l-carnitine (free carnitine) transports cytosolic long-chain fatty acids as acylcarnitines across the inner mitochondrial membrane for β-oxidation and subsequent ATP production in the mitochondria. The endogenous carnitine pool comprises free l-carnitine as well as short-, medium- and long-chain fatty acid esters (acyl-carnitines) and depends on absorption of l-carnitine from dietary sources, endogenous biosynthesis and renal tubular reabsorption from glomerular filtrate [1]. More than 99% of the carnitine pool is located outside of plasma. Carrier-mediated transport ensures high tissue-to-plasma concentration ratios in tissues that depend critically on fatty acid oxidation, such as heart and muscle. Myocytes have one of the highest intracellular carnitine concentrations in the body, and red meat is a rich source of dietary carnitine. Absorption of orally administered l-carnitine is very variable and ranges from 5 to 18% for pharmacological doses of up to 75% for dietary l-carnitine. The low bioavailability depends both on transport kinetics and on l-carnitine metabolism by intestinal bacteria [1, 2]. The renal clearance of l-carnitine (1–3 mL/min) is much lower than the glomerular filtration rate because of extensive (98–99%) tubular reabsorption. The lack of reabsorption during the haemodialysis procedure results in large losses of l-carnitine in dialysate. The existence of a threshold concentration for tubular reabsorption results in much higher renal clearances after intravenous administration of high doses [3].
In 1978 a dramatic reduction in carnitine concentration in muscle and plasma was observed after the haemodialysis session due to loss of carnitine into the dialysate [4]. By 1980 it was reported that intravenous l-carnitine for 14 weeks decreased serum triglycerides [5], and oral d,l-carnitine for 30 days decreased serum triglycerides and returned HDL cholesterol to normal in chronic haemodialysis patients with hypertriglyceridaemia [6]. Potential benefits of carnitine supplementation on cardiomyopathy, cardiac failure and atherosclerosis in renal failure patients were envisioned. By 2003, National Kidney Foundation practice recommendations offered an expert opinion on the use of l-carnitine in dialysis patients with disorders potentially related to carnitine deficiency, such as erythropoietin-resistant anaemia, intradialytic hypotension, cardiomyopathy and fatigability [7]. l-Carnitine administration may also ameliorate insulin resistance, hypertriglycedaemia, inflammation and protein wasting [8, 9]. Reimbursement for the intravenous and oral administration of l-carnitine for chronic renal failure anaemia and intradialytic hypotension was approved by the US Centers for Medicare & Medicaid Services in 2004 and 2012, respectively [10]. Even intraperitoneal supplementation in peritoneal dialysis fluid has been tested and reported to be associated with improving insulin sensitivity in nondiabetic peritoneal dialysis patients [11]. However, 35 years after the first reports on l-carnitine deficiency in haemodialysis patients, routine l-carnitine supplementation is not recommended by KDIGO or KDOQI clinical guidelines covering several aspects of the renal failure patient, based on lack of definitive evidence of benefit [8, 9, 12–14].
Chronic haemodialysis may lead to progressive l-carnitine deficiency through a combination of loss of l-carnitine in dialysate and decreased l-carnitine synthesis by the injured kidney. l-carnitine deficiency develops over time in haemodialysis patients and serum levels may not differ from controls in incident patients [15]. In a recent clinical trial, pre-dialysis serum carnitine levels decreased by ∼22% during the first year of haemodialysis in the placebo arm and carnitine deficiency developed in 30% of patients [16]. Furthermore, low renal clearance of short-chain acylcarnitines results in a high acylcarnitine:l-carnitine ratio in chronic kidney disease patients. In addition, a metabolomics approach identified higher concentrations of long-chain acylcarnitines in incident dialysis patients, and these high concentrations were a marker of cardiovascular mortality after multivariable adjustment [15]. While the haemodialysis session induces a decline in free, short-chain, medium-chain and dicarboxylic acylcarnitines, it does not affect long-chain acylcarnitines [17]. Thus, dialysis patients, especially those on haemodialysis, may present two abnormalities regarding carnitine levels: a carnitine deficiency and a high acylcarnitine/free carnitine ratio that may further limit free carnitine availability.
