Abstract
Objectives:
Scolopendra subspinipes mutilans (S. subspinipes mutilans) is known as a traditional medicine and includes various amino acids, peptides and proteins. The amino acids in the pharmacopuncture extracted from S. subspinipes mutilans by using derivatization methods were analyzed quantitatively and qualitatively by using high performance liquid chromatography (HPLC) over a 12 month period to confirm its stability.
Methods:
Amino acids of pharmacopuncture extracted from S. subspinipes mutilans were derived by using O-phthaldialdehyde (OPA) & 9-fluorenyl methoxy carbonyl chloride (FMOC) reagent and were analyzed using HPLC. The amino acids were detected by using a diode array detector (DAD) and a fluorescence detector (FLD) to compare a mixed amino acid standard (STD) to the pharmacopuncture from centipedes. The stability tests on the pharmacopuncture from centipedes were done using HPLC for three conditions: a room temperature test chamber, an acceleration test chamber, and a cold test chamber.
Results:
The pharmacopuncture from centipedes was prepared by using the method of the Korean Pharmacopuncture Institute (KPI) and through quantitative analyses was shown to contain 9 amino acids of the 16 amino acids in the mixed amino acid STD. The amounts of the amino acids in the pharmacopuncture from centipedes were 34.37 ppm of aspartate, 123.72 ppm of arginine, 170.63 ppm of alanine, 59.55 ppm of leucine and 57 ppm of lysine. The relative standard deviation (RSD %) results for the pharmacopuncture from centipedes had a maximum value of 14.95% and minimum value of 1.795% on the room temperature test chamber, the acceleration test chamber and the cold test chamber stability tests.
Conclusion:
Stability tests on and quantitative and qualitative analyses of the amino acids in the pharmacopuncture extracted from centipedes by using derivatization methods were performed by using HPLC. Through research, we hope to determine the relationship between time and the concentrations of the amino acids in the pharmacopuncture extracted from centipedes.
Keywords: amino acids, derivatization methods, high performance liquid chromatography (HPLC), pharmacopuncture, Scolopendra subspinipes mutilans
1. Introduction
Centipedes (Scolopendra subspinipes mutilans L. Koch) belong to the chilopod class that contains 3,500 species of centipedes [1]. Several centipede species are predators that inflict severe diseases in humans in the case of biting them, including hemoglobinuria and hematuria, hemorrhage, rhabdomyolysis, myocardial ischemia and infarction [2-4]. However, venom plays a key role in medical treatment. The composition and the structure of centipede venom, including serotonin, histamine, lipids, polysaccharides and polypeptides, have been identified in crude extracts of centipede venom in previous studies [5, 6]. The venoms also play significant biologic roles in the synthesis of proteins, peptides and enzymes. In a recent study, centipede venoms that contained about 500 proteins and peptides were involved in platelet aggregating activity, phospholipaseA2 activity, anticoagulant activity, trypsin inhibiting activity, voltage gated potassium channel activities, voltage gated sodium channel activities, and voltage gated calcium channel activities [6]. Another effective component in Scolopendra subspinipes mutilans (S. subspinipes mutilans) is phospholipase. The molecular weight of phospholipase in centipede venom was determined by using snake venom such as that of Bothrops neuwiedii, which has a known molecular weight of 13,364 Da [7].
In this study, pharmacopuncture extracted from the body part of centipedes was studied to determine how effective it was. We separated the head from the body of the centipede, and we performed stability tests and quantitative and qualitative analyses on the body parts, which are used as centipede pharmacopuncture in Korea.
2. Materials and Methods
Powders extracted from the bodies of centipedes were decocted in water for injection (WFI) at 50°C (250 g/1.5 L). This solution was extracted using 80% and 70% alcohol. Crude extracts were passed through a filter (0.1 ㎛, Sartorius, USA). To obtain freeze dried powders, we dried the crude extracts by using a lyophilizer. Freeze dried powders of centipedes and WFI were mixed in a tank (Fine FA, Korea) while adding 0.9% sodium chloride and adjusting to pH 7.0. After filtration (0.2 ㎛, Sartorius, USA), the end products as pharmacopuncture were stored at 4°C.
Analyses of the amino acids, which are component of amphoteric electrolyte composed of amine (-NH2) and carboxylic acid (-COOH) functional groups were done by using a diode array detector (DAD) and a fluorescence detector (FLD) [8]. For the analyses of the amino acids, derivatization of amino acids was performed using O-phthaldialdehyde (OPA) and 9-fluorenyl methoxy carbonyl chloride (FMOC) assay reagents (Agilent, USA). The OPA and the FMOC assay reagents were used for a comparison of the mixed amino acid standard (STD, Sigma-Aldrich) with the pharmacopuncture of centipedes prepared by using the method of the Korean Pharmacopuncture Institute (KPI).
The quantitative and qualitative analyses of the amino acid and the pharmacopuncture of centipedes were performed using high performance liquid chromatography (HPLC) with an Agilent 1200 series model on a ZORBAX Eclipse AAA system (4.6 mm × 150 mm, 5 μm, Agilent). The mobile phase composition was the A solvent: 40 mM sodium phosphate (di-basic) and 0.1% phosphoric acid pH 7.8 in water; the B solvent contained acetonitrile : methanol : DW = 45 : 45 : 10 (v/v). The HPLC analysis was done by using the gradient condition method (Table 1). It was used to sample a 1000 ppm mixed amino acid standard (Sigma, USA) that included 16 amino acid and the pharmacopuncture of centipedes (KPI, Korea) obtainable in markets in Korea. Fifty μL volumes of the amino acid standard and the pharmacopuncture sample were injected into the HPLC column. The flow rate was 2 mL/minutes. The procedures during the HPLC analysis were performed at 40°C. The DAD was set to 338 nm and 262 nm to detect primary and secondary amino acids. The FLD was set to Exposure: 340 nm, Emission: 450 nm and Ex: 266 nm, EM: 305 nm. For the derivatization reaction of the amino acids and the pharmacopuncture of centipedes, all of samples were analyzed by using the functions of the injector program (Table 2).
