Abstract
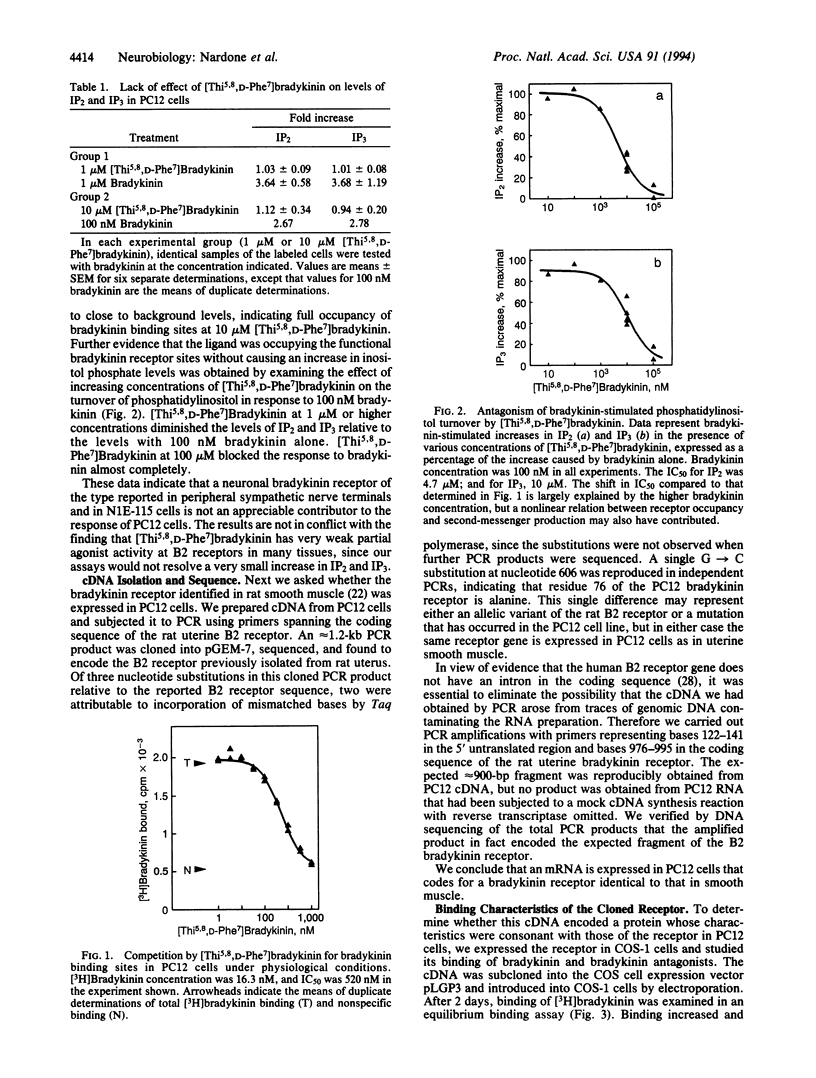
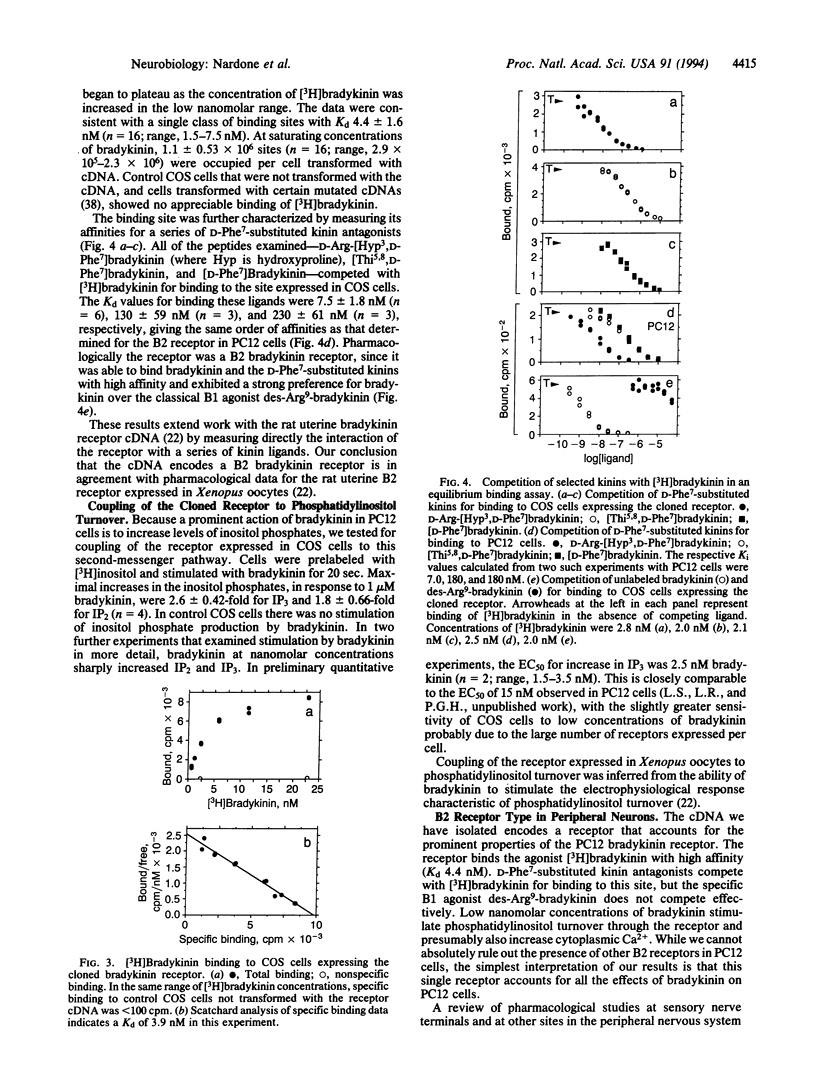
We have used rat PC12 pheochromocytoma cells, a clonal cell line closely related to sympathetic neurons, to investigate reports that the bradykinin receptor expressed in the peripheral nervous system is distinct from the well-characterized B2 bradykinin receptor of smooth muscle. Although there have been reports that [Thi5,8,D-Phe7]bradykinin [where Thi is beta-(2-thienyl)alanine] is a full agonist at some sites in the peripheral nervous system, we find that in PC12 cells [Thi5,8,D-Phe7]bradykinin behaves as a competitive antagonist of bradykinin-stimulated phosphatidylinositol turnover. In particular, sufficient concentrations of [Thi5,8,D-Phe7]bradykinin completely block the increase in inositol bisphosphate and trisphosphate in response to 100 nM bradykinin; [Thi5,8,D-Phe7]bradykinin alone, at up to 10 microM, does not appreciably increase inositol bisphosphate and trisphosphate. In contrast to the absence of evidence for a distinctive neuronal receptor, we have found convincing evidence that the bradykinin receptor previously identified in smooth muscle is present in PC12 cells. Using the polymerase chain reaction, we have isolated a full-length cDNA encoding a bradykinin receptor that is expressed in PC12 cells and verified that its nucleotide sequence is identical except at a single position to that of the rat uterine B2 bradykinin receptor. When expressed in COS cells this uterine bradykinin receptor exhibits the same high affinity for [3H]bradykinin (Kd 4.4 nM), the same relative affinities for a series of kinin antagonists, and the same efficient coupling to phosphatidylinositol turnover (EC50 2.5 nM) as the receptor in PC12 cells. We interpret our data, and the findings of a number of pharmacological studies, as strengthening the view that the B2 receptor expressed in PC12 cells and in certain cells of the peripheral nervous system is identical to the receptor in rat uterine smooth muscle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG D., DRY R. M. L., KEELE C. A., MARKHAM J. W. Observations on chemical excitants of cutaneous pain in man. J Physiol. 1953 May 28;120(3):326–351. doi: 10.1113/jphysiol.1953.sp004898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvier P. T., Chahl L. A., Ladd R. J. Modification by capsaicin and compound 48/80 of dye leakage induced by irritants in the rat. Br J Pharmacol. 1977 Jan;59(1):61–68. doi: 10.1111/j.1476-5381.1977.tb06977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccaglini P. I., Hogan P. G. Some rat sensory neurons in culture express characteristics of differentiated pain sensory cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):594–598. doi: 10.1073/pnas.80.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathon J. M., Proud D. Bradykinin antagonists. Annu Rev Pharmacol Toxicol. 1991;31:129–162. doi: 10.1146/annurev.pa.31.040191.001021. [DOI] [PubMed] [Google Scholar]
