Abstract
The extracellular matrix (ECM) plays a significant role in cellular and retinal health. The study of retinal tyrosine-sulfated proteins is an important first step toward understanding the role of ECM in retinal health and diseases. These secreted proteins are members of the retinal ECM. Tyrosine sulfation was shown to be necessary for the development of proper retinal structure and function. The importance of tyrosine sulfation is further demonstrated by the evolutionary presence of tyrosylprotein sulfotransferases, enzymes that catalyze proteins’ tyrosine sulfation, and the compensatory abilities of these enzymes. Research has identified four tyrosine-sulfated retinal proteins: fibulin 2, vitronectin, complement factor H (CFH), and opticin. Vitronectin and CFH regulate the activation of the complement system and are involved in the etiology of some cases of age-related macular degeneration. Analysis of the role of tyrosine sulfation in fibulin function showed that sulfation influences the protein's ability to regulate growth and migration. Although opticin was recently shown to exhibit anti-angiogenic properties, it is not yet determined what role sulfation plays in that function.
Future studies focusing on identifying all of the tyrosine-sulfated retinal proteins would be instrumental in determining the impact of sulfation on retinal protein function in retinal homeostasis and diseases.
Keywords: tyrosine sulfation, retina, extracellular matrix, retinal diseases
INTRODUCTION
Tyrosine is a non-essential amino acid that is a precursor for melanin, neurotransmitters, and hormones. As a member of a peptide, a tyrosine may undergo nitration, phosphorylation, or sulfation as post-translational modifications (PTMs) that ultimately influence the function of a protein. These tyrosine-specific PTMs (Figure 1) are made possible by the presence of a side chain hydroxyl group. Nitrotyrosine, a non-reversible modification, is formed from the interaction of reactive nitrogen species with peptide bound tyrosines. Nitrotyrosine is undetected in normal subjects, but detected, among others, in keratoconus (Buddi et al. 2002) and diabetes (Pacher et al. 2005). While nitration is a non-enzymatic reaction, both phosphorylation and sulfation occur through the actions of kinases and tyrosylprotein sulfotransferases (TPSTs), respectively. While tyrosine phosphorylation is involved in signal transduction, tyrosine sulfation is important for protein-protein interactions (Moore, 2003). Although tyrosine phosphorylation can be identified by state of the art mass spectroscopy, tyrosine sulfation is labile to mass spectrometry conditions (Wolfender et al. 1999; Önnerfjord et al 2004). Furthermore, unlike phosphorylation, tyrosine sulfation is a permanent modification. There is no known sulfatase that can remove the sulfate from a tyrosine.
Figure 1. Known biologically significant tyrosine-specific post-translational modification to proteins.
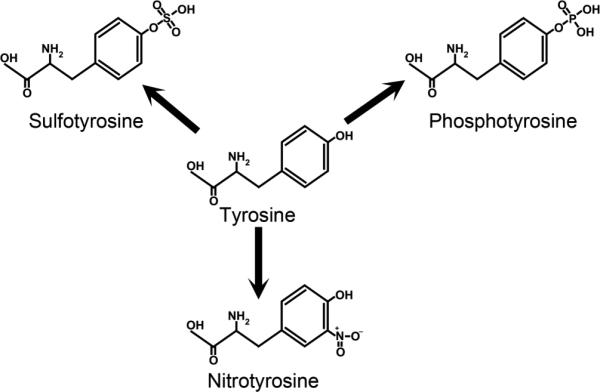
Tyrosine undergoes enzymatic sulfation and phosphorylation and non-enzymatic nitration that is mediated by reactive nitrogen species.
Although tyrosine sulfation is widely used by plants and animals (Moore, 2003), its biological significance is not fully understood, partly due to the difficulty in studying this process. What makes this PTM interesting is the fact that tyrosine-sulfated proteins are secreted to either 1) become independent members of the extracellular matrix, 2) to directly re-associate with the cells, or 3) to indirectly re-associate with the cells through interaction with membrane-bound proteins (Figure 2).
Figure 2. Pathway of tyrosine sulfation.
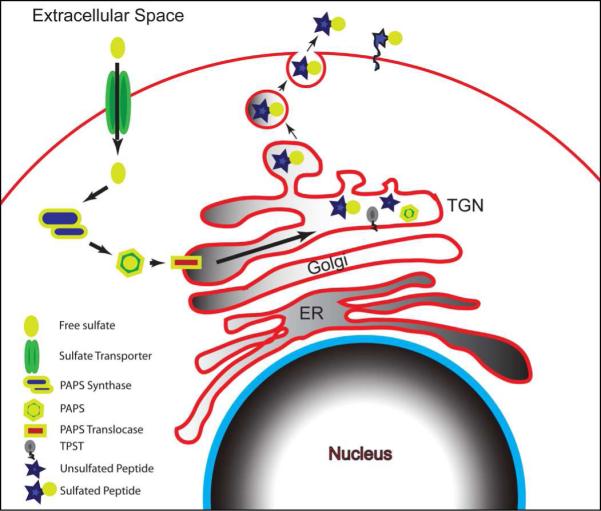
Free sulfate enter the cell via sulfate transporters and are rapidly converted to 3’-phosphoadenosine 5’-phosphosulfate (PAPS) by 3’-phosphoadenosine 5’-phosphosulfate synthase. 3’-phosphoadenosine 5’-phosphosulfate is then translocated into the trans-Golgi through 3’-phosphoadenosine 5’-phosphosulfate translocase. This allows the tyrosylprotein sulfotransferase to then add the free sulfate from the high energy source, 3’-phosphoadenosine 5’-phosphosulfate, to a newly synthesized protein. The tyrosine sulfated protein is secreted to become a free member of the extracellular matrix or re-associate with the producing cell directly or through interactions with membrane proteins.
