Abstract
There is an increasing awareness about the risks of arterial and venous thromboembolism (TE) in hospital patients and general public which has led to consideration of thrombosis prevention measures in earnest. Early recognition of the symptoms of TE disease has led to timely administration of antiplatelet and anticoagulant drugs, translating to better outcome in many of these patients. In this respect, patients with chronic kidney disease (CKD) represent a special group. They indeed represent a high-risk group for thrombosis both in the cardiovascular territory and also in the venous circulation. At the same time, abnormalities in the platelet membranes put them at risk of bleeding which is significantly more than other patients with chronic diseases. Anticoagulation may be ideal to prevent the former, but the co-existing bleeding risk and also that the commonly used drugs for inhibiting coagulation are eliminated by renal pathways pose additional problems. In this review, we try to explain the complex thrombotic-haemorrhagic state of chronic kidney disease patients, and practical considerations for the management of anticoagulation in them with a focus on heparins.
Keywords: anticoagulation, heparin, thrombosis, warfarin
Introduction
Patients with chronic kidney disease (CKD) present a dilemma in that they are simultaneously prothrombotic and haemorrhagic [1]. In this respect, anticoagulant drugs, unlike the general population with normal renal function, can have both beneficial and harmful effects. It is well established now that these patients are at risk of thrombotic events reflected in increased incidence of cardiovascular disease and also, venous thromboembolism (TE) [2, 3]. Arterial TE events include cerebrovascular disease, myocardial infarction and peripheral artery disease, while venous TE can involve in addition to deep vein thrombosis and pulmonary embolism, thrombosis of venous access sites and central lines [1–4]. Interestingly, there have been reports of venous TE being not just limited to severe CKD, but also CKD Stages 1 and 2, and CKD Stage 3 in the presence of albuminuria [5]. There is a suggestion that the risk of venous TE is more related to albuminuria than to impaired glomerular filtration rate (GFR), reflecting the predominance of this complication in nephrotic syndrome patients [5, 6].
The prothrombotic nature of CKD may be explained by several factors including (i) prothrombotic changes which develop in the vascular endothelium, (ii) increase in coagulation factors like fibrinogen, factor VIII and von Willebrand factor as part of the chronic inflammatory process, (iii) increase in antifibrinolytic proteins like plasminogen activator inhibitor, which inhibit the clot breakdown, (iv) hyperlipidaemia which predisposes the patients to cardiovascular thrombosis, (v) haemoconcentration with consequent effects on rheology and (vi) also changes in the platelet membrane which make it proaggregatory [1, 4, 7]. Interestingly, simultaneously, the same patients are at risk of bleeding. The predominant pathophysiological factor attributable to the bleeding risk is platelet dysfunction although anaemia, which can impact on the interaction of platelets with the vessel wall and comorbidities like stress ulcers and need for frequent interventions, can contribute in addition to drugs (antiplatelets) [1, 4, 8]. For the latter, a recent Cochrane systematic review showed that risks may outweigh benefits among people with low annual risks of cardiovascular events, including those with early stages of CKD who do not have clinically evident occlusive cardiovascular disease [9].
The anticoagulation options in CKD
As discussed before, patients with CKD are at increased risk of thrombosis and as such, prophylaxis with anticoagulant agents may be beneficial in high-risk thrombotic situations. The currently available options in this respect are (i) parenteral forms which include unfractionated heparin, low-molecular weight heparins (including fondaparinux) and direct thrombin inhibitors and (ii) oral formulations, the newer oral anticoagulants. For the treatment of TE, these drugs are used at higher doses and in addition to newer oral anticoagulants, warfarin may also be considered. In the following sections, we discuss these agents in their special relation to CKD patients. We will not cover anticoagulation in dialysis which has been the subject of several brilliant reviews recently [10, 11].
Unfractionated heparin
Pharmacological properties
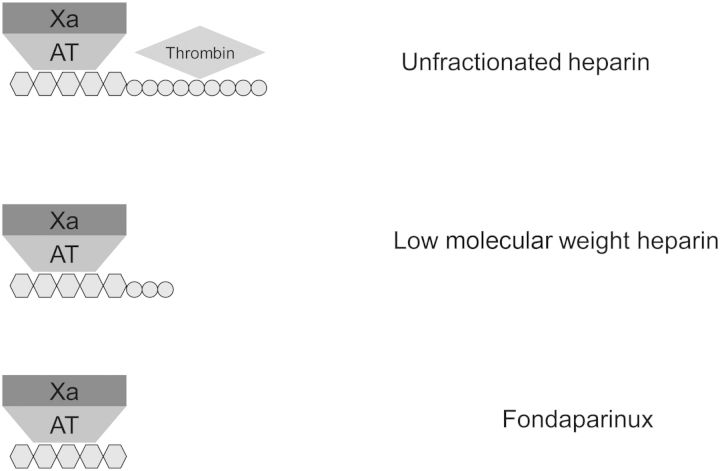
Unfractionated heparin (UFH) is a sulphated polysaccharide with a molecular weight of 3000–30 000 Da (mean, 15 000 Da, ∼45 monosaccharide chains). About one-third of UFH binds to antithrombin (AT) with high affinity, resulting in the inhibition of thrombin (IIa) and factor Xa in an equal ratio (1:1) (see Figure 1) [12]. UFH has a propensity to bind to positively charged surfaces, which can reduce its bioavailability and also lead to an unpredictable anticoagulant response. Although largely replaced in practice by the low-molecular weight heparins for the prophylaxis and treatment anticoagulation, UFH use in clinical practice is unlikely to become obsolete [13]. Their main limitations in practice are well documented, including (i) the unpredictable bioavailability and the subsequent need to stringently monitor activated partial thromboplastin time (APTT) levels to avoid sub- and supra-therapeutic anticoagulation, (ii) the immune mediated platelet activation and potentiating heparin-induced thrombocytopenia (HIT) and (iii) the adverse effects of long-term therapy such as osteopenia [14]. However, it is used as a first line treatment for anticoagulation in patients with high bleeding risks as they can be rapidly and completely neutralized by protamine. The short half-life of UFH [∼30 min at low doses (25 units/kg) increasing to 150 min at higher doses (400 units/kg) and in patients with severe renal dysfunction], allows anticoagulation effects to be removed promptly (1–4 h) [15–17].
