Abstract
BACKGROUND AND OBJECTIVE:
Few studies have tested the impact of motivational interviewing (MI) delivered by primary care providers on pediatric obesity. This study tested the efficacy of MI delivered by providers and registered dietitians (RDs) to parents of overweight children aged 2 through 8.
METHODS:
Forty-two practices from the Pediatric Research in Office Settings Network of the American Academy of Pediatrics were randomly assigned to 1 of 3 groups. Group 1 (usual care) measured BMI percentile at baseline and 1- and 2-year follow-up. Group 2 (provider only) delivered 4 MI counseling sessions to parents of the index child over 2 years. Group 3 (provider + RD) delivered 4 provider MI sessions plus 6 MI sessions from a RD. The primary outcome was child BMI percentile at 2-year follow up.
RESULTS:
At 2-year follow-up, the adjusted BMI percentile was 90.3, 88.1, and 87.1 for groups 1, 2, and 3, respectively. The group 3 mean was significantly (P = .02) lower than group 1. Mean changes from baseline in BMI percentile were 1.8, 3.8, and 4.9 across groups 1, 2, and 3.
CONCLUSIONS:
MI delivered by providers and RDs (group 3) resulted in statistically significant reductions in BMI percentile. Research is needed to determine the clinical significance and persistence of the BMI effects observed. How the intervention can be brought to scale (in particular, how to train physicians to use MI effectively and how best to train RDs and integrate them into primary care settings) also merits future research.
Keywords: obesity
What’s Known on This Subject:
Childhood obesity rates in the United States remain at historic highs. The pediatric primary care office represents an important, underutilized source of intervention. There is a need to test the efficacy of motivational interviewing for pediatric obesity in primary care.
What This Study Adds:
This is among the first large-scale randomized trials to show significant reductions in BMI and that motivational interviewing, delivered by trained providers in the primary care setting, can be an important and feasible part of addressing childhood obesity.
Rates of childhood obesity in the United States remain at historic highs.1 Consensus exists that ameliorating childhood obesity rates in this country will require concerted intervention at multiple levels and in multiple settings.2,3 The pediatric primary care office represents an important, underutilized intervention channel, as children have regular contact with their primary care providers (PCPs) during grade school. Pediatricians believe that they should be involved in the detection, prevention, and treatment of childhood overweight/obesity.4
Numerous family-based pediatric weight control interventions have been tested,5–11 but most were conducted outside the primary care setting. Few have been conducted where the PCP delivered the core intervention.7,12–18 Results of major outcome studies in primary care have not shown significant effects on BMI,19 with 1 recent exception.18 The latter study, conducted in Italy, comprised 5 PCP-delivered motivational interviewing (MI) sessions delivered to 372 overweight youth in primary care.
Coordinated care models recommend that dietitians be engaged through primary care to provide more intensive dietary intervention.3 In 2 studies in which registered dietitians (RDs) were included in the care team, positive effects on adiposity were reported.6,13
Null effects on adiposity in previous PCP counseling studies may be related to insufficient intervention delivery.14,15 Barriers to PCP counseling include lack of time and reimbursement.4,20,21 Even more pivotal appears to be PCPs’ perceived lack of counseling skills and the confidence to use those skills.22 One approach to improving parent motivation and PCP counseling skills is MI.
MI is a client-centered communication style used extensively to modify health behavior and is a recommended counseling style for pediatric obesity.23,24 However, its efficacy in treating pediatric obesity has been examined in only a few, generally small-scale studies,13,14,18,25–30 only 2 of which showed positive effects.13,18 There is a need to test the efficacy of MI for pediatric obesity in primary care in a fully powered trial.
This 3-group study was designed to test the efficacy of moderate-intensity (4 sessions) PCP MI-based counseling and the effect of adding 6 MI-based counseling sessions by trained dietitians delivered to parents of overweight youth aged 2 to 8 years recruited through primary care offices.
Methods
BMI2 (Brief Motivational Interviewing to reduce Body Mass Index) was a cluster-randomized, 3-group intervention trial with clinical practices serving as the unit of randomization and analysis. Group 1 (usual care) measured BMI percentile at baseline and at 1- and 2-year follow-up and provided routine care by the PCP, as well as standard educational materials for parents. Usual care PCPs and their study staff attended a half-day orientation session that included current treatment guidelines.24,31 Group 2 (PCP only) included the same assessment points as group 1. In addition, group 2 PCPs received 2 days of in-person training in MI and behavior therapy led by the first author as well as an interactive MI DVD training system focusing on pediatric obesity developed for this study. PCPs in group 2 were asked to schedule 3 counseling sessions with a parent of the index child in year 1 and 1 additional “booster” visit in year 2, although they were given latitude in their appointment scheduling. Group 3 (PCP+RD) included the same intervention components as group 2 but added MI-based counseling from a trained RD who was linked to the practice. RDs were asked to deliver 6 MI-based counseling sessions over 2 years. RDs were given flexibility in scheduling counseling sessions, although again they were encouraged to provide more visits toward the beginning of the intervention. The RD sessions were delivered either in-person or by telephone. Similar to PCPs, RDs received 2 days of in-person MI and behavior therapy training and the MI DVD.
