Abstract
Mild traumatic brain injury (mTBI) occurs from a closed-head impact. Often referred to as concussion, about 20% of cases complain of secondary psychological sequelae, such as disorders of attention and memory. Known as post-concussive symptoms (PCS), these problems can severely disrupt the patient's quality of life. Changes in local spectral power, particularly low-frequency amplitude increases and/or peak alpha slowing have been reported in mTBI, but large-scale connectivity metrics based on inter-regional amplitude correlations relevant for integration and segregation in functional brain networks, and their association with disorders in cognition and behaviour, remain relatively unexplored. Here, we used non-invasive neuroimaging with magnetoencephalography to examine functional connectivity in a resting-state protocol in a group with mTBI (n = 20), and a control group (n = 21). We observed a trend for atypical slow-wave power changes in subcortical, temporal and parietal regions in mTBI, as well as significant long-range increases in amplitude envelope correlations among deep-source, temporal, and frontal regions in the delta, theta, and alpha bands. Subsequently, we conducted an exploratory analysis of patterns of connectivity most associated with variability in secondary symptoms of mTBI, including inattention, anxiety, and depression. Differential patterns of altered resting state neurophysiological network connectivity were found across frequency bands. This indicated that multiple network and frequency specific alterations in large scale brain connectivity may contribute to overlapping cognitive sequelae in mTBI. In conclusion, we show that local spectral power content can be supplemented with measures of correlations in amplitude to define general networks that are atypical in mTBI, and suggest that certain cognitive difficulties are mediated by disturbances in a variety of alterations in network interactions which are differentially expressed across canonical neurophysiological frequency ranges.
Keywords: Mild traumatic brain injury (mTBI), Magnetoencephalography (MEG), Resting-state, Attention, Depression, Anxiety, Functional connectivity, Neural oscillations
Highlights
-
•
Patients with mTBI display increased connectivity in low-frequency resting state.
-
•
Elevated low-frequency power observed in temporal and deep-grey regions in mTBI
-
•
Frontal, temporal and deep-grey regions show increased amplitude correlations in mTBI.
-
•
Disorders of attention, anxiety and depression are associated with distinct, frequency-specific networks across the brain.
1. Introduction
Mild traumatic brain injury (mTBI) is due to considerable changes in acceleration and resultant impact forces on the brain (Niogi et al., 2008; Xu et al., 2007). These forces may damage white matter, comprising stretching, inflammation, disruption, and separation from grey matter structures (Ball et al., 1977; Gloor et al., 1977; Huang et al., 2007) and may also induce changes in cholinergic transmission (Huang et al., 2014). Commonly referred to as concussion, mTBI is reported in emergency rooms in less than 2% of the overall population, although it encompasses about 75% of all head injuries, with the additional 25% including moderate and more severe TBI. Post-concussive symptoms (PCS) are often observed (Levin et al., 1987), broadly defined by three psychological clusters in the behavioural, cognitive, and emotional domains. Particularly prevalent are complaints such as irritability and fatigue, inattention, impulsivity and memory deficits, and anxiety and depression (Ryan and Warden, 2003). These symptoms spontaneously subside in about 80% of cases in less than 3 months post-injury; however, the other 20% continue to experience secondary, chronic psychological sequelae that can fail to attenuate (Binder et al., 1997), severely disrupting the patient's quality of life.
Despite these psychological problems, structural neuroimaging is unremarkable and often fails in diagnostics due to the lack of conspicuous neural lesions. However, functional neuroimaging has proven its efficacy in delineating indicators of the disorder (Huang et al., 2007). One of the macroscopic functional imaging markers that tend to distinguish sufferers of mTBI is changes in local spectral power measured using electroencephalography (EEG) and magnetoencephalography (MEG), as low-frequency amplitude increases, and/or peak alpha oscillatory slowing, have been reported by multiple groups (Huang et al., 2009; Lewine et al., 2007; Lewine et al., 1999; Tarapore et al., 2013).
Technological advances in the analyses of intrinsic brain connectivity, examined using resting-state paradigms have proven suitable for mapping functional connectivity (FC) among brain regions. Using this translatable and readily operationalised protocol, researchers have mapped the intrinsic and dynamic communication patterns underlying the spatio-temporal coordination of information (Damoiseaux et al., 2006) required for complex goal-directed behaviour. FC is reflected in the coordination of neurophysiological activity among neural populations relevant for cortical computation and information integration (Wang, 2010) has shown to be useful in exploring cortical pathophysiology as well as neurological and neuropsychiatric disorders and their symptoms (Tewarie et al., 2013). MEG has proven particularly fruitful in this regard, and allows the patterns of brain activity from direct neural firing to be mapped with millisecond precision (Hari and Salmelin, 2012), supporting accurate noninvasive interrogation of coordinated oscillatory activity in the human brain relevant for cognition (Palva et al., 2005). Coordinated fluctuations in band-limited oscillatory amplitudes across brain regions have recently been shown to be an effective means for mapping the functional networks of the brain using MEG (Brookes et al., 2011a; Brookes et al., 2011b; Hipp et al., 2012). This technique provides an ideal opportunity, as there is little evidence of how mTBI modifies large-scale electrophysiological connectivity patterns of brain integration and segregation, and how this impacts cognition and behaviour. Given the hypothesised role of deafferentation of white matter in the disorder, axonal structures that mediate brain communication, changes in FC might be expected to be one of the principal outcomes and aetiology for PCS.
Prior studies have not investigated neurophysiological network connectivity in mTBI, or potential associations between altered network interactions in specific frequency ranges and the cognitive, affective and behavioural sequelae associated with this condition. To address this knowledge gap, we used non-invasive neuroimaging with MEG to examine FC in a resting-state protocol in a group with mTBI compared to a matched control group. We tested the hypotheses that mTBI patients would express atypical resting-state network amplitude correlations. Furthermore we performed an exploratory, data-driven analysis examining individual variability in certain cognitive-behavioural measures, and whether they correlated with connectivity measures and graph theoretical metrics. We selected connection weight, node strength and degree as our graph properties of interest, as they most directly correspond to network hyperconnectivity or hypoconnectivity, and thus correspond most closely with our hypothesis that patients with mTBI would show alterations in network-level neurophysiological interactions.
2. Methods and materials
2.1. Participants
Resting-state MEG data were recorded from 20 male participants with mTBI (less than 3 months post-injury, mean days since injury = 32.20, SD = 17.98, mean age at injury = 31.4 years, SD = 6.87). An age- and sex-matched control group without any history of TBI comprising 21 participants (mean age = 27.0 years, SD = 5) was also recruited.
Participants with mTBI were recruited from the emergency department of Sunnybrook Health Science Centre, Toronto. Inclusion criteria were: between 20 and 40 years of age; concussion symptoms whilst in emergency; less than 3 months since injury; if loss of consciousness occurred, then less than 30 min; if post-traumatic amnesia occurred, then less than 24 h; causes of head injury were clear (e.g. sustaining a force to the head); Glasgow Coma Scale ≥ 13 (within 24 h of injury); no skull fracture; unremarkable CT scan; no previous incidence of concussion. Every participant in the mTBI group completed the Symptom Checklist and Symptom Severity Score (Sports Concussion Assessment Tool 2; SCAT2); was able to tolerate enclosed space for MR brain imaging; English speaking, to comply with instructions to complete tasks during MEG and MR scans and able to give informed consent. The control group had no history of TBI (mild, moderate or severe) or neurological disorders. Exclusion criteria included ferrous metal inside the body that might be classified as MRI contraindications, or items that might interfere with MEG data acquisition; presence of implanted medical devices; seizures or other neurological disorders, or active substance abuse; certain ongoing medications (anticonvulsants, benzodiazepines, and/or GABA antagonists) known to directly or significantly influence electroencephalographic (EEG) findings.
