Abstract
BACKGROUND:
Diminished lung function and increased prevalence of asthma have been reported in children with a history of early lower respiratory illnesses (LRIs), including pneumonia. Whether these associations persist up to adulthood has not been established.
METHODS:
As part of the prospective Tucson Children's Respiratory Study, LRIs during the first 3 years of life were ascertained by pediatricians. Spirometry was performed at ages 11, 16, 22, and 26 years. The occurrence of asthma/wheeze during the previous year was ascertained at ages 11, 13, 16, 18, 22, 24, 26, and 29 years. Longitudinal random effects models and generalized estimating equations were used to assess the relation of LRIs to lung function and asthma.
RESULTS:
Compared with participants without early-life LRIs, those with pneumonia had the most severe subsequent lung function impairment, with mean ± SE deficits of −3.9% ± 0.9% (P < .001) and −2.5% ± 0.8% (P = .001) for pre- and post-bronchodilator FEV1:FVC ratio from age 11 to 26 years, respectively. Pneumonia was associated with increased risk for asthma (odds ratio [OR]: 1.95; 95% confidence interval [CI]: 1.11–3.44) and wheeze (OR: 1.94; 95% CI: 1.28–2.95) over the same age range. Early non-pneumonia LRIs were associated with mildly impaired pre-bronchodilator FEV1 (−62.8 ± 27.9mL, P = .024) and FEV1:FVC ratio (−1.1 ± 0.5%, P = .018), and wheeze (OR: 1.37; 95% CI: 1.09–1.72).
CONCLUSIONS:
Early pneumonia is associated with asthma and impaired airway function, which is partially reversible with bronchodilators and persists into adulthood. Early pneumonia may be a major risk factor for adult chronic obstructive pulmonary disease.
Keywords: early childhood pneumonia, lung function, asthma
What’s Known on This Subject:
Early-life lower respiratory illnesses, including pneumonia, are associated with increased prevalence of asthma and diminished lung function in children. Whether early-life pneumonia is associated with subsequent impaired lung function and asthma in adults is not yet clear.
What This Study Adds:
This is the first article providing strong data for an association between early-life pneumonia in an outpatient setting and airflow limitation and asthma into adulthood, supporting the hypothesis of the early-life origins of chronic obstructive pulmonary disease.
Several studies have reported an association between pneumonia in early childhood and the subsequent development of long-term respiratory sequelae, including asthma and wheeze up to adolescence1–4 and pulmonary function impairment in adults.5–9 Infants are at higher risk of sequelae after episodes of pneumonia than older children, which suggests the possibility that pneumonia may harm the growing lung.5,10–12 A recent meta-analysis concluded that 5.5% of cases of nonhospitalized pneumonia before 5 years of age were associated with at least 1 major long-term respiratory sequela, and the risk was even higher among hospitalized subjects.10 The occurrence of pneumonia in early childhood has been associated with both restrictive10 and obstructive5,12–14 pulmonary outcomes.
Whether these associations are due to lung injury caused by the severe respiratory infection underlying the pneumonia, or if pneumonia occurs in lungs the growth of which is already impaired before the episode occurs, or whether they represent a more proinflammatory phenotype or even a mild relative immune dysfunction favoring greater lung damage is currently unknown.
Most epidemiologic studies of the association of early childhood pneumonia with subsequent respiratory outcomes have been based on parental questionnaires8 or on reports by home health visitors.5,6,9 Only a few hospital-based follow-up studies of limited sample size are available, but in these latter studies, pneumonia was not assessed separately from other lower respiratory tract illnesses (LRIs).4,15–17
In previous analyses based on data from the Tucson Children’s Respiratory Study,1,18 we reported for the first time that radiologically ascertained pneumonia during the first 3 years of life was associated with persistent airway obstruction and asthma up to age 11 years. Here, we extend those studies in the same population up to age 29 years.
Methods
Study Design
The children who were included in this study were part of a birth cohort of 1246 healthy infants enrolled between 1980 and 1984 in the Tucson Children’s Respiratory Study.18 Parents were contacted shortly after their child was born and completed a questionnaire describing their race/ethnicity, history of physician-diagnosed asthma, years of education, current age, and current smoking habits. Participant race/ethnicity was categorized as both parents being non-Hispanic white, both parents being Hispanic white, or 1 non-Hispanic white parent and 1 Hispanic white parent; all other groups (African American, Asian American, Native American, and mixed) were combined into a single category (“other”). Parental history of asthma was defined as either parent report of a physician diagnosis of asthma.
Questionnaires
Physician-diagnosed asthma with active symptoms during the previous year (asthma attacks or episodes of wheeze) and active wheeze during the previous year were assessed prospectively by questionnaires completed by the participant’s parents at ages 11, 13, and 16 years and by the participant at ages 18, 22, 24, 26, and 29 years. Physician diagnosis of asthma was based on recall of what the physician said and activity during the past year as reported on the questionnaire. Self-reported current cigarette smoking was recorded at ages 16, 18, 22, 24, 26, and 29 years.
LRIs During Early Life
During the first 3 years of life, parents were instructed to take their children to their pediatricians whenever the children had LRI symptoms. Details of LRI ascertainment are provided in Supplemental Information 1. Children were classified as having pneumonia if the pediatrician specified this diagnosis and if there was radiologic evidence compatible with pneumonia, ie, presence of infiltrates and/or a radiologist’s diagnosis of bronchopneumonia or pneumonitis. Chest radiograms were not required in the study and were ordered for clinical reasons only. Children who had at least 1 LRI during the first 3 years of life and had a diagnosis of pneumonia and radiologic evidence of pneumonia for ≥1 of these LRIs were included in the “pneumonia” group. Children who had other LRIs were included in the “other LRIs” group, as were those with physician-diagnosed pneumonia but without radiologic evidence compatible with this diagnosis. All other children with full follow-up were included in the “no LRI” group. Among those with LRIs, 138 participants experienced multiple episodes of LRIs (see Supplemental Table 5).
