Abstract
This nontechnical review is focused upon educating the reader on optic nerve head biomechanics in both aging and disease along two main themes: what is known about how mechanical forces and the resulting deformations are distributed in the posterior pole and ONH (biomechanics) and what is known about how the living system responds to those deformations (mechanobiology). We focus on how ONH responds to IOP elevations as a structural system, insofar as the acute mechanical response of the lamina cribrosa is confounded with the responses of the peripapillary sclera, prelaminar neural tissues, and retrolaminar optic nerve. We discuss the biomechanical basis for IOP-driven changes in connective tissues, blood flow, and cellular responses. We use glaucoma as the primary framework to present the important aspects of ONH biomechanics in aging and disease, as ONH biomechanics, aging, and the posterior pole extracellular matrix (ECM) are thought to be centrally involved in glaucoma susceptibility, onset and progression.
I. The Optic Nerve Head (ONH) as a Biomechanical Structure
Glaucoma is primarily a disease of aging (Gordon, Beiser et al. 2002, Leske, Heijl et al. 2003) and is one of the leading causes of blindness in the developed world (Quigley and Broman 2006). ONH biomechanics and the posterior pole extracellular matrix (ECM) are also thought to be centrally involved in glaucoma susceptibility, as well as disease onset and progression (Zeimer and Ogura 1989). Hence, we will use glaucoma as the primary framework to present the important aspects of ONH biomechanics in aging and disease in this review.
The ONH is of particular interest from a biomechanical perspective because it is a weak spot within an otherwise strong corneo-scleral envelope. Overwhelming evidence suggests that the lamina cribrosa is the principal site of RGC axonal insult in glaucoma (Howell, Soto et al. 2012, Nickells, Howell et al. 2012). In this sense, glaucomatous optic neuropathy can be viewed as an axonopathy, where damage to the visual pathway is driven by insult to RGC axons as they exit the eye at the ONH (Howell, Soto et al. 2012, Nickells, Howell et al. 2012). Hence, neither neuroprotection of the RGC soma or neuroregeneration of the RGC axons is likely to be effective in preventing, slowing or reversing vision loss in glaucoma unless the pathologic environment in the ONH is also simultaneously addressed. As such, glaucoma prevention and treatment is a three-legged stool in which the health of the RGC soma, its axon, and the axonal pathway to the brain must be simultaneously supported and maintained to prevent vision loss. The mechanisms of RGC axonal insult at the ONH insult are poorly understood, but we present a framework of IOP-driven ONH biomechanics as a central mechanism in the pathophysiology of glaucoma in this review.
The lamina cribrosa provides structural and functional support to the RGC axons as they pass from the relatively high-pressure environment in the eye to a low-pressure region in the retrobulbar cerebrospinal space (Zeimer and Ogura 1989, Downs, Roberts et al. 2008). To protect the RGCs in this unique anatomic region, the lamina cribrosa in higher primates has developed into a complex structure composed of a three-dimensional (3D) network of flexible beams of connective tissue (Figure 1). The ONH is nourished by the short posterior ciliary arteries, which penetrate the immediate peripapillary sclera to feed capillaries contained within the laminar beams (Cioffi and Van Buskirk 1996). This intra-scleral and intra-laminar vasculature is unique in that it is encased in load-bearing connective tissue, either within the scleral wall adjacent to the lamina cribrosa, or within the laminar beams themselves. Glaucoma is a multifactorial disease, and we hypothesize that biomechanics not only determines the mechanical environment in the ONH, but also mediates IOP-related reductions in blood flow and cellular responses through various pathways (Figure 2). Consideration of the anatomy of the lamina cribrosa and peripapillary sclera alone suggests that the classic “mechanical” and “vascular” mechanisms of glaucomatous injury are inseparably intertwined (Figure 2) (Sigal, Roberts et al. 2010).
Figure 1. The optic nerve head (ONH) is a three-dimensional (3D) structure comprised of multiple interactive tissue systems that exist on different scales. This complexity has been a formidable deterrent to characterizing its mechanical environment.
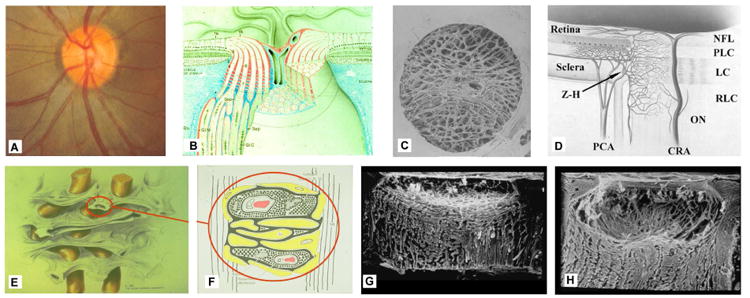
(A) While clinicians are familiar with the clinically visible surface of the optic nerve head (referred to as the optic disc), in fact the ONH (B) is a dynamic, 3D structure (seen here in an illustrated sectional view) in which the retinal ganglion cell (RGC) axons in bundles (white) surrounded by glial columns (red), pass through the connective tissue beams of the lamina cribrosa (light blue), isolated following trypsin digestion in an scanning electron micrograph (SEM) of the scleral canal in (C). The blood supply for the connective tissues of the lamina cribrosa (D) derives from the posterior ciliary arteries and the circle of Zinn-Haller (Z-H). (E-F) The relationship of the laminar beams to the axon bundles is shown in schematic form in (E). (F) Individual beams of the lamina cribrosa are lined by astrocytes and LC cells. Together they provide structural and metabolic support for the adjacent axon bundles. Within the lamina, the RGC axons have no direct blood supply. Axonal nutrition requires diffusion of nutrients from the laminar capillaries (solid red), across the endothelial basement membranes, through the extracellular matrix (ECM) of the laminar beam (stippled), across the basement membranes of the astrocytes (thick black), into the astrocytes (yellow), and across their processes (not shown) to the adjacent axons (vertical lines). Chronic age-related changes in the endothelial cell and astrocyte basement membranes, as well as intraocular pressure (IOP)-induced changes in the laminar ECM and astrocyte basement membranes may diminish nutrient diffusion to the axons in the presence of a stable level of laminar capillary volume flow. In advanced glaucoma, the connective tissues of the normal lamina cribrosa (G, sagittal view of the center of the ONH; vitreous above, orbital optic nerve below) change shape and remodel into a cupped and excavated configuration (H). Adapted from (Sigal, Roberts et al. 2010).
Figure 2. IOP-related stress and strain are a constant presence within the ONH at all levels of IOP.
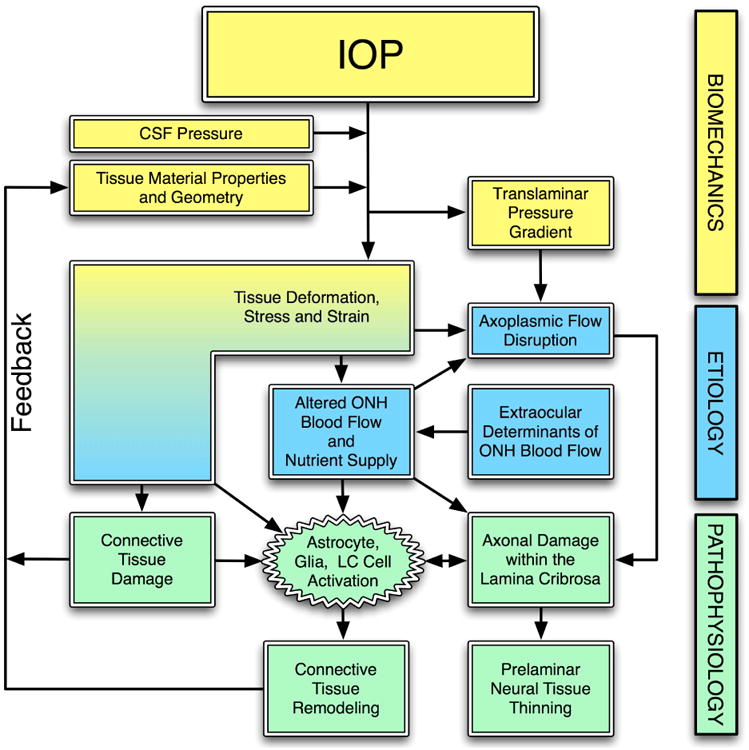
IOP and cerebrospinal fluid pressure act mechanically on the tissues of the eye, producing deformations, strain and stress within the tissues. These deformations depend on the eye-specific geometry and material properties of the individual eye. In a biomechanical paradigm, the stress and strain will alter the blood flow (primarily), and the delivery of nutrients (secondarily) through chronic alterations in connective tissue stiffness and diffusion properties. IOP-related stress and strain also induce connective tissue damage directly (laminar beam yield), or indirectly (cell mediated remodeling), which drives a connective tissue remodeling process that alters the tissues' geometry and mechanical response to loading. This feeds back directly onto the mechanical effects of IOP. Adapted from Sigal, Downs, et al. (Sigal, Roberts et al. 2010)
To incorporate these concepts into an overarching conceptual framework, we and others have proposed that the ONH is a biomechanical structure in which IOP-related stress (force/cross sectional area) and strain (local relative deformation of the tissues) are central determinants of both the physiology and pathophysiology of the ONH tissues and their blood supply at all levels of IOP (Burgoyne, Downs et al. 2005, Downs, Roberts et al. 2008, Sigal, Roberts et al. 2010, Campbell, Coudrillier et al. 2013) (Figure 2). IOP perturbations induce not only alterations to the load-bearing ECM of the lamina cribrosa and the peripapillary sclera, but also activate the resident cells of these tissues and damage the RGC axons in the ONH (Burgoyne 2011). Age-related changes to the cells and ECM also significantly affect the ONH biomechanical environment, so aging is an important pillar of the biomechanical framework (Burgoyne and Downs 2008). The prevalence of glaucoma is much higher in persons of African heritage compared to persons of European descent (Quigley and Broman 2006, Rudnicka, Mt-Isa et al. 2006). Recent studies have shown significant racial differences in scleral stiffening with age, which indicates that racial disparities in ocular biomechanics may play a role in glaucoma susceptibility.
