Abstract
Objective
NSABP R-04 was a randomized controlled trial of neoadjuvant chemoradiotherapy in patients with resectable stage II–III rectal cancer. We hypothesized that patients who underwent abdominoperineal resection (APR) would have a poorer quality of life than those who underwent sphincter-sparing surgery (SSS).
Methods
To obtain patient-reported outcomes (PROs) we administered two symptom scales at baseline and 1 year postoperatively: the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) and the European Organization for the Research and Treatment of Cancer module for patients with Colorectal Cancer Quality of Life Questionnaire (EORTC QLQ-CR38). Scoring was stratified by non-randomly assigned definitive surgery (APR vs SSS). Analyses controlled for baseline scores and stratification factors: age, gender, stage, intended surgery, and randomly assigned chemoradiotherapy.
Results
Of 1,608 randomly assigned patients, 987 had data for planned analyses; 62% underwent SSS; 38% underwent APR. FACT-C total and subscale scores were not statistically different by surgery at one year. For the EORTC-QLQ-CR38 functional scales, APR patients reported worse body image (70.3 vs 77.0, P=0.0005) at one year than did SSS patients. Males undergoing APR reported worse sexual enjoyment (43.7 vs 54.7, P=0.02) at one year than did those undergoing SSS. For the EORTC-QLQ-CR38 symptom scale scores, APR patients reported worse micturition symptoms than the SSS group at one year (26.9 vs 21.5, P=0.03). SSS patients reported worse GI tract symptoms than did the APR patients (18.9 vs 15.2, P<0.0001), as well as weight loss (10.1 vs 6.0, P=0.002).
Conclusions
Symptoms and functional problems were detected at one year by EORTC-QLQ-CR38, reflecting different symptom profiles in patients who underwent APR than those who underwent SSS. Information from these PROs may be useful in counseling patients anticipating surgery for rectal cancer.
Keywords: quality of life, rectal cancer, surgery
INTRODUCTION
An estimated 142,820 new cases of colorectal cancer will be diagnosed in 2013, and 50,830 deaths will occur from this disease. For rectal cancer, the five-year survival rate continues to improve, from 48% in 1975–1977 to 68% in 2002–2008.1 Despite the increasing number of rectal cancer survivors, little is known about the quality of life (QOL) and symptoms associated with the current multidisciplinary management of locally advanced rectal cancer. In 2005, the Institute of Medicine published a report, "From Cancer Patient to Cancer Survivor,"2 which highlighted the need for further research to determine the late effects on cancer survivors through prospective long-term follow-up.
Previous studies have evaluated QOL in patients with rectal cancer. but the majority of these studies either employed a small sample size3–5 or did not have a comparative surgical group.6 A 2005 Cochrane Review on QOL after resection for rectal cancer (with or without colostomy) identified 26 observational studies (with 3,675 patients) that used a validated QOL instrument with patients who underwent anterior resection or abdominoperineal resection (APR).7 Because of significant clinical heterogeneity among the studies, the authors could not definitively conclude if there was a difference in QOL between the APR and anterior resection groups and recommended that larger, well-designed prospective studies were needed to determine if there is a statistically significant difference in QOL between these two groups.
The contemporary management of rectal cancer is complex, and the decision for type of surgery depends on not only patient-related but also tumor-related factors and therefore cannot be randomly assigned. Our goal was to evaluate the comparative effectiveness of sphincter-sparing surgery (SSS) versus APR with respect to patient-reported outcomes (PROs) measured at one year after definitive surgery, controlling for baseline score (before neoadjuvant chemoradiotherapy) and other prognostic baseline factors. Our primary hypothesis was that APR patients would have poorer QOL than would patients who underwent SSS at one year after rectal cancer surgery.
METHODS
The National Surgical Adjuvant Breast and Bowel Project (NSABP) Protocol R-04 was a clinical trial comparing four different neoadjuvant chemoradiotherapy regimens in the treatment of patients who underwent surgery for rectal cancer. Patients were enrolled from July 2004 to August 2010.9
The R-04 protocol has been completely described elsewhere,8 but in brief, inclusion criteria required patients with surgically resectable adenocarcinoma of the rectum located within 12 centimeters from the anal verge.9 Enrolled patients were stratified based on gender, clinical stage, and intended surgery (surgeon-intended sphincter-sparing vs surgeon-intended non-sphincter-sparing surgery, which was documented at the time of study enrollment) and were then randomly assigned to four different chemoradiotherapy treatment arms: (1) infusional 5-fluorouracil with pelvic radiation; (2) infusional 5-fluorouracil, oxaliplatin, and pelvic radiation; (3) capecitabine with pelvic radiation; and (4) capecitabine, oxaliplatin, and pelvic radiation. Patients underwent surgical resection 6–8 weeks after completion of chemoradiation therapy and the surgery received was abstracted from the dictated operative notes to determine if the patient did or did not receive the intended surgery. As per the NSABP R-04 Protocol the choice of operative procedure was at the discretion of the surgeon but it was recommended by the protocol that the entire mesorectum be removed and that a distal rectal margin of at least 2 centimeters be obtained for patients undergoing sphincter-sparing surgery.