Table. 1. Gradient condition methods for the analysis of amino acids.
| Time (min) | B (%)* |
|---|---|
| 0.0 | 0 |
| 1.9 | 0 |
| 18.1 | 57 |
| 18.6 | 100 |
| 22.3 | 100 |
| 23.2 | 0 |
| 26.0 | 0 |
*A solvent: 40-mM sodium phosphate (Di-basic), 0.1% phosphoric acid pH 7.8 in water. B solvent: acetonitrile : methanol : DW = 45 : 45 : 10 (v/v).
Table. 2. Program methods of the HPLC injector.
| Function | Volume/time (μL/min) |
Position |
|---|---|---|
| Draw | 2.5 | Borate vial 1 |
| Draw | 0.5 | Sample vial |
| Mix | 3.0 | Washport 5 × |
| Wait | 0.2 | ─ |
| Draw | 0.5 | OPA vial 2 |
| Mix | 3.5 | Washport 6 × |
| Draw | 0.04 | FMOC vial 3 |
| Mix | 3.9 | Washport 10 × |
| Draw | 32 | Diluent vial 4 |
| Mix | 20 | Seat 8 × |
| Inject | ─ | ─ |
| Wait | 0.1 | ─ |
| Valve bypass | ─ | ─ |
*HPLC, high-performance liquid chromatography; OPA, O-phthaldialdehyde; FMOC, fluorenyl methoxy carbonyl chloride.
To confirm the stability of the pharmacopuncture of centipedes, we planned the fixation period to include 6 points: 1 month, 2 months, 3 months, 6 months, 9 months and 12 months. Three storage methods were used: storage in a room temperature test chamber at 25°C and 60% humidity, storage in an acceleration test chamber at 40°C and 80% humidity, and storage in a cold test chamber at 4°C (Sejong, Korea). Three vials that had been stored in each condition were analyzed by using HPLC on a C18 column (UG80 5 μm, 4.6 mm × 250 mm, Shiseido, Japan) during the period of the stability test. The mobile phase involved the A solvent, 0.1% formic acid in water (J. T. Baker, USA) and the B solvent, 0.1% formic acid in acetonitrile (J. T. Baker, USA). A volume of 30 μL was introduced into the column at a rate of 1 mL/minutes. The detector was set for a wavelength of 255 nm, and the column’s temperature was 35°C. The stability analysis was performed by using the gradient condition method during HPLC (Agilent, USA) (Table 3).
Table. 3. Gradient condition methods for the stability test.
| Time (min) | B (%)* |
|---|---|
| 10 | 1 |
| 40 | 90 |
| 50 | 90 |
*A solvent, 0.1% formic acid in water; B solvent, 0.1% formic acid in acetonitrile
3. Results
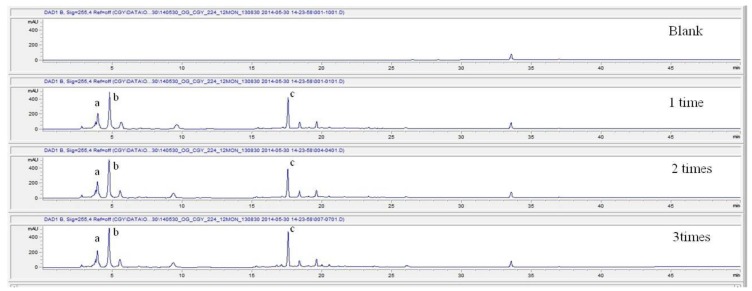
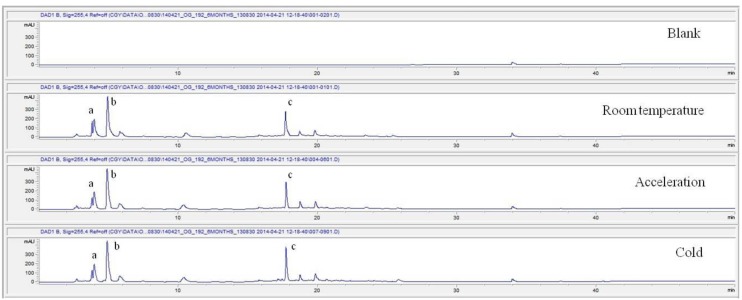
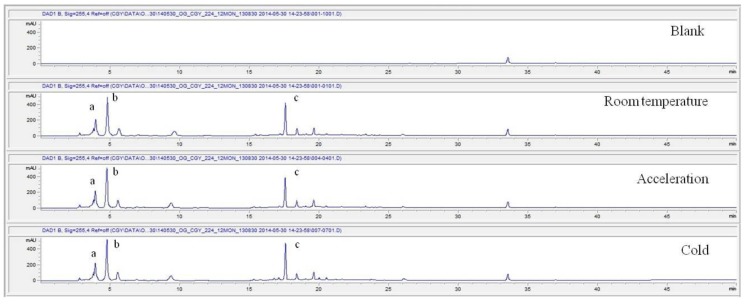
For the stability test, the pharmacopuncture extracted from the bodies of centipedes, which had been stored at 4°C, was analyzed by using HPLC. The pharmacopuncture of centipedes was designed through comparisons between the initial pharmacopuncture and the pharmacopuncture of centipedes fixed for periods of 1 month, 2 months, 3 months, 6 months, 9 months and 12 months. Initial data and fixation period data for the pharmacopuncture from the bodies of centipedes were confirmed from the HPLC chromatograms by using chemstation (Fig. 1). The retention time averages of the pharmacopuncture, which were the three biggest peaks of the chromatograms (130830), were 04.13, 05.12 and 18.08 minutes. The averages of the areas were 1761, 4278 and 2687. The area between a positive valued curve and the horizontal axis, measured between two values a and b (b is defined as the larger of the two values) on the horizontal axis, is given by the integral from a to b of the function that represents the curve. It is also called integral calculus and is written:
Fig. 1a. Initial chromatograms of the pharmacopuncture from centipedes manufactured on August 30, 2013 by using 3 repeated tests.