Although clinical IOP-lowering remains the only proven method of preventing the onset and progression of glaucoma, the role of IOP in the development and progression of the disease is not well understood. This largely arises from the clinical observation that significant numbers of patients with normal IOPs develop glaucoma (e.g., normal or low-tension glaucoma), while other individuals with elevated IOP show no signs of the disease. This could mean that IOP (or some factor driven by IOP) is a primary causative factor in glaucoma, and IOP vulnerability varies between individuals. Another possibility is that clinical characterization of mean IOP using infrequent snapshot measurements fails to capture exposure to injurious IOP fluctuations that are partly driving the disease in these normotensive glaucoma patients, which makes the IOP-glaucoma relationship murky.
Recent data indicate that IOP fluctuates as much as 5 mmHg day-to-day and hour-to-hour, and 15 to 40 mmHg second-to-second when measured continuously via telemetry in unrestrained, awake nonhuman primates (Downs, Burgoyne et al. 2011) (Figure 3). Very little is known about IOP fluctuations in humans and how the eye responds to those fluctuations, but IOP levels at all timescales have the potential to injure the RGC axons in the ONH (Cullen and LaPlaca 2006, Resta, Novelli et al. 2007). Interestingly, recent work has shown that the sclera (Coudrillier, Tian et al. 2012, Geraghty, Jones et al. 2012, Fazio, Grytz et al. 2014), lamina cribrosa (Albon, Purslow et al. 2000), and cornea (Knox Cartwright, Tyrer et al. 2011) stiffen significantly with age. One might assume this to be protective against axon damage, as stiffer connective tissues resist mechanical deformation better than more compliant tissues. However, stiffening of the corneoscleral shell also induces larger IOP spikes related to blinks, saccades, and vascular filling, in that the eye is less able to elastically expand to absorb IOP impulse (a measure of the mechanical insult delivered by IOP spikes). Hence, it may be that in the process of age-related connective tissue stiffening, the eye has remodeled to a state wherein the ONH is subjected to much larger IOP spikes.
Figure 3. High- and Low-frequency IOP Fluctuation in the Nonhuman primate.
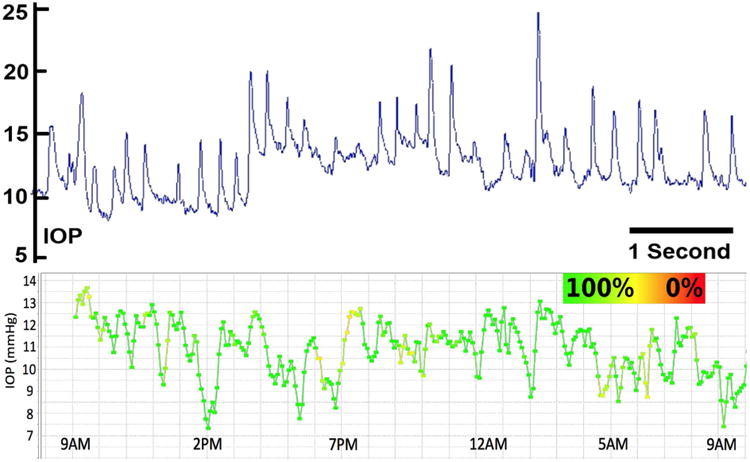
Top:Screen capture of approximately 7 seconds of the continuous IOP tracing from an unrestrained awake primate showing baseline mean IOP of ∼8-13 mmHg and IOP fluctuations up to 12 mmHg associated with blinks and saccadic eye movements. IOP fluctuations can be much larger and of longer duration, especially when the animal squints or is agitated or stressed. Bottom: Plot of the 10-minute time-window average of 24 hours of continuous IOP showing low frequency IOP fluctuation from a single nonhuman primate. The color of the plot points and lines indicate how much data were removed from each 10-minute window after post-hoc digital filtering of signal dropout and noise. Green indicates that 100% of the continuous IOP data were used in the 10-minute average IOP plotted in each point, and yellow indicates that 50% were eliminated due to signal dropout or noise. Note the fluctuations in IOP are substantial even when the high-frequency IOP spikes seen in the top plot are averaged out. Adapted from Downs, et al. (Downs, Burgoyne et al. 2011).
Whether it is mean IOP and/or IOP fluctuations that drive glaucomatous pathogenesis, there is a wide spectrum of individual susceptibility to IOP-related glaucomatous vision loss, and the biomechanical effects of IOP on the tissues of the ONH likely play a central role in the development and progression of the disease at all IOPs. The individual susceptibility of a particular patient's ONH to IOP insult is likely a function of the biomechanical response of the constituent tissues and the resulting mechanical, ischemic and cellular events driven by that response. Hence eyes with a particular combination of tissue geometry and material properties may be susceptible to damage at normal IOP, while others may have a combination of ONH tissue geometry and material properties that can withstand even high levels of IOP. Age-related changes in the ONH and peripapillary sclera alone may change the biomechanical environment of the ONH sufficiently that RGC axonal damage results, even in the absence of chronically elevated IOP. Recent studies have also suggested that vascular deficiencies (Flammer, Konieczka et al. 2013, Wang, Cull et al. 2014), as well as age-related loss of metabolic efficiency (Chrysostomou, Trounce et al. 2010, Kong, van Bergen et al. 2012) are also likely to contribute to the increased risk of glaucoma in the elderly.
In this review, we focus on ocular biomechanics along two main themes: what is known about how mechanical forces and the resulting deformations are distributed in the posterior pole and ONH (biomechanics) and what is known about how the living system responds to those deformations (mechanobiology) in both aging and disease.
2. Mechanical Environment of the Optic Nerve Head and Peripapillary Sclera
2.A. Basic Engineering Concepts
The following are fundamental terms and concepts from engineering mechanics (Timoshenko 1970) that may not be familiar to clinicians and non-engineering scientists. Stress is a measure of the load applied to, transmitted through, or carried by a material or tissue. Stress can be defined as the amount of force applied to a tissue divided by the cross sectional area over which it acts (e.g., pressure is a stress and can be expressed in pounds per square inch or psi). It is important to note that stress is a mathematical quantity that can be calculated, but cannot be directly measured, felt, or observed. Strain is a measure of the local deformation in a material or tissue induced by an applied stress. It is important to recognize that strain, unlike stress, may be observed and measured experimentally. The localized relative displacement described by the strain provides a measurable indicator of the level of micro-deformation (stretch, compression, or shearing) experienced by the tissue. Local strain is known to induce cellular mechanotransduction (Morrison 2006), and strain in collagen fibrils has been shown to passively protect the fibrils from enzymatic degradation by matrix metalloproteases (Flynn, Bhole et al. 2010). Both these strain-driven mechanisms likely play important roles in connective tissue remodeling.
The material properties of a tissue describe its ability to resist deformation under applied load and therefore relate stress to strain (i.e. load to deformation). Material properties can be thought of as the stiffness or compliance of a particular tissue or material that is intrinsic to the material itself. Hence, a stiff tissue such as sclera can have high stress, but low strain, while an equal volume of compliant tissue like retina might have high strain even at low levels of stress. The material properties of a tissue are characterized by the stiffness, morphology and interactions of its constituents (e.g. elastin, collagen fibrils, proteoglycans, and cells). Biological tissues are living matter and their material properties are not constant but change over the lifespan due to aging, remodeling, wound healing, and disease.
Another very useful concept in biomechanics is structural stiffness, which incorporates both the material properties and geometry of a complex load bearing structure into a composite measure of the structure's resistance to deformation. In the posterior pole, both the geometry and material properties of the sclera and lamina cribrosa contribute to structural stiffness, and hence determine the deformation of the ONH and peripapillary sclera when exposed to IOP. As such, individual ONH biomechanics is governed by the geometry (size and shape of the scleral canal, scleral thickness, regional laminar density and beam orientation) and the material properties (stiffness) of the lamina cribrosa and sclera. Hence, two eyes exposed to identical IOPs may exhibit very different strain fields due to differences in their structural stiffness (Figure 4).
Figure 4. Complexities in the Posterior Pole Biomechanical Response.
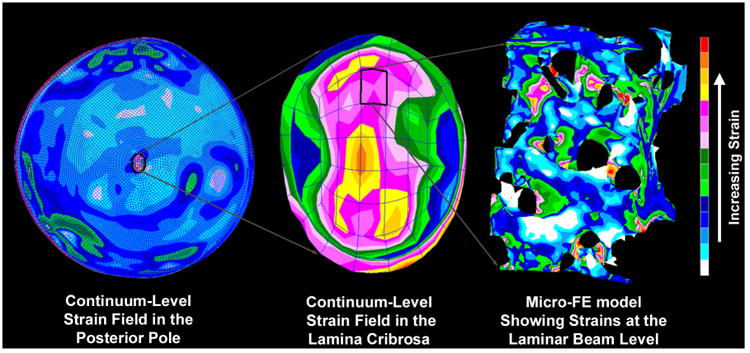
The image on the left shows the strain distribution in a macro-scale model of the connective tissues of the posterior pole of the eye. Note that thickness variations in the sclera give rise to a non-uniform distribution of strain within the scleral shell and that the strains are lower in the sclera than in the more compliant lamina cribrosa. The middle image shows a detail of the strain field within the macro-scale representation of the lamina cribrosa. While this portion of the model has been assigned regional material properties related to the amount and orientation of the laminar beams (based on 3D reconstruction data such as that in Figure 5), the continuum description represents a bulk homogenization of the specific microarchitecture in each element. The right image shows the distribution of mechanical strain at the micro-scale in the laminar beam microarchitecture, demonstrating that strains concentrate focally in individual beams and around individual pores.
2.B. Overview of the Mechanical Environment of the ONH and Peripapillary Sclera
The biomechanics of the ONH and peripapillary sclera are very complex (Figure 4). IOP-related stress generates strain patterns in the ONH and peripapillary sclera that are not only dependent on local connective tissue geometries (Figures 1 and 4) and material properties, but are also influenced by complex dynamic loading conditions (Figure 3). The important factors governing the ONH's response to IOP include the alignment and density of collagen fibrils in each tissue (stiffness and anisotropy) (Coudrillier, Boote et al. 2012, Pijanka, Coudrillier et al. 2012, Grytz, Fazio et al. 2014), the regional density and thickness of the tissue (Roberts, Grau et al. 2009, Roberts, Liang et al. 2010, Fazio, Grytz et al. 2012), the rate of change in IOP (via tissue viscoelasticity) (Downs, Burgoyne et al. 2011) and the level of IOP-related strain at the time of altered loading (via tissue nonlinearity) (Grytz, Fazio et al. 2014). In broad terms, the ONH connective tissues should be stiffer when there is already considerable strain present (elevated steady-state IOP) and/or if the IOP load is applied quickly (IOP spikes). Conversely, the ONH should be more compliant in response to slow changes in IOP and/or at low baseline levels of strain. In addition, studies have shown that mechanical strain is higher in regions of ONH where laminar density is lower (Roberts, Liang et al. 2010) (Figure 5).