The 2000 KDOQI Clinical Practice Guidelines for Nutrition in Chronic Renal Failure Work Group extensively reviewed the evidence regarding l-carnitine supplementation for diverse symptoms and complications of chronic dialysis and agreed that there was insufficient evidence to support or refute the routine use of l-carnitine for metabolic abnormalities in maintenance dialysis patients, including hypertriglyceridaemia, hypercholesterolaemia, and anaemia or for treatment for several symptoms or complications of dialysis, including intradialytic arrhythmias and hypotension, low cardiac output, interdialytic and post-dialytic symptoms of malaise or asthenia, general weakness or fatigue, skeletal muscle cramps and decreased exercise capacity or low peak oxygen consumption [8]. However, the workgroup indicated that in selected individuals who manifest the above symptoms or disorders and who have not responded adequately to standard therapies, a trial of l-carnitine may be considered.
In the same line of thought, the KDOQI Clinical Practice Guideline for Nutrition in Children with CKD 2008 Work Group could not recommend the use of carnitine, but it did not discourage a therapeutic trial of carnitine if the clinical symptoms are suggestive of the disorder, especially when the evaluation provides laboratory evidence compatible with a diagnosis of carnitine deficiency [12]. In this regard, the Work Group defined carnitine deficiency as an acyl:free carnitine ratio >0.4 (estimating acylcarnitine from total and free carnitine) or a total serum carnitine <40 μmol/L. However, they observed that most studies on carnitine deficiency in paediatric dialysis patients achieved an increase in plasma carnitine level but did not observe changes in symptoms.
The 2012 KDIGO Clinical Practice Guideline for Anaemia in CKD and the KDOQI commentary on those guidelines suggested not using adjuvants to erythropoiesis-stimulating agents, including l-carnitine [13, 14]. Indeed, in a recent clinical trial treatment of incident haemodialysis patients with intravenous l-carnitine did not improve their response to erythropoietin, despite increasing pre-dialysis l-carnitine levels 3-fold over baseline levels that were already in the normal range [16]. A 2014 systematic review and meta-analysis failed to confirm the previous findings regarding the effects of l-carnitine on haemoglobin and the erythropoietin dose but showed that l-carnitine significantly decreased serum LDL and C-reactive protein. The extent of the decrease in LDL was not clinically relevant, whereas the significant decrease in C-reactive protein was both statistically and clinically relevant, but has not been shown to impact outcomes [18].
Despite this lack of endorsement of the practice based on insufficient quality or contradictory evidence, many physicians prescribe l-carnitine supplementation routinely to haemodialysis patients given the biological plausibility, potential benefit and lack of adverse effects [19]. However, recent advances in the fields of the microbiota and metabolomics have cast doubts over the tenet that oral l-carnitine supplementation is free of potentially serious adverse effects. In this regard, a meta-analysis disclosed potential differences between the effects of oral and intravenous l-carnitine on serum cholesterol: oral l-carnitine increased serum cholesterol while intravenous l-carnitine did not [18].
In this issue of CKJ, Fukami et al. [20] report that in haemodialysis patients that had previously received oral l-carnitine supplements for 1-year, a switch to intravenous l-carnitine for 1 week significantly increased total, free and acyl carnitine levels. Pre-dialysis total carnitine levels increased by 40%, with a milder increase (33%) after the haemodialysis session. In addition, the switch decreased serum free fatty acids by 21% [20]. The decrease in free fatty acids was positively correlated with the decrease in the acyl/free carnitine ratio. Switching from the oral to the intravenous route of l-carnitine administration increased HDL cholesterol levels by 12% at four weeks but had no impact on LDL cholesterol or triglycerides. Confirming previous reports, total and free carnitine levels before initiating l-carnitine supplementation were 33% lower in haemodialysis patients than in matched healthy controls [20]. However, long-term oral carnitine had increased serum carnitine levels in l-carnitine-supplemented patients to values several fold higher than in healthy controls (free carnitine; 3-fold, acyl carnitine; 4-fold) and had increased LDL-cholesterol and triglyceride levels, but it did not affect HDL cholesterol values.
