Abstract
BACKGROUND
Recently, tangles and plaque-like aggregates have been identified in certain cases of dilated cardiomyopathy (DCM). This suggests a potential underlying cause for the one-third of cases, traditionally labeled idiopathic (iDCM), where there is no specific diagnostic test or targeted therapy.
OBJECTIVE
We sought to identify the make-up of myocardial aggregates to understand the molecular mechanisms of these cases of DCM; this strategy has been central to understanding Alzheimer’s disease.
METHODS
Aggregates were extracted from human iDCM samples with high congophilic reactivity (an indication of plaque presence) and the findings validated in a larger cohort of samples. We tested the expression, distribution, and activity of cofilin in human tissue and generated a cardiac-specific knockout mouse model to investigate the functional impact of the human findings. We also modeled cofilin inactivity in vitro using pharmacological and genetic gain and loss of function approaches.
RESULTS
Aggregates in the human myocardium were enriched for cofilin-2, an actin-depolymerizing protein known to participate in neurodegenerative diseases and nemaline myopathy. Cofilin-2 was predominantly phosphorylated, rendering it inactive. Cardiac-specific haploinsufficiency of cofilin-2 in mice recapitulated the human disease’s morphological, functional, and structural phenotype. Pharmacological stimulation of cofilin-2 phosphorylation and genetic overexpression of the phosphomimetic protein promoted the accumulation of “stress-like” fibers and severely impaired cardiomyocyte contractility.
CONCLUSIONS
Our study provides the first biochemical characterization of prefibrillar myocardial aggregates in humans and the first report to link cofilin-2 to cardiomyopathy. The findings suggest a common pathogenetic mechanism between certain iDCMs and other chronic degenerative diseases, laying the groundwork for new therapeutic strategies.
Keywords: adenovirus, heart failure, nemaline
In one-third of dilated cardiomyopathy (DCM) cases, the disease’s origin remains unknown. Consequently, for those cases traditionally labeled “idiopathic” (iDCM), there is no specific early screening, diagnostic test, or targeted therapies. With the advent of a new nomenclature for classifying cardiomyopathies (1), patients are being grouped based on morphofunctional phenotype, organ(s) involved, genetics, and disease etiology. In the new classification, MOGES (1), the iDCM cases would be described as dilated morphofunctional phenotype (M), involving the heart (O), in sporadic cases (G) of unknown etiology (E) and classified as MDOHGSEO (D = dilated; H = heart; S = sporadic; O = no known etiology; http://moges.biomeris.com).
Myofibrillar accumulation of β-sheet fibrils with structural and tintorial properties of amyloid fibers and their precursor seeds has been recently identified in certain iDCM hearts (2,3). They were not related to primary amyloid systemic disorders or to the normal aging process. With physiological aging, aggregates derived from defective folding or clearance of proteins accumulate as amyloid β-sheet fibrils, adversely affecting organ function (4,5). Their premature appearance underpins numerous degenerative diseases, affecting various organs.
In the heart, the currently known illnesses due to protein aggregates are systemic and senile amyloidosis or desmin and amylin cardiomyopathies. In these conditions, the major peptides composing the fibrillar aggregates are known (monoclonal immunoglobulin κ or λ light chains, wild type (WT) or mutated transthyretin, αβ-crystallin or desmin, and amylin respectively). Conversely, the constituents of the iDCM aggregates and their pathogenetic role remain unknown. A milestone in understanding the pathophysiology of the first described disease with protein aggregates, Alzheimer’s disease (AD), was recognizing that fragments of the amyloid precursor protein (Aβ42) are the primary constituents of the amyloid plaques (6,7). Therefore identifying the components of the iDCM deposits is critical to understanding the mechanisms of the disease.
In the current study we purified the soluble cardiac pre-amyloid seeds, that will be referred here as pre-amyloid oligomers (PAO), providing (to our knowledge) the first evidence that cofilin-2 is a critical component within the iDCM aggregates.
A 19kD protein, cofilin is a member of the ADF/cofilin family of actin-binding proteins named for its ability to form actin filaments (COFILamentous structures of actIN). It participates in the disassembly of the sarcomeric protein actin as well as the pathogenesis of nemaline skeletal myopathy and AD (8–10). There are 3 isoforms: cofilin-1, ubiquitously expressed; cofilin-2, expressed mainly in muscle cells; and destrin or ADF, expressed primarily in epithelial and endothelial cells (11). Cofilin-1 and cofilin-2 have overlapping functions and are both present in the heart (8,10,12).
Considering its known involvement in protein aggregate disorders in other organs such as brain (AD) and skeletal muscle (nemaline myopathy), and since mass spectroscopy (MS) identified cofilin with its functional interactome, we focused on the contribution of cofilin in the pathogenesis of iDCM. We validated the initial discovery in a larger cohort of human heart tissues in which we tested the expression and activity of cofilin-2 and its coexistence with the PAO. We then generated a cardiac-specific cofilin-2 (CSC2) heterozygous knockout mouse to model cofilin-2 reduced activity. The in vivo analysis at the organ level was complemented with in vitro measurements of contractility of isolated cardiomyocytes to provide a better understanding of the cellular defects caused by cofilin alterations.
To establish a direct causal link between the altered pattern of cofilin-2 phosphorylation and sarcomeric structure and function, we stimulated or inhibited upstream kinases pharmacologically and overexpressed the phosphomimetic (the constitutively active form of cofilin) in vitro using adenovirus.
METHODS
Frozen myocardial samples from the anterior wall of the left ventricle (LV) isolated from explanted failing hearts at the time of transplant (MOGES MDOHGSE0SIV; S refers to stage) and nonfailing donor hearts were used to purify protein aggregates under native conditions.
To resolve composition, we capitalized on the common conformation of the soluble PAO and on the availability of conformational antibodies (A11) to immunoprecipitate them. A11s have been made to recognize an epitope common to the conformation of PAO rather than the unique sequence of the specific protein forming them (13). We used MS to identify the components of the immunoprecipitate and validated the results by immunoblotting, immunohistochemistry, 2-dimensational (2D) gel electrophoresis, phosphate affinity assay (Phos-tag), and dot-blot.
For cardiac-specific expression, Cofilin-2-flox mice were crossed with αMHC-cre mice. Cardiomyocytes were isolated from 2-month-old CSC2 mice, WT littermates, and αMHC-Cre mice by enzymatic digestion, cultured, and analyzed as previously described (2,14).
The Online Appendix contains a detailed description of the methods.
STATISTICAL ANALYSIS
Continuous variables were reported as means ± SD or median and interquartile range as appropriate then compared using Student t test or Wilcoxon rank sum test if not normally distributed. Categorical variables were analyzed using Fisher’s exact test. Mixed effects model was used to compare cell-derived, continuous variables between WT and transgenic mice, using a random effect to account for data correlation within each mouse. Whisker lengths are covering the 5th to 95th percentile interval. A p value < 0.05 was considered significant. For multiple comparison of continuous normally distributed data, a post-hoc analysis was performed using Bonferroni method. The analysis was performed using STATA data analysis and statistical software (StataCorp LP, College Station, Texas).
RESULTS
HUMAN SAMPLES
We previously reported the detection, characterization, and distribution of myocardial aggregates positive for amyloid staining dyes in 25 explanted iDCM heart samples (2). From these samples, we selected 5 iDCM (Table 1A) with the highest presence of congophilic inclusions to isolate the PAO and characterize their biochemical composition. Three donor hearts were used as controls. A validation cohort consisting of 10 iDCM and 10 donor samples (Table 1B) was used to subsequently determine the expression and distribution of cofilin-2 in the myocardium. Myocardial tissue was also obtained from a patient diagnosed with nemaline cardiomyopathy (Table 1B).
TABLE 1.
Clinical Parameters of Donors and iDCM Patients
| Table 1A | |||||||
|---|---|---|---|---|---|---|---|
| Age (yrs) | Sex | Type | Disease | Associated Diseases | Cause of death | Heart Weight (g) | FS (%) |
| 73 | F | Donor | NA | 420 | 60 | ||
| 62 | BF | Donor | CVA | 420 | 60 | ||
| 42 | CM | Donor | Anoxic injury | 320 | 42 | ||
| 57 | CM | Transplant | iDCM | AF, DM, Obesity, HTN HL, HT | 620 | 20 | |
| 63 | CF | Transplant | iDCM | Anemia, AF, MVR, IDDM, HTN | 330 | 10 | |
| 53 | CF | Transplant | iDCM | Breast Carcinoma, esophageal reflux | 340 | 19 | |
| 59 | CM | Transplant | iDCM | HTN, HL, CAD, psoriasis, gout, HT, obesity, sleep apnea, embolic stroke | 710 | 30 | |
| 48 | AM | Transplant | iDCM | HT, VT, MR, AFl + AV block III, PMK, PFO | 393 | 12 | |
| Table 1B | |||||||
|---|---|---|---|---|---|---|---|
| Age (yrs) | Sex | Type | Disease | Associated Diseases | Cause of death | Heart Weight | FS (%) |
| 58 | F | Donor | NA | 353 | NA | ||
| 42 | CM | Donor | Anoxic injury | 320 | NA | ||
| 71 | CF | Donor | Seizure, Arthritis | ICH | 353 | NA | |
| 56 | CM | Donor | ICH, Trauma | 400 | NA | ||
| 65 | CM | Donor | SVT, MV prolapse | CVA, RA | 458 | NA | |
| 26 | CF | Donor | Astrocytoma | 240 | 25 | ||
| 58 | CM | Donor | Head Trauma | 486 | 55 | ||
| 54 | CF | Donor | Depression | DO | 380 | NA | |
| 35 | CM | Donor | GSW to head | 363 | 55 | ||
| 56 | CM | Donor | ICH | 630 | 76 | ||
| 60 | CM | Transplant | iDCM | NA | 16 | ||
| 36 | M | Transplant | iDCM | IDDM, HTN, CRI, Nephropathy | 450 | 41 | |
| 55 | CF | Transplant | iDCM | VT, Asthma, OB, PH, HTyr, TPTyr, AF | 250 | 22 | |
| 59 | CM | Transplant | iDCM | HTN, HL, CAD, Gout, HT, OB, ES | 710 | 30 | |
| 66 | CM | Transplant | iDCM | HT, CAD, HL, Gout, ETOH, AF | 380 | 20 | |
| 57 | CM | Transplant | iDCM | DM, AF, HTN, HL | 620 | 20 | |
| 63 | CF | Transplant | iDCM | Anemia, AF, MR, IDDM, HTN | 330 | 10 | |
| 31 | BF | Transplant | iDCM | Pneumonia, Asthma | 376 | 10 | |
| 65 | CF | Transplant | iDCM | AF, COPD, ETOH, HTyr | 570 | 10 | |
| 33 | CF | Transplant | iDCM | PHTN, OB, ETOH, NSVT, PE, renal dysfunction | 590 | 23 | |
| 19 | CF | LVAD | Nemaline | Congenital pulmonary airway malformation | 566 | 7 | |
(A) Clinical data of patients included in the initial cohort of heart samples used for PAO purification. (B) Clinical data of patients included in the validation cohort.