Tyrosine sulfation is catalyzed by two independent tyrosylprotein sulfotransferases (TPSTs) that are bound to the trans-Golgi network membrane, with their active sites residing in the lumen (Niehrs and Huttner, 1990; Niehrs et al. 1992). TPSTs transfer the sulfuryl group from the sulfate carrier, 3’-phosphoadenosine 5’-phosphosulfate, onto tyrosine residues in proteins (Figure 2; Suiko et al. 1992). Mice lacking either of the two TPSTs (Tpst1−/− or Tpst2−/−) have been generated and characterized (Borghei et al. 2006; Ouyang et al. 2002; Westmuckett et al. 2008). Tpst1−/− mice, on average, weigh approximately 5% less than controls, and the knockout females produce smaller litters because of embryonic deaths (Ouyang et al. 2002). Tpst2−/− mice, on the other hand, exhibit a moderate growth delay but achieve normal body weight by the age of 10 weeks. However, the males are infertile (Borghei et al. 2006). Although they exhibit minor developmental phenotypes, the life spans of both Tpst1−/− and Tpst2−/− mice are normal. However, Tpst1−/−/Tpst2−/− double knockout (Tpst/DKO) mice die soon after birth, and few survive beyond weaning (Westmuckett et al. 2008). These mice succumb to cardio-pulmonary insufficiency, demonstrating the importance of tyrosine sulfation for animal viability (Westmuckett et al. 2008).
Function of tyrosine sulfation
Tyrosine-sulfated proteins belong to multiple families, including coagulation factors, chemokine receptors, extracellular matrix proteins, hormone receptors, G-Protein coupled receptors, and serpins (Costagliola et al. 2002; Farrell et al. 1991; Hortin, 1990; Jukkola et al. 1986). In these proteins, the major function of tyrosine sulfation is protein-protein interaction (Costagliola et al. 2002; Nishimura et al. 2010; Rodgers et al. 2001; Stone et. al 2009; Zhu et al. 2011). In proteins, tyrosine sulfation introduces a negative charge, which can then interact with positively charged amino acids, forming salt bridges (Woods et al. 2007). Some examples of the role of tyrosine sulfation in protein-protein interaction are given below.
Binding of leukocyte P-selectin glycoprotein ligand-1 (PSGL-1) protein to P-Selectin
It has been previously shown that PSGL-1 binding to P-selectin on endothelial cells is responsible for optimal leukocyte rolling in blood vessels, and recruitment of leukocytes to inflamed tissue (Rodgers et al. 2001). Elimination of tyrosine sulfation in PSGL-1, by harvesting leukocytes from TPST knockout animals, resulted in impaired leukocyte rolling compared with rolling of leukocytes harvested from wild-type animals (Westmuckett et al. 2011). Rolling of leukocytes on endothelial cells, the result of rapid and balanced formation and breaking of selectin–ligand bonds, is an important step for the successful recruitment of those leukocytes into the tissue (Boehncke and Schön, 2003). In addition, antibody blocking experiments using anti-PSGL-1 antibody completely eliminated rolling of leukocytes in both TPST and wild-type animals (Westmuckett et al. 2011). Taken together, the results show that optimum binding of PSGL-1 to P-selectin is responsible for leukocyte rolling. Tyrosine sulfation enhances this binding.
Binding of PSGL-1 to enterovirus 71 (EV71)
Tyrosine sulfation of PSGL-1 in leukocytes is responsible for binding Enterovirus 71 (EV71) virus and mediating viral entry into leukocytes (Nishimura et al. 2010). This virus is responsible for a wide range of neurological diseases in children (Nishimura et al. 2010). Elimination of one or more sulfated tyrosines on PSGL-1 by site-directed mutagenesis or inhibition with chlorate resulted in elimination of optimum binding between PSGL-1 to EV71, and prevented viral entry and replication (Nishimura et al. 2010). These results demonstrate that tyrosine sulfation of the amino terminus of PSGL-1 is critical for EV71 infection.
Chemokine receptor binding to chemokines
Further evidence of tyrosine sulfation enhancing protein-protein interaction was discovered in studies of chemokine receptors. Chemokine receptors are G protein-coupled receptors which play an important role in regulating immune and inflammatory responses by mediating leukocyte migration (Nibbs and Graham, 2013).
All of the chemokine receptors have at least 3 tyrosines surrounded by acidic amino acids, a consensus associated with sulfated tyrosines (Kraemer et al. 2013; Zhu et al. 2013). Seven of these receptors, CCR5, CCR2, CCR8, CXCR3, CXCR4, DARC, and CX3CR1, have been shown to contain 3 sulfated tyrosines at the N-terminus (Bannert et al. 2001; Colvin et al. 2006; Rapp et al. 2013; Simpson 2009; Tan et al. 2013; Veldkamp et al. 2006; Zhu et al. 2011). Using sulfopeptides showing differential sulfation of tyrosine residues, it has been demonstrated that sulfation enhances binding of chemokines to their receptors compared with their unsulfated counterparts: differential sulfation of tyrosine residues modulates the selectivity and binding affinity of the receptor to different chemokines (Simpson et al. 2009).
Binding of CCR5 to HIV-1 gp120/CD4 complexes
Tyrosine sulfation of chemokine receptor CCR5 is also responsible for the binding of HIV-1 gp120/CD4 complexes and virus entry (Brower et al. 2009; Farzan et al. 1999). The generation of a tyrosine-sulfated peptide derived from the N-terminal region of CCR5 prevented the association of HIV-1 gp120 with CCR5 and thereby prevented HIV-1 entry, a result that was not seen with its unsulfated counterpart (Dorfman et al. 2006).
Hormone-receptor binding
Tyrosine sulfation also plays a role in protein-protein interaction in the three glycoprotein hormone receptors, follicle-stimulating hormone receptor (FSHr), luteinizing hormone receptor (LH), and thyrotrophin receptor (TSHr). Tyrosine sulfation has been detected in the C-terminus of these receptors. Using site-directed mutagenesis, it was shown that tyrosine sulfation of the receptor is needed for efficient hormone binding (Bonomi et al. 2006).
