Abstract
OBJECTIVES:
To examine patterns of associations between a broad range of mental and physical conditions by using a large, systematically obtained pediatric registry.
METHODS:
The sample included 9014 youth ages 8 to 21 years (4349 males and 4665 females; 3585 aged <13 years, 3678 aged 13 to 18 years, and 1751 aged 19 to 21 years) from the Philadelphia Neurodevelopmental Cohort identified through pediatric clinics at the Children’s Hospital of Philadelphia health care network by the Center for Applied Genomics. Measures were as follows: physical condition based on electronic medical records and interview data on 42 physical conditions of 14 organ systems/specialties and mental disorders based on an abbreviated version of the structured Kiddie-Schedule for Affective Disorders and Schizophrenia psychiatric diagnostic interview.
RESULTS:
There was a direct association between the severity of the physical condition and most classes of mental disorders, as well as with functional impairment. Models adjusted for sociodemographic correlates, other physical and mental disorders, and false discovery and revealed broad patterns of associations between neurodevelopmental disorders with behavior disorders (odds ratio [OR]: 1.5; 95% confidence interval [CI]: 1.3–1.8; P < .004) and attention-deficit/hyperactivity disorder (OR: 3.1; 95% CI: 2.7–3.6; P < .0001), and neurologic/central nervous system conditions (OR: 1.3; 95% CI: 1.1–1.9; P < .05) with mood disorders and attention-deficit/hyperactivity disorder (OR: 1.3; 95% CI: 1.1–1.5; P < .001), and autoimmune/inflammatory conditions with mood disorders (OR: 1.4; 95% CI: 1.1–1.8, P < .05).
CONCLUSIONS:
Findings show the strong overlap between physical and mental conditions and their impact on severity and functional impairment in youth. Specific patterns of comorbidity have important implications for etiology. Prospective tracking of cross-disorder morbidity will be important to establish more effective mechanisms for prevention and intervention.
Keywords: comorbidity, physical disorders, mental disorders, youth, registry
What’s Known on This Subject:
Although there is evidence regarding comorbidity of physical and mental disorders from clinical samples of specific disorders and treatment registries, there is limited evidence from systematic samples of youth with comprehensive information on the full range of mental and physical disorders.
What This Study Adds:
This report is the first study to investigate the specificity of associations between a broad range of mental and physical conditions by using a large, systematically obtained pediatric sample with enriched information from electronic medical records and direct interviews.
A recent comprehensive review of comorbidity of mental and physical conditions from the Robert Wood Johnson Foundation Synthesis Project1 documented the high prevalence, complex causal connections, and high individual and societal costs of comorbid disorders in adults. In fact, comorbidity is perhaps the major challenge to effective implementation of the Affordable Health Care Act in the United States.2 An evidence base regarding the patterns and correlates of comorbidity is therefore critical to our understanding of the pathogenesis of human chronic diseases and, consequently, central to their prevention and treatment.
Systematic reviews of specific physical and/or mental disorders,3–11 as well as studies of clinical12–16 and community samples,17–26 have also demonstrated both the co-occurrence and consequences of comorbidity in youth. However, much of the current evidence is based on cross-sectional assessment of small numbers of cases and self-reported disorders rather than systematic diagnostic assessments. Moreover, previous research is challenged by a lack of comparability of diagnostic information on mental and physical disorders, failure to include a comprehensive range of both mental and physical conditions, and perhaps most important, limited information on the order of onset and evolution of these conditions across the life span.
The Philadelphia Neurodevelopmental Cohort (PNC) provides a unique resource to fill this gap. The PNC is a Grand Opportunity National Institute of Mental Health–funded collaborative study that directly assessed a large sample of youth ascertained through the Children’s Hospital of Philadelphia (CHOP) network. This article presents the results on comorbidity of a comprehensive range of mental and physical disorders in the PNC. To our knowledge, this is the first study to investigate the specificity of associations between a broad range of mental and physical conditions by using a large, systematically obtained pediatric sample with enriched electronic medical record (EMR) and direct interview information.
Methods
Sample
The PNC study characterizes clinical and neurobehavioral phenotypes in a prospectively accrued community cohort of 8- to 21-year-old youth.27,28 The Center of Applied Genomics identified the sample through pediatric clinics within the CHOP health care network. Their team enrolled and genotyped 50 293 children who provided written informed consent/assent authorizing access to EMRs and to be recontacted for future studies. Seventy percent were ascertained through outpatient medicine and 30% through preoperative surgery clinics. Notably, participants were not recruited from psychiatric clinics. EMRs of a randomly selected sample of the total registry stratified by age, gender, and ethnicity were screened to select a quasi-epidemiologic sample of children representative of the greater Philadelphia area from all routine well-child and illness-related pediatric visits. Inclusion criteria were that the youth were ambulatory, in stable health, proficient in English, and able to complete the study procedures including computerized neurocognitive testing. Therefore, children with severe developmental delay, significant hearing loss, or limited mobility were not invited to participate in the study. A total of 19 161 youth met these criteria. Eligible participants were initially contacted by letter and a follow-up phone call, and they were enrolled consecutively between November 2009 and November 2011 consented to an interview at either the University of Pennsylvania or in their homes. The present study included 9014 children who provided complete information on mental and physical disorders. Of the remaining sample who met age and medical record eligibility criteria, reasons for noninterview included the following: our inability to locate the youth and/or their parents (43%), 18% declined, 10% had moved out of region, 9% were repeated no-shows, 5% were excluded due to age change or medical reasons or cognitive disturbances that precluded their ability to complete the study assessments, and 15% were still in the process of potential enrollment at the time of this work.
Mental Disorder Assessment
The interview (GO-ASSESS) included a timeline of life events, demographic characteristics, medical and medication history, an abbreviated structured interview on the full range of mental disorders, the Children’s Global Assessment Scale (CGAS),29 and interviewer observations. An abbreviated computerized version of the Kiddie version of the Schedule for Affective Disorders and Schizophrenia (K-SADS) using a structured screener30 was administered face-to-face to the youth and/or caretakers to collect diagnostic information to derive dimensional ratings of indices of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), mental disorders.31,32 More details on the training and interrater reliability of the interviewers are described in another publication on the study methods.33 After obtaining written legal guardian consent and youth assent, parents or caretakers were interviewed to provide diagnostic information on participating youth ages 8 to 17 years, and those aged 11 to 21 years were also interviewed directly. Disorder modules included 6 overarching categories: anxiety (agoraphobia, generalized anxiety disorder, obsessive compulsive disorder, panic disorder, posttraumatic stress disorder, separation anxiety disorder, social phobia, and specific phobia), mood (major depression, mania), behavior (conduct, oppositional defiant disorder), attention-deficit/hyperactivity disorder (ADHD), eating disorders (anorexia, bulimia), and subclincal psychotic spectrum symptoms (possible or definite hallucinations and/or delusions). Diagnostic algorithms for DSM-IV indices of mental disorders incorporated endorsement of screen questions, symptom count, duration, distress, and impairment. Disorders were rated on the following dimensional coding scheme: 0 = none, 1 = endorsed, 2 = subthreshold, 3 = threshold, and 4 = significant. Computerized algorithms were applied to diagnostic information that was obtained from the abbreviated K-SADS interviews to generate diagnosis variables. Comparison of the diagnostic variables based on the abbreviated K-SADS interview with those based on the full Composite International Diagnostic Interview–Adolescent version with the use of data from the National Comorbidity Survey–Adolescent Supplement yielded fair (ie, eating disorders) to excellent (ie, ADHD) area under the receiver operator characteristic curve values for the major classes of disorders30,32 (data available upon request). More extensive information on psychosis spectrum symptoms was obtained via embedded screening tools, including a revised version34 of the PRIME subpsychosis screen,35 and 6 negative/disorganized assessor-rated scales from the Structured Interview for Prodromal Syndromes (SIPS).36 These symptoms that have not been routinely collected in previous population or primary care samples in the United States were obtained to establish a baseline for subsequent follow-up by the University of Pennsylvania study team. The institutional review boards of the University of Pennsylvania and CHOP approved these procedures.