Abbreviations: CM= Caucasian male, CF=Caucasian female, BF=black female, AM=Asian male. AF = atrial fibrillation; AFl = atrial flutter; AV = atrioventricular; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; CRI=chronic renal insufficiency; CVA = cerebrovascular accident; DM = diabetes mellitus; DO = drug overdose; EF = ejection fraction; ES = embolic stroke; ETOH = alcohol; FS = fractional shortening; GSW = gun shot wound; HL = hyperlipidemia; HTyr = hypothyroidism; HPTyr = hyperparathyroidism; HTN = hypertension; ICH = intracranial hemorrhage; iDCM = idiopathic dilated cardiomyopathy; IDDM = insulin-dependent diabetes mellitus; LVAD = left ventricular assist device; MR = mitral regurgitation; MV = mitral valve; NA = not available; NSVT = nonsustained ventricular tachycardia; OB = obesity; PE = pulmonary embolism; PHTN = pulmonary hypertension; PMK = pacemaker; PFO = patent foramen ovale; PH = pulmonary hypertension; RA= rheumatoid arthritis; SVT = supraventricular tachycardia; VT = ventricular tachycardia.
Before acquiring the β-sheet amyloid structure, misfolded proteins undergo progressive maturation steps from monomers to multiple “mers-” generating PAO (up to 24 mers). These coexist with the mature fibers in the tissues (Online Figure 1) (15–17). In this process, proteins lose their sequence specificity and acquire a common conformation. By using A11-conformational antibodies (13), we enriched for soluble PAO from human hearts and confirmed their presence by electron microscopy (EM) (Figure 1A and 1B).
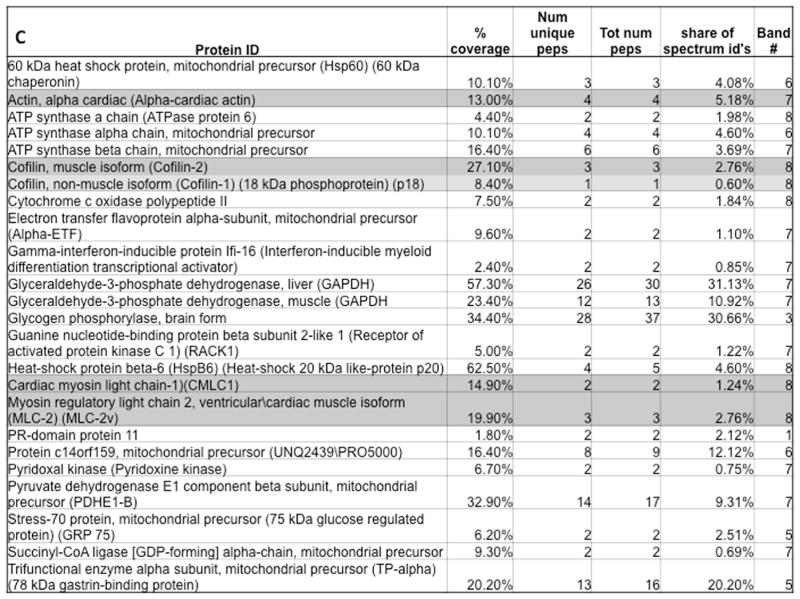
FIGURE 1. Cofilin-2 and Its Substrates.
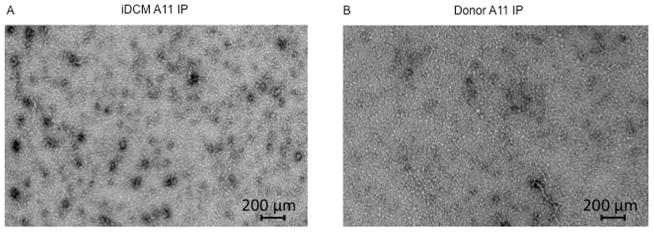
A11 antibody pull-down identifies cofilin-2 and its substrates within human myocardial pre-amyloid oligomers (PAO) via electron micrographs of purified PAO from (A) iDCM and (B) donor myocardium. PAOs are numerous in iDCM. (C) Proteins identified within human-extracted PAO. iDCM = idiopathic dilated cardiomyopathy. A11=structural antibodies anti-PAO
Following immunoprecipitation, denatured PAO components were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Seven bands differentially expressed in iDCM and 1 in donor hearts were excised and analyzed by MS. Within the peptides identified in the bands having higher expression intensity in iDCM cases, cofilin-2 and its substrates actin and MLC-II were present with high percent coverage by MS analysis (Figure 1C).
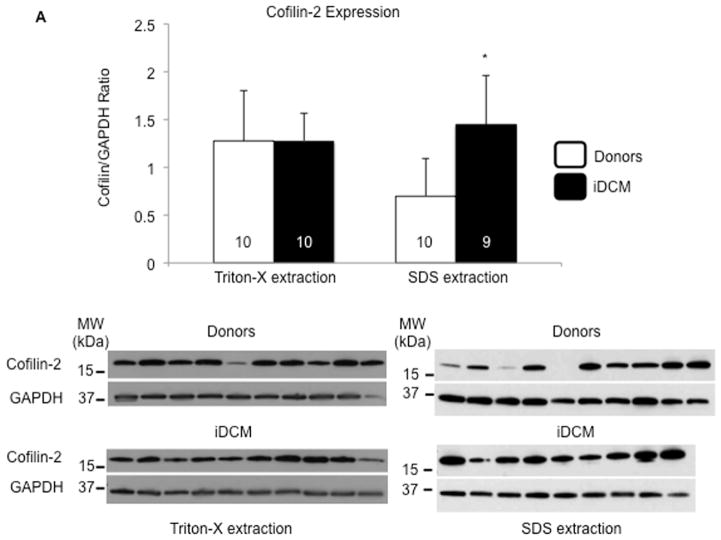
The expression of cofilin-2 in the human myocardium was evaluated by SDS-PAGE. The samples were prepared utilizing two lysis buffers, one containing the nonionic detergent Triton-X-100, able to extract the more soluble fraction, and one containing high percentage of the ionic detergent SDS to extract the less soluble aggregates. Cofilin-2 expression was similar in lysates from iDCM and donor hearts when Triton-X-100 was employed, but significantly higher in iDCM than in donor myocardium in the SDS extraction (Figure 2A). These data suggest that much of the cofilin-2 in the iDCM hearts we studied is in a Triton-X-100 insoluble form. Interestingly, once samples were plotted individually, it appeared that cofilin expression in the SDS fraction was increased in the donor hearts from older individuals (Online Figure 2 and Online Table).
FIGURE 2. Cofilin-2 Prominence in iDCM PAO.