All participants underwent cognitive-behavioural testing in addition to the MEG resting-state scan. These assessments included: estimates of IQ from the Wechsler Abbreviated Scale of Intelligence (WASI); the Alcohol Use Disorders Identification Test (AUDIT); Conners Attention-Deficit Hyperactivity Disorder Test; the Generalised Anxiety Disorder 7 test (GAD-7); Patient Health Questionnaire (PHQ9); and the Sports Concussion Assessment Tool 2 (SCAT2).
2.2. Procedure and MEG data acquisition
Resting-state MEG data were collected whilst participants were lying supine, and instructed to rest with eyes open and maintain visual fixation on an X within a circle on a screen 60 cm from the eyes. MEG data were collected inside a magnetically-shielded room on a CTF Omega 151 channel system (CTF Systems, Inc., Coquitlam, Canada) at The Hospital for Sick Children, at 600 Hz for 300 s. Throughout the run, head position was continuously recorded by three fiducial coils placed on the nasion, and left and right pre-auricular points.
After the MEG session, anatomical MRI images were acquired using the3 T MRI scanner (Magnetom Tim Trio, Siemens AG, Erlangen, Germany) in a suite adjacent to the MEG. Structural data were obtained as T1-weighted magnetic resonance images using resolution 3D MPRAGE sequences (repetition time [TR] = 2300 ms; echo time [TE] = 2.9 ms; flip angle [FA] = 9°; Field-of-view [FOV] = 28.8 × 19.2 cm; 256 × 256 matrix; 192 slices; 1 mm isovoxel) on a 12-channel head coil. MEG data were coregistered to the MRI structural images using the reference fiducial coil placements. A multi-sphere head model was constructed for each individual and brain space was normalised to a standard Montreal Neurological Institute (MNI) brain using SPM2.
2.3. MEG data processing
2.3.1. Seed definition and virtual electrode recording
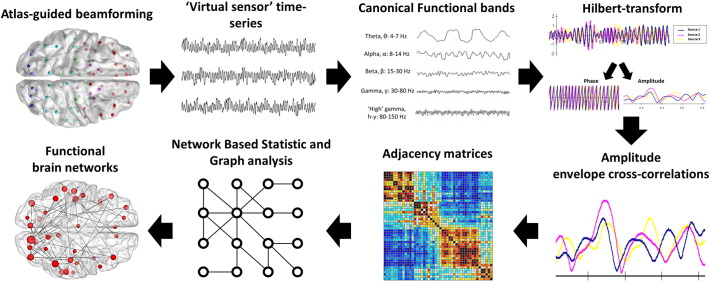
MEG data were band-pass filtered offline at 1–150 Hz, a notch filter applied at 60 Hz (8 Hz bandwidth) and a third-order spatial gradient environmental noise-cancellation applied to the recording. A priori sources (seeds) of interest in the cortex and sub-cortical regions were identified from the Automated Anatomical Labeling AAL (Tzourio-Mazoyer et al., 2002) atlas giving 90 locations for time-series to be extracted and analysed. Broadband time-series (‘virtual electrodes’) from these voxels were reconstructed using a vector beamformer on the basis of the 90 AAL coordinates for each subject and filtered into ‘classical’ EEG bandwidths for further analysis: Delta (1–4 Hz), theta (4–7 Hz), alpha (8–14 Hz), beta (15–30 Hz), low gamma (30–80 Hz) and high gamma (80–150 Hz) (see Fig. 1).
Fig. 1.
Analysis pipeline for resting-state functional connectivity. We employed an atlas-guided beamforming approach using all 90 cortical and subcortical regions in the AAL atlas to define seed regions. ‘Virtual-sensor’ time-series were reconstructed and filtered into canonical frequency bands, and a Hilbert transform is applied to derive estimates of instantaneous amplitude and phase. Each pairwise combination of time-series amplitude envelopes was then cross-correlated to define the degree of connectivity between seeds (‘amplitude envelope intrinsic coupling’). Adjacency matrices for each group were then compiled and submitted to a network based statistic analysis, and significant network differences are visualised using the BrainNet viewer toolbox.
Continuous time series were reconstructed from each of the 90 cortical and subcortical locations using beamformer analysis. Beamformers are a type of spatial filter used to suppress signals from unwanted noise sources, whilst being optimally sensitive to activity in a given brain location (in this particular case, the AAL seed locations). Individual weight vectors were applied to each sensor measurement and summated to give estimated source activity at a particular cortical seed location (Quraan and Cheyne, 2010). Additionally, MEG beamformers are effective at suppressing ocular artefacts generated by eye movements, which are a particularly problematic source noise in EEG, and non-ocular artefacts, such as cardiac and muscle activity (Muthukumaraswamy, 2013).
Each of the analysed frequency range data from each subject was then submitted to a functional connectivity analysis, by computing amplitude envelope cross-correlations across the entire resting-state run, based on the instantaneous amplitude estimate of each sample from the filtered time-series calculated using the Hilbert transform. The degree of amplitude cross-correlation between all pairwise combinations of the seeds varied between 1 (perfect correlation) and −1 (perfect anti-correlation). These values quantify the magnitude of the amplitude correlation between any two sources, referred to henceforth as functional connectivity.
2.3.2. Statistical analysis
Adjacency matrices with amplitude correlation values acting as edge weights for all sources were constructed, which resulted in a 90 × 90 matrix of weighted undirected graphs for each participant, within each analysed frequency range. Inferential statistics investigating group differences for edge weights were implemented using non-parametric permutation testing using the Network Based Statistic (NBS) Toolbox (Zalesky et al., 2010). Importantly, permutation testing does not require that the data distributions be normal.
NBS first applies an initial univariate threshold to each analysed edge (p < 0.05). The extent of connectivity components, defined as contiguous groups of nodes connected by suprathreshold connections, is then obtained. Group labels are then permuted and the extent of the largest component which occurs in this surrogated data is then recorded, and this process is repeated 5000 times to generate a null distribution. The ranking of connectivity components from the unshuffled data in the surrogate distribution is used to determine statistical confidence. Since the surrogate distribution considers the largest connectivity component that could occur, assuming the null hypothesis across the entire analysed network, this approach is controls for false positives due to multiple comparisons (see Zalesky et al., 2010; Zalesky et al., 2012). False positives due to multiple comparisons were controlled for using the NBS-Extent method, meaning that connectivity components were ranked according to the number of supra-threshold edges (inter-regional connections).
Network measures of node degree were used to assess the importance of a node within the connected graph component (integer sum of the number of significant edges connecting a node to the network), and these measures were obtained using Brain Connectivity Toolbox (Rubinov and Sporns, 2010). Brain networks were visualised and figures were produced using BrainNet Viewer (Xia et al., 2013). Further behavioural correlation analyses were conducted using MATLAB Statistics Toolbox (The Mathworks, Inc.).
