Abstract
Background
Outside of pregnancy, anti-glomerular basement membrane (GBM) antibody disease is associated with significant morbidity and mortality. However, there is limited knowledge regarding de novo anti-GBM disease in pregnancy.
Methods
A systematic review was performed to identify maternal, pregnancy and fetal outcomes in de novo anti-GBM disease in pregnancy. Studies were selected from PubMed, EMBASE, Cochrane Library databases and conference proceedings, without language restriction.
Results
Data from eight patients were derived from seven case reports and one unpublished case. Most (6/8) patients presented after the first trimester. During pregnancy, acute kidney injury (5/8), anemia (5/8), hematuria (8/8) and proteinuria (8/8) were common. When hemodialysis was required antepartum (5/8), renal function recovery to independence of renal replacement was unlikely (2/5). While pulmonary involvement was common (5/8), no permanent damage was reported (0/8). The majority of cases ended in live births (6/8) although prematurity (6/6), intrauterine growth restriction (2/6), small for gestational age (4/6) and complications of prematurity (1/6) were common. When anti-GBM levels were tested in the living newborn, they were detectable (2/5), but no newborn renal or lung disease was reported (0/6). Complications in pregnancy included gestational diabetes (3/8), hyperemesis gravidarum (2/8) and preeclampsia (2/8).
Conclusions
Live births can be achieved in de novo anti-GBM disease in pregnancy, but are commonly associated with adverse maternal, pregnancy and fetal outcomes. Only with awareness of common presentations, and management strategies can outcomes be optimized.
Keywords: anti-GBM, Goodpasture's disease, placenta, pregnancy, vasculitis
Introduction
Anti-glomerular basement membrane (anti-GBM) antibody disease commonly leads to rapidly progressive glomerulonephritis (RPGN) [1, 2], end-stage kidney disease and death [3]. Named eponymously for its discoverer in 1919 [4], Goodpasture's syndrome consists of pulmonary hemorrhage and glomerulonephritis [5] caused by an autoantibody to alveolar and glomerular basement membrane antigens [6].
Acute kidney injury (AKI) occurs in ∼1:15 000–1:20 000 pregnancies [7] and is associated with adverse maternal and fetal outcomes. A number of etiologies are well established for obstetric AKI [8], but there is limited knowledge regarding de novo glomerulonephritis, and in particular de novo Goodpasture's disease in pregnancy. Given the implications of systemic vasculitis and its management in maternal and fetal outcomes, literature on this topic is essential.
We performed a systematic review of de novo anti-GBM disease in pregnancy, reporting on clinical presentation, management and pregnancy outcomes.
Methods
Search strategy and selection criteria
Electronic databases (PubMed, EMBASE and the Cochrane Library) were searched up to 20 April 2014. Conference abstracts (American Society of Nephrology 2003–2013, European Renal Association 2002–2013) were searched. The search terms were ‘anti-GBM,’ ‘Glomerular basement membrane,’ ‘pregnancy,’ ‘gestation,’ ‘Goodpasture's disease,’ ‘Goodpasture's syndrome’. There were no restrictions on language, publication form or study type.
Studies and case reports were included if they met the following criteria: (i) Patient was pregnant during diagnosis or management of de novo anti-GBM disease. (ii) Anti-GBM disease was confirmed by positive anti-GBM antibody, renal biopsy findings considered diagnostic for anti-GBM disease, or both.
Data extraction and quality assessment
One investigator (B.T.) twice used the search strategy to identify potentially relevant articles. Full reports of potentially relevant studies were obtained and each was reviewed using predefined eligibility criteria. Data were abstracted for study and patient characteristics, management strategy as well as maternal, fetal and pregnancy outcome assessment.
Data synthesis and analysis
Patient characteristics including maternal and gestational age, obstetric history (gravidity and parity), and medical/surgical history were collected. Clinical, laboratory and radiologic features at presentation were recorded. Chest imaging was recorded when available. When renal biopsy was performed, the timing relative to pregnancy, and whether it was diagnostic for anti-GBM disease was recorded. Information regarding immunosuppressive management was collected.
Maternal outcomes included antepartum and post-partum (i) renal function, defined by serum creatinine, creatinine clearance or the need for renal replacement therapy, (ii) lung function, defined by the presence of cough, dyspnea, hemoptysis or the result of radiologic imaging (chest X-ray, ventilation-perfusion scan or pulmonary function tests). Pregnancy outcomes included (i) vaginal versus cesarean section delivery, (ii) presence of pregnancy-related complications, such as placenta praevia or abruptio, hyperemesis gravidarum, gestational diabetes, gestational hypertension or preeclampsia. Fetal outcomes included (i) gestational age at birth, (ii) intrauterine growth restriction or small for gestational age, (iii) live birth or non-live birth (therapeutic abortion, miscarriage, stillborn), (iv) presence of congenital abnormalities at birth, (v) newborn anti-GBM antibodies, (vi) newborn kidney or lung disease.
Results
Selected studies
Of 160 initial study abstracts reviewed, 12 qualified for full review (Figure 1). A total of seven case reports were identified that related to de novo anti-GBM disease in pregnancy. One case report was from the 2006 American Society of Nephrology annual conference abstracts [9], and was the first of two cases from our research group. When an additional case from our research group was added, there were eight total case reports of de novo anti-GBM disease in pregnancy.