Fig. 1.
Heparin binds to AT which potentiates its anti-Xa and AT effects. The AT-thrombin effect does require the long-chain, while the anti-XA effect requires only the pentasaccharide sequence.
Clearance
UFH is primarily excreted by reticuloendothelial systems in a rapid dose-dependent saturable mechanism [15–17]. Clear guidance in this respect for the extent of reticuloendothelial saturation from a practical point of view is difficult to ascertain. A secondary slower first-order clearance of UFH by renal excretion, results in non-linear UFH pharmacokinetics with anticoagulation increasing disproportionally at high therapeutic doses. Over-anticoagulation can occur in patients with moderate (CrCl 30–50 mL/min) to severe renal dysfunction (CrCl <30 mL/min), due to the impaired clearance at high therapeutic doses once saturation of reticuloendothelial clearance occurs. Inter-patient variability of accumulation is expected and the anticoagulant response is therefore unpredictable. Therefore, a conservative dosing of UFH is recommended in patients with severe renal impairment to avoid supra-therapeutic anticoagulation [18, 19].
Dosing
In our practice, the traditional 75–80 units/kg loading dose and 18 units/kg/h maintenance dose for treatment of a venous TE for patients with severe kidney dysfunction is associated with supra-therapeutic levels. A more conservative dose of 60 units/kg loading dose and 12 units/kg/h maintenance dose is thus chosen for patients with severe kidney dysfunction. This dosing regimen is borrowed from the recommended acute coronary syndrome (ACS) treatment guide where a lower target APTT range of 1.5–2 is desired [20]. This dose minimization is still expected to achieve minimum therapeutic anticoagulation [APTT >1.5] for patients treated for a venous TE, albeit at the lower end of the range, despite the 33% dose reduction. Subsequent doses should be adjusted to maintain a therapeutic level (APTT 1.5–2.5).
Renal perspectives
Despite the extensive experience with UFH in daily practice, little evidence is available to confirm the safety of UFH in this high-risk group. A post-hoc analysis of the large randomized controlled trials, ESSENCE and TIMI 11b, investigated the safety of enoxaparin and UFH treatment of ACS in patients with a CrCl < 30 mL/min [21]. A total of 143 patients previously randomized to UFH or low molecular weight heparin (LMWH), enoxaparin 1 mg/kg, were retrospectively analysed. The rates of major bleeding was 1.1 and 6.6% (P < 0.0001) in patients with CrCl >30 mL/min and <30 mL/min, respectively, with no difference in bleeding risks with UFH or unadjusted enoxaparin groups. The analysis demonstrates that the bleeding risk in patients with CKD (CrCl < 30 mL/min) is greater than in patients with CrCl >30 mL/min regardless of the anticoagulant used. All-cause mortality at 43 days was significantly increased in the severe renal disease subgroup compared with the control group (18.2 versus 3.2%, P < 0.001) despite no significant difference in recurrent myocardial infarction or urgent revascularisation within this time. The exact cause of increased mortality is not clearly defined within the study. Few patients from this renally impaired group were defined as severe CKD (or CKD 5). Additionally, no dose adjustment of enoxaparin was performed in concordance to the licencing, and a lower intensity UFH regimen was used (target APTT ratio 1.5–2), therefore lower bleeding rates are postulated if appropriate dose adjustments of the LMWH are performed, and conversely, UFH dosing for venous TE treatment (APTT range 1.5–2.5 and higher initial dosing) may correspond to higher bleeding rates.
A retrospective cohort study in a single teaching hospital reviewed the outcomes of UFH and LWMH treatment in 620 patients with mild-to-severe CKD [22]. Standard UFH protocol treatment was compared with enoxaparin 1 mg/kg BD (unadjusted dose) treatment and demonstrated a risk of major bleeding was higher in UFH group (22.3 versus 20.7 bleeds per 1000 treatment days; non-significant). Fifteen percent (n = 98) of the study had a CrCl <20 mL/min, and although the major bleeding risk was higher there was no significant difference between the two treatments (30.7 versus 36.6 bleeds per 1000 treatment days for UFH and enoxaparin respectively; non-significant). Minor bleeding, defined as non-retroperitoneal or intracranial bleeding or bleeding with the loss of <3 g/dL of haemoglobin, was higher in the enoxaparin group. This study was the first of many to suggest that higher risk of bleeding in severe CKD is not related to choice of anticoagulant used but due to baseline risk factors such as uraemia, increased age, concurrent treatment and comorbidities. Additionally, diagnosis of a major bleed after a patient receives >2 units of blood are likely to cause bias results. Due to renal anaemia and the lower baseline Hb in the severe CKD group, it is likely that the threshold for transfusion is much lower in this group.
In summary, the advantage of using UFH in patients with severe kidney disease is unlikely to be due to a lower bleeding risk, but rather due to the ease in which any episode of bleeding can be corrected with UFH's advantageous short therapeutic half-life and its complete reversal with protamine.
Low-molecular weight heparins
Pharmacological properties
LMWH are used routinely in practice for anticoagulation of patients and have largely replaced the use of UFH [23]. LWMHs are synthesized through chemical or enzymatic depolymerization of UFH, resulting in shorter heparin chains that show a stronger affinity for inhibiting factor Xa and lower specificity towards thrombin (see Figure 1). The proportional interaction with factor Xa and thrombin differs among different LMWH. The shorter heparin chains of LMWH also reduce the interaction with platelets and endothelial cells, reducing the incidence of HIT and osteopenia and other side effects, like alopecia, seen with UFH [24]. Due to the homogeneity of the polysaccharide chains, LMWHs have preferable pharmacodynamic and pharmacokinetic properties in comparison to UFHs. The advantages of LMWH include predictable pharmacokinetics and anticoagulation response, the convenience of once and twice daily dosing regimens (versus UFHs twice or thrice daily dosing or continuous infusion), lack of routine monitoring required and good track-record of safety and efficacy in practice. Due to their ease of use, LMWHs are more versatile and can be used by patients at home [25].
