Abstract
Objective
To test the hypothesis that umbilical cord arterial lactate is superior to pH for predicting short-term neonatal morbidity at term.
Methods
We conducted a prospective cohort study of all consecutive, nonanomalous, singleton, vertex, term births from 2009 to 2012 at Washington University Medical Center. Umbilical arterial lactate and pH were measured immediately after delivery, prior to knowledge of neonatal outcomes. The primary outcome was a composite neonatal morbidity consisting of neonatal death, intubation, mechanical ventilation, meconium aspiration syndrome, hypoxic encephalopathy, and need for hypothermic therapy. The predictive ability of lactate and pH were compared using receiver-operating characteristics curves. Optimal cut-off values of lactate and pH were estimated based on the maximal Youden index.
Results
Of 4,997 term deliveries during the study period, 4,910 met inclusion criteria. The composite neonatal morbidity occurred in 56 neonates (1.1%). The mean lactate level was nearly two-fold higher in neonates with the composite morbidity (6.49 vs 3.26 mmol/L, p<0.001), while mean pH values were less distinct (7.19 vs 7.29, p<0.001). Lactate was significantly more predictive of neonatal morbidity than pH (ROC curve area: 0.84 vs 0.78, p=0.03). The optimal cut-off value for prediciting neonatal morbidity was 3.90 mmol/L for lactate and 7.25 for pH. Corresponding sensitivities and specificities were also higher for lactate (83.9% and 74.1% vs 75.0% and 70.6%, respectively).
Conclusion
Results of this large prospective cohort study show that umbilical cord arterial lactate is a more discriminating measure of neonatal morbidity at term than pH.
INTRODUCTION
Umbilical cord blood acid-base analysis provides an objective assessment of newborn metabolic status. Accordingly, it is recommended that physicians attempt to obtain venous and arterial samples when there is high risk of neonatal compromise [1]. Cord gas analysis includes direct measurement of pH, pO2, pCO2, and calculation of base excess using various algorithms. Umbilical cord arterial pH falls and base excess increases when hydrogen ions from anaerobic metabolism overwhelm the buffer capacity of the fetus [2]. Several studies have linked umbilical arterial acidemia with perinatal morbidity and long-term adverse outcomes [3].
Lactate is a direct end product of anaerobic metabolism. Under hypoxic conditions glucose is broken down to pyruvate that is converted to lactate and hydrogen ions. The fetus has been implicated as the main contributor to lactate concentration in labor with little influence by maternal and uteroplacental production [4]. Data from animal models show that fetal lactate increases earlier in hypoxia, and persists longer than low pH after normoxia is restored [5]. A number of studies suggest that umbilical lactate may be at least equally predictive as pH, with the additional advantage of ease of measurement [6–11]. However, many studies are limited by their retrospective design, small sample sizes or indirect outcome measures such as Apgar scores. Further, there is the potential for selection bias since cord gases and lactate were measured only in selected neonates.
We conducted a prospective cohort study to test the hypothesis that umbilical cord arterial lactate is superior to pH for predicting short-term neonatal morbidity at term.
MATERIALS AND METHODS
This was a prospective cohort study of consecutive deliveries at Washington University in St. Louis Medical Center from 2009 to 2012. The study was approved by the Washington University School of Medicine Human Research Protection Office.
Women were eligible if they carried a singleton fetus at term and underwent labor prior to delivery. We excluded women with multiple gestations or fetal anomalies. Term pregnancy was defined as gestational age greater than or equal to 37 weeks. Pregnancies were dated by a woman’s last menstrual period and confirmed with first or second-trimester ultrasonography [12]. Trained research nurses abstracted demographic information from patients’ records including medical and surgical history, obstetric and gynecologic history, and prenatal history. We also obtained detailed labor and delivery information.
Umbilical cord blood was collected immediately after infant delivery, prior to knowledge of neonatal outcomes. A policy of universal umbilical cord gas and lactate measurement was instituted prior to the study. Under this protocol, both arterial and venous blood samples are obtained from a clamped segment of cord immediately after delivery. Blood gases and lactate were measured using whole blood in automated benchtop analyzers. The GEM Premier 4000 Analyzer (Instrumentation Laboratory) was used to measure blood gases while the DXC-800 Automated Chemistry Analyser (Beckman Coulter) was used for lactate assays. The coefficient of variation of the lactate assay from regular quality control testing in our laboratory is approximately 2.9%. Umbilical arterial blood samples were validated to be arterial by ensuring that pH was at least 0.02 lower in the artery than the vein [13]. This permitted exclusion of mixed up samples and double sampling from the same vessel.