- Beck P. W., Handwerker H. O. Bradykinin and serotonin effects on various types of cutaneous nerve fibers. Pflugers Arch. 1974 Mar 11;347(3):209–222. doi: 10.1007/BF00592598. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braas K. M., Manning D. C., Perry D. C., Snyder S. H. Bradykinin analogues: differential agonist and antagonist activities suggesting multiple receptors. Br J Pharmacol. 1988 May;94(1):3–5. doi: 10.1111/j.1476-5381.1988.tb11492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn S. J., Marley P. D., Livett B. G. Receptor stimulated formation of inositol phosphates in cultures of bovine adrenal medullary cells: the effects of bradykinin, bombesin and neurotensin. Neuropeptides. 1990 Apr;15(4):187–194. doi: 10.1016/0143-4179(90)90012-n. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Mullaney I., McNeill M., Dunn P. M., Rang H. P. Second messengers involved in the mechanism of action of bradykinin in sensory neurons in culture. J Neurosci. 1989 Sep;9(9):3314–3325. doi: 10.1523/JNEUROSCI.09-09-03314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J. M., Hammond B. R. The effects of sensory denervation on the responses of the rabbit eye to prostaglandin E1, bradykinin and substance P. Br J Pharmacol. 1980 Jul;69(3):495–502. doi: 10.1111/j.1476-5381.1980.tb07040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre T. J., Basbaum A. I., Levine J. D. Neural control of vascular permeability: interactions between primary afferents, mast cells, and sympathetic efferents. J Neurophysiol. 1989 Jul;62(1):48–58. doi: 10.1152/jn.1989.62.1.48. [DOI] [PubMed] [Google Scholar]
- ELLIOTT D. F., HORTON E. W., LEWIS G. P. Actions of pure bradykinin. J Physiol. 1960 Oct;153:473–480. doi: 10.1113/jphysiol.1960.sp006548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggerickx D., Raspe E., Bertrand D., Vassart G., Parmentier M. Molecular cloning, functional expression and pharmacological characterization of a human bradykinin B2 receptor gene. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1306–1313. doi: 10.1016/0006-291x(92)90445-q. [DOI] [PubMed] [Google Scholar]
