Abstract
Myofibroma and myofibromatosis is a well-recognized spindle cell neoplasm that occurs predominantly in infants and young children. They have been described under different names since 1951. These lesions are a benign fibroblast and myofibroblast proliferation containing a biphasic presentation of spindle shaped cells surrounding a central zone of less differentiated cells focally arranged in a hemangiopericytoma like pattern. Classically these lesions are described in children younger than two, with 2/3rd present at birth and rarely in adults. Controversy exists as to an autosomal dominant or recessive inheritance or to a sporadic occurrence. Presented here is a unique case of myofibroma involving the mandible in a 11 year-old male patient. Clinically it mimicked more like a beningn tumor and not exhibiting any of its classical signs. The diagnosis could be established only after complete excision of the lesion and histopathological examination. There was no recurrence after a follow up period of 4 months.
Keywords: Myofibroma, Spindle cell neoplasm, Smooth muscle
Introduction
Myofibroma is a solitary nodular tumor of the soft tissue, bone, or internal organs that affects all ages. The tumor may present as single or multiple nodules. In 1951, Williams and Schrum [1] first named the lesion congenital fibrosarcoma, subsequently Stout amended term to congenital generalized fibromatosis following a study of fibrous growth in children. These terms were used to denote a multicentric and multinodular beningn fibroblastic process composed of spindle cells. In 1965, Kauffman and Stout [2] divided this tumor into two types: those with a good prognosis that affect the skin, subcutaneous tissue, or skeleton; and those with a poor prognosis that affect the soft tissue, muscles, bones, or internal organs. In 1981, Chung and Enzinger [3] at the US Armed Forces Institute of Pathology studied 61 cases of both solitary and multicentric types and renamed the lesion as infantile myofibromatosis to indicate its myofibroblastic nature and its predilection for infants finally. In 1989 Smith et al. [4] and Daimaru et al. [5] reformed to the solitary variant in adults using the term myofibroma and myofibromatosis, respectively. The latter terms have been adopted by the WHO [6] to describe solitary (myofibroma) or multicentric (myofibromatosis) benign neoplasm.
The exact etiology of the condition is unknown with most cases reported as sporadic, however some reported cases suggest the possibility of a familial pattern of inheritance suggesting that myofibromatosis may be inherited as an autosomal dominant or recessive trait [7, 8].
These lesions can occur over a wide age range with many occurring in the 1st decade of life, according to some authors, 90% of cases manifest before the age of 2 years [9, 10]. The solitary form (myofibroma) occurs most often in the dermis and subcutis, with head and neck according for a majority of the lesions [11].
Reported here is a unique case report of a patient with a slow growing lesion in the left side of the mandible intra-orally. The case is unique in the sense both clinical, radiological features are non suggestive of a myofibroma and initial incisional biopsy could not establish the diagnosis. It was only after complete excision of the lesion followed by post operative histopathology that a diagnosis of myofibroma was established.
Case Report
A 11-year-old boy was referred to the Department of Oral and Maxillofacial Surgery regarding a left lower jaw mass (Fig. 1). The patient was healthy with a nonsignificant medical history including no use of medications, no known drug allergies, and no significant family history. The current lesion presented as a slowly enlarging, firm, soft tissue mass of the left mandibular vestibule (Fig. 2). According to his parents they noticed the swelling since 3–4 months. Evaluation showed an enlarged, firm, fibrotic, asymmetric, non-tender, somewhat fixed mass of the left mandibular vestibule, extending from the area of the first permanent molar to the deciduous canine with extension to the inferior border of the mandible. There was neither ulceration nor inflammation of the overlying mucosa. The neck was supple with minimal cervical lymphadenopathy. An occlusal radiograph showed buccal cortical plate erosion (Fig. 3). A panoramic radiograph showed that the lesion was not associated with the roots of the deciduous second molar. The upper border of the lesion was slightly demarcated, the lower border overlapped on the inferior alveolar nerve canal, and the remaining borders were ill-defined. Owing to the slow growing nature and other clinical and radiological features a differential diagnosis of peripheral ameloblastoma, fibroma, and epulis was considered. Aspiration biopsy revealed nothing. An incisional biopsy was carried out which was inconclusive. Patient was then taken up for surgery under general anaesthesia, the lesion was completely excised in total, showing involvement of the mucosa and periosteum of bone where it was slightly adherent (Fig. 4). The lesion appeared solid, soft, whitish non bleeding mass (Fig. 5). The entire mass was submitted for histological examination. Histologically section showed biphasic tumor with cells like spindle and round cells. Spindle cells are arranged in diffuse sheet in streaming and fascicular pattern with long blunt ended nuclei. There were small cells which are round with long nuclei and weakly eosinophilic. There was also slit like spaces and dilated blood vessels. Few areas of hyalinized stroma were present (Fig. 6).