Physical Conditions
Information from electronic searches of the CHOP EMRs and parent (for youth aged 8–17 years) or respondent (youth aged 18–21 years) interviews on subsequent diagnoses of physical conditions was obtained on 42 conditions that were classified into 14 organ systems/specialties (allergy/immunology, cardiology, endocrine/metabolism, ear/nose/throat, gastroenterology, hematology, nephrology, neurology, oncology, orthopedics, pediatrics, pulmonology/airways, surgery, and urology). For any condition with insufficient diagnostic information from electronic searches, manual review of the EMRs’ International Classification of Diseases, Ninth Revision, codes was completed by nurses and other qualified medical staff. Discrepant information, which occurred in ∼5% of cases, was reconciled by physician review. Parent report was also used to identify disorders that were diagnosed after the last evaluation at CHOP. An index of the overall severity of physical conditions was created with the following levels: 1 = none, no ongoing physical conditions requiring sustained intervention or interference with overall functioning; 2 = mild, conditions requiring pediatric visits and, at times, medications but mild in severity; 3 = moderate, multiple physical conditions requiring standing medications and monitoring; and 4 = severe, physical conditions that can be life-threatening requiring multiple procedures and monitoring.
Statistical Analysis
Statistical analyses were conducted with the SAS software package, version 9.3 (The SAS Inc., Cary, NC). Mental disorders were dichotomized as present (rating = 4, significant) and absent (rating = 0, 1, 2, or 3), whereas physical conditions were dichotomized as present (rating =3 or 4, moderate or severe) and absent (rating = 1 or 2, none or mild) for these analyses. Subclinical psychotic spectrum symptoms were defined as present if any of the screening symptoms with the diagnostic interview, SIPS, or PRIME screen were endorsed. These symptoms are not indicative of current psychosis but rather are used in aggregate as indicators of subsequent psychotic disorders. Cross-tabulations were used to calculate the lifetime prevalence of 14 organ system/specialty physical conditions, 5 major classes of mental disorders, and a CGAS ≤70, which indicates functional impairment in at least 1 domain (at home, at school, or with peers). Logistic regression analysis was used to examine demographic correlates and comorbidity of physical and mental disorders. The outcome variables included CGAS ≤70 and the major classes of mental disorders. The main predictor variables were physical conditions and their overall severity. Models predicting the CGAS ≤70 were based on multiple logistic regressions that simultaneously controlled for demographic characteristics (age, gender, race/ethnicity, and maternal education) and all 14 categories of physical conditions; models predicting major classes of mental disorders were additionally adjusted for comorbid mental disorders. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated from exponentiated logistic regression coefficients for ease of interpretation. The Benjamini-Hochberg false discovery rate (FDR) procedure was applied to account for false positives due to multiple significance testing.37 Statistical significance was based on 2-sided FDR-adjusted P values at a level of .05.
Results
The total and gender- and age-specific prevalence of physical and mental disorders, including the proportion with CGAS scores ≤70 and their associations with gender and age, are presented in Table 1. Nearly 1 in 5 youth experienced a neurologic/central nervous system (CNS; 19.6%) or developmental condition (18.0%). Fifteen percent had birth anomalies, including broad “birth defects” or complications during birth. Fewer were affected by disorders of other organ systems, ranging from endocrine/metabolic (6.1%) to autoimmune/inflammatory (8.9%) conditions. Although the prevalence of seasonal allergies or allergies to foods was high (61.7%), few had moderate or severe ratings (0.9%). CNS, developmental, gastroenterologic, and hematologic conditions were more common in males than in females, whereas females had higher rates of autoimmune/inflammatory, endocrine/metabolic, nephrology/urologic, and orthopedic conditions. Compared with the oldest age group (>18 years), younger age groups were more likely to have birth anomalies and developmental conditions, whereas older youth had higher rates of immunologic, cardiologic, gastroenterologic, or oncologic disorders relative to their younger counterparts.
TABLE 1.
