Abstract
Bone health and cardiovascular function are compromised in individuals with type 2 diabetes mellitus (T2DM). The purpose of the present study was to determine whether skeletal vascular control mechanisms are altered during the progression of T2DM in the Zucker diabetic fatty (ZDF) rat. Responses of the principal nutrient artery (PNA) of the femur from obese ZDF rats with prediabetes, short-term diabetes, and long-term diabetes to endothelium-dependent (acetylcholine) and –independent (sodium nitroprusside) vasodilation, and KCl, norepinephrine and myogenic vasoconstrictor were determined in vitro. Few changes in the PNA vasomotor responses occurred in the pre-diabetic and short-term diabetic conditions. Endothelium-dependent and –independent vasodilation were reduced, and norepinephrine and myogenic vasoconstriction were enhanced in obese ZDF rats with long-term diabetes relative to lean age-matched controls. Differences in endothelium-dependent vasodilation of the femoral PNA between ZDF rats and controls were abolished by the nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester. The passive pressure-diameter response of the femoral PNA was also lower across a range of intraluminal pressures with long-term T2DM. Regional bone and marrow perfusion and vascular conductance, measured in vivo using radiolabeled microspheres, were lower in obese ZDF rats with long-term diabetes. These findings suggest that the profound impairment of the bone circulation may contribute to the osteopenia found to occur in long bones with chronic T2DM.
Keywords: Diabetes, Bone blood flow, Endothelium, Vascular conductance, Zucker diabetic fatty rat
Introduction
The increase in fracture risk observed among patients with type 2 diabetes mellitus (T2DM) has been suggested to be associated with corollaries of the disease, such as peripheral neuropathy, decreased physical fitness, vision loss, and poor balance (Leslie, et al. 2012, Melton, et al. 2008, Schwartz. 2003). Emerging research also suggests a causal link between cardiovascular function and indices of bone health (Farhat & Cauley. 2008, Lampropoulos, et al. 2012, Parfitt. 2000, Prisby, et al. 2012). Cardiovascular disease is a leading complication of T2DM (Grundy, et al. 1999, World Health Organization. 2013), including impairment of endothelium-dependent vasodilation of large conduit arteries (Hogikyan, et al. 1998, Makimattila, et al. 1999, Rossi, et al. 2005, van de Ree, et al. 2001).
Previous findings using the obese Zucker diabetic fatty (ZDF) rats (Lesniewski, et al. 2008), an animal model of T2DM, demonstrate that an impairment of endothelium-dependent vasodilation through the nitric oxide (NO) signaling mechanism effectively changes the balance of vasomotor control in skeletal muscle arterioles to favor vasoconstriction. Decreases in BMD have also been shown to occur in obese ZDF rats with long-term T2DM (Prisby, et al. 2008) and is one basis for the decrease in mechanical strength of long bones in the hindlimb. It was hypothesized that one contributing factor to this decrease in BMD and mechanical strength could be a reduction in bone and marrow blood flow and impairment of coupling mechanisms linking endothelium-dependent vasodilation to bone cell remodeling activity (Prisby, et al. 2008). For example, reduced blood flow to the bone and marrow of the femur occurs in aged rats (Prisby, et al. 2007), and this is accompanied by an impairment of the ability of the femoral principal nutrient artery (PNA) to vasodilate by way of a NO signaling mechanism (Prisby, et al. 2007). In addition, prior experiments with young and old exercise trained rats (Dominguez, et al. 2010) and ovariectomized rats (Prisby, et al. 2012) suggest a coupling of endothelium-dependent vasodilation to measures of bone volume. Therefore, the purpose of the present study was to determine whether control mechanisms of the skeletal resistance vasculature, including endothelium-dependent vasodilation, are altered during the progression of T2DM when BMD is both increasing and decreasing (Prisby, et al. 2008). We hypothesized that endothelium-dependent vasodilation of the femoral PNA would be higher in pre-diabetic 7-wk old obese ZDF rats when femoral BMD is greater than that in 7-wk old lean ZDF rats, and that endothelium-dependent vasodilation would be impaired in long-term diabetic 20-wk old obese ZDF rats when femoral BMD is reduced. Results from these vascular studies demonstrated that endothelium-dependent vasodilation of the PNA was diminished with long-term diabetes, along with other vascular mechanisms that serve to regulate bone perfusion. Consequently, a secondary purpose of this study was to determine the effects of frank T2DM on hindlimb bone and marrow blood flow and vascular conductance. We hypothesized that bone and marrow perfusion would be lower in long-term diabetes relative to that in 20-wk old lean ZDF animals.
Materials and Methods
Animals
All experimental procedures were approved by the University of Florida’s Institutional Animal Care and Use Committee and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Eighth edition, 2011). Lean (371: +/?) and obese (370: fa/fa) ZDF rats were obtained from Charles River Laboratories (Kingston, NY, USA). Obese ZDF rats are homozygous for the fatty (fa) gene (Truett, et al. 1991) and when fed the Purina 5008 diet manifest obesity and hyperlipidemia (Leonard, et al. 2005). Seven-wk old obese ZDF rats manifest hyperinsulinemia and mild hyperglycemia and have been classified as pre-diabetic. The strain develops more severe hyperglycemia by 13 wks of age (short-term diabetes) and becomes normo- or hypoinsulinemic by 20 wks of age (long-term diabetes) (Etgen & Oldham. 2000, Peterson, et al. 1990). Animals from the present study were provided a Purina 5008 diet and water ad libitum, maintained on a 12:12 light-dark cycle, and studied at 7 wks (lean, n=28; obese, n=29), 13 wks (lean, n=28; obese, n=28), and 20 wks (lean, n=51; obese, n=51) of age to correspond to a pre-diabetic, short-term diabetic, and long-term diabetic state in the obese ZDF rats, respectively.
Isolated microvessels
Animals were anesthetized with isoflurane (2%/O2 balance), a thoracotomy was performed, and 2 mL of blood was withdrawn via cardiac puncture before excision of the heart. Immediately after euthanasia the hindlimbs were removed and placed into a dissecting bath containing 4°C physiological saline solution (PSS). Femoral PNAs, which regulate ~70% of the blood supply to the femoral bone marrow and cortex (Bridgeman & Brookes. 1996), were isolated, cannulated, and pressurized with PSS at 60 cmH2O (44 mmHg) as previously described (Dominguez, et al. 2010, Prisby, et al. 2007). This pressure was selected based upon intravascular arterial pressures measured within similarly sized skeletal muscle resistance arteries of 43–46 mmHg (Meininger, et al. 1984).
Experimental design
Endothelium-dependent vasodilation of femoral PNAs (n=14–15/group) was assessed by the cumulative addition of acetylcholine (ACh, 10−9–10−4 mol l−1). To determine the contribution of the NO signaling pathway to endothelium-dependent vasodilation, PNAs were incubated with the NO synthase (NOS) inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 10−5 mol l−1) (Dominguez, et al. 2010, Muller-Delp, et al. 2002, Prisby, et al. 2007), and the ACh dose-response was repeated. To investigate the contribution of prostaglandin signaling to endothelium-dependent vasodilation, PNAs were co-incubated with L-NAME and the cyclooxygenase (COX) inhibitor indomethacin (10−5 mol l−1) (Prisby, et al. 2007), and the ACh dose-response was repeated. Endothelium-independent vasodilator responsiveness was assessed via the cumulative addition of the NO donor sodium nitroprussside (SNP, 10−9–10−4 mol l−1). Lastly, maximal intraluminal diameter and medial wall thickness were determined after two 15 min incubations in Ca2+-free PSS supplemented with SNP (10−4 mol l−1) to achieve complete smooth muscle relaxation. Medial wall thickness was recorded as the average of three distinct wall measurements made with the video caliper as previously described (Stabley, et al. 2013).
In a separate set of rats (n=13–14/group), vasoconstrictor responses of PNAs to increasing concentrations of potassium chloride (KCl, 10–100 mmol l−1) were determined to investigate the contribution of voltage-gated Ca2+ channels. Concentrations of NaCl and KCl in PSS were balanced such that bath osmolarity was maintained and the desired isotonic K+ bath concentrations were achieved (Donato, et al. 2005). Vasoconstrictor responses to the cumulative addition of norephinephrine (NE, 10−9–10−4 mol l−1) were recorded to measure the contribution of alpha adrenoreceptors (Delp. 1999). In a separate group of 20-wk old animals (lean, n=11; obese, n=10), the endothelium of PNAs was removed by passing 3–5 mL of air through the lumen as previously described (Donato, et al. 2005). Administration of a bolus dose of ACh (10−5 mol l−1) was used to confirm the successful removal of the endothelium, and measurement of the PNA response to the cumulative addition of NE was determined.