Fig. 1.
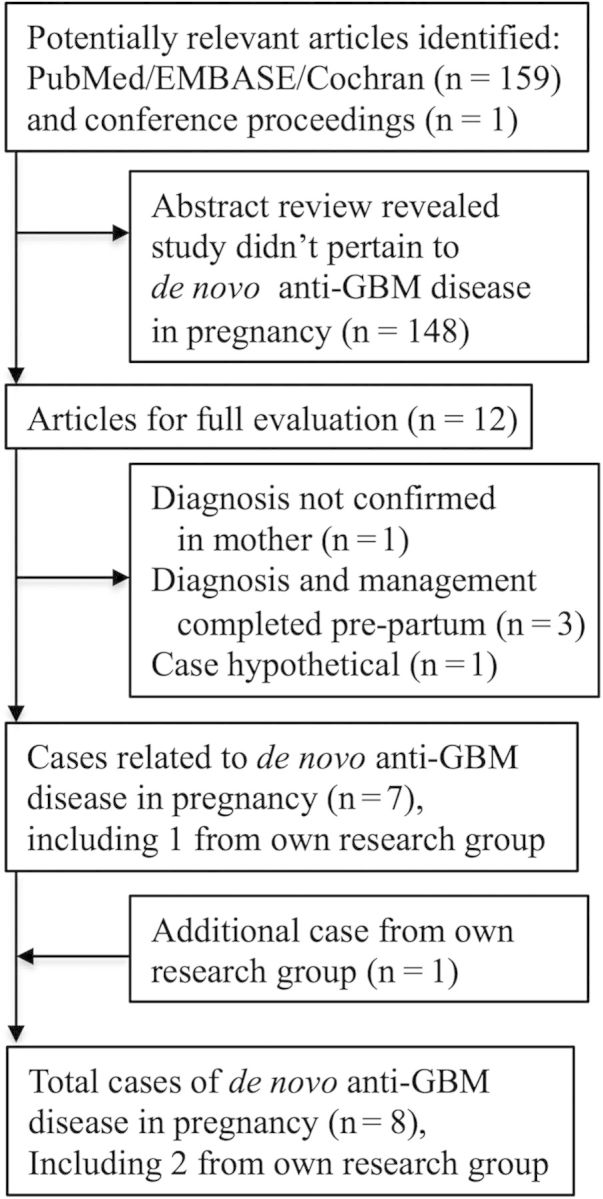
Flow and selection of studies through review. GBM, glomerular basement membrane.
Patient characteristics
Maternal age ranged from 19 to 34 (mean 27.3) years (Table 1). Most women presented in the second trimester (6/8) with only the case of Deubner et al. presenting at 12 weeks gestation and the case of Yankowitz et al. being diagnosed 3 months prior to pregnancy. Most (5/8) women had no prior pregnancies, two women had other living children. Underlying renal disease (‘possible IgA nephropathy’) was found in only one woman [15], and underlying hypertension only in London, 2013.
Table 1.
Patient characteristics and immunosuppressive management of de novo anti-GBM disease in pregnancy
| Nillsen [10] | Yankowitz [11] | Deubner [12] | Al-Harbi [13] | Vasiliou [14] | Nair [15] | Joseph [9] | London 2013 | |
|---|---|---|---|---|---|---|---|---|
| Patient characteristics | ||||||||
| Maternal age (years) | 19 | 28 | 21 | 30 | 34 | 23 | 33 | 30 |
| Gestational age (weeks) at presentation | 19 | 3 months pre-pregnancy | 12 | 28 | 18 | 13 | 15 | 25 |
| Previous obstetric history (gravida/parity) | G0 P0 | G0 P0 | G0 P0 | G7 P7 | G1 P0 | G0 P0 | G0 P0 | G2 P1 |
| Relevant past medical history | Possible IgA nephropathy | HTN | ||||||
| Immunosuppressive management | ||||||||
| Timing (pre-pregnancy, during or post-partum) | Post | Pre | Post | During | During | Post | During | During |
| Induction: corticosteroids | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) |
| Cyclophosphamide | (+) | (+) | (+) | (−) | (−) | (+) | (−) | (+) |
| Plasmapharesis | (+) (n = NR) | (+) (n = 5) | (+) (n = NR) | (+) (n = NR) | (+) (n = 6) | (+) (n = 14) | (+) (n = 20) | (+) (n = 32) |
| Azathioprine | (−) | (−) | (−) | (−) | (+) | (−) | (+) | (+) |
| Other | None | PO CYC and PRED continued in pregnancy | None | Post-partum management NR | None | None | Low-dose ASA | Azathioprine post-3 IV CYC doses, 32 weeks gestation |
ASA, acetylsalicylic acid; CYC, cyclophosphamide; HTN, hypertension; IV, intravenous; NR, not reported; PCOS, polycystic ovarian syndrome; PO, per oral; PRED, prednisone.