The R-04 Behavioral and Health Outcomes study examined PROs at three different time points: baseline (before chemoradiotherapy), after chemoradiotherapy (but before surgery), and one year after surgery. A secondary aim of the NSABP R-04 protocol was to describe the impact of the type of surgical management of rectal cancer on QOL at one year after cancer treatment. All patients were asked to complete the baseline QOL questionnaire after signing the informed consent; reasons for non-completion were patient refusal and patients who did not speak English, Spanish, or French. Patients who developed tumor recurrence or second primary were not expected to complete the one-year QOL questionnaire; reasons for non-completion were patient refusal and inability to contact patient via telephone or mail. It should also be noted that most patients had additional postsurgical adjuvant chemotherapy. Informed consent was obtained for neoadjuvant chemoradiotherapy treatment and collection of study data.
The QOL questionnaires used in the current analysis include the Functional Assessment of Cancer Therapy for patients with colorectal cancer (FACT-C)10 and the European Organization for the Research and Treatment of Cancer module for patients with colorectal cancer (EORTC-QLQ-CR38).11 The FACT-C includes a total score as well as subscales for physical well-being, social-family well-being, emotional well-being, functional well-being, and colorectal cancer-specific concerns. The EORTC-QLQ-CR38 has 38 questions: all patients complete 19 of the questions, and 19 are completed by subgroups of patients, depending on gender or presence of a stoma. The EORTC-QLQ-CR38 includes four functional scales (body image, sexual function, sexual enjoyment, future perspective) as well as eight symptom scales (micturition problems, symptoms in the gastrointestinal [GI] tract, chemotherapy side effects, defecation problems, stoma-related problems, male and female sexual problems, and weight loss). Of note, the sexual enjoyment functional scale is a single item and conditional on being sexually active.
Patient characteristics were compared between the SSS and APR groups using the chi-square test for difference between groups. FACT-C and EORTC-QLQ-CR38 scores were calculated at baseline (before chemoradiotherapy) and at one year postoperatively. Change from baseline to year one was tested using the paired t-test. The proportion of sexually active patients at baseline and at year one were compared between the two surgical groups using the chi-square test. Analyses comparing one-year scores between surgical groups (APR vs SSS) used linear models adjusted for baseline score as well as stratification factors including age, gender, clinical stage, intended surgery, and randomly assigned chemoradiotherapy. To compare one-year scores by surgery intent (received intended surgery vs did not receive intended surgery) our model adjusted for surgery actually received as well as the previously mentioned stratification variables. All statistical analyses were performed using SAS version 9.2; P values < 0.05 were considered statistically significant.