Blood Coagulation
Numerous proteins involved in the blood coagulation process, such as Factors V, VIII, IX, and fibrinogen, are tyrosine-sulfated (Arruda et al. 2001; Farrell et al. 1991; Hirose et al. 1988; Hortin, 1990; Leyte et al. 1991). Studies of Factor VIII have shown that sulfated tyrosine at position 1680 is required for interaction with von Willebrand factor (vWF) (Leyte et al. 1991). Peptides derived from Factor VIII, containing the sulfated tyrosine 1680 and its non-sulfated analogue using a chlorate inhibitor, showed drastically reduced binding to vWF (Leyte et al. 1991). Tyrosine sulfation sites on two other proteins, fibrinogen and hirugen, is also necessary for optimum binding to thrombin. This was determined by studying the binding of fibrin and hirugen peptides that either have sulfated tyrosines, or are unsulfated to thrombin (Meh et al. 2001; Skrzypczak-Jankun et al. 1991).
Tyrosine sulfation of extracellular proteins
Tyrosine sulfation in elastic tissue
Elastic fibers are extracellular matrix (ECM) structures that are composed of elastin, microfibrills, and their associated proteins, such as microfibril-associated glycoproteins (MAGPs), fibulins, and EMILIN-1. Microfibril-associated glycoprotein-1 (MAGP-1) and fibulin 2 have been shown to contain tyrosine-sulfated residues (Trask et al. 2001; Kanan et al. 2014a), while fibrillin 1 and 2 have been predicted to contain numerous tyrosine sulfate residues, according to the Sulfinator (Monigatti et al. 2002) (http://www.pdg.cnb.uam.es/cursos/BioInfo2004/pages/visualizacion/programas_manuales/spdbv_userguide/us.expasy.org/tools/sulfinator/index.html). The Sulfinator is a software tool that is used for the prediction of sulfated tyrosines based upon location within a sequence in the middle of a 25-amino acid window, as well as in domains when several tyrosines are present (Monigatti et al. 2002). Although it is proposed that it's predictions are with an overall accuracy of 98% (Monigatti et al. 2002), we have found that many of the predicted tyrosines are not sulfated and vice versa.
While MAGP-1 has been shown to bind tropoelastin and fibrillin (Clarke and Weiss, 2004; Werneck et al. 2004), fibulin 2 has been shown to bind several ECM proteins such as tropoelastin, fibronectin, nidogen 2, laminin 1, aggrecan, versican, and perlecan (Gu et al. 2000; Olin et al. 2001; Sasaki et al. 1999). While it has not been shown that tyrosine sulfation is responsible for these interactions, given the function of tyrosine sulfation in mediating protein-protein interaction, it is not impossible to conceive that tyrosine sulfation may play a role in overall elastic fiber integrity.
Tyrosine sulfation of collagens
Collagens are the most abundant proteins in the ECM. Procollagen V and Procollagen III have been shown to be tyrosine-sulfated (Fessler et al. 1986; Jukkola et al. 1986). Sulfinator analysis predicts that three human minor fibrillar collagens, Col5a1, Col11a1, and Col11a2, are tyrosine-sulfated (Fang et al. 2012). Since collagens bind to other proteins in the ECM, such as fibronectin (Chandrasekhar et al. 1983; Dzamba et al. 1993), integrins such as α1β1 and α2β1 (Nykvist et al. 2000), morphogenetic protein-2 (BMP-2; Morin et al. 2006), and hepatocyte growth factor (HGF; Schuppan et al. 1998), tyrosine sulfation on collagens may be involved in optimum binding to these ECM proteins.
Other tyrosine-sulfated proteins in the ECM
Class II leucine-rich repeat (LRR) proteins contain numerous sulfated tyrosines in the N-terminal region. These ECM proteins include fibromodulin (Antonsson et al. 1991), lumican (Onnerfjord et al. 2004), and osteoadherin (Onnerfjord et al. 2004). The function of tyrosine sulfation in these proteins is unknown. However, in other proteins, such as PSGL-1 and chemokine receptors CCR5, CCR2, CCR8, CXCR3, CXCR4, DARC, and CX3CR1, the presence of tyrosine sulfation at the N-terminus suggests a role in protein-protein interaction (Kehoe and Bertozzi, 2000). Therefore, its role in class II LRR proteins may be to interact with other proteins.
Function of tyrosine-sulfated proteins in ocular tissues
The analysis of bovine ocular tissues identified the presence of tyrosine-sulfated proteins in the cornea, aqueous humor, lens, iris, vitreous humor, neurosensory retina, retinal pigment epithelium (RPE), and sclera (Kanan et al. 2012). Using the anti-sulfotyrosine antibody PSG2, the vitreous humor was revealed as the richest source of tyrosine-sulfated proteins, while the lens had the fewest tyrosine-sulfated proteins (Kanan et al. 2012). Analysis of mouse neurosensory retinas in models such as Tpst1−/−, Tpst2−/−, and Tpst/DKO, showed that many tyrosine-sulfated proteins are still retained in each of the single knockout animals. Tyrosine-sulfated proteins are only completely eliminated in the double knockout animals, suggesting some compensation between the two TPST enzymes (Sherry et al. 2012; Sherry et al. 2010). The Tpst1−/− mice lived a normal lifespan with a mild developmental visual phenotype, wherein the scotopic “a” and “b” waves were slightly below wild type levels at early ages, but reached normal levels at P90 (Sherry et al. 2012). However, the Tpst2−/− mice exhibited similar developmental visual deficits that did not correct with age (Sherry et al. 2012). The rod outer segment structure and synaptic terminals in both Tpst1−/− and Tpst2−/− single knockout animals were normal (Sherry et al. 2012). Immunohistologic examinations of retinal sections from both single knockouts probed with PSG2 suggested that TPST-1 and -2 differentially sulfate most of their substrates but other substrates may be sulfated by both enzymes (Sherry et al. 2012).
The mild phenotypes observed in the single knockout mice is in contrast to the drastic visual functional and ultrastructural abnormalities observed in Tpst/DKO animals (Sherry et al. 2010). Postnatal (P) 21 Tpst/DKO mice exhibited 25% of wild-type electroretinographic (ERG) scotopic responses, and only 15% of wild-type photopic responses. Additionally, the rod outer segments were ultrastructurally abnormal, with large inter-discal and intradiscal spacing and projection of the disk membrane into the extracellular space (Sherry et al. 2010). Moreover, the synaptic terminals of the photoreceptors appeared to be structurally disorganized, though they still formed the triad structure seen in rod terminals and flat contacts seen in cone terminals (Sherry et al. 2010). Unfortunately, the Tpst/DKO mice do not survive long past weaning (Westmuckett et al. 2008), making it impossible to study the role of tyrosine-sulfated proteins in retinal maintenance in this model.