Investigations
Markedly elevated serum creatinine was reported in 5/8 cases (Table 2), and anemia in all cases that reported a serum hemoglobin (5/5). When reported, serum anti-GBM titer was elevated at presentation (6/7). Urinalysis consistently showed hematuria (6/6) and proteinuria (6/6). Urine microscopy confirmed red blood cells (rbc) (6/6) but only rarely revealed dysmorphic rbc (1/6) or cellular casts (1/6). When performed, chest X-ray commonly (3/4) found evidence of parenchymal disease suggestive of pulmonary hemorrhage. Renal biopsy was performed in most (7/8) cases, once pre-pregnancy and equally frequently during (3/7) and after pregnancy (3/7). Every kidney biopsy performed was diagnostic for anti-GBM disease (7/7).
Table 2.
Investigations in patients with de novo anti-GBM disease in pregnancy
| Nillsen [10] | Yankowitz [11] | Deubner [12] | Al-Harbi [13] | Vasiliou [14] | Nair [15] | Joseph [9] | London 2013 | |
|---|---|---|---|---|---|---|---|---|
| Laboratory investigations at presentation | ||||||||
| Creatinine (µmol/L) | NR | (CrCl = 198 mL/min) | 97 | 1217 | 1306 | 318 | 1132 | 1130 |
| Hemoglobin (g/dL) | NR | NR | NR | 6.6 | 7.8 | 7.3 | 6.7 | 5.9 |
| Anti-GBM antibody | NR | (+) | 12 U/mL (‘borderline’) | (−) | (+) | (+) | (+) | (+) |
| Urinalysis | (1+)HEM, (1+)PRO | (3+)HEM, (2+)PRO | NR | (3+)HEM, (2+)PRO | (1+)HEM, (1+)PRO | NR | (+)HEM (+)PRO | >3.0 g/L PRO Large Blood |
| Urine microscopy | NR | NR | 5-6 wbc/HPF, 15–20 rbc/HPF | >25 rbc/HPF, no casts | Dysmorphic rbc, no casts | 0–2 wbc/HPF ++ rbc/HPF no casts |
Rbc, Cellular casts | >100 rbc/HPF <10 wbc/HPF, granular casts |
| 24-h urine protein quantification (g/day) | NR | 0.196 | 3.2 | NR | NR | NR | NR | 0.91 |
| Chest imaging | NR | CXR: ‘Bilateral interstitial/nodular infiltrates’ | NR | CXR: ‘Congested lungs’ | NR | NR | Normal | CXR: ‘Congested’ VQ scan: ‘suggestive of pulmonary hemorrhage’ |
| Renal biopsy: performed | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (−) |
| Timing (pre-pregnancy, during or post-partum) | Post | Pre | Post | Post | During (18 weeks) | During (14 weeks) | During (15 weeks) | |
| Diagnostic of anti-GBM disease | (+) | (+) | (+) | (+) | (+) | (+) | (+) | |
| Crescents (±, %) | (+), ‘advanced’ | (+), NR | (+), 100% | (+), 100% | (+), 80% | (+), 60% | (+), 100% | |
CXR, chest X-ray; g, grams; HEM, hematuria; HPF, high powered field; mL, milliliter; L, liter; NR, not reported; PRO, proteinuria; rbc, red blood cell; U, units; VQ, ventilation-perfusion scan; wbc, white blood cell.
Immunosuppression
Immunosuppressive therapy was used during pregnancy in half (4/8) of the cases (Table 1). All immunosuppression regimes included corticosteroids (8/8). Cyclophosphamide exposure occurred during pregnancy in a minority of cases (2/8) and was delayed to the post-partum period in three cases. All patients received plasmapheresis during their treatment course, but the number of treatments varied dramatically (range 5–32). Azathioprine was used in the absence of cyclophosphamide induction in two cases.
Outcomes: maternal
Most (7/8) women required hemodialysis during their clinical course, with most (5/8) requiring hemodialysis during pregnancy (Table 3). Two women recovered renal function following termination of pregnancy to the point of not requiring hemodialysis, one after a therapeutic abortion at 15 weeks and the other after delivery at 26 weeks. While many (5/8) women presented with potential pulmonary hemorrhage, none were reported to have any permanent pulmonary disease.
Table 3.
Maternal, fetal and pregnancy-associated outcomes in de novo anti-GBM disease in pregnancy
| Nillsen [10] | Yankowitz [11] | Deubner [12] | Al-Harbi [13] | Vasiliou [14] | Nair [15] | Joseph [9] | London 2013 | |
|---|---|---|---|---|---|---|---|---|
| Outcomes: maternal | ||||||||
| Renal: antepartum | Normal | Normal | Proteinuria hematuria, mild RF |
RPGN, daily HD | RPGN, daily HD | RPGN, alternate day HD | RPGN, daily HD | RPGN, daily HD |
| Post-partum | RPGN, HD, RTx | Normal | HD, RTx | HD | Partial renal recovery | Full renal recovery | HD, RTx | HD |
| Lung: Antepartum | Normal | ‘Hemoptysis’ ‘Dyspnea’ | Normal | ‘Dyspnea’, CXR: ‘Congested’ |
‘Hemoptysis’ ‘cough’ | ‘Cough’ ‘Dyspnea’ | Normal | Cough, dyspnea, VQ: ‘pulmonary hemorrhage’ |
| Post-partum | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| Outcomes: pregnancy | ||||||||
| Delivery method | V | C | V | C | C | V | V | V |
| Pregnancy-related complications | (−) | (−) | PEC | (−) | GDM, PEC | HG | GDM, HG, PCP | GDM, HG |
| Outcomes: fetal | ||||||||
| Livebirth | (−)(SB) | (+) | (+) | (+) | (+)a | (−)(TA) | (+) | (+) |
| Delivery date (weeks gestation) | 28 | 37 | 35 | 34 | 26.5 | 15 | 35 | 35 |
| Intrauterine growth restriction | NR | (−) | NR | NR | (+) | NR | (+) | (−) |
| Small for gestational age | NR | (−) | NR | (+) | (+) | NR | (+) | (+) |
| Complication of prematurity | NR | (−) | NR | (−) | (+) | NR | (−) | (−) |
| Newborn anti-GBM antibodies | NR | NR | (−) | (−) | (−) | NR | (+) | (+) |
| Newborn kidney or lung disease | NR | (−) | (−) | (−) | (−) | NR | (−) | (−) |
CXR, chest X-ray; GDM, gestational diabetes mellitus; HD, hemodialysis; HG, hyperemesis gravidarum; NR, not reported; PCP, Pneumocystis carinii pneumonia; PEC, preeclampsia; RPGN, rapidly progressive glomerulonephritis; RTx, renal transplantation; SB, stillborn; TA, therapeutic abortion; VQ, ventilation-perfusion lung scan.