Given the low number of patients (n = 9) and the lack of a placebo-controlled group, this should be considered an exploratory study that should be confirmed in larger, controlled studies. However, these results may provide further insights into potential differential effects or oral and intravenous l-carnitine supplementation in light of recent advances reported in the medical literature. If confirmed, how can the additional effects of iv carnitine over oral carnitine be explained? Despite the low oral bioavailability of l-carnitine, the issue was not carnitine deficiency, since serum carnitine levels in patients receiving oral supplements were well above those of controls. A pharmacological effect of higher concentrations of l-carnitine should be postulated. As an alternative, the switch from oral to intravenous l-carnitine may have impacted on circulating TMAO levels.
Both intravenous and oral l-carnitine are licensed for use in haemodialysis patients [10]. However, there might be major differences between the oral and the intravenous route that go beyond assuring compliance and bypassing the low absorption of l-carnitine in the gut. Recent reports have highlighted oral l-carnitine processing by gut microbiota to trimethylamine (TMA) which is absorbed and further processed to trimethylamine-N-oxide (TMAO) by liver flavin monoxygenases (especially FMO3 and FMO1) [21] (Figure 1). Genetic deficiencies in FMO enzymes prevent conversion of malodorous TMA to odourless TMAO, resulting in trimethylaminuria or ‘fish odour syndrome’ [22]. Concerns had been raised 8 years ago about the potential toxicity of l-carnitine metabolites [3]. Dose-dependent increases in circulating TMA and TMAO levels were observed in healthy volunteers following oral l-carnitine dosing [3]. The accumulation of potentially toxic metabolites was more dramatic in haemodialysis patients dosed with oral l-carnitine for 12 days [23]. Plasma levels of TMA remained unaltered, but pre-dialysis plasma TMAO concentrations were continually rising and approximately doubled in 2 weeks. However, a recent report from Japan, a country where fish is an important dietary source of TMAO, observed an increase in circulating TMA levels following dosing with oral l-carnitine, but failed to appreciate an increase in TMAO [24]. Furthermore, pre-dialysis TMAO levels in Japanese haemodialysis patients were in the range found in healthy volunteers. However, TMAO had been reported to be increased in Australian patients on haemodialysis not supplemented with l-carnitine and in the absence of fish ingestion for 48 h [25], and TMAO is considered a uraemic toxin [26]. Besides the potential role of dietary fish or ethnic differences in FMAO activity as explanations for these discrepant results, recent developments have shed light both on the potential clinical consequences of higher TMAO levels and in the potential explanations for the diverse TMAO levels observed in haemodialysis patients [27–29]. In this regard, TMAO levels are very variable both along time in the same individual and in response to an oral load of precursors [30, 31]. This variability may depend on dietary content of TMAO or its precursors, the individual microbiota and on genetic differences in the activity of enzymes involved in TMAO metabolism [21, 22].
Fig. 1.
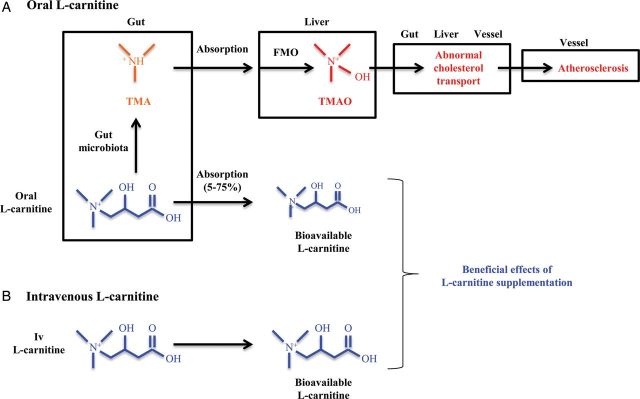
The different metabolism of l-carnitine depending on route of administration may underlie differences in therapeutic effects. (A) Oral l-carnitine is in part processed by the gut microbiota to generate trimethylamine (TMA). l-carnitine and TMA are absorbed in the gut. TMA is oxidized to trimethylamine-N-oxide (TMAO) in the liver by flavin monooxygenases (FMOs). TMAO interferes with cholesterol transport in different organs and tissues and is associated with higher cardiovascular risk. Potential effects of TMAO on free fatty acid metabolism have not been addressed. (B) By contrast, the bioavailability of intravenous l-carnitine is higher than oral l-carnitine (100 versus 5–75%, lower for pharmacological oral supplementation than for dietary l-carnitine) and intravenous l-carnitine administration does not result in TMAO generation. Different biological actions of oral versus intravenous l-carnitine may be related to higher l-carnitine availability and/or to lower serum TMAO levels following intravenous administration.