Active myogenic responses to stepwise increases in intraluminal pressure were determined by raising the height of both fluid reservoirs in 15 cmH2O increments from 0 cmH2O to 135 cmH2O. Intraluminal pressure was then decreased in 15 cmH2O increments back to 0 cmH2O. Passive myogenic responses to stepwise increases in intraluminal diameter were determined using the steps employed for the active myogenic response describe above except that the vessel bathing solution was replaced with Ca2+-free PSS plus SNP (10−4 mol l−1) as previously described (Delp. 1999).
Peripheral quantitative computed tomography (pQCT)
Tomographic scans were performed ex vivo on femoral mid-shafts and distal femora in PBS using a Stratec XCT Research-M device as previously reported (Prisby, et al. 2008). Reported measures of cortical BMD were derived from the femoral mid-shaft and reported total and cancellous BMD were derived from measures of the distal femora. For precision of the measurements, calibration of the Stratec XCT Research-M device was performed prior to scanning by use of a hydroxyapatite standard cone phantom. The distal metaphysis of the femur was scanned 4, 5, and 6 mm from the proximal plateau and the mid-diaphysis was scanned (on center) 8 mm from distal end of the lateral epicondyles. For both the distal metaphysis and mid-diaphysis, values from multiple slices were averaged. Scans were performed at 5 mm/s with voxel resolution of 0.07 × 0.07 × 0.5 mm. Additionally, analyses were performed using cut and peel modes of 3 and 2. According to the manufacturer’s data, machine precision is ±3.0 mg/cm3 for cancellous bone and ±9.0 mg/cm3 for cortical bone.
Surgical procedures
Blood flow was measured in a separate group of 20-wk old lean (n=12) and obese (n=12) ZDF rats as previously described (Colleran, et al. 2000, Prisby, et al. 2007). Animals were anesthetized with isoflourane (2–2.5%/O2 balance) and a polyethylene catheter filled with heparinized saline solution (100 U/mL) was implanted in the ascending aorta via the right carotid artery. Blood (~300 µL) was collected from this catheter to determine plasma insulin and glucose concentrations. A separate polyethylene catheter was implanted into the caudal artery of the tail for reference blood sample withdrawals (Delp & Armstrong. 1988). Each animal was allowed ≥3 hrs of recovery after wound closure and the cessation of anesthesia before experiments to measure tissue blood flow were performed. Previous research demonstrates that cardiovascular dynamics, regional blood flow, and acid-base status recover to normal levels within 3 hrs after anesthesia (Flaim & Zelis. 1980).
Blood flow determination
Bone blood flow using radiolabeled microspheres was measured during quiet standing in animals with long-term diabetes and 20 wk old age-matched lean controls as previously described (Colleran, et al. 2000, Prisby, et al. 2007, Stabley, et al. 2013). After completion of the blood flow experiment, animals were euthanized with Beuthanasia®-D Special (~0.6 mL/kg, Schering-Plough Animal Health, Union, NJ, USA) administered via the carotid artery catheter. Hindlimb bones were removed, cleared of muscle and tendon, and divided into regions as previously described (Colleran, et al. 2000, Dominguez, et al. 2010, Prisby, et al. 2007). Individual tissue and reference blood sample radioactivity were determined and individual tissue blood flow (mL/min/100 g) was calculated according to the reference sample microsphere method (Ishise, et al. 1980). Tissue vascular conductance (mL/min/100 g/mmHg) was calculated by dividing individual tissue blood flow by mean arterial pressure.
Statistical analyses
Vascular responses were calculated and expressed as a percentage of vasoconstriction or vasodilation as follows:
where Db was the initial baseline intraluminal diameter measured before experimental intervention, Ds was the steady-state intraluminal diameter measured after agonist addition, and Dm was the maximal diameter recorded at 60 cmH2O.
Basal tone was expressed as a percentage of the maximal diameter (Dm) as follows:
The significance of differences in body mass and bone tissue mass were determined via Student’s unpaired t-tests. Pressure-response and concentration-response curves were evaluated by using repeated-measures ANOVA with one within (intraluminal pressure or agonist concentration) and one between (experimental groups) factor. Planned contrasts were conducted at each intraluminal pressure or concentration level to determine whether differences existed between experimental groups (lean vs. obese). Regression analyses were used to individually investigate the relation between peak-endothelium-dependent vasodilation and cancellous BMD in the distal femur and total BMD and cortical BMD in the femur diaphysis. Differences in hindlimb bone blood flow and vascular conductance between experimental groups were determined by one-tailed independent-samples t-tests. An alpha level of 0.05 delineated significance.
Results
Animal and PNA characteristics
Body mass was greater in obese ZDF rats in the pre-diabetic, short-term and long-term diabetic conditions relative to the age-matched lean controls (Table 1). Blood glucose concentration was higher in ZDF rats with short- and long-term diabetes when compared to age-matched lean rats (Table 1), and blood insulin levels were higher in obese ZDF rats during the pre-diabetic and short-term diabetec conditions (Table 1).
Table 1.
Body mass, blood glucose and insulin concentrations, and femoral bone mineral density (BMD).
| 7 wk Control |
7 wk Pre- diabetic |
13 wk Control |
13 wk Short- term |
20 wk Control |
20 wk Long- term |
|
|---|---|---|---|---|---|---|
| Body Mass (g) | 128±5 | 174±6* | 276±8 | 341±14* | 369±5 | 402±13* |
| Glucose (mg/dL) | 86±4 | 104±8 | 88±5 | 219±17* | 94±4 | 242±14* |
| Insulin (ng/mL) | 1.3±0.2 | 4.7±1.0* | 1.2±0.1 | 5.2±1.4* | 1.5±0.1 | 1.6±0.1 |
| Total BMD (mg/cm3) | 459±4 | 471±6 | 562±7 | 513±16 | 617±11 | 487±7* |
| Cancellous BMD (mg/cm3) | 350±9 | 383±9* | 317±7 | 309±20 | 326±10 | 227±23* |
| Cortical BMD (mg/cm3) | 1,163±4 | 1,152±4 | 1,333±3 | 1,324±2* | 1,386±3 | 1,368±3* |
Values are mean ± S.E.
Denotes significant differences from age-matched lean controls (p< 0.05).
Maximal diameter of the femoral PNA did not differ between lean and obese ZDF rats in the pre-diabetic or short-term diabetic condition (Table 2). However, maximal diameter of the PNA was smaller in obese ZDF rats with long-term diabetes than that in age-matched lean controls (Table 2). Basal tone of the PNA did not differ between lean and obese ZDF rats at any age studied (Table 2).
Table 2.
Principal nutrient artery (PNA) maximal diameter and basal tone.
| 7 wk Control |
7 wk Pre- diabetic |
13 wk Control |
13 wk Short- term |
20 wk Control |
20 wk Long- term |
|
|---|---|---|---|---|---|---|
| PNA Maximal Diameter (µm) | 164±3 | 165±3 | 178±5 | 183±3 | 190±8 | 163±8* |
| PNA Basal Tone (%) | 50±4 | 44±5 | 41±5 | 33±5 | 37±3 | 35±4 |
Values are mean ± S.E.
Denotes significant differences from age-matched lean controls (p< 0.05).
PNA vasodilation
Endothelium-dependent vasodilation of the femoral PNA was greater in pre-diabetic ZDF rats when compared to age-matched lean ZDF animals (Fig. 1A). This difference was abolished by inhibition of NOS alone and by co-inhibition of NOS and COX (Fig. 1A). There was no difference in endothelium-dependent vasodilation between short-term diabetic animals and lean control ZDF rats (Fig. 1B). Endothelium-dependent vasodilation of the PNA in ZDF rats with long-term diabetes was lower than that of lean controls (Fig. 1C), and this difference was abolished with NOS inhibition alone and in combination with COX inhibition.
Figure 1.