Fig. 1b. Chromatograms of the pharmacopuncture from centipedes after 1 month.
Fig. 1c. Chromatograms of the pharmacopuncture from centipedes after 2 months.
Fig. 1d. Chromatograms of the pharmacopuncture from centipedes after 3 months.
Fig. 1e. Chromatograms of the pharmacopuncture from centipedes after 6 months.
Fig. 1f. Chromatograms of the pharmacopuncture from centipedes after 9 months.
Fig. 1g. Chromatograms of the pharmacopuncture from centipedes after 12 months.
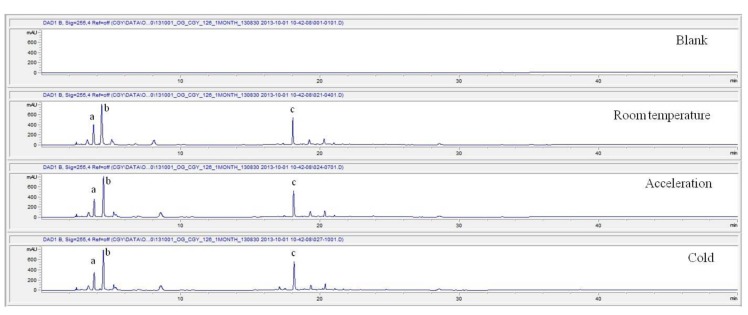
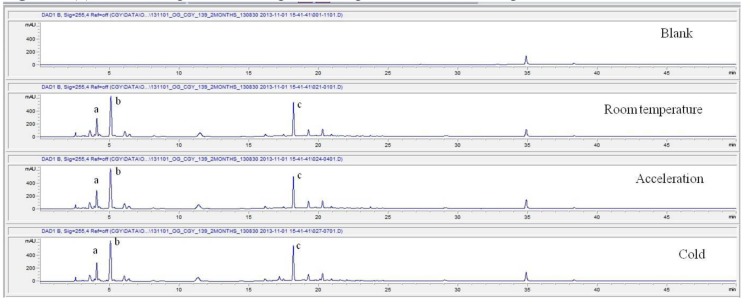
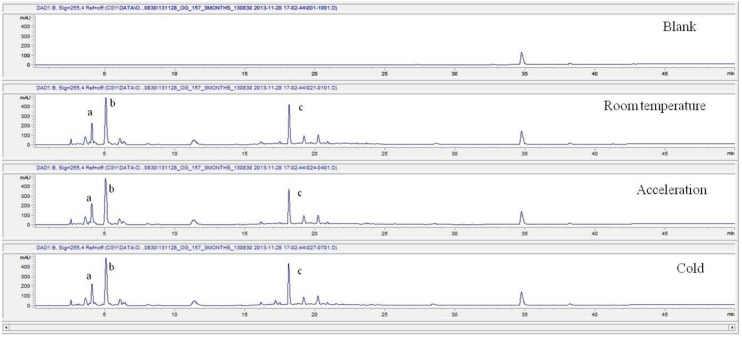
These values were determined by comparisons of the initial data and the data for the pharmacopunctures stored in the room temperature test chamber, in the acceleration test chamber, and in the cold test chamber for 12 months (Tables 4,5). The overall statistical data for the three retention times determined from the three peaks for the pharmacopuncture of centipedes are shown in (Table 6). The relative standard deviation (RSD %) for the pharmacopuncture of centipedes had a maximum of 14.95% and a minimum of 1.795% (Table 6).
Table. 4. Retention times (in minutes) and area values (in integral calculus) for the initial pharmacopuncture from centipedes prepared by using the method of the KPI on August 30, 2013.
| Repetitions | 1 Time | 2 Times | 3 Times | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak Points | A | B | C | A | B | C | A | B | C |
| Retentiontime | 4.09 | 5.03 | 17.99 | 4.16 | 5.18 | 18.12 | 4.15 | 5.17 | 18.13 |
| Area | 1661 | 4260 | 2739 | 1706 | 4288 | 2660 | 1738 | 4286 | 2663 |
KPI, Korean pharmacopuncture institute. A, about a 4-min retention time; B, about a 5-min retention time; C, about a 19-min retention time.