To study the role of tyrosine-sulfated proteins in retinal function and structure past weaning, it is essential to develop a conditional knockout model wherein TPST1 and 2 are both eliminated in the context of rods, cones, RPE, and other retinal cells using cell-type specific Cre-expressing mice. This will permit the study of the role of tyrosine-sulfated proteins in the function of each retinal cell type, the determination of the expressing cell type (following the identification of all tyrosine-sulfated retinal proteins), and the long term effects of the elimination of tyrosine sulfation on retinal structure and function.
Tyrosine-sulfated proteins and retinal diseases
The first step in determining the role of tyrosine-sulfated proteins in retinal diseases is the identification of all of those proteins. As initial steps towards that goal, extracts of bovine RPE and neurosensory retina were subjected to immunoaffinity column purification using the PSG2 antibody (Kanan et al. 2014a, 2014b). Three proteins, fibulin 2, vitronectin, and opticin, were identified (Kanan et al. 2014a, 2014b). In addition, data mining and tyrosine sulfation prediction analysis of the RPE secretome identified complement factor H (CFH) as another tyrosinesulfated protein (Kanan et al. 2014b). Further in vivo and in vitro analysis confirmed that all four proteins are tyrosine-sulfated (Kanan et al. 2014a, 2014b).
While the in vivo function of fibulin 2 has yet to be identified, this protein belongs to the fibulin family (De et al. 2009), several members of which have been implicated in ocular disease. Fibulin 3 is implicated in Malattia Leventinese or dominant Doyne honeycomb retinal degeneration (Marmorstein et al. 2002; Stone et al. 1999). Fibulin 5 and 6 have been associated with macular dystrophy (Schultz et al. 2003; Stone et al. 2004). Fibulin 1 has been implicated in recessive vitreoretinal dystrophy (Weigell-weber et al. 2003). Genome-wide homozygosity mapping with microsatellite markers mapped the candidate gene to 22q13, where fibulin 1 is located (Weigell-weber et al. 2003). Therefore, fibulin 2 may also be involved in ocular disease.
Analysis of the role of tyrosine sulfation in the function of fibulin 2 showed that fibulin 2 is upregulated in the RPE following retinal detachment (Kanan et al. 2014a), suggesting a role for fibulin 2 in the re-attachment process. Elimination of fibulin 2 sulfation did not influence fibulin 2 secretion, but reduced its ability to regulate cellular growth and migration (Kanan et al. 2014a). This is not surprising, knowing that tyrosine sulfation is important for protein-protein interaction.
Vitronectin and CFH are regulatory proteins of the complement system. While CFH inactivates the complement system by acting as a cofactor in Factor I-induced decay of C3-covertase (Pechtl et al. 2011), vitronectin inhibits the complement system by binding the final product of the activated complement system, membrane attack complex (MAC) C5b-9 (Podack et al. 1984). Mutations in CFH are implicated in some cases of age-related macular degeneration (AMD) resulting from uncontrolled complement activation (Edwards et al. 2005; Hageman et al. 2005; Haines et al. 2005; Klein et al. 2005). One of the clinical signs of AMD is the presence of drusen in the Bruch's membrane (Pauleikhoff et al. 1992). Vitronectin and C5b-9 are primary components of drusen (Hageman et al. 1999). It has been shown that complement activation of RPE cells results in the upregulation of both C5b-9 and vitronectin (Lueck et al. 2011; Wasmuth et al. 2009). Therefore, vitronectin may serve a protective purpose in drusen, namely, to inactivate the complement cascade by inhibiting the MAC complex. Future experiments should focus on identifying the role of sulfation in the functions of CFH and vitronectin, and whether modulation of tyrosine sulfation influences the uncontrolled activation of the complement system.
Opticin is another tyrosine-sulfated protein (Kanan et al. 2014b). This protein has recently been shown to prevent neovascularization in the oxygen-induced retinopathy model (OIR model) in mouse eyes (Le Goff et al. 2012a). Further studies have shown that the mechanism involves binding of opticin to collagen, thereby preventing the binding of endothelial cells’ integrins to collagens (Le Goff et al. 2012b). Future studies should determine the role of sulfation in opticin's ability to regulate neovascularization.
Current and future research focus
Since it impossible to study the role of tyrosine sulfation in adult Tpst/DKO mice, due to their limited survival, the best way to study the role of tyrosine sulfation is using a conditional knockout mouse model (Utomo et al. 1999). Conditional Cre expression has been demonstrated in rods (e.g. Le et al. 2006b), cones (e.g. Le et al. 2006a), Müller cells (e.g. Ueki et al. 2009), and RPE cells (e.g. Le at al. 2008; Fu et al. 2014). If the two TPSTs are knocked down in specific cells, these animal models can be used to study the role of tyrosine sulfation in these cells. Furthermore, since these mice should not have limited viability, age-related studies could also be performed.
Knockin experiments can also produce animal models in which tyrosine sulfation is eliminated in each known retinal tyrosine-sulfated protein. Although costly, these are informative models. The models can be generated by substituting the sulfated tyrosine with a phenylalanine that lacks the hydroxyl group, hence eliminating sulfation.
CONCLUSION
The study of tyrosine sulfation is in its infancy, and requires considerable effort to identify the involved proteins, their role in human diseases, and, ultimately, the role of tyrosine sulfation in the overall function of proteins and in diseases. Four tyrosine-sulfated retinal proteins have been identified: fibulin 2, CFH, vitronectin, and opticin. The role of sulfation in modulating fibulin function has been elucidated. Future studies should focus on the role of sulfation in CFH and vitronectin function, and should determine the role of tyrosine sulfation in complement activation and AMD.
Highlights.