aLivebirth in Vasiliou born with ‘massive intraventricular hemorrhage,’ developed neurodevelopmental delay, visual dysfunction, failure to thrive.
Outcomes: pregnancy
Vaginal and cesarean section delivery were equally performed for live births (3/6 for both) (Table 3). Gestational diabetes (3/8), preeclampsia (2/8), hyperemesis gravidarum (2/8) and severe infections (1/8) were reported complications in pregnancy.
Outcomes: fetal
Most (6/8) cases resulted in singleton live births (Table 3). However, all live births were premature (6/6 live births), with severe prematurity (26.5 weeks) in one case. When reported, intrauterine growth restriction (3/4) and small for gestational age (4/5) were common. One infant had severe complications of prematurity with intraventricular and intraparenchymal hemorrhage, and at 1 year continued to have neurocognitive delay [14]. Anti-GBM antibodies were measured (5/8) and present in only two newborns. However, no kidney or lung disease was reported in any of the newborns.
Discussion
Our study confirms that maternal, pregnancy and fetal outcomes in de novo anti-GBM disease in pregnancy are poor, comparable to systemic lupus erythematosus (SLE). From a maternal perspective, the chance of renal recovery is very low. Interestingly, one dialysis-dependent patient had complete recovery of renal function with aggressive treatment and therapeutic abortion at 15 weeks compared with all other patients who reached ESRD or had severe renal dysfunction despite coming off hemodialysis. Pregnancy may worsen the clinical course in SLE patients [16], and therapeutic abortion may be recommended in severe circumstances [17]. This may suggest that similar to SLE patients, pregnancy may worsen the clinical course. Pregnancy complications were also high in de novo anti-GBM disease treated with immunosuppression, with high rates of gestational diabetes (3/8), preeclampsia (2/8), hyperemesis gravidarum (2/8) and serious infection (1/8). Finally, fetal complications in de novo anti-GBM disease in pregnancy are high, with prematurity (6/6 live births), intrauterine growth restriction (3/4), small for gestational age (4/5) and congenital abnormalities (2/6 live births). Fetal demise or severe adverse fetal outcomes were present in three of eight. While live births are possible in de novo anti-GBM disease (6/8), understanding the risks of renal biopsy, immunosuppression and hemodialysis are crucial to fully inform the mother of her optimal management decisions.
Renal biopsy during pregnancy was performed in only a minority (3/8) of cases (Table 2). While initial reports of antepartum renal biopsies were associated with high rates of bleeding complications [18], this was confounded by concurrent hypertension and preeclampsia, conditions in which a bleeding complication is well recognized [19]. Indeed, more recent trials indicate similar complication rates between pregnant and non-pregnant patients [20, 21]. Furthermore, renal biopsy results can significantly alter management decisions. In Vasiliou et al. and Joseph et al. cases, renal biopsy revealed >50% crescentic glomeruli [14] suggesting renal recovery was quite unlikely [22]. Under such circumstances, immunosuppression with the potentially teratogenic cyclophosphamide could be avoided if the patient does not have persisting pulmonary hemorrhage. Nair et al. [15] renal biopsy results may have informed patient counseling and decision-making. Thus, renal biopsy during pregnancy should be performed with caution, but ultimately should be considered when it offers the opportunity to make a diagnosis other than severe preeclampsia (far) from term [23].
The goal of treatment in de novo Goodpasture's disease in pregnancy must be to induce remission, in light of the significant maternal and fetal morbidity and mortality associated with uncontrolled vasculitis [17, 24]. Induction therapy for de novo Goodpasture's disease may require a combination of corticosteroid with oral or intravenous cyclophosphamide (CYC), followed by maintenance immunosuppression with azathioprine [25–27]. Systemic corticosteroids are generally considered low risk for teratogenicity, with historical links to cleft palate [28, 29] although this association is unclear [30]. Reported associations with adverse pregnancy outcomes [30, 31] are difficult to disentangle from the effects of the disorder for which the pregnant women is being treated [28]. Of cases in which immunosuppression was started in pregnancy with a goal of a live birth, all received a high dose then tapering doses of corticosteroids. Corticosteroids increase the risk of gestational diabetes [32], a complication seen often in our review (3/8) [9, 11, 14]. Thus, it is important to monitor for adverse effects of corticosteroids while simultaneously inducing disease remission, particularly pulmonary hemorrhage, as a primary goal.