Prevalence and Association of Physical Conditions and Mental Disorders by Gender and Age Group
| Prevalence, % | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (N = 9014) | Gender | Age Group | Gender (Reference: Female)a | Age Group (Reference: >18 y)a | ||||||||
| Male (n = 4349) | Female (n = 4665) | <13 y (n = 3585) | 13–18 y (n = 3678) | >18 y (n = 1751) | <13 y | 13–18 y | ||||||
| aOR | (95% CI) | aOR | (95% CI) | aOR | (95% CI) | |||||||
| Medical specialty/systemsb | ||||||||||||
| Allergy/immunologic | ||||||||||||
| Severe allergies | 0.9 | 1.0 | 0.9 | 0.9 | 1.1 | 0.6 | 1.11 | (0.72–1.72) | 1.34 | (0.67–2.67) | 1.63 | (0.83–3.20) |
| Asthma/respiratory | 1.2 | 1.2 | 1.1 | 1.2 | 1.1 | 1.1 | 1.12 | (0.76–1.66) | 0.99 | (0.58–1.69) | 0.96 | (0.56–1.65) |
| Autoimmune/inflammatory | 8.9 | 7.9 | 9.8 | 7.3 | 9.9 | 9.9 | 0.77 | (0.67–0.90) | 0.70 | (0.57–0.85) | 0.96 | (0.79–1.17) |
| Cardiologic | 3.5 | 3.4 | 3.5 | 3.3 | 3.2 | 4.5 | 0.97 | (0.77–1.21) | 0.71 | (0.53–0.96) | 0.69 | (0.52–0.93) |
| Endocrine/metabolic | 6.1 | 5.4 | 6.8 | 4.8 | 7.7 | 5.6 | 0.76 | (0.64–0.90) | 0.84 | (0.65–1.09) | 1.38 | (1.09–1.76) |
| Eye/ear/nose/throat | 3.3 | 3.1 | 3.4 | 3.4 | 3.4 | 2.8 | 0.89 | (0.70–1.12) | 1.21 | (0.86–1.70) | 1.19 | (0.85–1.67) |
| Gastroenterologic | 6.9 | 7.6 | 6.2 | 5.4 | 7.5 | 8.6 | 1.24 | (1.05–1.46) | 0.55 | (0.44–0.70) | 0.81 | (0.66–1.00) |
| Hematologic | 3.9 | 4.3 | 3.6 | 3.5 | 4.2 | 4.3 | 1.26 | (1.02–1.56) | 0.79 | (0.59–1.06) | 0.97 | (0.73–1.29) |
| Nephrology/urologic | 2.6 | 2.1 | 3.1 | 2.2 | 2.7 | 3.1 | 0.67 | (0.51–0.87) | 0.73 | (0.51–1.04) | 0.86 | (0.61–1.20) |
| Neurologic | ||||||||||||
| Birth anomalies | 15.0 | 15.5 | 14.5 | 16.9 | 14.4 | 12.1 | 1.03 | (0.92–1.16) | 1.43 | (1.21–1.70) | 1.19 | (1.00–1.42) |
| CNS | 19.6 | 20.5 | 18.8 | 16.0 | 19.2 | 27.7 | 1.15 | (1.04–1.28) | 0.47 | (0.41–0.55) | 0.60 | (0.53–0.69) |
| Developmental | 18.0 | 21.4 | 14.8 | 19.8 | 18.0 | 14.0 | 1.53 | (1.38–1.71) | 1.47 | (1.25–1.73) | 1.33 | (1.13–1.56) |
| Oncologic | 3.1 | 3.2 | 3.0 | 2.6 | 2.9 | 4.6 | 1.07 | (0.84–1.36) | 0.55 | (0.41–0.75) | 0.60 | (0.45–0.81) |
| Orthopedic | 7.9 | 7.0 | 8.8 | 6.7 | 9.1 | 7.9 | 0.76 | (0.65–0.89) | 0.84 | (0.68–1.05) | 1.15 | (0.94–1.42) |
| Mental disordersc | ||||||||||||
| Anxiety disorders | 11.7 | 8.8 | 14.4 | 10.5 | 12.7 | 11.8 | 0.58 | (0.51–0.67) | 0.96 | (0.80–1.15) | 1.14 | (0.96–1.36) |
| ADHD | 17.6 | 22.5 | 12.9 | 23.2 | 17.5 | 6.2 | 1.88 | (1.68–2.11) | 4.38 | (3.55–5.41) | 3.18 | (2.57–3.93) |
| Behavior disorders | 18.2 | 20.9 | 15.7 | 15.5 | 22.9 | 13.8 | 1.51 | (1.35–1.69) | 1.19 | (1.01–1.41) | 2.01 | (1.71–2.35) |
| Mood disorders | 12.4 | 9.2 | 15.5 | 5.1 | 15.7 | 20.4 | 0.60 | (0.52–0.68) | 0.22 | (0.19–0.27) | 0.75 | (0.65–0.87) |
| Subclinical psychosis symptoms | 21.4 | 22.5 | 20.3 | 18.5 | 25.0 | 19.5 | 1.20 | (1.08–1.33) | 0.96 | (0.83–1.12) | 1.44 | (1.25–1.66) |
| CGAS score ≤70 | 23.3 | 24.7 | 22.0 | 19.4 | 25.2 | 27.5 | 1.23 | (1.12–1.36) | 0.65 | (0.57–0.74) | 0.92 | (0.81–1.05) |
aOR based on logistic regression models controlling for gender, age, race/ethnicity, and maternal education.
Physical conditions based on electronic medical chart review and parent informant report (or proband report for youth aged ≥18 y).
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition indices of mental disorders: diagnosis algorithms considered endorsement of screen question, symptom count, duration, distress, and impairment. Anxiety disorders = agoraphobia, generalized anxiety disorder, panic disorder, separation anxiety disorder; Behavior disorders = oppositional defiant disorder, conduct disorder; Mood disorders = major depressive disorder and mania; psychosis symptoms were based on subpsychotic positive symptoms (PRIME-Screen Revised), subthreshold negative/disorganized symptoms (6 SIPS ratings), and/or positive psychosis screen.
Behavior disorders were the most frequent mental disorder among youth (18.2%), followed by ADHD (17.6%), mood disorders (12.4%), and anxiety disorders (11.7%). A total of 21.4% of youth screened positive for subclinical psychosis spectrum symptoms. Relative to females, male adolescents had greater rates of ADHD, behavior disorders, and subclinical psychotic spectrum symptoms and lower rates of mood and anxiety disorders. The prevalence of mood disorders increased uniformly with age, whereas the prevalence of ADHD decreased with age by a similar magnitude. Relative to the oldest age group (>18 years), youth in the youngest age group (<13 years) and the middle age group (13–18 years) had higher rates of behavior disorders. Approximately 1 in 4 to 5 youth in the overall sample had at least minimal functional impairment.
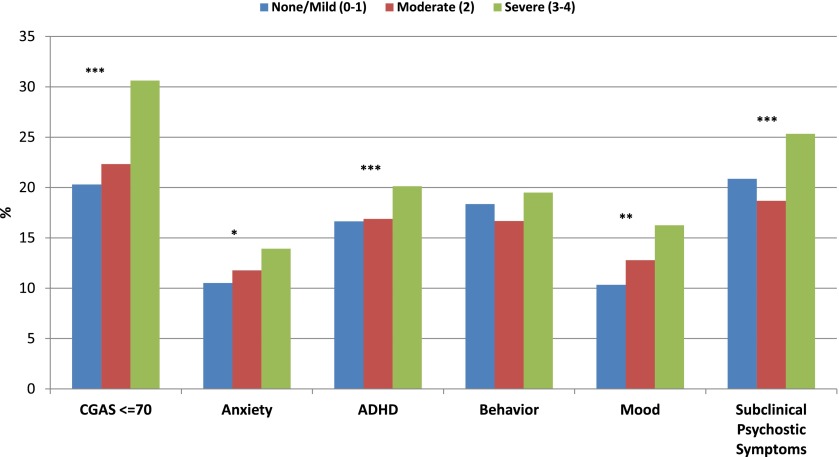
Figure 1 presents the proportion of youth with mental disorders by the severity of the physical condition. There was a direct increase in the prevalence of mental disorders by the severity of aggregate physical conditions. A 1.5-fold greater proportion of youth with severe physical conditions (ie, 30%) scored CGAS ≤70 indicating functioning impairment than those with mild or no physical conditions.
FIGURE 1.
Severity of physical conditions by classes of mental disorders. ***P < .001, **P < .01, *P < .05.
