Abstract
Background
Fibroblast Growth Factor-23 (FGF23) and cofactor Klotho are key regulators of mineral metabolism in chronic kidney disease (CKD), but little is known about the mechanisms that regulate their production. This study evaluates longitudinal changes of FGF23 and Klotho levels and their regulatory factors in children on chronic peritoneal dialysis (PD).
Methods
FGF23, Klotho, 25(OH) vitamin D, 1,25-dihydroxyvitamin D and parathyroid hormone (PTH) plasma concentrations were measured during 1 year of follow-up in PD children. Anthropometric and dialytical parameters were evaluated in addition to mineral metabolism variables.
Results
Thirty-one patients under chronic PD were followed for 12 months. FGF23 mean plasma levels at Month 1 were significantly increased compared with controls, 215.1 ± 303.6 versus 9.4 ± 5.7 pg/mL, respectively (P < 0.001). Baseline Klotho levels were 41% lower in patients compared with controls, 132.1 ± 58 versus 320 ± 119.4 pg/mL, respectively (P < 0.001), and did not correlate with FGF23 and phosphorus levels. At Month 12, FGF23 (195 ± 300 pg/mL) and Klotho levels (130 ± 34 pg/mL) remained similar to baseline values. Log-FGF23 correlated significantly with height/age Z score (r= −0.38) and residual renal function (r = −0.44), but no correlation was found with serum phosphorus, phosphate intake, PTH and vitamin D levels. The log-FGF23 strongly correlated with calcium levels at Months 1, 6 and 12, however, this relationship was blunted if serum phosphorus was >6 mg/dL. By multiple regression analysis, calcium was the strongest variable determining FGF23 levels.
Conclusions
In this longitudinal study, FGF23 levels are markedly increased, and Klotho levels are reduced in PD children compared with controls. FGF23 levels appeared to be regulated primarily by serum calcium, showing a significant correlation at each time of measurement. This relationship was lost in patients with phosphorus >6 mg/dL. These observations may have important consequences to the therapeutic management of phosphate homeostasis in CKD patients.
Keywords: calcium; chronic kidney disease, FGF23, Klotho; phosphorus
Introduction
The identification of the fibroblast growth factor-23 (FGF23)–Klotho axis has changed our understanding of mineral metabolism in chronic kidney disease (CKD) [1–5].
FGF23 is a phosphaturic hormone secreted by osteocytes and osteoblasts and collectively reduces circulating phosphate and 1,25-dihydroxyvitamin D [1,25(OH)2D] levels. FGF23 functions as an endocrine hormone, reaching its cellular targets in the kidney and parathyroid glands where it binds to the co-receptor Klotho, and converts the FGF receptor 1c into a specific FGF23 receptor [6]. In CKD, plasma FGF23 levels increase as CKD progresses to maintain normal phosphate balance attaining markedly elevated concentrations in patients receiving dialysis [7–9].
Klotho, originally identified as an anti-aging factor, is a transmembrane protein predominantly expressed in the kidney, parathyroid gland and choroid plexus [8]. Klotho binds to several FGF receptors, acting as an obligatory co-receptor for FGF23 [10, 11]. Klotho expression and circulating levels are decreased in CKD patients, probably secondary to reduced renal mass [9–12], and Klotho deficiency may contribute to vascular calcification in CKD. It has been suggested that Klotho protects these patients against vascular calcification promoting phosphaturia, preserving renal function, and through a direct effect on vascular smooth muscle cells [13–15]
The regulation of FGF23 synthesis remains incompletely understood. This is of special relevance in CKD, where increased FGF23 levels have been linked to adverse outcomes [16]. Dietary phosphorus, serum 1,25(OH)2D and serum phosphorus have been identified as the main regulators of FGF23 synthesis [17–19]. The tight relationship between calcium and phosphorus homeostasis raises the possibility of FGF23 regulation by calcium in some settings. Animals on high-calcium diets and increased serum calcium, display elevated FGF23 levels [20], while when fed a low-calcium diet, with resulting hypocalcemia, display low FGF23 despite high PTH and calcitriol levels. Hypocalcemia attenuated the association between FGF23 and phosphorus, and serum calcium was the only independent variable significantly associated with FGF23 [21]. Targeted inactivation of PTH and the Ca-sensing receptor have further delineated the existence of calcium-stimulated FGF23 production independent of other variables, and demonstrated that calcium-mediated increases in FGF23 levels required a given threshold level of serum phosphorus concentration [22]. The effects of calcium-sensing receptor agonists on serum FGF23 levels have further added evidence to the regulation of FGF23 by serum calcium [23, 24]. Most studies reporting elevated FGF23 levels in children are cross-sectional [7, 9, 25–27], and little is known about their longitudinal changes while on maintenance peritoneal dialysis (PD). In one study, FGF23 levels sustained an important increase after 8 months of observation, but the patients were on escalating doses of vitamin D receptor activators which contributes to elevate FGF23 concentrations, and the potential regulating effects of the changing serum calcium concentrations on FGF23 levels were not specifically studied [28]. Thus, the objectives of the present study were to evaluate longitudinal changes of circulating FGF23 and Klotho levels and to study their interactions with serum calcium, phosphorus, PTH and vitamin D metabolites as potential regulating factors of their levels in PD children.