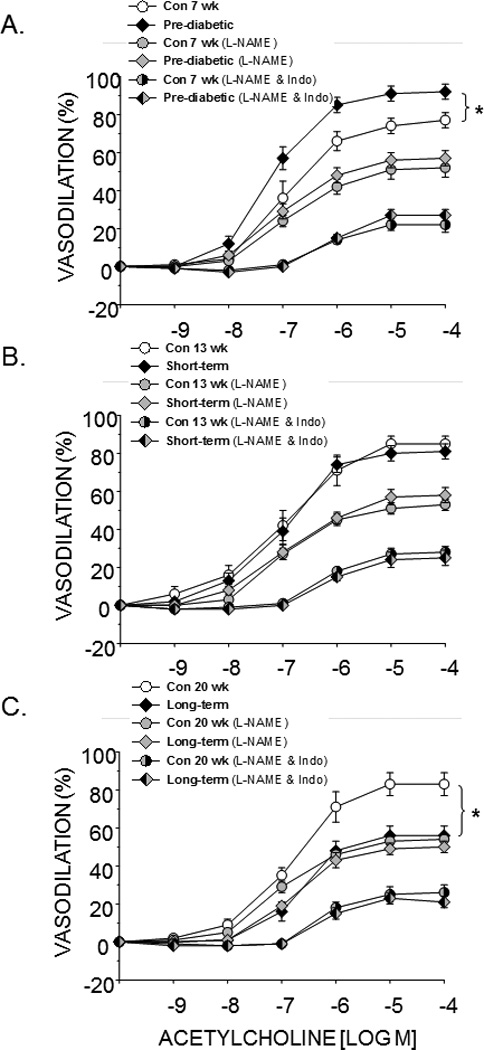
Effects of pre-diabetes (A), short-term diabetes (B), and long-term diabetes (C) on endothelium-dependent vasodilation of the femoral principal nutrient artery alone, in the presence of the nitric oxide synthase inhibitor L-NAME, and in the presence of L-NAME and the cyclooxygenase inhibitor indomethacin (Indo). Values are means ± SE, n = 14–15/group. *Mean is different from age-matched non-diabetic control animals (P<0.05).
Endothelium-independent vasodilation to SNP did not differ between lean ZDF rats and obese ZDF animals in the pre-diabetic and short-term diabetic conditions (Fig. 2). However, SNP-mediated vasodilation was attenuated in PNAs from long-term obese ZDF rats relative to lean controls (Fig. 2).
Figure 2.
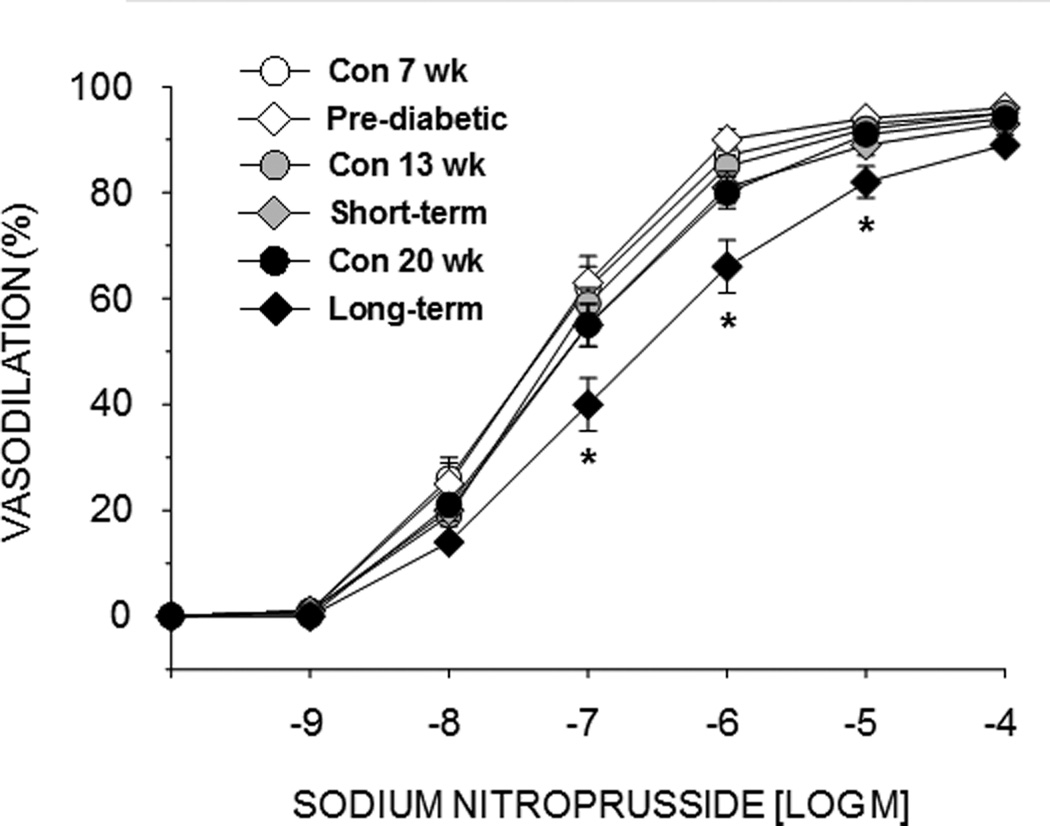
Effects of pre-diabetes, short-term diabetes, and long-term diabetes on sodium nitroprusside-induced vasodilation of the femoral principal nutrient artery (PNA). Values are means ± SE, n = 8–10/group. *Mean PNA response from 20 wk obese ZDF rats is different from that of 20 wk lean ZDF rats (P<0.05).
PNA vasoconstriction
Vasoconstrictor responsiveness to KCl was greater in pre-diabetic animals relative to lean ZDF rats (see supplemental materials). However, there were no differences in vasoconstrictor responses to KCl in PNAs from animals with short-term or long-term diabetes.
NE-induced vasoconstriction of the PNA was not different in animals with pre-diabetes or short-term diabetes relative to age-matched lean controls (see supplemental materials). However, NE-induced vasoconstriction was greater in animals with long-term diabetes at concentrations between 10−7 and 10−6 M (Fig. 3A). When the PNAs were denuded of the endothelium, differences in NE-mediated vasoconstriction between obese and lean ZDF rats were abolished (Fig. 3B).
Figure 3.
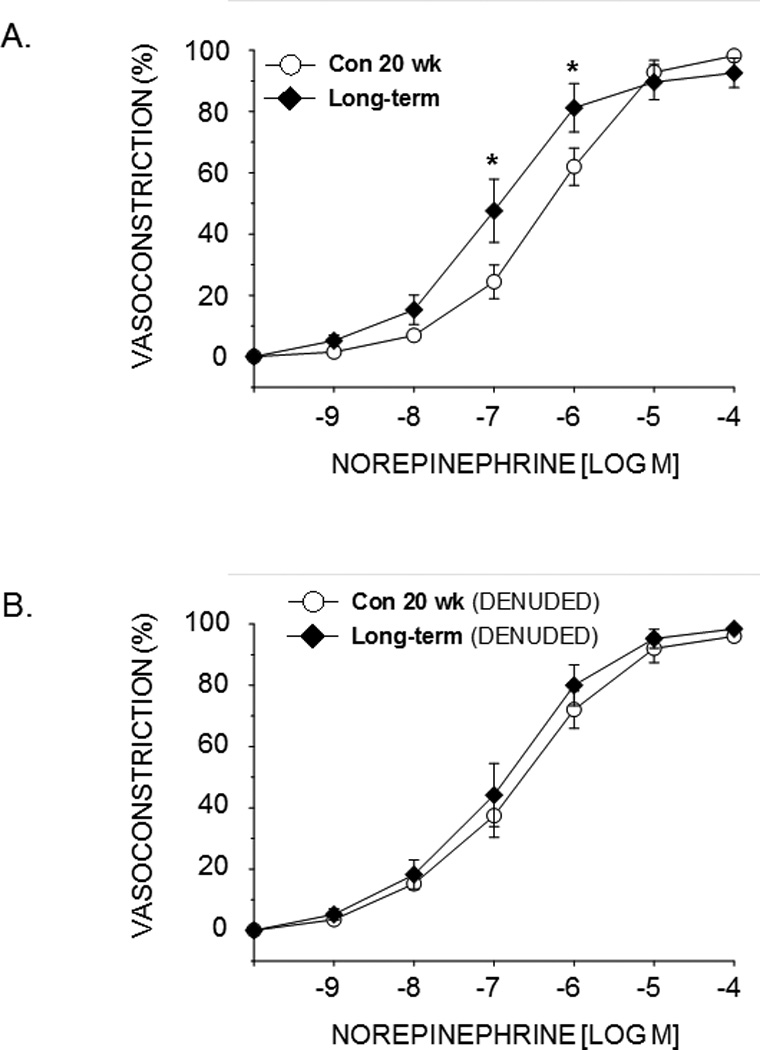
Effects of long-term diabetes on norepinephrine-induced vasoconstriction of the femoral principal nutrient artery (PNA) with the endothelium intact (A) and with the endothelium removed (B). Values are means ± SE, n = 10–14/group. *Mean PNA response from 20 wk obese ZDF rats is different from that of 20 wk lean ZDF rats (P<0.05).