Fig. 1.
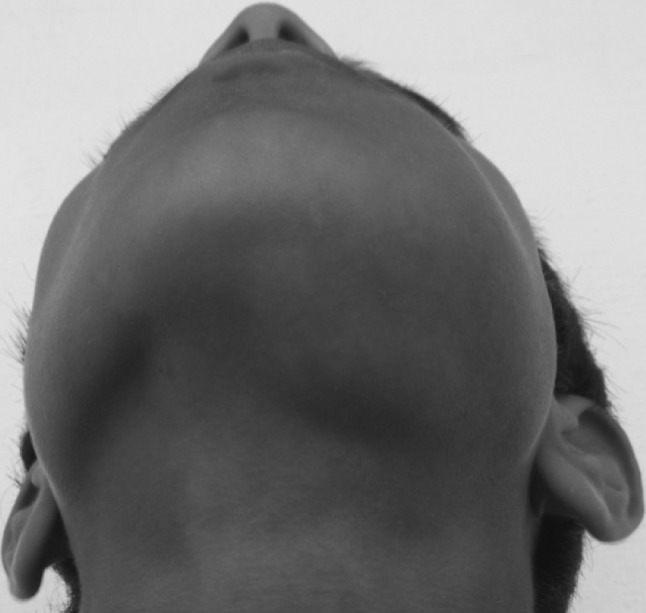
Extral oral view showing the swelling
Fig. 2.
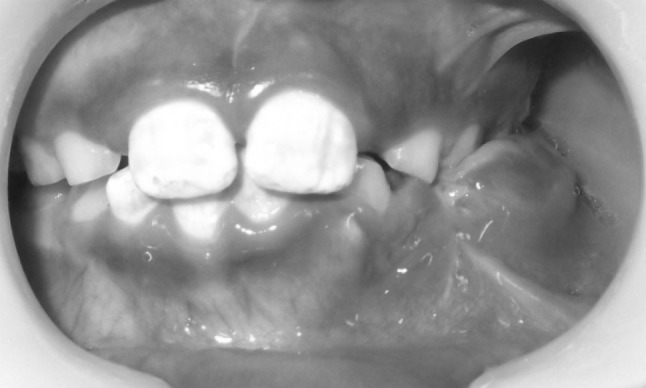
Intra oral view showing the swelling on the left side of the mandible
Fig. 3.
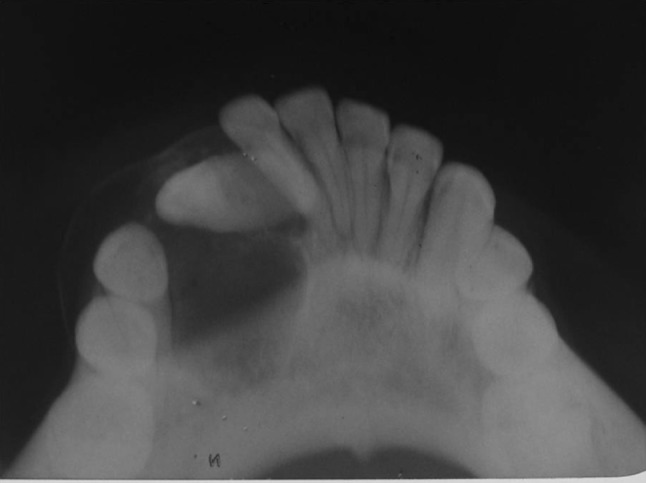
Occlusal radiograph showing cortical destruction
Fig. 4.