The associations of mental disorders and specific physical conditions are presented in Table 2. Youth with severe CNS (eg, 33.6%) and developmental (eg, 44.7%) disorders were significantly more likely to have a score of CGAS ≤70 indicating functional impairment than those without these conditions. Youth with CNS conditions were more likely to have ADHD (adjusted OR [aOR]: 1.3; 95% CI:1.1–1.5, FDR-adjusted P < .004) and mood disorders (aOR: 1.3; 95% CI: 1.1–1.5; P < .05); and those with developmental conditions had higher risks of most disorders, including ADHD (aOR: 3.1; 95% CI: 2.7–3.6; P < .0001), behavior disorders (aOR: 1.5; 95% CI: 1.3–1.8; P < .004), and psychosis spectrum symptoms (aOR: 1.7; 95% CI:1.5–2.0; P < .0001) than those who were not affected with these conditions. Those with autoimmune/inflammatory conditions were significantly more likely to have mood disorders than those without these conditions (aOR: 1.39; 95% CI: 1.1–1.8; P < .05). Of interest, those with developmental disorders were significantly less likely to have mood disorders than those without these conditions.
TABLE 2.
Associations of DSM-IV Indices of Mental Disorders and Physical Conditions Among Youth in Pediatric Services
| Physical Conditionsa and Rating | n | CGAS ≤70b | Mental Disordersc,d | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anxiety | ADHD | Behavior | Mood | Subclinical Psychosis Symptoms | |||||||||
| % | aOR (95% CI) | % | aOR (95% CI) | % | aOR (95% CI) | % | aOR (95% CI) | % | aOR (95% CI) | % | aOR (95% CI) | ||
| Allergy/immunology | |||||||||||||
| Severe allergies | |||||||||||||
| None/mild | 8932 | 23.3 | Ref | 11.7 | Ref | 17.5 | Ref | 18.2 | Ref | 12.3 | Ref | 21.4 | Ref |
| Mod/severe | 82 | 28.0 | 1.14 (0.68–1.91) | 13.4 | 0.99 (0.50–1.97) | 24.4 | 1.44 (0.81–2.55) | 15.9 | 0.65 (0.33–1.29) | 20.7 | 2.17 (1.17–4.03) | 20.7 | 0.78 (0.42–1.42) |
| Asthma/respiratory | |||||||||||||
| None/mild | 8910 | 23.3 | Ref | 11.8 | Ref | 17.6 | Ref | 18.3 | Ref | 12.4 | Ref | 21.5 | Ref |
| Mod/severe | 104 | 23.1 | 0.79 (0.48–1.31) | 3.8 | 0.29 (0.10–0.83) | 14.4 | 0.85 (0.47–1.56) | 8.7 | 0.51 (0.23–1.10) | 11.5 | 1.36 (0.70–2.66) | 11.5 | 0.53 (0.27–1.02) |
| Autoimmune/ inflammatory | |||||||||||||
| None/mild | 8213 | 23.4 | Ref | 11.6 | Ref | 17.6 | Ref | 18.5 | Ref | 12.0 | Ref | 21.6 | Ref |
| Mod/severe | 801 | 23.1 | 1.02 (0.85–1.24) | 12.9 | 1.05 (0.82–1.34) | 16.7 | 1.05 (0.84–1.31) | 15.2 | 1.00 (0.80–1.27) | 17.0 | 1.39 (1.11–1.75)* | 18.5 | 0.87 (0.71–1.08) |
| Cardiology | |||||||||||||
| None/mild | 8700 | 23.2 | Ref | 11.7 | Ref | 17.7 | Ref | 18.4 | Ref | 12.4 | Ref | 21.4 | Ref |
| Mod/severe | 314 | 27.7 | 1.05 (0.79–1.38) | 10.2 | 0.78 (0.52–1.17) | 13.7 | 0.69 (0.48–0.99) | 13.4 | 0.87 (0.60–1.26) | 14.0 | 1.16 (0.81–1.67) | 21.0 | 1.08 (0.79–1.47) |
| Endocrine/metabolic | |||||||||||||
| None/mild | 8463 | 23.3 | Ref | 11.7 | Ref | 17.6 | Ref | 18.2 | Ref | 12.4 | Ref | 21.5 | Ref |
| Mod/severe | 551 | 24.7 | 1.03 (0.83–1.28) | 12.0 | 1.04 (0.78–1.39) | 17.1 | 0.98 (0.76–1.28) | 17.6 | 1.17 (0.90–1.52) | 13.1 | 0.87 (0.65–1.17) | 19.6 | 0.95 (0.75–1.21) |
| Eye/ear/nose/throat | |||||||||||||
| None/mild | 8718 | 23.1 | Ref | 11.6 | Ref | 17.3 | Ref | 18.1 | Ref | 12.5 | Ref | 21.2 | Ref |
| Mod/severe | 296 | 31.1 | 1.23 (0.93–1.62) | 14.2 | 1.23 (0.86–1.76) | 24.3 | 1.22 (0.89–1.67) | 22.0 | 1.32 (0.95–1.82) | 10.1 | 0.56 (0.37–0.86) | 27.4 | 1.35 (1.01–1.81) |
| Gastroenterology | |||||||||||||
| None/mild | 8394 | 23.3 | Ref | 11.7 | Ref | 17.5 | Ref | 18.4 | Ref | 12.3 | Ref | 21.6 | Ref |
| Mod/severe | 620 | 23.4 | 1.03 (0.83–1.27) | 11.9 | 1.09 (0.83–1.45) | 18.2 | 1.17 (0.92–1.51) | 15.3 | 0.99 (0.76–1.28) | 14.2 | 0.99 (0.75–1.30) | 17.9 | 0.89 (0.70–1.14) |
| Hematology | |||||||||||||
| None/mild | 8660 | 23.3 | Ref | 11.6 | Ref | 17.6 | Ref | 18.1 | Ref | 12.4 | Ref | 21.1 | Ref |
| Mod/severe | 354 | 25.1 | 0.92 (0.70–1.19) | 13.8 | 1.15 (0.82–1.63) | 15.5 | 0.74 (0.53–1.03) | 20.3 | 1.01 (0.74–1.37) | 13.3 | 0.93 (0.65–1.33) | 28.2 | 1.32 (1.01–1.73) |
| Nephrology/urology | |||||||||||||
| None/mild | 8780 | 23.2 | Ref | 11.6 | Ref | 17.6 | Ref | 18.2 | Ref | 12.2 | Ref | 21.4 | Ref |
| Mod/severe | 234 | 28.2 | 1.14 (0.83–1.55) | 13.2 | 0.93 (0.61–1.42) | 17.5 | 0.98 (0.66–1.45) | 17.1 | 1.01 (0.68–1.49) | 19.7 | 1.57 (1.08–2.29) | 20.9 | 0.89 (0.62–1.28) |
| Neurology | |||||||||||||
| Birth anomalies | |||||||||||||
| None/mild | 7666 | 22.4 | Ref | 11.4 | Ref | 17.2 | Ref | 18.4 | Ref | 12.5 | Ref | 20.9 | Ref |
| Mod/severe | 1348 | 28.4 | 1.21 (1.04–1.40) | 13.2 | 1.16 (0.94–1.41) | 19.4 | 0.89 (0.74–1.06) | 16.8 | 0.88 (0.73–1.06) | 11.7 | 0.85 (0.68–1.05) | 24.0 | 1.24 (1.05–1.46) |
| CNS | |||||||||||||
| None/mild | 7248 | 20.8 | Ref | 11.1 | 16.2 | Ref | 17.7 | Ref | 11.3 | Ref | 20.2 | Ref | |
| Mod/severe | 1766 | 33.6 | 1.60 (1.42–1.81)*** | 14.0 | 1.10 (0.93–1.30) | 23.0 | 1.32 (1.14–1.53)** | 20.2 | 0.96 (0.82–1.12) | 16.9 | 1.30 (1.10–1.53)* | 26.3 | 1.20 (1.05–1.37) |
| Developmental | |||||||||||||
| None/mild | 7395 | 18.7 | Ref | 10.9 | 13.0 | Ref | 15.6 | Ref | 12.2 | Ref | 18.5 | Ref | |
| Mod/severe | 1619 | 44.7 | 3.34 (2.95–3.76)*** | 15.2 | 1.16 (0.97–1.39) | 38.4 | 3.12 (2.72–3.58)*** | 29.8 | 1.51 (1.30–1.75)*** | 13.5 | 0.76 (0.63–0.92)* | 34.3 | 1.73 (1.50–1.98)*** |
| Oncology | |||||||||||||
| None/mild | 8731 | 23.5 | Ref | 11.8 | Ref | 17.7 | Ref | 18.3 | Ref | 12.4 | Ref | 21.4 | Ref |
| Mod/severe | 283 | 19.1 | 0.79 (0.57–1.08) | 7.4 | 0.64 (0.40–1.03) | 12.4 | 0.75 (0.50–1.11) | 14.1 | 1.01 (0.70–1.47) | 12.0 | 1.04 (0.70–1.55) | 19.1 | 1.08 (0.77–1.49) |
| Orthopedics | |||||||||||||
| None/mild | 8300 | 23.2 | Ref | 11.6 | Ref | 17.5 | Ref | 18.5 | Ref | 12.1 | Ref | 21.5 | Ref |
| Mod/severe | 714 | 25.2 | 0.96 (0.79–1.16) | 12.9 | 1.04 (0.81–1.35) | 18.6 | 1.09 (0.87–1.38) | 14.6 | 0.82 (0.64–1.06) | 15.7 | 1.27 (0.99–1.62) | 19.3 | 0.84 (0.67–1.04) |
N = 9014. ***P < .0001, **P < .01, *P < .05 (FDR adjusted). DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; mod, moderate; Ref, reference.
Physical conditions based on electronic medical chart review and parent informant report (or proband report for youth aged ≥18 y).