Ethics approval was obtained from the University of Michigan and the American Academy of Pediatrics (AAP). Most PROS (Pediatric Research in Office Settings) practices (n = 38) operated under the AAP Institutional Review Board, whereas the remaining practices (n = 4), obtained local institutional review board approval. All parents gave written informed consent for their and their child’s participation.
Outcomes
The primary outcome was the child’s BMI percentile at 2-year follow-up.
BMI Percentile
PCPs and their office assistants were trained in proper assessment of height and weight and provided with print and online resources to convert heights and weights to BMI and BMI percentile. We ensured that all practices were accurately measuring height by sending a 36-inch calibration rod. If needed, a new stadiometer was provided. All practices were provided with a digital scale. Parent BMI was calculated from self-reported heights and weights.
Demographics
Parents reported household income by using 8 contiguous categories that were collapsed into <$40 000 and ≥$40 000. Education was assessed with 7 categories, collapsed into less than college graduate and college graduate or greater. We queried insurance coverage first by asking if the child had any insurance, and then by asking about specific types.
The target population was children aged 2 to 8 with a BMI ≥85th and ≤97th percentile.32
Exclusion criteria were type 1 or type 2 diabetes, non-English-speaking parent, no working telephone, chronic medical disorders, chromosomal disorders, syndromes and nonambulatory conditions (such as myelodysplasia, cerebral palsy), medications known to affect growth, enrollment in a weight loss program, or seen by weight loss specialist in past 12 months. Those enrolled by practices but subsequently found to be ineligible by the study team were allowed to continue in the study, but their data were excluded in all analyses.
Reimbursement and Incentives
PCPs in groups 2 and 3 and RDs in group 3 were compensated on a fee-per-service basis. PCPs received $50 per MI session. RDs were compensated $50 per in-person visit and $35 for telephone sessions. We provided $25 for missed appointments, up to $250 per provider or RD. There were also incentives for practice participation. Group 1 received $25 per child enrolled along with a start-up incentive of $250. Group 2 and 3 practices received $500 upon initiating the study. Practices received an initial $100 incentive before the onset of year 2 rechecks in their practice. Group 1 practices received $75 for each child completing a year 2 recheck, and group 2 and 3 practices received $50 for each child completing a year 2 recheck. Any practice retaining 50% of its cohort received an additional $400, plus $400 more if they reached 80% retention.
Study Sites
All practices were recruited from the AAP’s PROS network. Established in 1986, PROS is the largest US pediatric primary care research network, comprising 1676 practitioners from 712 pediatric practices. PROS practitioners are similar to their broader counterparts demographically and clinically.33–35
We approached PROS sites that had previously participated in at least 1 research project, excluding (1) sites offering a structured obesity treatment program and (2) clinicians with extensive experience with MI. Each practice identified an office staff member who served as the local study coordinator. This person attended the protocol training.
All practices were asked to enroll at least 20 and up to 25 eligible children. Given the higher rates of overweight and obesity in minority children, we oversampled practices with at least 25% black and/or Hispanic patients. PROS sites were matched on race and urban/suburban status, whenever possible, and then randomized to 1 of the 3 treatment arms. For the final 5 sites, we randomized using a ratio of 1:2:2 to compensate for higher dropout from the first cohort in group 2 and 3 practices.
Recruiting RDs
RDs for group 3 were selected from a registry within the Academy of Nutrition and Dietetics’ Practice-Based Research Network. RDs were paired with a practice. Potential RDs were interviewed to assess their potential for implementing MI by using a simulated patient encounter. Fifteen RDs were recruited and trained in MI.
MI Intervention
MI is a patient-centered communication style that uses specific techniques such as reflective listening, autonomy support, shared decision-making, and eliciting change talk. Practitioners in groups 2 and 3 provided MI-based counseling, by using a 3-phase model developed by the lead author36–38 that helps clinicians transition from building motivation to planning a course of action. Our MI training focused on building PCP and RD skills, including extensive practice using reflective listening and eliciting change talk. Additional details can be found elsewhere.13
Assessing Practitioner Fidelity
At the end of the 2-day training, all PCPs and RDs counseled a standardized patient. These encounters were videotaped and rated with a standardized MI fidelity scale (available from the first author). While at the training, the clinicians received detailed feedback from study staff about their counseling encounter. Practitioners were offered an additional supervision session by telephone.