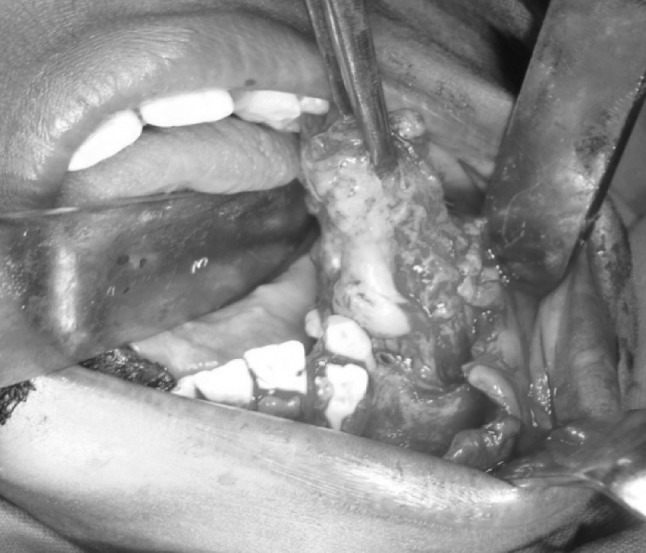
Intra operative picture showing the lesion
Fig. 5.
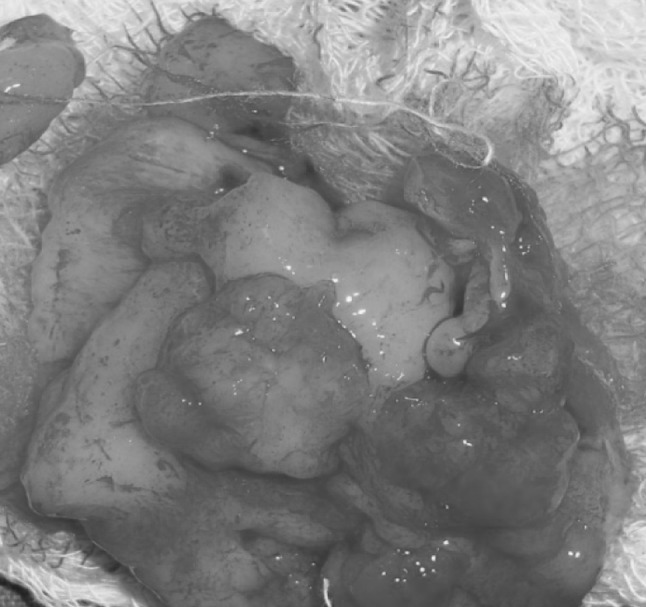
Excised lesion
Fig. 6.
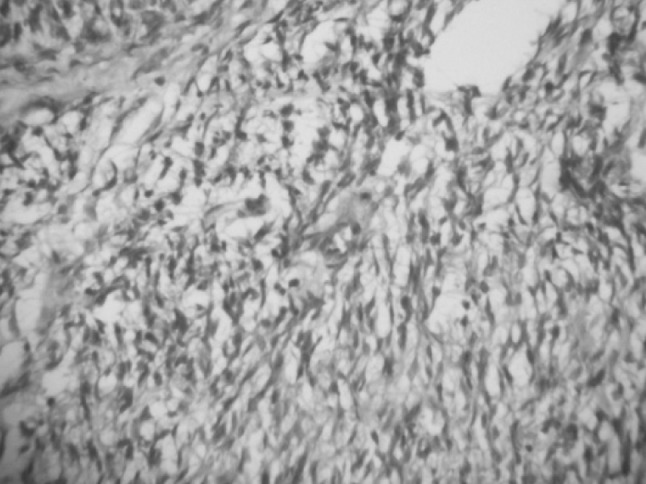
Histopathological slide showing myofibroblast
Discussion
Myofibroma (unifocal) and myofibromatosis (multifocal) are rare spindle cell neoplasms composed of myofibroblasts, i.e., cells with characteristics intermediate between smooth muscle, fibroblasts, and undifferentiated cells. These lesions typically appear in children and infants. Nonetheless both solitary and multicentric lesions can occur at any age [9, 10, 12]. In adults the solitary lesion is the most common form [9].Clinically these lesions show a predilection for the head and neck, with the oral lesions typically presenting in the mandible, lips, cheek, and tongue [12].