3. Results
3.1. Cognitive-behavioural assessments and mTBI clinical information
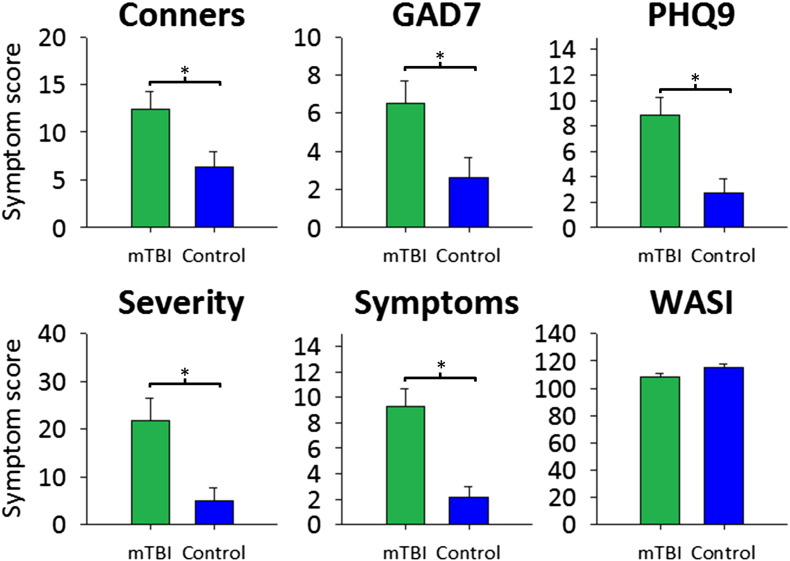
Measures for the cognitive-behavioural assessments using the Conners ADHD, GAD7, PHQ9, SCAT2 symptoms and severity, and WASI test outcomes in the mTBI group compared with the control group are presented in Fig. 2. The mTBI group showed significantly greater scores on attentional problems, anxiety, depression, number of concussion symptoms, and severity of concussion symptoms (p < 0.05). The groups were found to be matched on measures of intelligence. Table 1 shows mTBI patient demographic and clinical information, including symptom number and severity, days since injury, whether loss of consciousness occurred and for how long, Glasgow Coma Scale, post-traumatic amnesia, and mechanism of injury.
Fig. 2.
Mean and SD scores for the cognitive-behavioural assessments for the mTBI (green) and control (blue) groups. Conners: attention deficit and hyperactivity disorder; GAD7: Generalised Anxiety Disorder 7-item scale; PHQ9: Personalised Health Questionnaire 9-scale item for depression; severity: severity of concussion symptomology; symptoms: number of symptoms; WASI: Wechsler's Abbreviated Scale of Intelligence. *p < 0.05.
Table 1.
Patient demographics and clinical data.
| ID | Age | Symptoms | Severity | Days since injury | LOC | GCS | PTA | Mechanism |
|---|---|---|---|---|---|---|---|---|
| PT01 | 32 | 15 | 36 | 9 | No | 14 | No | Sports |
| PT02 | 41 | 0 | 0 | 27 | Altered | 14 | <24 h | Sports |
| PT03 | 22 | 2 | 9 | 30 | Yes | 14 | <24 h | MVC |
| PT04 | 27 | 5 | 8 | 47 | Yes | 15 | No | Pedestrian/car |
| PT05 | 21 | 7 | 9 | 38 | No | 15 | No | MVC |
| PT07 | 35 | 6 | 18 | 49 | No | 15 | No | MVC |
| PT08 | 26 | 5 | 8 | 22 | Yes (30 s) | 14 | Yes | Sports |
| PT09 | 22 | 1 | 1 | 51 | Yes | 15 | No | Fall |
| PT10 | 32 | 4 | 6 | 53 | Altered | 14 | No | Sports |
| PT11 | 40 | 13 | 27 | 62 | Yes (2 min) | 13 | Yes | Fall |
| PT12 | 38 | 22 | 75 | 45 | Yes (<1 min) | 15 | No | MVC |
| PT13 | 24 | 14 | 26 | 18 | No | 15 | Yes | Sports |
| PT14 | 44 | 5 | 6 | 34 | Yes (<1 min) | 14 | <24 h | Fall |
| PT15 | 37 | 8 | 18 | 26 | Yes (2 min) | 15 | No | MVC |
| PT16 | 35 | 20 | 74 | 10 | Yes (5 min) | 14 | <20 min | MVC |
| PT17 | 28 | 9 | 12 | 7 | Yes (2 min) | 15 | Yes | Sports |
| PT18 | 33 | 13 | 35 | 60 | No | 15 | No | Fall |
| PT19 | 25 | 12 | 27 | 35 | No | 15 | Yes | Work |
| PT20 | 30 | 10 | 16 | 11 | Altered | 14 | No | Accident |
| PT22 | 36 | 15 | 24 | 10 | Yes (2 min) | 14 | No | Sports |
| Mean | 31.40 | 9.30 | 21.75 | 32.20 | NA | 14.45 | NA | NA |
| SD | 6.85 | 6.11 | 20.88 | 17.98 | NA | 0.60 | NA | NA |
LOC, Loss of consciousness; GCS, Glasgow Coma Scale; PTA, Post-traumatic amnesia.
3.2. Resting-state spectral power
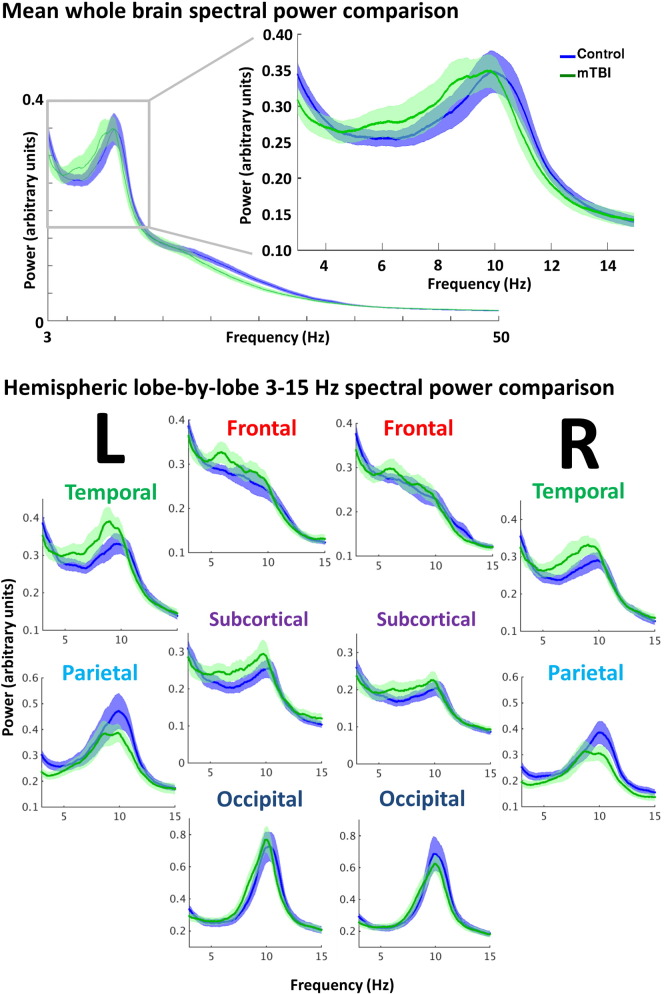
Spectral power estimates for 1–150 Hz were computed from each of the 90 seed regions for every participant, and averaged to derive mean, whole-brain power spectrum with which to compare the groups (Fig. 3, top). Visual assessment suggested minor, relative increases in spectral power for low-frequency oscillations for the mTBI group versus the control group, particularly in the 4–10 Hz range. Furthermore, averaging by lobe revealed comparative increases in the 4–10 Hz range that appeared pronounced in the temporal and subcortical regions; frontal and occipital spectral power were barely distinguishable between the groups, whilst the parietal regions showed relative decreases in power in the peak alpha frequency for the mTBI group (Fig. 3, bottom). Group differences in peak frequency and power in the 4–10 Hz range at the whole-brain level were not significant (Student's t, p > 0.05), but indicated a trend toward increased low-frequency power and slower peak oscillatory frequency in the mTBI group.