Target Behaviors and Intervention Strategies in Groups 2 and 3
Both PCPs and RDs focused their counseling on discrete behaviors, assessed through a parent questionnaire, that have been shown to affect children’s weight39: snack foods, sweetened beverages, fruits, vegetables, television/screen time, and physical activity/exercise. PCPs were asked to provide positive feedback for healthy behaviors and then, collaboratively with the parent, identify behaviors that might be modified. RDs received a copy of the parent baseline questionnaire responses before their first session. Group 3 PCPs and RDs were given a form to record their patient encounters, which were shared by providers.
Educational Materials
Usual care parents received a set of educational materials that addressed healthy eating and exercise. For groups 2 and 3, preexisting or new materials written in a style consistent with MI and self-determination theory40 were used. Content emphasized child choice in making behavior change. Groups 2 and 3 also were offered self-monitoring logs for the child and/or parent to complete. For groups 2 and 3, clinicians offered parents only the educational materials and logs that were either requested by the parent or that related to the target behavior change chosen by the family.
Sample-Size Calculations
The study was powered to detect a 3-point difference in BMI percentile between any pair of study groups at 2-year follow-up, with an assumed SD for BMI percentile between 4 and 6: power of 0.80 and 2-tailed α of .05. We inflated our sample size to account for practice-level clustering,41 assuming a practice-level intraclass correlation between 0.01 and 0.05. On the basis of these assumptions and a projected 25% to 30% attrition at 2-year follow-up, we required 10 to 12 practices per arm (30–36 total) and an average of 15 to 20 children per practice at baseline.
Outcome Analysis
The primary outcome was BMI percentile at 2-year follow-up. To control for cluster randomization effects, we used mixed-effects regression with children nested within their practice. Although the primary analyses are based on intention to treat, we also provide post hoc exploratory results stratified by “low-dose” and “high-dose” MI received for groups 2 and 3. We used 75% of the expected dose (3 sessions for group 2 and 8 sessions for group 3) as the cutoff for low and high MI exposure. Initial covariates included child age, gender, parent BMI, and child baseline BMI either because they differed between groups at baseline or have been shown to affect BMI changes over time. We also initially included days from baseline to follow-up BMI assessment, but this was subsequently removed because its inclusion did not affect our results.
Results
Sample Description
Mean baseline BMI percentile was 91.9, with values similar across the 3 experimental groups (Table 1). Mean age was 5.1, with groups 2 and 3 recruiting older children than group 1. Parent-reported BMI was highest in group 2. The child sample was 57% female, and 91% of the responding parents were mothers. Groups 2 and 3 had a greater percentage of mothers as respondents than group 1. With regard to ethnicity/race, the cohort was 60% white, 22% Hispanic, 7% black, and 6% Asian, and the 3 groups differed significantly with regard to ethnic/racial composition. Overall, ∼68% of parents reported household income at or above $40 000 per year, with group 2 significantly less likely to report >$40 000 income. Approximately 39% of the sample reported at least a college education, with group 2 having lower rates than groups 1 and 3. Group 2 was less likely to have private insurance and more likely to have Medicaid coverage.
TABLE 1.
Baseline Sample Description: BMI2
| Group 1 (Usual Care) (n = 198) | Group 2 (Provider Only) (n = 212) | Group 3 (Provider + RD) (n = 235) | Total (n = 645) | |
|---|---|---|---|---|
| Mean child age (SD)a | 4.9 (1.7) | 5.1 (1.9) | 5.3 (1.8) | 5.1 (1.8) |
| Mean child BMI percentile (SD) | 91.5 (3.3) | 92.2 (3.3) | 92.1 (3.4) | 91.9 (3.3) |
| Mean parent BMI (SD)a | 28.4 (6.8) | 30.1 (7.4) | 28.5 (6.4) | 29.0 (6.9) |
| Child gender, % | ||||
| Male | 47.0 | 42.9 | 39.6 | 43.0 |
| Female | 53.0 | 57.1 | 60.4 | 57.1 |
| Parent respondent, %b | ||||
| Mother | 87.2 | 92.4 | 91.7 | 90.5 |
| Father | 12.2 | 4.3 | 7.5 | 7.9 |
| Other | 0.5 | 3.3 | 0.9 | 1.6 |
| Child race, %b | ||||
| White | 67.9 | 53.6 | 59.1 | 60.0 |
| Black | 2.6 | 11.0 | 6.09 | 6.6 |
| Hispanic | 13.3 | 30.14 | 20.9 | 21.6 |
| Asian | 6.6 | 1.44 | 8.7 | 5.7 |
| Other | 9.7 | 3.83 | 5.2 | 6.1 |
| Household income, %a | ||||
| <$40 000 | 27.2 | 38.6 | 29.8 | 31.9 |
| ≥$40 000 | 72.8 | 61.4 | 70.2 | 68.1 |
| Parent education, %b | ||||
| Less than college | 61.8 | 70.1 | 52.6 | 61.1 |
| College or higher | 38.2 | 29.9 | 47.4 | 38.9 |
| Child insurance coverage, % | ||||
| Any | 99.5 | 98.1 | 97.4 | 98.3 |
| Privatea | 74.0 | 59.8 | 65.9 | 66.4 |
| Medicaidb | 17.4 | 36.4 | 23.0 | 25.7 |
Study groups differ P < .05, based on analysis of variance for continuous and χ2 for categorical variables.
