosal closure was achieved. Sutures were removed on the 10th day post-op and surgical site healing was satisfactory.
Fig. 4.
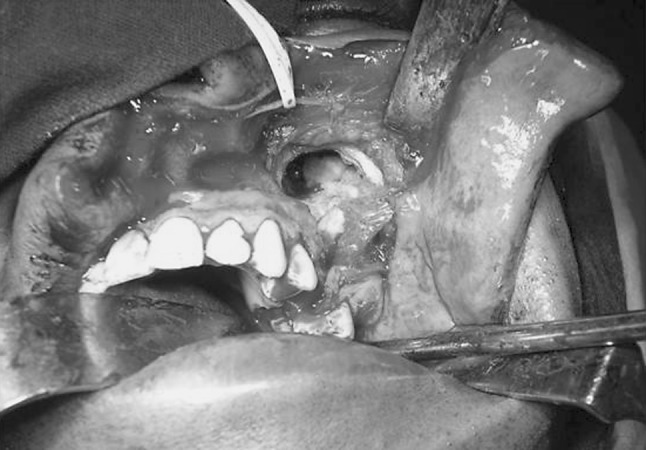
Tumour seen bulging into the left maxillary sinus, without involving the walls of the sinus (except lateral wall)
Fig. 5.
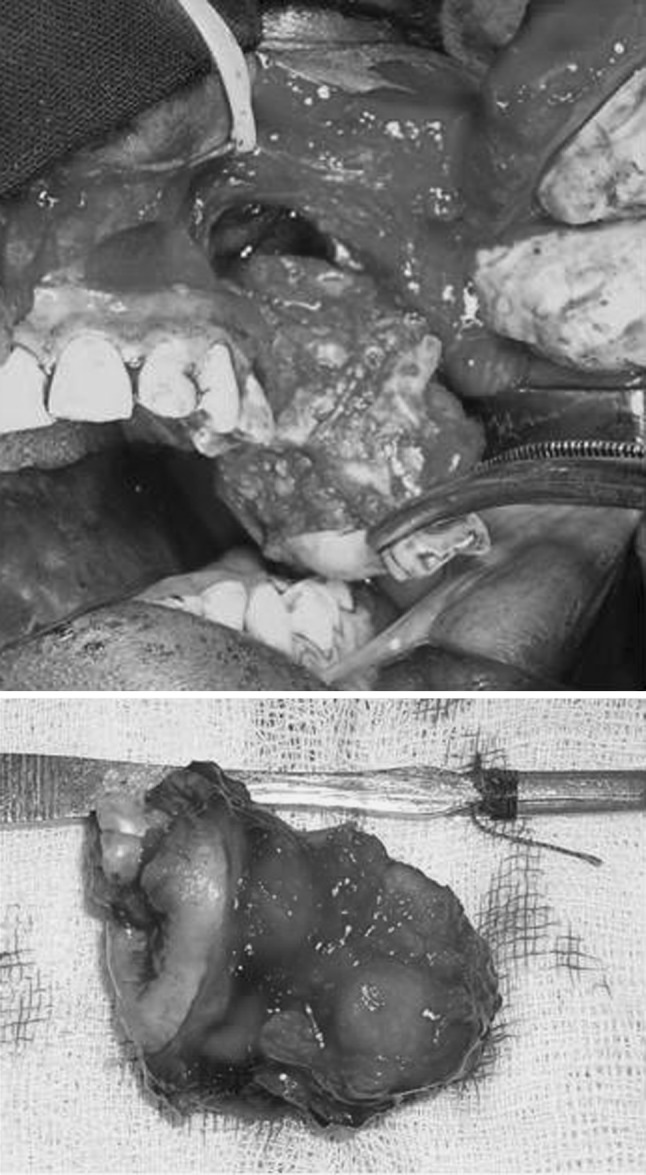
Tumour excision in toto
The resected specimen measured 4 × 3 × 2.2 cm which was sent for HPE. It was reported as osteoblastic variant of osteosarcoma with focal chondroid differentiation with positive margins. Based on the excisional biopsy report the patient was referred to medical and radiation oncologist for opinion and further management. Chemotherapy was concurrent in intent for attempted increased control of disease combined with radiation therapy (RT). Patient underwent five cycles of chemotherapy with 60 mg Cisplatin and RT for the same. However he discontinued RT for a period of one month due to personal reasons. He returned after four months with ptosis of left eye and numbness of left temporal region. CT brain and PNS with contrast revealed lesion in the left infratemporal fossa suggesting recurrence and also secondaries in brain. Patient is currently undergoing chemotherapy with ifosfamide and doxorubicin. Although prognosis appears grave he is still under periodic follow up.