Materials and Methods
A 12-month, prospective, observational study in children treated with chronic PD was performed at the Division of Pediatric Nephrology, Luis Calvo Mackenna Children's Hospital, University of Chile. Inclusion criteria: >3 months on stable PD. Exclusion criteria: 25(OH) vitamin D (25(OH)D) levels <20 ng/mL, active nephrotic syndrome, non-renal bone disease, peritonitis within 2 months prior to inclusion, steroid treatment and noncompliance. Anthropometric and nutritional parameters and routine blood determinations were obtained monthly. Blood FGF23, Klotho, 25(OH)D and 1,25(OH)2D were measured at Months 1, 6 and 12. Height, weight and body mass index measurements were expressed as Z-score values. Caloric and protein intake were supervised by a renal dietitian following Recommended Dietary Allowances (RDA) and KDOQI guidelines [29]. The percentage of age-recommended intake (adequacy of intake) for protein, calcium and phosphorus were calculated monthly. The protein equivalent of urea nitrogen appearance (nPNA) was calculated monthly as previously published [29, 30]. Dialysis adequacy was adjusted according to standard guidelines [31]. The minimal target total dialysis dose (Kt/V) was 2.1. A peritoneal equilibration test (PET) was performed at the start and at the end of the observation period as previously reported [31]. All patients were prescribed phosphate binders as calcium carbonate, and most were treated with calcitriol and erythropoietin as clinically indicated.
Biochemical determinations: routine chemistries were measured using the Vitros® 4600 Chemistry System. Serum parathyroid hormone (PTH, normal range 10–65 pg/mL) was measured by a first-generation immunometric assay (Immutopics, San Clemente, CA). 25(OH)D was measured by radioimmunoassay (RIA), (DiaSorin®, Italy) and 1,25(OH)2D by RIA (DIAsource Immunoassays SA®, Belgium). Plasma FGF23 levels (pg/mL) were determined by a second-generation enzyme-linked immunosorbent assay (ELISA), recognizing the intact molecule (Immutopics, San Clemente, CA). Klotho levels were measured by a solid-phase sandwich assay (ELISA, Cusabio, China). Normal values for serum FGF23 and Klotho levels were obtained from 45 pediatric patients with normal kidney function undergoing elective surgery.
The study was approved by the Ethical Committee, Faculty of Medicine, University of Chile, and informed consent/assent was obtained from parents, patients and controls.
Statistical Analysis
At least 14 case subjects and 20 controls were estimated to provide a power of 90%, to detect a standardized difference of 0.9 in mean FGF-23 levels, assuming a two-sided type I error of 5%. Controls were a reference group with only one basal time point to estimate the normal value of circulating FGF23 and Klotho in healthy children, as well as to calculate the size of the sample. Mean ± standard deviation (SD), median/ranges and log-transformed values were used when appropriate. Pearson correlation coefficient and t-test were used for bivariate analysis. Multiple linear regression with log-transformed FGF23 levels as the dependent variable was used to examine the association with potential clinical and biochemical parameters based on clinical judgment. A P < 0.05 was considered significant. All analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
Results
The mean age was 8.3 ± 4.6 years in the 34 patients (18 males) and 6.5 ± 3.0 years in 45 (24 males) control children. Causes of CKD were kidney dysplasia (n = 11), obstructive or reflux nephropathy (n = 6), nephrotic syndrome (n = 4), glomerulopathies (n = 2), hereditary nephropaties (n = 4), hemolytic uremic syndrome (n = 3), and unknown etiology (n = 4).
Vitamin D metabolites, FGF23, and Klotho measurements were obtained at entry (n = 31), and at Months 6 (n = 25) and 12 (n = 15); biochemical, nutritional and dialysis parameters were obtained monthly. The number of patients decreased during the observation period because of transplantation (n = 11), death due to sepsis (n = 2), and noncompliance (3).
Biochemical levels at 6 and 12 months remained similar to baseline values (Table 1). Isolated hypercalcemic episodes resolved promptly by adjusting the dose of calcium carbonate or calcitriol. Serum phosphorus was maintained within age-adjusted ranges with the use of calcium carbonate [32]. Circulating 1,25(OH)2D levels were markedly lower compared with reference control values. Although the levels of 25(OH)D at Months 6 and 12 were not significantly different from baseline, the values tended to diminish throughout the study period, but remained above the cut-off inclusion level of 20 ng/mL (Table 1).
Table 1.