Fig. 3.
Spectral power content for the mTBI and control groups. Top: mean, whole-brain spectral power content with ±1 standard error bars, showing a minor increase in 4–10 Hz power (arbitrary units) in the mTBI. Bottom: mean spectral power content for the low-frequency 3–15 Hz range, divided by hemisphere and lobe for the mTBI versus control group. Minor alterations in spectral power were found in left and right temporal, subcortical, and parietal regions in the mTBI group.
3.3. Resting-state functional connectivity analyses
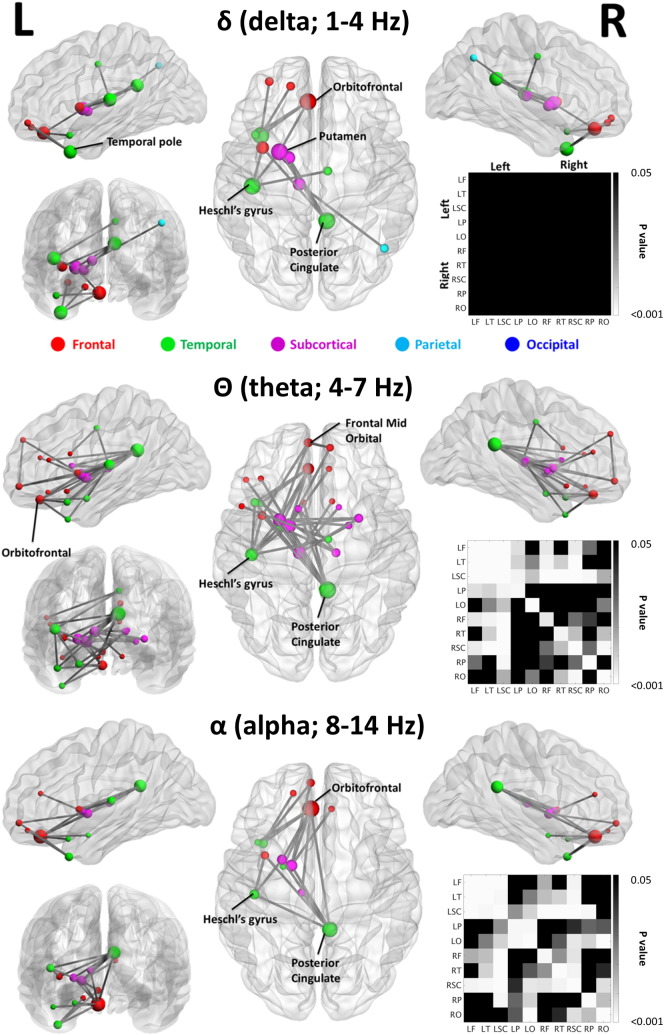
Increased delta, theta and alpha resting-state network amplitude envelope correlations were observed in the mTBI group compared to controls (p < 0.05, Fig. 4; node radius scaled by degree, or alternatively, ‘number of connections’); the higher frequency bands showed no group effects. The increases in amplitude correlations were found in similar networks across delta, theta and alpha frequency ranges, with the elevated theta-mediated network comprising the greatest number of significant nodes and edge weights. In the delta band, significant increases in amplitude correlations were observed in the left hemisphere, with connectivity differences in important nodes noted in the left frontal, left temporal, and subcortical regions, as well as the right posterior cingulate cortex (PCC). Similar network topologies are evident in both the theta (Fig. 4, middle) and alpha (Fig. 4, bottom) bands, although the theta band network displayed the greatest number of significant nodes and edges, with extensive alterations once again in left frontal, left temporal, and left subcortical regions, and right PCC. No significant differences were noted for Control > mTBI. Adjacency matrices depicting significant differences in lobe–lobe interactions for mTBI > Control can be seen accompanying the functional maps (Bonferroni-corrected at p < 0.05; null distributions generated using 100,000 permutations).
Fig. 4.
Functional connectivity maps of frequency-specific increases in amplitude envelope correlations in mTBI compared to controls, for the delta (1–4 Hz; top), theta (4–7 Hz; middle) and alpha (8–14 Hz; bottom) frequency bands. All images derived from the NBS-Extent method (p < 0.05, corrected). Additionally, adjacency matrices of p values for contrasts of intra- and inter-hemispheric lobe-by-lobe connectivity measures for mTBI versus control are shown to the right in each panel; grey-to- white indicates significant interactions for mTBI > Controls (Bonferroni-corrected at p < 0.05), black indicates no significant difference (p > 0.05, corrected).
3.4. Edge weight and seed strength correlates of cognitive-behavioural outcomes
In addition to these main MEG findings, we also conducted an exploratory analysis of patterns of connectivity most associated with variability in secondary symptoms of mTBI. A whole-brain, data-driven approach was implemented to quantify the relation between resting-state connectivity and cognitive-behavioural outcomes in the mTBI group. Specifically, we examined symptoms of ADHD (and in particular the inattention subscale), anxiety, and depression. Data were not corrected for multiple comparisons given the exploratory, hypothesis-free nature of the analysis, but images were thresholded with moderate-to-high correlation values of r > 0.6, and an uncorrected p < 0.01.
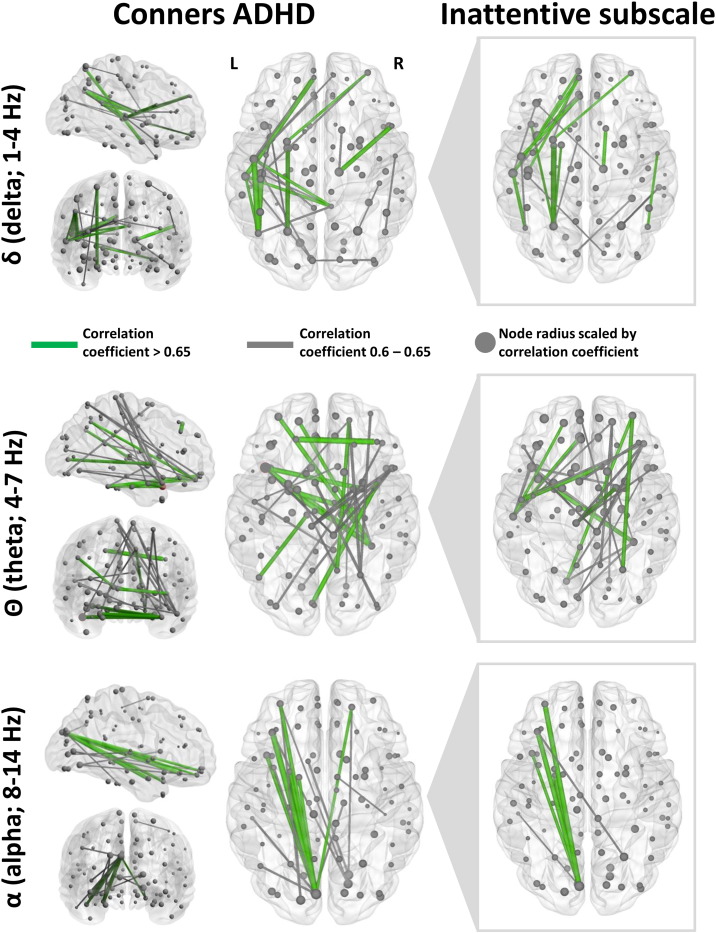
Edge weight (amplitude envelope cross-correlation coefficient) and node strength (summation of undirected edge weights of seed to the network) were correlated against the Conners ADHD outcome, and results are shown in Fig. 5 (green connections: correlation coefficient > 0.65, uncorrected p < 0.01; grey connections, correlation coefficient > 0.6, uncorrected p < 0.01; node radius scaled by correlation coefficient). Associations between neurophysiological network connectivity and Conners ADHD cognitive outcome were found in the delta, theta and alpha frequency bands. In the delta band, problems with attention and hyperactivity were associated with increased amplitude coupling in the left parietal, temporal and frontal regions. Furthermore, these patterns of spatial correlations were found to be most strongly associated with the Inattentive Subscale of the Conners ADHD assessment, suggesting that deficits in concentration ability and focus were driving the associations between increased network connectivity and Conners ADHD scores in mTBI. In the theta band, correlations were observed between Conners ADHD outcome and amplitude correlations in right frontal, left fronto-temporal, and right parietal regions. Network connectivity correlation patterns appear markedly different in those observed for the alpha range, with left occipito-frontal connectivity most associated with attentional problems in this frequency band. This exploratory analysis suggests that multiple network and frequency-specific alterations in large scale brain connectivity may contribute to overlapping cognitive sequelae in mTBI.