Study groups differ P < .01, based on analysis of variance for continuous and χ2 for categorical variables.
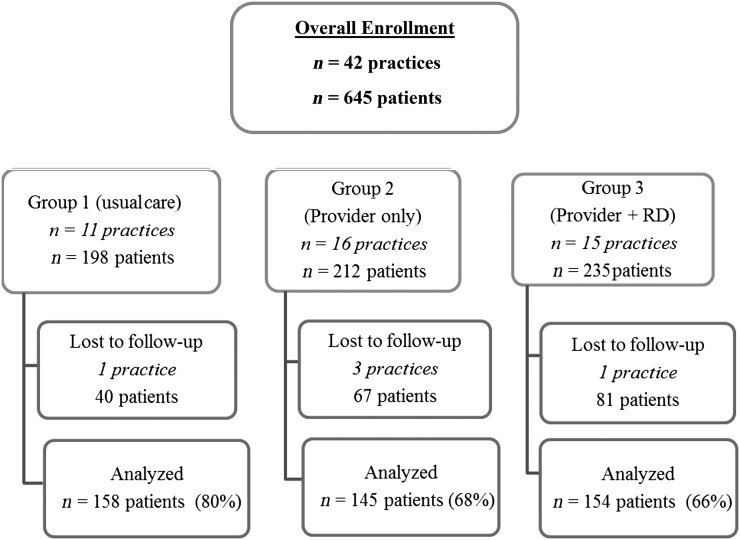
A total of 674 participants were recruited (Fig 1). Of these, 29 were ineligible because their BMI percentile, when verified by study staff, was outside the eligible range. Of the original 42 practices, 3 group 2 practices dropped out (1 PCP died, 1 retired, and 1 declined to recruit any patients), a group 3 practice dropped out because of medical illness, and a usual care practice was excluded for not following the study protocol.
FIGURE 1.
Study overview.
Of the 645 eligible baseline children, 2-year follow-up BMI data were obtained for 457 (71%). The retained cohort was similar to those lost to follow-up with regard to BMI percentile, age, and gender (Table 2). However, those lost to follow-up were significantly more likely to be black or Hispanic patients and to come from households with <$40 000 income and lower parental education. They were also more likely to have Medicaid. Parents lost to follow-up had higher baseline self-reported BMI. The intraclass correlation of year 2 BMI percentile due to practice level clustering was 0.04.
TABLE 2.
Comparison of Cohort and Dropouts: BMI2
| Cohort (n = 457) | Dropouts (n = 188) | |
|---|---|---|
| Mean child age (SD) | 5.1 (1.8) | 5.1 (1.9) |
| Mean child BMI percentile (SD) | 91.8 (3.4) | 92.2 (3.1) |
| Parent BMI (SD)a | 28.5 (6.8) | 30.3 (7.0) |
| Child gender, % | ||
| Male | 45.1 | 37.8 |
| Female | 54.9 | 62.2 |
| Parent completing questionnaire, % | ||
| Mother | 90.9 | 89.6 |
| Father | 7.5 | 8.8 |
| Other | 1.5 | 1.6 |
| Child race, %a | ||
| White | 64.0 | 50.0 |
| Black | 5.5 | 9.3 |
| Hispanic | 18.3 | 29.7 |
| Asian | 6.4 | 3.8 |
| Other | 5.7 | 7.1 |
| Household income, %a | ||
| <$40 000 | 27.7 | 42.5 |
| ≥$40 000 | 72.3 | 57.5 |
| Parent education, %a | ||
| Less than college | 55.8 | 74.4 |
| College or higher | 44.2 | 25.6 |
| Child insurance coverage, % | ||
| Anyb | 99.3 | 95.6 |
| Privatea | 72.4 | 51.4 |
| Medicaida | 22.7 | 33.2 |
Dropouts defined as missing 2-year follow-up data.
Cohort and dropout groups differ P < .01, based on analysis of variance for continuous and χ2 for categorical variables.
Cohort and dropout groups differ P < .05, based on analysis of variance for continuous and χ2 for categorical variables.
MI Dose in Groups 2 and 3
The expected dose in group 2 was 4 PCP contacts, and in group 3, the expected dose was 4 PCP contacts plus 6 by RDs (10 total). The mean MI dose for PCPs was 3.4 and 3.3 (out of 4) in groups 2 and 3, respectively (Table 3). For group 3, the mean dose for RD contacts was 2.7 (out of 6). For group 2, 73% of PCPs delivered all 4 sessions, and 10% delivered 3. For group 3, the corresponding rates for PCPs were 68% and 8%. For group 3 RDs, 12% delivered all 6 sessions, and another 14% and 6% delivered 4 or 5 sessions. RDs and parents were given a choice as to in-person or telephone for conducting contacts 2 through 6, and the majority of these contacts (79%) were by completed by telephone.