In 2011 a metabolomics approach identified plasma TMAO as a predictor of cardiovascular risk that was confirmed in an independent large clinical cohort [27]. Supplementation with TMAO promoted atherosclerosis in mice. Genetic variations controlling expression of FMO segregated with atherosclerosis in hyperlipidaemic mice. This study demonstrated the critical role of the gut microbiota in dietary choline-induced TMAO production and atherosclerosis. Indeed, in humans a phosphatidylcholine challenge (ingestion of two hard-boiled eggs) increased plasma TMAO [28]. This response was suppressed by the administration of antibiotics and reappeared after withdrawal of antibiotics.
In 2013 the role of dietary l-carnitine as a precursor of TMAO was specifically addressed [21]. Intestinal microbiota metabolism of dietary l-carnitine was shown to produce TMAO and to accelerate atherosclerosis in mice. Specific bacterial taxa in the gut were associated with higher TMAO levels. Dietary l-carnitine itself induced the microbiota capacity to metabolize l-carnitine into TMA that eventually originates TMAO. In this regard, omnivorous humans (eating red meat) produced more TMAO upon oral l-carnitine challenge than did vegans or vegetarians. Indeed, circulating TMAO levels were higher in onmivorous than in vegans or vegetarians. However, an oral 250 mg labelled l-carnitine challenge, although followed by an increase in labelled circulating TMAO, had little impact on endogenous TMAO levels in subjects with normal renal function [21]. In mice, chronic dietary l-carnitine supplementation altered the caecal microbiota, markedly enhanced synthesis of TMA and TMAO, and increased atherosclerosis. The increase in atherosclerosis burden occurred in the absence of pro-atherogenic changes in plasma lipids, lipoproteins, glucose or insulin levels, although l-carnitine tended to increase serum cholesterol by 10% while TMAO tended to decrease serum cholesterol by 10%. The effect of l-carnitine or TMAO supplementation on free fatty acids was not studied. Thus, the potential impact of TMAO levels on the changes in free fatty acids observed by Fukami et al. [20] after switching the route of l-carnitine administration remains unexplored. Antibiotic-mediated suppression of intestinal microbiota prevented these effects and resulted in higher circulating l-carnitine levels following oral supplementation, presumably because of lower bacterial catabolism into TMA. Given the large quantities of l-carnitine in red meats, it was proposed that l-carnitine and the microbiota may contribute to the causal link between high consumption of red meat and cardiovascular risk [21]. However, as is often the case in medicine, new studies raise new questions. The conundrum posed by the novel concept of supplemental l-carnitine as a source of atherosclerosis-promoting TMAO was recently reviewed [29].
The pro-atherogenic effect of TMAO may be related to upregulation of macrophage scavenger receptors linked to atherosclerosis, promoting foam cell differentiation [27] or to reduced reverse cholesterol transport and reduced expression of Cyp7a1, the rate limiting step in the catabolism of cholesterol [21]. The intimate molecular mechanisms of TMAO actions is yet unknown, but TMAO may stabilize the folded state of diverse proteins, functioning as a chemical chaperone [32].
Plasma l-carnitine levels predicted incident major adverse cardiac events in a large cohort only among subjects with concurrently high TMAO levels [21]. High circulating TMAO was recently confirmed to be associated with incident cardiovascular events over a 3-year period in >4000 individuals after adjustment for traditional risk factors, as well as in lower-risk subgroups [28].
While the relationship between serum TMAO and adverse cardiovascular events was established in the general population, TMAO metabolism in CKD has not been completely clarified. In this regard, high circulating TMAO levels in haemodialysis patients are efficiently removed during a single haemodialysis session [25] while increased urinary TMAO levels were observed in CKD patients [33]. These increased urinary levels may represent local renal TMAO synthesis or renal excretion of circulating TMAO.