Fig. 5.
Correlations of amplitude envelope coupling (edge weights) and node network strength versus Conners ADHD attentional outcome measures in mTBI for the delta, theta and alpha bands. Left panels show correlations for the Conners ADHD and connectivity correlations, and right panels show the inattentive subscale correlations. Note the high degree of overlap between the correlational networks, suggesting inattention problems in the mTBI are driving the correlational networks shown in the Conners ADHD outcome measure. Node radius is scaled by correlation coefficient. Edge weights show correlation coefficients with uncorrected p < 0.01; grey edges correlation coefficients > 0.6, green edges correlation coefficients > 0.65.
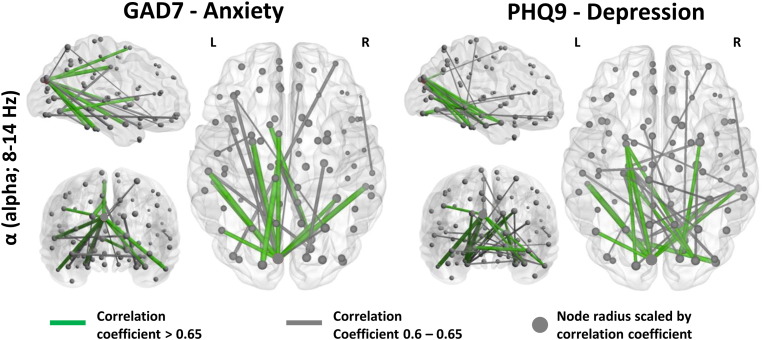
Additionally, Fig. 6 shows associations between increased neurophysiological network connectivity and GAD7 measures of anxiety and PHQ9 scores for depression in participants with mTBI, although these networks were largely absent in the delta and theta bands (as before, green connections: correlation coefficient > 0.65, uncorrected p < 0.01; grey connections, correlation coefficient > 0.6, uncorrected p < 0.01; node radius scaled by correlation coefficient). These networks appeared similar in spatial extent and distribution, with a high-degree node correlation observed in the left occipital cortex that was connected with bilateral temporal and subcortical regions for both cognitive outcomes.
Fig. 6.
Correlations of amplitude envelope coupling (edge weights) and node network strength versus GAD7 (anxiety; left panels) and PHQ9 (depression; right panels) cognitive-behavioural outcome measures in the alpha band in the mTBI group. These correlational networks appear similar, and are comparable to the Conners ADHD network in the alpha band. Node radius is scaled by correlation coefficient. Edge weights show correlation coefficients with uncorrected p < 0.01; grey edges correlation coefficients > 0.6, green edges correlation coefficients > 0.65.
4. Discussion
4.1. Summary
This study provides the first evidence that band-limited alterations in neuronal network amplitude coupling are associated with mild traumatic brain injury. First, we report a trend toward increased low-frequency amplitude in patients with mTBI, and this spectral alteration appeared most prominently in temporal and deep-grey regions, consistent with alpha ‘slowing’ which has previously been shown in numerous studies to signify brain injury (Huang et al., 2014; Lewine et al., 1999; Lewine et al., 2007). Moreover, alpha spectral showed a trend toward being reduced in parietal regions in mTBI. Second, we uniquely demonstrate that mTBI patients show increased low-frequency amplitude envelope correlations among brain regions compared to healthy controls, known as ‘amplitude envelope intrinsic coupling modes’ (Engel et al., 2013), and that temporal and subcortical regions were again involved, along with a number of frontal areas. Third, we conducted an exploratory analysis (uncorrected for multiple comparisons) to examine correlations of amplitude covariations, and how these were related to cognitive-behavioural outcomes, whereby we observed a number of frequency bands that were potentially related to individual variability in disorders of attention, a common complaint of patients (Max et al., 1998; Whyte et al., 1995; Whyte and DiPasquale, 1995). Additional correlational networks between connectivity and symptoms were also identified in the alpha band for anxiety and depression, and interestingly, these comorbid correlational networks were strikingly similar across both cognitive conditions.
In terms of the cognitive difficulties associated with altered network connectivity in mTBI, our results suggest that attentional problems may be mediated by a number of distinct systems across various canonical frequency bands, which serve to highlight the complexity of attentional regulation and its dysfunction. When examining networks that seem related to anxiety and depression, these were principally found in the alpha band, and these systems appeared topologically equivalent in this rhythm. Together this suggests that (1) at the behavioural level, the symptoms measured here highly covary in those with mTBI (high comorbidity), and (2), at the neurophysiological level, attentional control and its dysfunction reside in a variety of broad-band neuronal networks, whilst anxiety and depression are mediated by comparable frequency-specific neural systems.
More generally, these results indicate that some of the disorders of cognition in mTBI may not be associated with just one particular region, or even a specific network, but a variety of connections spanning multiple frequencies, as well as topological and spatial scales. To our knowledge, this is the first evidence that altered network interactions in the low-frequency rhythms distinguish mTBI from healthy brain function, and that these interactions are associated with individual variability in mTBI in disorders of anxiety, depression, and attention in particular.
4.2. Cognitive-behavioural outcome and spectral power content
In relation to our cognitive-behavioural assessment measures, and consistent with other studies (Bryant et al., 2010), we observed increased incidences of attentional problems (Max et al., 1998; Whyte et al., 1995; Whyte and DiPasquale, 1995), anxiety (Hiott and Labbate, 2002; Landre et al., 2006; Moore et al., 2006), and depression (Guskiewicz et al., 2007; Jorge et al., 2004; Kreutzer et al., 2001) in participants with mTBI, whilst estimates of IQ appeared comparable between the groups. In contrast to the non-mTBI controls, the mTBI group presented spectral power content alterations in low-frequency rhythms, with prominent increased theta and alpha power in temporal and deep-grey regions, corroborating prior reports (Huang et al., 2009; Lewine et al., 1999; Lewine et al., 2007; Tarapore et al., 2013). Recently, other groups have used atypical slow-wave power content to accurately and objectively identify mTBI on a single-subject basis, and found that its presence in cortical regions has been shown to be related to some of the secondary psychological sequelae evident in the disorder (Huang et al., 2014), including personality changes, trouble concentrating, and depression. In terms of the neurophysiological mechanisms that give rise to such modulation of spectral power content (particularly slow-wave generation), it has been hypothesised to be related to white matter structural alterations, such as deafferentation as a result of axonal injury (Ball et al., 1977; Gloor et al., 1977; Huang et al., 2007), which is thought to disrupt thalamo- and cortico-cortical communication. This proposal was supported recently when it was shown that abnormal MEG slow-waves generated in the grey matter of patients with mTBI were connected by white matter that showed reduced diffusion tensor imaging (DTI) measures of fractional anisotropy (FA) (Davenport et al., 2012).