The Food and Drug Administration (FDA) has evaluated cyclophosphamide as Category D, indicating positive evidence of human fetal risk, but potential benefits may warrant use of the drug in pregnant women despite potential risks [33]. Use of cyclophosphamide during the first trimester, during organogenesis, has been associated with malformation [34, 35] and miscarriages [35]. Growth retardation, suppression of fetal hematopoiesis and impaired neurological development have been reported with use later in pregnancy in the second and third trimesters [34, 35]. Cyclophosphamide use during pregnancy has been associated with adverse outcomes in SLE [36–38]. Cyclophosphamide exposure increases long-term risk of malignancies in patients treated with the drug [39] and papillary thyroid cancer and neuroblastoma was reported in a child exposed in utero to cyclophosphamide [40]. It is difficult to extrapolate the effects of cyclophosphamide during pregnancy in de novo anti-GBM disease in pregnancy from our study, since only one of our cases was exposed. Potential benefits may warrant use of the drug in select pregnant women with severe disease despite the known potential fetal risks. The decision whether or not to initiate cyclophosphamide should be made after considering the stage of pregnancy, weighing the relative efficacy of alternative therapies, and counseling of patients regarding maternal and fetal risks.
The use of azathioprine has been established as safe throughout pregnancy in a variety of clinical settings; transplant, inflammatory bowel and SLE literature [41, 42]. A meta-analysis did not find an association between maternal thiopurine exposure for inflammatory bowel disease and congenital defects [43]. Lower birth weight and higher prematurity rate have been reported but may be an effect of maternal underlying disease [44]. While ineffective for induction of severe anti-GBM disease, use of azathioprine may be appropriate in mild de novo anti-GBM disease in pregnancy. Moreover, use of azathioprine in combination with plasmapheresis may permit deferral of cyclophosphamide use until after pregnancy. Azathioprine-associated bone marrow suppression should be monitored [45]. Maternal thiopurine use has been associated with anemia and thrombocytopenia [46, 47] and leucopenia [48] in newborns. Thus, judicious use of azathioprine, with close monitoring for associated side effects, may be appropriate to optimize maternal and fetal outcomes.
In non-pregnant patients with incident Goodpasture's disease, plasmapheresis may be indicated in the presence of pulmonary hemorrhage [49] or AKI with a reasonable likelihood of recovery [27]. Typically, daily or alternate daily plasma exchanges with albumin are performed for 2–3 weeks [22, 26, 49], unless ongoing bleeding is present, in which case fresh frozen plasma may be used to replace blood clotting factors [25]. In combination with immunosuppression, plasmapheresis reduces titers of anti-GBM antibodies. Most mothers in our series (6/8) had detectable anti-GBM antibodies during pregnancy, and the levels were significantly lowered with plasmapheresis. Plasmapheresis is generally considered safe in pregnancy [50].
There is strong evidence that anti-GBM antibodies bind human placental antigens [51]; however, the clinical impact of this is controversial. Deubner et al. [12] hypothesized a protective effect of non-specific placental binding of anti-GBM antibodies, leading to decreased transfer to the fetus, and decreased total anti-GBM antibody maternal levels.
Joseph et al. [9] performed placental testing of IgG antibodies and showed diffuse binding of IgG antibodies; unfortunately, specific testing for anti-GBM antibodies could not be done. They postulated that anti-GBM binding to placenta may increase the risk of placental insufficiency and ultimately preeclampsia if not treated aggressively similar to Vasillou et al. [14]. Anti-GBM antibodies are IgG antibodies and as such are actively transported across the placenta via placental transporters; therefore, it is expected that the fetus would be exposed to all maternal IgG antibodies including anti-GBM antibodies.
Newborn anti-GBM antibody levels were commonly negative in our series (3/5) [12–14]. However, maternal anti-GBM levels in these three cases were negative [13], ‘decreasing,’ [14] or ‘borderline’ when measured closer to delivery [12]. To determine whether anti-GBM antibodies transfer across the placenta, simultaneous collection of maternal, neonatal and cord blood must be performed at a time when maternal anti-GBM titers are detectable. Simultaneous evaluation at delivery confirmed anti-GBM titers equivalent (mother = 4.3 U/mL, newborn = 6.5 U/mL, cord blood = 5.8 U/mL) [9] or lower in the mother (London, 2013: mother < 2.5 U/mL, newborn = 9.0 U/mL). Thus, anti-GBM antibodies cross the placenta, and it is unlikely that the placenta plays a protective role. While there remain no reported cases of newborn anti-GBM disease, this provides only some reassurance given only six described live births from mothers with de novo anti-GBM disease in pregnancy (Table 3). Moreover, transplacental antibody transmission has been linked to neonatal pulmonary-renal syndrome from myeloperoxidase-ANCA antibodies [52, 53], and neonatal lupus erythematosus from a variety of lupus antibodies [54].