Skin-Prick Test
Skin-prick tests to local aeroallergens (Bermuda grass, Alternaria alternata, careless weed, house dust mix, and mesquite, mulberry, and olive tree pollens) were performed at age 6 years as previously described.19 Atopy was defined as ≥1 positive skin-prick test.
Lung Function Assessments
Methods to assess lung function at ages 11, 16, 22, and 26 years are described in Supplemental Information 2. Spirometry indices included forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and forced expiratory flow between 25% and 75% of the FVC (FEF25–75). Values were adjusted for gender, height, and race/ethnicity.
Statistical Methods
Proportions were compared by using χ2 or Fisher's exact test as appropriate. A longitudinal random-effects model was used to assess the relation of radiologically ascertained pneumonia and other LRIs before age 3 to lung function from ages 11 through 26 years. Initially, a marginal analysis was performed at ages 11, 16, 22, and 26 years to assess the significance of covariates believed to possibly influence the relation of early LRIs to lung function. Covariates that had a P value ≤.1 or that were considered important on the basis of existing literature were retained for the longitudinal random-effects models. Akaike’s information criteria were compared between models to determine the best-fitting model.20 All models included age, gender, height, race/ethnicity, and history of early LRIs (the base model), plus current physician-diagnosed asthma, maternal smoking, paternal smoking, and any remaining significant covariates. The relation of radiologically ascertained pneumonia and other LRIs before age 3 to subsequent physician-diagnosed asthma and wheeze from ages 11 through 29 years was assessed by using longitudinal generalized estimating equations, which are extension of the generalized linear mixed models.21,22 A marginal analysis was performed initially at ages 11, 13, 16, 18, 22, 24, 26, and 29 years to assess the significance of covariates believed to influence the relation of early LRIs to occurrence of asthma or wheeze on the basis of the literature and previous analyses conducted by our group. Covariates that had a P value ≤.1 or that were considered important on the basis of the literature were retained for the longitudinal generalized estimating equations. All best-fitting models included age and history of early LRIs plus maternal smoking, paternal smoking, and any remaining significant covariates. Two-sided P values <.05 were considered significant. Statistical analyses were carried out by using SPSS for Windows 22.0 (IBM SPSS Statistics, IBM Corporation, Armonk, NY) and Stata 13.0 (StataCorp, College Station, TX). Informed consent was obtained from the parents for their children or by the enrollees themselves, and the Institutional Review Board of the University of Arizona approved the study.
Results
Characteristics of Participants With Complete and Incomplete Data
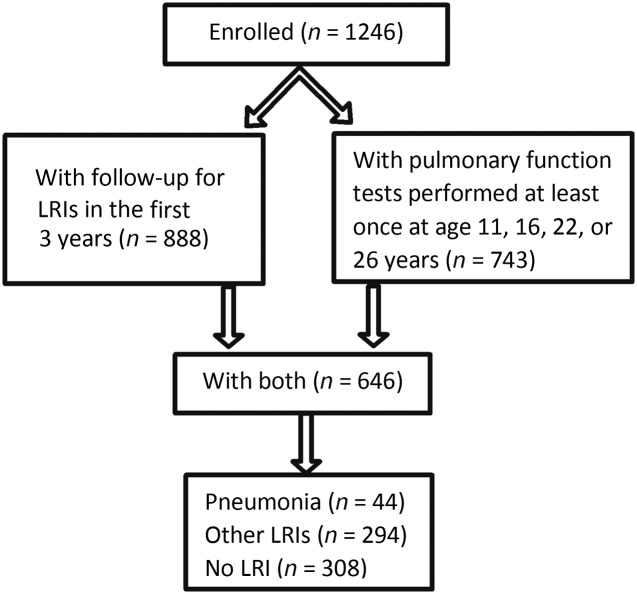
Participants included in the current study were required to have complete follow-up for LRIs during the first 3 years of life as well as ≥1 pulmonary function test completed at ages 11, 16, 22, or 26 years (n = 646; Fig 1). When compared with participants with incomplete data, those with complete data were more likely to be non-Hispanic white or mixed non-Hispanic/Hispanic white and to have parents who were older and with more years of education (Table 1).
FIGURE 1.
Numbers of subjects enrolled, with complete follow-up for LRIs in the first 3 years of life, and for whom data were available for pulmonary function tests performed at least once at ages 11, 16, 22, or 26 years.
TABLE 1.
Characteristics of Subjects With and Without Complete LRI Data and Any Pulmonary Function Data at Ages 11, 16, 22, or 26 Years
| Subjects With Complete LRIa and PFT Data (N = 646) | Subjects With Complete LRI Data but No PFT Data (N = 242) | Subjects Without Complete LRI and PFT Data (N = 358) | Pb | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Participant characteristics | |||||||
| Male gender | 646 | 50.8 | 242 | 47.1 | 358 | 47.8 | .507 |
| Race/ethnicityc | |||||||
| NHW | 646 | 60.5 | 242 | 63.6 | 358 | 52.8 | <.001 |
| HW | 11.9 | 12.4 | 7.5 | ||||
| Mixed NHW/HW | 14.4 | 9.1 | 5.9 | ||||
| Other | 13.2 | 14.9 | 33.8 | ||||
| One or more positive skin-prick test at 6 years | 572 | 39.3 | 82 | 35.4 | 108 | 33.3 | .434 |
| Active wheeze at 6 years | 633 | 28.1 | 187 | 24.6 | 196 | 21.9 | .193 |
| Active physician-diagnosed asthma at 6 years | 630 | 9.4 | 189 | 9.0 | 196 | 10.2 | .913 |
| Maternal characteristics | |||||||
| Active smoking | 646 | 15.9 | 241 | 19.5 | 356 | 19.7 | .241 |
| Education ≤12 years | 646 | 27.7 | 241 | 32.4 | 354 | 38.7 | .002 |
| Age >28 years | 646 | 41.0 | 242 | 42.6 | 357 | 31.1 | .003 |
| Positive for physician-diagnosed asthma | 638 | 10.3 | 233 | 12.9 | 284 | 10.9 | .571 |
| Paternal characteristics | |||||||
| Active smoking | 638 | 28.7 | 238 | 33.2 | 350 | 35.1 | .090 |
| Education ≤12 years | 633 | 26.4 | 238 | 29.4 | 346 | 35.8 | .008 |
| Age >28 years | 637 | 59.3 | 239 | 56.9 | 353 | 41.4 | <.001 |
| Positive for physician-diagnosed asthma | 609 | 13.1 | 223 | 12.1 | 262 | 9.5 | .328 |
HW, Hispanic white; NHW, Non-Hispanic white; PFT, pulmonary function test.