Not all LMWH are created equal?
The LMWH routinely used in the UK are enoxaparin, dalteparin and tinzaparin, with each having different dosing schedules and licenced indications for the treatment of ACS and venous TE. The individual LMWH's molecular weight affects the specificity each drug has to binding to factor II and factor Xa with smaller LWMH selectively targeting factor Xa in comparison to factor II (see Table 1). Additionally, renal clearance is indirectly proportional to the molecular weight of the drug (see Table 1). Thus, an LMWH with a lower molecular weight are is more dependent on renal clearance and therefore may accumulate in patients with renal dysfunction and may be more pronounced with the smaller LMWH [29].
Table 1.
Dosing and pharmacological characteristics of three low-molecular weight heparins in chronic kidney disease
| Dalteparin [26] | Enoxaparin [27] | Tinzaparin [28] | |
|---|---|---|---|
| Treatment indications (dose) | ACS (120 IU/kg BD), VTE (200 IU/kg OD) | ACS (1 mg/kg BD), VTE (1.5 mg/kg OD) | VTE (175 IU/kg OD) |
| Dosing advice in CKD 4/5 | No dose adjustment advised except with anti-Xa level for eGFR <30 mL/min | Dose reduction to 1 mg/kg once daily if eGFR <30 mL/min | No dose reduction needed if eGFR >20 mL/min. Dose adjust as per anti-Xa level if <20 mL/min |
| Anti-Xa:IIa ratio | 2.2 | 3.9 | 2.8 |
| Average molecular weight (Da) | 5000 | 4500 | 5500–7500 |
OD, once daily; BD, twice daily; VTE, venous thromboembolism.
Renal perspectives
Due to the expected accumulation of all LMWH in severe renal dysfunction, patients with renal dysfunction have been excluded from the majority of the large randomized control trials performed with LMWHs. Without these results and the subsequent lack of safety and efficacy data, caution is required when dosing the LWMHs in patients with renal impairment. A dose reduction and monitoring of anti-factor Xa levels is generally advised.
The majority of the evidence for treatment dose LMWH in severe renal impairment is based on smaller open label studies where outcomes such as anti-Xa levels are reviewed and not the patient orientated outcomes such as mortality, disease progression and major bleeding rates. Some analysis of subgroups of CKD patients in larger randomised controlled trials has been performed and has proved valuable. Due to the latter analysis exclusion criteria, the majority of patients defined as having severe CKD will have a renal function just below CrCl 30 mL/min and are not representative of patients with end-stage renal disease. The pharmacokinetic properties of the three licenced LMWH vary as does the evidence base for treatment of ACS and venous TE in severe CKD (Table 2).
Table 2.
Published pharmacokinetic data on low molecular weight heparin use in CKD 4/5
| Authors | Dose | Population | Results | Comment |
|---|---|---|---|---|
| Enoxaparin | ||||
| Spinler et al. [10] | 1 mg/kg BD (versus UFH) | Randomized control trial subgroup | Endpoints (mortality, MI and urgent revascularization) 18.8 versus 32.4% in enoxaparin and UFH arms (ns) | Bleeding rates increased in severe RI group regardless of anticoagulant used |
| Thorevska et al. [11] | 1 mg/kg BD (versus UFH) | Retrospective cohort study (n = 620), <60 mL/min | Major bleed rate per 1000 person days 30.7 versus 36.6 in UFH and enoxaparin groups (ns) | |
| Collet et al. 2003 [30] | 0.65 mg/kg BD (in CrCl <30 mL/min) | Registry data (n = 62), <30 mL/min | Adjusted anti-Xa levels similar in severe RI group and normal function group (0.85 ± 0.05 versus 0.95 ± 0.02, ns). Bleeding rates similar in two groups | One-third dose reduction of enoxaparin safe in severe RI patients |
| Fox et al. 2006 [31] | 1 mg/kg OD (in CrCl <30 mL/min) | Randomized control trial subgroup (n = 212 with <30 mL/min) | Similar results with adjusted enoxaparin versus UFH therapy in CrCl <30 mL/min. Non-significant difference is mortality or recurrent MI (33 versus 37.7%) and in bleeding risk (5.7 versus 2.8%) in enoxaparin and UFH respectively | Kidney function, regardless of anticoagulation used, is the main risk for increased mortality, recurrent disease and bleeding. |
| Chow et al. 2003 [32] | 1 mg/kg BD | Prospective cohort study n = 18 A: >30 mL/min B: ≤30 mL/min |
Adjusted anti-Xa levels after at least 3 doses A: 0.91 B: 1.34 (P<0.05) |
Author recommends dose reduction in 30 mL/min |
| Bazinet et al. 2005 [33] | 1.5 mg/kg OD, 1 mg/kg BD and 75% dose in dialysis patients | Prospective non-randomized trial A: >50 mL/min n = 106 B: 30–50 mL/min n = 54 C: 11–30 mL/min n = 36 D: HDx n = 18 |
Peak anti-Xa on Day 2 or 3, mean (95% CI) A: OD, 1.10 (1.0–1.2); BD, 1.06 (0.99–1.14) B: OD, 1.21 (1.09–1.33); BD, 1.25 (1.12–1.39) C: OD, 1.18 (0.92–1.44); BD, 1.27 (1.15–1.4) D: OD 1.04 (0.79–1.3); BD, 1.03 (0.45–1.61) |
Dose adjustments recommended in RI with the BD dosing |
| Dalteparin | ||||
| Shprecher et al. 2005 [34] | 100 IU/kg BD | Prospective cohort study A:<80 mL/min (n = 11) B: >40 mL/min (n = 7) |
No significant differences in anti-Xa values A: 0.55 ± 0.20 B: 0.47 ± 0.25 |
|
| Schmid et al. 2009 [35] | 100 IU/kg BD | Prospective cohort study A: >60 mL/min (n = 18) B: 30–59 mL/min (n = 9) C: 10–29 mL/min (n = 5) |
Adjusted anti-Xa values on Day 6 (median) A: 0.57 (0.30–0.69) B: 0.66 (0.47–0.69) C: 1.21 (0.99–1.41) |
Non-significant trend (P = 0.22) suggestive of accumulation of anti-Xa in CKD4/5 |
| Tinzaparin | ||||
| Siguret et al. 2000 [36] | 175 IU/kg OD for minimum of 10 days | Prospective cohort N = 30 Mean (40.6 ± 15.3 mL/min); range 20–72 mL/min |
Adjusted anti-Xa values (mean) No toxicity (anti-Xa >1.5 IU/mL) No accumulation seen (mean anti-Xa 0.66 ± 0.2 IU/mL) |
Authors recommend no dose adjustment for CrCl >20 mL/min |
| Pautus et al. 2002 [37] | 175 IU/kg OD up to 30 days | Prospective cohort N = 200 Mean (51.2 ± 22.9 mL/min) |
No correlation was found between anti-Xa activity and creatinine clearance or age. Rate of major bleeding was low (1.5%) and treatment was generally well tolerated | No dose adjustment recommended for CrCl >20 mL/min |
OD, once daily; BD, twice daily.