Tyrosine sulfation, a post-translational modification, occurs on ECM proteins
All ocular tissues express tyrosine sulfated proteins but identities are unknown
Although a common post-translational modification, tyrosine sulfation is understudied
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Antonsson P, Heinegard D, Oldberg A. Posttranslational modifications of fibromodulin. J.Biol.Chem. 1991;266:16859–61. [PubMed] [Google Scholar]
- Arruda VR, Hagstrom JN, Deitch J, Heiman-Patterson T, Camire RM, Chu K, Fields PA, Herzog RW, Couto LB, Larson PJ, High KA. Posttranslational modifications of recombinant myotube-synthesized human factor IX. Blood. 2001;97:130–8. doi: 10.1182/blood.v97.1.130. [DOI] [PubMed] [Google Scholar]
- Bannert N, Craig S, Farzan M, Sogah D, Santo NV, Choe H, Sodroski J. Sialylated O-glycans and sulfated tyrosines in the NH2-terminal domain of CC chemokine receptor 5 contribute to high affinity binding of chemokines. J.Exp.Med. 2001;194:1661–73. doi: 10.1084/jem.194.11.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehncke W-H, Schön M. Interfering with leukocyte rolling-a promising therapeutic approach in inflammatory skin disorders? Trends Pharmacol. Sci. 2003;24:49–52. doi: 10.1016/s0165-6147(02)00039-1. [DOI] [PubMed] [Google Scholar]
- Bonomi M, Busnelli M, Persani L, Vassart G, Costagliola S. Structural differences in the hinge region of the glycoprotein hormone receptors: evidence from the sulfated tyrosine residues. Mol.Endocrinol. 2006;20:3351–63. doi: 10.1210/me.2005-0521. [DOI] [PubMed] [Google Scholar]
- Borghei A, Ouyang YB, Westmuckett AD, Marcello MR, Landel CP, Evans JP, Moore KL. Targeted disruption of tyrosylprotein sulfotransferase-2, an enzyme that catalyzes post-translational protein tyrosine O-sulfation, causes male infertility. J.Biol.Chem. 2006;281:9423–31. doi: 10.1074/jbc.M513768200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower ET, Schon A, Klein JC, Freire E. Binding thermodynamics of the N-terminal peptide of the CCR5 coreceptor to HIV-1 envelope glycoprotein gp120. Biochemistry. 2009;48:779–85. doi: 10.1021/bi8021476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buddi R, Lin B, Atilano SR, Zorapapel NC, Kenney MC, Brown DJ. Evidence of oxidative stress in human corneal diseases. J. Histochem. Cytochem. 2002;50:341–51. doi: 10.1177/002215540205000306. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar S, Sorrentino JA, Millis AJ. Interaction of fibronectin with collagen: age-specific defect in the biological activity of human fibroblast fibronectin. Proc.Natl.Acad.Sci.U.S.A. 1983;80:4747–51. doi: 10.1073/pnas.80.15.4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AW, Weiss AS. Microfibril-associated glycoprotein-1 binding to tropoelastin: multiple binding sites and the role of divalent cations. Eur.J.Biochem. 2004;271:3085–90. doi: 10.1111/j.1432-1033.2004.04246.x. [DOI] [PubMed] [Google Scholar]
- Colvin RA, Campanella GS, Manice LA, Luster AD. CXCR3 requires tyrosine sulfation for ligand binding and a second extracellular loop arginine residue for ligand-induced chemotaxis. Mol.Cell Biol. 2006;26:5838–49. doi: 10.1128/MCB.00556-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costagliola S, Panneels V, Bonomi M, Koch J, Many MC, Smits G, Vassart G. Tyrosine sulfation is required for agonist recognition by glycoprotein hormone receptors. EMBO J. 2002;21:504–13. doi: 10.1093/emboj/21.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de VS, Iwamoto T, Yamada Y. Fibulins: multiple roles in matrix structures and tissue functions. Cell Mol.Life Sci. 2009;66:1890–902. doi: 10.1007/s00018-009-8632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman T, Moore MJ, Guth AC, Choe H, Farzan M. A tyrosine-sulfated peptide derived from the heavy-chain CDR3 region of an HIV-1-neutralizing antibody binds gp120 and inhibits HIV-1 infection. J.Biol.Chem. 2006;281:28529–35. doi: 10.1074/jbc.M602732200. [DOI] [PubMed] [Google Scholar]
- Dzamba BJ, Wu H, Jaenisch R, Peters DM. Fibronectin binding site in type I collagen regulates fibronectin fibril formation. J.Cell Biol. 1993;121:1165–72. doi: 10.1083/jcb.121.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AO, Ritter R, III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- Fang M, Jacob R, McDougal O, Oxford JT. Minor fibrillar collagens, variable regions alternative splicing, intrinsic disorder, and tyrosine sulfation. Protein Cell. 2012;3:419–33. doi: 10.1007/s13238-012-2917-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell DH, Mulvihill ER, Huang SM, Chung DW, Davie EW. Recombinant human fibrinogen and sulfation of the gamma' chain. Biochemistry. 1991;30:9414–20. doi: 10.1021/bi00103a004. [DOI] [PubMed] [Google Scholar]
- Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard NP, Gerard C, Sodroski J, Choe H. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell. 1999;96:667–76. doi: 10.1016/s0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- Fessler LI, Brosh S, Chapin S, Fessler JH. Tyrosine sulfation in precursors of collagen V. J.Biol.Chem. 1986;261:5034–40. [PubMed] [Google Scholar]
- Fu S, Zhu M, Wang C, Le YZ. Efficient induction of productive Cre-mediated recombination in retinal pigment epithelium. Mol.Vis. 2014;20:480–7. [PMC free article] [PubMed] [Google Scholar]