Subjects with complete LRI data had complete follow-up during the first 3 years of life.
P values (2-sided) based on Pearson χ2 statistic.
Participant race/ethnicity determined from parental information provided at enrollment.
Adult Lung Function and Pneumonia During Early Life
Compared with children who did not have early LRIs, those who had pneumonia during the first 3 years of life had a significantly lower prebronchodilator FEV1 at ages 11, 16, and 22 years and a significantly lower prebronchodilator FEV1:FVC ratio, FEF25–75, and FEF25–75:FVC ratio at ages 11, 16, 22, and 26 years (Table 2). For the postbronchodilator spirometry, pneumonia was associated with a significantly lower FEV1 at age 22 years and a significantly lower FEV1:FVC ratio, FEF25–75, and FEF25–75:FVC ratio at ages 11, 16, 22, and 26 years (Table 2). There was no significant relation between pneumonia during early life and either pre- or postbronchodilator FVC at any age.
TABLE 2.
Regression Models for Cross-sectional Lung Function and LRIs During the First 3 Years of Life
| LRIs | FEV1, mL | FVC, mL | FEV1:FVC Ratio, % | FEF25–75, mL/s | FEF25–75:FVC Ratio, % | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| B (SE) | P | B (SE) | P | B (SE) | P | B (SE) | P | B (SE) | P | |
| Prealbuterol lung function | ||||||||||
| Age 11 years (N = 548) | ||||||||||
| No LRI | Ref | Ref | Ref | Ref | Ref | |||||
| Pneumonia | −109.74 (46.71) | .019 | 2.05 (53.75) | .970 | −4.30 (1.07) | <.001 | −415.45 (105.29) | <.001 | −16.46 (4.07) | <.001 |
| Other LRIs | −64.87 (23.46) | .006 | −56.37 (27.00) | .037 | −0.72 (0.54) | .179 | −147.40 (52.88) | .006 | −4.10 (2.04) | .045 |
| Age 16 years (N = 434) | ||||||||||
| No LRI | Ref | Ref | Ref | Ref | Ref | |||||
| Pneumonia | −158.51 (76.81) | .040 | 56.10 (92.44) | .544 | −4.20 (1.25) | .001 | −460.97 (149.66) | .002 | −10.94 (3.77) | .004 |
| Other LRIs | −83.61 (40.64) | .040 | −19.18 (48.91) | .695 | −1.48 (0.66) | .026 | −212.63 (79.43) | .008 | −4.79 (2.00) | .017 |
| Age 22 years (N = 397) | ||||||||||
| No LRI | Ref | Ref | Ref | Ref | Ref | |||||
| Pneumonia | −260.93 (87.45) | .003 | −97.42 (97.22) | .317 | −3.84 (1.42) | .007 | −523.42 (187.23) | .005 | −9.42 (4.27) | .028 |
| Other LRIs | −27.90 (43.38) | .521 | 33.92 (48.23) | .482 | −1.30 (0.70) | .065 | −117.09 (92.87) | .208 | −3.70 (2.12) | .082 |
| Age 26 years (N = 297) | ||||||||||
| No LRI | Ref | Ref | Ref | Ref | Ref | |||||
| Pneumonia | −163.02 (99.30) | .102 | −23.87 (110.57) | .829 | −3.23 (1.51) | .034 | −507.60 (216.73) | .020 | −11.14 (4.91) | .024 |
| Other LRIs | −119.77 (52.08) | .022 | −119.18 (57.99) | .041 | −0.63 (0.79) | .427 | −151.45 (113.67) | .184 | −1.92 (2.57) | .456 |
| Postalbuterol lung function | ||||||||||
| Age 11 years (N = 534) | ||||||||||
| No LRI | Ref | Ref | Ref | Ref | Ref | |||||
| Pneumonia | −43.33 (44.40) | .330 | 16.66 (53.00) | .753 | −2.09 (1.01) | .038 | −281.22 (101.84) | .006 | −11.15 (4.09) | .007 |
| Other LRIs | −22.39 (22.62) | .323 | −16.53 (26.99) | .540 | −0.40 (0.51) | .434 | −103.83 (51.87) | .046 | −3.73 (2.08) | .073 |
| Age 16 years (N = 425) | ||||||||||
| No LRI | Ref | Ref | Ref | Ref | Ref | |||||
| Pneumonia | −116.67 (76.90) | .130 | 42.11 (91.63) | .646 | −3.17 (1.03) | .002 | −427.17 (149.75) | .005 | −10.55 (3.72) | .005 |
| Other LRIs | −66.62 (41.07) | .106 | −35.04 (48.98) | .475 | −0.86 (0.55) | .119 | −187.95 (80.08) | .019 | −4.13 (1.99) | .039 |
| Age 22 years (N = 393) | ||||||||||
| No LRI | Ref | Ref | Ref | Ref | Ref | |||||
| Pneumonia | −234.25 (80.84) | .004 | −92.95 (96.86) | .338 | −3.14 (1.16) | .007 | −589.87 (178.22) | .001 | −10.27 (4.25) | .016 |
| Other LRIs | −24.05 (40.27) | .551 | 11.09 (48.25) | .818 | −0.83 (0.58) | .154 | −138.40 (89.05) | .121 | −3.63 (2.12) | .088 |
| Age 26 years (N = 286) | ||||||||||
| No LRI | Ref | Ref | Ref | Ref | Ref | |||||
| Pneumonia | −142.10 (97.46) | .146 | 0.70 (144.20) | .995 | −3.03 (1.32) | .022 | −522.03 (225.51) | .021 | −11.39 (5.20) | .029 |
| Other LRIs | −119.02 (50.93) | .020 | −125.12 (59.68) | .037 | −0.23 (0.69) | .742 | −110.35 (118.21) | .351 | −0.13 (2.72) | .963 |
All models included gender, height, race/ethnicity, and LRIs. B, model coefficient; Ref, reference group in the model.