Enoxaparin
Enoxaparin is the most studied LMWH in patients with renal dysfunction, primarily due to its licenced dose reduction (1 mg/kg once daily) for patients with severe renal disease (CrCl < 30 mL/min). The reduced dose enoxaparin is the most robustly studied LMWH regime in patients with renal dysfunction (see table). Care must be taken however to avoid sub-therapeutic treatment in the renal patients. Bleeding risks should be minimized but not at the risk of increasing further ACS or Venous ThromboEmbolism (VTE) events. Under-dosing, defined as peak anti-Xa levels <0.5 IU/mL have been shown to occur in approximately one-fourth of patients on the dose adjusted enoxaparin treatment (1 mg/kg ONCE daily) [38]. Anti-Xa monitoring is therefore essential for enoxaparin and any other LMWH treatment in this high-risk population.
Dalteparin
The current practice of dalteparin treatment of ACS and VTE in severe CKD is very limited, with pharmacokinetic investigations completed on only 12 patients across two studies to date (see Table 2). Data on treatment outcomes, such as mortality and bleeding complications, is not detailed in this high-risk population. This highlights the risks of full dose dalteparin treatment in this group but further studies are required before dalteparin can be recommended.
Tinzaparin
The current licence for tinzaparin recommend a dose of 175 IU/kg once daily for patients with a CrCl >30 mL/min, however, available evidence does indicate that no dose reductions are required down to a CrCl 20 mL/min (see Table 2). Tinzaparin is the largest molecular weight licenced LMWH and clearance is less dependent on the kidney function. Despite the expected benefits of tinzaparin in patients with renal dysfunction, little evidence is available to confirm the safety of VTE treatment in this population, particularly in patients with CrCl <20 mL/min.
Dosing frequency of LMWH in CKD
The use of a twice daily dosing regime is desirable in patients with high bleeding risks. The splitting of the total dose into two equal doses will reduce the peak exposure and the theoretically increased bleeding risks associated with these peaks. The traditional use of once daily dosing over twice daily dosing for treatment of VTE for all LMWH was instigated primarily for patient convenience. The convenience of once daily dosing outweighs the potential for a lower efficacy as identified in a Cochrane review [39]. While bleeding rates were similar between the two dosing regimens, this is based on patients with normal renal function and therefore this must not be assumed in patient with severe renal impairment. Thus, due to the concerns of accumulation and consequential bleeding, an initial dose reduction of treatment dose LMWH is anticipated and anti-Xa monitoring should influence further dosing.
Anti-Xa monitoring
The laboratory test for low-molecular weight heparin is anti-factor Xa activity. Anti-Xa activity can differ between different LMWH and as such, agent-specific calibrators should be used in the laboratory to ensure accuracy. A fondaparinux-specific anti-Xa assay is also available but not widely used. Also, anti-Xa assays are now available for new oral anticoagulants, rivaroxaban and apixaban, but are not available in every laboratory. Performance of this assay is based on the principle that endogenous factor Xa that is not complexed with the anticoagulant can cleave a chromophore off a substrate and produce a colour change which can be detected by a spectrophotometer, Results are reported as anti-factor Xa level. Currently accepted values for prophylactic dosing are 0.1–0.3 U/mL of anti-Xa activity, while the same for therapeutic dosing will be 0.4–1.0 U/mL. It needs to be borne in mind that these values can change depending on the laboratory and calibrator used and discussion with the laboratory is important.
Our practice of monitoring LMWH in patients with CKD is given in Table 3. A useful guide for initiating low-molecular weight heparin for treatment of venous TE is given in Table 4. In this context, we need to stress that the choice of dalteparin is based on hospital choice of anticoagulation for all non-renal patients and is not evidence-based in CKD.
Table 3.
Monitoring of anti-Xa levels with low molecular weight heparin for anticoagulation in renal impairment
| Initial anti-Xa test | |
|
Before and after the third dose |
| Subsequent monitoring once in therapeutic range | (Peak and trough) twice a week |
| Target anti-Xa level | Pre-dose level (trough) <0.1–0.3 IU/mLa2–4 h post-dose (peak) 0.5–1 IU/mLa |
aAnti-Xa levels out of range should be discussed with haematologist.
Table 4.
The hospital protocol for the use of low-molecular weight heparin for use in chronic kidney disease patients
| GFR >40 mL/min | GFR 30–39 mL/min | GFR <30 mL/min | |
|---|---|---|---|
| Recommended dose | 100% of licenced dose once daily | 80–90% of licenced dose once daily | UFH or 60% of licenced dose twice daily |
| Pre- and post- dose anti-Xa levels are essential for the use of low-molecular weight heparin in patients with a GFR <50 mL/min to avoid supra- or sub-therapeutic anticoagulation. | |||
The choice of low molecular weight heparin is not based on trials in renal patients. We advise the practitioners to alter the above according to the local protocol of choice of anticoagulant.