- Gu YC, Nilsson K, Eng H, Ekblom M. Association of extracellular matrix proteins fibulin-1 and fibulin-2 with fibronectin in bone marrow stroma. Br.J.Haematol. 2000;109:305–13. doi: 10.1046/j.1365-2141.2000.02011.x. [DOI] [PubMed] [Google Scholar]
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc.Natl.Acad.Sci.U.S.A. 2005;102:7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman GS, Mullins RF, Russell SR, Johnson LV, Anderson DH. Vitronectin is a constituent of ocular drusen and the vitronectin gene is expressed in human retinal pigmented epithelial cells. FASEB J. 1999;13:477–84. doi: 10.1096/fasebj.13.3.477. [DOI] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Hirose S, Oda K, Ikehara Y. Tyrosine O-sulfation of the fibrinogen gamma B chain in primary cultures of rat hepatocytes. J.Biol.Chem. 1988;263:7426–30. [PubMed] [Google Scholar]
- Hortin GL. Sulfation of tyrosine residues in coagulation factor V. Blood. 1990;76:946–52. [PubMed] [Google Scholar]
- Jukkola A, Risteli J, Niemela O, Risteli L. Incorporation of sulphate into type III procollagen by cultured human fibroblasts. Identification of tyrosine O-sulphate. Eur.J.Biochem. 1986;154:219–24. doi: 10.1111/j.1432-1033.1986.tb09382.x. [DOI] [PubMed] [Google Scholar]
- Kanan Y, Brobst D, Han Z, Naash MI, Al-Ubaidi MR. Fibulin 2, a tyrosine O-sulfated protein, is up-regulated following retinal detachment. J Biol Chem . 2014a) doi: 10.1074/jbc.M114.562157. ‘In press’. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanan Y, Siefert JC, Al-Ubaidi MR. Complement factor H, Vitronectin and Opticin are tyrosine sulfated proteins of the retinal pigmented epithelium. 2014b doi: 10.1371/journal.pone.0105409. ‘Unpublished results’. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanan Y, Hamilton RA, Moore KL, Al-Ubaidi MR. Protein tyrosine-O-sulfation in bovine ocular tissues. Adv.Exp.Med.Biol. 2012;723:835–41. doi: 10.1007/978-1-4614-0631-0_107. [DOI] [PubMed] [Google Scholar]
- Kehoe JW, Bertozzi CR. Tyrosine sulfation: a modulator of extracellular protein-protein interactions. Chem.Biol. 2000;7:R57–R61. doi: 10.1016/s1074-5521(00)00093-4. [DOI] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer S, Alampour-Rajabi S, El BO, Bernhagen J. Hetero-oligomerization of chemokine receptors: diversity and relevance for function. Curr.Med.Chem. 2013;20:2524–36. doi: 10.2174/09298673113209990117. [DOI] [PubMed] [Google Scholar]
- Le Goff MM, Lu H, Ugarte M, Henry S, Takanosu M, Mayne R, Bishop PN. The vitreous glycoprotein opticin inhibits preretinal neovascularization. Invest Ophthalmol.Vis.Sci. 2012a;53:228–34. doi: 10.1167/iovs.11-8514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goff MM, Sutton MJ, Slevin M, Latif A, Humphries MJ, Bishop PN. Opticin exerts its anti-angiogenic activity by regulating extracellular matrix adhesiveness. J.Biol.Chem. 2012b;287:28027–36. doi: 10.1074/jbc.M111.331157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le YZ, Ash JD, Al-Ubaidi MR, Chen Y, Ma JX, Anderson RE. Conditional gene knockout system in cone photoreceptors. Adv.Exp.Med.Biol. 2006a;572:173–8. doi: 10.1007/0-387-32442-9_26. [DOI] [PubMed] [Google Scholar]
- Le YZ, Zheng L, Zheng W, Ash JD, Agbaga MP, Zhu M, Anderson RE. Mouse opsin promoter-directed Cre recombinase expression in transgenic mice. Mol.Vis. 2006b;12:389–98. [PubMed] [Google Scholar]
- Le YZ, Zheng W, Rao PC, Zheng L, Anderson RE, Esumi N, Zack DJ, Zhu M. Inducible expression of cre recombinase in the retinal pigmented epithelium. Invest Ophthalmol.Vis.Sci. 2008;49:1248–53. doi: 10.1167/iovs.07-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyte A, van Schijndel HB, Niehrs C, Huttner WB, Verbeet MP, Mertens K, van Mourik JA. Sulfation of Tyr1680 of human blood coagulation factor VIII is essential for the interaction of factor VIII with von Willebrand factor. J.Biol.Chem. 1991;266:740–6. [PubMed] [Google Scholar]
- Lueck K, Wasmuth S, Williams J, Hughes TR, Morgan BP, Lommatzsch A, Greenwood J, Moss SE, Pauleikhoff D. Sub-lytic C5b-9 induces functional changes in retinal pigment epithelial cells consistent with age-related macular degeneration. Eye (Lond) 2011;25:1074–82. doi: 10.1038/eye.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein LY, Munier FL, Arsenijevic Y, Schorderet DF, McLaughlin PJ, Chung D, Traboulsi E, Marmorstein AD. Aberrant accumulation of EFEMP1 underlies drusen formation in Malattia Leventinese and age-related macular degeneration. Proc.Natl.Acad.Sci.U.S.A. 2002;99:13067–72. doi: 10.1073/pnas.202491599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meh DA, Siebenlist KR, Brennan SO, Holyst T, Mosesson MW. The amino acid sequence in fibrin responsible for high affinity thrombin binding. Thromb.Haemost. 2001;85:470–4. [PubMed] [Google Scholar]
- Monigatti F, Gasteiger E, Bairoch A, Jung E. The Sulfinator: predicting tyrosine sulfation sites in protein sequences. Bioinformatics. 2002;18:769–70. doi: 10.1093/bioinformatics/18.5.769. [DOI] [PubMed] [Google Scholar]
- Moore KL. The biology and enzymology of protein tyrosine O-sulfation. J.Biol.Chem. 2003;278:24243–6. doi: 10.1074/jbc.R300008200. [DOI] [PubMed] [Google Scholar]
- Morin R, Kaplan D, Perez-Ramirez B. Bone morphogenetic protein-2 binds as multilayers to a collagen delivery matrix: an equilibrium thermodynamic analysis. Biomacromolecules. 2006;7:131–8. doi: 10.1021/bm050461i. [DOI] [PubMed] [Google Scholar]