Active myogenic and passive pressure-diameter responses were not different between animals with pre-diabetes or short-term diabetes and their lean counterparts (see supplemental materials). However, active myogenic vasoconstriction was greater in animals with long-term diabetes relative to lean control rats (Fig. 4A), and the passive pressure-diameter responses were lower in long-term diabetic rats compared to lean controls (Fig. 4B).
Figure 4.
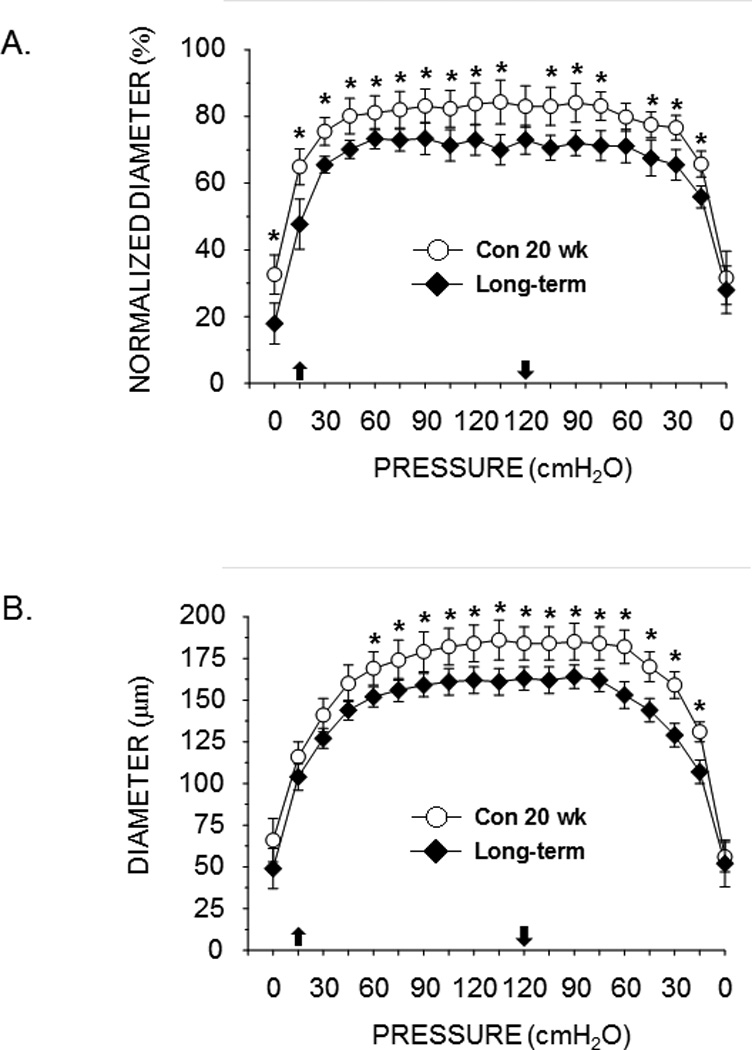
Effects of long-term diabetes on active myogenic vasoconstriction (A) and the passive pressure-diameter relation (B) of the femoral principal nutrient artery. Upward pointing arrow indicates where pressure began to be increased; downward arrow indicates where pressure began to be lowered. Values are means ± SE, n = 13–14/group. *Mean PNA response from 20 wk obese ZDF rats is different from that of 20 wk lean ZDF rats (P<0.05).
Femoral BMD
Total and cortical BMD were the same in pre-diabetic rats versus lean controls, whereas cancellous BMD of the distal femur was greater in the pre-diabetic condition (Table 1). With short-term diabetes, total and cancellous BMD were not different between obese and lean ZDF rats, while cortical BMD was lower in obese ZDF rats (Table 1). Rats in both of these age groups still exhibit skeletal growth, so the effects of factors present in the pre-diabetic and short-term diabetic states may occur as a result of skeletal growth. In long-term obese ZDF rats, where the animals are more skeletally mature, total, cancellous and cortical BMD were lower than that in lean ZDF animals (Table 1).
Relation between endothelium-dependent vasodilation and femoral BMD
Cancellous BMD of the femur was positively associated with peak endothelium-dependent vasodilation of the PNA in prediabetes, short-term diabetes, and long-term diabetes (Figs. 5A–C). In addition, endothelium dependent-vasodilation was positively correlated with total BMD (Fig. 6A) and cortical BMD (Fig. 6B) of the femur in long-term diabetes.
Figure 5.
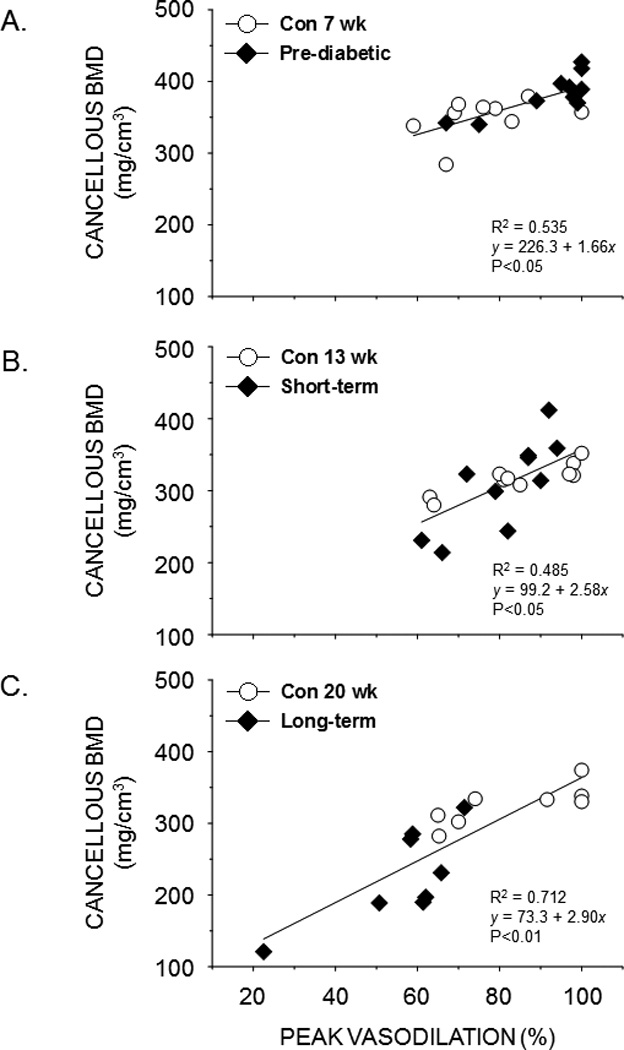
Scattergrams showing the relation between cancellous bone mineral density (BMD) in the distal femur and peak endothelium-dependent vasodilation of the femoral PNA from lean and obese ZDF rats during pre-diabetes (A), short-term diabetes (B), and long-term diabetes (C). A significant linear relation (P< 0.05) exists between cancellous BMD and peak PNA vasodilation in each condition.
Figure 6.