The case presented here represents a unique case of myofibroma of the mandible. The parents of the patient have noticed only 3–4 months before. Also clinically the lesion never appeared like a myofibroma. The lesion typically appears as a painless mass that can show rapid enlargement and ulceration. Again in our case none of such phenomena could be noticed which lead to insufficient diagnosis prior to the excision.
Chung and Enzinger [3] also reported a series of 61 cases of infantile myofibromatosis (45 solitary and 16 multiple cases) with a frequency of 32.8% in the head and neck region. The solitary form was more common in males (69%), primarily affecting the tissues of the head, neck, and trunk. The multicentric form was more common in females (63%), being found in the soft tissues, skeleton, and viscera.
Myofibroma of the mandible is a tumor typically diagnosed in children (mean 7.2 years, median 6 years), usually in the first decade of life, with a definite male predominance (male/female ratio 2.3:1). These features vary considerably from those presented by the myofibroma of the oral mucosa, which is usually diagnosed in an older age group (mean 21.7 years, median 13 years) with a female predominance (1.6:1) (M. Vered, March 2006, unpublished data). Patients with myofibroma of the mandible usually present with asymptomatic jaw swelling, occasionally accompanied by an intraoral soft tissue mass lined by normal-appearing mucosa. This is the result of perforation of the cortical plates by the central tumor. Oral mucosa myofibroma is also frequently manifested as a painless swelling. However, in some cases the overlying oral epithelium is secondarily ulcerated (M. Vered, March 2006, unpublished data). Duration of the central lesions of myofibroma is difficult to accurately assess, ranging from 3 days to several years. The absence of symptoms, even when lesions expand and/or perforate the cortical plates and manifest as an oral mass, is an important factor in the delay of the diagnosis. Furthermore, the presence of lesions in infants is totally dependent on the parents’ awareness, since lesions are usually asymptomatic. Generally, these central lesions are slow growing in nature, when confined to the mandible [8], becoming more rapid after perforation [13].
Radiologic features of myofibroma of the mandible revealed a radiolucent lesion, frequently unilocular (70%) with well-defined borders (67%). Occasionally, cortical expansion and/or perforation were shown. Based on these radiologic features, together with the young age of the patients, the leading differential diagnosis included ameloblastic fibroma, central giant cell granuloma, keratocystic odontogenic tumor (odontogenic keratocyst), and unicystic ameloblastoma. Lesions less likely to be considered were the radiolucent varieties of calcifying cystic odontogenic tumor (calcifying odontogenic cyst) and central odontogenic fibroma. For the multilocular appearance of myofibroma, differential diagnosis should be extended to include central hemangioma and aneurysmal bone cyst. Approximately one-third of the central myofibromas appeared with an ill-defined radiolucency. In these cases, aggressive lesions, such as desmoplastic fibroma, solitary eosinophilic granuloma, or malignant bone tumors, such as fibrosarcoma and Ewing’s sarcoma, can be considered in the differential diagnosis in the young age group. Metastatic bone lesions are not commonly encountered in this group of patients, but metastatic neuroblastoma could be considered.