Discussion
Osteosarcoma is a bone tumour which is thought to arise from a primitive mesenchymal bone forming cell and is characterized by production of osteoid. It can occur in any bone but most commonly occurs in the long bones of the extremities near metaphyseal growth plates [7]. Craniofacial osteosarcomas, most often located in the mandible or maxilla, account for only 6–13% of all osteosarcomas [3].
In addition to definitive incisional biopsy, basic diagnostic evaluation should include a clear history, physical examination, plain radiograph of affected jaw including those of chest, and CT or magnetic resonance imaging of affected area. In the jaws, the biologic behavior of osteosarcoma differs from tumours involving the other skeletal bones: the average age of onset is 10–20 years later than for skeletal lesions [3–5, 8, 9], the histopathological variables are more favourable, distant metastases occur less frequently; and survival rates are higher. Also general manifestations of disease, such as fever or considerable weight loss are rare [8]. Similarly our patient was in his early forties without history of fever or weight loss and there was no evidence of distant metastases.
Comparable to literary findings, the patient presented to us with symptoms of pain, swelling and paresthesia with history of tooth mobility. As these symptoms could also be produced by other lesions of oral cavity, a rare malignant tumour like osteosarcoma was not suspected. Although the OPG revealed classical garrington’s sign in relation to upper left impacted third molar, it was initially interpreted as a sign of apical periodontitis. It is important to note that some cases of osteosarcoma may present with no evident radiological changes, while others may exhibit variable appearances including sclerotic, laminated and the classical sunburst patterns [8]. However because of calcified cartilage or distention of reactive periosteum, other malignancies will also produce the sunray appearance. Even some benign tumours or infections causing reactive periosteal distention can produce this appearance. A widening of PDL space is seen in several mesenchymal malignancies as an early finding but is most commonly seen in osteosarcoma [4]. While most osteosarcomas arise de novo, many arise post RT [3, 6, 10], in paget’s disease, osteomyelitis and in pre-existing benign osseous tumours [10]. In our case the lesion appeared de novo and there was no history of previous RT or any other pre existing condition.
The CT findings of osteosarcoma of maxilla vary from predominantly radiolucent, poorly delineated lesions to dense radiopaque masses with sunburst appearance. Extension to the orbit, destruction of the septum with extension to the opposite side and involvement of the skull base can also be noted [9]. In our case there was no orbital or intracranial extension noted during the initial pre-operative CT scan and the affected bone was poorly delineated. However CT brain with contrast taken during the fourth month follow up visit revealed lesion in infratemporal fossa and secondaries in brain. Chest X-ray was taken as a part of pre-operative work up and also following excisional biopsy report. They were normal with no significant findings.
Histologically osteosarcoma is characterized by the proliferation of both atypical osteoblasts and their less differentiated precursors. A number of histological types of osteosarcoma exist, based on the predominant features of the cells (i.e. osteoblastic, chondroblastic, fibroblastic), though the subtypes are clinically indistinguishable. In general, the characteristic feature of osteosarcoma is presence of osteoid formed by malignant osteoblasts in the lesion, even at sites distant from bone (e.g. the lung). Stromal cells may be spindle shaped and atypical with irregular shaped nuclei [7].
In this case the incisional biopsy specimen was reported as chondromyxoid fibroma. This was due to the presence of hypocellular lobules with a myxochondroid appearance separated by bands of cellular tissue composed of fibroblasts-like spindle cells and giant cells (touton giant cells). There was mild degree of pleomorphism, however mitotic figures were not seen. Also seen was buccal mucosal stratified squamous epithelium which was hyperplastic (refer Fig. 6).
Fig. 6.
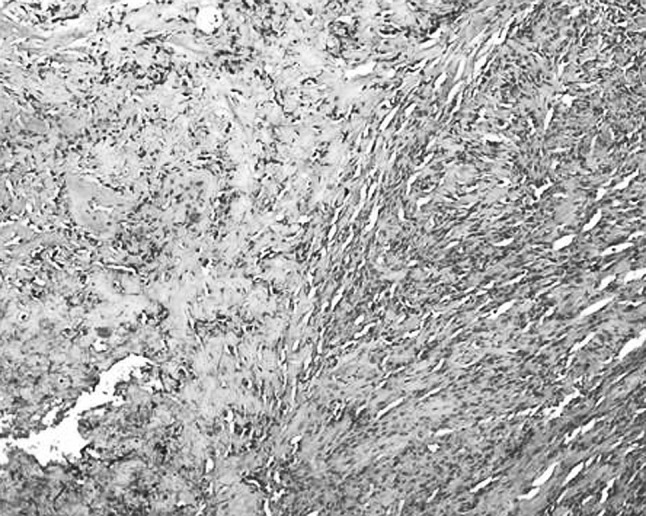
Microphotograph of sections from incisional biopsy showing spindle cell and myxoid areas. H&E × 100
However the histopthological examination of excisional biopsy specimen was reported as osteoblastic variant of osteosarcoma with focal chondroid differentiation. Microscopically the tumour cells were arranged discretely and at places in clusters which were moderately pleomorphic and showed vesicular to hyperchromatic nuclei with prominent nucleoli, occasional mitotic figures were seen. The tumour cells had scant to moderate amount of eosinophilic cytoplasm. A few bizarre tumour giant cells were also seen along with intervening lace-like arrangement of tumour osteoid (refer Fig. 7). There were areas of chondroid and myxoid change. The pathologists felt that the incisional biopsy was taken from the periphery of the lesion and therefore the exact picture was not revealed.