aORs based on logistic regression models simultaneously controlling for gender, age, race/ethnicity, maternal education, and other physical disorders shown in the table (not adjusted for mental disorders).
Diagnosis algorithms for DSM-IV indices of mental disorders considered endorsement of screen question, symptom count, duration, distress, and impairment. Anxiety = agoraphobia, generalized anxiety disorder, panic disorder, separation anxiety disorder; Behavior = oppositional defiant disorder, conduct disorder; Mood = major depressive disorder and mania; subclinical psychosis symptoms were based on subpsychotic positive symptoms (PRIME-Screen Revised), subthreshold negative/disorganized symptoms (6 SIPS ratings), and/or positive psychosis screen.
aORs based on logistic regression models simultaneously controlling for gender, age, race/ethnicity, maternal education, other DSM-IV indices of mental disorders, and other physical disorders shown in the table (not adjusted for CGAS score).
Discussion
This study documents the pervasive comorbidity between clinically diagnosed physical conditions and the full range of mental disorders from the PNC, the largest cross-sectional study of mental and physical comorbidity in the United States to date. There is a direct association between the severity of physical conditions and most classes of mental disorders, subclinical psychotic symptoms, as well as with functional impairment. The specific links between mood disorders with inflammatory/immunologic conditions, as well as the broader associations between neurologic disorders that affect brain systems with behavioral disorders, have major implications for future studies of etiology and interventions. However, prospective information will be necessary to distinguish between alternative explanations including common underlying etiologic mechanisms (eg, immunologic factors precipitating allergies/asthma or depression)12,38,39 or causal relationships (eg, depression as a consequence of disability secondary to epilepsy or its treatment)14,17,40–42 for physical-mental comorbidity.
Mood Disorders
There has been a recent resurgence of interest in the long-suspected association between depression and immunologic factors/disorders in light of the results of large-scale genetic studies implicating the human major histocompatibility complex in schizophrenia43 and prenatal maternal-fetal interactions underlying autism.44 In the current study, the robust association between immunologic/inflammatory conditions and mood disorders in youth demonstrates the importance of evaluation of immunologic/inflammatory systems as a possible pathway to depression. Indeed, these findings confirm growing evidence from population-based samples that have demonstrated prospective links between immunologic/inflammatory conditions and the subsequent development of clinical depression.45,46
Behavior Disorders
Advances in our understanding of the neural correlates of ADHD implicate dysfunction at a network and connectivity level rather than regional anatomic abnormalities.47 Therefore, the associations between other developmental problems and ADHD observed in this study are plausible given that disruption in any component of the circuitry involved in attention and activity could lead to diverse neurobehavioral problems. The strong comorbid association between ADHD with CNS/neurologic disorders, particularly seizures and epilepsy,48 that are likely to result from common underlying genetic49 and/or environmental50,51 risk factors confirms this well-established association from clinical samples. The availability of measures of neurocognitive function and neuroimaging in a subset of this sample will facilitate our investigation of possible mechanisms for these associations.
Neurodevelopmental problems, including neurologic soft signs, learning problems, and ADHD, were also associated with behavior disorders and subclinical psychotic spectrum symptoms, as previously documented in numerous prospective studies of conduct disorder52–54 and schizophrenia.55,56 In addition to early developmental difficulties, children with severe behavior problems have elevated rates of mood disorders that often go undetected.57 Such youth also have a striking elevation in risk of the subsequent development of alcohol58 and drug59 dependence in later adolescence and adulthood. When coupled with the abundant evidence for the salient role of social determinants of conduct disorder and its progression,60 these findings provide a compelling rationale for the early identification of developmental problems among youth to minimize their educational and social sequelae.
Limitations
Limitations of the current study should be considered in the interpretation of these findings. First, because the associations observed between physical conditions and mental health disorders are cross-sectional in nature, the temporal precedence among these conditions cannot be established. Although physical conditions served as predictors in this analysis, it is also possible that mental health disorders contributed to the development of these conditions.61 Second, comprehensive diagnostic interviews were not feasible due to the large sample size and the restricted duration of the study funding period. Even though the abbreviated clinical psychiatric diagnostic interview had strong prognostic value in comparison with a standard diagnostic interview, additional clinical information would strengthen the validity of the diagnostic information. Third, these findings only pertain to youth with moderate or severe physical problems. Future analyses will assess associations between milder manifestations of physical disorders with the full range of mental disorders (eg, allergies and asthma with anxiety disorders). Fourth, the prevalence rates of physical disorders are not representative of those in the general population because of the pediatric referral source of the sample. Therefore, the results of this study may not apply to youth in the general population of the United States. Future follow-up of subsets of this sample will be informative in confirming the classification of physical and mental disorders and examining the longitudinal stability and directional links of these associations.