The primary outcome was a composite neonatal morbidity consisting of any of the following: neonatal death, intubation, mechanical ventilation, meconium aspiration syndrome, hypoxic-ischemic encephalopathy, and need for hypothermic therapy. Only one morbidity was counted per patient. We calculated baseline characteristics for the entire cohort and then compared them between women with and without the composite neonatal morbidity. Continuous variables were compared using the Student’s t test while categorical variables were compared using the chi-square or Fisher’s exact test as appropriate. Normality of distribution of the continuous variables was confirmed using the Kolmogorov-Smirnov test.
Receiver-operating characteristics (ROC) curves were constructed to assess the predictive ability of umbilical arterial pH and lactate for the composite neonatal morbidity. Areas under the ROC curves were compared using the nonparametric method of Delong et al. [14]. To objectively compare the predictive characteristics of lactate and pH, we used the maximal Youden index to estimate their ‘optimal’ cut-off values for predicting the composite neonatal morbidity. The Youden index is a function of sensitivity and specificity that is used to estimate the diagnostic effectiveness of cut-off values on ROC curves [15].This index ranges between 0 and 1, with values close to 1 indicating the effectiveness of a given cut-off value is relatively large while values close to 0 indicate limited effectiveness. The maximal Youden index is the maximum difference that is achievable between the ROC curve and the diagonal or chance line. Thus, the corresponding cut-off value maximizes the number of correctly classified individuals [16]. We calculated sensitivities and specificities based on these ‘optimal’ cut-off values.
Since base excess is also a marker of metabolic acidosis, we compared the predictive characteristics of lactate to base excess. Finally, we calculated predictive characteristics of different cut-off values of lactate, pH and base excess. The selected cut-off values were based on the 95th or 5th percentiles from our data, as well as other values suggested in the literature.
We included all consecutive subjects meeting inclusion criteria; no a priori sample size estimation was performed. Statistical tests were all 2-tailed and P<0.05 was considered significant. All statistical analyses were completed using STATA software package, version 12, Special Edition (College Station, TX).
RESULTS
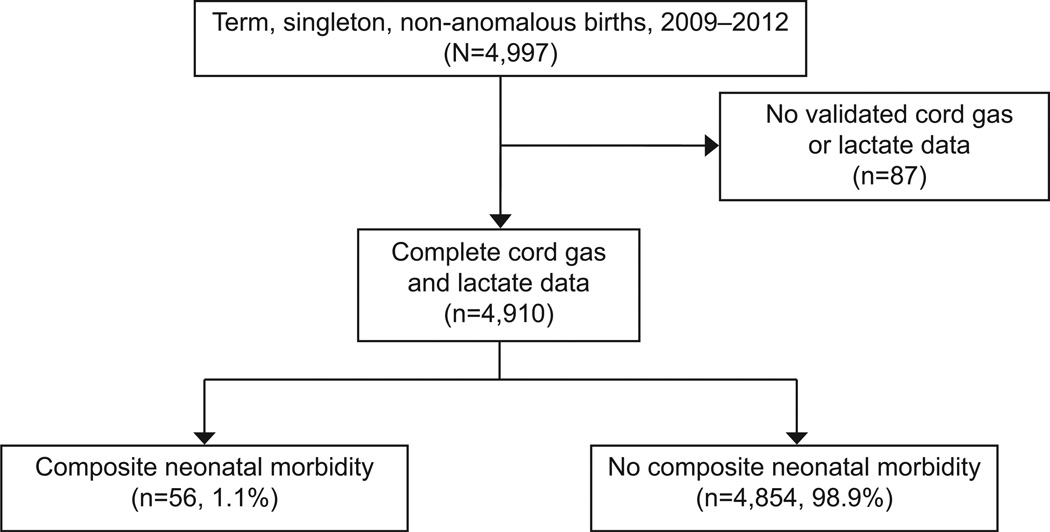
A total of 4,997 singleton, term, non-anomalous births occurred during the study period. Eighty-seven neonates, constituting 1.7% of the cohort had no validated cord gas or lactate information and were excluded. A total of 4,910 were included in the final analysis (Figure 1). The composite outcome was diagnosed in 56 neonates, constituting 1.1% of the cohort. Frequencies of components of the composite neonatal morbidity are shown in Table 1. Notably, some neonates were diagnosed with more than one morbidity. The most prevalent morbidities were endotracheal intubation 39 (69.6%) and mechanical ventilation 36 (64.3%), followed by need for hypothermic therapy 26 (46.4%) hypoxic encephalopathy 22 (39.3%) and meconium aspiration syndrome 12 (21.4%).