Figure 5. Regional differences in laminar microarchitecture in a normal eye, and the predicted relationship between regional laminar density and strain.
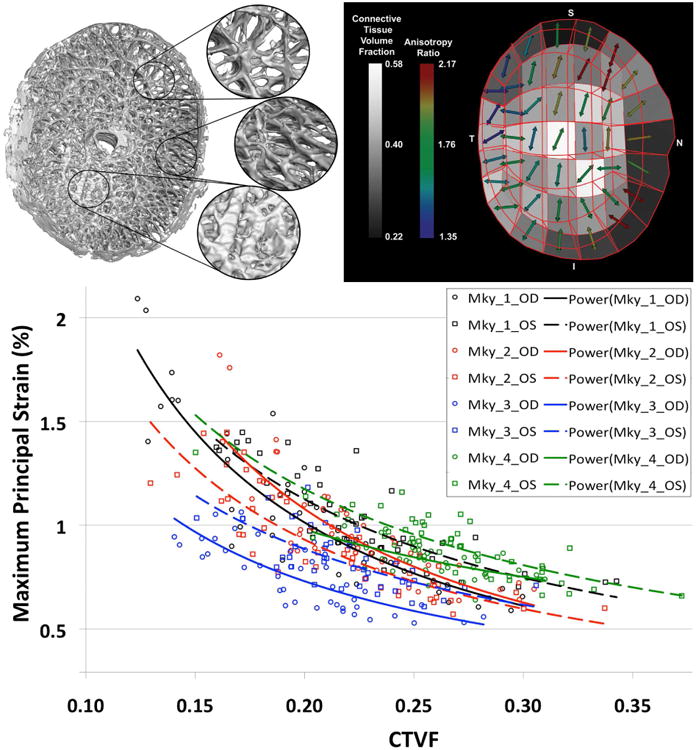
Characterization of the laminar microarchitecture (Left) utilizes the element boundaries of a continuum finite mesh to partition the lamina cribrosa connective tissue into forty-five sub-regions (Right). The connective tissue volume fraction (CTVF) for each region is expressed as a percentage and mapped to a grayscale value in the background. The arrows indicate the predominant orientation of the laminar beams in each region, with higher values (color-coded) indicating regions in which the beams are more highly oriented. Note that in the peripheral regions of the lamina, the beams are tethered radially into the scleral canal wall. (Bottom) FE model simulations in both eyes of four nonhuman primates show that strains are highest in areas where the laminar density is lowest, and strains are lowest in areas where laminar density is highest (Roberts, Liang et al. 2010). The symbols represent strains in each of the elements, colored by eye and animal, and the lines represent the nonlinear fit to those data.
It is important to note that the ONH responds to IOP elevations as a structural system, so the acute mechanical response of the lamina cribrosa is confounded with the responses of the peripapillary sclera, prelaminar neural tissues, and retrolaminar optic nerve. The lamina cribrosa lies underneath the prelaminar neural tissues and the structural responses of these two tissues to acute IOP elevations are quite different; hence, laminar deformation cannot be directly measured from imaging the surface topography of the ONH (Agoumi, Sharpe et al. 2011).
It's important to keep in mind that cerebrospinal fluid pressure works in concert with IOP to determine the translaminar pressure gradient (a biomechanical stress; Figure 2) that must be borne by the lamina cribrosa (Morgan, Chauhan et al. 2002). Emerging evidence suggests a link between glaucoma and low cerebral spinal fluid pressure in patients, further bolstering the evidence that laminar biomechanics plays a central role in glaucoma (Berdahl, Allingham et al. 2008, Berdahl, Ethier et al. 2009, Berdahl and Allingham 2010, Fleischman, Berdahl et al. 2012). A recent groundbreaking study showed glaucomatous ONH changes in nonhuman primates subjected to experimental lowering of cerebrospinal pressure via lumbar shunt, without any perturbation in IOP (Yang, Fu et al. 2014). Even though glaucomatous ONH changes only occurred in half the primates subjected to CSFP lowering, this study definitively shows that an increase in the translaminar pressure difference, either by increasing IOP or decreasing CSFP, can induce glaucoma-like changes in the ONH and coincident axonal loss (Yang, Fu et al. 2014).
Intuitively, it may seem that for a given acute increase in IOP, the lamina cribrosa should deform posteriorly. Recent histologic and in vivo OCT imaging studies have shown that the lamina cribrosa does not consistently deform posteriorly when IOP increases (Yang, Downs et al. 2009, Agoumi, Sharpe et al. 2011, Strouthidis, Fortune et al. 2011). In some eyes, the lamina moves anteriorly with respect to Bruch's membrane opening after acute IOP elevation (Yang, Downs et al. 2009, Agoumi, Sharpe et al. 2011, Strouthidis, Fortune et al. 2011). Thus, our current understanding of the aggregate response of the ONH to acute IOP elevation is that expansion of the scleral canal pulls the lamina taut within the plane of the sclera to a variable degree, making it more resistant to posterior deformation out of that plane (Sigal, Roberts et al. 2010, Sigal, Yang et al. 2011, Sigal, Yang et al. 2011). It is important to note that the apparent lack of anterior-to-posterior laminar deformation with acute IOP elevation does not mean that the lamina is not strained. In this scenario, the expansion of the canal stretches the lamina cribrosa within the plane of the sclera generating substantial strain within the laminar beams.
The cells that maintain the ocular connective tissues are biologically active, and the ONH and sclera are constant state of remodeling. Grytz and colleagues have proposed the notion that the cells are constantly expressing factors conducive to ECM degradation and synthesis in an effort to maintain a homeostatic mechanical environment (Grytz, Sigal et al. 2012). ONH cells express the pro-fibrotic growth factors TGFb2, CTGF, and gremlin, which have been shown to increase ECM production (Wordinger, Agarwal et al. 2002, Zode, Clark et al. 2009, Fuchshofer 2011, Zode, Sethi et al. 2011, Wallace, Clark et al. 2013, Wallace, Murphy-Ullrich et al. 2014). Interestingly, both TGFb2 and gremlin are elevated in the glaucomatous ONH. As such, the geometry and material properties of the sclera and lamina cribrosa change in response to both physiologic (age) and pathologic (IOP-related damage) factors. These processes are in part driven by the natural aging processes that induce collagen crosslinking (Sady, Khosrof et al. 1995, Birch, Bailey et al. 1999, Vasan, Foiles et al. 2003), and vascular (Sawabe 2010) and cellular (Chrysostomou, Trounce et al. 2010, Kong, van Bergen et al. 2012) changes. The eye is also exposed to ever changing loading conditions because IOP is extremely dynamic, with short-term and long-term fluctuations ranging from blinks and eye rubs to circadian rhythms (Downs, Burgoyne et al. 2011) (Figure 3). Liu and colleagues have recently shown that stiffening even small areas of the ocular coat leads to increased IOP spikes associated with controlled microvolumetric fluid injections in porcine eyes (Liu and He 2009). Also, IOP spike amplitude has been shown to be positively correlated with scleral stiffness (Morris, Tang et al. 2013). Hence, IOP dynamics, and resulting dynamic mechanical stress and strain, also change with aging because the corneoscleral shell is less able to elastically deform to absorb energy as it stiffens with age (Coudrillier, Tian et al. 2012, Fazio, Grytz et al. 2014), resulting in larger IOP spikes in the elderly even when steady-state IOP remains unchanged with age.
2.C. The Contribution of the Sclera to ONH Biomechanics
The results described above, as well as the closed form analyses and computational models described in the next section suggest that the sclera plays an important role in ONH biomechanics. The peripapillary sclera provides the mechanical boundary conditions for the ONH. By this we mean that the peripapillary sclera is the tissue through which load and deformation are transmitted to the ONH, and that the structural stiffness of the peripapillary sclera, therefore, influences how the lamina deforms (Figure 6). This can be understood from the discussion above in which a compliant sclera allows the scleral canal to expand following an acute IOP elevation, pulling the lamina taut within the canal and thereby increasing laminar resistance to posterior deformation. In contrast, a rigid sclera allows less IOP-driven expansion of the scleral canal or none at all, forcing the structural stiffness of the lamina alone to bear the IOP-related stress. Hence, characterization of both components of scleral structural stiffness (geometry and material properties) is essential to understanding the effects of IOP on the ONH.
Figure 6. The Influence of Scleral Mechanics on ONH Mechanics.
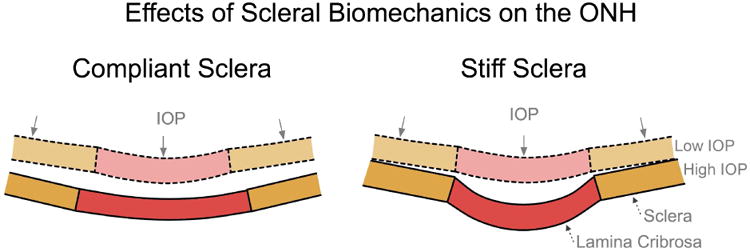
IOP induces large scleral canal expansions in eyes with compliant sclera (left) that pulls the contained lamina taut despite the direct posterior force of IOP on the laminar surface. Conversely, a stiff sclera allows relatively little canal expansion with IOP elevation (right) and less stretching of the contained lamina, thus allowing the lamina to be displaced posteriorly by the direct action of IOP on its anterior surface. Adapted from (Sigal, Roberts et al. 2010).
2.C.1 Scleral Geometry
Maps of the thickness variation for the posterior pole of human (Olsen, Aaberg et al. 1998, Norman, Flanagan et al. 2010) eyes show extreme spatial variation in scleral thickness, with very thin regions near the equator (as low as 300 μm in the human). The peripapillary sclera is notably thicker (1000 μm in the human), and the biomechanics of the peripapillary sclera has been shown to directly influence ONH biomechanics in computational simulations (Norman, Flanagan et al. 2011). Interestingly, this thick ring of peripapillary sclera is absent in the nasal quadrant of nonhuman primate eyes due to the oblique nasal insertion of the optic nerve through the scleral canal (Downs, Blidner et al. 2002). Such variation in peripapillary scleral thickness, whether they occur naturally or in pathologic conditions such as myopia, certainly influence biomechanics (Figure 4) and may be important in assessing individual susceptibility to glaucomatous damage.