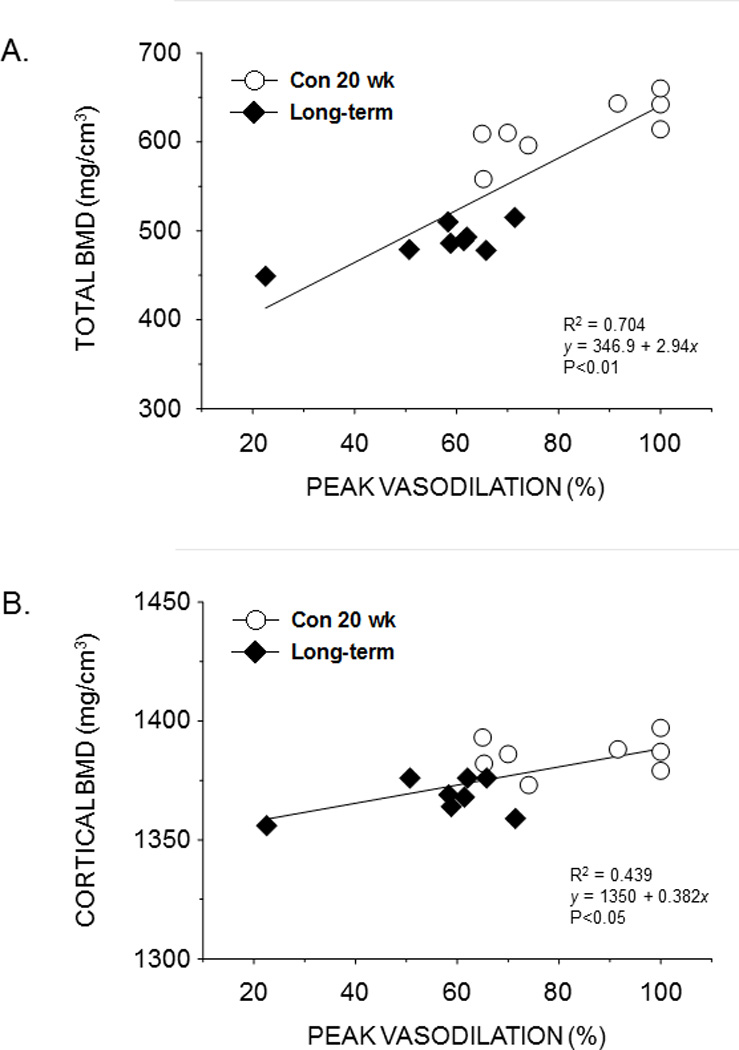
Scattergrams showing the relation between total bone mineral density (BMD) of the distal femur (A) and cortical BMD of the femoral mid-shaft (B) with peak endothelium-dependent vasodilation of the femoral PNA from lean and obese ZDF rats with long-term diabetes. A significant linear relation (P< 0.05) exists between total and cortical BMD and peak PNA vasodilation.
Blood flow, arterial pressure and vascular conductance
Blood flow was lower in all regions of the femur (Fig. 7A) and in all other skeletal regions of the hindlimb (see supplemental materials) in 20-wk old ZDF rats with long-term diabetes. Mean arterial pressure was not different between ZDF rats with long-term diabetes (160 ± 6 mmHg) and lean controls (160 ± 4 mmHg). Consequently, vascular conductance was lower in the femur (Fig. 7B) and all regions of the hindlimb skeleton of 20-wk old obese ZDF rats with long-term diabetes (see supplemental materials).
Figure 7.
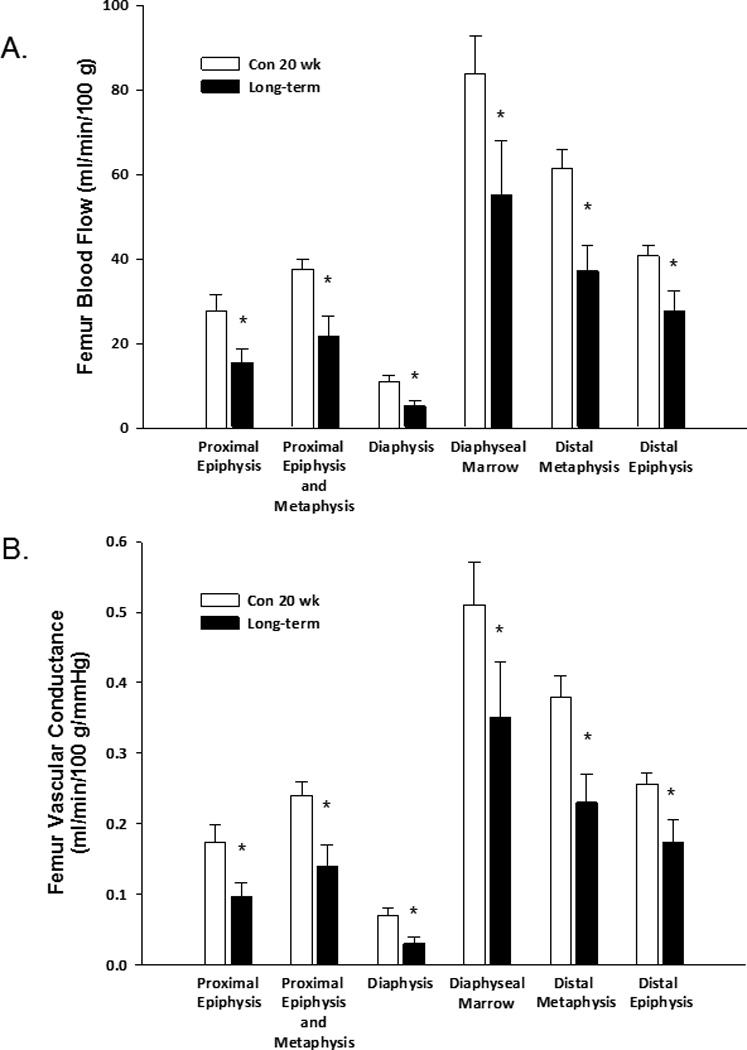
Effects of long-term diabetes on regional femoral blood flow (A) and vascular conductance (B). Values are means ± SE, n = 12/group. *Mean is different from age-matched, non-diabetic control animals (P< 0.05).
Discussion
Previously work has shown a deterioration of both bone health and general cardiovascular function during the progression of T2DM (Grundy, et al. 1999, Leslie, et al. 2012, Prisby, et al. 2008). However, little is known regarding the effects of T2DM on the bone circulation. Thus, the primary purpose of the present study was to determine whether the progression of T2DM through a pre-diabetic, short-term diabetic and more chronic diabetic state alters mechanisms of vascular control in bone resistance arteries. The results show few adverse effects of the pre-diabetic and short-term diabetic conditions on the vasodilator or vasoconstrictor properties of the femoral PNA. However, with long-term diabetes the PNA shifts toward a more pro-vasoconstrictor phenotype. This is evidenced by a decrement in endothelium-dependent (Fig. 1C) and –independent (Fig. 2) vasodilation, an enhanced noradrenergic (Fig. 3A) and myogenic (Fig. 4A) vasoconstrictor responsiveness, as well as a decrease in the passive mechanical distensibility (Fig. 4B) of the PNA. Consequently, a secondary purpose of the present study was to determine whether this shift toward a more pro-vasoconstrictor phenotype in the bone resistance vasculature diminishes in vivo bone and marrow perfusion and vascular conductance. The results demonstrate an impairment of bone and marrow blood flow (Fig. 7A) and vascular conductance (Fig. 7B) with long-term T2DM. Given the potential coupling of bone vascular signaling and blood flow with skeletal remodeling (Farhat & Cauley. 2008, Lampropoulos, et al. 2012, Parfitt. 2000, Prisby, et al. 2012), the present results suggest that the profound impairment of the bone circulation could contribute to the osteopenia found to occur in long bones with chronic T2DM.
T2DM is a physiologically devastating disease whose deleterious effects on bone and the cardiovascular system are multifaceted. For example, the hyperglycemia and hyperlipidemia associated with T2DM can stimulate mitochondrial free radical production and alter redox balance to promote endothelial dysfunction in the vasculature (Schalkwijk & Stehouwer. 2005, van den Oever, et al. 2010). Impaired endothelium-dependent vasodilation of conduit arteries is present in adolescents (Naylor, et al. 2011) and adults (Bruno, et al. 2012, Hogikyan, et al. 1998, Kotb, et al. 2012, Makimattila, et al. 1999) with T2DM, and has been shown to be associated with elevated oxidative stress and the uncoupling of endothelial NOS (Pannirselvam, et al. 2002), resulting in diminished NO bioavailability to induce vascular smooth muscle cell relaxation. Although T2DM-induced decreases in conduit artery endothelium-dependent vasodilation (Makimattila, et al. 1999, Rossi, et al. 2005) do not necessarily reflect changes in the microvasculature (Meyer, et al. 2008), results from the present study also demonstrate endothelial dysfunction in the bone microcirculation with T2DM (Fig. 1C). Further, the results suggest that this is due to an impairment of endothelial NO signaling, as indicated by the elimination of differences in endothelium-dependent vasodilation between PNAs from obese ZDF rats and age-matched lean controls when NOS inhibition is present.