Implications
This project begins to fill the gap in population-based data on the prevalence of physical conditions and their interrelationships with mental disorders in US youth, a gap that is striking when compared with other developed countries. We demonstrate the feasibility of a large-scale multidisciplinary collaborative effort to obtain comprehensive data on a systematic pediatric sample that can inform science and health care delivery. However, this cross-sectional work only represents an initial step in establishing such data; prospective tracking of cross-disorder morbidity will be essential to establish causal links and to develop more effective strategies for prevention and service.
Recent advances in our understanding of disease pathogenesis across many fields of medicine, such as the shift from classification of cancer by organ system to the tumor’s molecular signature,62,63 diverse immunologic conditions/diseases resulting from shared pathogenic influences,64 and the strong link between CNS disorders and the gut microbiota,65 have revealed the importance of dismantling boundaries between medical specialties. Likewise, emerging evidence from genetics and neuroscience has implicated common environmental/pathogenetic factors (eg, neurodevelopmental, immunologic, and infectious) and genes underlying schizophrenia,43,66,67 autism,49,68 and bipolar disorder.69 The pervasive patterns of comorbidity identified in this study confirm the importance of cross-specialty integration. The identification of common pathophysiologic domains underlying purportedly distinct disorders may inform etiologic processes for these disorders, particularly if detected early in development. In particular, our finding of clustering of both neurodevelopmental and mood disorders with neurologic disturbances provides compelling support for closer integration of psychiatry with neurology and other medical specialties. A small number of combined child medical board programs in the United States are a step forward; however, in light of the high magnitude of these problems, broader initiatives are needed.
The increase in severity and disability among youth with physical-mental comorbidity17 also indicates the need for better integration of mental health and medical specialty care in children to address this “hidden morbidity.”70 Funding for services in the current US system is based on single diagnostic entities for youth with emotional and behavioral disorders (eg, learning disability, autism spectrum disorders, serious emotional disturbances, etc), which fails to incorporate the pervasive comorbidity demonstrated herein. The limited coordination across treatment sectors, including primary care, mental health, education, social services, and justice, reduces our ability to make informed decisions for resource allocation and implementation of interventions. Moreover, there is a glaring gap in evidence-based programs for comorbid disorders. Although there are growing local and national efforts to facilitate mental health care for youth identified in pediatrics and primary care settings,71,72 far more comprehensive approaches at the national level will be required. A shift to earlier identification and intervention with a coordinated multisector effort could have tremendous economic and public health impact. Community-wide prevention programs for anxiety and depression, early psychosis, suicide, smoking, and obesity in Australia73–76 provide a sound paradigm for such a shift.
Conclusions
We demonstrate pervasive comorbidity between physical and mental conditions that has a substantial impact on severity and functional impairment in youth. Specific patterns of comorbidity have important implications for both our understanding of the etiology and development of effective interventions for these disorders. These findings highlight the importance of capturing the roots of both mental and physical disorders in childhood to trace their evolution into chronic diseases with major public health impact in adulthood. Prospective tracking of cross-disorder morbidity will be important to infer causal mechanisms and to establish more effective mechanisms for prevention and intervention.
Footnotes
Dr Merikangas participated in the study concept and design, developed plans for analysis and interpreted the data, and prepared the first draft and all revisions of the manuscript; Drs Calkins, Burstein, Lateef, R.C. Gur, and Lehner participated in the study concept and design, provided input into the analyses and interpretation, and revised the manuscript; Mr He participated in the study concept and design, carried out the analyses, and reviewed and revised the manuscript; Ms Chiavacci participated in the study concept and design, reviewed and coded all medical records, reviewed the manuscript, and provided input into the study methods; Ms Ruparel participated in the design, oversaw the data management, and reviewed the accuracy of the manuscript; Dr Hakonarson established the entire genetics cohort, participated in the concept and design of the neurodevelopmental cohort, and reviewed the manuscript; Dr R.E. Gur was the principal investigator of the neurodevelopmental cohort, reviewed plans and data analyses, and critically revised drafts of the manuscript; and all authors approved the final manuscript as submitted.
The views and opinions expressed in this article are those of the authors and should not be construed to represent the views of any of the sponsoring organizations, agencies, or the US government.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by National Institute of Mental Health grants 5RC2MH089983 (Drs Gur and Hakonarson, principal investigators) and K08MH079364 (Dr Calkins) and grant Z-01-MH002931 from the Intramural Research Program of the National Institute of Mental Health (Dr Merikangas). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Druss BG, Walker ER. Mental disorders and medical comorbidity. Synth Proj Res Synth Rep. 2011. Feb(21):1–26 [PubMed]
- 2.Affordable Care Act. 2010. Pub L No. 111-148, 124 Stat 119. Available at: www.gpo.gov/fdsys/pkg/PLAW-111publ148/pdf/PLAW-111publ148.pdf. Accessed October 30, 2014
- 3.Combs-Orme T, Heflinger CA, Simpkins CG. Comorbidity of mental health problems and chronic health conditions in children. J Emot Behav Disord. 2002;10(2):116–125 [Google Scholar]
- 4.Friedman AH, Morris TL. Allergies and anxiety in children and adolescents: a review of the literature. J Clin Psychol Med Settings. 2006;13(3):323–335 [Google Scholar]
- 5.LeBovidge JS, Lavigne JV, Donenberg GR, Miller ML. Psychological adjustment of children and adolescents with chronic arthritis: a meta-analytic review. J Pediatr Psychol. 2003;28(1):29–39 [DOI] [PubMed] [Google Scholar]
- 6.Mackner LM, Sisson DP, Crandall WV. Review: psychosocial issues in pediatric inflammatory bowel disease. J Pediatr Psychol. 2004;29(4):243–257 [DOI] [PubMed] [Google Scholar]
- 7.McQuaid EL, Kopel SJ, Nassau JH. Behavioral adjustment in children with asthma: a meta-analysis. J Dev Behav Pediatr. 2001;22(6):430–439 [DOI] [PubMed] [Google Scholar]