Biochemical parameters of mineral metabolism in children treated with chronic peritoneal dialysis
| Month 1 (n:31) | Month 6 (n:25) | Month 12 (n:15) | |
|---|---|---|---|
| Calcium (mg/dL) 9.4–10.3a | 9.9 ± 1.1 | 9.8 ± 0.8 | 9.4 ± 0.9 |
| Phosphorus (mg/dL) 3.6–5.8a | 5.4 ± 1.2 | 5.7 ± 1.6 | 5.3 ± 1.3 |
| 1,25 (OH)D (pg/mL) 43 ± 2b | 26.7 ± 22.2 | 27.5 ± 21.4 | NA |
| 25(OH)D (ng/mL) >30c | 33.7 ± 6.8 | 24.9 ± 8.2 | 24.1 ± 5.6 |
| Parathyroid hormone (pg/mL) 200–300a | 330.8 ± 273.4 | 349.9 ± 283.3 | 320.8 ± 205.1 |
| FGF23 (pg/mL) 9.4 ± 5.7d | 215.1 ± 303.6 | 229.8 ± 252.6 | 194.8 ± 300.9 |
| FGF23 log | 1.98 ± 0.6 | 2.01 ± 0.6 | 1.77 ± 0.7 |
| Klotho (pg/mL) 320 ± 119.4d | 132.1 ± 58 | 133.3 ± 29.2 | 130.3 ± 34.4 |
At entry, FGF23 levels were 215.1 ± 303.6 versus 9.4 ± 5.7 pg/mL in the control group (P < 0.001). Compared with controls, FGF23 levels were elevated >20-fold normal controls at all measured time-points (P < 0.001) (Table 1). Baseline Klotho levels were 41% lower in patients compared with controls, 132.1 ± 58 versus 320 ± 119.4 pg/mL, respectively (P < 0.001), and remained virtually unchanged throughout the observation period (Table 1). Baseline and final average height (Table 2) and weight Z scores (not shown) did not show significant differences along the follow-up. The adequacy intake for calcium, phosphorus, protein and the estimated PNA at Months 1, 6 and 12 are displayed in Table 2. Calcium intake tended to decline after 1 year of observation, but the differences from baseline and Month 6 were not significant. Intake of phosphorus and protein remained virtually unchanged. In 21 patients that were treated with calcitriol (Rocaltrol®), the average dose of 1,25(OH)2D administered was 0.05 ± 0.07 mcg/kg/week.
Table 2.
Nutritional and dialytical parameters in children under chronic peritoneal dialysis at 12 months of follow-upa
| Variable | Month 1 (n:31) | Month 6 (n:25) | Month 12 (n:15) |
|---|---|---|---|
| Height/age SDS | −1.88 ± 1.18 | −1.65 ± 1.07 | −1.78 ± 1.15 |
| nPNA | 1.03 ± 0.3 | 0.97 ± 0.3 | 1.01 ± 0.3 |
| Adequacy of calcium intake (%) | 99.1 ± 44.5 | 90.7 ± 25.9 | 69.4 ± 27.7 |
| Adequacy of phosphorus intake (%) | 100.7 ± 70.9 | 91.4 ± 35.1 | 87 ± 34.2 |
| Adequacy of protein intake (%) | 117.8 ± 31.9 | 132.1 ± 50.2 | 139.2 ± 36.7 |
| Total KtV | 2.9 ± 1.4 | 2.9 ± 1.7 | 2.8 ± 1.7 |
| Residual KtV | 1.27 ± 1,5 | 1.02 ± 1.4 | 1.2 ± 1.8 |
aAnalysis of variance for repeated measures. All comparisons are non-significant
Values are expressed as mean ± SD.
nPNA: normalized protein equivalent of urea nitrogen appearance; Kt/V: weekly dialysis dose
Mean residual Kt/V at Months 1 and 12 represented 43 and 42% of the total Kt/V, respectively (Table 2). At baseline, 2-h creatinine D/P and Glucose D2/D0 values were 0.33 and 0.67, respectively.
Bi-variate analysis: Baseline PTH levels correlated with serum calcium (r = −0.39, P < 0.05) and with phosphorus (r = 0.42, P < 0.05), while log FGF23 correlated with height/age Z score (r = −0.38, P < 0.05) and residual Kt/V (r = −0.44, P < 0.05).
The log-FGF23 values correlated with the prevalent plasma calcium levels at all sequential measurements during Months 1, 6 and 12 (Figure 1a–c). FGF23 did not correlate with serum phosphorus, adequacy of phosphorus intake, adequacy of calcium intake, 25OHD, 1,25(OH)2D or Klotho levels at any time.
Fig. 1.