Fig. 7.
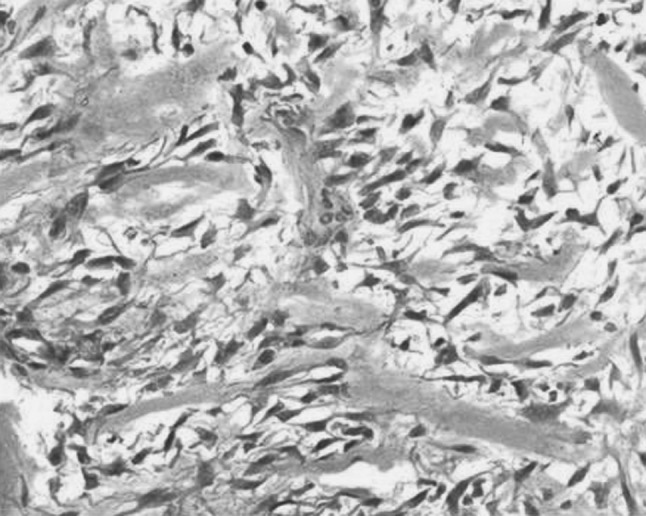
Microphotograph of sections from resected tumour showing anaplastic tumour cells with intervening osteoid. H&E × 400
An analysis by Jasnau et al. confirms the paramount importance of complete surgical resection. None of their 24 patients who achieved a permanent surgical remission developed metastases, and all survived. In contrast, failure to achieve a surgical remission or local recurrence after seemingly complete resection both heralded poor outcomes. Local failure is infrequent in extremity osteosarcoma, with local control rates well above 90%. However, maxillary and especially extragnathic tumours are inherently difficult to resect due to their close proximity to the orbit and intracranial cavities, whereas mandibular tumours are more amenable to wide resection [3].
In this case, wide local excision was performed as our intent was excision of a benign tumour based on the incisional biopsy report. Had the patient been diagnosed with malignant pathology pre-operatively, our surgical plan would have been more aggressive and we would have performed a hemi-maxillectomy. Nevertheless, the tumour mass was successfully excised. The resected specimen was reported as having positive margins histopathologically, although we felt that we had given adequate surgical clearance. This could have been because the tumour was ballooning into the maxillary sinus without involving the sinus walls, so normal tissue could not be obtained adjacent to pathological tissue. The specimen was easily separable as the upper part of the tumour was just projecting into the sinus. Hence the walls of the maxillary sinus, except the lateral wall were left intact and were visibly free of any pathological tissue.
However, the medical and radiation oncologists at our centre were in favour of post-operative chemo and radiotherapy based on the histopathology report. The patient was referred for the same with the intent of preventing recurrence and improving the prognosis, keeping in mind the importance of surgical remission.
The benefits of chemotherapy in the treatment of craniofacial osteosarcomas is being debated [1]. While chemotherapy improves the prognosis of extremity osteosarcoma dramatically, its impact on craniofacial osteosarcoma is still controversial [3]. However available evidence indicates that there has been a remarkable improvement in the survival for patients treated with multiple drug chemotherapy as a routine in conjunction with surgery [8]. Guidance according to cooperative osteosarcoma study group (COSS) was generally as follows: the use of chemotherapy encouraged for patients with inoperable tumours, among patients with operable lesions, the use of chemotherapy was strongly recommended for tumors of the skull and not recommended for those with mandibular primaries. Patients with osteosarcomas of the maxillofacial bones were to receive preoperative chemotherapy according to the current regimen for extremity tumors if respectability was questionable. All COSS chemotherapy protocols included high-dose methotrexate with leucovorin rescue. In addition doxorubicin, cisplatin, ifosfamide, and bleomycin, cyclophosphamide and dactinomycin were used in varying combinations. The scheduled duration of chemotherapy ranged from 24–38 weeks [3]. Most medical oncologists apply strategies used in the treatment of long bone osteosarcomas to the treatment of the maxillofacial counterpart. The choice between induction chemotherapy and post-operative chemotherapy remains unresolved. Induction chemotherapy may certainly convert an inoperable tumour to one that is operable and may also allow a less mutilating or functionally destructive operation. However, there is no apparent survival benefit between pre and post-operative therapy for long bone osteosarcomas [5].