Figure 1.
Flow chart of study participants.
Table 1.
Distribution of components of the composite neonatal morbidity (n=56)
| Component | *n | Percentage of neonates with composite morbidity ^ |
|---|---|---|
| Neonatal death | 3 | 5.4 |
| Hypoxic encephalopathy | 22 | 39.3 |
| Hypothermic therapy | 26 | 46.4 |
| Endotracheal intubation | 39 | 69.6 |
| Mechanical ventilation | 36 | 64.3 |
| Meconium aspiration syndrome | 12 | 21.4 |
Ten neonates were diagnosed with one complication and 46 had multiple complications (23 had two, 13 had three, 7 had four and 3 had five)
Percentage of neonates with composite morbidity (n=56) who were diagnosed with each specific morbidity.
Baseline characteristics of the study cohort were examined for the entire cohort and then compared between those with and without the composite neonatal morbidity (Table 2). The mean maternal age for the entire cohort was 26.0 years. The majority of the women were African American (65.2%) and obese. In all, 17% of the neonates were delivered by cesarean and an additional 6.5% underwent operative vaginal delivery. The mean gestational age was 38.9 weeks. There were no significant differences in baseline characteristics between women with and without neonatal morbidity, with the exception of mode of delivery. Patients with neonatal morbidity were significantly more likely to have been delivered by cesarean or operative vaginal delivery (55.3% versus 22.2%, p<0.001).
Table 2.
Baseline characteristics of study participants (n=4910)
| Variable | Entire Cohort (N=4910) |
Neonatal Composite Morbidity (n=56) |
No Neonatal Composite Morbidity (n=4,854) |
P |
|---|---|---|---|---|
| Maternal age, years, mean (sd) | 26.0 (6.0) | 26.0 (7.1) | 26.0 (5.9) | 0.97 |
| Race, n(%) | ||||
| African-American | 3201 (65.2) | 41 (73.2) | 3160 (65.1) | 0.44 |
| Caucasian | 1038 (21.1) | 9 (16.1) | 1029 (21.2) | |
| Hispanic | 401 (8.2) | 5 (8.9) | 396 (8.2) | |
| Other | 270 (5.5) | 1 (1.8) | 32 (7.3) | |
| Body mass index, Kg/m2, mean (sd) | 32.0 (7.3) | 33.9 (7.8) | 32.0 (7.3) | |
| Epidural, n (%) | 4369 (89.0) | 48 (85.7) | 4321 (89.0) | 0.43 |
| Primiparous, n (%) | 1635 (33.3) | 24 (42.9) | 1611 (33.2) | 0.13 |
| Smoking, n(%) | 694 (14.1) | 9 (16.1) | 685 (14.1) | 0.68 |
| Alcohol, n(%) | 52 (1.1) | 2 (3.6) | 50 (1.0) | 0.07 |
| Chronic hypertension, n(%) | 199 (6.8) | 5 (11.1) | 194 (6.7) | 0.24 |
| Diabetes, n(%) | 170 (5.8) | 5 (11.1) | 165 (5.7) | 0.12 |
| Mode of delivery, n (%) | ||||
| Spontaneous vaginal | 3800 (77.4) | 25 (44.6) | 3775 (77.8) | <0.01 |
| Operative vaginal | 271 (6.5) | 4 (7.1) | 267 (5.5) | |
| Cesarean | 839 (17.1) | 27 (48.2) | 812 (16.7) | |
| Gestational age, weeks, mean (sd) | 38.9 (1.2) | 38.8 (1.3) | 38.9 (1.2) | 0.66 |
| Birth weight, grams, mean (sd) | 3241 (462) | 3218 (515) | 3242 (461) | 0.70 |
| Small for gestational age, n(%) | 628 (12.8) | 12 (21.4) | 616 (12.7) | 0.05 |
| Male infant, n (%) | 2579 (12.8) | 33 (58.9) | 2546 (52.5) | 0.34 |
n (%), number and percentage of subjects having a given categorical characteristic.