- Nibbs RJ, Graham GJ. Immune regulation by atypical chemokine receptors. Nat Rev Immunol. 2013;13:815–829. doi: 10.1038/nri3544. [DOI] [PubMed] [Google Scholar]
- Niehrs C, Huttner WB. Purification and characterization of tyrosylprotein sulfotransferase. EMBO J. 1990;9:35–42. doi: 10.1002/j.1460-2075.1990.tb08077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C, Stinchcombe JC, Huttner WB. Two membrane-bound forms of tyrosylprotein sulfotransferase as revealed by phase partitioning in Triton X-114. Eur.J.Cell Biol. 1992;58:35–43. [PubMed] [Google Scholar]
- Nishimura Y, Wakita T, Shimizu H. Tyrosine sulfation of the amino terminus of PSGL-1 is critical for enterovirus 71 infection. PLoS.Pathog. 2010;6:e1001174. doi: 10.1371/journal.ppat.1001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykvist P, Tu H, Ivaska J, Kapyla J, Pihlajaniemi T, Heino J. Distinct recognition of collagen subtypes by alpha(1)beta(1) and alpha(2)beta(1) integrins. Alpha(1)beta(1) mediates cell adhesion to type XIII collagen. J.Biol.Chem. 2000;275:8255–61. doi: 10.1074/jbc.275.11.8255. [DOI] [PubMed] [Google Scholar]
- Olin AI, Mörgelin M, Sasaki T, Timpl R, Heinegård D, Aspberg A. The proteoglycans aggrecan and Versican form networks with fibulin-2 through their lectin domain binding. J Biol Chem. 2001;276:1253–1261. doi: 10.1074/jbc.M006783200. [DOI] [PubMed] [Google Scholar]
- Onnerfjord P, Heathfield TF, Heinegard D. Identification of tyrosine sulfation in extracellular leucine-rich repeat proteins using mass spectrometry. J.Biol.Chem. 2004;279:26–33. doi: 10.1074/jbc.M308689200. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Crawley JT, Aston CE, Moore KL. Reduced body weight and increased postimplantation fetal death in tyrosylprotein sulfotransferase-1-deficient mice. J.Biol.Chem. 2002;277:23781–7. doi: 10.1074/jbc.M202420200. [DOI] [PubMed] [Google Scholar]
- Pacher P, Obrosova IG, Mabley JG, Szabó C. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr. Med. Chem. 2005;12:267–75. doi: 10.2174/0929867053363207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauleikhoff D, Chen J, Bird AC, Wessing A. [The Bruch membrane and choroid. Angiography and functional characteristics in age-related changes]. Ophthalmologe. 1992;89:39–44. [PubMed] [Google Scholar]
- Pechtl IC, Kavanagh D, McIntosh N, Harris CL, Barlow PN. Disease-associated N-terminal complement factor H mutations perturb cofactor and decay-accelerating activities. J.Biol.Chem. 2011;286:11082–90. doi: 10.1074/jbc.M110.211839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack ER, Preissner KT, Muller-Eberhard HJ. Inhibition of C9 polymerization within the SC5b-9 complex of complement by S-protein. Acta Pathol.Microbiol.Immunol.Scand.Suppl. 1984;284:89–96. [PubMed] [Google Scholar]
- Rapp C, Snow S, Laufer T, McClendon CL. The role of tyrosine sulfation in the dimerization of the CXCR4:SDF-1 complex. Protein Sci. 2013;22:1025–36. doi: 10.1002/pro.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers SD, Camphausen RT, Hammer DA. Tyrosine sulfation enhances but is not required for PSGL-1 rolling adhesion on P-selectin. Biophys.J. 2001;81:2001–9. doi: 10.1016/S0006-3495(01)75850-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Gohring W, Miosge N, Abrams WR, Rosenbloom J, Timpl R. Tropoelastin binding to fibulins, nidogen-2 and other extracellular matrix proteins. FEBS Lett. 1999;460:280–4. doi: 10.1016/s0014-5793(99)01362-9. [DOI] [PubMed] [Google Scholar]
- Schultz DW, Klein ML, Humpert AJ, Luzier CW, Persun V, Schain M, Mahan A, Runckel C, Cassera M, Vittal V, Doyle TM, Martin TM, Weleber RG, Francis PJ, Acott TS. Analysis of the ARMD1 locus: evidence that a mutation in HEMICENTIN-1 is associated with age-related macular degeneration in a large family. Hum.Mol.Genet. 2003;12:3315–23. doi: 10.1093/hmg/ddg348. [DOI] [PubMed] [Google Scholar]
- Schuppan D, Schmid M, Somasundaram R, Ackermann R, Ruehl M, Nakamura T, Riecken EO. Collagens in the liver extracellular matrix bind hepatocyte growth factor. Gastroenterology. 1998;114:139–52. doi: 10.1016/s0016-5085(98)70642-0. [DOI] [PubMed] [Google Scholar]
- Sherry DM, Kanan Y, Hamilton R, Hoffhines A, Arbogast KL, Fliesler SJ, Naash MI, Moore KL, Al-Ubaidi MR. Differential developmental deficits in retinal function in the absence of either protein tyrosine sulfotransferase-1 or -2. PLoS.One. 2012;7:e39702. doi: 10.1371/journal.pone.0039702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry DM, Murray AR, Kanan Y, Arbogast KL, Hamilton RA, Fliesler SJ, Burns ME, Moore KL, Al-Ubaidi MR. Lack of protein-tyrosine sulfation disrupts photoreceptor outer segment morphogenesis, retinal function and retinal anatomy. Eur.J.Neurosci. 2010;32:1461–72. doi: 10.1111/j.1460-9568.2010.07431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson LS, Zhu JZ, Widlanski TS, Stone MJ. Regulation of chemokine recognition by site-specific tyrosine sulfation of receptor peptides. Chem.Biol. 2009;16:153–61. doi: 10.1016/j.chembiol.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypczak-Jankun E, Carperos VE, Ravichandran KG, Tulinsky A, Westbrook M, Maraganore JM. Structure of the hirugen and hirulog 1 complexes of alpha-thrombin. J.Mol.Biol. 1991;221:1379–93. [PubMed] [Google Scholar]