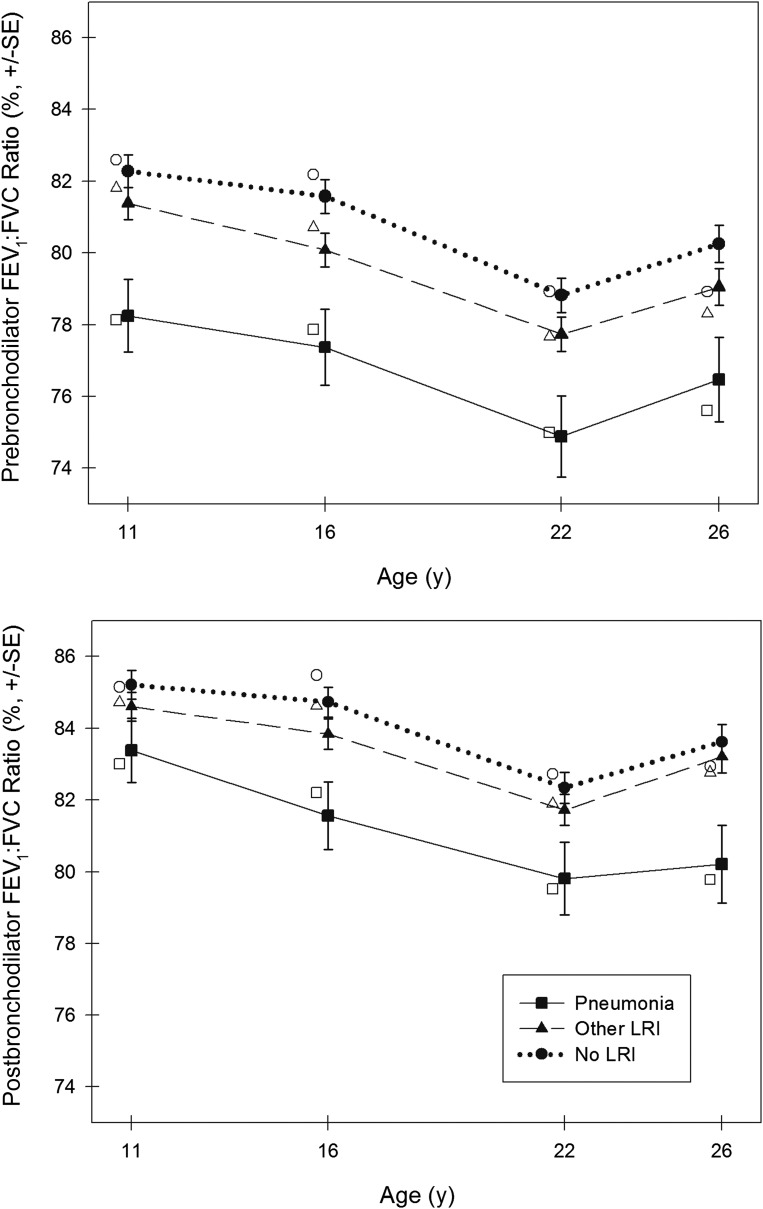
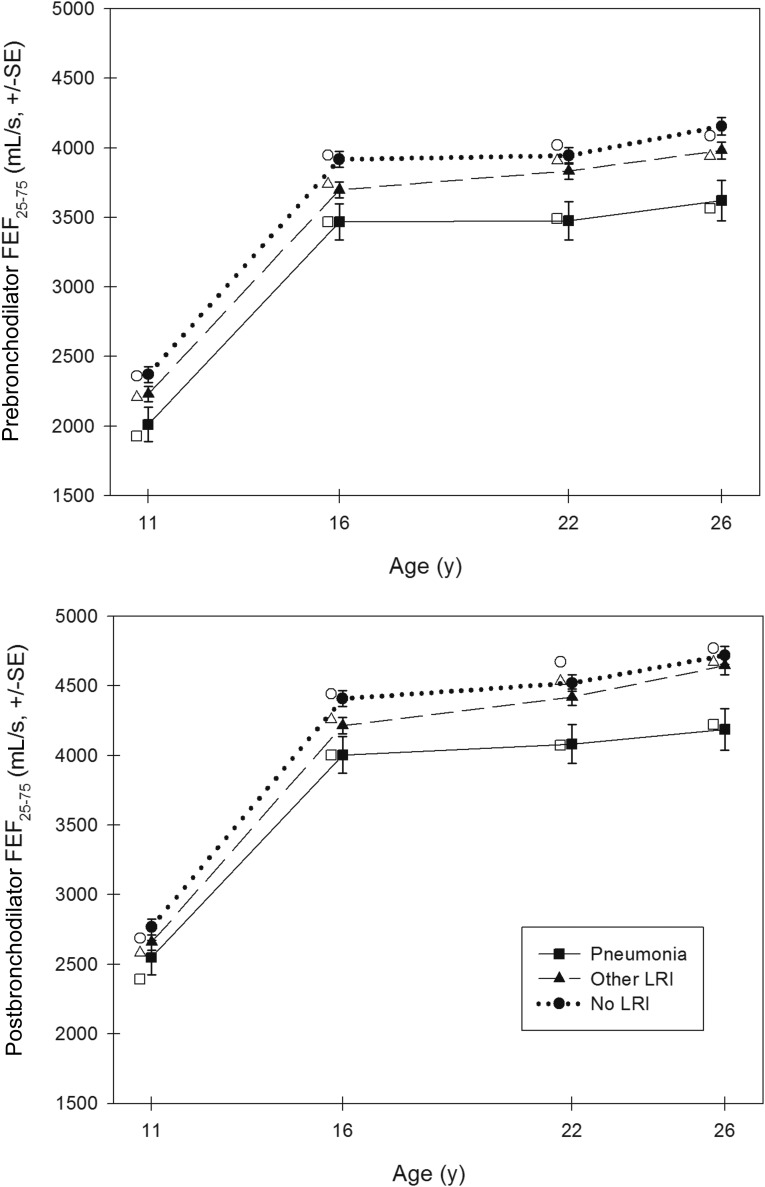
In longitudinal random-effects models, after adjusting for age, gender, height, and race/ethnicity, early pneumonia was associated with a significantly lower prebronchodilator FEV1 (P = .033), FEV1:FVC ratio (P < .001), FEF25–75 (P < .001), and FEF25–75:FVC ratio (P < .001) up to age 26 years (Table 3). Early pneumonia was also associated with a significantly lower postbronchodilator FEV1:FVC ratio (P = .001), FEF25–75 (P = .002), and FEF25–75:FVC ratio (P = .002). Figures 2 and 3 show the predicted mean values for the FEV1:FVC ratio and FEF25–75 derived from our base models in non-Hispanic white male participants at ages 11, 16, 22, and 26 years. Models were not appreciably different for females. Further adjustment for participants’ current smoking, current wheeze, current physician-diagnosed asthma, maternal history of physician-diagnosed asthma, maternal smoking, and paternal smoking did not notably modify the results (see Supplemental Table 6). Among those early pneumonia cases, 68.2% (30 of 44) had concurrent wheeze; however, the association between early pneumonia and subsequent impaired lung function was similar for those with wheezy pneumonia versus nonwheezy pneumonia (data not shown).