Heparin-induced thrombocytopenia
HIT is a life threatening complication associated with heparin treatment, where antibodies to heparin–platelet complexes can lead to platelet activation and aggregation [14]. Since the antibodies can cross-react, any form of heparin including LMWH or heparin flushes should be avoided in these patients. However, since it is a prothrombotic state, an alternative parenteral anticoagulant is indicated.
The choice of alternative anticoagulation is influenced by the patient's renal function. Patients with HIT are recommended to use non-heparin anticoagulants, such as lepirudin, argatrogan, danaparoid and fondaparinux (Table 5) [14, 40–42]. Lepirudin is extensively metabolized and excreted through the kidneys, and aggressive dose reductions are required for therapeutic dosing in patients with renal impairment. Due to the complex dosing adjustments, lepirudin is usually avoided in this population [14]. More recently, lepirudin has been withdrawn from the market. Danaparoid and fondaparinux (unlicensed use) can be used in patients with mild-to-moderate renal impairment (CrCl >30 mL/min); however, accumulation and increased risk of bleeding is possible [41, 42]. The risk of bleeding with fondaparinux increases with increasing renal impairment (mild 4.4%, moderate 6.6% and severe renal impairment 14.5%) [42]. In patients with severe renal impairment (CrCl <30 mL/min), argatroban is the preferred choice of anticoagulant. It is extensively cleared by the hepatobiliary system and no dose adjustment (0.5–2 µg/kg/min) is required for initial dosing. Regular APTT monitoring is used to direct subsequent dosing [40].
Table 5.
Characteristic of anticoagulants used to treat patients with HIT [14]
| Lepirudin | Argatroban [40] | Danaparoid [41] | Fondaparinux [42] | |
|---|---|---|---|---|
| Coagulation target | Thrombin | Thrombin | Factor Xa | Factor Xa |
| Half-life (h) | 1.2 (48 in CKD 4/5) | 0.6–0.8 | 24 (>31 in CKD 4/5) | 17 (35–85 in CKD4/5) |
| Kidney clearance | 45% (+metabolism in kidney) | 15% | 40–50% | 64–77% |
| Approved for CrCl <30 mL/min | No | Yes | No | No |
Oral anticoagulation: warfarin
Warfarin has traditionally been the oral anticoagulant of choice due to clinician familiarity. However, in general population, warfarin use is complicated by a narrow therapeutic index and multiple drug–drug and drug–food interactions. In addition, there are some unique renal specific factors which should be borne in mind with the use of warfarin in CKD patients.
Lower dose requirements: Limdi et al. [43] have shown patients with severe renal dysfunction require a significantly lower daily dose of warfarin to achieve therapeutic INR in comparison to control of normal kidney function. Patients with a CrCl of 30–59 mL/min/1.73 m2 tend to need a 10% lower maintenance dose, while those with levels of <30 mL/min/1.73 m2 a 20% lower dose. This is probably related to the down-regulation of cytochrome P450 in CKD.
Labile INRs: CKD patients spent a longer time outside of the target INR and were at highest risk of supra-therapeutic anticoagulation (INR >4). This in the setting of bleeding risk put such patients at higher risk of bleeding [44].
In a very recent study, warfarin treatment was associated with a lower 1-year risk for the composite outcome of death, myocardial infarction and ischaemic stroke without a higher risk of bleeding. This association was not related to the severity of concurrent CKD [45].
Risk of vascular calcification: renal failure is associated with hyperphosphatemia which can stimulate vascular smooth muscles in the arterial walls to develop osteoblastic characteristic, leading to vascular calcification [46]. This process is normally inhibited by matrix G1a protein, which requires vitamin K, which in turn is blocked by warfarin.
Warfarin-related nephropathy [47]: this is an unexplained increase in serum creatinine ≥0.3 mg/dL within 1 week of an INR reading of ≥3.0. It is thought to be due to glomerular haemorrhage and tubular obstruction by red cell casts. Compared with 16% of non-CKD patients, 33.0% of those with CKD developed this complication and had a 1-year mortality which increased from 18.9 to 31%.
Despite high-bleeding risks and lack of high quality evidence in this population, warfarin is still recommended for VTE and atrial fibrillation (AF) treatment. A start-low go-slow approach towards dosing of warfarin is advised to avoid over-anticoagulation of patients with severe kidney dysfunction.
New oral anticoagulants
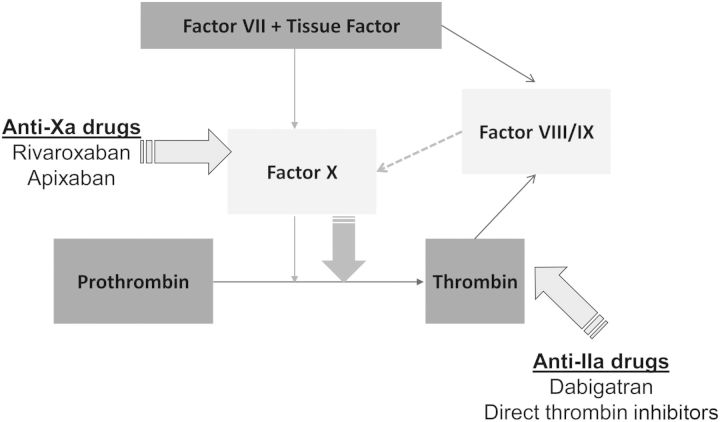
In recent years, there has been an influx in new oral anticoagulants to the market for the prevention and treatment of VTE and stroke prevention in AF patients (see Figure 2). The main advantage of the new oral anticoagulants is their predictable therapeutic effect and the monitoring-free therapy this provides. Differences exist with the three new oral anticoagulants, with varying licences, different pharmacodynamic targets and a variable pharmacokinetic clearance from the kidneys (Table 6). Since dabigatran and to a lesser extent, rivaroxaban and apixaban are excreted by the kidneys, drug accumulation which can translate into accentuated anticoagulant effects [48–51].
Fig. 2.