Literature regarding hemodialysis in pregnancy has expanded since the first report of de novo anti-GBM disease in pregnancy, in 1986 [10]. Comprehensive reviews of this topic are available [55, 56]. Adverse maternal and fetal outcomes in women with end-stage kidney disease on hemodialysis are common, including maternal hypertension, anemia and preeclampsia, and fetal intrauterine growth restriction, polyhydramnios, prematurity and death [57, 58]. Ultimately, great attention to calcium and phosphate balance, nutrition, iron supplementation and anemia, dialysis duration, target weight, and blood pressure is required [55, 56], to optimize outcomes.
Pregnancies complicated by vasculitis, the need for hemodialysis and immunosuppression require special expertise using a multidisciplinary approach to optimize maternal, pregnancy and fetal outcomes. Input from high risk obstetrics, dietician, social work, clinical pharmacy, neonatology, nephrology, pediatrics and anesthesia are all beneficial. The need for this expertise necessitates patient admission to an academic hospital with the resources to facilitate management.
In conclusion, de novo anti-GBM disease in pregnancy is associated with poor maternal and fetal outcomes. Given the complexity of issues and decisions surrounding renal biopsy and immunosuppression, a multidisciplinary team is needed to provide comprehensive patient counseling and to optimize maternal, pregnancy and fetal outcomes.
Conflict of interest statement
None declared.
References
- 1.Couser WG. Rapidly progressive glomerulonephritis: classification, pathogenetic mechanisms, and therapy. Am J Kidney Dis. 1988;11:449–464. doi: 10.1016/s0272-6386(88)80079-9. [DOI] [PubMed] [Google Scholar]
- 2.Andrassy K, Kuster S, Waldherr R, et al. Rapidly progressive glomerulonephritis: analysis of prevalence and clinical course. Nephron. 1991;59:206–212. doi: 10.1159/000186552. [DOI] [PubMed] [Google Scholar]
- 3.Kambham N. Crescentic Glomerulonephritis: an update on Pauci-immune and Anti-GBM diseases. Adv Anat Pathol. 2012;19:111–124. doi: 10.1097/PAP.0b013e318248b7a1. [DOI] [PubMed] [Google Scholar]
- 4.Goodpasture EW. The significance of certain pulmonary lesions in relation to the etiology of influenza. Am J Med Sci. 1919;158:863–870. doi: 10.1097/MAJ.0b013e31818fff94. [DOI] [PubMed] [Google Scholar]
- 5.Benoit FL, Rulon DB, Theil GB, et al. Goodpasture's syndrome: a clinicopathologic entity. Am J Med. 1964;37:424–444. doi: 10.1016/0002-9343(64)90199-8. [DOI] [PubMed] [Google Scholar]
- 6.Bolton WK. Goodpasture's syndrome. Kidney Int. 1996;50:1753–1766. doi: 10.1038/ki.1996.495. [DOI] [PubMed] [Google Scholar]
- 7.Gammill HS, Jeyabalan A. Acute renal failure in pregnancy. Crit Care Med. 2005;33:S372–S384. doi: 10.1097/01.ccm.0000183155.46886.c6. [DOI] [PubMed] [Google Scholar]
- 8.Krane NK. Acute renal failure in pregnancy. Arch Intern Med. 1988;148:2347–2357. [PubMed] [Google Scholar]
- 9.Joseph G, Edris F, Martens M, et al. SA-PO750: A Pregnancy Complicated by RPGN Secondary to Anti-GBM Disease. San Diego, CA, USA: American Society of Nephrology; 2006. p. 732A. Journal of the American Society of Nephrology 2006: [Google Scholar]
- 10.Nilssen DE, Talseth T, Brodwall EK. The many faces of Goodpasture's syndrome. Acta Med Scand. 1986;220:489–491. doi: 10.1111/j.0954-6820.1986.tb02800.x. [DOI] [PubMed] [Google Scholar]
- 11.Yankowitz J, Kuller JA, Thomas RL. Pregnancy complicated by Goodpasture syndrome. Obstet Gynecol. 1992;79:806–808. [PubMed] [Google Scholar]
- 12.Deubner H, Wagnild JP, Wener MH, et al. Glomerulonephritis with anti-glomerular basement membrane antibody during pregnancy: potential role of the placenta in amelioration of disease. Am J Kidney Dis. 1995;25:330–335. doi: 10.1016/0272-6386(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 13.Al-Harbi A, Malik GH, Al-Mohaya SA, et al. Anti-glomerular basement membrane antibody disease presenting as acute renal failure during pregnancy. Saudi J Kidney Dis Transpl. 2003;14:516–521. [PubMed] [Google Scholar]
- 14.Vasiliou DM, Maxwell C, Shah P, et al. Goodpasture syndrome in a pregnant woman. Obstet Gynecol. 2005;106:1196–1199. doi: 10.1097/01.AOG.0000161061.35611.98. [DOI] [PubMed] [Google Scholar]
- 15.Nair S, George J, Kumar S, et al. A case of Goodpasture's syndrome complicating pregnancy with dialysis requiring renal failure responding to plasmapheresis and termination of pregnancy. Ren Fail. 2013;35:1173–1175. doi: 10.3109/0886022X.2013.815566. [DOI] [PubMed] [Google Scholar]