RESULTS
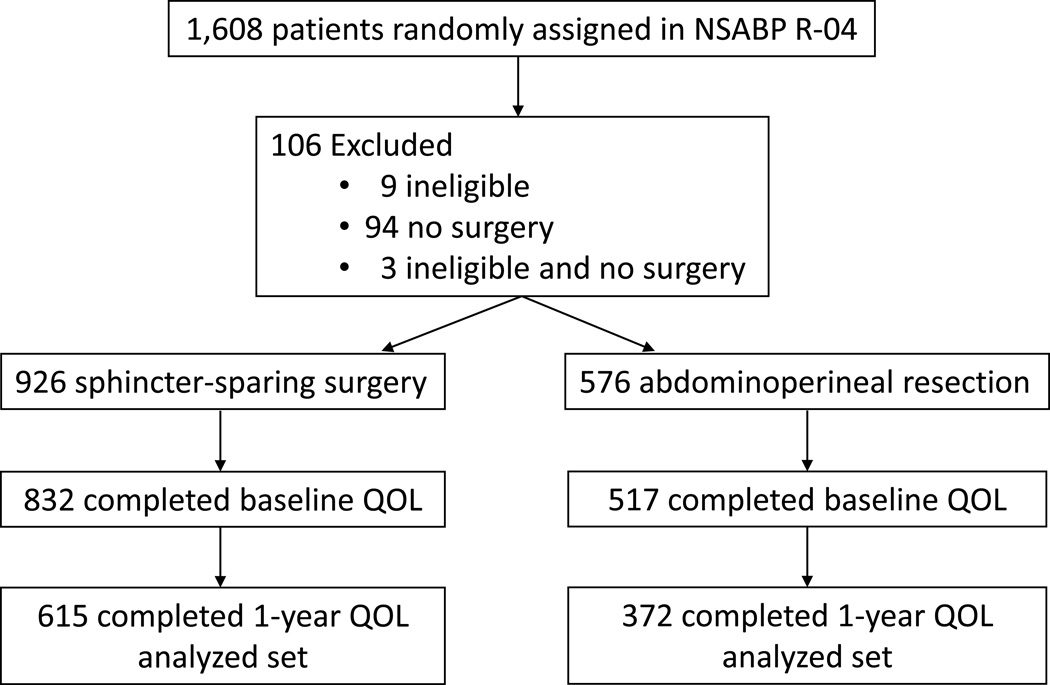
There were 1,608 patients who were randomly assigned to the arms of the NSABP R-04 study, 106 of whom were excluded from the study due to ineligibility and/or no surgery performed. Of the remaining evaluable 1,502 patients, 926 underwent SSS and 576 underwent APR. In both surgical groups, 89.8% completed the baseline QOL questionnaire; completion rates for the one-year QOL questionnaire were similar between the two groups (73.9% for SSS and 72% for APR, respectively). The flow diagram for patients included in this analysis is shown in Figure 1.
Figure 1.
Flow Diagram Showing Patients Included in the NSABP R-04 Study.
A comparison of the demographics between the 987 patients included in the current study and the 621 patients without one-year QOL data revealed no statistically significant differences between the two groups for age, gender, or intended surgery. There were some differences between the two groups by stage, with a higher proportion of stage II rectal cancer in the 987 patients who completed the one-year QOL questionnaire (61.5% vs 55.7%, P value 0.04), which is at least partially explained by higher recurrence rates in stage III patients, because patients with a documented cancer recurrence or second primary cancer are not expected to complete QOL questionnaires after that event (N=194). The reasons for missing QOL form at one year after surgery included: staff oversight or understaffing, staff concern for participant’s medical or emotional condition, participant stating he/she was too ill or upset, participant refused to complete the questionnaire, participant was unavailable, staff was unable to contact participant by phone, or questionnaire was mailed but participant did not return it.
Of the 987 patients available for analysis in the current study, 62% underwent SSS, and 38% underwent APR. Of those who underwent SSS, 69% had an ileostomy created at the time of surgery, 22% had no ostomy created, and 9% had a colostomy created. Patient characteristics stratified by type of surgery are shown in Table 1. There were no statistically significant differences in gender, rectal cancer stage, receipt of adjuvant chemotherapy, or tumor size between the APR and SSS groups (the duration of postoperative chemotherapy is not known). There was a statistically significant difference in respect to receipt of intended surgery between the APR and SSS groups (27% intended APR, whereas 38% actually had APR, P value < 0.0001). Of the patients who received APR, only 56.7% were intended to undergo this surgery. In contrast, of the patients who received SSS, 91.1% were intended to undergo SSS.
Table 1.