In summary, complex interactions between diet, genetics and the microbiota may impact on processing of oral l-carnitine into pro-atherogenic metabolites such as TMAO that regulate lipid metabolism. The differential effect of oral versus intravenous l-carnitine supplementation observed by Fukami et al. [20] may be related to either higher final l-carnitine levels or to reduced production of metabolites such as TMAO. If Fukami et al. have biobanked serum from their study, it would be interesting to analyse the impact of switching from oral to intravenous l-carnitine on serum TMAO levels and the relationship between TMAO and the observed changes in free fatty acid and HDL levels.
Acknowledgements
The authors are supported by the grants FIS PI13/00047, ISCIII-RETIC REDinREN RD12/0021, Comunidad de Madrid S2010/BMD-2378, CYTED IBERERC, Programa Intensificación Actividad Investigadora (ISCIII) to A.O., and FIS Sara Borrell to M.D.S.-N.
Conflict of interest statement
None declared.
(See related article by Fukami et al. Effects of switching from oral administration to intravenous injection of l-carnitine on lipid metabolism in hemodialysis patients. Clin Kidney J 2014; 7: 470–474)
References
- 1.Evans A. Dialysis-related carnitine disorder and levocarnitine pharmacology. Am J Kidney Dis. 2003;41(Suppl 4):S13–S26. doi: 10.1016/s0272-6386(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 2.Evans AM, Fornasini G. Pharmacokinetics of l-carnitine. Clin Pharmacokinet. 2003;42:941–967. doi: 10.2165/00003088-200342110-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bain MA, Milne RW, Evans AM. Disposition and metabolite kinetics of oral l-carnitine in humans. J Clin Pharmacol. 2006;46:1163–1170. doi: 10.1177/0091270006292851. [DOI] [PubMed] [Google Scholar]
- 4.Bohmer T, Bergrem H, Eiklid K. Carnitine deficiency induced during intermittent haemodialysis for renal failure. Lancet. 1978;1:126–128. doi: 10.1016/s0140-6736(78)90422-1. [DOI] [PubMed] [Google Scholar]
- 5.Guarnieri GF, Ranieri F, Toigo G, et al. Lipid-lowering effect of carnitine in chronically uremic patients treated with maintenance hemodialysis. Am J Clin Nutr. 1980;33:1489–1492. doi: 10.1093/ajcn/33.7.1489. [DOI] [PubMed] [Google Scholar]
- 6.Lacour B, Di Giulio S, Chanard J, et al. Carnitine improves lipid anomalies in haemodialysis patients. Lancet. 1980;2:763–764. doi: 10.1016/s0140-6736(80)90384-0. [DOI] [PubMed] [Google Scholar]
- 7.Eknoyan G, Latos DL, Lindberg J. Practice recommendations for the use of l-carnitine in dialysis-related carnitine disorder. National Kidney Foundation Carnitine Consensus Conference. Am J Kidney Dis. 2003;41:868–876. doi: 10.1016/s0272-6386(03)00110-0. [DOI] [PubMed] [Google Scholar]
- 8.Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis. 2000;35(6 Suppl 2):S1–140. doi: 10.1053/ajkd.2000.v35.aajkd03517. [DOI] [PubMed] [Google Scholar]
- 9.Wasserstein AG. l-carnitine supplementation in dialysis: treatment in quest of disease. Semin Dial. 2013;26:11–15. doi: 10.1111/sdi.12041. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; end-stage renal disease quality incentive program. Final Rule. Fed Regist. 2011;76:627–646. [PubMed] [Google Scholar]
- 11.Bonomini M, Di Liberato L, Del Rosso G, et al. Effect of an L-carnitine-containing peritoneal dialysate on insulin sensitivity in patients treated with CAPD: a 4-month, prospective, multicenter randomized trial. Am J Kidney Dis. 2013;62:929–938. doi: 10.1053/j.ajkd.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 12.KDOQI Work Group. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am J Kidney Dis. 2009;53(3 Suppl 2):S11–104. doi: 10.1053/j.ajkd.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl. 2012;2:279–233. [Google Scholar]