TABLE 3.
Longitudinal Random-Effects Models (Base Models) for Lung Function From Ages 11–26 Years and LRIs During the First 3 Years of Life
| LRIs | FEV1, mL | FVC, mL | FEV1:FVC Ratio, % | FEF25–75, mL/s | FEF25–75:FVC Ratio, % | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| B (SE) | P | B (SE) | P | B (SE) | P | B (SE) | P | B (SE) | P | |
| Prealbuterol lung function at ages 11–26 years | ||||||||||
| No LRI | Ref | Ref | Ref | Ref | Ref | |||||
| Pneumonia | −117.22 (54.94) | .033 | 46.34 (64.12) | .470 | −3.92 (0.93) | <.001 | −417.77 (114.02) | <.001 | −12.38 (3.17) | <.001 |
| Other LRIs | −62.79 (27.87) | .024 | −21.90 (32.53) | .501 | −1.12 (0.47) | .018 | −160.14 (57.79) | .006 | −4.11 (1.60) | .010 |
| Postalbuterol lung function at ages 11–26 years | ||||||||||
| No LRI | Ref | Ref | Ref | Ref | Ref | |||||
| Pneumonia | −67.23 (53.68) | .210 | 55.83 (64.80) | .389 | −2.54 (0.79) | .001 | −348.36 (111.54) | .002 | −9.49 (3.14) | .002 |
| Other LRIs | −40.83 (27.28) | .134 | −18.18 (32.92) | .581 | −0.64 (0.40) | .111 | −125.19 (56.66) | .027 | −3.20 (1.59) | .044 |
All base models included age, gender, height, race/ethnicity, and LRIs. The number of observations for the prealbuterol FEV1 model was 1676; the number of individuals included in the analysis was 646, with an average number of observations per individual of 2.6. B, model coefficient; Ref, reference group in the model.
FIGURE 2.
Predicted mean values for FEV1:FVC ratio in non-Hispanic white males at ages 11, 16, 22, and 26 years by LRIs. Closed symbols (plotted) represent the predicted mean values for lung function for non-Hispanic white male participants based on longitudinal random-effects models using base models adjusted for age, gender, height, race/ethnicity, and LRIs. All predicted mean values for lung function were standardized to mean heights for male participants at ages 11, 16, 22, and 26 years (ie, 144.5, 176.5, 178.2, and 179.0 cm, respectively). Open symbols (adjacent to the plotted symbols) represent the predicted mean values for lung function for non-Hispanic white male participants based on cross-sectional linear regression models (adjusted for gender, height, and race/ethnicity) at ages 11, 16, 22, and 26 years.
FIGURE 3.
Predicted mean values for FEF25–75 in non-Hispanic white males at ages 11, 16, 22, and 26 years by LRIs. Closed symbols (plotted) represent the predicted mean values for lung function for non-Hispanic white male participants based on longitudinal random-effects models using base models adjusted for age, gender, height, race/ethnicity, and LRIs. All predicted mean values for lung function were standardized to mean heights for male participants at ages 11, 16, 22, and 26 years (ie, 144.5, 176.5, 178.2, and 179.0 cm, respectively). Open symbols (adjacent to the plotted symbols) represent the predicted mean values for lung function for non-Hispanic white male participants based on cross-sectional linear regression models (adjusted for gender, height, and race/ethnicity) at ages 11, 16, 22, and 26 years.
Adult Lung Function and Other LRIs During Early Life
Compared with participants without early LRIs, those with other LRIs but no pneumonia during the first 3 years of life had a significantly lower prebronchodilator FEV1:FVC ratio at age 16 years only, and there was no significant association of other LRIs with postbronchodilator FEV1:FVC ratio at any age (Table 2). Other LRIs were associated with a significantly lower prebronchodilator FEV1 at ages 11, 16, and 26 years and a significantly lower postbronchodilator FEV1 at age 26 years only (Table 2).
In longitudinal random-effects models, other LRIs were associated with significantly lower prebronchodilator FEV1 (P = .024), FEV1:FVC ratio (P = .018), FEF25–75 (P = .006), and FEF25–75:FVC ratio (P = .010) and lower postbronchodilator FEF25–75 (P = .027) and FEF25–75:FVC ratio (P = .044) up to age 26 years (Table 3). There was no significant relation between other LRIs and either pre- or postbronchodilator FVC (Table 3). Adjustment for other covariates did not notably modify these results (see Supplemental Table 6).