- Smithies O, Gregg RG, Boggs SS, Koralewski MA, Kucherlapati RS. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985;317:230–4. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Stone EM, Braun TA, Russell SR, Kuehn MH, Lotery AJ, Moore PA, Eastman CG, Casavant TL, Sheffield VC. Missense variations in the fibulin 5 gene and age-related macular degeneration. N.Engl.J.Med. 2004;351:346–53. doi: 10.1056/NEJMoa040833. [DOI] [PubMed] [Google Scholar]
- Stone EM, Lotery AJ, Munier FL, Heon E, Piguet B, Guymer RH, Vandenburgh K, Cousin P, Nishimura D, Swiderski RE, Silvestri G, Mackey DA, Hageman GS, Bird AC, Sheffield VC, Schorderet DF. A single EFEMP1 mutation associated with both Malattia Leventinese and Doyne honeycomb retinal dystrophy. Nat.Genet. 1999;22:199–202. doi: 10.1038/9722. [DOI] [PubMed] [Google Scholar]
- Stone MJ, Chuang S, Hou X, Shoham M, Zhu JZ. Tyrosine sulfation: an increasingly recognised post-translational modification of secreted proteins. N.Biotechnol. 2009;25:299–317. doi: 10.1016/j.nbt.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Suiko M, Fernando PH, Sakakibara Y, Nakajima H, Liu MC, Abe S, Nakatsu S. Post-translational modification of protein by tyrosine sulfation: active sulfate PAPS is the essential substrate for this modification. Nucleic Acids Symp.Ser. 1992:183–4. [PubMed] [Google Scholar]
- Tan JH, Ludeman JP, Wedderburn J, Canals M, Hall P, Butler SJ, Taleski D, Christopoulos A, Hickey MJ, Payne RJ, Stone MJ. Tyrosine sulfation of chemokine receptor CCR2 enhances interactions with both monomeric and dimeric forms of the chemokine monocyte chemoattractant protein-1 (MCP-1). J.Biol.Chem. 2013;288:10024–34. doi: 10.1074/jbc.M112.447359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask BC, Broekelmann T, Ritty TM, Trask TM, Tisdale C, Mecham RP. Posttranslational modifications of microfibril associated glycoprotein-1 (MAGP-1). Biochemistry. 2001;40:4372–80. doi: 10.1021/bi002738z. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Ash JD, Zhu M, Zheng L, Le YZ. Expression of Cre recombinase in retinal Muller cells. Vision Res. 2009;49:615–21. doi: 10.1016/j.visres.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utomo AR, Nikitin AY, Lee WH. Temporal, spatial, and cell type-specific control of Cre-mediated DNA recombination in transgenic mice. Nat.Biotechnol. 1999;17:1091–6. doi: 10.1038/15073. [DOI] [PubMed] [Google Scholar]
- Veldkamp CT, Seibert C, Peterson FC, Sakmar TP, Volkman BF. Recognition of a CXCR4 sulfotyrosine by the chemokine stromal cell-derived factor-1alpha (SDF-1 alpha/CXCL12). J.Mol.Biol. 2006;359:1400–9. doi: 10.1016/j.jmb.2006.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmuth S, Lueck K, Baehler H, Lommatzsch A, Pauleikhoff D. Increased vitronectin production by complement-stimulated human retinal pigment epithelial cells. Invest Ophthalmol.Vis.Sci. 2009;50:5304–9. doi: 10.1167/iovs.08-3326. [DOI] [PubMed] [Google Scholar]
- Weigell-Weber M, Sarra GM, Kotzot D, Sandkuijl L, Messmer E, Hergersberg M. Genomewide homozygosity mapping and molecular analysis of a candidate gene located on 22q13 (fibulin-1) in a previously undescribed vitreoretinal dystrophy. Arch.Ophthalmol. 2003;121:1184–8. doi: 10.1001/archopht.121.8.1184. [DOI] [PubMed] [Google Scholar]
- Werneck CC, Trask BC, Broekelmann TJ, Trask TM, Ritty TM, Segade F, Mecham RP. Identification of a major microfibril-associated glycoprotein-1-binding domain in fibrillin-2. J Biol Chem. 2004;279:23045–23051. doi: 10.1074/jbc.M402656200. [DOI] [PubMed] [Google Scholar]
- Westmuckett AD, Hoffhines AJ, Borghei A, Moore KL. Early postnatal pulmonary failure and primary hypothyroidism in mice with combined TPST-1 and TPST-2 deficiency. Gen.Comp Endocrinol. 2008;156:145–53. doi: 10.1016/j.ygcen.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmuckett AD, Thacker KM, Moore KL. Tyrosine sulfation of native mouse Psgl-1 is required for optimal leukocyte rolling on P-selectin in vivo. PLoS.One. 2011;6:e20406. doi: 10.1371/journal.pone.0020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfender JL, Chu F, Ball H, Wolfender F, Fainzilber M, Baldwin MA, Burlingame AL. Identification of tyrosine sulfation in Conus pennaceus conotoxins alpha-PnIA and alpha-PnIB: further investigation of labile sulfo- and phosphopeptides by electrospray, matrix-assisted laser desorption/ionization (MALDI) and atmospheric pressure MALDI mass spectrometry. J.Mass Spectrom. 1999;34:447–54. doi: 10.1002/(SICI)1096-9888(199904)34:4<447::AID-JMS801>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Woods AS, Wang HY, Jackson SN. Sulfation, the up-and-coming post-translational modification: its role and mechanism in protein-protein interaction. J.Proteome.Res. 2007;6:1176–82. doi: 10.1021/pr060529g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JZ, Millard CJ, Ludeman JP, Simpson LS, Clayton DJ, Payne RJ, Widlanski TS, Stone MJ. Tyrosine sulfation influences the chemokine binding selectivity of peptides derived from chemokine receptor CCR3. Biochemistry. 2011;50:1524–34. doi: 10.1021/bi101240v. [DOI] [PubMed] [Google Scholar]
- Zhu L, Zhao Q, Wu B. Structure-based studies of chemokine receptors. Curr.Opin.Struct.Biol. 2013;23:539–46. doi: 10.1016/j.sbi.2013.05.003. [DOI] [PubMed] [Google Scholar]