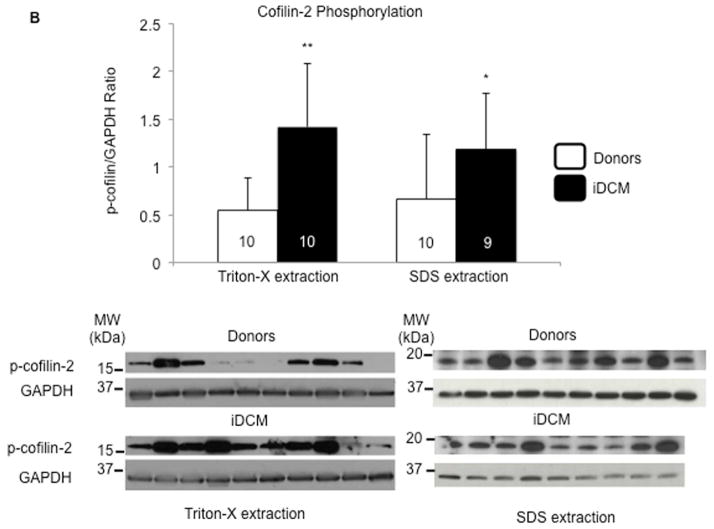
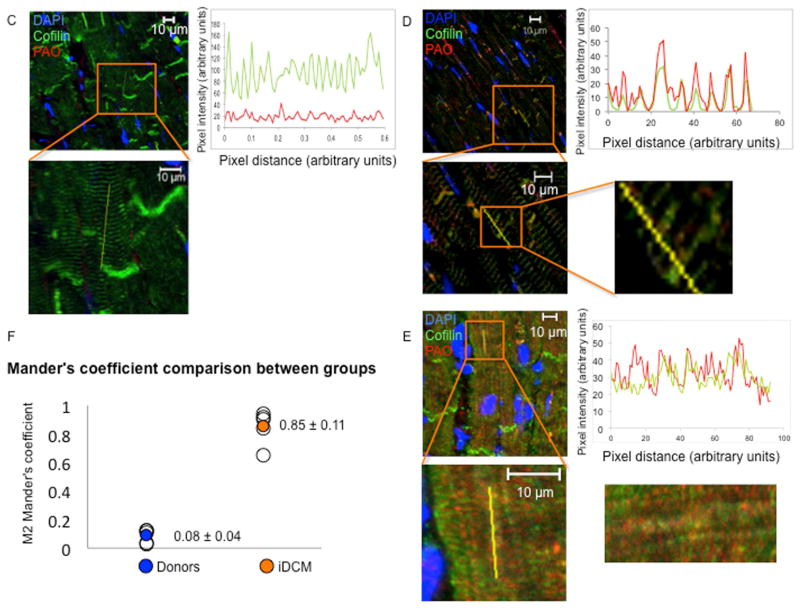
(A) Expression of total cofilin-2 in donor (white bars) and iDCM (black bars) human hearts following extraction with Triton X-100 (left panels) or SDS detergents (right panels). Cofilin-2 quantity is increased in iDCM with SDS detergent. *p < 0.05. (B) Phospho-cofilin-2 increased significantly in iDCM by Triton-X-100 and SDS extraction. *p < 0.05, **p < 0.01. Values are mean ± SD; number of samples indicated in the bars. MW= molecular weight; kDa=kilo-Dalton. (C) Colocalization of cofilin-2 and PAO in donor and (D–E) iDCM hearts. Pixel intensity and distance overlap in iDCM but not donor hearts; the boxes define areas at higher magnification. Cofilin-2, green; A11-stained PAO, red; nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI), blue. (D) iDCM sample with less A11-positive PAOs. Here, A11 stain colocalizes in the area of cofilin still within sarcomeres. (E) iDCM sample with more abundant PAO clustered in larger aggregates (more images in Online Figure 7). (F) Colocalization was quantified by Mander’s correlation coefficient (M). M1 represents the percentage of colocalization of cofilin-2 with the PAO; M2 represents colocalization of PAOs with cofilin-2; M2 Mander’s coefficient was calculated from multiple random images collected from 5 donor and 5 iDCM hearts. p = 0.000029. Values in each sample were pooled and averaged. Open symbols are individual samples; solid symbols are the mean values for donor (blue) and iDCM (orange) hearts. GAPDH = glyceraldehyde 3-phosphate dehydrogenase; SDS = sodium dodecyl sulfate; other abbreviations as in Figure 1.
Cofilin activity is regulated by reversible phosphorylation on ser3. Phosphorylation inactivates and dephosphorylation activates cofilin, regulating its ability to bind G- or F-actin (Online Figure 3) (8,18–21). The relative amount of ser3-phosphorylated cofilin-2 was quantified in donor and iDCM tissue to determine how much of the total pool is unable to interact with actin. Phospho-cofilin-2 increased significantly in iDCM by either Triton-X-100 or SDS extraction (Figure 2B). We acknowledge the limitation of using human heart samples that present inevitable variability. However, the difference in cofilin expression/activity between iDCM and donor samples was statistically significant despite the relatively small number of cases. We also measured the fraction of total cofilin-2 that is phosphorylated, by 2D western and Phos-tag (Online Figure 4 and 5). The Phos-tag confirmed cofilin phosphorylation increased in iDCM hearts. Finally, following A11 antibody pull-down, an increased amount of α-sarcomeric actin was confirmed in iDCM hearts compared to donor (Online Figure 6), indicating that actin co-precipitates with cofilin in the aggregates, validating the MS data.
To obtain further evidence of the presence of cofilin-2 within PAO in the myocardium of patients with iDCM, we stained frozen tissue sections for A11 reactive PAO and cofilin-2. As shown in Figure 2, there was little A11 staining (in red) in the donor sample. Cofilin (in green) did not colocalize with the PAO (Figure 2C). In iDCM hearts, PAOs were scattered within the sarcomeres in the less damaged case (Figure 2D) and were more abundant and clumped in larger deposits in the more damaged case (Figure 2E and Online Figure 7). The extent of colocalization was quantified from randomly chosen confocal images taken from each section of the iDCM and donor hearts using the Mander’s overlap coefficient (MOC) (22). The average MOC-M2 represents the percent of PAO staining colocalized with cofilin-2 in the region analyzed. We found that cofilin-2 colocalized with PAO in 70% of the iDCM samples we studied but none of the donor hearts. The calculated average M2 value was significantly higher in the images from iDCM versus the donor samples (p = 0.000029; n = 5 each group) (Figure 2F).
IN VIVO MODEL
The results in human specimens suggest that loss in cofilin-2 actin-binding activity may have important implications in cardiac structural and functional disarray found in iDCM with protein aggregates. However, the assays possible in human hearts are limited. While severe inactivation or depletion of cofilin causes early-onset myopathy in skeletal muscle, our series of cases consisted of adult-onset iDCM, where the inactivation of cofilin by phosphorylation was incomplete. This poses the question of whether a partial defect in cofilin activity would contribute to development of contractile dysfunction and cardiac muscle myopathy. Hence, we created a mouse model of cardiac-specific cofilin-2 haploinsufficiency (CSC2).
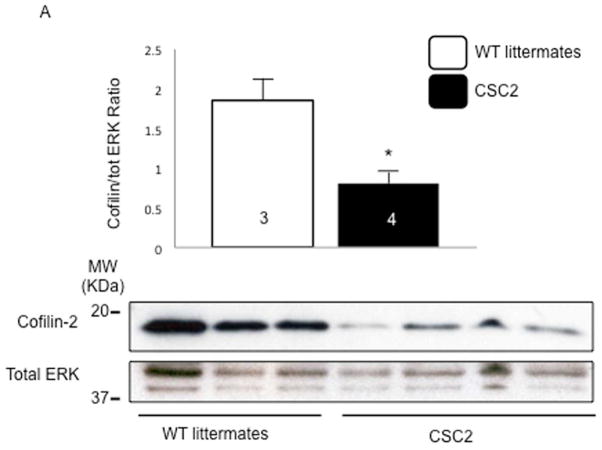
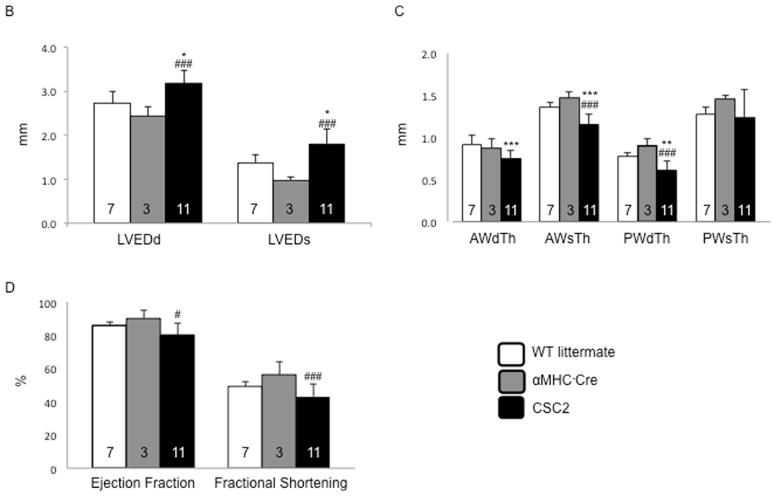
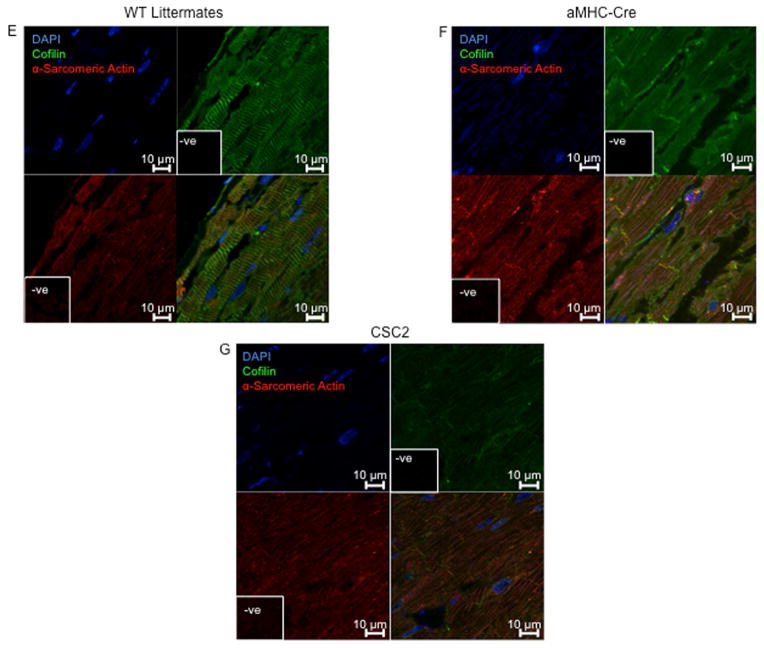
When compared to their WT littermates, heterozygous CSC2 showed a 60% reduction in cofilin-2 expression (Figure 3A). By echocardiography, 2-month-old CSC2 mice already manifested a marked decrease in LV wall thickness, ejection fraction (EF), and fractional shortening (FS), as well as increased LV end-diastolic diameter (LVEDD) and LV end-systolic diameter (LVESD) (Figure 3B–D and Online Figure 8). The echocardiographic data were confirmed by Bonferroni post-hoc analysis (Online Appendix). At this stage, interstitial fibrosis was not detected (Online Figure 9). Myofibrils were altered in CSC2 animals showing abnormal actin pattern, lack of proper orientation, and poorly organized sarcomeres as revealed by immunohistochemistry (Figure 3E–G) and EM (Figure 3N insert 2 and Online Figure 10). The I and M bands were frequently absent in sarcomeres, which were often hypercontracted, and the Z lines interrupted (Figure 3L insert 2 and arrowheads). Mice also displayed structures similar to actin-rods described in nemaline myopathy (23) (inserts 1 Figure 3L and N). Worth noting: by EM, the pathological features of the CSC2 mice myocardium closely recapitulated the pathology shown in a case of human nemaline cardiomyopathy (Figure 3M and Online Figure 10).