LRIs During Early Life and Subsequent Asthma and Wheeze
In the best-fitting longitudinal generalized estimating equation for asthma and after adjusting for age, atopy at age 6 years, current smoking, maternal asthma, paternal asthma, maternal smoking, paternal smoking, and paternal age, participants with early pneumonia had a significantly higher risk of active physician-diagnosed asthma (odds ratio [OR]: 1.95; 95% confidence interval [CI]: 1.11–3.44) during the previous year up to age 29 years compared with those with no LRI during early life (Table 4). Other LRIs in early life were not associated with a significantly higher risk of subsequent asthma. After adjusting for the same covariates, early pneumonia was associated with an increased risk of active wheeze during the previous year up to age 29 years (OR: 1.94; 95% CI: 1.28–2.95) as were other LRIs, although the association with the latter was much weaker than that for pneumonia (OR: 1.37; 95% CI: 1.09–1.72) (Table 4).
TABLE 4.
Best-Fitting Multivariate Longitudinal Generalized Estimating Equation Models for Physician-Diagnosed Asthma and Active Wheeze From Ages 11–29 Years and LRIs During the First 3 Years of Life
| LRIs | Symptoms | |||
|---|---|---|---|---|
| Asthmaa | Active Wheezeb | |||
| OR (95% CI) | P | OR (95% CI) | P | |
| No LRI | Reference | Reference | ||
| Pneumonia | 1.95 (1.11–3.44) | .021 | 1.94 (1.28–2.95) | .002 |
| Other LRI | 1.30 (0.93–1.80) | .122 | 1.37 (1.09–1.72) | .007 |
Best-fitting model for asthma included age, LRIs, atopy at 6 years of age, current smoking, maternal asthma, paternal asthma, maternal smoking, paternal smoking, and paternal age. The number of observations for asthma outcome was 4150; the number of individuals included in the analysis was 738, with an average number of observations per individual of 5.6.
Best-fitting model for active wheeze included age, LRIs, atopy at 6 years of age, current smoking, maternal asthma, paternal asthma, maternal smoking, paternal smoking, and maternal education. The number of observations for active wheeze outcome was 4160; the number of individuals included in the analysis was 738, with an average number of observations per individual of 5.6.
Discussion
Our results indicate that having had an episode of radiologically ascertained pneumonia in early life is associated with long-lasting respiratory impairment and morbidity. By their late 20s, participants who had pneumonia before age 3 had deficits of −3.9% ± 0.9% (P < .001) and −2.5% ± 0.8% (P = .001) for pre- and postbronchodilator FEV1:FVC ratios, respectively, and these deficits were independent of participants’ concomitant wheeze and smoking. Early-life pneumonia was also associated with a doubling of the risk of active asthma and wheeze up to age 29 years. Associations between LRIs other than pneumonia and subsequent respiratory outcomes were weaker and less consistent, indicating that long-term sequelae may be specific for pneumonia.
Our group previously reported that, in this same cohort and when compared with children without LRIs, those with early pneumonia had lower maximal flows at functional residual capacity at age 6 years and lower levels of FEV1 and FEF25–75 at age 11 years.1 In the large longitudinal British National Child Development Study, early pneumonia was associated with deficits in both FEV1 and FVC at the age of 34 or 35 years.8 Shaheen et al12 showed that early pneumonia before the age of 2 years was associated with reduced mean FEV1 and FVC at a mean age of 57.6 years. No change in FEV1:FVC ratio was observed. Pneumonia or bronchitis in infancy was associated with reduced mean FEV1 in Hertfordshire 60-year-olds.5 In a recent meta-analysis, early pneumonia under the age of 5 years was associated with a 5.4% (95% CI: 2.5%–10.2%) risk of restrictive lung disease and a 2.8% (95% CI: 0.8%–6.4%) risk of obstructive lung disease.10 In contrast, our results revealed clear evidence of an obstructive spirometric pattern but no evidence of a restrictive pattern in adult participants with radiologically ascertained pneumonia before age 3. What may determine these discrepancies is unknown, but differences in ascertainment methods, such as recruiting hospitalized versus nonhospitalized children and the fact that only in our study were radiograms used to confirm clinical pneumonia in nonhospitalized children, may have influenced the results. In addition, most cases of pneumonia ascertained in this study were associated with respiratory viruses,1 whereas microbiology of the episode of pneumonia was not available for previous studies.5,8,10,12 Whether bacterial pneumonia was more frequent in those studies than in ours and if such etiology is more likely to be associated with restrictive pulmonary sequelae are currently unknown.
We also previously reported in this same cohort that early pneumonia was associated with physician-diagnosed asthma and wheeze at ages 6 and 11 years.1 In the large longitudinal British National Child Development Study by Anderson et al,3 early pneumonia by the age of 7 years was associated with increased risk of developing asthma or wheeze at age 11 and 16 years. Another longitudinal study by Kusel et al2 showed that early febrile LRI during infancy was associated with higher risk of developing asthma (risk ratio: 2.57; 95% CI: 1.33–4.98) and persistent wheeze (risk ratio: 2.12; 95% CI: 1.14–3.95) at age 10 years. In contrast, an 8-year follow-up study by Korppi et al4 failed to show a significant association between early pneumonia with subsequent development of asthma, probably due to small sample size. However, they did find increased bronchial hyperreactivity after infantile pneumonia and bronchiolitis. Our longitudinal study is by far the largest birth cohort with the longest follow-up period in which the relation between radiologically ascertained pneumonia and subsequent asthma and wheeze was assessed up to adult life.
The main strength of our study is that ascertainment was population-based and the diagnosis of pneumonia and LRIs was made by study pediatricians on the basis of a preestablished set of standardized criteria. Moreover, the additional requirement of radiologic evidence of pneumonia further enhanced the diagnostic accuracy. The complete 3-year follow-up of all participants ensured that all LRI episodes during the first 3 years of life were captured in our study. In contrast to most of the available literature, which mostly reported cross-sectional mean lung function estimates, we conducted our longitudinal analysis on the basis of all available lung function data at ages 11, 16, 22, and 26 years. Nevertheless, the study has potential limitations. Only 71.3% (888 of 1246) of participants had full follow-up for LRIs in early life and, of these, 646 also had available lung function data. The main differences between included and excluded participants were in socioeconomic status and ethnicity, and the influence of these differences on the associations under study is unknown.