Patient Characteristics Stratified by Surgical Treatment: NSABP R-04
| Characteristic | Abdomino- Perineal Resection |
Sphincter- Saving Surgery |
Total | P value* |
|---|---|---|---|---|
| (percent) | (N=372) | (N=615) | (N=987) | |
| Age Group | ||||
| ≤ 59 | 53.5 | 60.3 | 57.8 | 0.04 |
| ≥ 60 | 46.5 | 39.7 | 42.3 | |
| Sex | ||||
| Male | 66.4 | 66.7 | 66.6 | 0.94 |
| Female | 33.6 | 33.3 | 33.4 | |
| Stage | ||||
| II | 57.8 | 63.7 | 61.5 | 0.07 |
| III | 42.2 | 36.3 | 38.5 | |
| Intended Surgery | ||||
| Abdominoperineal resection | 56.7 | 8.9 | 27 | < 0.0001 |
| Sphincter saving | 43.3 | 91.1 | 73.1 | |
| Adjuvant Chemotherapy | ||||
| 5-FU | 10.8 | 9.6 | 10 | 0.72 |
| Capecitabine | 14.5 | 15.5 | 15.1 | |
| Irinotecan | 0 | 0.3 | 0.2 | |
| 5-FU/Leucovorin | 10 | 8 | 8.7 | |
| 5-FU/Leucovorin/Irinotecan | 1.1 | 1.3 | 1.2 | |
| Other/none | 63.7 | 65.4 | 64.7 | |
| Location of tumor | N=356 | N=571 | N=927 | |
| Upper | 1.1 | 10.7 | 7 | < 0.0001 |
| Middle | 20.8 | 50.6 | 39.2 | |
| Lower | 78.1 | 38.7 | 53.8 | |
| Tumor Size | N=311 | N=483 | N=794 | |
| 0.0 – 3.0 | 75.9 | 81 | 79 | 0.23 |
| 3.1 – 6.0 | 21.9 | 17.4 | 19.1 | |
| 6.1 – 10.0 | 2.3 | 1.7 | 1.9 |
Chi-square test for difference between surgical treatment groups
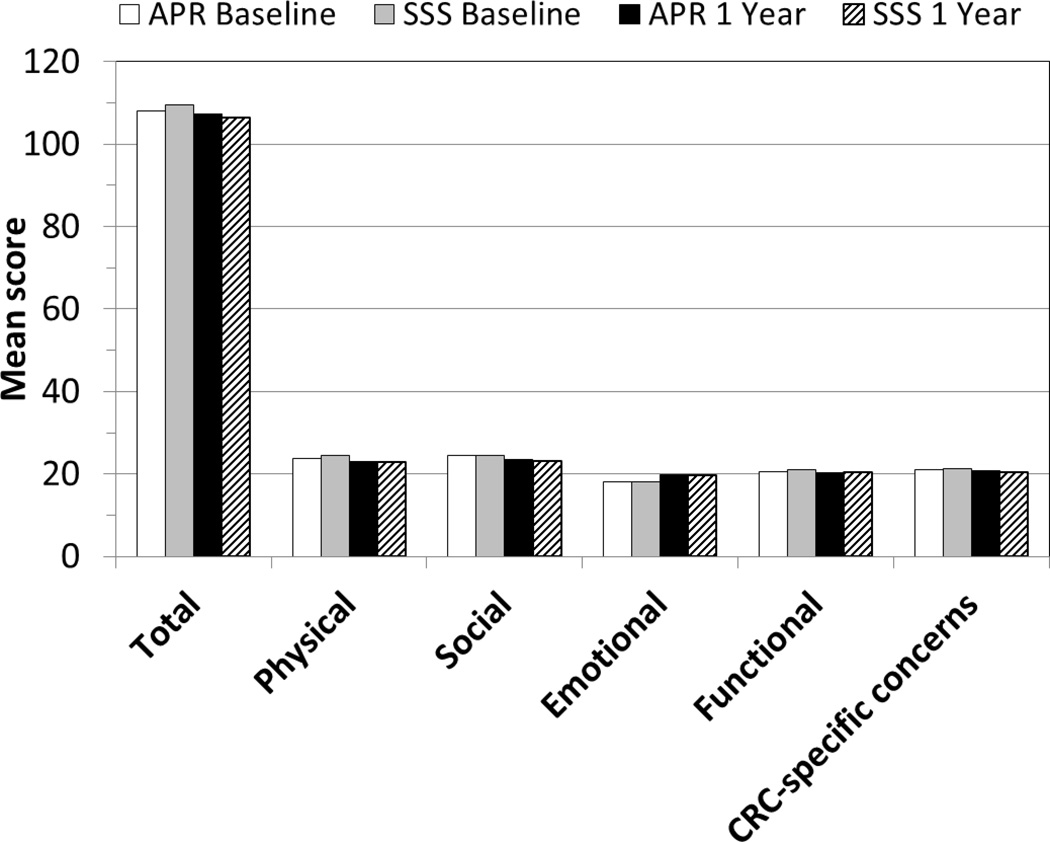
The FACT-C scores stratified by type of surgery (APR vs SSS) and time (baseline vs one year postoperatively) are shown in Figure 2. There is no statistically significant difference by type of surgery for the FACT-C total score or for any of the subscales (physical well-being, social-family well-being, emotional well-being, functional well-being, or cancer-specific concerns) at one year after surgery.
Figure 2.
FACT-C scores stratified by type of surgery (APR vs SSS) and time (baseline vs one year after rectal cancer surgery) - - NSABP R-04 Study.
FACT-C indicates Functional Assessment of Cancer Therapy-Colorectal quality of life instrument; APR, abdominoperineal resection; SSS, sphincter-sparing surgery; CRC, colorectal cancer.