- 8.Pinquart M. Associations of general parenting and parent-child relationship with pediatric obesity: a meta-analysis. J Pediatr Psychol. 2014;39(4):381–393 [DOI] [PubMed] [Google Scholar]
- 9.Pinquart M. Achievement of developmental milestones in emerging and young adults with and without pediatric chronic illness—a meta-analysis. J Pediatr Psychol. 2014;39(6):577–587 [DOI] [PubMed] [Google Scholar]
- 10.Pinquart M, Oslejsek B, Teubert D. Efficacy of systemic therapy on adults with mental disorders: a meta-analysis. Psychother Res. 2014;Jul 17:1–17 [DOI] [PubMed] [Google Scholar]
- 11.Rodenburg R, Stams GJ, Meijer AM, Aldenkamp AP, Deković M. Psychopathology in children with epilepsy: a meta-analysis. J Pediatr Psychol. 2005;30(6):453–468 [DOI] [PubMed] [Google Scholar]
- 12.Freilinger M, Reisel B, Reiter E, Zelenko M, Hauser E, Seidl R. Behavioral and emotional problems in children with epilepsy. J Child Neurol. 2006;21(11):939–945 [DOI] [PubMed] [Google Scholar]
- 13.Bjorgaas HM, Hysing M, Elgen I. Psychiatric disorders among children with cerebral palsy at school starting age. Res Dev Disabil. 2012;33(4):1287–1293 [DOI] [PubMed] [Google Scholar]
- 14.Ferro MA, Landgraf JM, Speechley KN. Factor structure of the Child Health Questionnaire Parent Form-50 and predictors of health-related quality of life in children with epilepsy. Qual Life Res. 2013;22(8):2201–2211 [DOI] [PubMed] [Google Scholar]
- 15.Letitre SL, de Groot EP, Draaisma E, Brand PL. Anxiety, depression and self-esteem in children with well-controlled asthma: case-control study. Arch Dis Child. 2014;99(8):744–748 [DOI] [PubMed] [Google Scholar]
- 16.Pinquart M, Pfeiffer JP. Alcohol use in students with and without hearing loss. J Deaf Stud Deaf Educ. 2015. Jan;20(1):82–90 [DOI] [PubMed] [Google Scholar]
- 17.Cadman D, Boyle M, Szatmari P, Offord DR. Chronic illness, disability, and mental and social well-being: findings of the Ontario Child Health Study. Pediatrics. 1987;79(5):805–813 [PubMed] [Google Scholar]
- 18.LeBlanc LA, Goldsmith T, Patel DR. Behavioral aspects of chronic illness in children and adolescents. Pediatr Clin North Am. 2003;50(4):859–878 [DOI] [PubMed] [Google Scholar]
- 19.Haarasilta L, Marttunen M, Kaprio J, Aro H. Major depressive episode and physical health in adolescents and young adults: results from a population-based interview survey. Eur J Public Health. 2005;15(5):489–493 [DOI] [PubMed] [Google Scholar]
- 20.Ford T, Collishaw S, Meltzer H, Goodman R. A prospective study of childhood psychopathology: independent predictors of change over three years. Soc Psychiatry Psychiatr Epidemiol. 2007;42(12):953–961 [DOI] [PubMed] [Google Scholar]
- 21.Merikangas KR, Ames M, Cui L, et al. The impact of comorbidity of mental and physical conditions on role disability in the US adult household population. Arch Gen Psychiatry. 2007;64(10):1180–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyce WF, Davies D, Raman SR, et al. Emotional health of Canadian and Finnish students with disabilities or chronic conditions. Int J Rehabil Res. 2009;32(2):154–161 [DOI] [PubMed] [Google Scholar]
- 23.Gregory AM, Caspi A, Moffitt TE, Milne BJ, Poulton R, Sears MR. Links between anxiety and allergies: psychobiological reality or possible methodological bias? J Pers. 2009;77(2):347–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hysing M, Elgen I, Gillberg C, Lundervold AJ. Emotional and behavioural problems in subgroups of children with chronic illness: results from a large-scale population study. Child Care Health Dev. 2009;35(4):527–533 [DOI] [PubMed] [Google Scholar]
- 25.Janszky I, Ahnve S, Lundberg I, Hemmingsson T. Early-onset depression, anxiety, and risk of subsequent coronary heart disease: 37-year follow-up of 49,321 young Swedish men. J Am Coll Cardiol. 2010;56(1):31–37 [DOI] [PubMed] [Google Scholar]
- 26.Goodwin RD. Association between infection early in life and mental disorders among youth in the community: a cross-sectional study. BMC Public Health. 2011. Nov 21;11:878–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gur RC, Calkins ME, Satterthwaite TD, et al. Neurocognitive growth charting in psychosis spectrum youths. JAMA Psychiatry. 2014;71(4):366–374 [DOI] [PubMed] [Google Scholar]
- 28.Satterthwaite TD, Elliott MA, Ruparel K, et al. Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage. 2014. Feb 1;86:544–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaffer D, Gould MS, Brasic J, et al. A children’s global assessment scale (CGAS). Arch Gen Psychiatry. 1983;40(11):1228–1231 [DOI] [PubMed] [Google Scholar]
- 30.Merikangas K, Avenevoli S, Costello J, Koretz D, Kessler RC. National comorbidity survey replication adolescent supplement (NCS-A): I. Background and measures. J Am Acad Child Adolesc Psychiatry. 2009;48(4):367–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merikangas KR, Dierker LC, Szatmari P. Psychopathology among offspring of parents with substance abuse and/or anxiety disorders: a high-risk study. J Child Psychol Psychiatry. 1998;39(5):711–720 [PubMed] [Google Scholar]
- 32.Kessler RC, Avenevoli S, Green J, et al. National comorbidity survey replication adolescent supplement (NCS-A): III. Concordance of DSM-IV/CIDI diagnoses with clinical reassessments. J Am Acad Child Adolesc Psychiatry. 2009;48(4):386–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calkins ME, Moore TM, Merikangas KR, et al. The psychosis spectrum in a young U.S. community sample: findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry. 2014;13(3):296–305 [DOI] [PMC free article] [PubMed]
- 34.Kobayashi H, Nemoto T, Koshikawa H, et al. A self-reported instrument for prodromal symptoms of psychosis: testing the clinical validity of the PRIME Screen-Revised (PS-R) in a Japanese population. Schizophr Res. 2008;106(2-3):356–362 [DOI] [PubMed] [Google Scholar]
- 35.Miller TJ, Zipursky RB, Perkins D, et al. The PRIME North America randomized double-blind clinical trial of olanzapine versus placebo in patients at risk of being prodromally symptomatic for psychosis. II. Baseline characteristics of the “prodromal” sample. Schizophr Res. 2003;61(1):19–30 [DOI] [PubMed] [Google Scholar]
- 36.McGlashan TH, Zipursky RB, Perkins D, et al. The PRIME North America randomized double-blind clinical trial of olanzapine versus placebo in patients at risk of being prodromally symptomatic for psychosis. I. Study rationale and design. Schizophr Res. 2003;61(1):7–18 [DOI] [PubMed] [Google Scholar]
- 37.Benjamini Y, Horchberg Y. Controlling for false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser A Stat Soc. 1995;B57(1):289–300 [Google Scholar]
- 38.Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013. Sep 12;11:200215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol Psychiatry. 2012;72(1):34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garg N, Silverberg JI. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. 2014;112(6):525–532 [DOI] [PubMed] [Google Scholar]