Interestingly, a gradation of effect was seen in our results, in that radiologically ascertained pneumonia was associated with more severe deficits in airway function than those observed for other LRIs. We previously reported that the detection frequencies for viruses we found associated with pneumonia were not very different from those observed for other LRIs.1 It is thus possible that there is a spectrum of severity of viral LRI, with those LRIs that are associated with more severe symptoms being more likely to be diagnosed as pneumonia and to be associated with radiologic changes compatible with this diagnosis. Distinguishing between patchy infiltrates and local atelectasis in early-life radiograms is not straightforward, and the possibility cannot be excluded that many episodes diagnosed as pneumonia could be more severe airway obstruction associated with viral respiratory infections.
The factors that determine the association between pneumonia in early life and the respiratory outcomes observed in this and other studies are unknown. One possible mechanism is direct airway damage attributable to the severe lung infection causing the pneumonia. Interference of pneumonia with normal lung growth has been shown in animal models.23 Furthermore, infancy is a period of rapid airway and alveolar growth in humans, and early pneumonia may have impaired the normal development of lung structure and function during this crucial period.24,25 FEF25–75 and FEF25–75:FVC are indices of small airway function.26,27 The dominant obstructive deficit, especially in small airways, observed in our study suggests that the growth of airways was disproportionately affected. Viruses known to cause pneumonia in infancy and early childhood, such as adenovirus and influenza A, can cause severe, irreversible structural damage to the developing lung in susceptible subjects.28–30 It is thus possible that a range of susceptibilities may exist in the population such that some young children may develop less severe impairment, as observed in this study.
Alternatively, individuals with early pneumonia may already have a lower premorbid lung function before their LRIs. In this scenario, pneumonia may be a marker of a preexisting small airway caliber, which predisposes to both airflow limitation and asthma into adult life.31–33 Our group has previously shown that premorbid maximal expiratory flow at functional residual capacity (VmaxFRC) measured before age 1 and before any LRI was lower in children with subsequent early pneumonia and with other LRIs than in those with no LRI, although the difference only reached statistical significance for those with other LRIs, likely due to the small sample size.1 Other studies from Norway and Perth have shown that schoolchildren with asthma have deficits in lung function that may already be present shortly after birth.34,35 It is reasonable to surmise that both preexisting deficits in lung function and the potential damaging effects of severe acute respiratory illnesses may have separate and complementary influences on the association between early pneumonia and subsequent deficits in lung function.
Conclusions
Radiologically ascertained pneumonia before age 3 years is associated with asthma and impaired airway function that is only partially reversible with bronchodilators and that persists into adult life. Because there is considerable evidence that asthma associated with airflow limitation is a strong risk factor for subsequent chronic obstructive pulmonary disease,36 the prevention of early-life pneumonia and of the factors that determine low lung function in infancy32 may contribute significantly to decrease the public health burden of chronic obstructive pulmonary disease.
Supplementary Material
Acknowledgments
We gratefully acknowledge the contributions of Dr Lynn Taussig, who started the Tucson Children’s Respiratory Study in 1980. We also thank Mr Bruce Saul for data management; our study nurses, Marilyn Lindell, RN, Lydia de la Ossa, RN, and Nicole Pargas, RN, for data collection and participant follow-up; and Ms Shelley Radford for her expertise in pulmonary function testing.
Footnotes
Dr Chan analyzed the data under the direction of Dr Martinez, carried out the analyses, interpreted the data, drafted the initial manuscript, and revised the manuscript; Ms Stern carried out the analyses, interpreted the data, and reviewed and revised the manuscript; Dr Guerra interpreted the data and reviewed and revised the manuscript; Drs Wright and Morgan interpreted the data and reviewed and revised the manuscript; Dr Martinez conceptualized and designed the study, supervised the analyses, interpreted the data, reviewed and revised the manuscript, had full access to all of the data in the study, and had final responsibility for the decision to submit for publication; and all authors approved the final manuscript as submitted.
The funding source had no role in the study design, data collection, data analysis, data interpretation, or writing of the article.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This work was supported by National Heart, Lung, and Blood Institute grant HL056177. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Castro-Rodríguez JA, Holberg CJ, Wright AL, et al. Association of radiologically ascertained pneumonia before age 3 yr with asthmalike symptoms and pulmonary function during childhood: a prospective study. Am J Respir Crit Care Med. 1999;159(6):1891–1897 [DOI] [PubMed] [Google Scholar]
- 2.Kusel MM, Kebadze T, Johnston SL, Holt PG, Sly PD. Febrile respiratory illnesses in infancy and atopy are risk factors for persistent asthma and wheeze. Eur Respir J. 2012;39(4):876–882 [DOI] [PubMed] [Google Scholar]
- 3.Anderson HR, Bland JM, Peckham CS. Risk factors for asthma up to 16 years of age: evidence from a national cohort study. Chest. 1987;91(6 suppl):127S–130S [DOI] [PubMed] [Google Scholar]
- 4.Korppi M, Kuikka L, Reijonen T, Remes K, Juntunen-Backman K, Launiala K. Bronchial asthma and hyperreactivity after early childhood bronchiolitis or pneumonia. An 8-year follow-up study. Arch Pediatr Adolesc Med. 1994;148(10):1079–1084 [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303(6804):671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britten N, Davies JM, Colley JR. Early respiratory experience and subsequent cough and peak expiratory flow rate in 36 year old men and women. Br Med J (Clin Res Ed). 1987;294(6583):1317–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gold DR, Tager IB, Weiss ST, Tosteson TD, Speizer FE. Acute lower respiratory illness in childhood as a predictor of lung function and chronic respiratory symptoms. Am Rev Respir Dis. 1989;140(4):877–884 [DOI] [PubMed] [Google Scholar]