FIGURE 3. Cofilin-2 Expression and Cardiac Phenotype in CSC2 Haploisufficient Mice.
(A) The expression of cofilin was reduced in cardiac- specific cofilin-2 (CSC2) mice. *p < 0.05; values are mean ± SD; number of samples indicated in the bars; MW= molecular weight; kDa=kilo-Dalton. (B) Echocardiographic measurements of CSC2 mice (black bars) show a dilated LV chamber (LVEDD and LVESD), with (C) thinning of the LV wall. (D) CSC2 mice also show reduced contractile function measured as EF and FS. *p < 0.05, **p < 0.01, and ***p < 0.005 CSC2 versus WT; #p < 0.05, ##p < 0.01, and ###p < 0.005 CSC2 versus αMHC-Cre; p values are calculated using the Bonferroni method; number of mice indicated in the bars; data are mean ± SD (E) Myocardial tissue showing normal actin pattern in WT littermates and (F) in αMHC-Cre mice; (G) myocardial tissue showing actin disarray in CSC2 mice; color staining as in Figure 2. (H–L) EM images showing normal myocardial structure in WT littermates (H) and αMHC-Cre mice (I). In CSC2 mice (J, L), sarcomeric Z line disruption (arrowheads) and disorganized sarcomeres (arrows) are seen; boxed areas are shown at higher magnification: accumulation of aggregates similar to the skeletal muscle rods (insert 1), sarcomeric disarray and Z line interruption (insert 2). The mice pathology recapitulates closely structural changes observed in the myocardium of a case of human nemaline cardiomyopathy (K) (more images in Online Figure 10). AWdTh = anterior wall thickness in diastole; AWsTh = anterior wall thickness in systole; EF = ejection fraction; EM = electron microscopy; ERK = extracellular signal-regulated kinase; FS = fractional shortening; LVEDD = LV end-diastolic diameter; LVESD = LV end-systolic diameter; PWdTh = posterior wall thickness in diastole; PWsTh = posterior wall thickness in systole; WT = wild type; other abbreviations as in Figure 2.
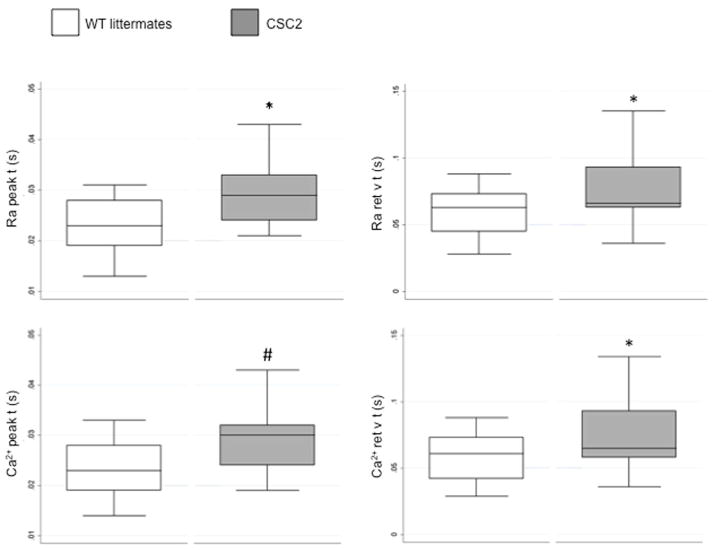
The morphological defects of the myocardium of CSC2 were accompanied by abnormalities in cell function. In comparison with WT, CSC2 cardiomyocytes showed a prolongation in cell shortening and Ca2+ transient velocities (Figure 4 and Table 2).
FIGURE 4. Reduced Velocities of Rise and Decline of Ca2+ Transients in CSC2 Cardiomyocytes.
Box plot of the Ca2+ transient peak and return velocity times expressed as Fura-2 340/380 ratio (Ra peak t and Ra ret v t) and nM Ca2+ (Ca2+ peak t and Ca2+ ret v t). Isolated cardiomyocytes from CSC2 show significant slower transient parameters; measurements were obtained from 31 cells from 4 WT littermate mice (open boxes), and 14 cells from 2 CSC2 mice (solid boxes). Mixed model statistical analysis was applied to allow the statistical analysis of two CSC2 mice. *p < 0.05; #p < 0.005; data are mean ± SD. Other abbreviations as in Figure 3.
Table 2.
Cardiomyocyte Contractility and Ca2+ Transient Parameters: Slower Contraction and Relaxation Velocities
| WT | CSC2 | ||||
|---|---|---|---|---|---|
| Average | SD | Average | SD | p Value | |
| Cell length | |||||
| Dep v t, s | 0.017 | 0.005 | 0.019 | 0.007 | 0.240 |
| Bl%peak h | 2.813 | 2.808 | 2.731 | 2.571 | 0.890 |
| Peak t, s | 0.044 | 0.011 | 0.048 | 0.012 | 0.330 |
| Ret v t, s | 0.066 | 0.020 | 0.072 | 0.021 | 0.480 |
| T to bl 50.0% | 0.076 | 0.020 | 0.078 | 0.022 | 0.770 |
| T to bl 90.0% | 0.122 | 0.032 | 0.107 | 0.020 | 0.400 |
| Sin exp tau, s | 0.049 | 0.025 | 0.043 | 0.022 | 0.640 |
| Sarcomere length | |||||
| Dep v t, s | 0.018 | 0.005 | 0.023 | 0.009 | 0.040 |
| Bl%peak h | 0.047 | 0.037 | 0.064 | 0.052 | 0.630 |
| Peak t, s | 0.045 | 0.009 | 0.053 | 0.014 | 0.045 |
| Ret v t, s | 0.068 | 0.017 | 0.078 | 0.022 | 0.160 |
| T to bl 50.0% | 0.076 | 0.018 | 0.084 | 0.024 | 0.270 |
| T to bl 90.0% | 0.124 | 0.025 | 0.112 | 0.029 | 0.300 |
| Sin exp tau, s | 0.047 | 0.023 | 0.048 | 0.019 | 0.850 |
| F0/F1 ratio | |||||
| Bl | 1.923 | 0.290 | 1.710 | 0.159 | 0.120 |
| Dep v t, s | 0.008 | 0.004 | 0.009 | 0.004 | 0.500 |
| Peak | 2.858 | 0.589 | 2.542 | 0.282 | 0.410 |
| Peak t, s | 0.023 | 0.005 | 0.030 | 0.008 | 0.003 |
| Ret v t, s | 0.059 | 0.018 | 0.077 | 0.024 | 0.008 |
| T to bl 50.0% | 0.079 | 0.017 | 0.084 | 0.012 | 0.590 |
| T to bl 90.0% | 0.136 | 0.033 | 0.144 | 0.017 | 0.800 |
| Sin exp tau, s | 0.107 | 0.038 | 0.232 | 0.436 | 0.100 |
| Calcium | |||||
| Bl, nM | 303.753 | 78.286 | 308.103 | 52.351 | 0.850 |
| Dep v t, s | 0.008 | 0.003 | 0.009 | 0.004 | 0.280 |
| Peak, nM | 617.551 | 298.323 | 610.833 | 111.381 | 0.970 |
| Peak t, s | 0.023 | 0.005 | 0.030 | 0.007 | 0.005 |
| Ret v t, s | 0.058 | 0.017 | 0.072 | 0.027 | 0.030 |
| T to bl 50.0% | 0.077 | 0.016 | 0.086 | 0.010 | 0.280 |
| T to bl 90.0% | 0.136 | 0.031 | 0.145 | 0.011 | 0.570 |
| Sin exp tau, s | 0.105 | 0.039 | 0.108 | 0.027 | 0.740 |
Significant p values indicated in bold.
Contractile parameters from the whole cell length, sarcomere shortening, and Ca2+ transient measured as Fura-2 ratio and nM Ca2+. Bl = diastolic Ca2+ levels; Bl%peak h = cell shortening expressed as % of resting length; CSC2 = cardiac-specific cofilin-2 mice; Dep v t = departure velocity time; Peak = systolic Ca2+ levels; Peak t = time to peak; Ret v t = return velocity time; sin exp tau = index of isovolumic relaxation; t to bl 50.0% and t to bl 90.0% = times to 50% and 90% relaxation times; WT = wild-type mice.
Knockout mice models have previously served well to model the biological and pathological effect of a prevalent inactive cofilin-1 (24,25). However, the ratio cofilin/actin may also modulate formation of actin bundles and rods, thus affecting the sarcomeric machinery structure and function (10). Therefore, we determined the impact of altered patterns of cofilin-2 phosphorylation on cardiomyocyte structure and function by pharmacologic manipulation of cofilin upstream signaling and genetic modulation of cofilin phosphorylation.