Table. 5. Retention times (in minutes) and area values (in integral calculus) for the initial pharmacopuncture from centipedes prepared by using the method of the KPI on August 30, 2013.
| Period | Room temperature chamber | The worst stress chamber | Cold chamber | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peak points | A | B | C | A | B | C | A | B | C | |
| 1 month | Retention time | 4.04 | 4.91 | 18.24 | 4.06 | 5.05 | 18.49 | 4.06 | 5.14 | 18.64 |
| Area | 2078 | 4708 | 3149 | 1718 | 4618 | 3005 | 1707 | 4870 | 3190 | |
| 2 months | Retention time | 4.05 | 5.05 | 18.18 | 4.04 | 4.03 | 18.17 | 4.04 | 4.02 | 18.16 |
| Area | 1386 | 4301 | 2608 | 1485 | 4382 | 2427 | 1486 | 4336 | 2728 | |
| 3 months | Retention time | 4.04 | 5.03 | 18.14 | 4.03 | 5.02 | 18.12 | 4.04 | 5.04 | 18.12 |
| Area | 1489 | 4387 | 2559 | 1480 | 4509 | 2279 | 1471 | 4284 | 2724 | |
| 6 months | Retention time | 3.79 | 4.91 | 17.68 | 3.95 | 4.89 | 17.73 | 3.95 | 4.89 | 17.73 |
| Area | 1797 | 4594 | 2413 | 1721 | 4571 | 2104 | 1741 | 4646 | 2752 | |
| 9 months | Retention time | 3.93 | 4.77 | 17.56 | 3.9 | 4.73 | 17.54 | 3.90 | 4.73 | 17.56 |
| Area | 1713 | 4630 | 2437 | 1665 | 4563 | 2212 | 1728 | 4656 | 2693 | |
| 12 months | Retention time | 4.02 | 4.73 | 18.24 | 4.06 | 4.86 | 18.28 | 4.07 | 4.96 | 18.45 |
| Area | 1908 | 4528 | 2715 | 1920 | 4518 | 2384 | 1844 | 4768 | 2912 | |
A, about a 4-min retention time; B, about a 5-min retention time; C, about a 19-min retention time.
Table. 6. Overall statistical data for the values of the area value for 3 conditions over 12 months.
| Retentiontime | Room temperature chamber | The worst stress chamber | Cold chamber | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | A | B | C | |
| 1 month | 2078 | 4708 | 3149 | 1718 | 4618 | 3005 | 1707 | 4870 | 3190 |
| 2 months | 1386 | 4301 | 2608 | 1485 | 4382 | 2427 | 1486 | 4336 | 2728 |
| 3 months | 1489 | 4387 | 2559 | 1480 | 4509 | 2279 | 1471 | 4284 | 2724 |
| 6 months | 1797 | 4594 | 2413 | 1721 | 4571 | 2104 | 1741 | 4646 | 2752 |
| 9 months | 1713 | 4630 | 2437 | 1665 | 4563 | 2212 | 1728 | 4656 | 2693 |
| 12 months | 1908 | 4528 | 2715 | 1920 | 4518 | 2384 | 1844 | 4768 | 2912 |
| Statistical data for pharmacopuncture from centipedes during the stability test | |||||||||
| Initial Average area | 1701 | 4278 | 2687 | 1701 | 4278 | 2687 | 1701 | 4278 | 2687 |
| Average area | 1728.50 | 4524.67 | 2646.83 | 1664.83 | 4526.83 | 2401.83 | 1662.83 | 4593.33 | 2833.17 |
| S.D. area | 258.45 | 153.95 | 270.09 | 165.89 | 81.17 | 317.68 | 150.47 | 234.80 | 191.18 |
| RSD (%) area | 14.95 | 3.40 | 10.20 | 9.96 | 1.79 | 13.23 | 9.05 | 5.11 | 6.75 |
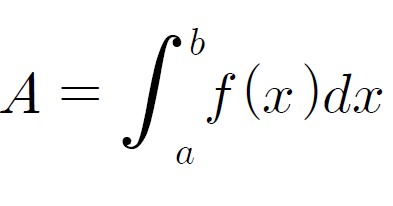 |
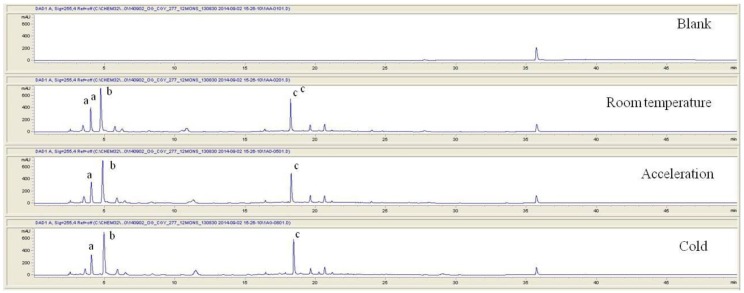
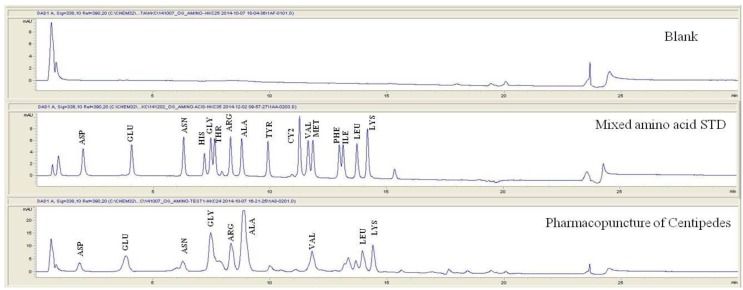
For the qualitative amino acid test, the pharmacopuncture was prepared by using the method of KPI, and a 1000 ppm mixed amino acid STD (1 mg/mL, water) was prepared. After that, the pharmacopuncture and the 1000 ppm mixed amino acid STD obtained by using the derivatization methods (Table 2) were analyzed in accordance with the injector program method of HPLC with the DAD set to 338 nm. When comparing chromatographs between the pharmacopuncture and the 1000 ppm mixed amino acid STD, aspartate (Asp), glutamate (Glu), asparagines (Asn), glycine (Gly), arginine (Arg), alanine (Ala), valine (Val), leucine (Leu), and lysine (Lys) for the same retention time as that of the mixed amino acid STD were found to be contained in the pharmacopuncture of centipedes (Fig. 2, Table 7).