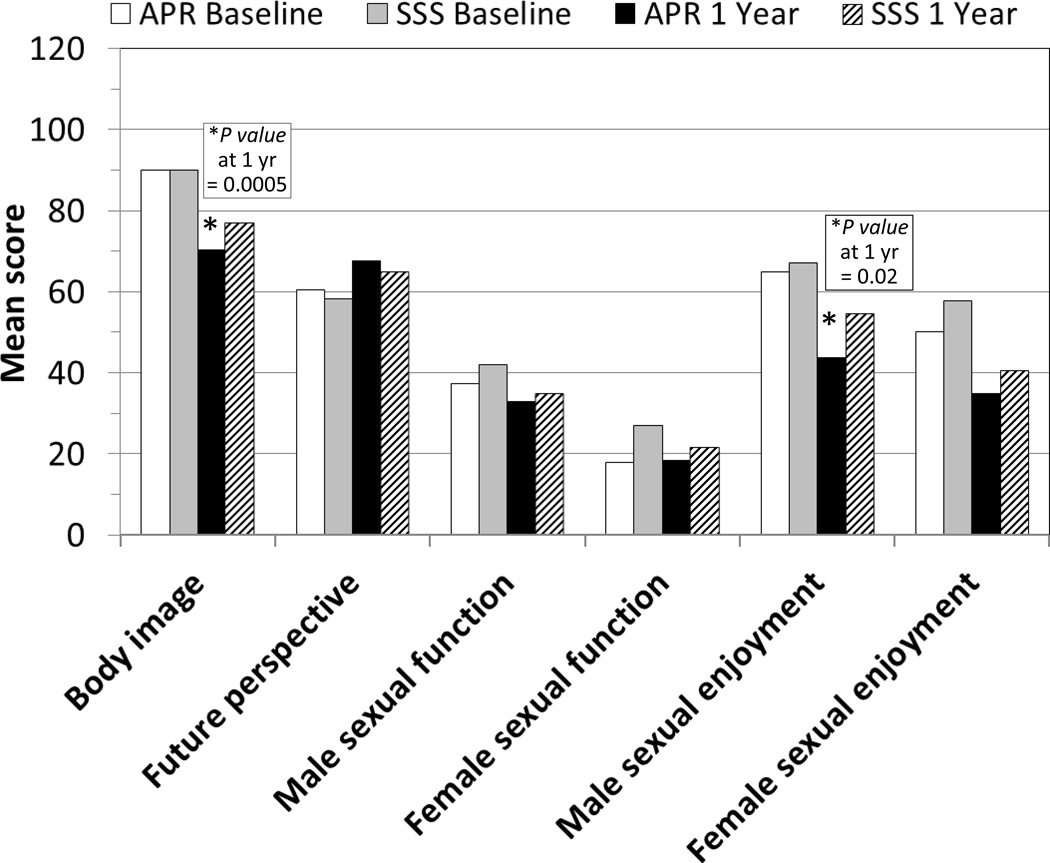
The EORTC-QLQ-CR38 functional scale scores stratified by type of surgery (APR vs SSS) and time (baseline vs one year postoperatively) are shown in Figure 3. There were significant differences between the two surgical groups. At one year, APR-receiving patients reported worse body image and male sexual enjoyment than did those who received SSS. There were no differences reported between the two surgical groups at one year for the functional scales of: future perspective, male or female sexual function, and female sexual enjoyment.
Figure 3.
EORTC-QLQ-CR38 Functional Scale scores stratified by type of surgery (APR vs SSS) and time (baseline vs one year after rectal cancer surgery) - - NSABP R-04 Study.
EORTC-QLQ-CR38 indicates European Organization for the Research and Treatment of Cancer module for patients with colorectal cancer; APR, abdominoperineal resection; SSS, sphincter-sparing surgery.
Sexual activity was calculated based on patient responses to the sexual enjoyment functional scale questionaire, which is conditional on being sexually active. Patients who underwent APR were less sexually active, not only at baseline (57.1% vs 64.6%, P value 0.03) but also at the one-year (49.1% vs 58.3%, P value 0.01) time periods than were patients who underwent SSS. When stratified by gender, the proportion of sexually active males was similar in the APR and SSS groups (65.8% vs 69.5% at baseline, P value 0.22; 55.0% vs 62.5% at one year, P value 0.07); however, females who underwent APR had significantly lower sexual activity compared with females who underwent SSS (39.2% vs 54.3% at baseline, P value 0.02; 35.6% vs 49.4% at one year, P value 0.03).