Coagulation starts by tissue factor binding to factor VII. This activates some factor X which will cause a small, initial thrombin burst. This thrombin will activate factors VIII and IX, which will activate further factor X leading to huge thrombin burst. The sites of action of the newer oral anticoagulants and direct thrombin inhibitors are shown.
Table 6.
Characteristics of the new oral anticoagulants
| Rivaroxaban [48] | Apixiban [49] | Dabigatran [50] | |
|---|---|---|---|
| Coagulation target | Factor Xa | Factor Xa | Thrombin |
| Bioavailability (%) | 80–100 | 50 | 6.5 |
| Protein binding (%) | 92–95 | 87 | 35 |
| Half-life (h) | 5–13 | 12 | 12–14 (27 in CKD4/5) |
| Renal clearance (%) | 33 | 27 | 85 |
| Approved for CrCl <30 mL/min | Yes. Reduce dose in CrCl 15–29 mL/min |
Yes. Reduce dose in CrCl 15–29 mL/min |
No |
| Interactions | Cyp 3A4 and P-gp | Cyp 3A4 and P-gp | P-gp |
One of the key issues in this area is the fact that eGFR and CrCl are not interchangeable, although eGFR could provide some guidance. The summary of product characteristics of each new oral anticoagulant recommends that ‘Cockcroft and Gault’ formula is used for dosing and monitoring. A recent study showed that if the MDRD-derived eGFR was used instead of Cockcroft–Gault in prescribing these new agents, many elderly patients with AF would either incorrectly become eligible for them or would receive too high a dose [52]. There is the additional concern that the risk of major bleeding would be increased in patients with unsuspected renal impairment [52]. The eGFR calculated using the modification of diet in renal disease equation is now considered the standard test for assessing renal function. However, historically, CrCl estimation using the Cockcroft and Gault equation was used to make decisions on dosing drugs in relation to kidney function. Although the British National Formulary recommends eGFR for monitoring kidney function, they state that calculation with the Cockcroft and Gault equation should be used for low therapeutic index and high-risk drugs. In other cases, eGFR is an adequate estimate of the CrCl.
Since no antidote currently exists to reverse active bleeding associated with these new oral anticoagulants and also the expected prolongation of the therapeutic half-life in CKD4/5 patients, rivaroxaban and apixiban, these agents should be used with caution in this population.
The following practical points may be considered:
Patients must have a baseline renal function test before initiating these agents.
Renal function can decline while on treatment hence monitor annually or more often in high-risk patients.
Acute illness often transiently affects renal function (infections, acute heart failure, etc.), and therefore should trigger re-evaluation. Care should be taken when prescribing other drugs which may be nephrotoxic in such situations.
The summary of product characteristics for dabigatran recommends that this agent is contraindicated in patients with a CrCl <30 mL/min in the UK. Patients at increased risk of haemorrhage and all patients of ≥80 years should be given a lower dose (110 mg twice daily) and this reduced dose is recommended for all [50]. US licencing advises a reduced dose (75 mg twice daily) for patients with CrCl 15–30 mL/min but this remains an off-label indication in the UK at time of print.
Rivaroxaban is contraindicated in patients with a CrCl of <15 mL/min and the dose should be reduced from 20 to 15 mg daily in those with a CrCl of 15–49 mL/min [48].
In patients with CrCl 15–29 mL/min, apixaban should be given at 2.5 mg twice daily [49].
New oral anticoagulant therapy should be avoided and VKAs may be a more suitable alternative for now in AF patients on haemodialysis. Whilst warfarin therapy remains unpredictable and is not without its complications, it can be quickly and effectively reversed in this high-bleeding risk group, an essential property of an anticoagulant for use in the severe renal dysfunction population.
Conclusions
In summary, management of anticoagulation in CKD requires special considerations to minimize complications from bleeding from over-anticoagulation while at the same time, adequate dosing can be problematic. Close liaison with the haematologists may be beneficial in this regard and setting up local hospital protocols which are strictly adhered to and audited also helps. Further prospective trials are necessary before this can be addressed but a recent editorial summarizes this issue as ‘It is ironic that we are routinely treating many patients with renal disease and atrial fibrillation every day with great uncertainty as to benefit or harm without their consent, and at the same time, major regulatory and ethical barriers exist that prevent efficient enrolment of patients into clinical trials that are needed to answer this (and other) important questions’ [53].
Conflict of interest statement
None declared.