Fig. 2. Chromatograms of mixed amino acid STD and pharmacopuncture extracted from centipedes by using derivatization methods.
STD, standard; ASP, aspartate; GLU, glutamate; ASN, asparagine; HIS, histidine; GLY, glycine; THR, threonine; ARG, arginine; ALA, alanine; TYR, tyrosine; CY2, cystine; VAL, valine; MET, methionine; PHE, phenylalanine; ILE, isoleucine; LEU, leucine; LYS, lysine.
Table. 7. Retention time of mixed amino acid STD and pharmacopuncture extracted from centipedes by using derivatization methods.
| Mixed amino acid STD | Pharmacopuncture from centipedes | ||
|---|---|---|---|
| Retention time | Amino acid name | Retention time | Amino acid name |
| 1.97 | ASP | 1.86 | ASP |
| 4.03 | GLU | 3.84 | GLU |
| 6.34 | ASN | 6.27 | ASN |
| 7.20 | HIS | ─ | ─ |
| 7.52 | GLY | 7.47 | GLY |
| 7.70 | THR | ─ | ─ |
| 8.26 | ARG | 8.33 | ARG |
| 8.85 | ALA | 8.85 | ALA |
| 9.97 | TYR | ─ | ─ |
| 11.32 | CY2 | ─ | ─ |
| 11.78 | VAL | 11.79 | VAL |
| 11.97 | MET | ─ | ─ |
| 13.11 | PHE | ─ | ─ |
| 13.30 | ILE | ─ | ─ |
| 13.89 | LEU | 13.95 | LEU |
| 14.28 | LYS | 14.39 | LYS |
STD, standard; ASP, aspartate; GLU, glutamate; ASN, asparagine; HIS, histidine; GLY, glycine; THR, threonine; ARG, arginine; ALA, alanine; TYR, tyrosine; CY2, cystine; VAL, valine; MET, methionine; PHE, phenylalanine; ILE, isoleucine; LEU, leucine; LYS, lysine.
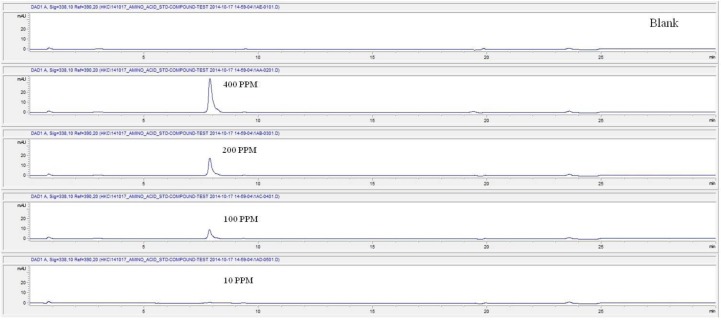
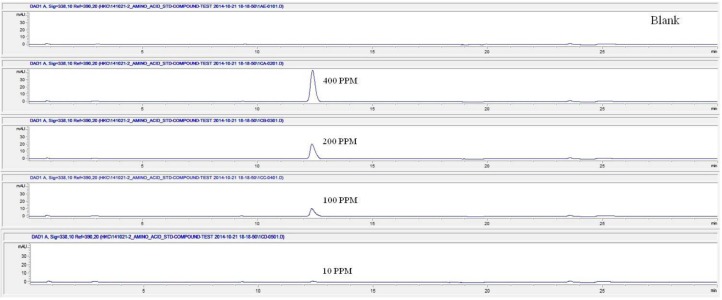
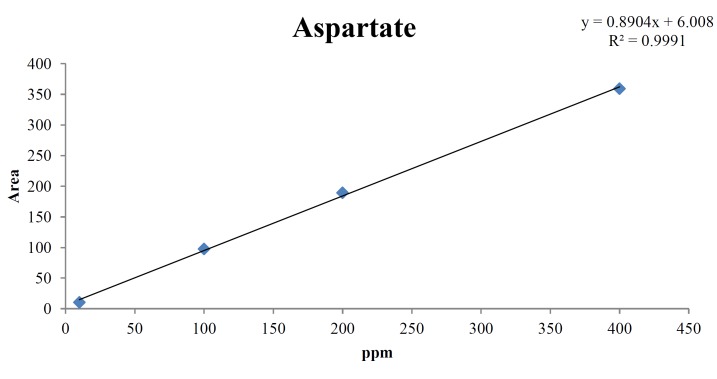
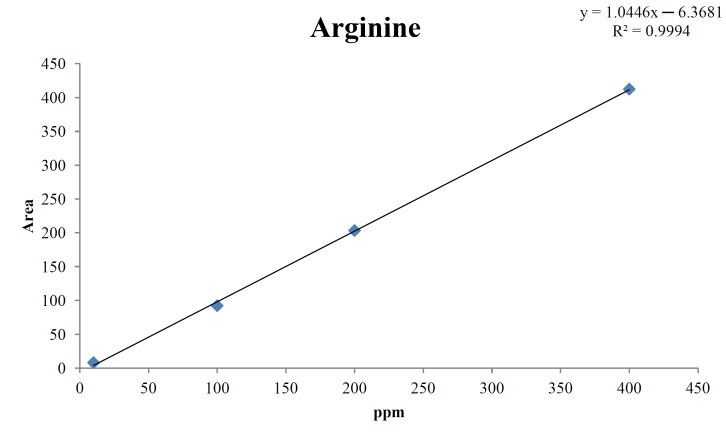
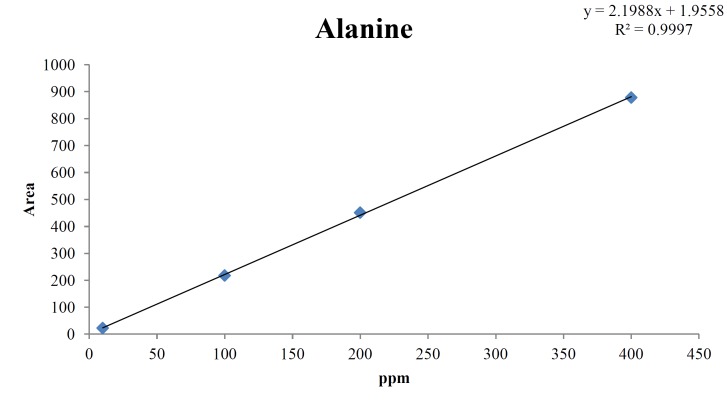
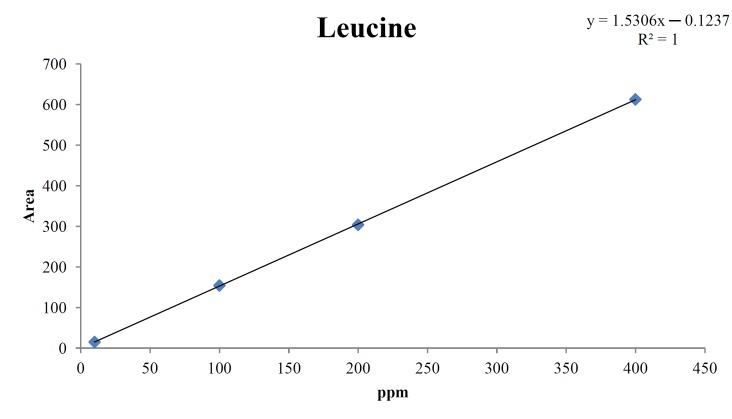
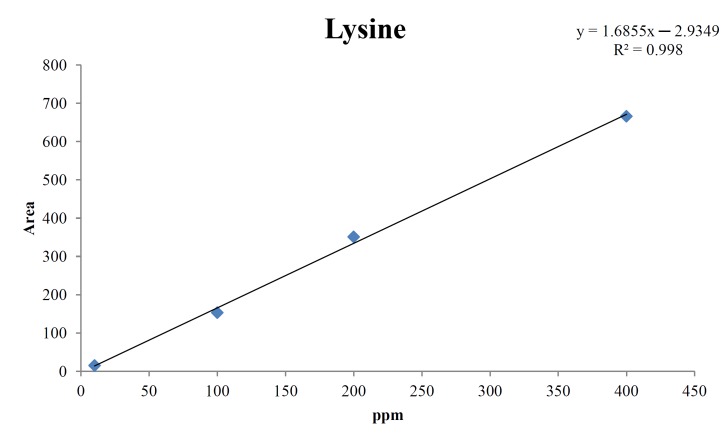