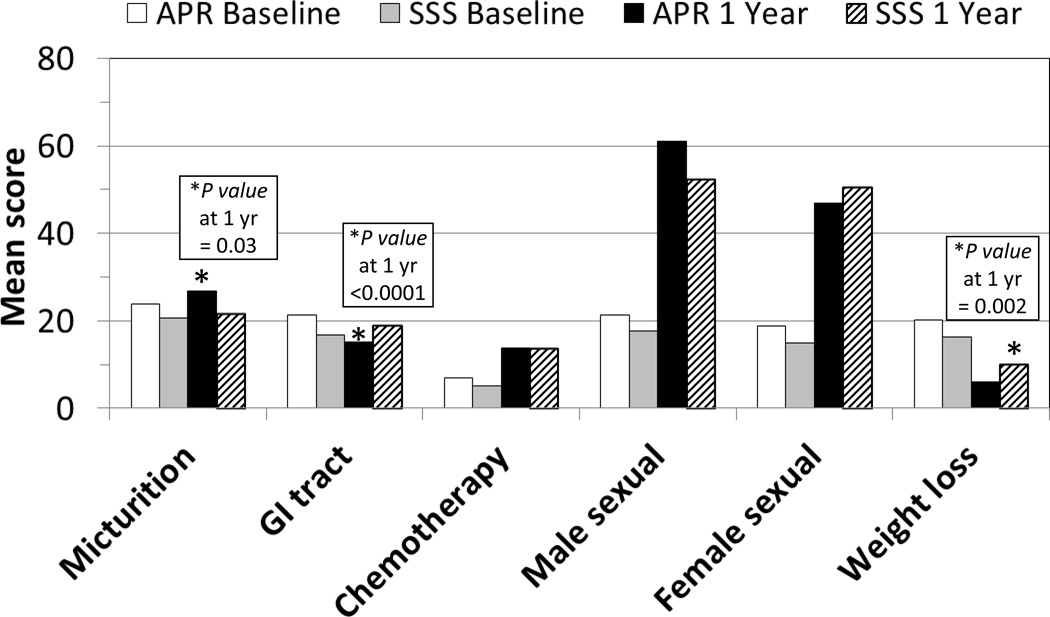
The EORTC-QLQ-CR38 symptom scale scores stratified by type of surgery (APR vs SSS) and time (baseline vs one year postoperatively) are shown in Figure 4. There were significant differences between the two surgical groups. At one year, APR-receiving patients reported worse micturition symptoms than did those who received SSS. Additionally, those receiving SSS also reported worse symptoms in the GI tract and weight loss than did those who received APR at one year. There were no differences reported between the two surgical groups at one year for the symptom scales of: future perspective, male or female sexual function, and female sexual enjoyment. Chemotherapy side effects, male sexual problems, and female sexual problems are all reported as worse at one year compared with baseline, and there was no difference between the APR and SSS groups.
Figure 4.
EORTC-QLQ-CR38 Symptom Scale scores stratified by type of surgery (APR vs SSS) and time (baseline vs one year after rectal cancer surgery) - - NSABP R-04 Study
EORTC-QLQ-CR38 indicates European Organization for the Research and Treatment of Cancer module for patients with colorectal cancer; APR, abdominoperineal resection; SSS, sphincter-sparing surgery; GI, gastrointestinal.
For the stoma-related problems symptom scale, patients who underwent SSS with ileostomy had the most severe symptoms (41.4) in comparison to SSS patients with colostomy (34.0) or APR (34.9), but this difference was not statistically significant. In comparison, for patients who underwent SSS with no ostomy, the mean score for the defecation symptom scale at one year was 27.8 (95% confidence interval 24.6 – 32.1).
Both the FACT-C total score and subscales, as well as the EORTC-QLQ-CR38 functional and symptom scale scores, were analyzed by receipt of intended surgery. There were no differences in any of the scores at baseline or one year between patients who received intended surgery (regardless of whether APR or SSS) versus patients who did not receive intended surgery. Similarly, there were no differences at one year for patients who received APR (when comparing intended APR/received APR vs intended APR/received SSS) and SSS (when comparing intended SSS/received SSS vs intended SSS/received APR).