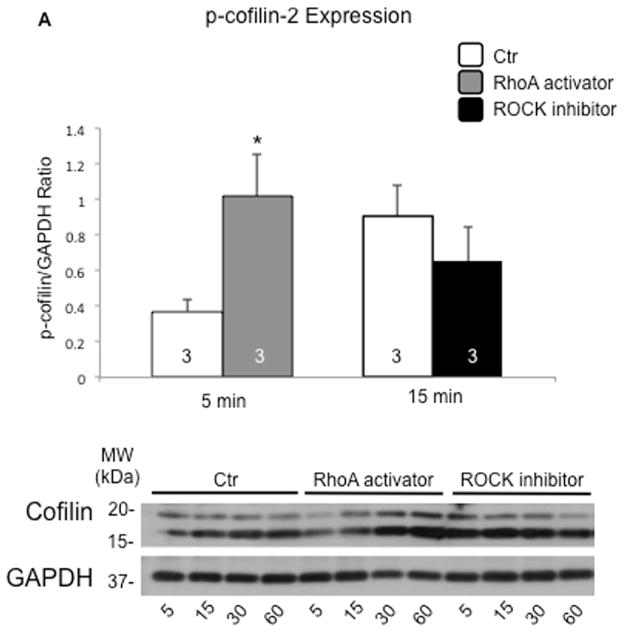
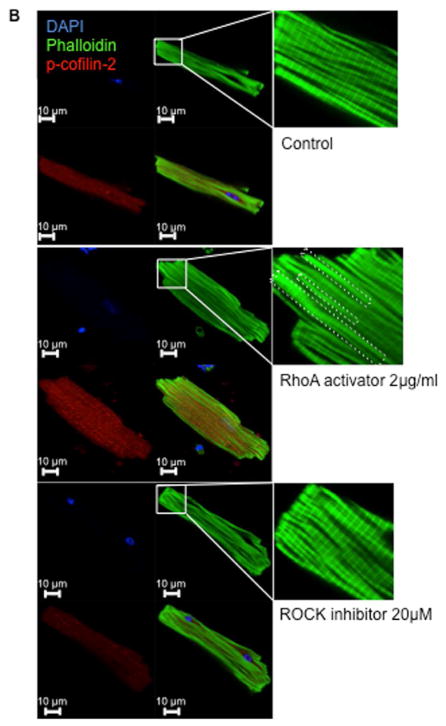
Cofilin-1 and -2 are phosphorylated on ser3 by LIM-domain (LIMK) and TES (TESK) kinases; both are downstream of the Ras-homolog gene family member A (RhoA) and the Rho-associated-protein-kinase (ROCK) (Online Figure 11) (18). RhoA stimulation significantly increased the phosphorylation levels of cofilin2 and led to the formation of “stress-like” fibers in adult mice cardiomyocytes (p < 0.05) (26). Conversely, “stress-like” fibers were not observed with inhibition of ROCK that reduced cofilin-2 phosphorylation (although the p value did not reach statistical significance; p = 0.09) (Figure 5A and 5B).
FIGURE 5. Pharmacological Phosphorylation of Cofilin-2 Induces Formation of Stress-like Fibers in Cardiomyocytes.
(A) Phosphorylation of cofilin-2 is increased following stimulation of RhoA (grey bars) (*p < 0.05) and reduced by ROCK inhibition (black bars) compared to control vehicle (white bars); values are mean ± SD; number of samples indicated in the bars. MW= molecular weight; kDa=kilo-Dalton. (B) RhoA stimulation and ROCK inhibition respectively stimulate and protect from “stress-like” fibers formation in adult mice cardiomyocytes compared to controls (26). Stress-like fibers are indicated by the dotted lines. Phalloidin staining of F-actin, green; phospho-cofilin, red; nuclei stained with DAPI, blue. DAPI = 4′,6-diamidino-2-phenylindole; RhoA = Ras-homolog gene family member A; ROCK = Rho-associated-protein-kinase; other abbreviations as in Figure 2.
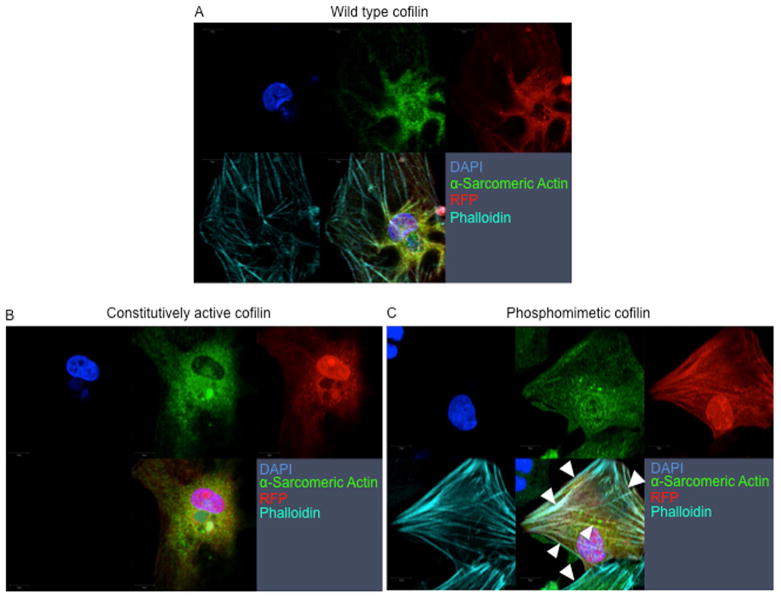
Since pharmacological stimulation may lead to specificity issues with dosing, we used an adenoviral (Ad.) gene transfer approach to express the phosphomimetic (Ad.S3E), constitutively active (Ad.S3A), and WT form of cofilin-1 in neonatal rat cardiomyocytes (Figure 6). When compared to adenoviral overexpression of the WT or S3A, only Ad.S3E induced the formation of “stress-like” fibers in cardiomyocytes (Figure 6C). Overexpressing the phosphomimetic form of cofilin-2 (Ad.S3E) abrogated contractile function and impaired survival of adult cardiomyocytes impeding functional measurement.
FIGURE 6. Genetic Overexpression of Phosphomimetic Ser3-Cofilin-2 Induces Formation of Stress-like Fibers in Cardiomyocytes.
(A) WT cofilin-1, (B) Ad.S3A (constitutively active), or (C) Ad.S3E (phosphomimetic) infected neonatal cardiomyocytes. Adenoviral expression of the phosphomimetic cofilin-1 increases the formation of “stress-like” fibers (arrowheads). Red-fluorescence-protein reporter gene (RFP) indicates cardiomyocytes infected with Ad.WT, Ad.S3A or Ad.S3E, red; α-Sarcomeric Actin, green; phalloidin staining of F-actin, teal; nuclei stained with DAPI, blue. Other abbreviations as in Figure 2.
DISCUSSION
Lack of knowledge regarding the mechanisms initiating the myocardial defect in iDCM is the critical barrier to early screening, prevention, and ultimately a cure for this devastating disease. The recent discovery of the presence, in iDCM, of plaque- and tangle-like aggregates that impair cell function in cardiomyocytes (2,3) identifies a new pathogenesis for at least some cases. However, the aggregates’ composition, which has contributed to the understanding of the pathogenesis and consequently progress towards new AD therapies, is a critical but unresolved issue in the pathogenesis of iDCM.
Our study demonstrates that the actin-depolymerizing protein cofilin-2, along with its interacting proteins actin and MLC-II, are comprised within the iDCM aggregates. In the majority of our human iDCM samples, cofilin-2 expression was increased, and most was found within the aggregates recognized by the A11-structural antibody (Central Illustration). Moreover, cofilin-2 activity in iDCM tissue was decreased, owing to its enhanced degree of phosphorylation. Together, cofilin-2 sequestration in the aggregates and inactivation by enhanced phosphorylation will interfere with its critical function in maintaining actin filament homeostasis, affecting myocyte contractility.
Cofilin contributes to the dynamic turnover of actin composing the thin filaments in contractile cells and microtubules in noncontractile cells (10). Consequently, cofilin is an essential protein that maintains the myofilament architecture needed for the mechanical properties of the sarcomeres, cell motility, and intracellular transport (8,10,27). Abnormalities at this level will critically hamper cardiomyocyte function, as was shown for skeletal myocytes and neurons. In fact, cofilin is known to be involved in the pathogenesis of neurodegenerative diseases (including corticobasal degeneration, William’s syndrome, fragile X syndrome, and spinal muscular atrophy), and skeletal muscle myopathy, where it accumulates as cofilin/actin rods (9,23,28,29). Consistent with these findings, the presence of cofilin-2, actin, and MLC-II in the PAO-enriched lysate in iDCM points towards similar structures forming in cardiomyocytes. Interestingly the difference in cofilin sequestration between donor and iDCM hearts was reduced with age. These data are consistent with the hypothesis that iDCM, like AD, is a pathology bearing an anticipated aging phenotype.