On gross examination, these lesions present as firm circumscribed non encapsulated nodules, often with lobulation. Histologically, interlacing bundles of spindle cells with tapered and blunt ended nuclei and eosinophilic cytoplasm suggest smooth muscle and fibroblast differentiation. A biphasic or zonation pattern is commonly seen composed of nodular fascicles of spindle cells surrounding cellular zones of undifferentiated cells with small round basophilic nuclei. The latter exhibit scattered normal mitotic figures and are organized in central vascular hemangiopericytoma—like areas. Immunohistochemical studies reveal positivity for smooth muscle actin, muscle specific actin (HHF-35), vimentin and pac-actin whereas desnin, S-100, epithelial membrane antigen, and cytokinin are negative.
A study by Jorden and Regizi [14] on 307 cases of benign and malignant oral spindle cell tumors showed sharp differences and underlined the difficulties in diagnosing these lesions. The reason for the difficulty in accurately diagnosing the lesions is their rarity and overlapping subtle histological patterns, often non lesions show an aggressive histological pattern with necrosis, vascular invasion and nuclear pleomorphism leading to misdiagnosis [15]. This could be the reason for the inconclusive histopathological result following the initial biopsy in our case.
Treatment of myofibroma of the mandible was usually conservative excision (75%). In only a few cases (25%), an aggressive surgical procedure of segmental jaw resection was performed to remove extensive and destructive tumors. Since myofibroma of the mandible mainly affects young patients, the outcome of this procedure, which usually requires additional reconstructive operations [16], could be detrimental at the functional, esthetic, and psychological levels. Given that none of the reported lesions recurred, it is suggested that the available potential anti-myofibroblast pharmacological therapeutic agents be considered to spare these patients from aggressive surgical procedures. Pharmacological agents that have been used with a fair degree of clinical success in another more aggressive myofibroblastic lesion, the fibromatoses, are the selective estrogen receptor modulators, i.e., tamoxifen, raloxifene, and toremifene [17, 18]. Their mechanism of action on myofibroblasts is not dependent on the presence of estrogen receptors. It is proposed that in the future, these agents, either alone, or in combination with limited surgery, be used in cases of large and destructive myofibroma of the mandible.
In summary, myofibroma of the mandible is a typical tumor of childhood and adolescents located solely in the mandible. Radiologically, it is generally a unilocular radiolucent lesion frequently with well-defined borders. Its slow growth rate and tendency to thin and expand the cortical plates mimic the biologic behavior of an odontogenic lesion. Microscopically, myofibroma can be misdiagnosed with a variety of benign tumors (e.g., smooth muscle and neural tissue origin), aggressive borderline lesions (e.g., desmoplastic fibroma), and malignant spindle cell tumors (e.g., fibrosarcoma). Increased awareness of the presence of myofibroma within the mandible may lower the possibility of inaccurate diagnoses. Treatment of choice for myofibroma is local excision.
In the present case, no recurrences were reported. However, it is suggested that future studies should consider alternative therapeutic options that target myofibroblasts to reduce the outcome of aggressive surgery.
In the case presented here, the lesion appeared clinically as a beningn tumor with no clinical, radiological or histological evidence of myofibroma and was treated by excision with no recurrence till to date. Both periapical, occlusal and OPG were used to show the extent of the involvement and aided us in successful complete resection. It was only in post op histopathology that a correct diagnosis of a myofibroma could be established. The rarity of the lesion lead to misdiagnosis initially, however the treatment pattern remained same. The total follow up of the case is 4 months and the case will be under our constant observation till further.