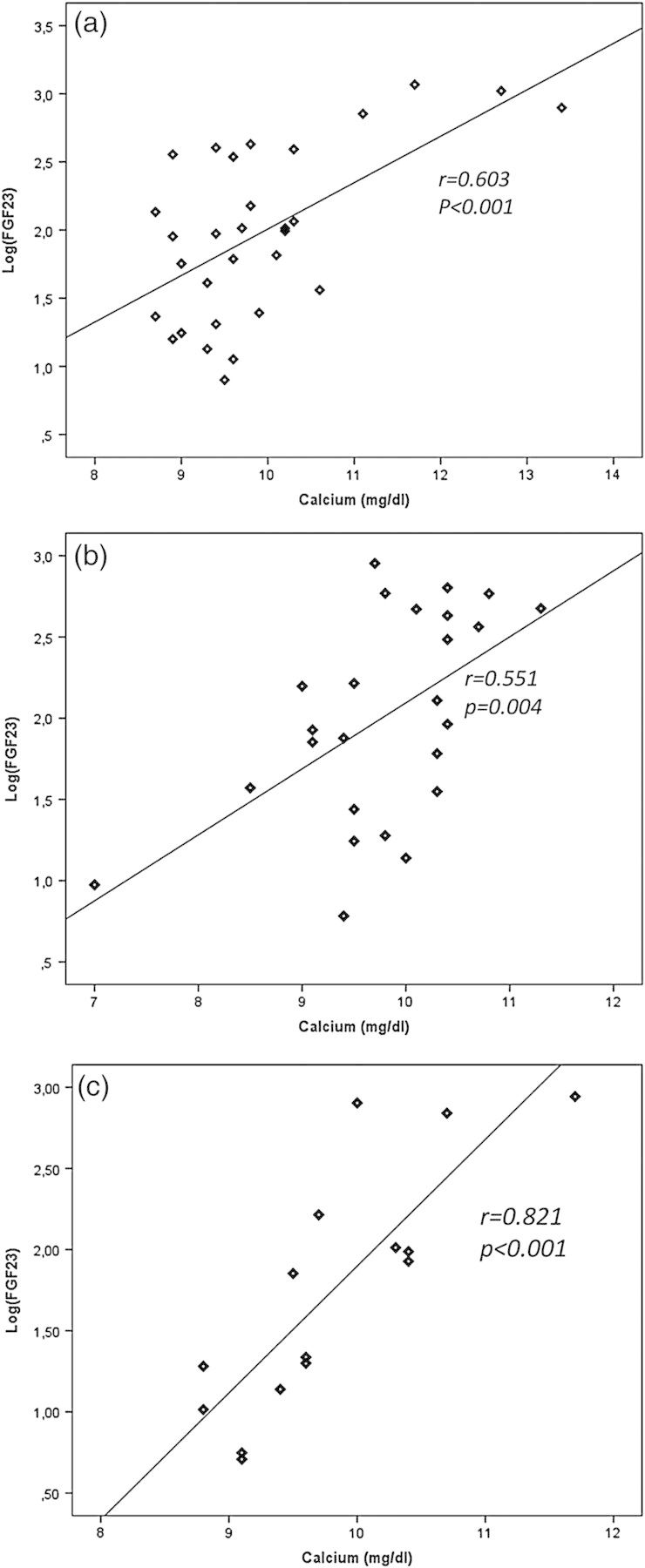
Linear correlation between serum calcium and serum FGF23 at Months 1 (a, n:31), 6 (b, n:25) and 12 (c, n:15) in children under chronic peritoneal dialysis.
Serum Klotho levels did not show a significant correlation with serum calcium, phosphorus, vitamin D and FGF23 at any time of measurement.
The calcium–phosphorus product showed a significant correlation with log FGF23 values at Months 1, 6 and 12 (all P < 0.05). To evaluate the potential interactions of calcium and phosphorus as possible determinants of FGF23, the correlation of calcium with log-FGF23 was reevaluated employing a cut-off plasma phosphorus concentration of 6 mg/dL (Figure 2). As shown, the significant associations were maintained at all three time-points when phosphorus was <6 mg/dL but were consistently lost with phosphorus concentrations >6 mg/dL (P < 0.05 for all comparisons).
Fig. 2.
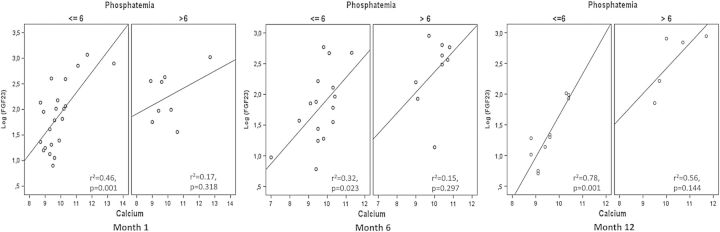
The direct correlation between serum calcium and serum FGF23 at Months 1 (a, n:31), 6 (b, n:25) and 12 (c, n:15) was evaluated according plasma phosphorus, at a cut-off level of 6 mg/dL. It could be observed that the significant association was mantained in patients with a plasma phosphorus <6 mg/dL, but it was consistently lost in patients with phosphemia >6 mg/dL.
In the multiple regression analysis, calcium was the strongest variable determining FGF23 levels at Months 1, 6 and 12 (Table 3). Although plasma phosphorus did correlate significantly with FGF23 at Month 12, its significance was lost when calcium was removed from the model. Including residual KtV in the multivariate analysis did not change the correlation of Ca with FGF23.
Table 3.