It has further been posited that slow-wave generation might be induced by cholinergic alteration (Huang et al., 2014). This supposition was based on research that indicated slow-wave increases could be induced by administration of atropine, an antagonist of acetylcholine receptors and a blocker of cholinergic transmission (Schaul, 1998; Schaul et al., 1978). Given this, Huang and colleagues theorised that a blockage in and/or limitations to cholinergic transmission (in addition to axonal injury) might also contribute to slow-wave generation, connectivity deficits, and subsequent cognitive sequelae (Huang et al., 2014).
We also observed a trend toward relative decreases in parietal alpha for mTBI, and prior studies have shown that alpha power reflects cognitive components frequently affected in mTBI, such as response inhibition (Roche et al., 2004), working memory (Kumar et al., 2009; McAllister et al., 2001) and attentional control (Dockree et al., 2004). Normative studies have highlighted the importance of alpha-band connectivity in the fronto-parietal control network (Sadaghiani et al., 2012) and working memory (Klimesch, 1999; Klimesch et al., 1993; Klimesch et al., 1994), and these phenomenological observations support the thesis that cognitive deficits in these patients are due to defective alpha generators. These studies and our own results serve to highlight the critical nature of alpha regulation in efficient cognitive control and how this is impacted in mTBI.
4.3. Increased inter-regional amplitude correlations in mTBI
We provide the first evidence that mTBI patients show increased inter-regional correlations in the amplitude resting-state oscillatory activity compared to controls. In particular, regional amplitude coupling, putatively termed ‘amplitude envelope intrinsic coupling modes’, or ICMs, (Engel et al., 2013), was greater in the delta, theta and alpha bands. The role of this particular mechanism in cortical communication can be exemplified by contrasting its role with neuronal phase synchronisation, or ‘phase intrinsic coupling modes’ (Engel et al., 2013), a communication mode mediated by phase coupling rather than regional amplitude correlations.
Regarding the roles of these apparently subtle, but functionally-distinct, mechanisms, amplitude envelope ICMs have been hypothesised to be largely related to structural connectivity (Greicius et al., 2009; Vincent et al., 2007), with a low dependence on cognitive state, and for functional roles, be a regulatory mechanism for the temporal coordination of activation in brain regions. In contrast to this, ‘phase ICMs’ have a highly variable relation to the physical structure of the network (Engel and Fries, 2010; Siegel et al., 2012), and are for the most part implicated in functional brain network alterations (as opposed to structural) (Honey et al., 2007), as well as displaying a high cognitive state-dependence, and are thought to be regulatory in the integration of cognitive contents across regions (Dunkley et al., 2014; Engel et al., 2013). Therefore, in terms of the group differences we witness in amplitude ICM elevations in mTBI, we propose that these alterations are closely related to structural changes that subsequently impart secondary alterations to cognition; this is discussed below.
4.4. Brain–behaviour associations
In the mTBI group we observed associations between inter-regional amplitude coupling and cognitive outcome, expressed across a variety of canonical frequencies. First, network metrics that correlate with disorders of attention were found across distinct frequency and spatial scales, with highly variable patterns of connections that were associated with attention (and foremost, difficulties in concentration). Second, we observed patterns of correlational inter-regional amplitude coupling that were similarly related to anxiety and depression, in this case being expressed within the alpha frequency band.
The spatial distribution and non-frequency specific connectivity correlates of attentional control serve to highlight the complex nature of its network substrates. A number of studies have implicated a widely-distributed set of regions, in a variety of networks, across multiple components of attention (Posner and Dehaene, 1994; Raz, 2004; Raz and Buhle, 2006), and across multiple frequency bands (Fan et al., 2007; Fan et al., 2009). Taken together, these studies and our own indicated that head injury results in diffuse, heterogeneous alterations to network connectivity. The high-degree of similarity between anxiety and depression is likely related to the high-degree of comorbidity/incidence of the two disorders and their overlapping neural substrates (Bjelland et al., 2002; Brady and Kendall, 1992; Clarke et al., 1991; Wittchen et al., 2002).
4.5. Future directions
Here we present evidence that mTBI is associated with increased inter-regional correlations between spontaneous neuromagnetic oscillations. However, it is known that there are large dynamic changes that occur during the extended recovery period after injury which includes acute, sub-acute and chronic stages of concussion and comorbities. Variability within these sub-stages of injury and recovery may contribute to the altered network topologies observed here, and these alterations may further distinguish these subgroups; collapsing across these sub-stages may weaken any potential hallmarks of recovery. To address this problem, further study using a larger cohort in a longitudinal design will be required to investigate the relation between injury, recovery, other clinical variables and MEG resting-state connectivity. Further investigation with a larger cohort would also permit a more comprehensive and rigorous investigation of relations between spontaneous MEG networks, functional outcome and relevant clinical factors.
4.6. Conclusions
This study provides the first evidence that mTBI patients can be distinguished from a matched control population by alterations to regional connectivity in low-frequency inter-regional amplitude correlations. Additionally, we used a whole-brain data-driven approach to demonstrate that outcome measures examining disorders of attention, anxiety problems and depression in those with head injury correlate with a variety of distinct frequency-specific networks across the brain, which more generally serves to highlight the complex nature of atypical connectivity that can contribute to these psychological sequelae often seen in mTBI.
Conflicts of interest
The authors declare no competing financial interests or potential conflicts of interest.
Acknowledgements
Thanks are given to Amanda Robertson and Marc Lalancette for their help in the data collection, and Daniel Cassel for his help with the data analysis pipeline. This work was supported by funding from Defence Research and Development Canada (DRDC) and the Canadian Forces Health Services to MJT and EWP (contract # W7719-135182/001/TOR).