The enhanced adrenergic vasoconstriction of the femur resistance vasculature in the present results (Fig. 3A) coincides with observations of greater adrenergic vasoconstriction in the forearm of humans with T2DM (Hogikyan, et al. 1999). Such changes in vasoconstrictor responsiveness could be mediated through the α-adrenergic receptor signaling pathway in smooth muscle cells that elicits vasoconstriction, or the modulatory influence of α-adrenergic receptor signaling in endothelial cells that promotes vasodilation. Results in bone resistance arteries indicate the latter effect, since removal of the endothelium abolished differences in PNA responses to NE (Fig. 3B). Previous research with skeletal muscle resistance arteries has shown a similar endothelium-dependent effect on NE-mediated vasoconstriction with long-term diabetes (Lesniewski, et al. 2008). Together, the results of endothelium-dependent vasodilation and NE-mediated vasoconstriction point to a critical role for the endothelium in the transition of the bone resistance arteries to a more pro-vasoconstrictor phenotype with T2DM.
Alterations in the bone resistance vasculature with T2DM are not limited to changes in endothelial cell signaling, however. Endothelium-independent vasodilation to SNP is impaired (Fig. 2), active myogenic vasoconstriction is enhanced (Fig. 4A), and the passive distensibility of the PNA is decreased with long-term diabetes (Fig. 4B). Each of these changes would also contribute to greater vasoconstrictor responsiveness, which correspond to reductions in vascular conductance in vivo (Fig. 7B).
The decrements in SNP-mediated vasodilation (Fig. 2) of the PNA suggest stiffer bone arteries with long-term diabetes. One potential mechanism for enhanced stiffness is an increase in vascular calcification. Previous work has shown that there is an increased risk for arterial calcification in T2DM (Kreines, et al. 1985), and that vascular calcification in diabetes is associated with decreased muscle blood flow (Christensen. 1968). Several studies also demonstrate positive associations between vascular calcification and osteoporosis or low BMD (Adragao, et al. 2009, Bandeira, et al. 2012, Choi, et al. 2009, Hak, et al. 2000, Hyder, et al. 2009, Reddy, et al. 2008). In particular, iliac artery vascular calcification is positively associated with lumbar spine and femoral neck osteoporosis in men with T2DM (Bandeira, et al. 2012).
The association between vascular alterations and bone loss are likely impacted by other factors beyond changes in arterial calcification. Evidence suggests an active role of the vasculature in coupling vascular signaling and bone remodeling (Colleran, et al. 2000, Dominguez, et al. 2010, Prisby, et al. 2012). For instance, the basic multicellular unit (BMU) that drives bone remodeling activity may be regulated via signals from core capillary endothelial cells to constituent osteoblasts and osteoclasts as the BMU advances through bone (Parfitt. 2000). The present results demonstrate that femur BMD is positively associated with endothelium-dependent vasodilation of the femoral PNA in the pre-diabetic (Fig. 5A), short-term diabetic (Fig. 5B), and long-term diabetic (Figs. 5A&6) states. The association between endothelium-dependent vasodilation of the femoral PNA and bone volume has been previously demonstrated among young and old sedentary and exercise trained rats (Dominguez, et al. 2010), as well as among young and old ovariectomized and estrogen replaced animals (Prisby, et al. 2012). Important endothelium-derived signaling molecules involved in local vascular control, including NO, prostacyclin (PGI2), and prostaglandin E2 (PGE2), have also been shown to be important in bone biology (MacIntyre, et al. 1991, Pead & Lanyon. 1989). For example, NO is a potent inhibitor of osteoclast driven bone resorption (MacIntyre, et al. 1991) and has a positive effect on osteoblastic differentiation (Hikiji, et al. 1997), while PGI2 has been shown to be a powerful inhibitor of osteoclastic bone resorption (Chambers & Ali. 1983) and PGE2 has been reported to increase osteoblast and decrease osteoclast numbers (Jee, et al. 1987). Results from the present study indicate that NO signaling from the vasculature is the more important molecule in the apparent coupling of endothelium-dependent vasodilation to BMD, given that differences in endothelium-dependent vasodilation in PNAs from obese and lean ZDF rats were abolished by NOS inhibition alone (Figs. 1 A&C).
An additional factor impacting the relation between the vasculature and bone health is bone perfusion. Bone blood flow facilitates the generation of differences in hydrostatic pressure between the medullary cavity and bone capillary efferents that drive centrifugal interstitial fluid flow (Montgomery, et al. 1988). Resultant transcortical fluid flow stimulates osteocytes and osteoblasts (Knothe Tate. 2003) and subsequent bone formation (Riddle & Donahue. 2009, Turner. 1999). Thus, the decreases in bone and marrow blood flow found in the present study (Fig. 7A) could also compromise important flow and pressure stimuli that affect bone formation.
The selection of an animal model of T2DM is important since no single model will permit the investigation of all disease-related questions (Peterson, et al. 1990). For example, the Goto-Kakizaki rat is a valuable model for investigating effects of T2DM in the absence of a corresponding increase in adiposity (Akash, et al. 2013). Therein, the model fails to replicate the typical clinical T2DM phenotype that includes obesity. The Zucker obese rat is a valuable model of the metabolic syndrome (Kurtz, et al. 1989) with impaired glucose tolerance and insulin resistance, but it fails to demonstrate hyperglycemia (Peterson, et al. 1990). The ZDF rat utilized in the present study was originally derived from the Zucker obese rat strain via select inbreeding of animals that spontaneously demonstrated unusually high blood glucose levels (Peterson, et al. 1990). The ZDF rat displays a well-defined T2DM disease progression (Etgen & Oldham. 2000, Kawaguchi, et al. 1999) with deleterious changes in obesity and blood lipid profiles (Leonard, et al. 2005) that correspond with human T2DM. Since glucose and insulin levels have important outcomes on bone health (Inzerillo & Epstein. 2004) and resistance artery function (Lesniewski, et al. 2008), the present study was designed to leverage known changes in ZDF rat T2DM disease progression to investigate their effects on potential bone-vascular interactions.
In conclusion, the present study demonstrates that few vasomotor alterations occur in the bone resistance vasculature in the pre-diabetic and short-term diabetic states. However, the femoral PNA shifts toward a more pro-vasoconstrictor phenotype with long-term diabetes, as indicated by decreases in endothelium-dependent (Fig. 1C) and –independent (Fig. 2) vasodilation, enhanced noradrenergic (Fig. 3A) and myogenic (Fig. 4A) vasoconstrictor responsiveness, and a decrease in the passive mechanical distensibility (Fig. 4B). The enhanced vasoconstrictor responsiveness of the bone resistance vasculature was evident in vivo from decreases in bone and marrow blood flow (Fig. 7A) and vascular conductance (Fig. 7B) with long-term T2DM. Given the potential coupling of bone vascular signaling molecules (Figs. 5&6) and bone fluid dynamics with skeletal remodeling (Farhat & Cauley. 2008, Lampropoulos, et al. 2012, Parfitt. 2000, Prisby, et al. 2012), the present results suggest that the impairment of the bone circulation could contribute to the bone loss found to occur during the progression of T2DM.
Supplementary Material
Acknowledgments
Funding
This study was supported by grants from the National Aeronautics and Space Administration (NNX12AL41G) and National Institutes of Health (AG-31317).
Footnotes
Declaration of interest
The authors state that they have no conflicts of interest.
Author contributions
Authors’ roles: Study design: JNS, RDP and MDD. Study conduct: JNS, RDP and BJB. Data collection: JNS, RDP and BJB. Data analysis: JNS, RDP and MDD. Data interpretation: JNS, RDP, BJB and MDD. Drafting manuscript: JNS and MDD. Revising manuscript content: JNS, RDP, BJB and MDD. Approving final version of manuscript: JNS, RDP, BJB and MDD. MDD takes responsibility for the integrity of the data analysis.