- Farmer S. G., Burch R. M. Biochemical and molecular pharmacology of kinin receptors. Annu Rev Pharmacol Toxicol. 1992;32:511–536. doi: 10.1146/annurev.pa.32.040192.002455. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H., Moncada S., Vane J. R. Prostaglandins and the mechanism of analgesia produced by aspirin-like drugs. Br J Pharmacol. 1973 Sep;49(1):86–97. doi: 10.1111/j.1476-5381.1973.tb08270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales R., Goldyne M. E., Taiwo Y. O., Levine J. D. Production of hyperalgesic prostaglandins by sympathetic postganglionic neurons. J Neurochem. 1989 Nov;53(5):1595–1598. doi: 10.1111/j.1471-4159.1989.tb08557.x. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbacher T., Lembeck F. Effect of bradykinin antagonists on bradykinin-induced plasma extravasation, venoconstriction, prostaglandin E2 release, nociceptor stimulation and contraction of the iris sphincter muscle in the rabbit. Br J Pharmacol. 1987 Oct;92(2):333–340. doi: 10.1111/j.1476-5381.1987.tb11328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J. F., Borkowski J. A., Young G. S., Strader C. D., Ransom R. W. Cloning and pharmacological characterization of a human bradykinin (BK-2) receptor. Biochem Biophys Res Commun. 1992 Apr 15;184(1):260–268. doi: 10.1016/0006-291x(92)91187-u. [DOI] [PubMed] [Google Scholar]
- Kaufman M. P., Iwamoto G. A., Longhurst J. C., Mitchell J. H. Effects of capsaicin and bradykinin on afferent fibers with ending in skeletal muscle. Circ Res. 1982 Jan;50(1):133–139. doi: 10.1161/01.res.50.1.133. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Mizumura K. The polymodal receptors in the testis of dog. Brain Res. 1977 Nov 18;136(3):553–558. doi: 10.1016/0006-8993(77)90080-4. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Levine J. D., Lau W., Kwiat G., Goetzl E. J. Leukotriene B4 produces hyperalgesia that is dependent on polymorphonuclear leukocytes. Science. 1984 Aug 17;225(4663):743–745. doi: 10.1126/science.6087456. [DOI] [PubMed] [Google Scholar]
- Levine J. D., Taiwo Y. O., Collins S. D., Tam J. K. Noradrenaline hyperalgesia is mediated through interaction with sympathetic postganglionic neurone terminals rather than activation of primary afferent nociceptors. Nature. 1986 Sep 11;323(6084):158–160. doi: 10.1038/323158a0. [DOI] [PubMed] [Google Scholar]
- Llona I., Vavrek R., Stewart J., Huidobro-Toro J. P. Identification of pre- and postsynaptic bradykinin receptor sites in the vas deferens: evidence for different structural prerequisites. J Pharmacol Exp Ther. 1987 May;241(2):608–614. [PubMed] [Google Scholar]