DISCUSSION
PROs are becoming an increasingly recognized endpoint after not only medical but also surgical treatments. For example, the Patient-Centered Outcomes Research Institute (PCORI) was established in 2010 through the Affordable Care Act with the goal of funding research that provides patients and clinicians with the information they need in order to compare the effectiveness of medical treatments.12 Patients with locally advanced rectal cancer are an ideal population in which to study PROs because the treatment is multi-modality and has the potential to impact not only overall QOL but also specifically bladder, bowel, and sexual function. Additionally, there are two very distinct surgical treatments with very different functional outcomes based on the presence of a permanent colostomy with APR or the possibility of altered bowel function with SSS. Finally, a recent five-year prospective study of QOL after colorectal cancer demonstrated that both rectal cancer and presence of a permanent stoma are risk factors for poorer QOL.13
The primary finding from the current study is that there is no difference in overall QOL as measured by the FACT-C total score between the APR and SSS groups at one year after surgery. However, there were specific differences with respect to bladder, bowel, and sexual function between the two surgical groups both at baseline and at one year after surgery. At baseline, sexual function and symptoms in the GI tract were worse in the patients who underwent APR, which is presumably due to the low location of the rectal tumor. However, there was no difference in sexual function between the APR and SSS groups at one year, whereas the patients who underwent SSS had worse GI symptoms after surgery than did those who underwent APR. With respect to bladder function, there was no difference in the two groups at baseline, whereas patients who underwent APR had significantly worse micturition symptoms at one year after surgery. Those who underwent APR reported significantly worse body image and sexual enjoyment at one year (either of which may be related to permanent stoma), whereas those who underwent SSS had significantly worse weight loss symptoms at one year.
Several recent studies also attempt to evaluate QOL differences between APR and low anterior resection (LAR) groups. How et al5 compared the one-year QOL in patients after APR (N=30) with LAR for low rectal cancer (N=32). Similar to the current study, patients who underwent LAR were younger and had worse GI function (including issues with incontinence). In contrast, patients who underwent LAR reported better sexual function, whereas there was no difference for this factor between SSS and APR in the current study. This difference may be due to the fact that patients who underwent LAR in the study by How et al were less likely to receive neoadjuvant therapy, whereas all patients in the R-04 study received neoadjuvant chemoradiotherapy. Kasparek et al6 evaluated the long-term sexual and urinary function after APR and demonstrated impaired QOL and sexual function, especially in younger and male patient groups. However, these findings must be interpreted in light of the lack of baseline QOL data, the lack of a comparison group undergoing rectal surgery, and the variation in receipt of pelvic radiotherapy. Finally, Pucciarelli et al4 prospectively collected QOL data in 99 patients who underwent preoperative chemoradiotherapy for rectal cancer. Unfortunately, that cohort included only a small number of APR patients, and therefore conclusions could not be drawn about the differences in QOL between the two surgical treatments for rectal cancer. The results of the current study should add significantly to the body of literature on PROs after rectal cancer surgery because of the large sample size in both surgical groups (N=372 in APR group and N=615 in SSS group), the collection of baseline QOL data, standardized neoadjuvant chemoradiotherapy regimens, and the inclusion of patients from multiple different surgeons and hospitals.
Despite the strengths of the current study, there are several limitations. First, the surgical treatment could not be randomly assigned and both the intended surgery (preoperative assessment) and surgery actually received were at the discretion of the surgeon. Second, although the one-year QOL analysis was a planned secondary endpoint in the R-04 protocol, not all patients completed the one-year QOL questionnaire. However, an analysis of the characteristics between patients who did and did not complete the one-year follow-up showed a lower percentage of stage III patients who completed the QOL questionnaire, which may reflect the likelihood that rectal cancer patients with a worse stage may have had poorer health, greater post-operative treatment, and/or less interest in participating in the QOL follow-up study. Third, we do not know the technical reasons that such a large proportion of the patients intended for SSS ultimately received APR (of those who underwent APR, 43.3% were intended to undergo SSS). Although there do not seem to be any statistically significant QOL differences between patients who received the intended surgery versus those who did not, we plan to review the operative reports in a future study to try to better understand the technical reasons for the change in surgical decision-making. Fourth, we do not have information regarding preoperative stoma education or siting, which may impact QOL outcomes for patients with either a temporary or permanent stoma. Finally, while there are some statistically significant differences in one-year QOL between the APR and SSS groups due to our large sample size, these differences may not be clinically meaningful.
In summary, this is the largest prospective study to date to evaluate QOL in rectal cancer survivors at one year after surgery. Given the large number of rectal cancer patients enrolled in the NSABP R-04 clinical trial, we were able to compare the effectiveness of APR to SSS with respect to PROs. Although there is no difference in overall QOL between the two surgical procedures at one year after surgery, there are procedure-specific differences not only in function but also with symptoms as measured by the EORTC-QLQ-CR38. These interesting early findings provided the impetus for amending the R-04 protocol to collect long-term QOL outcome data at five years after surgery. In this way, we hope to examine the evolution and possible resolution of symptoms that were present at one year after surgery.