- 16.Lateef A, Petri M. Managing lupus patients during pregnancy. Best Pract Res Clin Rheumatol. 2013;27:435–447. doi: 10.1016/j.berh.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 18.Schewitz LJ, Friedman IA, Pollak VE. Bleeding after renal biopsy in pregnancy. Obstet Gynecol. 1965;26:295–304. [PubMed] [Google Scholar]
- 19.Wide-Swensson D, Strevens H, Willner J. Antepartum percutaneous renal biopsy. Int J Gynaecol Obstet. 2007;98:88–92. doi: 10.1016/j.ijgo.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 20.Packham D, Fairley KF. Renal biopsy: indications and complications in pregnancy. Br J Obstet Gynaecol. 1987;94:935–939. doi: 10.1111/j.1471-0528.1987.tb02266.x. [DOI] [PubMed] [Google Scholar]
- 21.Day C, Hewins P, Hildebrand S, et al. The role of renal biopsy in women with kidney disease identified in pregnancy. Nephrol Dial Transplant. 2008;23:201–206. doi: 10.1093/ndt/gfm572. [DOI] [PubMed] [Google Scholar]
- 22.Levy JB, Turner AN, Rees AJ, et al. Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med. 2001;134:1033–1042. doi: 10.7326/0003-4819-134-11-200106050-00009. [DOI] [PubMed] [Google Scholar]
- 23.Kuller JA, D'Andrea NM, McMahon MJ. Renal biopsy and pregnancy. Am J Obstet Gynecol. 2001;184:1093–1096. doi: 10.1067/mob.2001.114917. [DOI] [PubMed] [Google Scholar]
- 24.Nalli C, Iodice A, Andreoli L, et al. The effects of lupus and antiphospholipid antibody syndrome on foetal outcomes. Lupus. 2014;23:507–517. doi: 10.1177/0961203313501402. [DOI] [PubMed] [Google Scholar]
- 25.Lockwood CM, Rees AJ, Pearson TA, et al. Immunosuppression and plasma-exchange in the treatment of Goodpasture's syndrome. Lancet. 1976;1:711–715. doi: 10.1016/s0140-6736(76)93089-0. [DOI] [PubMed] [Google Scholar]
- 26.Jindal KK. Management of idiopathic crescentic and diffuse proliferative glomerulonephritis: evidence-based recommendations. Kidney Int Suppl. 1999;70:S33–S40. doi: 10.1046/j.1523-1755.1999.07005.x. [DOI] [PubMed] [Google Scholar]
- 27.Johnson JP, Moore J, Jr, Austin HA, 3rd, et al. Therapy of anti-glomerular basement membrane antibody disease: analysis of prognostic significance of clinical, pathologic and treatment factors. Medicine (Baltimore) 1985;64:219–227. doi: 10.1097/00005792-198507000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Fraser FC, Sajoo A. Teratogenic potential of corticosteroids in humans. Teratology. 1995;51:45–46. doi: 10.1002/tera.1420510107. [DOI] [PubMed] [Google Scholar]
- 29.Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385–392. doi: 10.1002/1096-9926(200012)62:6<385::AID-TERA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 30.Bermas BL. Non-steroidal anti inflammatory drugs, glucocorticoids and disease modifying anti-rheumatic drugs for the management of rheumatoid arthritis before and during pregnancy. Curr Opin Rheumatol. 2014;26:334–340. doi: 10.1097/BOR.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 31.Reinisch JM, Simon NG, Karow WG, et al. Prenatal exposure to prednisone in humans and animals retards intrauterine growth. Science. 1978;202:436–438. doi: 10.1126/science.705336. [DOI] [PubMed] [Google Scholar]
- 32.Vejrazkova D, Vcelak J, Vankova M, et al. Steroids and insulin resistance in pregnancy. J Steroid Biochem Mol Biol. 2014;139:122–129. doi: 10.1016/j.jsbmb.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Sannerstedt R, Lundborg P, Danielsson BR, et al. Drugs during pregnancy: an issue of risk classification and information to prescribers. Drug Saf. 1996;14:69–77. doi: 10.2165/00002018-199614020-00001. [DOI] [PubMed] [Google Scholar]
- 34.Baer AN, Witter FR, Petri M. Lupus and pregnancy. Obstet Gynecol Surv. 2011;66:639–653. doi: 10.1097/OGX.0b013e318239e1ee. [DOI] [PubMed] [Google Scholar]
- 35.Lannes G, Elias FR, Cunha B, et al. Successful pregnancy after cyclophosphamide therapy for lupus nephritis. Arch Gynecol Obstet. 2011;283:61–65. doi: 10.1007/s00404-011-1859-0. [DOI] [PubMed] [Google Scholar]
- 36.Clowse ME, Magder L, Petri M. Cyclophosphamide for lupus during pregnancy. Lupus. 2005;14:593–597. doi: 10.1191/0961203305lu2169oa. [DOI] [PubMed] [Google Scholar]
- 37.Brucato A, Cimaz R, Caporali R, et al. Pregnancy outcomes in patients with autoimmune diseases and anti-Ro/SSA antibodies. Clin Rev Allergy Immunol. 2011;40:27–41. doi: 10.1007/s12016-009-8190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smyth A, Oliveira GH, Lahr BD, et al. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol. 2010;5:2060–2068. doi: 10.2215/CJN.00240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radis CD, Kahl LE, Baker GL, et al. Effects of cyclophosphamide on the development of malignancy and on long-term survival of patients with rheumatoid arthritis. A 20-year follow-up study. Arthritis Rheum. 1995;38:1120–1127. doi: 10.1002/art.1780380815. [DOI] [PubMed] [Google Scholar]