Functionally, cofilin regulation of actin dynamics is controlled by reversible phosphorylation. Cofilin phosphorylation on ser3 by TESK or LIMK inactivates, and dephosphorylation by slingshot or chronophin phosphatases 14-3-3 activates, cofilin to bind either G- or F-actin (18). Consequently, the proper balance of phosphorylated/dephosphorylated cofilin is required for cytoskeleton organization, sarcomeric homeostasis, and contractile function. Here we add pristine evidence of unbalanced cofilin-2 phosphorylation in iDCM heart samples. Lack of cofilin activity would lead to the accumulation of polymerized F-actin filaments and impaired contractility as shown here, either directly by the pharmacological stimulation of cofilin phosphorylation and by genetic expression of the phosphomimetic cofilin mutant in cardiomyocytes, or indirectly by means of the CSC2 mouse model. In vitro cardiomyocyte pharmacological stimulation of the endogenous protein phosphorylation resulted in fibrillar accumulation. Likewise, genetic expression of human phosphomimetic cofilin generated “stress-like” fibers and hampered cardiomyocyte contractility. The mouse model of cardiac-specific cofilin-2 haploinsufficiency aptly recapitulated the human cardiac phenotype. Disorganized myofibrils, immature hypercontracted sarcomeres and aggregates, and disruption of the Z-bands, typical of the nemaline myopathic skeletal and cardiac muscle, were apparent in CSC2 cardiomyocytes, accounting for a decline in these cells’ contribution to cardiac function. Indeed, the co-existence of intact and disrupted sarcomeres can be expected to interfere with sarcomere mechanics, impacting cardiac function.
The in vitro modulation of cofilin activity and the structural abnormalities developed in the CSC2 mouse may not entirely reproduce the pathological changes found in human iDCM. The pathology of the CSC2 mouse myocardium recapitulates the pathology of human nemaline (cardio)myopathy. Likewise, cofilin abnormalities may not represent the pathogenetic factor in all cases of iDCM. However, cofilin may participate in the pathogenesis of iDCM in a subgroup of patients, as is the case of other amyloid-related cardiomyopathies. Additional large-scale studies are warranted to determine how many cases of iDCM with amyloid-like aggregates have cofilin-2 defects as a pathological substrate.
Notably, active cofilin competitively inhibits myosin-II binding to F-actin and loss of this modulatory mechanism in cofilin-depleted cells increases myosin-II/actin assembly leading to further accumulation of abnormal F-actin (30). Interestingly, both actin and MLC-II were found enriched in cardiac PAO. Thus, in addition to the effect of loss of cofilin-2 function on actin and MLC-II binding to F-actin, changes in the amount of MLC-II could contribute to the disorganization of the contractile apparatus, further impairing cardiomyocyte function (2,27,31). Furthermore, by accumulating in PAO, cofilin-2, actin, and MLC-II will alter myocardial function through PAO direct toxicity. Hence, cofilin pathology may alter myocardial contractility by a triple mechanism: “aggregate-independent” loss of function of the sequestered sarcomeric protein(s); “aggregate-dependent” gain of PAO toxicity; and mechanical disruption of sarcomeric integrity, as previously shown for other misfolded prone proteins (2,32).
Besides contractility, cofilin regulates several other actin-related cellular processes, including trafficking of intracellular molecules, directional motility, cell division, and viability (10,33). As a main structural component of the cytoskeletal network, actin also binds to signal transduction proteins at the cytosolic side of the cell membrane, mediating intracellular signaling in response to extracellular matrix deformation and mechanical stress. Furthermore, the upstream regulator of cofilin activity, RhoA, cooperatively coordinates cell migration and motility with the small GTPase RhoA subfamily member, Rac. Their reciprocal function in mediating cytoskeletal plasticity may regulate cell geometry and rigidity in response to extracellular stress and force generation (34). Finally, actomyosin dynamic polymerization coordinates the appropriate distribution of the duplicated chromosomes during cytokinesis (27,30,31,35,36). Thus, abnormalities in cofilin regulation of actin networks may also negatively affect the replication capacity, shaping, and maturation of the developing heart. At the same time, although not univocally accepted (24,37), when in its active form, cofilin promotes the translocation of Bax to the mitochondria, triggering the cell death pathway. This process of cell clearance is impaired in the presence of increased phospho-cofilin-2, interfering with its beneficial effects on cellular survival and aging. Thus, changes in normal cofilin pattern may further contribute to the development of cardiomyopathy by affecting tissue remodeling and impairing the myocardial ability to respond to stress.
LIMITATIONS
We acknowledge the limitation of using human heart samples that present inevitable variability. We minimized the variability by matching the samples at best for age/gender ethnicity between iDCM and control samples. We acknowledge the difference in the structural appearance of the stress-like and the rod-like structures, which warrant further studies.
Whether cofilin appears to provide with a new pathogenetic mechanism for iDCM its primary role in the development of iDCM could not be proven in the human samples, as at present there are no known mutations in CFL leading to human iDCM.
CONCLUSIONS
Our study demonstrates that cofilin-2 and its interactome are present in human and experimental myocardial aggregates, and abnormalities in this protein complex alter myocyte structure and function. Moreover, cofilin-2 activity is decreased in iDCM tissue due to its enhanced degree of phosphorylation. Therefore, cofilin-2 sequestration in aggregates, in tandem with its inactivation, is likely to interfere with cofilin-2 critical function in the maintenance of actin filament homeostasis for effective myocyte contraction and relaxation. Thus, abnormal cofilin regulation provides a novel pathogenetic mechanism for iDCM and a novel conceptual ground for future development of personalized therapies.
Supplementary Material
Online Figure 1. Schematic simplified cartoon of the aggregate-prone protein maturation to amyloid fibers.
Aggregate-prone proteins that lose the native conformation become progressively larger structures from monomer, dimer-trimers, PAOs (>24 mers), protofibrils and amyloid fibers reducing the free energy. These structures are common for all aggregate-prone proteins. A11 antibodies recognize the PAOs structure.
Online Figure 2. Cofilin is sequestered in the aggregates in the aging hearts
Expression levels of cofilin-2 in human myocardial samples extracted with Triton-X or SDS from (A) donors and (B) iDCM samples. The results plotted individually show how, in the samples where the levels of cofilin-2 expression is lower in the Triton-X fraction, the protein is more sequestered in the SDS one (red diamond) and vice-versa (red circles). The samples were ordered by age showing how in the donor hearts at older age cofilin also accumulates in the SDS fraction supporting the hypothesis that iDCM is a disease with an anticipated aging phenotype. In two iDCM samples (red asterisk) cofilin was not found increased in the SDS fraction. In two other samples (blue asterisk) the level of cofilin-2 in the SDS extraction was not high underlying that not all iDCM may be due to cofilin pathology. M=molecular weight marker. Full blots are shown in the upper panels of Figure 2B (C, D): the middle panels are the gels (E, F), the lower panels are the blots after transfer used for normalization for total proteins (G, H). Stain-free gels were used for this WB. Normalization was done on the band at approximately the same molecular weight.
Online Figure 3. Schematic simplified cartoon of active and inactive (phosphorylated) cofilin effect on actin treadmilling.
The globular form of actin (G-actin) polymerizes into filamentous actin (F-actin), which undergoes a dynamic turnover of assembly and disassembly. At steady state in non-muscle cells, G-actin (ATP-actin in red) is added to the plus end of the actin filament and ADP-actin (in blue) is depolymerized by cofilin from the minus end (9,10). De-phosphorylated cofilin cooperatively binds to F-actin to increase the turnover (treadmilling) of actin filaments maintaining the pool of G-actin. The actin subunits depolymerizing from cofilin-bound filaments are recycled to filaments lacking cofilin. Actin is depicted with the pointed (plus) and barbed (minus) ends. Cofilin is in green and phospho-cofilin in light blue. The yellow dot represents the phosphorylated residue.
Online Figure 4. 2D western shows increased phosphorylation of cofilin in iDCM.
2D western displayed an increase in the ratio of phospho/total cofilin in iDCM. Top panels show representative 2D blots for iDCM and control samples. Quantification of phospho-cofilin as % of total cofilin in the lower panel. White bar: donor, black bar: iDCM hearts. Number of samples is indicated in the bars. The panel on the right shows the overlay of the 2D western with the 2D markers (red boxes). NS due to sample selection.
Online Figure 5. Phos-tag measurement of cofilin phosphorylation indicates increased phosphorylation in iDCM.
Control Western Blot (left upper panel) and mobility shift for phosphorylated proteins (Phos-tag) (right upper panel). Coomassie stained gels for loading control (middle panels). Densitometric analysis of the phospho/total cofilin-2 ratio (lower panel). *p<0.05. A major band below the 22 kDa marker, compatible with the un-phosphorylated form of cofilin-2 was detected by western blot of both Phos-tag and non Phos-tag control gels. Another major band at ≈30 kDa apparent molecular weight (non Phos-tag control gels) was delayed in Phos-tag gels (to ≈36 kDa apparent molecular weight), indicating protein phosphorylation. Additional bands were observed at 50 and 100 kDa. It is possible that protein-protein interactions involving phosphorylated cofilin-2 may account for the increased molecular weight, also in the non Phos-tag control gels. The band at 100 kDa is compatible with actin-cofilin oligomerization possibly involving non-phosphorylated cofilin-2 as there is no noticeable difference with or without Phos-tag
Online Figure 6. Immunoprecipitation with structural antibodies demonstrates that the cofilin substrate, actin, is also enriched in the PAOs aggregates in iDCM
Dot-blot for α-sarcomeric actin after immunoprecipitation with A11-antibody; Actin is increased in the PAOs complex in iDCM.
Online Figure 7. Cofilin co-localizes with PAOs in human iDCM
A) Donor and B) iDCM human sample showing the individual confocal channels of the images in Figure 2C and 2D. C) The iDCM human sample with high PAOs content as in Figure 2D shows diffuse dotted staining as well as larger deposits. By IHC some larger deposits (indicated by the arrows) are selected to quantify the co-localization. The four panels show the individual channels and the overlay. Upper left nuclei stained blue with DAPI, upper right cofilin, lower left A11-PAOs and lower right the overlay. D) In the same iDCM sample the colocalization was quantified in three different areas. Below the images are the graphs of colocalization and the individual channels of the areas for cofilin and PAOs at higher magnification. The arrow indicate the area analyzed.