Multiple regression analysis between log-FGF23 level versus serum calcium, phosphorus, PTH and 25(OH)vitamin D at Months 1, 6 and 12 of follow-up
| Dependent variable | Independent variables | Regression coefficient | SEM | t-test value H0≠0 | P value | IC 95% lower limit | IC 95% upper limit |
|---|---|---|---|---|---|---|---|
| Log FGF23 Month 1 | Intercept | −2.259 | 1.003 | −2.251 | 0.033 | −4.325 | −0.192 |
| Calcium | 0.372 | 0.093 | 3.996 | 0.001 | 0.180 | 0.564 | |
| Phosphorus | −0.021 | 0.099 | −0.210 | 0.835 | −0.225 | 0.183 | |
| Parathyroid Hormone | 0.001 | 0.000 | 1.290 | 0.209 | 0.000 | 0.001 | |
| 25 (OH) D | 0.015 | 0.014 | 1.100 | 0.282 | −0.013 | 0.044 | |
| Log FGF23 Month 6 | Intercept | −6.476 | 3.309 | −1.957 | 0.086 | −14.105 | 1.154 |
| Calcium | 0.884 | 0.340 | 2.603 | 0.031 | 0.101 | 1.667 | |
| Phosphorus | −0.132 | 0.162 | −0.817 | 0.438 | −0.506 | 0.241 | |
| Parathyroid Hormone | 0.001 | 0.001 | 1.808 | 0.108 | 0.000 | 0.003 | |
| 25 (OH)vit D | −0.005 | 0.027 | −0.189 | 0.855 | −0.068 | 0.057 | |
| Log FGF23 Month 12 | Intercept | −5.257 | 1.504 | −3.495 | 0.005 | −8.567 | −1.946 |
| Calcium | 0.566 | 0.161 | 3.514 | 0.005 | 0.212 | 0.921 | |
| Phosphorus | 0.322 | 0.101 | 3.194 | 0.009 | 0.100 | 0.544 | |
| Parathyroid Hormone | −0.001 | 0.001 | −1.457 | 0.173 | −0.002 | 0.001 | |
| 25 (OH)vit D | 0.005 | 0.017 | 0.295 | 0.774 | −0.032 | 0.042 |
Calcemia was found to be the strongest variable determining FGF23 plasma levels during the observation period. At Month 12, phosphemia showed a statistical significance, however, it was lost when serum calcium was removed from the model.
Discussion
In the present study, we confirm that elevated levels of FGF23 do not correlate with plasma phosphorus in children on dialysis. We herein demonstrate a significant relationship between FGF23 levels and the prevailing calcium concentrations, independent of PTH and phosphorus concentrations, yet modifiable by the prevailing phosphorus concentrations. These findings suggest important regulating effects of circulating calcium on FGF23 levels.
Studies in adults demonstrated that FGF23 plasma levels increase earlier than phosphorus or PTH in CKD stages 2–4 [34]. Similar findings have been reported in children, with the highest levels observed in patients on dialysis [7, 9].
As predicted, we also observed high FGF23 levels in our dialysis patients. Throughout 12 months of observation, average levels were 20-fold higher than age-matched controls. These values are similar to those reported by other investigators employing different assays [7, 9, 35–37]. While these assays are not identical, those recognizing predominantly the full-length intact molecule such as the one we employed are used extensively by researchers in the clinical field [16, 27, 37, 38]. Specifically, the Immutopics intact assay used in our study has been shown to have a strong linear correlation with the C-terminal Immutopics assay, and both measure reliably the bioactive hormone [16, 37].
Residual renal function (RRF) is another factor to consider when interpreting FGF23 levels [36], and could be a variable explaining the higher FGF23 levels reported by Seeherunvong et al. [35]. We observed an inverse correlation between residual Kt/V and FGF23, similar to adults on dialysis [36]. Anuric patients showed higher FGF23 levels despite higher peritoneal phosphate removal, lower phosphorus intake and higher doses of phosphate binders, suggesting a preeminent role of RRF on FGF23 levels [36]. We observed stable and significantly unchanged levels of FGF23 on sequential measurements after 12 months of prospective follow-up, while others have reported progressively increasing levels in patients on peritoneal dialysis [7, 9, 28]. These differences could be explained in part by the escalating and higher doses of the vitamin D receptor activators given to those patients compared with the rather low and stable dose of calcitriol received by our patients [28]. These contrasting findings may have relevant therapeutic implications on the use of calcitriol or vitamin D analogs, since higher FGF23 concentrations may contribute to higher rates of ventricular hypertrophy and cardiovascular morbidity and mortality in patients with CKD [16, 19, 35].
We also observed an inverse relationship between FGF23 levels and height Z scores, a finding not previously reported in children under PD. Previous investigators have reported a positive relationship between FGF23 and statural growth in CKD children [38], but those and our studies are not comparable. The study of Wesseling-Perry et al. [38] included only CKD stage 2–5 patients, not yet on dialysis, while our patients were all receiving maintenance dialysis, and most were treated with calcium carbonate and activated vitamin D which may have divergent effects on bone histology (including variable degrees of adynamic bone disease) and FGF23 levels, and thus have different consequences on the patient's growth rates. A relationship between FGF23 and IGF1 was reported by Bachetta et al. [27], however, only children with a GFR > 30 mL/min/1.73 m2 were included, none of them under dialysis therapy.
Klotho levels in our patients were reduced on average 2.5-fold compared with healthy controls. This finding suggests that CKD is a state of Klotho deficiency [9, 12–15]. In addition to its role in phosphorus homeostasis, Klotho exhibits beneficial effects on the cardiovascular system including protection against oxidative stress, renal fibrosis and vascular calcification [14, 39, 40]. The latter is of particular relevance to young CKD patients, where cardiovascular mortality rates are 100 times greater than healthy controls [41] probably in part due to early coronary calcification [42]. The association of reduced plasma Klotho levels with markers of arterial stiffness and coronary calcification suggest that maintaining higher Klotho levels could improve the vascular abnormalities in CKD [43].