2.C.2 Characterization of Scleral Material Properties
Uniaxial testing of scleral strips has been used to estimate scleral material properties in various species. However, uniaxial testing of scleral strips is limited in its ability to describe the nonlinear and anisotropic responses of the sclera, which led to the development of new approaches to measure scleral strain in 3D under inflation. Girard and Fazio have used a customized scleral shell pressurization apparatus, precise IOP control, and laser-based electronic speckle pattern interferometry to measure the IOP-induced 3D deformation of the entire posterior scleral shell in nonhuman primates (Girard, Downs et al. 2007) and humans (Fazio, Grytz et al. 2012). Their results show that posterior sclera is highly nonlinear (it gets stiffer as IOP increases) and anisotropic (the underlying collagen fibril distribution is non-uniform and changes throughout the scleral shell, which affects directional stiffness) (Girard, Downs et al. 2007). In addition, Fazio has measured the regional strains in the posterior human eye, and found that peripapillary strains are highest in the temporal and inferior quadrants, and are in general higher than those further away from the ONH (Fazio, Grytz et al. 2012). Two groups have used inflation testing to show that human scleral strain significantly decreases with age, i.e., the sclera exhibits significant age-related stiffening (Coudrillier, Tian et al. 2012, Fazio, Grytz et al. 2014, Fazio, Grytz et al. 2014, Grytz, Fazio et al. 2014) and another study showed a similar result in scleral strips (Geraghty, Jones et al. 2012). A computational study using inverse modeling to fit scleral material properties to experimental inflation data has shown that this age-related stiffening is likely due to due to a higher shear stiffness and a lower level of stretch at which the collagen fibrils uncrimp and stiffen (Grytz, Fazio et al. 2014). The sclera is also stiffer in human donor eyes with glaucoma (Coudrillier, Tian et al. 2012), and studies in the nonhuman primate have shown that the scleral shell stiffens in response to chronic IOP exposure (Girard, Suh et al. 2011).
Many studies have shown peripapillary scleral biomechanics to be an important determinant of the ONH biomechanical environment, but there is little evidence that peripapillary scleral strain is directly related to axonal homeostasis in the ONH. Recent studies have shown that the stiffness of the immediate peripapillary sclera changes significantly with age, but that the response varies by location (Fazio, Grytz et al. 2014). The resulting maps of the coefficients of scleral stiffness change with age in normal eyes match well with maps of neural rim area measurement change with age published for normal human eyes (See, Nicolela et al. 2009) (Figure 7). While this may be entirely coincidental, it does provide some support for the notion that age-related changes in scleral stiffness may impact normal age-related loss of axons in adjacent regions of the ONH.
Figure 7. A possible link between age-related changes in scleral stiffness and age-related loss of axons in normal eyes?
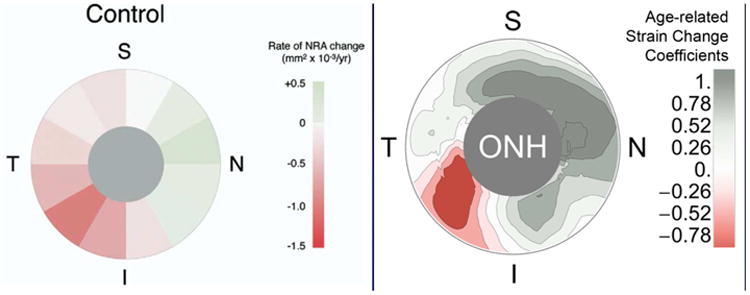
Maps showing the rate of change in neural retinal rim area measurement with age in normal human eyes (See, Nicolela et al. 2009) and the age-related change in underlying peripapillary scleral structural stiffness (note negative coefficients indicate increased scleral compliance with age and positive coefficients indicate increased stiffness with age) (Fazio, Grytz et al. 2014). While these results are independent and there has not been a causative link established between these phenomena, one might speculate that the age-related changes in scleral stiffness contribute to the pattern of age-related axon loss in normal eyes.
2.D. Estimating Stress and Strain in the ONH and Peripapillary Sclera
Attempts to mathematically model the mechanical environment of the ONH generally fall into two broad categories – closed form solutions and numerical simulations. In closed form solutions, engineering principles are used to derive equations that can be analyzed to understand the effects of selected biological parameters. However, closed form solutions are of limited utility because they cannot capture the complexity of the ONH and peripapillary scleral tissues (e.g., the non-uniform and asymmetric geometry and material properties). To overcome the inherent limitations of closed form solutions, researchers often utilize numerical simulation methods to study more complex biological systems. One of the most powerful of these is FE analysis. In FE analysis, complex load-bearing structures are broken into small, regularly shaped elements (Figure 4). Stress and strain within each element is calculated and then superposed to predict the mechanical response of the entire structure.
The power of FE analysis lies in its ability to model structures with highly complex geometries using material properties with varying levels of complexity as warranted (e.g., inhomogeneous, anisotropic, nonlinear, or viscoelastic material descriptions). The three inputs necessary for FE models are the 3D geometry of the tissue structure to be modeled, the material properties of the different tissues in the model, and appropriate loading and boundary conditions. These requirements have spurred the development of methodologies to isolate and describe the 3D geometry of the ONH and peripapillary sclera and experimentally characterize their constituent material properties (Figure 5).
2.D.1. Parametric and Eye-specific Simulations of ONH Biomechanics
Parametric modeling involves computing stress and strain in average, idealized geometries that do not conform to any individual's particular anatomy. Within these models, parameters such as peripapillary scleral thickness and laminar stiffness can be varied independently to gauge that parameter's effects on ONH biomechanics as a whole (Sigal, Flanagan et al. 2005). To address the limitations of idealized geometric and simplified material property descriptions inherent in parametric FE models, individual-specific FE models can be created from the reconstructed geometries of particular eyes (Sigal, Flanagan et al. 2007, Roberts, Liang et al. 2010). At present, individual-specific modeling is based on high-resolution 3D reconstructions of nonhuman primate and human cadaver eyes (Figure 4), with a long-term goal to build models based on clinical imaging of living eyes so as to use them in the assignment of target IOP in clinical management of glaucoma. This is especially important given that the 3D geometry of the scleral canal and peripapillary sclera largely determine the stress and strain transmitted to the contained ONH. Anatomically accurate 3D models are necessary to capture the biomechanics of anisotropic scleral material properties (varying collagen fibril orientation), scleral canals that are non-circular and have varying optic nerve insertion angles (i.e. the optic nerve inserts from the nasal side resulting in a thinner peripapillary sclera in that quadrant), and regional variations in laminar density and beam orientation. When modeling an ONH with anatomic fidelity, the tissue geometries can be constructed either by serial histologic methods or 3D imaging, and material properties are generally determined through direct mechanical testing. Unfortunately, imaging of the lamina in vivo is not yet possible at the resolutions required for modeling, and no technology exists for experimental biomechanical testing of laminar beams. As a result, ONH FE models are typically constructed from eyes that are perfusion or immersion fixed at a selected IOP, and then undergo ex vivo 3D reconstruction of their connective tissues.
Burgoyne and colleagues developed a histologic technique to reconstruct the 3D trabeculated structure of the lamina cribrosa from individual nonhuman primate eyes that were perfusion fixed at varying levels of IOP (Burgoyne, Downs et al. 2004). The resulting 3D data sets form the geometries of individual-specific FE models of the ONH at the macro- and micro-scale. Roberts and co-workers have developed macro-scale continuum FE models of the posterior pole and ONH connective tissues from individual nonhuman primate eyes (Figure 5) (Roberts, Liang et al. 2010). In these models, the laminar microarchitecture is modeled using a continuum approach, with anisotropic material properties assigned to each FE in the ONH based on the connective tissue volume fraction and the predominant beam orientation of the contained laminar microarchitecture (Figure 5). Regional differences in connective tissue volume fraction and predominant orientation are translated into similar distributions in local oriented stiffness so that regions of higher and lower porosity reflect greater and lesser compliance, respectively. The inclusion of regional laminar material properties (connective tissue volume fraction and beam orientation) into FE models has a pronounced effect on the ONH's response to IOP (Figure 5). This indicates that the regional variations in laminar geometry and structural stiffness must be represented in models to fully capture the biomechanical behavior of the ONH. Furthermore, these results show that regional laminar density is significantly associated with regional stress and strain, with areas of high laminar density showing less strain and regions of low laminar density exhibiting high strains (Roberts, Liang et al. 2010) (Figure 5).
2.D.2. Biomechanics of the Laminar Microstructure
We have also used the 3D reconstruction and continuum modeling approaches to characterize and explore laminar beam biomechanics (Downs, Roberts et al. 2007). This micro-scale modeling approach utilizes a substructuring technique based on parent macro-scale FE models to calculate the IOP-related stress and strain fields in laminar beams (Downs, Roberts et al. 2007) (Figure 4). This technique reveals a complexity of IOP-related strains and stresses within the lamina cribrosa microarchitecture that is not available through macro-scale FE modeling. There have been several important results from this work. First, stress and strain in the laminar microarchitecture are likely higher than predicted by macro-scale models of the ONH. Second, even at normal levels of IOP, the micro-FE models predict that while the majority of laminar beams are within physiologic strain ranges, there are individual laminar beams with levels of IOP-related strain that are likely pathologic. Third, mean strain within the laminar beams of different nonhuman primates varies greatly, and is generally dependent on the 3D geometry of each eye's ONH connective tissues. (Roberts, Liang et al. 2010)This approach holds the possibility of testing hypotheses about failure mechanisms and cellular responses at the level of the laminar beams.
3. Remodeling of the Optic Nerve Head With Age and Disease
3.A. Aging
The ONH connective tissues are exposed to substantial levels of IOP-related stress and strain at normal levels of IOP (Figure 4). Physiologic levels of stress and strain experienced over a lifetime induce a broad spectrum of changes in both the connective tissues and vasculature that are central to normal aging. Thus, the restructuring and remodeling of glaucomatous damage (described in the following sections), should be understood to occur in the setting of the physiologic restructuring and remodeling inherent in normal aging.