References
- 1.Lutz J, Menke J, Sollinger D, et al. Haemostasis in chronic kidney disease. Nephrol Dial Transplant. 2014;29:29–40. doi: 10.1093/ndt/gft209. [DOI] [PubMed] [Google Scholar]
- 2.Ronco C, McCullough P, Anker SD, et al. The clinical management of the Cardio-Renal syndromes: work group statements from the 7th ADQI consensus conference. Nephrol Dial Transplant. 2010;25:2077–2089. doi: 10.1093/ndt/gfq252. [DOI] [PubMed] [Google Scholar]
- 3.Wattanakit K, Cushman M, Stehman-Breen C, et al. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol. 2008;19:135–140. doi: 10.1681/ASN.2007030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavord S, Myers B. Bleeding and thrombotic complications of kidney disease. Blood Rev. 2011;25:271–278. doi: 10.1016/j.blre.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Ocak G, Verduijn M, Vossen CY, et al. Chronic kidney disease stages 1–3 increase the risk of venous thrombosis. J Thromb Haemost. 2010;8:2428–2435. doi: 10.1111/j.1538-7836.2010.04048.x. [DOI] [PubMed] [Google Scholar]
- 6.Barbano B, Gigante A, Amoroso A, et al. Thrombosis in nephrotic syndrome. Semin Thromb Hemost. 2013;39:469–476. doi: 10.1055/s-0033-1343887. [DOI] [PubMed] [Google Scholar]
- 7.Wattanakit K, Cushman M. Chronic kidney disease and venous thromboembolism: epidemiology and mechanisms. Curr Opin Pulm Med. 2009;15:408–412. doi: 10.1097/MCP.0b013e32832ee371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boccardo P, Remuzzi G, Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost. 2004;30:579–589. doi: 10.1055/s-2004-835678. [DOI] [PubMed] [Google Scholar]
- 9.Palmer SC, Di Micco L, Razavian M, et al. Antiplatelet agents for chronic kidney disease. Cochrane Database Syst Rev. 2013;2:CD008834. doi: 10.1002/14651858.CD008834.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Krüger T, Brandenburg V, Schlieper G, et al. Sailing between Scylla and Charybdis: oral long-term anticoagulation in dialysis patients. Nephrol Dial Transplant. 2013;28:534–541. doi: 10.1093/ndt/gfs485. [DOI] [PubMed] [Google Scholar]
- 11.Davenport A. What are the anticoagulation options for intermittent hemodialysis? Nat Rev Nephrol. 2011;7:499–508. doi: 10.1038/nrneph.2011.88. [DOI] [PubMed] [Google Scholar]
- 12.Casu B, Oreste P, Torri G, et al. The structure of heparin oligosaccharide fragments with high anti-(factor Xa) activity containing the minimal antithrombin III-binding sequence. Chemical and 13C nuclear-magnetic-resonance studies. Biochem J. 1981;197:599–609. doi: 10.1042/bj1970599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia DA, Baglin TP, Weitz JI, et al. Parenteral anticoagulants. Chest. 2012;141(Suppl 2):e24S–e43S. doi: 10.1378/chest.11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linkins L-A, Dans AL, Moores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia: antithrombotic therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(Suppl 2):e495S–e530S. doi: 10.1378/chest.11-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjornsson TD, Wolfram KM, Kitchell BB. Heparin kinetics determined by three assay methods. Clin Pharmacol Ther. 1982;31:104–113. doi: 10.1038/clpt.1982.16. [DOI] [PubMed] [Google Scholar]
- 16.de Swart CA, Nijmeyer B, Roelofs JM, et al. Kinetics of intravenously administered heparin in normal humans. Blood. 1982;60:1251–1258. [PubMed] [Google Scholar]
- 17.Olsson P, Lagergren H, Ek S. The elimination from plasma of intravenous heparin. An experimental study on dogs and humans. Acta Med Scand. 1963;173:619–630. doi: 10.1111/j.0954-6820.1963.tb17446.x. [DOI] [PubMed] [Google Scholar]
- 18.Baglin T, Barrowcliffe TW, Cohen A, et al. Guidelines on the use and monitoring of heparin. Br J Haematol. 2006;133:19–34. doi: 10.1111/j.1365-2141.2005.05953.x. www.bcshguidelines.com/ (29 January 2014, date last accessed) [DOI] [PubMed] [Google Scholar]
- 19.National Institute for Health and Clinical Excellence (NICE) Venous thromboembolic diseases: the management of venous thromboembolic diseases and the role of thrombophilia testing. 2012 NICE full clinical guideline 144 June www.nice.org.uk. (29 January 2014, date last accessed) [PubMed] [Google Scholar]
- 20.Braunwald E, Antman E, Beasley J, et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2000;36:970–1062. doi: 10.1016/s0735-1097(00)00889-5. [DOI] [PubMed] [Google Scholar]
- 21.Spinler SA, Inverso SM, Cohen M, et al. Safety and efficacy of unfractionated heparin versus enoxaparin in patients who are obese and patients with severe renal impairment: analysis from the ESSENCE and TIMI 11B studies. Am Heart J. 2003;146:33–41. doi: 10.1016/S0002-8703(03)00121-2. [DOI] [PubMed] [Google Scholar]
- 22.Thorevska N, Amoateng-Adjepong Y, Sabahi R, et al. Anticoagulation in hospitalized patients with renal insufficiency: a comparison of bleeding rates with unfractionated heparin vs enoxaparin. Chest. 2004;125:856–863. doi: 10.1378/chest.125.3.856. [DOI] [PubMed] [Google Scholar]
- 23.Anderson DR, O'Brien BJ, Levine MN, et al. Efficacy and cost of low-molecular-weight heparin compared with standard heparin for the prevention of deep vein thrombosis after total hip arthroplasty. Ann Intern Med. 1993;119:1105–1112. doi: 10.7326/0003-4819-119-11-199312010-00008. [DOI] [PubMed] [Google Scholar]
- 24.Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med. 1995;332:1330–1335. doi: 10.1056/NEJM199505183322003. [DOI] [PubMed] [Google Scholar]
- 25.Handeland GF, Abildgaard U, Holm HA, et al. Dose adjusted heparin treatment of deep venous thrombosis: a comparison of unfractionated and low molecular weight heparin. Eur J Clin Pharmacol. 1990;39:107–112. doi: 10.1007/BF00280041. [DOI] [PubMed] [Google Scholar]
- 26.Pfizer Ltd; Summary of Product Characteristics—Fragmin (dalteparin) 10,000 IU/0.4 ml solution for injection. http://www.medicines.org.uk/emc/medicine/26901/SPC/ (4 December 2014, date last accessed) [date of revision of the text October 2012] [Google Scholar]
- 27.Sanofi Aventis; Summary of Product Characteristics—Clexane (enoxaparin) Forte Syringes. http://www.medicines.org.uk/emc/medicine/10054/SPC/ (4 December 2014, date last accessed) [date of revision of the text 19 February 2014] [Google Scholar]