TABLE 3.
Number and Percent of MI Sessions Completed: BMI2
| Intervention Group | n (%) of Parents Completing MI Sessions | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Group 2 (n = 145) | 3 (2.1) | 14 (9.7) | 8 (5.5) | 14 (9.7) | 106 (73.1) | NA | NA |
| Group 3 providers (n = 154) | 3 (1.9) | 18 (11.7) | 17 (11.0) | 12 (7.8) | 104 (67.5) | NA | NA |
| Group 3 RDs (n = 154) | 21 (13.6) | 24 (15.6) | 29 (18.8) | 30 (19.5) | 22 (14.3) | 9 (5.8) | 19 (12.3) |
NA, not applicable.
BMI Percentile Results
At 2-year follow-up, the adjusted BMI percentile was 90.3, 88.1, and 87.1 for usual care, group 2, and group 3, respectively (Table 4). There was an overall group effect, P = .049. Planned post hoc contrasts showed that the group 3 mean was significantly (P = .02) lower than the usual care group. Using the difference in BMI percentile (baseline – year 2), means were 1.8, 3.8, and 4.9 BMI percentile units across groups 1, 2, and 3, respectively, with significance patterns virtually identical to that observed by using BMI percentile. The net difference between groups 3 and 1 was 3.1 BMI percentile units and 2.0 percentile units between groups 2 and 1. Using raw BMI units, the difference between the usual care group and groups 2 and 3 was 0.4 and 0.6 BMI units, respectively (data not shown). There was no significant interaction of intervention group by child gender, child age, child race, baseline BMI, parent income, parent education, or parent BMI.
TABLE 4.
Two-Year BMI Percentile and BMI Percentile Change by Study Group
| Study Group | n | Year 2 BMI Percentilea (SE) | BMI Percentile Differencea,b (SE) |
|---|---|---|---|
| Group 1 | 158 | 90.3c (0.94) | 1.8c (0.98) |
| Group 2 | 145 | 88.1 (0.94) | 3.8 (0.96) |
| Group 3 | 154 | 87.1c (0.92) | 4.9c (0.99) |
Adjusted for age, race, gender, baseline BMI, household income, parent BMI, provider age, and practice effects (clustering).
Subtracting year-2 BMI percentile from baseline BMI percentile.
Groups with common superscript differ P < .05.
Exploratory “completers” analyses indicated that across the 5 groups, (ie, usual care, “low” group 2 dose, “high” group 2 dose, “low” group 3 dose, and “high” group 3 dose), the mean changes in BMI percentile scores were 1.7, 3.2, 4.2, 4.6, and 5.5 (Table 5). Both group 3 high- and low-dose means were significantly greater than the usual care group. Neither group 2 high or low means differed from usual care.
TABLE 5.
BMI Percentile Change by MI Dose Received: BMI2
| Study Group | n | Mean BMI Percentile Changea (SE) |
|---|---|---|
| Group 1 | 149 | 1.7b,c (0.94) |
| Group 2, low dose, <3 MI | 23 | 3.2 (2.1) |
| Group 2, high dose, ≥3 MI | 112 | 4.2 (1.0) |
| Group 3, low dose, <8 MI | 104 | 4.6c (1.03) |
| Group 3, high dose, ≥8 MI | 37 | 5.5b (1.6) |
Group 2 had a maximum of 4 provider sessions; group 3 had a maximum of 10 sessions (4 providers/6 RDs).
Adjusted for age, race, gender, baseline BMI, parent gender, household income, parent BMI, and practice effects (clustering).
Groups with common superscript significantly differ; P < .05.
Groups with common superscript significantly differ; P < .05.
Discussion
Overweight children, whose parents received MI counseling from their PCPs supplemented by RD counseling, showed a significant reduction in BMI percentile over 2 years compared with children whose parents received usual care. The net difference in BMI reduction between these 2 groups was 3.1 BMI percentile units. This is among the first counseling interventions using MI and delivered in primary care to yield significant effects on adiposity.14,15,18,19 The 1 previous positive MI study in primary care yielded a 0.3 net effect on raw BMI, whereas our net effect on raw BMI between usual care and groups 2 and 3 was 0.4 and 0.6 BMI units, respectively; their positive results were limited to girls.18 The larger effects herein may be because that study used only PCPs to deliver the MI intervention, whereas our study also used RD counseling.
The group that received only the PCP counseling in our study showed a net difference of 2.0 BMI percentile units compared with usual care; however, this difference was not statistically significant. Whereas a 2.0 percentile reduction in BMI may confer health benefits, there are no published studies that have specifically examined the clinical impact of this size change.