For the quantitative test of the amino acids, Asp, Arg, Ala, Leu and Lys, whose presence had been confirmed by using the chromatogram of the 1000 ppm mixed amino acid STD, were diluted with water to 400 ppm, 200 ppm, 100 ppm, and 10 ppm. Then, HPLC was performed by using the above method (Fig. 3). The concentrations of Asp, Arg, Ala, Leu and Lys, which had been determined by using the qualitative test on the amino acid STD, were plotted on an analytical calibration curve in order to confirm the results from the quantitative test that had been performed to determine the concentrations of the amino acids in the pharmacopuncture. As a result, the concentrations of Asp, Arg, Ala, Leu and Lys contained in the pharmacopuncture of centipedes were 34.37, 123.72, 170.63, 59.55 and 57 ppm, respectively (Fig. 4, Table 8).
Fig. 3a. Chromatograms of aspartate STD for concentrations of 400, 200, 100, and 10 ppm.
Fig. 3b. Chromatograms of arginine STD for concentrations of 400, 200, 100, and 10 ppm.
Fig. 3c. Chromatograms of alanine STD for concentrations of 400, 200, 100, and 10 ppm.
Fig. 3d. Chromatograms of aspartate STD for concentrations of 400, 200, 100, and 10 ppm.
Fig. 3e. Chromatograms of lysine STD for concentrations of 400, 200, 100, and 10 ppm.
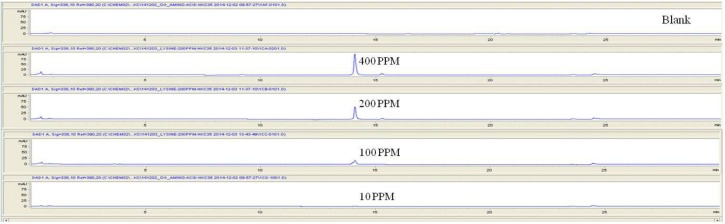
Fig. 4a. Analytical calibration curve of aspartate STD for concentrations of 400, 200, 100, and 10 ppm.
Fig. 4b. Analytical calibration curve of arginine STD for concentrations of 400, 200, 100, and 10 ppm.
Fig. 4c. Analytical calibration curve of alanine STD for concentrations of 400, 200, 100, and 10 ppm.
Fig. 4d. Analytical calibration curve of leucine STD for concentrations of 400, 200, 100, and 10 ppm.
Fig. 4e. Analytical calibration curve of lysine STD for concentrations of 400, 200, 100, and 10 ppm.
STD, standard.