- 14.Kliger AS, Foley RN, Goldfarb DS, et al. KDOQI US commentary on the 2012 KDIGO Clinical Practice Guideline for Anemia in CKD. Am J Kidney Dis. 2013;62:849–859. doi: 10.1053/j.ajkd.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Kalim S, Clish CB, Wenger J, et al. A plasma long-chain acylcarnitine predicts cardiovascular mortality in incident dialysis patients. J Am Heart Assoc. 2013;2:e000542. doi: 10.1161/JAHA.113.000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mercadal L, Coudert M, Vassault A, et al. l-carnitine treatment in incident hemodialysis patients: the multicenter, randomized, double-blinded, placebo-controlled CARNIDIAL trial. Clin J Am Soc Nephrol. 2012;7:1836–1842. doi: 10.2215/CJN.12431211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirolli V, Rossi C, Di Castelnuovo A, et al. Toward personalized hemodialysis by low molecular weight amino-containing compounds: future perspective of patient metabolic fingerprint. Blood Transfus. 2012;10(Suppl 2):s78–s88. doi: 10.2450/2012.012S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Abbate M, Tang L, et al. l-Carnitine supplementation for adults with end-stage kidney disease requiring maintenance hemodialysis: a systematic review and meta-analysis. Am J Clin Nutr. 2014;99:408–422. doi: 10.3945/ajcn.113.062802. [DOI] [PubMed] [Google Scholar]
- 19.Molyneux R, Seymour AM, Bhandari S. Value of carnitine therapy in kidney dialysis patients and effects on cardiac function from human and animal studies. Curr Drug Targets. 2012;13:285–293. doi: 10.2174/138945012799201595. [DOI] [PubMed] [Google Scholar]
- 20.Fukami K, Yamagishi S, Sakai K, et al. Effects of switching from oral administration to intravenous injection of L-carnitine on lipid metabolism in hemodialysis patients. Clin Kidney J. 2014;7:470–474. doi: 10.1093/ckj/sfu082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira F, Esteves S, Almeida LS, et al. Trimethylaminuria (fish odor syndrome): genotype characterization among Portuguese patients. Gene. 2013;527:366–370. doi: 10.1016/j.gene.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Bain MA, Faull R, Milne RW, et al. Oral l-carnitine: metabolite formation and hemodialysis. Curr Drug Metab. 2006;7:811–816. doi: 10.2174/138920006778520561. [DOI] [PubMed] [Google Scholar]
- 24.Ozasa H, Shimizu M, Koizumi A, et al. Trimethylamine generation in patients receiving hemodialysis treated with l-carnitine. Clin Kidney J. 2014;7:329. doi: 10.1093/ckj/sfu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bain MA, Faull R, Fornasini G, et al. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant. 2006;21:1300–1304. doi: 10.1093/ndt/gfk056. [DOI] [PubMed] [Google Scholar]
- 26.Duranton F, Cohen G, De Smet R, et al. European Uremic Toxin Work Group. Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012;23:1258–1270. doi: 10.1681/ASN.2011121175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang WHW, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ussher JR, Lopaschuk GD, Arduini A. Gut microbiota metabolism of l-carnitine and cardiovascular risk. Atherosclerosis. 2013;231:456–461. doi: 10.1016/j.atherosclerosis.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Miller CA, Corbin KD, da Costa KA, et al. Effect of egg ingestion on trimethylamine-N-oxide production in humans: a randomized, controlled, dose-response study. Am J Clin Nutr. 2014;100:778–786. doi: 10.3945/ajcn.114.087692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McEntyre CJ, Lever M, Chambers ST, et al. Variation of betaine, N,N-dimethylglycine, choline, glycerophosphorylcholine, taurine and trimethylamine-N-oxide in the plasma and urine of overweight people with type 2 diabetes over a two-year period. Ann Clin Biochem. 2014 doi: 10.1177/0004563214545346. pii: 0004563214545346 (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 32.Ma J, Pazos IM, Gai F. Microscopic insights into the protein-stabilizing effect of trimethylamine N-oxide (TMAO) Proc Natl Acad Sci USA. 2014;111:8476–8481. doi: 10.1073/pnas.1403224111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Posada-Ayala M, Zubiri I, Martin-Lorenzo M, et al. Identification of a urine metabolomics signature in patients with advanced-stage chronic kidney disease. Kidney Int. 2014;85:103–111. doi: 10.1038/ki.2013.328. [DOI] [PubMed] [Google Scholar]