In general, PCPs in both groups 2 and 3 delivered most of the recommended dose of MI counseling. The mean MI session completion rates for PCPs in groups 2 and 3 were 3.4 and 3.3 (out of 4), respectively. However, intervention completion by RDs was less successful. Out of the expected 6 RD sessions, the mean number completed was only 2.7 (out of 6). Dose-response analyses suggest that even larger effects on BMI might have been achieved had either the PCP or RD counseling been delivered at a higher rate.
Inability of RDs to complete their counseling sessions was likely due to several factors. At study’s end, we interviewed 7 of the project RDs. Although RDs attempted to reach parents during the evening, parents were often too busy with family responsibilities. Anecdotal data from parents also indicted that scheduling difficulties limited their ability to complete the RD counseling. Several RDs noted that their PCPs did not share their counseling summaries, and RDs did not feel fully integrated into the care team. RDs were added to the practice solely for this study and, unlike the PCPs, were not treating their own patients. They also felt some PCPs did not adequately encourage patients to participate in the RD counseling.
One somewhat surprising finding was the relatively large BMI reduction in the usual care group: 1.7 BMI units. A few factors likely contributed to usual care response. First, the study attracted PCPs who had an interest in pediatric obesity and MI. Consequently, we recruited practitioners who were motivated to treat their overweight patients and improve their counseling skills. Usual care practitioners, based on a baseline provider survey, were younger than those in the 2 intervention arms combined: 48 vs 54 years, respectively. They were more likely to be women: 80% compared with 70% among group 2 and 3 practitioners. Finally, they were more likely to perceive their weight management counseling as effective. Debriefing interviews also found that some practices added an RD to their staff during the trial, and others noted that their patients were motivated to lose weight “to make their doctor look good.” Together these factors at least partially explain the higher than anticipated usual care group changes.
Although the effects on BMI observed in group 2 were slightly better than the usual care group, they were not statistically significant. The policy and practice implications of this finding are somewhat unclear. Had the usual care group exhibited the degree of change we expected, then these effects would have achieved statistical significance. Thus, it is possible that the PCP intervention alone, perhaps with slightly increased dose, merits further exploration.
We lost ∼30% of the baseline sample. Although this was in the anticipated range of attrition and consistent with previous studies,42 the fact that those lost to follow-up differed on several demographic variables (eg, race, income, and education) limits generalizability. The less-than-anticipated completion of the RD counseling complicates interpretation of our results. Although completer analyses suggest that a higher dose might yield greater BMI response, such analyses are potentially confounded. Reverse causality may explain the effects among completers (ie, those families doing better may have been more likely to participate in the RD calls). Another generalizability issue is that we only enrolled PROS practices that had previously completed a research protocol. How our findings generalize to other PROS practices and the general pediatric profession merits study.
There was no attention control for the MI counseling. Thus, we cannot discern whether the effects observed in groups 2 and 3 were due to generic attention effects rather than MI per se.
Conclusions
This is among the first large-scale trials to show statistically significant reductions in BMI by using MI delivered by PCPs and RDs. Research is needed to determine the clinical significance and persistence of the BMI effects observed. Given the relatively modest dose, the intervention appears to have considerable dissemination potential, which can be explored in future studies. How the intervention can be brought to scale (in particular, how to train physicians to effectively use MI and how best to train RDs and integrate them into primary care settings) merits future research.