Table. 8a. Quantity of aspartate in the pharmacopuncture from centipedes.
| Aspartate STD | |
|---|---|
| ppm | Area |
| 400 | 359.1 |
| 200 | 189.2 |
| 100 | 97.5 |
| 10 | 10.4 |
| Quantity of pharmacopuncture from centipedes in 1.86 min | |
| 34.37 ppm | 27.62 |
Table. 8b. Quantity of arginine in the pharmacopuncture of centipedes.
| Arginine STD | |
|---|---|
| ppm | Area |
| 400 | 412.4 |
| 200 | 203.4 |
| 100 | 92.3 |
| 10 | 8.1 |
| Quantity of pharmacopuncture from centipedes in 8.33 min | |
| 123.72 ppm | 129.81 |
Table. 8c. Quantity of alanine in pharmacopuncture from centipedes.
| Arginine STD | |
|---|---|
| ppm | Area |
| 400 | 878 |
| 200 | 450.9 |
| 100 | 217.6 |
| 10 | 22.5 |
| Quantity of pharmacopuncture from centipedes in 8.55 min | |
| 170.63 ppm | 169.74 |
Table. 8d. Quantity of leucine in pharmacopuncture from centipedes.
| Leucine STD | |
|---|---|
| ppm | Area |
| 400 | 612.8 |
| 200 | 303.9 |
| 100 | 154.3 |
| 10 | 15.2 |
| Quantity of pharmacopuncture from centipedes in 13.95 min | |
| 59.55 ppm | 59.63 |
Table. 8e. Quantity of lysine in pharmacopuncture from centipedes.
| Lysine STD | |
|---|---|
| ppm | Area |
| 400 | 666 |
| 200 | 351 |
| 100 | 153 |
| 10 | 15 |
| Quantity of pharmacopuncture from centipedes in 14.39 min | |
| 57 ppm | 58.74 |
STD, standard.
4. Discussion
The centipede is known to contain substances including 5-hydroxytryptamine, histamine, lipids, and polysaccharides, as well as various enzymes and effective amino acids [5, 9]. Traditional medicines that contain the effective substances of the centipede have been used in China for several hundred years to treat thrombotic diseases [10]. S. subspinipes mutilans extracted in the ethanol has also been studied for its marked cytotoxic activity to prevent human cancer [11]. Thus, the pharmacological and the biochemical characteristics of the substances contained in centipedes have been studied [12, 13], but analyses of those substances have not been performed because of the lack of sufficient quantities of the venom and the unstable nature of centipede parts and components. Thus, the composition and the structure of centipede venom remain unknown. However, previous studies have found serotonin, histamine, lipids, and polysaccharides in that venom [5]. Also, polypeptides have been reported to be effective components in crude extracts from centipede venom glands [6]. Known to be one of the polypeptides is Factor Xa (FXa) inhibiting peptide, which is a serine endopeptidase that plays a significant role in blood coagulation [14]. A novel FXa inhibitor, a natural peptide containing the sequence Thr-Asn-Gly-Tyr-Thr (TNGYT), was isolated from the venom of S. subspinipes mutilans by using a combination of size exclusion and reverse phase chromatography [15]. Another attractive target has been the development of an anti-thrombotic drug with a primary peptide structure Ser-Gln-Leu (SQL) from S. subspinipes mutilans [16].
In this study, pharmacopuncture extracted from the body portion of centipedes was identified by using reverse phase chromatography. We made an effort to determine quantitatively and qualitatively, through comparisons with 16 standard amino acids, the concentrations of the amino acids found in the pharmacopuncture of centipedes prepared by using the method of KPI. Qualitatively, 9 of the 16 standard amino acids were identified in the pharmacopuncture, but the concentrations of only 5 of those 9 amino acids, Asp, Arg, Ala, Leu and Lys, were determined quantitatively because of limitations on the analyses. However, this study has importance. Although the essential amino acids are synthesized in the human body for biological activity, they are not easily synthesized and, therefore, exist in the human body in limited quantities; thus, their natural concentrations in the human body must be increased through the intake of food. The quantitative and qualitative analysis of the amino acids in the pharmacopuncture of centipedes showed the presence of the essential amino acids Val, Leu and Lys, which are involved in collagen formation, tissue damage repair and muscle lose suppression. Thus, when the pharmacopuncture of centipedes is injected into the blood vessels in certain parts of the human body, the effective absorption rate of these essential amino acids is expected to be larger than it would be if they were being taken in through the consumption of food [17].
So far, the pharmacopuncture of centipedes has been quantitatively and qualitatively shown to contain several essential amino acids, and we plan to continue research aimed at determining if serotonin, histamine, lipids, polysaccharides, polypeptides, and other unknown components are present in the pharmacopuncture of centipedes by using HPLC-Q time of flight (TOF, Agilent, USA). We also studied the stability of the pharmacopuncture of centipedes through testing conducted over a 12 month period. The results showed that compared to the initial values of the 3 major peaks in the pharmacopuncture of centipedes, the area values those 3 major peaks, corresponding to retention times of about 4 minutes, 5 minutes and 19 minutes, respectively, in the room temperature test chamber, the acceleration test chamber, and the cold test chamber did not vary, within experimental error, during the 12 month stability test. In the future, the variations in those values for the pharmacopuncture of centipedes will be confirmed under environmental conditions such as direct sunlight and for longer periods of 24 and 36 months. Henceforward, through stability and expiration date testing, we will develop a pharmacopuncture of centipedes that will be more effective and be capable of long-term storage.