- 28.Leo Laboratories Ltd; Summary of Product Characteristics—Innohep (tinzaparin) 20,000 IU/ml and Innohep syringe 20,000 IU/ml. https://www.medicines.org.uk/emc/medicine/2623/SPC. (4 December 2014, date last accessed) [date of revision of the text 24 February 2014] [Google Scholar]
- 29.Palm M, Mattsson C. Pharmacokinetics of heparin and low molecular weight heparin fragment (Fragmin) in rabbits with impaired renal or metabolic clearance. Thromb Res. 1985;40:129–133. [PubMed] [Google Scholar]
- 30.Collet JP, Montalescot G, Fine E, et al. Enoxaparin in unstable angina patients who would have been excluded from randomized pivotal trials. J Am Coll Cardiol. 2003;41:8–14. doi: 10.1016/s0735-1097(02)02664-5. [DOI] [PubMed] [Google Scholar]
- 31.Fox KA, Antman EM, Montalescot G, et al. The impact of renal dysfunction on outcomes in the ExTRACT-TIMI 25 trial. J Am Coll Cardiol. 2007;49:2249–2255. doi: 10.1016/j.jacc.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 32.Chow SL, Zammit K, West K, et al. Correlation of antifactor Xa concentrations with renal function in patients on enoxaparin. J Clin Pharmacol. 2003;43:586–590. [PubMed] [Google Scholar]
- 33.Bazinet A, Almanric K, Brunet C, et al. Dosage of enoxaparin among obese and renal impairment patients. Thromb Res. 2005;116:41–50. doi: 10.1016/j.thromres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Shprecher AR, Cheng-Lai A, Madsen EM, et al. Peak antifactor Xa activity produced by dalteparin treatment in patients with renal impairment compared with controls. Pharmacotherapy. 2005;25:817–822. doi: 10.1592/phco.2005.25.6.817. [DOI] [PubMed] [Google Scholar]
- 35.Schmid P, Brodmann D, Odermatt Y, et al. Study of bioaccumulation of dalteparin at a therapeutic dose in patients with renal insufficiency. J Thromb Haemost. 2009;7:1629–1632. doi: 10.1111/j.1538-7836.2009.03556.x. [DOI] [PubMed] [Google Scholar]
- 36.Siguret V, Pautas E, Fevrier M, et al. Elderly patients treated with tinzaparin (Innohep®) administered once daily (175 anti-Xa IU/kg): anti-Xa and anti-IIa activities over 10 days. Thromb Haemost. 2000;84:800–804. [PubMed] [Google Scholar]
- 37.Pautas E, Gouin I, Bellot O, et al. Safety profile of tinzaparin administered once daily at a standard curative dose in two hundred very elderly patients. Drug Safety. 2002;25:725–733. doi: 10.2165/00002018-200225100-00005. [DOI] [PubMed] [Google Scholar]
- 38.Montalescot G, Collet JP, Tanguy ML, et al. Anti-Xa activity relates to survival and efficacy in unselected acute coronary syndrome patients treated with enoxaparin. Circulation. 2004;110:392–398. doi: 10.1161/01.CIR.0000136830.65073.C7. [DOI] [PubMed] [Google Scholar]
- 39.van Dongen CJ, van den Belt AG, Prins MH, et al. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev. 2004;4 doi: 10.1002/14651858.CD001100.pub2. CD001100. [DOI] [PubMed] [Google Scholar]
- 40.Summary of Product Characteristics—Exembol (Argatroban) Mitsubishi Pharma Europe. https://www.medicines.org.uk/emc/medicine/26622/SPC. (4 December 2014, date last accessed) [date of revision of the text 28 June 2012]
- 41.A Summary of Product Characteristics—Orgaran (Danaparoid) Merck Sharp and Dohme LTD. https://www.medicines.org.uk/emc/medicine/1386/SPC. (4 December 2014, date last accessed) [date of revision of the text 31 August 2013]
- 42.UK: GlaxoSmithKline; Summary of Product Characteristics—Arixtra (fondaparinux) 5 mg, 7.5 mg, 10 mg pre-filled syringe. https://www.medicines.org.uk/emc/medicine/15937/SPC. (4 December 2014, date last accessed) [date of revision of the text 2 May 2013] [Google Scholar]
- 43.Limdi NA, Limdi MA, Cavallari L, et al. Warfarin dosing in patients with impaired kidney function. Am J Kidney Dis. 2010;56:823–831. doi: 10.1053/j.ajkd.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan KE, Lazarus JM, Thadhani R, et al. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20:2223–2233. doi: 10.1681/ASN.2009030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carrero JJ, Evans M, Szummer K, et al. Warfarin, kidney dysfunction, and outcomes following acute myocardial infarction in patients with atrial fibrillation. JAMA. 2014;311:919–28. doi: 10.1001/jama.2014.1334. [DOI] [PubMed] [Google Scholar]
- 46.El-Abbadi M, Giachelli CM. Mechanisms of vascular calcification. Adv Chronic Kidney Dis. 2007;14:54–66. doi: 10.1053/j.ackd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Brodsky SV, Nadasdy T, Rovin BH, et al. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011;80:181–189. doi: 10.1038/ki.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bayer PLC; Summary of Product Characteristics—Xarelto (Rivaroxaban) https://www.medicines.org.uk/emc/medicine/21265/SPC. (4 December 2014, date last accessed) [date of revision of the text 6 January 2014] [Google Scholar]
- 49.Bristol-Myers Squibb-Pfizer; Summary of Product Characteristics—Eliquis (Apixiban) https://www.medicines.org.uk/emc/medicine/27220/SPC. (4 December 2014, date last accessed) [date of revision of the text 1 October 2013] [Google Scholar]
- 50.Boehringer Ingelheim Limited; Summary of Product Characteristics—Pradaxa (Dagibatran) https://www.medicines.org.uk/emc/medicine/20760/SPC. (4 December 2014, date last accessed) [date of revision of the text 28 January 2014] [Google Scholar]
- 51.MHRA. Drug safety update. Dabigatran (Pradaxa): risk of serious haemorrhage—contraindications clarified and reminder to monitor renal function. Med Healthc Prod Regul Agency. 2012;5 http://www.mhra.gov.uk/Safetyinformation/DrugSafetyUpdate/CON175429. (4 December 2014, date last accessed) [date of revision of the text 21 july 2014] [Google Scholar]
- 52.Harper P, Young L, Merriman E. Bleeding risk with dabigatran in the frail elderly. N Engl J Med. 2012;366:864–866. doi: 10.1056/NEJMc1112874. [DOI] [PubMed] [Google Scholar]
- 53.Granger CB, Chertow GM. A pint of sweat will save a gallon of blood: a call for randomized trials of anticoagulation in end stage renal disease. Circulation. 2014;129:1190–1192. doi: 10.1161/CIRCULATIONAHA.113.007549. [DOI] [PubMed] [Google Scholar]