Footnotes
Dr Resnicow conceptualized and designed the study; drafted the initial manuscript and incorporated all edits from the authorship team; drafted the final manuscript; and oversaw data analysis, recruitment, and data collection; Dr McMaster reviewed and revised the manuscript, and conceptualized and participated in study strategy development and data collection at all project sites; Ms Bocian reviewed and revised the manuscript and oversaw data analysis, study recruitment, and data collection at the American Academy of Pediatrics (AAP) and all project sites; Ms Harris reviewed and revised the manuscript, coordinated study recruitment, and coordinated data collection at AAP and all project sites; Ms Zhou carried out the data analyses and reviewed and revised the manuscript; Dr Snetselaar critically reviewed and revised the manuscript, conceptualized and participated in study strategy development, and oversaw study implementation; Drs Schwartz, Myers, Gotlieb, and Woolford critically reviewed and revised the manuscript, conceptualized and participated in study strategy development, and oversaw study implementation; Ms Foster critically reviewed and revised the manuscript and conceptualized and participated in study strategy development; Ms Hollinger, Ms Smith, and Ms Mueller critically reviewed and revised the manuscript and conceptualized and participated in study strategy development; Dr Wasserman critically reviewed and revised the manuscript and conceptualized and oversaw study recruitment strategies, data collection, and data analysis; and all authors approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifier NCT01335308).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by a grant (HL085400) from the US National Institutes of Health National Heart, Lung, and Blood Institute. The Pediatric Research in Office Settings Network receives core funding from the US Health Resources and Services Administration Maternal and Child Health Bureau and the American Academy of Pediatrics. The funders had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found on page 757, and online at www.pediatrics.org/cgi/doi/10.1542/peds.2015-0495.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307(5):483–490 [DOI] [PMC free article] [PubMed]
- 2.Lee WW. An overview of pediatric obesity. Pediatr Diabetes. 2007;8(suppl 9):76–87 [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Accelerating Progress in Obesity Prevention: Solving the Weight of the Nation. Washington, DC: The National Academies Press; 2012 [Google Scholar]
- 4.Story MT, Neumark-Stzainer DR, Sherwood NE, et al. Management of child and adolescent obesity: attitudes, barriers, skills, and training needs among health care professionals. Pediatrics. 2002;110(1):210–214 [PubMed]
- 5.Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychol. 2007;26(4):381–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magarey AM, Perry RA, Baur LA, et al. A parent-led family-focused treatment program for overweight children aged 5 to 9 years: the PEACH RCT. Pediatrics. 2011;127(2):214–222 [DOI] [PubMed] [Google Scholar]
- 7.Nowicka P, Flodmark CE. Family in pediatric obesity management: a literature review. Int J Pediatr Obes. 2008;3(suppl 1):44–50 [DOI] [PubMed] [Google Scholar]
- 8.Kalarchian MA, Levine MD, Arslanian SA, et al. Family-based treatment of severe pediatric obesity: randomized, controlled trial. Pediatrics. 2009;124(4):1060–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Resnicow K, Yaroch AL, Davis A, et al. GO GIRLS!: results from a nutrition and physical activity program for low-income, overweight African American adolescent females. Health Educ Behav. 2000;27(5):616–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Resnicow K, Taylor R, Baskin M. Results of Go Girls: a nutrition and physical activity intervention for overweight African American adolescent females conducted through black churches. Obes Res. 2005;13(10):1739–1748 [DOI] [PubMed] [Google Scholar]
- 11.Epstein LH, Paluch RA, Beecher MD, Roemmich JN. Increasing healthy eating vs. reducing high energy-dense foods to treat pediatric obesity. Obesity (Silver Spring). 2008;16(2):318–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taveras EM, Hohman KH, Price SN, et al. Correlates of participation in a pediatric primary care-based obesity prevention intervention. Obesity (Silver Spring). 2011;19(2):449–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz RP, Hamre R, Dietz WH, et al. Office-based motivational interviewing to prevent childhood obesity: a feasibility study. Arch Pediatr Adolesc Med. 2007;161(5):495–501 [DOI] [PubMed]
- 14.Taveras EM, Gortmaker SL, Hohman KH, et al. Randomized controlled trial to improve primary care to prevent and manage childhood obesity: the High Five for Kids study. Arch Pediatr Adolesc Med. 2011;165:714–722 [DOI] [PMC free article] [PubMed]
- 15.McCallum Z, Wake M, Gerner B, et al. Outcome data from the LEAP (Live, Eat and Play) trial: a randomized controlled trial of a primary care intervention for childhood overweight/mild obesity. Int J Obes (Lond). 2007;31(4):630–636 [DOI] [PubMed] [Google Scholar]
- 16.Woolford SJ, Sallinen BJ, Clark SJ, Freed GL. Results from a clinical multidisciplinary weight management program. Clin Pediatr (Phila). 2011;50(3):187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saelens BE, Sallis JF, Wilfley DE, Patrick K, Cella JA, Buchta R. Behavioral weight control for overweight adolescents initiated in primary care. Obes Res. 2002;10(1):22–32 [DOI] [PubMed] [Google Scholar]