References
- Ball G.J., Gloor P., Schaul N. The cortical electromicrophysiology of pathological delta waves in the electroencephalogram of cats. Electroencephalogr. Clin. Neurophysiol. 1977;43(3):346–361. doi: 10.1016/0013-4694(77)90258-9. 70336 [DOI] [PubMed] [Google Scholar]
- Binder L.M., Rohling M.L., Larrabee G.J. A review of mild head trauma. Part I: meta-analytic review of neuropsychological studies. J. Clin. Exp. Neuropsychol. 1997;19(3):421–431. doi: 10.1080/01688639708403870. 9268816 [DOI] [PubMed] [Google Scholar]
- Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J. Psychosom. Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. 11832252 [DOI] [PubMed] [Google Scholar]
- Brady E.U., Kendall P.C. Comorbidity of anxiety and depression in children and adolescents. Psychol. Bull. 1992;111(2):244–255. doi: 10.1037/0033-2909.111.2.244. 1557475 [DOI] [PubMed] [Google Scholar]
- Brookes M.J., Hale J.R., Zumer J.M., Stevenson C.M., Francis S.T., Barnes G.R., Owen J.P., Morris P.G., Nagarajan S.S. Measuring functional connectivity using MEG: methodology and comparison with fcMRI. Neuroimage. 2011;56(3):1082–1104. doi: 10.1016/j.neuroimage.2011.02.054. 21352925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes M.J., Woolrich M., Luckhoo H., Price D., Hale J.R., Stephenson M.C., Barnes G.R., Smith S.M., Morris P.G. Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc. Natl. Acad. Sci. U. S. A. 2011;108(40):16783–16788. doi: 10.1073/pnas.1112685108. 21930901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R.A., O'Donnell M.L., Creamer M., McFarlane A.C., Clark C.R., Silove D. The psychiatric sequelae of traumatic injury. Am. J. Psychiatry. 2010;167(3):312–320. doi: 10.1176/appi.ajp.2009.09050617. 20048022 [DOI] [PubMed] [Google Scholar]
- Clarke D.M., Minas I.H., Stuart G.W. The prevalence of psychiatric morbidity in general hospital inpatients. Aust. N. Z. J. Psychiatry. 1991;25:322–329. doi: 10.3109/00048679109062632. 1845255 [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A., Barkhof F., Scheltens P., Stam C.J., Smith S.M. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U S A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. 16945915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport N.D., Lim K.O., Armstrong M.T., Sponheim S.R. Diffuse and spatially variable white matter disruptions are associated with blast-related mild traumatic brain injury. Neuroimage. 2012;59(3):2017–2024. doi: 10.1016/j.neuroimage.2011.10.050. 22040736 [DOI] [PubMed] [Google Scholar]
- Dockree P.M., Kelly S.P., Roche R.A., Hogan M.J., Reilly R.B., Robertson I.H. Behavioural and physiological impairments of sustained attention after traumatic brain injury. Brain Res. Cogn. Brain Res. 2004;20(3):403–414. doi: 10.1016/j.cogbrainres.2004.03.019. 15268918 [DOI] [PubMed] [Google Scholar]
- Engel A.K., Fries P. Beta-band oscillations — signalling the status quo? Curr. Opin. Neurobiol. 2010;20(2):156–165. doi: 10.1016/j.conb.2010.02.015. 20359884 [DOI] [PubMed] [Google Scholar]
- Engel A.K., Gerloff C., Hilgetag C.C., Nolte G. Intrinsic coupling modes: multiscale interactions in ongoing brain activity. Neuron. 2013;80(4):867–886. doi: 10.1016/j.neuron.2013.09.038. 24267648 [DOI] [PubMed] [Google Scholar]
- Fan J., Byrne J., Worden M.S., Guise K.G., McCandliss B.D., Fossella J. The relation of brain oscillations to attentional networks. J. Neurosci. 2007;27(23):6197–6206. doi: 10.1523/JNEUROSCI.1833-07.2007. 17553991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Gu X., Guise K.G., Liu X., Fossella J., Wang H. Testing the behavioral interaction and integration of attentional networks. Brain Cogn. 2009;70(2):209–220. doi: 10.1016/j.bandc.2009.02.002. 19269079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor P., Ball G., Schaul N. Brain lesions that produce delta waves in the EEG. Neurology. 1977;27(4):326–333. doi: 10.1212/wnl.27.4.326. 557774 [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Supekar K., Menon V., Dougherty R.F. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex. 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. 18403396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz K.M., Marshall S.W., Bailes J., McCrea M., Harding H.P., Jr., Matthews A. Recurrent concussion and risk of depression in retired professional football players. Med. Sci. Sports Exerc. 2007;39(6):903–909. doi: 10.1249/mss.0b013e3180383da5. 17545878 [DOI] [PubMed] [Google Scholar]
- Hari R., Salmelin R. Magnetoencephalography: from SQUIDs to neuroscience. Neuroimage 20th anniversary special edition. Neuroimage. 2012;61(2):386–396. doi: 10.1016/j.neuroimage.2011.11.074. 22166794 [DOI] [PubMed] [Google Scholar]
- Hiott D.W., Labbate L. Anxiety disorders associated with traumatic brain injuries. Neurorehabilitation. 2002;17(4):345–355. 12547982 [PubMed] [Google Scholar]
- Hipp J.F., Hawellek D.J., Corbetta M., Siegel M., Engel A.K. Large-scale cortical correlation structure of spontaneous oscillatory activity. Nat. Neurosci. 2012;15(6):884–890. doi: 10.1038/nn.3101. 22561454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey C.J., Kötter R., Breakspear M., Sporns O. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc. Natl. Acad. Sci. U S A. 2007;104(24):10240–10245. doi: 10.1073/pnas.0701519104. 17548818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.X., Nichols S., Baker D.G., Robb A., Angeles A., Yurgil K.A. Single-subject-based whole-brain MEG slow-wave imaging approach for detecting abnormality in patients with mild traumatic brain injury. Neuroimage Clin. 2014;5:109–119. doi: 10.1016/j.nicl.2014.06.004. 25009772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.X., Song T., Hagler D.J., Jr., Podgorny I., Jousmaki V., Cui L. A novel integrated MEG and EEG analysis method for dipolar sources. Neuroimage. 2007;37(3):731–748. doi: 10.1016/j.neuroimage.2007.06.002. 17658272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.X., Theilmann R.J., Robb A., Angeles A., Nichols S., Drake A. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J. Neurotrauma. 2009;26(8):1213–1226. doi: 10.1089/neu.2008.0672. 19385722 [DOI] [PubMed] [Google Scholar]
- Dunkley B.T., Doesburg S.M., Sedge P.A., Grodecki R.J., Shek P.N., Pang E.W., Taylor M.J. Resting-state hippocampal connectivity correlates with symptom severity in post-traumatic stress disorder. Neuroimage Clin. 2014;5:377–384. doi: 10.1016/j.nicl.2014.07.017. 25180157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge R.E., Robinson R.G., Moser D., Tateno A., Crespo-Facorro B., Arndt S. Major depression following traumatic brain injury. Arch. Gen. Psychiatry. 2004;61(1):42–50. doi: 10.1001/archpsyc.61.1.42. 14706943 [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Brain Res. Rev. 1999;29(2–3):169–195. doi: 10.1016/s0165-0173(98)00056-3. 10209231 [DOI] [PubMed] [Google Scholar]
- Klimesch W., Schimke H., Pfurtscheller G. Alpha frequency, cognitive load and memory performance. Brain Topogr. 1993;5(3):241–251. doi: 10.1007/BF01128991. 8507550 [DOI] [PubMed] [Google Scholar]
- Klimesch W., Schimke H., Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalogr. Clin. Neurophysiol. 1994;91(6):428–441. doi: 10.1016/0013-4694(94)90164-3. 7529682 [DOI] [PubMed] [Google Scholar]
- Kreutzer J.S., Seel R.T., Gourley E. The prevalence and symptom rates of depression after traumatic brain injury: a comprehensive examination. Brain Inj. 2001;15(7):563–576. doi: 10.1080/02699050010009108. 11429086 [DOI] [PubMed] [Google Scholar]