Age-related alterations of the laminar ECM have been reported to include increased collagen deposition, thickening of astrocyte basement membranes (Morrison, Jerdan et al. 1989, Morrison, Dorman-Pease et al. 1990) and increased rigidity (Albon, Karwatowski et al. 1995). Age-related changes in the scleral and laminar ECM significantly reshapes the biomechanical environment of the ONH (Albon, Karwatowski et al. 1995, Fazio, Grytz et al. 2014). But aging not only stiffens the connective tissues (Bailey, Paul et al. 1998); it should also diminish nutrient diffusion from the laminar capillaries through the laminar ECM, across the astrocyte basement membranes, and into the adjacent axons. Thus, in addition to the effects of age-related changes in ONH biomechanics, axonal nutrition in the aged eye may be further impaired as a result of diminished nutrient diffusion from the laminar capillaries to the center of the axon bundles. Glaucoma is primarily a disease of aging (Gordon, Beiser et al. 2002, Leske, Heijl et al. 2003), and recent work has shown that the sclera and lamina cribrosa stiffen significantly with age (Albon, Karwatowski et al. 1995, Coudrillier, Tian et al. 2012, Geraghty, Jones et al. 2012, Fazio, Grytz et al. 2014). One might assume this to be protective against mechanical strain, in that stiffer connective tissues resist mechanical strain better than more compliant tissues. It is important to note however that this stiffening also induces larger IOP spikes related to blinks, saccades, and vascular filling, in that the eye is less able to elastically expand to absorb IOP energy. Hence, it may be that the process of age-related connective tissue stiffening has remodeled the eye to a state that is actually more susceptible to IOP spikes. A recent study in human cadaver eyes has shown that the peripapillary sclera stiffens significantly with age in donors of European descent, but stiffens much more rapidly with age and over a larger area of the posterior scleral shell in donors of African heritage (Fazio, Grytz et al. 2014). IOP spikes should therefore be higher in the elderly, and even higher in persons of African descent, which may contribute to the increased glaucoma prevalence in these at risk populations. Recent work in an experimental mouse model of glaucoma supports this view, in that a stiffer sclera was associated with increased ganglion cell loss (Nguyen, Cone et al. 2013, Pease, Oglesby et al. 2014, Steinhart, Cone-Kimball et al. 2014). Also, Vande Geest and coworkers observed that the local orientation of the collagen fibrils changes through the thickness of the sclera and was found to be different between donors of African heritage versus European heritage, although no significant changes were seen with age (Yan, McPheeters et al. 2011).
3.B. IOP-driven Alterations of the ONH in Glaucoma
We have proposed a comprehensive framework within which to understand the biomechanically driven remodeling of the lamina cribrosa that results in glaucomatous ONH cupping (Downs, Roberts et al. 2011) (Figure 2). Pathophysiologic stress and strain induce pathologic changes in cell synthesis and tissue microarchitecture that exceed the effects of aging and underlie the two governing pathophysiologies in glaucoma: 1) growth and/or remodeling of the load-bearing connective tissues of the ONH, and 2) progressive damage to the adjacent axons through multiple pathways (Figure 2).
Early glaucomatous damage has not been rigorously studied in humans because human cadaver eyes with well-characterized early damage are rare. In nonhuman primates, following moderate experimental IOP elevations, we have described the following changes in ONH and peripapillary scleral connective tissue architecture and material properties at the onset of confocal scanning laser tomography-detected ONH surface change (clinical cupping): 1) enlargement and elongation of the neural canal (Downs, Yang et al. 2007); 2) posterior deformation and thickening of the lamina cribrosa (Yang, Downs et al. 2007); 3) outward migration of the posterior lamina insertion point (Yang, Williams et al. 2011) and significant but less pronounced outward migration of the anterior lamina insertion point (Yang, Williams et al. 2011); 4) alterations in the elastic and viscoelastic material properties of the peripapillary sclera (Downs, Suh et al. 2003, Girard, Suh et al. 2011). The increase in laminar thickness in these early glaucoma nonhuman primate eyes is likely due to connective tissue remodeling and new connective tissue synthesis. Quantification of the amount of connective tissue within 3D reconstructions of the lamina showed an increase in connective tissue volume of 44% to 82% in early glaucoma compared to their contralateral control eyes, which is at least partially driven by the recruitment of retrolaminar septa into the load-bearing 3D laminar structure (Roberts, Grau et al. 2009). These data strongly support the notion that connective tissue remodeling and new connective tissue synthesis are very active in this early stage of the neuropathy. Furthermore, the work of Yang, Burgoyne and co-workers has shown that the lamina cribrosa migrates posteriorly in the neural canal during glaucomatous progression, and that process starts early in the disease (Yang, Williams et al. 2011). Laminar depth was significantly larger in glaucoma patients with younger age, higher untreated IOP, and lower RNFL thickness as measured with OCT in a recent crossectional study (Jung, Jung et al. 2014). In a recent comprehensive review, we proposed a framework that supports biomechanics-driven progressive laminar remodeling and migration as the central mechanism underlying the change in lamina cribrosa morphology from normal to the cupped and excavated shape typical of glaucoma (Downs, Roberts et al. 2011).
In vivo OCT imaging studies in which the laminar position was measured relative to Bruch's Membrane opening at baseline and after acute IOP elevation in nonhuman primates with experimental glaucoma have revealed changes in the structural stiffness of the lamina cribrosa that occur in early glaucoma. Preliminary results indicate that the lamina cribrosa deforms significantly more posteriorly in response to an acute IOP elevation from 10 to 30 mmHg in most glaucoma eyes compared to their contralateral normal controls, and that laminar compliance is significantly related to the peak IOP measured after chronic IOP elevation was induced. This suggests that IOP-driven remodeling is altering laminar structural stiffness as glaucoma progresses. Modeling studies in nonhuman primate early glaucoma have supported this hypothesis, and predict that even though the lamina cribrosa adds a significant volume of connective tissue through remodeling very early in the disease, the laminar connective tissue is weakened considerably during that process, resulting in a more substantial lamina that is still structurally more compliant than its contralateral control eye (Roberts, Sigal et al. 2010). These results lend credibility to the notion that the remodeling cascade in the laminar ECM begins with a reorganization process that weakens the tissues very early in the disease process, which is followed by a consolidation and stiffening process.
It has been proposed that ONH astrocytes and lamina cribrosa cells play a central role in mediating the laminar ECM remodeling response and the resulting axonal insult (Hernandez, Luo et al. 1989, Hernandez and Ye 1993, Hernandez 2000, Kirwan, Fenerty et al. 2005, Balaratnasingam, Morgan et al. 2008, Wallace, Murphy-Ullrich et al. 2014). Cell activity associated with ECM remodeling has been observed in response to glaucoma in humans and exposure to chronically elevated IOP in animal models. Agapova and colleagues showed that matrix metalloproteases (MMPs) are elevated in the lamina cribrosa of nonhuman primates with experimental glaucoma, but not those with optic nerve transection (Agapova, Kaufman et al. 2003). These compounds are known to break down the ECM and allow cells to migrate and rebuild the matrix (Hernandez 2000).
Recent numerical growth and remodeling simulations have given us additional confidence in the hypothesis that local biomechanics is driving ONH remodeling in glaucoma. Grytz and colleagues performed a FE simulation study based on a homeostatic control mechanisms that predicted that the lamina cribrosa has to thicken by about 40% to maintain optimal load-bearing conditions at the collagen fibril level for a chronic IOP elevation from 15 to 25 mmHg (Grytz, Sigal et al. 2012). Their study also suggested that the thickening of the LC is mainly due to the recruitment of pre- and retro-laminar tissue into the lamina cribrosa, which agrees with previous experimental studies (Roberts, Grau et al. 2009). Their results also suggest that the lamina is biologically optimized to withstand IOP and constantly remodels to maintain strain within a homeostatic range.
Axoplasmic transport blockade in the ONH has been associated with acute (Quigley and Anderson 1976, Quigley and Anderson 1977) and chronic IOP elevations (Quigley and Addicks 1980), which indicates that IOP and its mechanical effects on the load-bearing tissues, vasculature (Geijer and Bill 1979, Radius 1980), and/or cells directly affects axonal homeostasis.
Studies of alterations in blood flow in early glaucoma are only just beginning. Recent studies by Wang and co-workers have shown that basal blood flow in the ONH is significantly decreased in early experimental glaucoma in nonhuman primates (Wang, Cull et al. 2012). They also reported changes in the time course of blood flow autoregulation (Cull, Burgoyne et al. 2013), which indicate that chronic alterations in blood flow accompany the alteration in connective tissue architecture and material properties described above. Together, these studies paint a complex picture of glaucomatous pathogenesis that involves simultaneous alterations in the connective tissues, cells and vasculature. The unifying theme that ties these mechanisms together is the involvement of ONH biomechanics in the disease cascade.
3.B.1. The Biomechanical Mechanisms Underlying Collagen Growth and Remodeling
The previously discussed numerical simulations were designed to estimate the stress and strain environment in the ONH for a given material or collagen architecture. Recent advances in numerical remodeling allow us gain insight into the origin of these anisotropic collagen structures in the eye. In these studies, stress or strain is not merely a predicted variable but is also used to predict the collagen fibril architecture (anisotropy) based on a remodeling rule. Biomechanically induced remodeling of tissue anisotropy was estimated by allowing collagen fibers to be adaptively reoriented towards optimal load-bearing conditions based on the IOP-related tissue stress. This numerical approach was used to predict the physiological collagen fiber architecture in the peripapillary sclera and lamina cribrosa (Grytz, Fazio et al. 2014). The numerical results suggest that the anisotropic collagen fibril architecture in the peripapillary sclera and lamina cribrosa evolved to establish optimal load-bearing conditions in the connective tissues. Furthermore, the numerical remodeling simulation provides insight into the significant effects of these underlying collagen fiber orientations on the IOP-related deformation of the ONH. The simulations show that the circumpapillary ring of collagen fibers protects the ONH from large scleral canal expansions and as such shields the lamina cribrosa and neural canal tissues from high tensile stresses. In contrast, the radial alignment of fibers in the periphery of the lamina cribrosa seems to reinforce the lamina cribrosa against posterior deformations and high transversal shear stresses.