In this longitudinal study, sequential measurements revealed stable Klotho levels up to 12 months of follow-up. Reduced Klotho levels may involve upregulation of the renal renin-angiotensin system (RAS) pathway, since angiotensin II negatively regulates the renal expression of Klotho in CKD [44–46]. Activated vitamin D or its analogs suppress the renal RAS [47, 48] and may help upregulate Klotho expression and maintain its secretion [49]. Since most patients were treated with calcitriol, this intervention may have contributed to maintain levels of Klotho particularly in patients with preserved RRF. Our study was not designed to evaluate the effects of Klotho on the vascular system; therefore future studies are needed to evaluate the potential effects of stratified Klotho levels on noninvasive surrogate markers of cardiovascular disease.
As shown by others [9], we also did not find a correlation between phosphorus and FGF23 levels, except for the bivariate analysis at Month 12. While previous studies in hemodialysis children did not find FGF-23 levels to correlate with those of phosphorus, nor with calcium, PTH, 25(OH)D and 1,25(OH)2D concentrations [35], others have observed phosphorus and FGF23 correlations in pre-dialysis [7] and in hemodialysis patients [50, 51].
Average plasma phosphate concentrations during one year of follow-up reflected a satisfactory control of hyperphosphatemia [32], and the adequacy of phosphorus intake [29] indicated appropriate nutritional compliance. Since dietary phosphorus intake, rather than blood phosphorus levels, is the primary stimulus for FGF23 secretion [17, 18], it could explain in part the lack of association between plasma phosphorus and FGF23 levels in our patients.
We observed a significant association of calcium with FGF23 levels at all time points. As shown in Table 3, this direct correlation remained the strongest independent variable associated with FGF23 plasma levels in the multiple regression model. In a recent study in children with CKD, serum Ca correlated with log FGF23 in the univariate analysis, but the analysis included patients with all stages of CKD and the correlation did not persist on the multiple regression analysis [9]. The regulation of FGF23 by calcium or vice versa is still poorly understood, and has only recently been studied in animal models. Stimulation of the 1,25(OH)2D-VDR pathway induces the expression of FGF23, as evidenced by increased FGF23 levels after 1,25(OH)2D administration [52], and VDR-null mice showed undetectable FGF23 levels. In addition, normalization of plasma calcium and phosphate levels by dietary means increased FGF23 levels in VDR-null mice, indicating that FGF23 expression is also regulated by a VDR-independent pathway [52]. Double knock-out mice for PTH and calcium-sensing receptor (CaSR) showed little FGF23 response to the increase in plasma phosphorus during phosphate loading, probably secondary to the severe hypocalcemia, suggesting that the latter prevents a normal FGF23 secretion by the bone [22]. Our observations of the lack of correlation between phosphorus and FGF23 on the bivariate analysis, coupled to the extinguished correlation of phosphorus and FGF23 at Month 12 by extracting plasma Ca from the regression model (Table 3), suggest a possible calcium-dependent effect. Experimental observations demonstrate that plasma calcium levels regulate the production of FGFG23 in low- or high-calcium conditions [21, 22], in agreement with the clinical findings outlined in the present study. Besides the isolated roles of calcium and phosphorus on FGF23 production, an apparent reciprocal relationship between both ions and FGF23 was also observed experimentally whereby FGF23 levels increased sharply when phosphorus was >5 mg/dL if plasma calcium was >8 mg/dL, but this relationship was lost when calcium was <8 mg/dL [22]. These observations suggest that plasma phosphorus does not regulate FGF23 under hypocalcemic conditions.
Similarly, while calcium and FGF23 correlated directly at a calcium concentration >8 mg/dL and phosphorus >5 mg/dL, this association was lost when phosphorus was <5 mg/dL, suggesting the existence of a threshold for both ions which reciprocally limits the effect on FGF23 by each one of them [22]. We observed the loss of the linear relationship between calcium and FGF23 when the phosphorus was >6 mg/dL, a finding consistently observed at all three study-points. While plasma calcium clearly correlates with, and presumably increases production of FGF23, the optimal range of plasma phosphorus at which this effect is consistent needs further testing [7, 21, 22, 36, 38].
The precise molecular mechanisms regulating FGF23 secretion remain elusive. Although it appears that neither the full-length CaS receptor nor PTH, 1,25(OH)2D or serum phosphorus appear to be exclusively and independently involved in the regulation of serum FGF23 [22], other possibilities to consider include the existence of yet unidentified cell surface receptors for serum calcium and phosphorus on osteoblasts and osteocytes capable of modulating FGF23 secretion, or that alterations in bone cell activity and mineralization coupled with changes in serum Ca and phosphorus concentration may result in changes of FGF23 secretion by other sensing mechanisms. Further studies will be required to unravel these possibilities.