References
- 1.Stout AP. Juvenile fibromatosis. Cancer. 1954;7:953–978. doi: 10.1002/1097-0142(195409)7:5<953::AID-CNCR2820070520>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 2.Kanfman SL, Stout AP. Congenital mesenchymal tumors. Cancer. 1965;18:460–476. doi: 10.1002/1097-0142(196504)18:4<460::AID-CNCR2820180410>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 3.Chung EB, Enzinger FM. Infantile fibromatosis. Cancer. 1981;48:1807–1818. doi: 10.1002/1097-0142(19811015)48:8<1807::AID-CNCR2820480818>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Slootweg PJ, Muller H. Localized infantile myofibromatosis: report of a case originating in the mandible. J Maxillofac Surg. 1984;12:86–89. doi: 10.1016/S0301-0503(84)80217-9. [DOI] [PubMed] [Google Scholar]
- 5.Daimaru Y, Hashimoto H, Enjoji M. Myofibromatosis in adults (adult counterpart of infantile myofibromatosis) Am J Surg Pathol. 1989;13:859–865. doi: 10.1097/00000478-198910000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher CDM, Unit KK, Martens F. WHO classification of tumors. Pathology and genetics. Tumors of soft tissue and bone. Lyon: IARC Press; 2002. pp. 59–61. [Google Scholar]
- 7.Sahin AA, Ro JY, Ordonez NG, et al. Myofibroblastoma of the tongue. Am J Clin Pathol. 1990;94:773–777. doi: 10.1093/ajcp/94.6.773. [DOI] [PubMed] [Google Scholar]
- 8.Jennings TA, Duray PH, Collins FS, Sabetta J, Enzinger FM. Infantile myofibromatosis, evidence for an autosomal-dominant disorder. Am J Surg Pathol. 1984;8:529–538. doi: 10.1097/00000478-198407000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Kempson RL, Fletcher CDM, Evans HL, Hendrickson MR, Sibley RK. Atlas of tumor pathology. Tumors of soft tissue. Bethesda: Armed Forces Institute of Pathology; 1998. pp. 64–67. [Google Scholar]
- 10.Foss RD, Ellis GL. Myofibromas and myofibromatosis of the oral region: a clinicopathologic analysis of 79 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:57–65. doi: 10.1067/moe.2000.102569. [DOI] [PubMed] [Google Scholar]
- 11.Enzinger FM, Weiss SW, Goldblum JR. Enzinger and Weiss soft tissue tumors. St Louis: Mosby; 2001. pp. 357–363. [Google Scholar]
- 12.Neville BW, Damm DD, Allen CM, Bouquot J. Oral & maxillofacial pathology. Philadelphia: WB Saunders; 2001. p. 518. [Google Scholar]
- 13.Odell EW, Aldred M, Carlos R, Curran A, Haikinheimo K, Hille J, et al. Clinicopathological conference 2002. Ann Acad Med Singapore. 2004;33:53S–58S. [PubMed] [Google Scholar]
- 14.Jordan RCK, Regizi JA. Oral spindle cell neoplasms: a review of 307 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:717–724. doi: 10.1067/moe.2003.1400. [DOI] [PubMed] [Google Scholar]
- 15.Hartig G, Koopmann C, Esclamado R. Infantile myofibromatosis: a commonly misdiagnosed entity. Otolaryngol Head Neck Surg. 1993;109:753–757. doi: 10.1177/019459989310900420. [DOI] [PubMed] [Google Scholar]
- 16.Troulis MJ, Williams WB, Kaban LB. Staged protocol for resection, skeletal reconstruction, and oral rehabilitation of children with jaw tumors. J Oral Maxillofacial Surg. 2004;62:335–343. doi: 10.1016/j.joms.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Picariello I, Tonelli F, Brandi ML. Selective estrogen receptor modulators in desmoids tumors. Expert Opin Investig Drugs. 2004;13:1457–1468. doi: 10.1517/13543784.13.11.1457. [DOI] [PubMed] [Google Scholar]
- 18.Balducci C, Licci C, Stabellini G, Marinucci L, Giustozzi G, Becchetti A, et al. (2005) Human desmoids fibroblasts: matrix metalloproteinases, their inhibitors and modulation by Toremifene. BMC Cancer 5:22. http://www.biomedcentral.com/1471-2407/5/22. Accessed Mar 2006 [DOI] [PMC free article] [PubMed]