References
- Adragao T, Herberth J, Monier-Faugere MC, Branscum AJ, Ferreira A, Frazao JM, Dias Curto J, Malluche HH. Low bone volume--a risk factor for coronary calcifications in hemodialysis patients. Clinical journal of the American Society of Nephrology : CJASN. 2009;4:450–455. doi: 10.2215/CJN.01870408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akash MS, Rehman K, Chen S. Goto-kakizaki rats: Its suitability as non-obese diabetic animal model for spontaneous type 2 diabetes mellitus. Current diabetes reviews. 2013;9:387–396. doi: 10.2174/15733998113099990069. [DOI] [PubMed] [Google Scholar]
- Bandeira E, Neves AP, Costa C, Bandeira F. Association between vascular calcification and osteoporosis in men with type 2 diabetes. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2012;15:55–60. doi: 10.1016/j.jocd.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Bridgeman G, Brookes M. Blood supply to the human femoral diaphysis in youth and senescence. Journal of anatomy. 1996;188(Pt 3):611–621. [PMC free article] [PubMed] [Google Scholar]
- Bruno RM, Penno G, Daniele G, Pucci L, Lucchesi D, Stea F, Landini L, Cartoni G, Taddei S, Ghiadoni L, et al. Type 2 diabetes mellitus worsens arterial stiffness in hypertensive patients through endothelial dysfunction. Diabetologia. 2012;55:1847–1855. doi: 10.1007/s00125-012-2517-1. [DOI] [PubMed] [Google Scholar]
- Chambers TJ, Ali NN. Inhibition of osteoclastic motility by prostaglandins I2, E1, E2 and 6-oxo-E1. The Journal of pathology. 1983;139:383–397. doi: 10.1002/path.1711390313. [DOI] [PubMed] [Google Scholar]
- Choi SH, An JH, Lim S, Koo BK, Park SE, Chang HJ, Choi SI, Park YJ, Park KS, Jang HC, et al. Lower bone mineral density is associated with higher coronary calcification and coronary plaque burdens by multidetector row coronary computed tomography in pre- and postmenopausal women. Clinical endocrinology. 2009;71:644–651. doi: 10.1111/j.1365-2265.2009.03535.x. [DOI] [PubMed] [Google Scholar]
- Christensen NJ. Muscle blood flow, measured by xenon133 and vascular calcifications in diabetics. Acta Medica Scandinavica. 1968;183:449–454. doi: 10.1111/j.0954-6820.1968.tb10506.x. [DOI] [PubMed] [Google Scholar]
- Colleran PN, Wilkerson MK, Bloomfield SA, Suva LJ, Turner RT, Delp MD. Alterations in skeletal perfusion with simulated microgravity: A possible mechanism for bone remodeling. Journal of applied physiology (Bethesda, Md.: 1985) 2000;89:1046–1054. doi: 10.1152/jappl.2000.89.3.1046. [DOI] [PubMed] [Google Scholar]
- Delp MD. Myogenic and vasoconstrictor responsiveness of skeletal muscle arterioles is diminished by hindlimb unloading. Journal of applied physiology (Bethesda, Md.: 1985) 1999;86:1178–1184. doi: 10.1152/jappl.1999.86.4.1178. [DOI] [PubMed] [Google Scholar]
- Delp MD, Armstrong RB. Blood flow in normal and denervated muscle during exercise in conscious rats. The American Journal of Physiology. 1988;255:H1509–H1515. doi: 10.1152/ajpheart.1988.255.6.H1509. [DOI] [PubMed] [Google Scholar]
- Dominguez JM, 2nd, Prisby RD, Muller-Delp JM, Allen MR, Delp MD. Increased nitric oxide-mediated vasodilation of bone resistance arteries is associated with increased trabecular bone volume after endurance training in rats. Bone. 2010;46:813–819. doi: 10.1016/j.bone.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Lesniewski LA, Delp MD. The effects of aging and exercise training on endothelin-1 vasoconstrictor responses in rat skeletal muscle arterioles. Cardiovascular research. 2005;66:393–401. doi: 10.1016/j.cardiores.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Etgen GJ, Oldham BA. Profiling of zucker diabetic fatty rats in their progression to the overt diabetic state. Metabolism: clinical and experimental. 2000;49:684–688. doi: 10.1016/s0026-0495(00)80049-9. [DOI] [PubMed] [Google Scholar]
- Farhat GN, Cauley JA. The link between osteoporosis and cardiovascular disease. Clinical cases in mineral and bone metabolism : the official journal of the Italian Society of Osteoporosis, Mineral Metabolism, and Skeletal Diseases. 2008;5:19–34. [PMC free article] [PubMed] [Google Scholar]
- Flaim SF, Zelis RF. Regional distribution of cardiac output in conscious rats at rest and during exercise. effects of diltiazem. Chest. 1980;78:187–192. [PubMed] [Google Scholar]
- Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr, Sowers JR. Diabetes and cardiovascular disease: A statement for healthcare professional from the American Heart Association. 1999. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- Hak AE, Pols HA, van Hemert AM, Hofman A, Witteman JC. Progression of aortic calcification is associated with metacarpal bone loss during menopause: A population-based longitudinal study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:1926–1931. doi: 10.1161/01.atv.20.8.1926. [DOI] [PubMed] [Google Scholar]
- Hikiji H, Shin WS, Oida S, Takato T, Koizumi T, Toyo-oka T. Direct action of nitric oxide on osteoblastic differentiation. FEBS letters. 1997;410:238–242. doi: 10.1016/s0014-5793(97)00597-8. [DOI] [PubMed] [Google Scholar]
- Hogikyan RV, Galecki AT, Halter JB, Supiano MA. Heightened norepinephrine-mediated vasoconstriction in type 2 diabetes. Metabolism: clinical and experimental. 1999;48:1536–1541. doi: 10.1016/s0026-0495(99)90242-1. [DOI] [PubMed] [Google Scholar]
- Hogikyan RV, Galecki AT, Pitt B, Halter JB, Greene DA, Supiano MA. Specific impairment of endothelium-dependent vasodilation in subjects with type 2 diabetes independent of obesity. The Journal of clinical endocrinology and metabolism. 1998;83:1946–1952. doi: 10.1210/jcem.83.6.4907. [DOI] [PubMed] [Google Scholar]
- Hyder JA, Allison MA, Wong N, Papa A, Lang TF, Sirlin C, Gapstur SM, Ouyang P, Carr JJ, Criqui MH. Association of coronary artery and aortic calcium with lumbar bone density: The MESA abdominal aortic calcium study. American Journal of Epidemiology. 2009;169:186–194. doi: 10.1093/aje/kwn303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzerillo AM, Epstein S. Osteoporosis and diabetes mellitus. Reviews in endocrine & metabolic disorders. 2004;5:261–268. doi: 10.1023/B:REMD.0000032415.83124.20. [DOI] [PubMed] [Google Scholar]
- Ishise S, Pegram BL, Yamamoto J, Kitamura Y, Frohlich ED. Reference sample microsphere method: Cardiac output and blood flows in conscious rat. The American Journal of Physiology. 1980;239:H443–H449. doi: 10.1152/ajpheart.1980.239.4.H443. [DOI] [PubMed] [Google Scholar]
- Jee WS, Ueno K, Kimmel DB, Woodbury DM, Price P, Woodbury LA. The role of bone cells in increasing metaphyseal hard tissue in rapidly growing rats treated with prostaglandin E2. Bone. 1987;8:171–178. doi: 10.1016/8756-3282(87)90017-2. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Koshimura K, Murakami Y, Tsumori M, Gonda T, Kato Y. Antihypertensive effect of insulin via nitric oxide production in the zucker diabetic fatty rat an animal model for non-insulin-dependent diabetes mellitus. European journal of endocrinology / European Federation of Endocrine Societies. 1999;140:341–349. doi: 10.1530/eje.0.1400341. [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML. "Whither flows the fluid in bone?" an osteocyte's perspective. Journal of Biomechanics. 2003;36:1409–1424. doi: 10.1016/s0021-9290(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Kotb NA, Gaber R, Salah W, Elhendy A. Relations among glycemic control, circulating endothelial cells, nitric oxide, and flow mediated dilation in patients with type 2 diabetes mellitus. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2012;120:460–465. doi: 10.1055/s-0032-1306349. [DOI] [PubMed] [Google Scholar]
- Kreines K, Johnson E, Albrink M, Knatterud GL, Levin ME, Lewitan A, Newberry W, Rose FA. The course of peripheral vascular disease in non-insulin-dependent diabetes. Diabetes care. 1985;8:235–243. doi: 10.2337/diacare.8.3.235. [DOI] [PubMed] [Google Scholar]