In summary, this is the first study evaluating simultaneously longitudinal changes of circulating FGF23 and Klotho concentrations, and their relationships to concurrent concentrations of calcium, phosphorus and various calciotropic hormones in children on dialysis. Plasma calcium emerged as the strongest regulating factor determining FGF23 levels, particularly when the prevailing concentrations of phosphorus remain <6 mg/dL. This may have important clinical implications for the use of calcium-containing phosphate binders which remain widely used for the management of hyperphosphatemia. Stable blood Klotho levels even after 1 year of dialysis therapy, possibly in part as a result of treatment with activated vitamin D, may antagonize the otherwise deleterious effects of CKD on the cardiovascular system. Future studies are needed to further delineate the intricate interactions between calcium and phosphorus in the regulation of FGF23 and Klotho in CKD patients.
Acknowledgements
This study was supported by Fondecyt Grant 1110226, CONICYT, Government of Chile.
Conflict of interest statement. None declared.
References
- 1.Kuro-o M. Overview of the FGF23-Klotho axis. Pediatr Nephrol. 2010;25:583–590. doi: 10.1007/s00467-009-1260-4. [DOI] [PubMed] [Google Scholar]
- 2.Nabeshima Y. The discovery of a-Klotho and FGF23 unveiled new insight into calcium and phosphate homeostasis. Cell Mol Life Sci. 2008;65:3218–3230. doi: 10.1007/s00018-008-8177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Winer K, Econs MJ, et al. FGF-23 is elevated by chronic hyperphosphatemia. J Clin Endocrinol Metab. 2004;89:4489–4492. doi: 10.1210/jc.2004-0724. [DOI] [PubMed] [Google Scholar]
- 4.Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Dov I, Galitzer H, Lavi-Moshayoff V, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;7:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 7.Wesseling-Perry K, Pereira R, Wang H, et al. Relationship between plasma fibroblast growth factor 23 concentration and bone mineralization in children with renal failure in peritoneal dialysis. J Clin Endocrinol Metab. 2009;94:511–517. doi: 10.1210/jc.2008-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 9.Wan M, Smith C, Shah V, Gullet A, et al. Fibroblast growth factor 23 and soluble Klotho in chidren with chronic Kidney disease. Nephrol Dial Transplant. 2013;28:153–156. doi: 10.1093/ndt/gfs411. [DOI] [PubMed] [Google Scholar]
- 10.Kuro-O M, Matsumura Y, Aizawa H, et al. Mutation of the mouse Klotho gene leads to a syndrome resembling aging. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 11.Tsujikawa H, Kurotaki Y, Fujimori T, et al. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 12.Koh N, Fujimori T, Nishiguchi S, et al. Severely reduced production of Klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280:1015–1020. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 13.Chang Hu M, Shi M, Zang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ming-Chang H, Kuro-O M, Moe O. Klotho and kidney disease. J Nephrol. 2010;23:S136–S144. [PMC free article] [PubMed] [Google Scholar]
- 16.Gutiérrez O, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med, 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90:1519–1524. doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 18.Antoniucci DM, Yamashita T, Portale A. Dietary phosphorus regulates plasma fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91:3144–3149. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez O, Wolf M, Taylor E. Fibroblast growth factor 23, cardiovascular disease risk factors, and phosphorus Intake in the health professionals follow-up study. Clin J Am Soc Nephrol. 2011;6:2871–2878. doi: 10.2215/CJN.02740311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimada T, Yamazaki Y, Takahashi M, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol. 2005;289:F1088–F1F95. doi: 10.1152/ajprenal.00474.2004. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez-Ortiz M, López I, Muñoz-Castañeda J, et al. Calcium deficiency reduces circulating levels of FGF23. J Am Soc Nephrol. 2012;23:1190–1197. doi: 10.1681/ASN.2011101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinn S, Thomsen A, Pang J, et al. Interactions between calcium and phosphorus in the regulation of the production of fibroblast growth factor 23 in vivo. Am J Physiol Endocrinol and Metab. 2013;304:E310–E320. doi: 10.1152/ajpendo.00460.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koizumi M, Komaba H, Nakanishi S, et al. Cinacalcet treatment and plasma FGF23 levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol Dial Transplant. 2012;27:784–790. doi: 10.1093/ndt/gfr384. [DOI] [PubMed] [Google Scholar]
- 24.Martin K, Bell G, Pickthorn K, et al. Velcalcetide (AMG 416), a novel peptide agonist of the calcium sensing receptor, reduces plasma parathyroid hormone and FGF23 levels in healthy male subjects. Nephrol Dial Transplant. 2014;29:385–392. doi: 10.1093/ndt/gft417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinha M, Turner C, Dalton RN, et al. Investigating FGF-23 concentrations and its relationship with declining renal function in paediatric patients with pre-dialysis CKD Stages 3–5. Nephrol Dial Transplant. 2012;0:1–7. doi: 10.1093/ndt/gfs109. [DOI] [PubMed] [Google Scholar]