Online Figure 8. Echocardiography representative images: Echocardiography representative images of WT littermates, αMHC Cre and CSC2 mice showing a dilated phenotype. The images show that the images for the measurements were taken at equivalent heart rate.
Online Figure 9: There is no evidence of increased fibrosis in the myocardium of heterozygous, 2-month-old, CSC2 mice.
Trichrome staining showing normal level of fibrosis in the CSC2 mice.
Online Figure 10. CSC2 recapitulate the human pathology
Upper panels: myocardial section of an explanted heart from a case diagnosed with nemaline cardiomyopathy showing “rods” (white boxes) and Z band interruption (red arrowheads). Lower panels: CSC2 mice showing actin “rod-like” scattered throughout the tissue (white boxes), myofibrillar disarray (red arrows) and Z band interruption (red arrowheads) recapitulating the human pathology.
Online Figure 11. Schematic simplified cartoon of cofilin activation signaling pathways.
Simplified RhoA/ROCK/LIMK/TESK signaling pathways controlling cofilin activity on the disassembly of actin fibers. LIM or TES kinases inactivate cofilin whereas slingshot (SSH) or chronophin phosphatases activate it. All signaling are downstream effectors of the G-protein coupled receptor GPCR/RhoA signaling known to activate the intracellular response to extracellular stimuli and stress. Green arrows indicate the pathways activated, and the red lines the pathways blocked by phosphorylation.
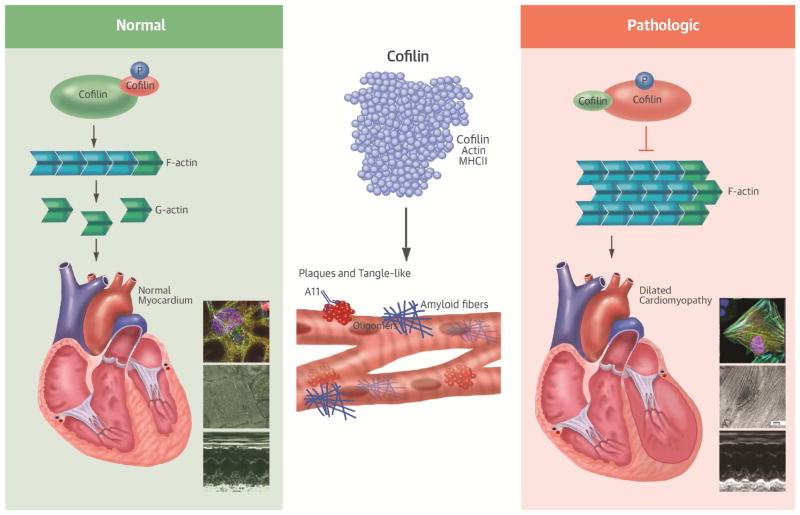
Figure 7. Central Illustration. Cofilin in Cardiac Plaques: New Mechanisms for Dilated Cardiomyopathy.
Plaques- and tangle-like aggregates accumulate in the myocardium in some cases of iDCM. The actin depolymerizing protein cofilin is enriched in the aggregates together with its binding partners actin and MHCII. Cofilin is for the most phosphorylated, thus inactivated in iDCM. The prevalent inhibition of cofilin activity in conjunction with the damage to the myofibrillar integrity and the accumulation of fibrillar aggregates interfere with the proper function of the sarcomeres ultimately contributing to cardiac dysfunction in these cases of iDCM.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
The actin-depolymerizing protein cofilin occurs in the cellular aggregates in nemaline skeletal myopathy and Alzheimer’s disease, and in recently identified aggregates in patients with iDCM. This suggests a common pathogenic mechanism linking inactivation and sequestration of cofilin in cerebral and skeletal muscle and cardiac tissue.
TRANSLATIONAL OUTLOOK
Identification of systemic or multicentric factors that contribute to iDCM pathogenesis could inform development of targeted therapies.
Acknowledgments
Funding sources: The study was supported by BIDMC departmental funds and by grants from the National Institute of Health and the America Heart Association to FdM (R21HL102716, R01HL098468, IRG18980028), the National Institute of Health to PBA (K08 AR055072).
We thank Dr. Alexander Ivanov at Northeastern University for assistance with mass spectrometry; Dr. Towia Libermann at BIDMC for critical interpretation of mass spectrometry data; the BWH for assistance with confocal microscopy and echocardiography; Dr. Dale Abel at the University of Iowa for the generous donation of the αMHC-Cre mice; and Alisa Shaw at University of Colorado with assistance with adenovirus constructs.
Abbreviations and Acronyms
- AD
Alzheimer’s disease
- ADF
actin depolymerizing factor
- CSC2
cardiac-specific cofilin-2 knockout mouse
- iDCM
idiopathic dilated cardiomyopathy
- PAO
pre-amyloid oligomers
- S3A
constitutively active cofilin mutant
- S3E
phosphomimetic cofilin mutant
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arbustini E, Narula N, Tavazzi L, et al. The MOGE(S) classification of cardiomyopathy for clinicians. J Am Coll Cardiol. 2014;64:304–18. doi: 10.1016/j.jacc.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 2.Gianni D, Li A, Tesco G, et al. Protein aggregates and novel presenilin gene variants in idiopathic dilated cardiomyopathy. Circulation. 2010;121:1216–26. doi: 10.1161/CIRCULATIONAHA.109.879510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanbe A, Osinska H, Saffitz JE, et al. Desmin-related cardiomyopathy in transgenic mice: a cardiac amyloidosis. Proc Natl Acad Sci U S A. 2004;101:10132–6. doi: 10.1073/pnas.0401900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David DC. Aging and the aggregating proteome. Front Genet. 2012;3:247. doi: 10.3389/fgene.2012.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naidoo N. ER and aging-Protein folding and the ER stress response. Ageing Res Rev. 2009;8:150–9. doi: 10.1016/j.arr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Alzheimer A. A new disease of the cortex. Allg Z Psych. 1907;64:146–8. [Google Scholar]
- 7.Glenner GG, Harada M, Isersky C. The purification of amyloid fibril proteins. Prep Biochem. 1972;2:39–51. doi: 10.1080/00327487208061451. [DOI] [PubMed] [Google Scholar]
- 8.Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- 9.Minamide LS, Striegl AM, Boyle JA, Meberg PJ, Bamburg JR. Neurodegenerative stimuli induce persistent ADF/cofilin-actin rods that disrupt distal neurite function. Nat Cell Biol. 2000;2:628–36. doi: 10.1038/35023579. [DOI] [PubMed] [Google Scholar]
- 10.Bamburg JR, Bernstein BW. Roles of ADF/cofilin in actin polymerization and beyond. F1000 Biol Rep. 2010;2:62. doi: 10.3410/B2-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vartiainen MK, Mustonen T, Mattila PK, et al. The three mouse actin-depolymerizing factor/cofilins evolved to fulfill cell-type-specific requirements for actin dynamics. Mol Biol Cell. 2002;13:183–94. doi: 10.1091/mbc.01-07-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skwarek-Maruszewska A, Hotulainen P, Mattila PK, Lappalainen P. Contractility-dependent actin dynamics in cardiomyocyte sarcomeres. J Cell Sci. 2009;122:2119–26. doi: 10.1242/jcs.046805. [DOI] [PubMed] [Google Scholar]
- 13.Kayed R, Glabe CG. Conformation-dependent anti-amyloid oligomer antibodies. Methods Enzymol. 2006;413:326–44. doi: 10.1016/S0076-6879(06)13017-7. [DOI] [PubMed] [Google Scholar]
- 14.Graham EL, Balla C, Franchino H, Melman Y, del Monte F, Das S. Isolation, culture, and functional characterization of adult mouse cardiomyoctyes. J Vis Exp. 2013:e50289. doi: 10.3791/50289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–90. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 16.Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426:900–4. doi: 10.1038/nature02264. [DOI] [PubMed] [Google Scholar]
- 17.del Monte F, Agnetti G. Protein post-translational modifications and misfolding: new concepts in heart failure. Proteomics Clin Appl. 2014;8:534–42. doi: 10.1002/prca.201400037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang N, Higuchi O, Ohashi K, et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–12. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 19.Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002;108:233–46. doi: 10.1016/s0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- 20.Gohla A, Birkenfeld J, Bokoch GM. Chronophin, a novel HAD-type serine protein phosphatase, regulates cofilin-dependent actin dynamics. Nat Cell Biol. 2005;7:21–9. doi: 10.1038/ncb1201. [DOI] [PubMed] [Google Scholar]
- 21.Huang TY, DerMardirossian C, Bokoch GM. Cofilin phosphatases and regulation of actin dynamics. Curr Opin Cell Biol. 2006;18:26–31. doi: 10.1016/j.ceb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Manders EEMV, FJ, Aten JA. Measurement of co-localization of objects in dual-colour confocal images. J Microsc. 1993;169:375–82. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal PB, Joshi M, Savic T, Chen Z, Beggs AH. Normal myofibrillar development followed by progressive sarcomeric disruption with actin accumulations in a mouse Cfl2 knockout demonstrates requirement of cofilin-2 for muscle maintenance. Hum Mol Genet. 2012;21:2341–56. doi: 10.1093/hmg/dds053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rehklau K, Gurniak CB, Conrad M, Friauf E, Ott M, Rust MB. ADF/cofilin proteins translocate to mitochondria during apoptosis but are not generally required for cell death signaling. Cell Death Differ. 2012;19:958–967. doi: 10.1038/cdd.2011.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodson M, Rust MB, Witke W, et al. Cofilin-1: a modulator of anxiety in mice. PLoS genetics. 2012;8:e1002970. doi: 10.1371/journal.pgen.1002970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aoki H, Izumo S, Sadoshima J. Angiotensin II activates RhoA in cardiac myocytes: a critical role of RhoA in angiotensin II-induced premyofibril formation. Circ Res. 1998;82:666–76. doi: 10.1161/01.res.82.6.666. [DOI] [PubMed] [Google Scholar]