In the present case, the question of pre-operative chemotherapy did not arise as incisional biopsy report was chondromyxoid fibroma, a benign tumour. While it is well known that jaw osteosarcoma is particularly resistant to RT, it has been shown that there is a role for RT combined with chemotherapy in the treatment of advanced osteosarcoma since it can be used to palliate the bulky primary tumour when the patient presents with metastatic disease [8]. But primary RT is of no avail [7]. Prognostic factors in overall survival include tumour size, location and histologic grade. In comparison with the osteosarcoma of the extremities, jaw osteosarcoma is characterized by an increased 5 year survival rate and a lower incidence of metastases although, there is no difference in histological degree of malignancy of the disease in the two sites, at least with respect to the mitotic activity [8]. Dissemination to the lung appears to occur less often with maxillary osteosarcomas than with those of long bones [11]. Consequently, local recurrence, inaccessibility of the tumour and intracranial invasion are the main causes of death in patients with osteosarcoma of the maxilla [10, 11].
In conclusion we would like to emphasize that an element of suspicion for malignant tumours has to be present when the incisional biopsy report of benign tumour is given especially for mixed tumours like chondromyxoid fibroma and treatment has to be planned accordingly. Early diagnosis and adequate surgical treatment at an early stage is the most important step one can take to achieve a good result. Also good cooperation and communication between surgeons, pathologists and oncologists is equally essential for an effective outcome.
Acknowledgments
Conflict of Interest
none declared.
Funding
none.
References
- 1.Thiele OC, Frier K, Bacon C, Egerer G, Hofele CM. Interdisciplinary combined treatment of craniofacial osteosarcoma with neoadjuvant and adjuvant chemotherapy and excision of the tumour: a retrospective study. Br J Oral Maxillofac Surg. 2008;46:533–536. doi: 10.1016/j.bjoms.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Godoy RLMR, Garcia AM, Taylor AM, Salazar JDG. Well-differentiated intraosseous osteosarcoma of the jaws: experience of two cases from the Institute Nacional de Cancerologia, Mexico. Oral Oncol. 1999;35:530–533. doi: 10.1016/S1368-8375(99)00005-6. [DOI] [PubMed] [Google Scholar]
- 3.Jasnau S, Meyer U, Potratz J, Jundt G, Kevric M, Joos UK, et al. Craniofacial osteosarcoma experience of the cooperative German–Austrian–Swiss osteosarcoma study group. Oral Oncol. 2008;44:286–294. doi: 10.1016/j.oraloncology.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Marx RE, Stern D (2003) Malignant neoplasms of bone. In: Oral & maxillofacial pathology: a rationale for diagnosis and treatment. Quintessence, Illinois, pp 799–828
- 5.Rosenthal MA, Mougos S, Wiesenfeld D. High-grade maxillofacial osteosarcoma: evolving strategies for a curable cancer. Oral Oncol. 2003;39:402–404. doi: 10.1016/S1368-8375(02)00147-1. [DOI] [PubMed] [Google Scholar]
- 6.Mendenhall WM, Fernandes R, Werning JW, Vaysberg M, Malyapa RS, Mendenhall NP. Head and neck osteosarcoma. Am J Otolaryngol. 2011;32(6):597–600. doi: 10.1016/j.amjoto.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Rajendran R (2009) Benign & malignant tumours of oral cavity. In: Shafer WG, Hine MK, Levy BM (eds) Shafer’s textbook of oral pathology, 6th edn. Elsevier, India, pp 80–218
- 8.Chindia ML. Osteosarcoma of the jaw bones. Oral Oncol. 2001;37:545–547. doi: 10.1016/S1368-8375(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 9.Panda NK, Jain A, Reddy CEE. Osteosarcoma and chondrosarcoma of the maxilla. Br J Oral Maxillofac Surg. 2003;41:329–333. doi: 10.1016/S0266-4356(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 10.Warnock G (2006) Malignant neoplasms of the gnathic bones. In: Thomson DR (ed) Head & neck pathology. A volume in the series foundations in diagnostic pathology. Churchill Livingstone, Philadelphia, pp 492–515
- 11.Okinaka Y, Takahashi M. Osteosarcoma of the maxilla: report of a case and review of the literature concerning metastasis. J Oral Maxillofac Surg. 1997;55:1177–1181. doi: 10.1016/S0278-2391(97)90304-9. [DOI] [PubMed] [Google Scholar]