- 40.Zemlickis D, Lishner M, Erlich R, et al. Teratogenicity and carcinogenicity in a twin exposed in utero to cyclophosphamide. Teratog Carcinog Mutagen. 1993;13:139–143. doi: 10.1002/tcm.1770130304. [DOI] [PubMed] [Google Scholar]
- 41.Schramm C, Herkel J, Beuers U, et al. Pregnancy in autoimmune hepatitis: outcome and risk factors. Am J Gastroenterol. 2006;101:556–560. doi: 10.1111/j.1572-0241.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 42.Moskovitz DN, Bodian C, Chapman ML, et al. The effect on the fetus of medications used to treat pregnant inflammatory bowel-disease patients. Am J Gastroenterol. 2004;99:656–661. doi: 10.1111/j.1572-0241.2004.04140.x. [DOI] [PubMed] [Google Scholar]
- 43.Akbari M, Shah S, Velayos FS, et al. Systematic review and meta-analysis on the effects of thiopurines on birth outcomes from female and male patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:15–22. doi: 10.1002/ibd.22948. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein LH, Dolinsky G, Greenberg R, et al. Pregnancy outcome of women exposed to azathioprine during pregnancy. Birth Defects Res A Clin Mol Teratol. 2007;79:696–701. doi: 10.1002/bdra.20399. [DOI] [PubMed] [Google Scholar]
- 45.Patel AA, Swerlick RA, McCall CO. Azathioprine in dermatology: the past, the present, and the future. J Am Acad Dermatol. 2006;55:369–389. doi: 10.1016/j.jaad.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 46.Jharap B, de Boer NK, Stokkers P, et al. Intrauterine exposure and pharmacology of conventional thiopurine therapy in pregnant patients with inflammatory bowel disease. Gut. 2014;63:451–457. doi: 10.1136/gutjnl-2012-303615. [DOI] [PubMed] [Google Scholar]
- 47.de Boer NK, de Meij T, van Bodegraven AA. Thiopurines during pregnancy in inflammatory bowel disease: is there a risk for the (unborn) child? Expert Rev Gastroenterol Hepatol. 2013;7:669–671. doi: 10.1586/17474124.2013.841541. [DOI] [PubMed] [Google Scholar]
- 48.Davison JM, Dellagrammatikas H, Parkin JM. Maternal azathioprine therapy and depressed haemopoiesis in the babies of renal allograft patients. Br J Obstet Gynaecol. 1985;92:233–239. doi: 10.1111/j.1471-0528.1985.tb01088.x. [DOI] [PubMed] [Google Scholar]
- 49.Savage CO, Pusey CD, Bowman C, et al. Antiglomerular basement membrane antibody mediated disease in the British Isles 1980–4. Br Med J (Clin Res Ed) 1986;292:301–304. doi: 10.1136/bmj.292.6516.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watson WJ, Katz VL, Bowes WA., Jr Plasmapheresis during pregnancy. Obstet Gynecol. 1990;76:451–457. [PubMed] [Google Scholar]
- 51.Derry CJ, Pusey CD. Tissue-specific distribution of the Goodpasture antigen demonstrated by 2-D electrophoresis and western blotting. Nephrol Dial Transplant. 1994;9:355–361. [PubMed] [Google Scholar]
- 52.Schlieben DJ, Korbet SM, Kimura RE, et al. Pulmonary-renal syndrome in a newborn with placental transmission of ANCAs. Am J Kidney Dis. 2005;45:758–761. doi: 10.1053/j.ajkd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Bansal PJ, Tobin MC. Neonatal microscopic polyangiitis secondary to transfer of maternal myeloperoxidase-antineutrophil cytoplasmic antibody resulting in neonatal pulmonary hemorrhage and renal involvement. Ann Allergy Asthma Immunol. 2004;93:398–401. doi: 10.1016/S1081-1206(10)61400-7. [DOI] [PubMed] [Google Scholar]
- 54.Porcel Chacon R, Tapia Ceballos L, Diaz Cabrera R, et al. Neonatal lupus erythematosis: a five-year case review. Reumatol Clin. 2013;10:170–173. doi: 10.1016/j.reuma.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 55.Nadeau-Fredette AC, Hladunewich M, Hui D, et al. End-stage renal disease and pregnancy. Adv Chronic Kidney Dis. 2013;20:246–252. doi: 10.1053/j.ackd.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 56.Furaz-Czerpak KR, Fernandez-Juarez G, Moreno-de la Higuera MA, et al. Pregnancy in women on chronic dialysis: a review. Nefrologia. 2012;32:287–294. doi: 10.3265/Nefrologia.pre2012.Jan.11319. [DOI] [PubMed] [Google Scholar]
- 57.Piccoli GB, Conijn A, Consiglio V, et al. Pregnancy in dialysis patients: is the evidence strong enough to lead us to change our counseling policy? Clin J Am Soc Nephrol. 2010;5:62–71. doi: 10.2215/CJN.05660809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malik GH, Al-Harbi A, Al-Mohaya S, et al. Pregnancy in patients on dialysis—experience at a referral center. J Assoc Physicians India. 2005;53:937–941. [PubMed] [Google Scholar]