- 26.Van Husen M, Fischer AK, Lehnhardt A, et al. Fibroblast growth factor 23 and bone metabolism in children with chronic kidney disease. Kidney Int. 2010;78:200–206. doi: 10.1038/ki.2010.107. [DOI] [PubMed] [Google Scholar]
- 27.Bacchetta J, Dubourg L, Harambat J, et al. The influence of glomerular filtration rate and age on fibroblast growth factor 23 pLasma levels in pediatric chronic kidney disease. J Clin Endocrinol Metab. 2010;95:1741–1748. doi: 10.1210/jc.2009-1576. [DOI] [PubMed] [Google Scholar]
- 28.Wesseling-Perry K, Pereira R, Sahney S, et al. Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int. 2011;79:112–119. doi: 10.1038/ki.2010.352. [DOI] [PubMed] [Google Scholar]
- 29.KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 Update. Am J Kidney Dis. 2009;53:S1–S124. doi: 10.1053/j.ajkd.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Cano F, Azocar M, Cavada G, et al. Kt/V and nPNA in pediatric peritoneal dialysis: a clinical or a mathematical association? Pediatr Nephrol. 2006;21:114–118. doi: 10.1007/s00467-005-2048-9. [DOI] [PubMed] [Google Scholar]
- 31.National Kidney Foundation: K/DOQI clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis. 2001;37:S65–S136. doi: 10.1016/s0272-6386(01)70006-6. [DOI] [PubMed] [Google Scholar]
- 32.KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. Am J Kidney Dis. 2003;42:S1–S201. [PubMed] [Google Scholar]
- 33.Chesney R, Rosen J, Hamstra A, DeLuca H. Serum 1,25-Dihydroxivitamin D levels in normal children and in vitamin D disorders. Am J Dis Child. 1980;134:135–139. doi: 10.1001/archpedi.1980.02130140009004. [DOI] [PubMed] [Google Scholar]
- 34.Isakova T, Wahl P, Vargas G, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seeherunvong W, Abitbol C, Chandar Y, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in children on dialysis. Pediatr Nephrol. 2012;27:2129–2136. doi: 10.1007/s00467-012-2224-7. [DOI] [PubMed] [Google Scholar]
- 36.Isakova T, Xie H, Barchi-Chung A, et al. Fibroblast growth factor 23 in patients undergoing peritoneal dialysis. Clin J Am Soc Nephrol. 2011;6:2688–2695. doi: 10.2215/CJN.04290511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burnett SM, Gunawardene SC, Bringhurst FR, et al. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21:1187–1196. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 38.Wesseling-Perry K, Pereira R, Tseng C, et al. Early skeletal and biochemical alterations in pediatric chronic kidney disease. Clin J Am Soc Nephrol. 2012;7:146–152. doi: 10.2215/CJN.05940611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vervloet AG, Larson TE. Fibroblast growth factor-23 and Klotho in chronic kidney disease. Kidney Inter Suppl. 2011;1:130–135. [Google Scholar]
- 40.John G, Cheng C, Kuro-o M. Role of Klotho in aging, phosphate metabolism, and CKD. Am J Kidney Dis. 2011;58:127–134. doi: 10.1053/j.ajkd.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(S3):S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 42.Srivaths PR, Goldstein SL, Silverstein DM, et al. Elevated FGF 23 and phosphorus are associated with coronary calcification in hemodialysis patients. Pediatr Nephrol. 2011;26:945–951. doi: 10.1007/s00467-011-1822-0. [DOI] [PubMed] [Google Scholar]
- 43.Kitagawa M, Sugiyama H, Morinaga H, et al. A decreased level of plasma soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One. 2013;8:e56695. doi: 10.1371/journal.pone.0056695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Borst M, Vervloet M, ter Wee P, et al. Cross talk between the Renin–Angiotensin–Aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol. 2011;22:1603–1609. doi: 10.1681/ASN.2010121251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitani H, Ishizaka N, Aizawa T, et al. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension. 2002;39:838–843. doi: 10.1161/01.hyp.0000013734.33441.ea. [DOI] [PubMed] [Google Scholar]
- 46.Yoon HE, Ghee JY, Piao S, et al. Angiotensin II blockade upregulates the expression of klotho the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrol Dial Transplant. 2011;26:800–813. doi: 10.1093/ndt/gfq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsujikawa H, Kurotaki Y, Fujimori T, et al. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 48.Freundlich M, Quiroz Y, Zhang Z, et al. Suppression of renin–angiotensin gene expression in the kidney by paricalcitol. Kidney Int. 2008;74:1394–1402. doi: 10.1038/ki.2008.408. [DOI] [PubMed] [Google Scholar]
- 49.Lau W, Leaf E, Hu M, et al. Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kid Int. 2012;82:1261–1270. doi: 10.1038/ki.2012.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srivaths PR, Goldstein SL, Silverstein DM, et al. Elevated FGF 23 and phosphorus are associated with coronary calcification in hemodialysis patients. Pediatr Nephrol. 2011;26:945–951. doi: 10.1007/s00467-011-1822-0. [DOI] [PubMed] [Google Scholar]
- 51.Imanishi Y, Inaba M, Nakatsuka K, et al. FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int. 2004;65:1943–1946. doi: 10.1111/j.1523-1755.2004.00604.x. [DOI] [PubMed] [Google Scholar]
- 52.Martin A, David V, Quarles D. Regulation and function of the FGF23/Klotho Endocrine pathways. Physiol Rev. 2012;92:131–155. doi: 10.1152/physrev.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