- Lundberg J. M., Saria A. Capsaicin-induced desensitization of airway mucosa to cigarette smoke, mechanical and chemical irritants. Nature. 1983 Mar 17;302(5905):251–253. doi: 10.1038/302251a0. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Patterson P. H. Primary cultures of dissociated sympathetic neurons. I. Establishment of long-term growth in culture and studies of differentiated properties. J Cell Biol. 1973 Nov;59(2 Pt 1):329–345. doi: 10.1083/jcb.59.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachern A. E., Shelton E. R., Bhakta S., Obernolte R., Bach C., Zuppan P., Fujisaki J., Aldrich R. W., Jarnagin K. Expression cloning of a rat B2 bradykinin receptor. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7724–7728. doi: 10.1073/pnas.88.17.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee D. S., Goy M. F., Oxford G. S. Involvement of the nitric oxide-cyclic GMP pathway in the desensitization of bradykinin responses of cultured rat sensory neurons. Neuron. 1992 Aug;9(2):315–324. doi: 10.1016/0896-6273(92)90170-i. [DOI] [PubMed] [Google Scholar]
- Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol. 1977 May;267(1):75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumura K., Minagawa M., Tsujii Y., Kumazawa T. The effects of bradykinin agonists and antagonists on visceral polymodal receptor activities. Pain. 1990 Feb;40(2):221–227. doi: 10.1016/0304-3959(90)90072-L. [DOI] [PubMed] [Google Scholar]
- Nardone J., Hogan P. G. Delineation of a region in the B2 bradykinin receptor that is essential for high-affinity agonist binding. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4417–4421. doi: 10.1073/pnas.91.10.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen P. J., Plevin R., Boarder M. R. Characterization of bradykinin-stimulated release of noradrenaline from cultured bovine adrenal chromaffin cells. J Pharmacol Exp Ther. 1989 Mar;248(3):1231–1236. [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta J. N., Saha J. K., Goyal R. K. Differential sensitivity to bradykinin of esophageal distension-sensitive mechanoreceptors in vagal and sympathetic afferents of the opossum. J Neurophysiol. 1992 Oct;68(4):1053–1067. doi: 10.1152/jn.1992.68.4.1053. [DOI] [PubMed] [Google Scholar]
- Steranka L. R., Manning D. C., DeHaas C. J., Ferkany J. W., Borosky S. A., Connor J. R., Vavrek R. J., Stewart J. M., Snyder S. H. Bradykinin as a pain mediator: receptors are localized to sensory neurons, and antagonists have analgesic actions. Proc Natl Acad Sci U S A. 1988 May;85(9):3245–3249. doi: 10.1073/pnas.85.9.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suidan H. S., Murrell R. D., Tolkovsky A. M. Carbachol and bradykinin elevate cyclic AMP and rapidly deplete ATP in cultured rat sympathetic neurons. Cell Regul. 1991 Jan;2(1):13–25. doi: 10.1091/mbc.2.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yandell D. W., Dryja T. P. Detection of DNA sequence polymorphisms by enzymatic amplification and direct genomic sequencing. Am J Hum Genet. 1989 Oct;45(4):547–555. [PMC free article] [PubMed] [Google Scholar]