- 8.Johnston ID, Strachan DP, Anderson HR. Effect of pneumonia and whooping cough in childhood on adult lung function. N Engl J Med. 1998;338(9):581–587 [DOI] [PubMed] [Google Scholar]
- 9.Shaheen SO, Barker DJ, Shiell AW, Crocker FJ, Wield GA, Holgate ST. The relationship between pneumonia in early childhood and impaired lung function in late adult life. Am J Respir Crit Care Med. 1994;149(3 pt 1):616–619 [DOI] [PubMed] [Google Scholar]
- 10.Edmond K, Scott S, Korczak V, et al. Long term sequelae from childhood pneumonia; systematic review and meta-analysis. PLoS ONE. 2012;7(2):e31239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glezen P, Denny FW. Epidemiology of acute lower respiratory disease in children. N Engl J Med. 1973;288(10):498–505 [DOI] [PubMed] [Google Scholar]
- 12.Shaheen SO, Sterne JA, Tucker JS, Florey CD. Birth weight, childhood lower respiratory tract infection, and adult lung function. Thorax. 1998;53(7):549–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston ID. Effect of pneumonia in childhood on adult lung function. J Pediatr. 1999;135(2 pt 2):33–37 [PubMed] [Google Scholar]
- 14.Samet JM, Tager IB, Speizer FE. The relationship between respiratory illness in childhood and chronic air-flow obstruction in adulthood. Am Rev Respir Dis. 1983;127(4):508–523 [DOI] [PubMed] [Google Scholar]
- 15.Mok JY, Simpson H. Outcome of acute lower respiratory tract infection in infants: preliminary report of seven-year follow-up study. Br Med J (Clin Res Ed). 1982;285(6338):333–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mok JY, Waugh PR, Simpson H. Mycoplasma pneuminia infection: a follow-up study of 50 children with respiratory illness. Arch Dis Child. 1979;54(7):506–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pullan CR, Hey EN. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. Br Med J (Clin Res Ed). 1982;284(6330):1665–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taussig LM, Wright AL, Morgan WJ, Harrison HR, Ray CG. The Tucson Children’s Respiratory Study. I. Design and implementation of a prospective study of acute and chronic respiratory illness in children. Am J Epidemiol. 1989;129(6):1219–1231 [DOI] [PubMed] [Google Scholar]
- 19.Halonen M, Stern DA, Wright AL, Taussig LM, Martinez FD. Alternaria as a major allergen for asthma in children raised in a desert environment. Am J Respir Crit Care Med. 1997;155(4):1356–1361 [DOI] [PubMed] [Google Scholar]
- 20.Sherrill D, Viegi G. On modeling longitudinal pulmonary function data. Am J Respir Crit Care Med. 1996;154(6 pt 2):S217–S222 [DOI] [PubMed] [Google Scholar]
- 21.Ballinger GA. Using generalized estimating equations for longitudinal data analysis. Organ Res Methods. 2004;7(2):127–150 [Google Scholar]
- 22.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22 [Google Scholar]
- 23.Castleman WL, Sorkness RL, Lemanske RF, Grasee G, Suyemoto MM. Neonatal viral bronchiolitis and pneumonia induces bronchiolar hypoplasia and alveolar dysplasia in rats. Lab Invest. 1988;59(3):387–396 [PubMed] [Google Scholar]
- 24.Shaheen SO, Barker DJ, Holgate ST. Do lower respiratory tract infections in early childhood cause chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 1995;151(5):1649–1651; discussion 1651–1652 [DOI] [PubMed] [Google Scholar]
- 25.Thurlbeck WM. Postnatal growth and development of the lung. Am Rev Respir Dis. 1975;111(6):803–844 [DOI] [PubMed] [Google Scholar]
- 26.Gelb AF, Zamel N. Simplified diagnosis of small-airway obstruction. N Engl J Med. 1973;288(8):395–398 [DOI] [PubMed] [Google Scholar]
- 27.Hanrahan J, Silverman M, Tepper RS. Clinical epidemiology and future directions. In: Stocks J, Sly PD, Tepper RS, Morgan WJ, eds. Infant Respiratory Function Testing. 1st ed. New York, NY: Wiley-Liss; 1996:551–562 [Google Scholar]
- 28.Laraya-Cuasay LR, DeForest A, Huff D, Lischner H, Huang NN. Chronic pulmonary complications of early influenza virus infection in children. Am Rev Respir Dis. 1977;116(4):617–625 [DOI] [PubMed] [Google Scholar]
- 29.Similä S, Linna O, Lanning P, Heikkinen E, Ala-Houhala M. Chronic lung damage caused by adenovirus type 7: a ten-year follow-up study. Chest. 1981;80(2):127–131 [DOI] [PubMed] [Google Scholar]
- 30.Sly PD, Soto-Quiros ME, Landau LI, Hudson I, Newton-John H. Factors predisposing to abnormal pulmonary function after adenovirus type 7 pneumonia. Arch Dis Child. 1984;59(10):935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez FD, Morgan WJ, Wright AL, Holberg CJ, Taussig LM. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. N Engl J Med. 1988;319(17):1112–1117 [DOI] [PubMed] [Google Scholar]
- 32.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370(9589):758–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young S, O’Keeffe PT, Arnott J, Landau LI. Lung function, airway responsiveness, and respiratory symptoms before and after bronchiolitis. Arch Dis Child. 1995;72(1):16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Håland G, Carlsen KC, Sandvik L, et al. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355(16):1682–1689 [DOI] [PubMed] [Google Scholar]
- 35.Mullane D, Turner SW, Cox DW, Goldblatt J, Landau LI, le Souëf PN. Reduced infant lung function, active smoking, and wheeze in 18-year-old individuals [published correction appears in JAMA Pediatr. 2013;167(9):873]. JAMA Pediatr. 2013;167(4):368–373 [DOI] [PubMed] [Google Scholar]
- 36.Martinez FD. The origins of asthma and chronic obstructive pulmonary disease in early life. Proc Am Thorac Soc. 2009;6(3):272–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright AL, Taussig LM, Ray CG, Harrison HR, Holberg CJ. The Tucson Children’s Respiratory Study. II. Lower respiratory tract illness in the first year of life. Am J Epidemiol. 1989;129(6):1232–1246 [DOI] [PubMed] [Google Scholar]
- 38.Morgan WJ, Stern DA, Sherrill DL, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172(10):1253–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.