- 18.Davoli AM, Broccoli S, Bonvicini L, et al. Pediatrician-led motivational interviewing to treat overweight children: an RCT. Pediatrics. 2013;132(5). Available at: www.pediatrics.org/cgi/content/full/132/5/e1236 [DOI] [PubMed] [Google Scholar]
- 19.Whitlock EP, O’Connor EA, Williams SB, Beil TL, Lutz KW. Effectiveness of weight management interventions in children: a targeted systematic review for the USPSTF. Pediatrics. 2010;125(2). Available at: www.pediatrics.org/cgi/content/full/125/2/e396 [DOI] [PubMed] [Google Scholar]
- 20.Kolagotla L, Adams W. Ambulatory management of childhood obesity. Obes Res. 2004;12(2):275–283 [DOI] [PubMed] [Google Scholar]
- 21.Perrin EM, Flower KB, Garrett J, Ammerman AS. Preventing and treating obesity: pediatricians’ self-efficacy, barriers, resources, and advocacy. Ambul Pediatr. 2005;5(3):150–156 [DOI] [PubMed] [Google Scholar]
- 22.Jelalian E, Boergers J, Alday CS, Frank R. Survey of physician attitudes and practices related to pediatric obesity. Clin Pediatr (Phila). 2003;42(3):235–245 [DOI] [PubMed] [Google Scholar]
- 23.Davis MM, Gance-Cleveland B, Hassink S, Johnson R, Paradis G, Resnicow K. Recommendations for prevention of childhood obesity. Pediatrics. 2007;120(suppl 4):S229–S253 [DOI] [PubMed]
- 24.Spear BA, Barlow SE, Ervin C, et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120(suppl 4):S254–S288 [DOI] [PubMed] [Google Scholar]
- 25.Resnicow K, Davis R, Rollnick S. Motivational interviewing for pediatric obesity: conceptual issues and evidence review. J Am Diet Assoc. 2006;106(12):2024–2033 [DOI] [PubMed] [Google Scholar]
- 26.Flattum C, Friend S, Neumark-Sztainer D, Story M. Motivational interviewing as a component of a school-based obesity prevention program for adolescent girls. J Am Diet Assoc. 2009;109(1):91–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brennan L, Walkley J, Fraser SF, Greenway K, Wilks R. Motivational interviewing and cognitive behaviour therapy in the treatment of adolescent overweight and obesity: study design and methodology. Contemp Clin Trials. 2008;29(3):359–375 [DOI] [PubMed] [Google Scholar]
- 28.Carels RA, Darby L, Cacciapaglia HM, et al. Using motivational interviewing as a supplement to obesity treatment: a stepped-care approach. Health Psychol. 2007;26(3):369–374 [DOI] [PubMed] [Google Scholar]
- 29.Irby M, Kaplan S, Garner-Edwards D, Kolbash S, Skelton JA. Motivational interviewing in a family-based pediatric obesity program: a case study. Fam Syst Health. 2010;28(3):236–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walpole B, Dettmer E, Morrongiello BA, McCrindle BW, Hamilton J. Motivational interviewing to enhance self-efficacy and promote weight loss in overweight and obese adolescents: a randomized controlled trial. J Pediatr Psychol. 2013;38(9):944–953 [DOI] [PubMed]
- 31.Barlow SE, Expert Committee . Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192 [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control. BMI percentile calculator for child and teen. English version. Available at: http://apps.nccd.cdc.gov/dnpabmi. Accessed September 2, 2011
- 33.Wasserman RC, Croft CA, Brotherton SE, American Academy of Pediatrics . Preschool vision screening in pediatric practice: a study from the Pediatric Research in Office Settings (PROS) Network. Pediatrics. 1992;89(5 pt 1):834–838 [PubMed] [Google Scholar]
- 34.Forrest CB, Glade GB, Baker AE, Bocian AB, Kang M, Starfield B. The pediatric primary-specialty care interface: how pediatricians refer children and adolescents to specialty care. Arch Pediatr Adolesc Med. 1999;153(7):705–714 [DOI] [PubMed] [Google Scholar]
- 35.Slora EJ, Thoma KA, Wasserman RC, Pedlow SE, Bocian AB. Patient visits to a national practice-based research network: comparing pediatric research in office settings with the National Ambulatory Medical Care Survey. Pediatrics. 2006;118(2). Available at: www.pediatrics.org/cgi/content/full/118/2/e228 [DOI] [PubMed] [Google Scholar]
- 36.Resnicow K, Rollnick S. Motivational interviewing in health promotion and behavioral medicine settings. In: Cox WM, Klinger E, eds. Handbook of Motivational Counseling: Motivating People for Change. 2nd ed. Sussex, UK: Wiley and Sons; 2011:581–595 [Google Scholar]
- 37.Resnicow K, McMaster F. Motivational interviewing: moving from why to how with autonomy support. Int J Behav Nutr Phys Act. 2012;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Resnicow K, McMaster F, Rollnick S. Action reflections: a client-centered technique to bridge the WHY-HOW transition in motivational interviewing. Behav Cogn Psychother. 2012;40(4):474–480 [DOI] [PubMed] [Google Scholar]
- 39.Academy of Nutrition and Dietetics. Factors associated with childhood overweight. Academy of Nutrition and Dietetics Evidence Analysis Library. Available at: http://andevidencelibrary.com/topic.cfm?cat=4156. Accessed November 18, 2013
- 40.Vansteenkiste M, Williams GC, Resnicow K. Toward systematic integration between self-determination theory and motivational interviewing as examples of top-down and bottom-up intervention development: autonomy or volition as a fundamental theoretical principle. Int J Behav Nutr Phys Act. 2012;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray D. Design and Analysis of Group Randomized Trials. Vol. 27 New York, NY: Oxford University Press; 1998 [Google Scholar]
- 42.Skelton JA, Beech BM. Attrition in paediatric weight management: a review of the literature and new directions. Obesity Rev. 2011;12(5):e273–e281 [DOI] [PMC free article] [PubMed]