- 27.Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–12. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maloney MT, Minamide LS, Kinley AW, Boyle JA, Bamburg JR. Beta-secretase-cleaved amyloid precursor protein accumulates at actin inclusions induced in neurons by stress or amyloid beta: a feedforward mechanism for Alzheimer’s disease. J Neurosci. 2005;25:11313–21. doi: 10.1523/JNEUROSCI.3711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agrawal PB, Greenleaf RS, Tomczak KK, et al. Nemaline myopathy with minicores caused by mutation of the CFL2 gene encoding the skeletal muscle actin-binding protein, cofilin-2. Am J Hum Genet. 2007;80:162–7. doi: 10.1086/510402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiggan O, Shaw AE, DeLuca JG, Bamburg JR. ADF/cofilin regulates actomyosin assembly through competitive inhibition of myosin II binding to F-actin. Dev Cell. 2012;22:530–43. doi: 10.1016/j.devcel.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tojkander S, Gateva G, Lappalainen P. Actin stress fibers--assembly, dynamics and biological roles. J Cell Sci. 2012;125:1855–64. doi: 10.1242/jcs.098087. [DOI] [PubMed] [Google Scholar]
- 32.Gandy S, Doeven MK, Poolman B. Alzheimer disease: presenilin springs a leak. Nat Med. 2006;12:1121–3. doi: 10.1038/nm1006-1121. [DOI] [PubMed] [Google Scholar]
- 33.Bernstein BW, Bamburg JR. ADF/cofilin: a functional node in cell biology. Trends Cell Biol. 2010;20:187–95. doi: 10.1016/j.tcb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reffay M, Parrini MC, Cochet-Escartin O, et al. Interplay of RhoA and mechanical forces in collective cell migration driven by leader cells. Nat Cell Biol. 2014;16:217–23. doi: 10.1038/ncb2917. [DOI] [PubMed] [Google Scholar]
- 35.Gunsalus KC, Bonaccorsi S, Williams E, Verni F, Gatti M, Goldberg ML. Mutations in twinstar, a Drosophila gene encoding a cofilin/ADF homologue, result in defects in centrosome migration and cytokinesis. J Cell Biol. 1995;131:1243–59. doi: 10.1083/jcb.131.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagaoka R, Kusano K, Abe H, Obinata T. Effects of cofilin on actin filamentous structures in cultured muscle cells. Intracellular regulation of cofilin action. J Cell Sci. 1995;108(Pt 2):581–93. doi: 10.1242/jcs.108.2.581. [DOI] [PubMed] [Google Scholar]
- 37.Posadas I, Perez-Martinez FC, Guerra J, Sanchez-Verdu P, Cena V. Cofilin activation mediates Bax translocation to mitochondria during excitotoxic neuronal death. J Neurochem. 2012;120:515–27. doi: 10.1111/j.1471-4159.2011.07599.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Figure 1. Schematic simplified cartoon of the aggregate-prone protein maturation to amyloid fibers.
Aggregate-prone proteins that lose the native conformation become progressively larger structures from monomer, dimer-trimers, PAOs (>24 mers), protofibrils and amyloid fibers reducing the free energy. These structures are common for all aggregate-prone proteins. A11 antibodies recognize the PAOs structure.
Online Figure 2. Cofilin is sequestered in the aggregates in the aging hearts
Expression levels of cofilin-2 in human myocardial samples extracted with Triton-X or SDS from (A) donors and (B) iDCM samples. The results plotted individually show how, in the samples where the levels of cofilin-2 expression is lower in the Triton-X fraction, the protein is more sequestered in the SDS one (red diamond) and vice-versa (red circles). The samples were ordered by age showing how in the donor hearts at older age cofilin also accumulates in the SDS fraction supporting the hypothesis that iDCM is a disease with an anticipated aging phenotype. In two iDCM samples (red asterisk) cofilin was not found increased in the SDS fraction. In two other samples (blue asterisk) the level of cofilin-2 in the SDS extraction was not high underlying that not all iDCM may be due to cofilin pathology. M=molecular weight marker. Full blots are shown in the upper panels of Figure 2B (C, D): the middle panels are the gels (E, F), the lower panels are the blots after transfer used for normalization for total proteins (G, H). Stain-free gels were used for this WB. Normalization was done on the band at approximately the same molecular weight.
Online Figure 3. Schematic simplified cartoon of active and inactive (phosphorylated) cofilin effect on actin treadmilling.
The globular form of actin (G-actin) polymerizes into filamentous actin (F-actin), which undergoes a dynamic turnover of assembly and disassembly. At steady state in non-muscle cells, G-actin (ATP-actin in red) is added to the plus end of the actin filament and ADP-actin (in blue) is depolymerized by cofilin from the minus end (9,10). De-phosphorylated cofilin cooperatively binds to F-actin to increase the turnover (treadmilling) of actin filaments maintaining the pool of G-actin. The actin subunits depolymerizing from cofilin-bound filaments are recycled to filaments lacking cofilin. Actin is depicted with the pointed (plus) and barbed (minus) ends. Cofilin is in green and phospho-cofilin in light blue. The yellow dot represents the phosphorylated residue.
Online Figure 4. 2D western shows increased phosphorylation of cofilin in iDCM.
2D western displayed an increase in the ratio of phospho/total cofilin in iDCM. Top panels show representative 2D blots for iDCM and control samples. Quantification of phospho-cofilin as % of total cofilin in the lower panel. White bar: donor, black bar: iDCM hearts. Number of samples is indicated in the bars. The panel on the right shows the overlay of the 2D western with the 2D markers (red boxes). NS due to sample selection.
Online Figure 5. Phos-tag measurement of cofilin phosphorylation indicates increased phosphorylation in iDCM.
Control Western Blot (left upper panel) and mobility shift for phosphorylated proteins (Phos-tag) (right upper panel). Coomassie stained gels for loading control (middle panels). Densitometric analysis of the phospho/total cofilin-2 ratio (lower panel). *p<0.05. A major band below the 22 kDa marker, compatible with the un-phosphorylated form of cofilin-2 was detected by western blot of both Phos-tag and non Phos-tag control gels. Another major band at ≈30 kDa apparent molecular weight (non Phos-tag control gels) was delayed in Phos-tag gels (to ≈36 kDa apparent molecular weight), indicating protein phosphorylation. Additional bands were observed at 50 and 100 kDa. It is possible that protein-protein interactions involving phosphorylated cofilin-2 may account for the increased molecular weight, also in the non Phos-tag control gels. The band at 100 kDa is compatible with actin-cofilin oligomerization possibly involving non-phosphorylated cofilin-2 as there is no noticeable difference with or without Phos-tag
Online Figure 6. Immunoprecipitation with structural antibodies demonstrates that the cofilin substrate, actin, is also enriched in the PAOs aggregates in iDCM
Dot-blot for α-sarcomeric actin after immunoprecipitation with A11-antibody; Actin is increased in the PAOs complex in iDCM.
Online Figure 7. Cofilin co-localizes with PAOs in human iDCM
A) Donor and B) iDCM human sample showing the individual confocal channels of the images in Figure 2C and 2D. C) The iDCM human sample with high PAOs content as in Figure 2D shows diffuse dotted staining as well as larger deposits. By IHC some larger deposits (indicated by the arrows) are selected to quantify the co-localization. The four panels show the individual channels and the overlay. Upper left nuclei stained blue with DAPI, upper right cofilin, lower left A11-PAOs and lower right the overlay. D) In the same iDCM sample the colocalization was quantified in three different areas. Below the images are the graphs of colocalization and the individual channels of the areas for cofilin and PAOs at higher magnification. The arrow indicate the area analyzed.
Online Figure 8. Echocardiography representative images: Echocardiography representative images of WT littermates, αMHC Cre and CSC2 mice showing a dilated phenotype. The images show that the images for the measurements were taken at equivalent heart rate.
Online Figure 9: There is no evidence of increased fibrosis in the myocardium of heterozygous, 2-month-old, CSC2 mice.
Trichrome staining showing normal level of fibrosis in the CSC2 mice.
Online Figure 10. CSC2 recapitulate the human pathology
Upper panels: myocardial section of an explanted heart from a case diagnosed with nemaline cardiomyopathy showing “rods” (white boxes) and Z band interruption (red arrowheads). Lower panels: CSC2 mice showing actin “rod-like” scattered throughout the tissue (white boxes), myofibrillar disarray (red arrows) and Z band interruption (red arrowheads) recapitulating the human pathology.
Online Figure 11. Schematic simplified cartoon of cofilin activation signaling pathways.
Simplified RhoA/ROCK/LIMK/TESK signaling pathways controlling cofilin activity on the disassembly of actin fibers. LIM or TES kinases inactivate cofilin whereas slingshot (SSH) or chronophin phosphatases activate it. All signaling are downstream effectors of the G-protein coupled receptor GPCR/RhoA signaling known to activate the intracellular response to extracellular stimuli and stress. Green arrows indicate the pathways activated, and the red lines the pathways blocked by phosphorylation.