3.B.2. Experimental Assessment of Collagen Remodeling
Experimental studies have shown that collagen fibril synthesis, degeneration and remodeling in soft tissues is modulated by mechanical stress and strain (Flynn, Bhole et al. 2010, Foolen, van Donkelaar et al. 2010). Furthermore, recent findings suggest that growth and remodeling mechanisms occur in soft tissues to establish and maintain optimal load-bearing conditions and these optimal conditions seem to be defined at the collagen fibril level (Flynn, Bhole et al. 2010, Foolen, van Donkelaar et al. 2010). Based on these findings, Grytz and colleagues developed a numerical growth and remodeling method and applied it to the ONH (Grytz, Sigal et al. 2012). This study predicted the formation of a lamina cribrosa in human eyes is necessary to establish optimal load-bearing conditions at the collagen fibril level. The simulation also suggests that smaller eyes such as those in rodents might not need a collagenous lamina cribrosa due to their small scleral canal, for which a cellular ‘lamina’ is sufficient (Sun, Lye-Barthel et al. 2009).
Taken together, these results support the hypothesis that elevated IOP, and presumably mechanical insult to the cells and/or reduced blood flow in the laminar region, underlie the significant ECM remodeling observed in glaucomatous eyes. Interestingly, these mechanisms are driven by exposure to IOP, a biomechanical insult, and are not simply a secondary effect of axonal damage and death.
3.C. Clinical Implications
3.C.1. How Might We Assess Biomechanical Risk to the ONH Clinically?
There are currently no science-based tools to predict at what level of IOP an individual ONH will be damaged. Eventually, knowing the relationship between IOP, IOP fluctuations, mechanical strain- or stress-driven remodeling, ONH blood flow, and astrocyte and axonal homeostasis will drive the clinical assessment of safe target IOP, although the technologies to assess these factors have yet to materialize. Once developed, clinical characterization of the actual IOP insult through continuous, telemetric IOP monitoring will eventually allow us to understand the biomechanical loads in the eye, a critical component to understanding the forces driving glaucoma pathophysiology. In vivo imaging has the ability to resolve laminar beams to some extent, and therefore regional laminar density could serve as a biomarker for areas that are under increased strain relative to neighboring regions (Roberts, Liang et al. 2010, Sredar, Ivers et al. 2013, Nadler, Wang et al. 2014, Sigal, Grimm et al. 2014) (Roberts, Liang et al. 2010) (Figure 5). Also, there is increasing evidence that the lamina remodels very early in glaucoma (Roberts, Grau et al. 2009, Yang, Williams et al. 2011), and that the biomechanical behavior of the lamina cribrosa is also altered in this process (Roberts, Sigal et al. 2010). Also, blood flow autoregulation changes are associated with early disease (Wang, Cull et al. 2014), and clinical detection is on the horizon. Once clinically detectable, early stabilization and perhaps reversal of these pathophysiologic changes will become new patient-specific endpoints to determine a safe target IOP.
Highlights of “Optic nerve head biomechanics in aging and disease”:
Nontechnical review focused on educating the reader in optic nerve head biomechanics
Focus on how mechanical forces and the resulting deformations are distributed in the posterior pole and ONH (biomechanics)
Focus on how the living system responds to those deformations (mechanobiology)
Focus on how ONH responds to IOP elevations as a structural system
Discuss the biomechanical basis for IOP-driven changes in connective tissues, blood flow, and cellular responses
Use glaucoma as the primary framework to present the important aspects of ONH biomechanics in aging and disease
Acknowledgments
Much of the work presented herein was funded by NIH Grant R01 EY-018926.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agapova OA, Kaufman PL, Lucarelli MJ, Gabelt BT, Hernandez MR. Differential expression of matrix metalloproteinases in monkey eyes with experimental glaucoma or optic nerve transection. Brain Res. 2003;967(1-2):132–143. doi: 10.1016/s0006-8993(02)04234-8. [DOI] [PubMed] [Google Scholar]
- Agoumi Y, Sharpe GP, Hutchison DM, Nicolela MT, Artes PH, Chauhan BC. Laminar and prelaminar tissue displacement during intraocular pressure elevation in glaucoma patients and healthy controls. Ophthalmology. 2011;118(1):52–59. doi: 10.1016/j.ophtha.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Albon J, Karwatowski WS, Avery N, Easty DL, Duance VC. Changes in the collagenous matrix of the aging human lamina cribrosa. Br J Ophthalmol. 1995;79(4):368–375. doi: 10.1136/bjo.79.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albon J, Purslow PP, Karwatowski WS, Easty DL. Age related compliance of the lamina cribrosa in human eyes. Br J Ophthalmol. 2000;84(3):318–323. doi: 10.1136/bjo.84.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev. 1998;106(1-2):1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- Balaratnasingam C, Morgan WH, Bass L, Ye L, McKnight C, Cringle SJ, Yu DY. Elevated pressure induced astrocyte damage in the optic nerve. Brain Res. 2008;1244:142–154. doi: 10.1016/j.brainres.2008.09.044. [DOI] [PubMed] [Google Scholar]
- Berdahl JP, Allingham RR. Intracranial pressure and glaucoma. Curr Opin Ophthalmol. 2010;21(2):106–111. doi: 10.1097/ICU.0b013e32833651d8. [DOI] [PubMed] [Google Scholar]
- Berdahl JP, Allingham RR, Johnson DH. Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology. 2008;115(5):763–768. doi: 10.1016/j.ophtha.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Berdahl JP, Ethier CR, Allingham RR. Cerebrospinal fluid pressure and glaucomatous optic disc cupping. Graefes Arch Clin Exp Ophthalmol. 2009;247(9):1289–1290. doi: 10.1007/s00417-009-1110-x. author reply 1291-1284. [DOI] [PubMed] [Google Scholar]
- Birch HL, Bailey JV, Bailey AJ, Goodship AE. Age-related changes to the molecular and cellular components of equine flexor tendons. Equine Vet J. 1999;31(5):391–396. doi: 10.1111/j.2042-3306.1999.tb03838.x. [DOI] [PubMed] [Google Scholar]
- Burgoyne CF. A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma. Exp Eye Res. 2011;93(2):120–132. doi: 10.1016/j.exer.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC. Premise and Prediction—How Optic Nerve Head Biomechanics Underlies the Susceptibility and Clinical Behavior of the Aged Optic Nerve Head. J Glaucoma. 2008;17(4):318–328. doi: 10.1097/IJG.0b013e31815a343b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC, Bellezza AJ, Francis Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24(1):39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC, Bellezza AJ, Hart RT. Three-dimensional reconstruction of normal and early glaucoma monkey optic nerve head connective tissues. Invest Ophthalmol Vis Sci. 2004;45(12):4388–4399. doi: 10.1167/iovs.04-0022. [DOI] [PubMed] [Google Scholar]
- Campbell IC, Coudrillier B, Ethier CR. Biomechanics of the posterior eye: A critical role in health and disease. J Biomech Eng. 2013 doi: 10.1115/1.4026286. [DOI] [PubMed] [Google Scholar]
- Chrysostomou V, Trounce IA, Crowston JG. Mechanisms of retinal ganglion cell injury in aging and glaucoma. Ophthalmic Res. 2010;44(3):173–178. doi: 10.1159/000316478. [DOI] [PubMed] [Google Scholar]
- Cioffi GA, Van Buskirk EM. Vasculature of the anterior optic nerve and peripapillary choroid. In: Ritch R, Shields MB, Krupin Mosby T, Louis St, editors. The Glaucomas. Basic Sciences; 1996. pp. 177–197. [Google Scholar]
- Coudrillier B, Boote C, Quigley HA, Nguyen TD. Scleral anisotropy and its effects on the mechanical response of the optic nerve head. Biomech Model Mechanobiol. 2012 doi: 10.1007/s10237-012-0455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudrillier B, Tian J, Alexander S, Myers KM, Quigley HA, Nguyen TD. Biomechanics of the human posterior sclera: age- and glaucoma-related changes measured using inflation testing. Invest Ophthalmol Vis Sci. 2012;53(4):1714–1728. doi: 10.1167/iovs.11-8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull G, Burgoyne CF, Fortune B, Wang L. Longitudinal Hemodynamic Changes within the Optic Nerve Head in Experimental Glaucoma. Invest Ophthalmol Vis Sci. 2013 doi: 10.1167/iovs.13-12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen DK, LaPlaca MC. Neuronal response to high rate shear deformation depends on heterogeneity of the local strain field. J Neurotrauma. 2006;23(9):1304–1319. doi: 10.1089/neu.2006.23.1304. [DOI] [PubMed] [Google Scholar]
- Downs JC, Blidner RA, Bellezza AJ, Thompson HW, Hart RT, Burgoyne CF. Peripapillary scleral thickness in perfusion-fixed normal monkey eyes. Invest Ophthalmol Vis Sci. 2002;43(7):2229–2235. [PMC free article] [PubMed] [Google Scholar]
- Downs JC, Burgoyne CF, Seigfreid WP, Reynaud JF, Strouthidis NG, Sallee V. 24-hour IOP telemetry in the nonhuman primate: implant system performance and initial characterization of IOP at multiple timescales. Invest Ophthalmol Vis Sci. 2011;52(10):7365–7375. doi: 10.1167/iovs.11-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JC, Roberts MD, Burgoyne CF. Mechanical environment of the optic nerve head in glaucoma. Optom Vis Sci. 2008;85(6):425–435. doi: 10.1097/OPX.0b013e31817841cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JC, Roberts MD, Burgoyne CF, Hart RT. Finite element modeling of the lamina cribrosa microstructure in normal and early glaucoma monkey eyes. Invest Ophthalmol Vis Sci 2007 [Google Scholar]