- Kumar S., Rao S.L., Chandramouli B.A., Pillai S.V. Reduction of functional brain connectivity in mild traumatic brain injury during working memory. J. Neurotrauma. 2009;26(5):665–675. doi: 10.1089/neu.2008.0644. 19331523 [DOI] [PubMed] [Google Scholar]
- Landre N., Poppe C.J., Davis N., Schmaus B., Hobbs S.E. Cognitive functioning and postconcussive symptoms in trauma patients with and without mild TBI. Arch. Clin. Neuropsychol. 2006;21(4):255–273. doi: 10.1016/j.acn.2005.12.007. 16716563 [DOI] [PubMed] [Google Scholar]
- Levin H.S., Amparo E., Eisenberg H.M., Williams D.H., High W.M., Jr., McArdle C.B. Magnetic resonance imaging and computerized tomography in relation to the neurobehavioral sequelae of mild and moderate head injuries. J. Neurosurg. 1987;66(5):706–713. doi: 10.3171/jns.1987.66.5.0706. 3572497 [DOI] [PubMed] [Google Scholar]
- Lewine J.D., Davis J.T., Bigler E.D., Thoma R., Hill D., Funke M. Objective documentation of traumatic brain injury subsequent to mild head trauma: multimodal brain imaging with MEG, SPECT, and MRI. J. Head Trauma Rehabil. 2007;22(3):141–155. doi: 10.1097/01.HTR.0000271115.29954.27. 17510590 [DOI] [PubMed] [Google Scholar]
- Lewine J.D., Davis J.T., Sloan J.H., Kodituwakku P.W., Orrison W.W., Jr. Neuromagnetic assessment of pathophysiologic brain activity induced by minor head trauma. A.J.N.R. Am. J. Neuroradiol. 1999;20(5):857–866. 10369357 [PMC free article] [PubMed] [Google Scholar]
- Max J.E., Arndt S., Castillo C.S., Bokura H., Robin D.A., Lindgren S.D. Attention-deficit hyperactivity symptomatology after traumatic brain injury: a prospective study. J. Am. Acad. Child Adolesc. Psychiatry. 1998;37(8):841–847. doi: 10.1097/00004583-199808000-00014. 9695446 [DOI] [PubMed] [Google Scholar]
- McAllister T.W., Sparling M.B., Flashman L.A., Guerin S.J., Mamourian A.C., Saykin A.J. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14(5):1004–1012. doi: 10.1006/nimg.2001.0899. 11697932 [DOI] [PubMed] [Google Scholar]
- Moore E.L., Terryberry-Spohr L., Hope D.A. Mild traumatic brain injury and anxiety sequelae: a review of the literature. Brain Inj. 2006;20(2):117–132. doi: 10.1080/02699050500443558. 16421060 [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D. High-frequency brain activity and muscle artifacts in MEG/EEG: a review and recommendations. Front. Hum. Neurosci. 2013;7:138. doi: 10.3389/fnhum.2013.00138. 23596409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi S.N., Mukherjee P., Ghajar J., Johnson C., Kolster R.A., Sarkar R. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3 T diffusion tensor imaging study of mild traumatic brain injury. A.J.N.R. Am. J. Neuroradiol. 2008;29(5):967–973. doi: 10.3174/ajnr.A0970. 18272556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva J.M., Palva S., Kaila K. Phase synchrony among neuronal oscillations in the human cortex. J. Neurosci. 2005;25(15):3962–3972. doi: 10.1523/JNEUROSCI.4250-04.2005. 15829648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M.I., Dehaene S. Attentional networks. Trends Neurosci. 1994;17(2):75–79. doi: 10.1016/0166-2236(94)90078-7. 7512772 [DOI] [PubMed] [Google Scholar]
- Quraan M.A., Cheyne D. Reconstruction of correlated brain activity with adaptive spatial filters in MEG. Neuroimage. 2010;49(3):2387–2400. doi: 10.1016/j.neuroimage.2009.10.012. 19850135 [DOI] [PubMed] [Google Scholar]
- Raz A. Anatomy of attentional networks. Anat. Rec. B New Anat. 2004;281(1):21–36. doi: 10.1002/ar.b.20035. 15558781 [DOI] [PubMed] [Google Scholar]
- Raz A., Buhle J. Typologies of attentional networks. Nat. Rev. Neurosci. 2006;7(5):367–379. doi: 10.1038/nrn1903. 16760917 [DOI] [PubMed] [Google Scholar]
- Roche R.A., Dockree P.M., Garavan H., Foxe J.J., Robertson I.H., O'Mara S.M. EEG alpha power changes reflect response inhibition deficits after traumatic brain injury (TBI) in humans. Neurosci. Lett. 2004;362(1):1–5. doi: 10.1016/j.neulet.2003.11.064. 15147767 [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. 19819337 [DOI] [PubMed] [Google Scholar]
- Ryan L.M., Warden D.L. Post concussion syndrome. Int. Rev. Psychiatry. 2003;15(4):310–316. doi: 10.1080/09540260310001606692. 15276952 [DOI] [PubMed] [Google Scholar]
- Sadaghiani S., Scheeringa R., Lehongre K., Morillon B., Giraud A.L., D'Esposito M. Alpha-band phase synchrony is related to activity in the fronto-parietal adaptive control network. J. Neurosci. 2012;32(41):14305–14310. doi: 10.1523/JNEUROSCI.1358-12.2012. 23055501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaul N. The fundamental neural mechanisms of electroencephalography. Electroencephalogr. Clin. Neurophysiol. 1998;106(2):101–107. doi: 10.1016/s0013-4694(97)00111-9. 9741769 [DOI] [PubMed] [Google Scholar]
- Schaul N., Gloor P., Ball G., Gotman J. The electromicrophysiology of delta waves induced by systemic atropine. Brain Res. 1978;143(3):475–486. doi: 10.1016/0006-8993(78)90358-x. 647373 [DOI] [PubMed] [Google Scholar]
- Siegel M., Donner T.H., Engel A.K. Spectral fingerprints of large-scale neuronal interactions. Nat. Rev. Neurosci. 2012;13(2):121–134. doi: 10.1038/nrn3137. 22233726 [DOI] [PubMed] [Google Scholar]
- Tarapore P.E., Findlay A.M., Lahue S.C., Lee H., Honma S.M., Mizuiri D. Resting state magnetoencephalography functional connectivity in traumatic brain injury. J. Neurosurg. 2013;118(6):1306–1316. doi: 10.3171/2013.3.JNS12398. 23600939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewarie P., Schoonheim M.M., Stam C.J., van der Meer M.L., van Dijk B.W., Barkhof F. Cognitive and clinical dysfunction, altered MEG resting-state networks and thalamic atrophy in multiple sclerosis. PLOS One. 2013;8(7):e69318. doi: 10.1371/journal.pone.0069318. 23935983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. 11771995 [DOI] [PubMed] [Google Scholar]
- Vincent J.L., Patel G.H., Fox M.D., Snyder A.Z., Baker J.T., Van Essen D.C. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. 17476267 [DOI] [PubMed] [Google Scholar]
- Wang X.J. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol. Rev. 2010;90(3):1195–1268. doi: 10.1152/physrev.00035.2008. 20664082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte J., DiPasquale M.C. Assessment of vision and visual attention in minimally responsive brain injured patients. Arch. Phys. Med. Rehabil. 1995;76(9):804–810. doi: 10.1016/s0003-9993(95)80543-5. 7668949 [DOI] [PubMed] [Google Scholar]
- Whyte J., Polansky M., Fleming M., Coslett H.B., Cavallucci C. Sustained arousal and attention after traumatic brain injury. Neuropsychologia. 1995;33(7):797–813. doi: 10.1016/0028-3932(95)00029-3. 7477808 [DOI] [PubMed] [Google Scholar]
- Wittchen H.U., Kessler R.C., Beesdo K., Krause P., Höfler M., Hoyer J. Generalized anxiety and depression in primary care: prevalence, recognition, and management. J. Clin. Psychiatry. 2002;63(Suppl. 8):24–34. 12044105 [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet viewer: a network visualization tool for human brain connectomics. PLOS One. 2013;8(7):e68910. doi: 10.1371/journal.pone.0068910. 23861951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Rasmussen I.A., Lagopoulos J., Håberg A. Diffuse axonal injury in severe traumatic brain injury visualized using high-resolution diffusion tensor imaging. J. Neurotrauma. 2007;24(5):753–765. doi: 10.1089/neu.2006.0208. 17518531 [DOI] [PubMed] [Google Scholar]
- Zalesky A., Cocchi L., Fornito A., Murrary M.M., Bullmore E.T. Connecticity differences in brain networks. NeuroImage. 2012;60(2):1055–1062. doi: 10.1016/j.neuroimage.2012.01.068. [DOI] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53(4):1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. 20600983 [DOI] [PubMed] [Google Scholar]