5. Conclusion
Pharmacopuncture extracted from the bodies of centipedes was matched with 9 amino acid through a comparative analysis of the pharmacopuncture of centipedes and a mixed amino acid STD. Five amino acids were found in the pharmacopuncture of centipedes: 34.37 ppm of Asp, 123.72 ppm of Arg, 170.63 ppm of Ala, 59.55 ppm of Leu, and 57 ppm of Lys. In a 12 month stability test, the RSD % of the 3 major peaks, corresponding to retention times of about 4 minutes, 5 minutes and 19 minutes, respectively, had a maximum values of 14.95% and a minimum value of 1.795% in the room temperature test chamber, the acceleration test chamber, and the cold test chamber. In this study, the amino acids present in the pharmacopuncture of centipedes were identified quantitatively and qualitatively, and tests conducted on all of the components found in that pharmacopuncture showed those components to be stable under three storage conditions.
Footnotes
Conflicts of interest. The authors declare that there are no conflict of interest.
References
- 1.Negrea S, Minelli A. Chilopoda. In: Decu V, Juberthie C (eds) Encyclopedia bioespeologica. France: Moulis; 1995. p. 1995:249–254. [Google Scholar]
- 2.Gomes A, Datta A, Sarangi B, Kar PK, Lahiri SC. Pharmacodynamics of venom of the centipede Scolopendra subspinipes dehaani brandt. Indian J Exp Biol. 1982;20(8):615–618. [PubMed] [Google Scholar]
- 3.Acosta MD, Cazorla D. Centipede (Scolopendra sp.) envenomation in a rural village of semi-arid region from falcon state, venezuela. Rev Investi Clin. 2004;56(6):712–717. [PubMed] [Google Scholar]
- 4.Medeiros CR, Susaki TT, Knysak I, Cardoso JL, Malaque CM, Fan HW et al. Epidemiologic and clinical survey of victims of centipede stings admitted to hospital vital brazil (sao paulo, brazil) Toxicon. 2008;52(5):606–610. doi: 10.1016/j.toxicon.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Gomes A, Datta A, Sarangi B, Kar PK, Lahiri SC. Isolation, purification and pharmacodynamics of a toxin from the venom of the centipede Scolopendra subspinipes dehaani brandt. Indian J Exp Biol. 1983;21(4):203–207. [PubMed] [Google Scholar]
- 6.Liu ZC, Zhang R, Zhao F, Chen ZM, Liu HW, Wang YJ et al. Venomic and transcriptomic analysis of centipede Scolopendra subspinipes dehaani. J Proteome Res. 2012;11(12):6197–6212. doi: 10.1021/pr300881d. [DOI] [PubMed] [Google Scholar]
- 7.Vidal JC, Cattaneo P, Stoppani AOM. Some characteristic properties of phospholipases A2 from bothrops neuwiedii venom. Arch Biochem Biophys. 1972;151(1):168–179. doi: 10.1016/0003-9861(72)90485-7. [DOI] [PubMed] [Google Scholar]
- 8.Michal G. Biochemical pathways: an atlas of biochemistry and molecular biology. Wiley; NewYork: 1999. p. 5. [Google Scholar]
- 9.Knysak I, Martins R, Bertim CR. Epidemiological aspects of centipede bites registered in greater S. Paulo,SP, Brazil. Rev Saude Publica. 1998;32(6):514–518. doi: 10.1590/s0034-89101998000600003. [DOI] [PubMed] [Google Scholar]
- 10.Pemberton RW. Insects and other arthropods used as drugs in Korean traditional medicine. J Ethnopharmacol. 1999;65(3):207–216. doi: 10.1016/S0378-8741(98)00209-8. [DOI] [PubMed] [Google Scholar]
- 11.Moon SS, Cho NS, Shin JH, Seo YW, Lee CO, Choi SU. Jineol, a cytotoxic alkaloid from the centipede Scolopendra subspinipes. J Nat Prod. 1996;59(8):777–779. [Google Scholar]
- 12.Mohamed AH, Abu-Sinna G, El-Shabaka HA, El-Aal A. Proteins, lipids, lipoproteins and some enzyme characterizations of the venom extract from the centipede Scolopendra moristans. Toxicon. 1983;21(3):371–377. doi: 10.1016/0041-0101(83)90093-4. [DOI] [PubMed] [Google Scholar]
- 13.Stankiewicz M, Hamon A, Benkhalifa R, Kadziela W, Hue B, Lucas S et al. Effects of a centipede venom fraction on insect nervous system, a native xenopus oocyte receptor and on an expressed drosophila muscarinic receptor. Toxicon. 1999;37(10):1431–1445. doi: 10.1016/S0041-0101(99)00089-6. [DOI] [PubMed] [Google Scholar]
- 14.Simkhada JR, Cho SS, Mander P, Choi YH, Yoo JC. Purification, biochemical properties and antithrombotic effect of a novel streptomyces enzyme on carrageenan- induced mice tail thrombosis model. Thromb Res. 2012;129(2):176–182. doi: 10.1016/j.thromres.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Yi Kong, Yu Shao, Hao Chen, Xin Ming, Jin-Bin Wang, Zhi-Yu Li et al. A novel factor xa-inhibiting peptide from centipedes venom. Int J Pept Res Ther. 2013;19:303–311. doi: 10.1007/s10989-013-9353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong Y, Huang SL, Shao Y, Li S, Wei JF. Purification and characterization of a novel antithrombotic peptide from Scolopendra subspinipes mutilans. J Ethnopharmacol. 2013;145:182–186. doi: 10.1016/j.jep.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 17.Fürst P, Stehle P. What are the essential elements needed for the determination of amino acid requirements in humans? J Nutr. 2004;134(S6):1558–1565. doi: 10.1093/jn/134.6.1558S. [DOI] [PubMed] [Google Scholar]