- Downs JC, Roberts MD, Sigal IA. Glaucomatous cupping of the lamina cribrosa: a review of the evidence for active progressive remodeling as a mechanism. Exp Eye Res. 2011;93(2):133–140. doi: 10.1016/j.exer.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JC, Suh JK, Thomas KA, Bellezza AJ, Burgoyne CF, Hart RT. Viscoelastic characterization of peripapillary sclera: material properties by quadrant in rabbit and monkey eyes. J Biomech Eng. 2003;125(1):124–131. doi: 10.1115/1.1536930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JC, Yang H, Girkin C, Sakata L, Bellezza A, Thompson H, Burgoyne CF. Three-dimensional histomorphometry of the normal and early glaucomatous monkey optic nerve head: neural canal and subarachnoid space architecture. Invest Ophthalmol Vis Sci. 2007;48(7):3195–3208. doi: 10.1167/iovs.07-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio MA, Grytz R, Bruno L, Girard MJ, Gardiner S, Girkin CA, Downs JC. Regional variations in mechanical strain in the posterior human sclera. Invest Ophthalmol Vis Sci. 2012;53(9):5326–5333. doi: 10.1167/iovs.12-9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio MA, Grytz R, Morris JS, Bruno L, Gardiner SK, Girkin CA, Downs JC. Age-related changes in human peripapillary scleral strain. Biomech Model Mechanobiol. 2014;13(3):551–563. doi: 10.1007/s10237-013-0517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio MA, Grytz R, Morris JS, Bruno L, Girkin CA, Downs JC. Human scleral structural stiffness increases more rapidly with age in donors of african descent compared to donors of European descent. Invest Ophthalmol Vis Sci. 2014;55(11):7189–7198. doi: 10.1167/iovs.14-14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flammer J, Konieczka K, Bruno RM, Virdis A, Flammer AJ, Taddei S. The eye and the heart. Eur Heart J. 2013;34(17):1270–1278. doi: 10.1093/eurheartj/eht023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman D, Berdahl JP, Zaydlarova J, Stinnett S, Fautsch MP, Allingham RR. Cerebrospinal fluid pressure decreases with older age. PLoS One. 2012;7(12):e52664. doi: 10.1371/journal.pone.0052664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn BP, Bhole AP, Saeidi N, Liles M, Dimarzio CA, Ruberti JW. Mechanical strain stabilizes reconstituted collagen fibrils against enzymatic degradation by mammalian collagenase matrix metalloproteinase 8 MMP-8. PLoS One. 2010;5(8):e12337. doi: 10.1371/journal.pone.0012337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foolen J, van Donkelaar CC, Soekhradj-Soechit S, Ito K. European Society of Biomechanics S.M. Perren Award 2010: an adaptation mechanism for fibrous tissue to sustained shortening. J Biomech. 2010;43(16):3168–3176. doi: 10.1016/j.jbiomech.2010.07.040. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R. The pathogenic role of transforming growth factor-beta2 in glaucomatous damage to the optic nerve head. Exp Eye Res. 2011;93(2):165–169. doi: 10.1016/j.exer.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Geijer C, Bill A. Effects of raised intraocular pressure on retinal, prelaminar, laminar, and retrolaminar optic nerve blood flow in monkeys. Invest Ophthalmol Vis Sci. 1979;18(10):1030–1042. [PubMed] [Google Scholar]
- Geraghty B, Jones SW, Rama P, Akhtar R, Elsheikh A. Age-related variations in the biomechanical properties of human sclera. J Mech Behav Biomed Mater. 2012;16:181–191. doi: 10.1016/j.jmbbm.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Girard M, Downs JC, Burgoyne CF, Bottlang M, Suh JKF. Anisotropic and nonlinear mechanical behavior of monkey posterior sclera under intraocular pressure. Invest Ophthalmol Vis Sci 2007 [Google Scholar]
- Girard MJ, Suh JK, Bottlang M, Burgoyne CF, Downs JC. Biomechanical changes in the sclera of monkey eyes exposed to chronic IOP elevations. Invest Ophthalmol Vis Sci. 2011;52(8):5656–5669. doi: 10.1167/iovs.10-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Kass MA. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714–720. doi: 10.1001/archopht.120.6.714. discussion 829-730. [DOI] [PubMed] [Google Scholar]
- Grytz R, Fazio MA, Girard MJ, Libertiaux V, Bruno L, Gardiner S, Girkin CA, Downs JC. Material properties of the posterior human sclera. J Mech Behav Biomed Mater. 2014;29:602–617. doi: 10.1016/j.jmbbm.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grytz R, Fazio MA, Libertiaux V, Bruno L, Gardiner S, Girkin CA, Downs JC. Age- and race-related differences in human scleral material properties. Invest Ophthalmol Vis Sci. 2014;55(12):8163–8172. doi: 10.1167/iovs.14-14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grytz R, Girkin CA, Libertiaux V, Downs JC. Perspectives on biomechanical growth and remodeling mechanisms in glaucoma. Mech Res Commun. 2012;42:92–106. doi: 10.1016/j.mechrescom.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grytz R, Sigal IA, Ruberti JW, Meschke G, Downs JC. Lamina Cribrosa Thickening in Early Glaucoma Predicted by a Microstructure Motivated Growth and Remodeling Approach. Mech Mater. 2012;44:99–109. doi: 10.1016/j.mechmat.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez MR. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res. 2000;19(3):297–321. doi: 10.1016/s1350-9462(99)00017-8. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Luo XX, Andrzejewska W, Neufeld AH. Age-related changes in the extracellular matrix of the human optic nerve head. Am J Ophthalmol. 1989;107(5):476–484. doi: 10.1016/0002-9394(89)90491-1. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Ye H. Glaucoma: changes in extracellular matrix in the optic nerve head. Ann Med. 1993;25(4):309–315. doi: 10.3109/07853899309147290. [DOI] [PubMed] [Google Scholar]
- Howell GR, Soto I, Libby RT, John SW. Intrinsic axonal degeneration pathways are critical for glaucomatous damage. Exp Neurol. 2012 doi: 10.1016/j.expneurol.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KI, Jung Y, Park KT, Park CK. Factors affecting plastic lamina cribrosa displacement in glaucoma patients. Invest Ophthalmol Vis Sci. 2014;55(12):7709–7715. doi: 10.1167/iovs.14-13957. [DOI] [PubMed] [Google Scholar]
- Kirwan RP, Fenerty CH, Crean J, Wordinger RJ, Clark AF, O'Brien CJ. Influence of cyclical mechanical strain on extracellular matrix gene expression in human lamina cribrosa cells in vitro. Mol Vis. 2005;11:798–810. [PubMed] [Google Scholar]
- Knox Cartwright NE, Tyrer JR, Marshall J. Age-related differences in the elasticity of the human cornea. Invest Ophthalmol Vis Sci. 2011;52(7):4324–4329. doi: 10.1167/iovs.09-4798. [DOI] [PubMed] [Google Scholar]
- Kong YX, van Bergen N, Bui BV, Chrysostomou V, Vingrys AJ, Trounce IA, Crowston JG. Impact of aging and diet restriction on retinal function during and after acute intraocular pressure injury. Neurobiol Aging. 2012;33(6):1126.e1115–1125. doi: 10.1016/j.neurobiolaging.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hussein M, B B, H L, K E E. M. G. T. Group. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121(1):48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- Liu J, He X. Corneal stiffness affects IOP elevation during rapid volume change in the eye. Invest Ophthalmol Vis Sci. 2009;50(5):2224–2229. doi: 10.1167/iovs.08-2365. [DOI] [PubMed] [Google Scholar]
- Morgan WH, Chauhan BC, Yu DY, Cringle SJ, Alder VA, House PH. Optic disc movement with variations in intraocular and cerebrospinal fluid pressure. Invest Ophthalmol Vis Sci. 2002;43(10):3236–3242. [PubMed] [Google Scholar]
- Morris HJ, Tang J, Cruz Perez B, Pan X, Hart RT, Weber PA, Liu J. Correlation between biomechanical responses of posterior sclera and IOP elevations during micro intraocular volume change. Invest Ophthalmol Vis Sci. 2013;54(12):7215–7222. doi: 10.1167/iovs.13-12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JC. Integrins in the optic nerve head: potential roles in glaucomatous optic neuropathy an American Ophthalmological Society thesis. Trans Am Ophthalmol Soc. 2006;104:453–477. [PMC free article] [PubMed] [Google Scholar]
- Morrison JC, Dorman-Pease ME, Dunkelberger GR, Quigley HA. Optic nerve head extracellular matrix in primary optic atrophy and experimental glaucoma. Arch Ophthalmol. 1990;108(7):1020–1024. doi: 10.1001/archopht.1990.01070090122053. [DOI] [PubMed] [Google Scholar]
- Morrison JC, Jerdan JA, Dorman ME, Quigley HA. Structural proteins of the neonatal and adult lamina cribrosa. Arch Ophthalmol. 1989;107(8):1220–1224. doi: 10.1001/archopht.1989.01070020286040. [DOI] [PubMed] [Google Scholar]
- Nadler Z, Wang B, Schuman JS, Ferguson RD, Patel A, Hammer DX, Bilonick RA, Ishikawa H, Kagemann L, Sigal IA, Wollstein G. In vivo three-dimensional characterization of the healthy human lamina cribrosa with adaptive optics spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55(10):6459–6466. doi: 10.1167/iovs.14-15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C, Cone FE, Nguyen TD, Coudrillier B, Pease ME, Steinhart MR, Oglesby EN, Jefferys JL, Quigley HA. Studies of scleral biomechanical behavior related to susceptibility for retinal ganglion cell loss in experimental mouse glaucoma. Invest Ophthalmol Vis Sci. 2013;54(3):1767–1780. doi: 10.1167/iovs.12-10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickells RW, Howell GR, Soto I, John SW. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci. 2012;35:153–179. doi: 10.1146/annurev.neuro.051508.135728. [DOI] [PubMed] [Google Scholar]
- Norman RE, Flanagan JG, Rausch SM, Sigal IA, Tertinegg I, Eilaghi A, Portnoy S, Sled JG, Ethier CR. Dimensions of the human sclera: Thickness measurement and regional changes with axial length. Exp Eye Res. 2010;90(2):277–284. doi: 10.1016/j.exer.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Norman RE, Flanagan JG, Sigal IA, Rausch SM, Tertinegg I, Ethier CR. Finite element modeling of the human sclera: influence on optic nerve head biomechanics and connections with glaucoma. Exp Eye Res. 2011;93(1):4–12. doi: 10.1016/j.exer.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Olsen TW, Aaberg SY, Geroski DH, Edelhauser HF. Human sclera: thickness and surface area. Am J Ophthalmol. 1998;125(2):237–241. doi: 10.1016/s0002-9394(99)80096-8. [DOI] [PubMed] [Google Scholar]