- Kurtz TW, Morris RC, Pershadsingh HA. The zucker fatty rat as a genetic model of obesity and hypertension. Hypertension. 1989;13:896–901. doi: 10.1161/01.hyp.13.6.896. [DOI] [PubMed] [Google Scholar]
- Lampropoulos CE, Papaioannou I, D'Cruz DP. Osteoporosis--a risk factor for cardiovascular disease? Nature reviews. Rheumatology. 2012;8:587–598. doi: 10.1038/nrrheum.2012.120. [DOI] [PubMed] [Google Scholar]
- Leonard BL, Watson RN, Loomes KM, Phillips AR, Cooper GJ. Insulin resistance in the zucker diabetic fatty rat: A metabolic characterisation of obese and lean phenotypes. Acta Diabetologica. 2005;42:162–170. doi: 10.1007/s00592-005-0197-8. [DOI] [PubMed] [Google Scholar]
- Leslie WD, Rubin MR, Schwartz AV, Kanis JA. Type 2 diabetes and bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27:2231–2237. doi: 10.1002/jbmr.1759. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Donato AJ, Behnke BJ, Woodman CR, Laughlin MH, Ray CA, Delp MD. Decreased NO signaling leads to enhanced vasoconstrictor responsiveness in skeletal muscle arterioles of the ZDF rat prior to overt diabetes and hypertension. American journal of physiology. Heart and circulatory physiology. 2008;294:H1840–H1850. doi: 10.1152/ajpheart.00692.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre I, Zaidi M, Alam AS, Datta HK, Moonga BS, Lidbury PS, Hecker M, Vane JR. Osteoclastic inhibition: An action of nitric oxide not mediated by cyclic GMP. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:2936–2940. doi: 10.1073/pnas.88.7.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makimattila S, Liu ML, Vakkilainen J, Schlenzka A, Lahdenpera S, Syvanne M, Mantysaari M, Summanen P, Bergholm R, Taskinen MR, et al. Impaired endothelium-dependent vasodilation in type 2 diabetes. relation to LDL size, oxidized LDL, and antioxidants. Diabetes care. 1999;22:973–981. doi: 10.2337/diacare.22.6.973. [DOI] [PubMed] [Google Scholar]
- Meininger GA, Harris PD, Joshua IG. Distributions of microvascular pressure in skeletal muscle of one-kidney, one clip, two-kidney, one clip, and deoxycorticosterone-salt hypertensive rats. Hypertension. 1984;6:27–34. doi: 10.1161/01.hyp.6.1.27. [DOI] [PubMed] [Google Scholar]
- Melton LJ, 3rd, Leibson CL, Achenbach SJ, Therneau TM, Khosla S. Fracture risk in type 2 diabetes: Update of a population-based study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008;23:1334–1342. doi: 10.1359/JBMR.080323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MF, Lieps D, Schatz H, Pfohl M. Impaired flow-mediated vasodilation in type 2 diabetes: Lack of relation to microvascular dysfunction. Microvascular research. 2008;76:61–65. doi: 10.1016/j.mvr.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Montgomery RJ, Sutker BD, Bronk JT, Smith SR, Kelly PJ. Interstitial fluid flow in cortical bone. Microvascular research. 1988;35:295–307. doi: 10.1016/0026-2862(88)90084-2. [DOI] [PubMed] [Google Scholar]
- Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. American journal of physiology. Heart and circulatory physiology. 2002;283:H1662–H1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- Naylor LH, Green DJ, Jones TW, Kalic RJ, Suriano KL, Shah M, Hopkins N, Davis EA. Endothelial function and carotid intima-medial thickness in adolescents with type 2 diabetes mellitus. The Journal of pediatrics. 2011;159:971–974. doi: 10.1016/j.jpeds.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Pannirselvam M, Verma S, Anderson TJ, Triggle CR. Cellular basis of endothelial dysfunction in small mesenteric arteries from spontaneously diabetic (db/db −/−) mice: Role of decreased tetrahydrobiopterin bioavailability. British journal of pharmacology. 2002;136:255–263. doi: 10.1038/sj.bjp.0704683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt AM. The mechanism of coupling: A role for the vasculature. Bone. 2000;26:319–323. doi: 10.1016/S8756-3282(00)80937-0. [DOI] [PubMed] [Google Scholar]
- Pead MJ, Lanyon LE. Indomethacin modulation of load-related stimulation of new bone formation in vivo. Calcified tissue international. 1989;45:34–40. doi: 10.1007/BF02556658. [DOI] [PubMed] [Google Scholar]
- Peterson RG, Shaw WN, Neel M, Little LA, Eichberg J. Zucker diabetic fatty rat as a model for non-insulin-dependent diabetes mellitus. ILAR Journal. 1990;32:16–19. doi: 10.1093/ilar.32.3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisby RD, Dominguez JM, 2nd, Muller-Delp J, Allen MR, Delp MD. Aging and estrogen status: A possible endothelium-dependent vascular coupling mechanism in bone remodeling. PloS one. 2012;7:e48564. doi: 10.1371/journal.pone.0048564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisby RD, Swift JM, Bloomfield SA, Hogan HA, Delp MD. Altered bone mass, geometry and mechanical properties during the development and progression of type 2 diabetes in the zucker diabetic fatty rat. The Journal of endocrinology. 2008;199:379–388. doi: 10.1677/JOE-08-0046. [DOI] [PubMed] [Google Scholar]
- Prisby RD, Ramsey MW, Behnke BJ, Dominguez JM, 2nd, Donato AJ, Allen MR, Delp MD. Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO bioavailability in rats. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22:1280–1288. doi: 10.1359/jbmr.070415. [DOI] [PubMed] [Google Scholar]
- Reddy J, Bilezikian JP, Smith SJ, Mosca L. Reduced bone mineral density is associated with breast arterial calcification. The Journal of clinical endocrinology and metabolism. 2008;93:208–211. doi: 10.1210/jc.2007-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle RC, Donahue HJ. From streaming-potentials to shear stress 25 years of bone cell mechanotransduction. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2009;27:143–149. doi: 10.1002/jor.20723. [DOI] [PubMed] [Google Scholar]
- Rossi R, Cioni E, Nuzzo A, Origliani G, Modena MG. Endothelial-dependent vasodilation and incidence of type 2 diabetes in a population of healthy postmenopausal women. Diabetes care. 2005;28:702–707. doi: 10.2337/diacare.28.3.702. [DOI] [PubMed] [Google Scholar]
- Schalkwijk CG, Stehouwer CD. Vascular complications in diabetes mellitus: The role of endothelial dysfunction. Clinical science (London, England : 1979) 2005;109:143–159. doi: 10.1042/CS20050025. [DOI] [PubMed] [Google Scholar]
- Schwartz AV. Diabetes mellitus: Does it affect bone? Calcified tissue international. 2003;73:515–519. doi: 10.1007/s00223-003-0023-7. [DOI] [PubMed] [Google Scholar]
- Stabley JN, Prisby RD, Behnke BJ, Delp MD. Chronic skeletal unloading of the rat femur: Mechanisms and functional consequences of vascular remodeling. Bone. 2013;57:355–360. doi: 10.1016/j.bone.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truett GE, Bahary N, Friedman JM, Leibel RL. Rat obesity gene fatty (fa) maps to chromosome 5: Evidence for homology with the mouse gene diabetes (db) Proceedings of the National Academy of Sciences of the United States of America. 1991;88:7806–7809. doi: 10.1073/pnas.88.17.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CH. Site-specific skeletal effects of exercise: Importance of interstitial fluid pressure. Bone. 1999;24:161–162. doi: 10.1016/s8756-3282(98)00184-7. [DOI] [PubMed] [Google Scholar]
- van de Ree MA, Huisman MV, de Man FH, van der Vijver JC, Meinders AE, Blauw GJ. Impaired endothelium-dependent vasodilation in type 2 diabetes mellitus and the lack of effect of simvastatin. Cardiovascular research. 2001;52:299–305. doi: 10.1016/s0008-6363(01)00379-0. [DOI] [PubMed] [Google Scholar]
- van den Oever IA, Raterman HG, Nurmohamed MT, Simsek S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators of inflammation. 2010;2010:792393. doi: 10.1155/2010/792393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Deaths from CVD and diabetes. 2013
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