- 41.Reilly C, Atkinson P, Das KB, et al. Neurobehavioral comorbidities in children with active epilepsy: a population-based study. Pediatrics 2014;133(6). Available at: www.pediatrics.org/cgi/content/full/133/6/e1586 [DOI] [PubMed]
- 42.Fisher HL, Caspi A, Poulton R, et al. Specificity of childhood psychotic symptoms for predicting schizophrenia by 38 years of age: a birth cohort study. Psychol Med. 2013;43(10):2077–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kodavali CV, Watson AM, Prasad KM, et al. HLA associations in schizophrenia: are we re-discovering the wheel? Am J Med Genet B Neuropsychiatr Genet. 2014;165B(1):19–27 [DOI] [PubMed] [Google Scholar]
- 44.Parker-Athill EC, Tan J. Maternal immune activation and autism spectrum disorder: interleukin-6 signaling as a key mechanistic pathway. Neurosignals. 2010;18(2):113–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen P, Pine DS, Must A, Kasen S, Brook J. Prospective associations between somatic illness and mental illness from childhood to adulthood. Am J Epidemiol. 1998;147(3):232–239 [DOI] [PubMed] [Google Scholar]
- 46.Benros ME, Waltoft BL, Nordentoft M, et al. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry. 2013;70(8):812–820 [DOI] [PubMed] [Google Scholar]
- 47.Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp. 2010;31(6):904–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chou IC, Chang YT, Chin ZN, et al. Correlation between epilepsy and attention deficit hyperactivity disorder: a population-based cohort study. PLoS ONE. 2013;8(3):e57926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Talkowski ME, Rosenfeld JA, Blumenthal I, et al. Sequencing chromosomal abnormalities reveals neurodevelopmental loci that confer risk across diagnostic boundaries. Cell. 2012;149(3):525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mick E, Biederman J, Prince J, Fischer MJ, Faraone SV. Impact of low birth weight on attention-deficit hyperactivity disorder. J Dev Behav Pediatr. 2002;23(1):16–22 [DOI] [PubMed] [Google Scholar]
- 51.Indredavik MS, Vik T, Evensen KA, Skranes J, Taraldsen G, Brubakk AM. Perinatal risk and psychiatric outcome in adolescents born preterm with very low birth weight or term small for gestational age. J Dev Behav Pediatr. 2010;31(4):286–294 [DOI] [PubMed] [Google Scholar]
- 52.Loeber R, Dishion T. Early predictors of male delinquency: a review. Psychol Bull. 1983;94(1):68–99 [PubMed] [Google Scholar]
- 53.Odgers CL, Caspi A, Broadbent JM, et al. Prediction of differential adult health burden by conduct problem subtypes in males. Arch Gen Psychiatry. 2007;64(4):476–484 [DOI] [PubMed] [Google Scholar]
- 54.Trzesniewski KH, Moffitt TE, Caspi A, Taylor A, Maughan B. Revisiting the association between reading achievement and antisocial behavior: new evidence of an environmental explanation from a twin study. Child Dev. 2006;77(1):72–88 [DOI] [PubMed] [Google Scholar]
- 55.Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed). 1987;295(6600):681–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewis SW, Murray RM. Obstetric complications, neurodevelopmental deviance, and risk of schizophrenia. J Psychiatr Res. 1987;21(4):413–421 [DOI] [PubMed] [Google Scholar]
- 57.Teplin LA, Abram KM, McClelland GM, Dulcan MK, Mericle AA. Psychiatric disorders in youth in juvenile detention. Arch Gen Psychiatry. 2002;59(12):1133–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knop J, Penick EC, Nickel EJ, et al. Childhood ADHD and conduct disorder as independent predictors of male alcohol dependence at age 40. J Stud Alcohol Drugs. 2009;70(2):169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fergusson DM, Horwood LJ, Ridder EM. Show me the child at seven: the consequences of conduct problems in childhood for psychosocial functioning in adulthood. J Child Psychol Psychiatry. 2005;46(8):837–849 [DOI] [PubMed] [Google Scholar]
- 60.Villodas MT, Pfiffner LJ, McBurnett K. Prevention of serious conduct problems in youth with attention deficit/hyperactivity disorder. Expert Rev Neurother. 2012;12(10):1253–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen H, Cohen P, Crawford TN, Kasen S, Guan B, Gorden K. Impact of early adolescent psychiatric and personality disorder on long-term physical health: a 20-year longitudinal follow-up study. Psychol Med. 2009;39(5):865–874 [DOI] [PubMed] [Google Scholar]
- 62.Michailidou K, Hall P, Gonzalez-Neira A, et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet 2013 Apr;45(4):353–361, 361e1–361e2 [DOI] [PMC free article] [PubMed]
- 63.Pharoah PD, Tsai YY, Ramus SJ, et al. GWAS meta-analysis and replication identifies three new susceptibility loci for ovarian cancer. Nat Genet. 2013 Apr;45(4):362–370, 370e1–370e2 [DOI] [PMC free article] [PubMed]
- 64.Zhernakova A, van Diemen CC, Wijmenga C. Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat Rev Genet. 2009;10(1):43–55 [DOI] [PubMed] [Google Scholar]
- 65.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712 [DOI] [PubMed] [Google Scholar]
- 66.Suvisaari J, Mantere O. Inflammation theories in psychotic disorders: a critical review. Infect Disord Drug Targets. 2013. Feb;13(1):59–70 [DOI] [PubMed] [Google Scholar]
- 67.Lehner T. The genes in the major histocompatibility complex as risk factors for schizophrenia: de omnibus dubitandum. Biol Psychiatry. 2012;72(8):615–616 [DOI] [PubMed] [Google Scholar]
- 68.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204(2):313–321 [DOI] [PubMed] [Google Scholar]
- 69.Smoller JW, Craddock N, Kendler K, et al. Cross-Disorder Group of the Psychiatric Genomics Consortium . Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Costello EJ, Edelbrock C, Costello AJ, Dulcan MK, Burns BJ, Brent D. Psychopathology in pediatric primary care: the new hidden morbidity. Pediatrics. 1988;82(3 pt 2):415–424 [PubMed] [Google Scholar]
- 71.Aupont O, Doerfler L, Connor DF, Stille C, Tisminetzky M, McLaughlin TJ. A collaborative care model to improve access to pediatric mental health services. Adm Policy Ment Health. 2013;40(4):264–273 [DOI] [PubMed] [Google Scholar]
- 72.Blau GM, Huang LN, Mallery CJ. Advancing efforts to improve children’s mental health in America: a commentary. Adm Policy Ment Health. 2010;37(1-2):140–144 [DOI] [PubMed] [Google Scholar]
- 73.Christensen H, Pallister E, Smale S, Hickie IB, Calear AL. Community-based prevention programs for anxiety and depression in youth: a systematic review. J Prim Prev. 2010;31(3):139–170 [DOI] [PubMed] [Google Scholar]
- 74.Calear AL, Christensen H. Systematic review of school-based prevention and early intervention programs for depression. J Adolesc. 2010;33(3):429–438 [DOI] [PubMed] [Google Scholar]
- 75.Hickie IB, Scott EM, Hermens DF, et al. Applying clinical staging to young people who present for mental health care. Early Interv Psychiatry. 2013;7(1):31–43 [DOI] [PubMed] [Google Scholar]
- 76.McGorry P. Beyond DSM: early stages of disorder pose predictable and modifiable risk for persistent disorder. Aust N Z J Psychiatry. 2013. Sep;47(9):880–881 [DOI] [PubMed] [Google Scholar]