- Pease ME, Oglesby EN, Cone-Kimball E, Jefferys JL, Steinhart MR, Kim AJ, Hanes J, Quigley HA. Scleral permeability varies by mouse strain and is decreased by chronic experimental glaucoma. Invest Ophthalmol Vis Sci. 2014;55(4):2564–2573. doi: 10.1167/iovs.13-13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijanka JK, Coudrillier B, Ziegler K, Sorensen T, Meek KM, Nguyen TD, Quigley HA, Boote C. Quantitative mapping of collagen fiber orientation in non-glaucoma and glaucoma posterior human sclerae. Invest Ophthalmol Vis Sci. 2012;53(9):5258–5270. doi: 10.1167/iovs.12-9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley H, Anderson DR. The dynamics and location of axonal transport blockade by acute intraocular pressure elevation in primate optic nerve. Invest Ophthalmol. 1976;15(8):606–616. [PubMed] [Google Scholar]
- Quigley HA, Addicks EM. Chronic experimental glaucoma in primates. II. Effect of extended intraocular pressure elevation on optic nerve head and axonal transport. Invest Ophthalmol Vis Sci. 1980;19(2):137–152. [PubMed] [Google Scholar]
- Quigley HA, Anderson DR. Distribution of axonal transport blockade by acute intraocular pressure elevation in the primate optic nerve head. Invest Ophthalmol Vis Sci. 1977;16(7):640–644. [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radius RL. Optic nerve fast axonal transport abnormalities in primates. Occurrence after short posterior ciliary artery occlusion. Arch Ophthalmol. 1980;98(11):2018–2022. doi: 10.1001/archopht.1980.01020040870017. [DOI] [PubMed] [Google Scholar]
- Resta V, Novelli E, Vozzi G, Scarpa C, Caleo M, Ahluwalia A, Solini A, Santini E, Parisi V, Di Virgilio F, Galli-Resta L. Acute retinal ganglion cell injury caused by intraocular pressure spikes is mediated by endogenous extracellular ATP. Eur J Neurosci. 2007;25(9):2741–2754. doi: 10.1111/j.1460-9568.2007.05528.x. [DOI] [PubMed] [Google Scholar]
- Roberts MD, Grau V, Grimm J, Reynaud J, Bellezza AJ, Burgoyne CF, Downs JC. Remodeling of the connective tissue microarchitecture of the lamina cribrosa in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2009;50(2):681–690. doi: 10.1167/iovs.08-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MD, Liang Y, Sigal IA, Grimm J, Reynaud J, Bellezza A, Burgoyne CF, Downs JC. Correlation between local stress and strain and lamina cribrosa connective tissue volume fraction in normal monkey eyes. Invest Ophthalmol Vis Sci. 2010;51(1):295–307. doi: 10.1167/iovs.09-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MD, Sigal IA, Liang Y, Burgoyne CF, Downs JC. Changes in the biomechanical response of the optic nerve head in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51(11):5675–5684. doi: 10.1167/iovs.10-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicka AR, Mt-Isa S, Owen CG, Cook DG, Ashby D. Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Invest Ophthalmol Vis Sci. 2006;47(10):4254–4261. doi: 10.1167/iovs.06-0299. [DOI] [PubMed] [Google Scholar]
- Sady C, Khosrof S, Nagaraj R. Advanced Maillard reaction and crosslinking of corneal collagen in diabetes. Biochem Biophys Res Commun. 1995;214(3):793–797. doi: 10.1006/bbrc.1995.2356. [DOI] [PubMed] [Google Scholar]
- Sawabe M. Vascular aging: from molecular mechanism to clinical significance. Geriatr Gerontol Int. 2010;10 Suppl 1:S213–220. doi: 10.1111/j.1447-0594.2010.00603.x. [DOI] [PubMed] [Google Scholar]
- See JL, Nicolela MT, Chauhan BC. Rates of neuroretinal rim and peripapillary atrophy area change: a comparative study of glaucoma patients and normal controls. Ophthalmology. 2009;116(5):840–847. doi: 10.1016/j.ophtha.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Flanagan JG, Ethier CR. Factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2005;46(11):4189–4199. doi: 10.1167/iovs.05-0541. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Predicted extension, compression and shearing of optic nerve head tissues. Exp Eye Res. 2007;85(3):312–322. doi: 10.1016/j.exer.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Grimm JL, Jan NJ, Reid K, Minckler DS, Brown DJ. Eye-specific IOP-induced displacements and deformations of human lamina cribrosa. Invest Ophthalmol Vis Sci. 2014;55(1):1–15. doi: 10.1167/iovs.13-12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal IA, Roberts MD, Girard MJA, Burgoyne CF, Downs JC. Chapter 20: Biomechanical Changes of the Optic Disc. In: Levin LA, Albert DM, editors. Ocular Disease: Mechanisms and Management. London: Elsevier; 2010. pp. 153–164. [Google Scholar]
- Sigal IA, Yang H, Roberts MD, Burgoyne CF, Downs JC. IOP-induced lamina cribrosa displacement and scleral canal expansion: an analysis of factor interactions using parameterized eye-specific models. Invest Ophthalmol Vis Sci. 2011;52(3):1896–1907. doi: 10.1167/iovs.10-5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal IA, Yang H, Roberts MD, Grimm JL, Burgoyne CF, Demirel S, Downs JC. IOP-induced lamina cribrosa deformation and scleral canal expansion: independent or related? Invest Ophthalmol Vis Sci. 2011;52(12):9023–9032. doi: 10.1167/iovs.11-8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sredar N, Ivers KM, Queener HM, Zouridakis G, Porter J. 3D modeling to characterize lamina cribrosa surface and pore geometries using in vivo images from normal and glaucomatous eyes. Biomed Opt Express. 2013;4(7):1153–1165. doi: 10.1364/BOE.4.001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart MR, Cone-Kimball E, Nguyen C, Nguyen TD, Pease ME, Chakravarti S, Oglesby EN, Quigley HA. Susceptibility to glaucoma damage related to age and connective tissue mutations in mice. Exp Eye Res. 2014;119:54–60. doi: 10.1016/j.exer.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strouthidis NG, Fortune B, Yang H, Sigal IA, Burgoyne CF. Effect of acute intraocular pressure elevation on the monkey optic nerve head as detected by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(13):9431–9437. doi: 10.1167/iovs.11-7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Lye-Barthel M, Masland RH, Jakobs TC. The morphology and spatial arrangement of astrocytes in the optic nerve head of the mouse. J Comp Neurol. 2009;516(1):1–19. doi: 10.1002/cne.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timoshenko SJ. Theory of Elasticity. New York: McGraw-Hill; 1970. [Google Scholar]
- Vasan S, Foiles P, Founds H. Therapeutic potential of breakers of advanced glycation end product-protein crosslinks. Arch Biochem Biophys. 2003;419(1):89–96. doi: 10.1016/j.abb.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Clark AF, Lipson KE, Andrews D, Crean JK, O'Brien CJ. Anti-connective tissue growth factor antibody treatment reduces extracellular matrix production in trabecular meshwork and lamina cribrosa cells. Invest Ophthalmol Vis Sci. 2013;54(13):7836–7848. doi: 10.1167/iovs.13-12494. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Murphy-Ullrich JE, Downs JC, O'Brien CJ. The role of matricellular proteins in glaucoma. Matrix Biol. 2014 doi: 10.1016/j.matbio.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Wang L, Cull G, Burgoyne CF, Thompson S, Fortune B. Longitudinal Alterations in the Dynamic Autoregulation of Optic Nerve Head Blood Flow Revealed in Experimental Glaucoma. Invest Ophthalmol Vis Sci. 2014 doi: 10.1167/iovs.14-14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cull GA, Piper C, Burgoyne CF, Fortune B. Anterior and posterior optic nerve head blood flow in nonhuman primate experimental glaucoma model measured by laser speckle imaging technique and microsphere method. Invest Ophthalmol Vis Sci. 2012;53(13):8303–8309. doi: 10.1167/iovs.12-10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordinger RJ, Agarwal R, Talati M, Fuller J, Lambert W, Clark AF. Expression of bone morphogenetic proteins BMP, BMP receptors, and BMP associated proteins in human trabecular meshwork and optic nerve head cells and tissues. Mol Vis. 2002;8:241–250. [PubMed] [Google Scholar]
- Yan D, McPheeters S, Johnson G, Utzinger U, Vande Geest JP. Microstructural differences in the human posterior sclera as a function of age and race. Invest Ophthalmol Vis Sci. 2011;52(2):821–829. doi: 10.1167/iovs.09-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Fu J, Hou R, Liu K, Jonas JB, Wang H, Chen W, Li Z, Sang J, Zhang Z, Liu S, Cao Y, Xie X, Ren R, Lu Q, Weinreb RN, Wang N. Optic neuropathy induced by experimentally reduced cerebrospinal fluid pressure in monkeys. Invest Ophthalmol Vis Sci. 2014;55(5):3067–3073. doi: 10.1167/iovs.13-13657. [DOI] [PubMed] [Google Scholar]
- Yang H, Downs JC, Girkin C, Sakata L, Bellezza A, Thompson H, Burgoyne CF. 3-D histomorphometry of the normal and early glaucomatous monkey optic nerve head: lamina cribrosa and peripapillary scleral position and thickness. Invest Ophthalmol Vis Sci. 2007;48(10):4597–4607. doi: 10.1167/iovs.07-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Downs JC, Sigal IA, Roberts MD, Thompson H, Burgoyne CF. Deformation of the normal monkey optic nerve head connective tissue after acute IOP elevation within 3-D histomorphometric reconstructions. Invest Ophthalmol Vis Sci. 2009;50(12):5785–5799. doi: 10.1167/iovs.09-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Williams G, Downs JC, Sigal IA, Roberts MD, Thompson H, Burgoyne CF. Posterior outward migration of the lamina cribrosa and early cupping in monkey experimental glaucoma. Invest Ophthalmol Vis Sci. 2011;52(10):7109–7121. doi: 10.1167/iovs.11-7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeimer RC, Ogura Y. The relation between glaucomatous damage and optic nerve head mechanical compliance. Arch Ophthalmol. 1989;107(8):1232–1234. doi: 10.1001/archopht.1989.01070020298042. [DOI] [PubMed] [Google Scholar]
- Zode GS, Clark AF, Wordinger RJ. Bone morphogenetic protein 4 inhibits TGF-beta2 stimulation of extracellular matrix proteins in optic nerve head cells: role of gremlin in ECM modulation. Glia. 2009;57(7):755–766. doi: 10.1002/glia.20803. [DOI] [PubMed] [Google Scholar]
- Zode GS, Sethi A, Brun-Zinkernagel AM, Chang IF, Clark AF, Wordinger RJ. Transforming growth factor-beta2 increases extracellular matrix proteins in optic nerve head cells via activation of the Smad signaling pathway. Mol Vis. 2011;17:1745–1758. [PMC free article] [PubMed] [Google Scholar]