Acknowledgments
Source(s) of Funding: Supported by: NCI-U10-CA-12027, U10-CA-37377, U10-CA-69974, U10-CA-69651, and U10-CA-21661, by Roche Laboratories Inc., a full member of the Roche Group of companies, and by Sanofi-Synthelabo Inc.
Footnotes
This work is an original review that has not been published elsewhere. Previous related works are as follows:
Ganz PA, Lopa SH, Yothers G, Ko CY, Arora A, Atkins JN, Bahary N, Soori GS, Robertson JM, Eakle JF, Marchello BT, Wozniak TF, Wolmark N. Comparative effectiveness of sphincter-sparing surgery (SSS) vs abdomino-perineal resection (APR) in rectal cancer: Patient reported outcomes (PROs) from NSABP R-04. J Clin Oncol/ASCO 2012; 30(15 Suppl). Abstr 3545.
Yothers G, Ganz PA, Lopa SH, Ko CY, Wickerham DL, Wolmark N. Patient reported outcomes (PROs) comparison of 5-FU and capecitabine (cape) with concurrent radiotherapy (RT) for neoadjuvant treatment of rectal cancer: Results of NSABP R-04. J Clin Oncol/ASCO GI Cancers Symposium 2012; 30(4 Suppl). Abstr 391.
Potential Conflict(s) of Interest:
All authors declare support from NCI-U10-CA-12027, U10-CA-37377, U10-CA-69974, U10-CA-69651, and U10-CA-21661, by Roche Laboratories Inc., a full member of the Roche Group of companies, and by Sanofi-Synthelabo Inc.
Patricia A. Ganz, MD – declares support for travel for NSABP group meetings. Greg Yothers, PhD – declares support for provision of writing assistance, medicines, equipment, or administrative support (Past).
All other authors declare no other potential conflict(s) of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 3.Allal AS, Bieri S, Pelloni A, et al. Sphincter-sparing surgery after preoperative radiotherapy for low rectal cancers: feasibility, oncologic results and quality of life outcomes. Br J Cancer. 2000;82:1131–1137. doi: 10.1054/bjoc.1999.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pucciarelli S, Del Bianco P, Efficace F, et al. Patient-reported outcomes after neoadjuvant chemoradiotherapy for rectal cancer: a multicenter prospective observational study. Ann Surg. 2011;253:71–77. doi: 10.1097/SLA.0b013e3181fcb856. [DOI] [PubMed] [Google Scholar]
- 5.How P, Stelzner S, Branagan G, et al. Comparative quality of life in patients following abdominoperineal excision and low anterior resection for low rectal cancer. Dis Colon Rectum. 2012;55:400–406. doi: 10.1097/DCR.0b013e3182444fd1. [DOI] [PubMed] [Google Scholar]
- 6.Kasparek MS, Hassan I, Cima RR, et al. Long-term quality of life and sexual and urinary function after abdominoperineal resection for distal rectal cancer. Dis Colon Rectum. 2012;55:147–154. doi: 10.1097/DCR.0b013e31823d2606. [DOI] [PubMed] [Google Scholar]
- 7.Pachler J, Wille-Jorgensen P. Quality of life after rectal resection for cancer, with or without permanent colostomy. Cochrane Database Syst Rev. 2005:CD004323. doi: 10.1002/14651858.CD004323.pub3. [DOI] [PubMed] [Google Scholar]
- 8. [accessed November 7, 2013];Radiation Therapy and Either Capecitabine or Fluorouracil With or Without Oxaliplatin Before Surgery in Treating Patients With Resectable Rectal Cancer. http://clinicaltrials.gov/ct2/show/NCT00058474?term=NSABP+R-04&rank=1.
- 9. [accessed November 7, 2013];NSABP Clinical Trials Overview: Protocol R-04. http://www.nsabp.pitt.edu/R-04.asp.
- 10.Ward WL, Hahn EA, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality of life instrument. Qual Life Res. 1999;8:181–195. doi: 10.1023/a:1008821826499. [DOI] [PubMed] [Google Scholar]
- 11.Sprangers MA, te Velde A, Aaronson NK. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38). European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Eur J Cancer. 1999;35:238–247. doi: 10.1016/s0959-8049(98)00357-8. [DOI] [PubMed] [Google Scholar]
- 12. [accessed November 7, 2013];Patient-Centered Outcomes Research Institute. http://www.pcori.org. [Google Scholar]
- 13.Chambers SK, Meng X, Youl P, et al. A five-year prospective study of quality of life after colorectal cancer. Qual Life Res. 2012;21:1551–1564. doi: 10.1007/s11136-011-0067-5. [DOI] [PubMed] [Google Scholar]