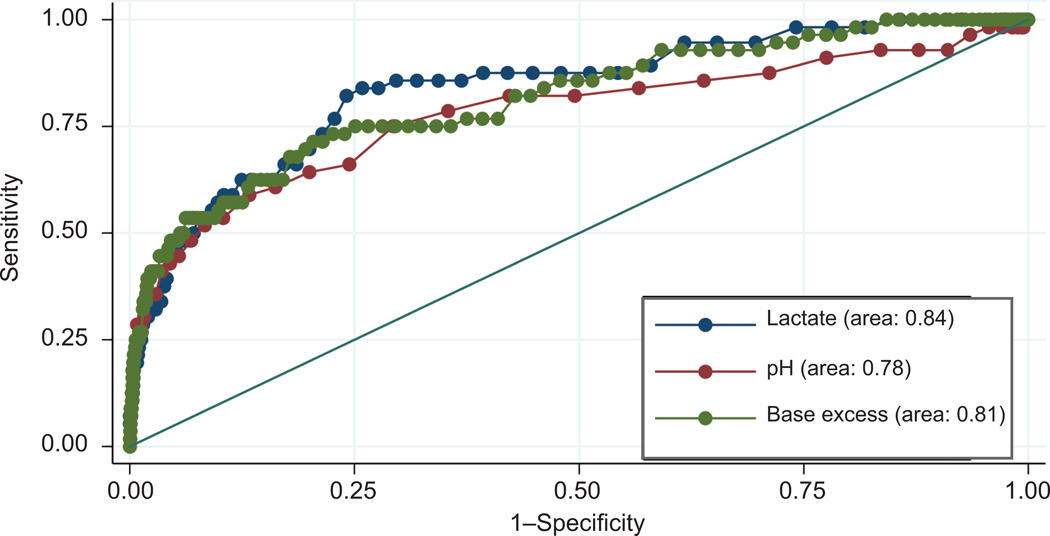
Average arterial lactate and pH were significantly different in neonates with and without the composite morbidity. The difference was much greater for lactate than for pH. Mean lactate was nearly 2-fold higher in neonates with the composite neonatal morbidity (6.49 ± 3.38 vs 3.26 ± 1.48 mmol/L, p<0.001), while mean pH values were less distinct (7.19 ± 0.13 vs 7.29 ± 0.06, p<0.001). The ROC curves of lactate and pH for predicting the composite neonatal morbidity are shown in Figure 2. Lactate was significantly more predictive of neonatal morbidity than pH (ROC curve area: 0.84 vs 0.78, p=0.03). Based on the maximal Youden index the ‘optimal’ cut-off value was 3.90 mmol/L for lactate and 7.25 for pH (Table 3). Both sensitivity and specificity were higher for lactate compared to pH (83.8% and 74.1% versus 75.0% and 70.6%, respectively). Predictive characteristics of different cut-off values of lactate and pH are shown in Table 4. Of all the cut-off values examined, the optimal cut-off value for lactate demonstrated the best balance of sensitivity and specificity for the composite morbidity.
Figure 2.
Receiver-operating characteristics curves of lactate, pH, and base excess for prediciting composite neonatal morbidity (N=4,910).
Table 3.
Predictive characteristics of ‘optimal cut-offs’ of umbilical arterial lactate and pH for predicting composite neonatal morbidity (N=4910)
| Lactate | pH | |
|---|---|---|
| ‘Optimal’ cut-off | >3.9 mmol/L | <7.25 |
| Sensitivity (95% CI) | 83.9 (71.9, 92.4) | 75.0 (61.6, 85.6) |
| Specificity (95% CI) | 74.1 (72.9, 75.4) | 70.6 (69.3, 71.9) |
| Positive predictive value (95% CI) | 3.6 (2.7, 4.8) | 2.9 (2.1, 3.9) |
| Negative predictive value (95% CI) | 99.8 (99.5, 99.9) | 99.6 (99.3, 99.8) |
Table 4.
Predictive characteristics of different cut-offs of lactate, pH and base excess for the composite neonatal morbidity (n=4910)
| Sensitivity (95% CI) |
Specificity (95% CI) |
|
|---|---|---|
| Lactate (mmol/L) | ||
| >3.9 (‘optimal’ cut-off from ROC curve) | 83.9 (71.7, 92.4) | 74.1 (72.9, 75.4) |
| >4.8 [27] | 62.5(48.5, 75.1) | 87.6 (86.6, 88.5) |
| >6.0 [7] | 46.4 (33.0, 60.3 | 94.9(94.2, 95.5) |
| >6.3 (>95th percentile) | 0.0 (0.0, 6.4) | 99.9 (99.8, 100) |
| pH | ||
| <7.00[28] | 8.9(3.0, 19.6) | 99.8 (99.6, 99.9) |
| <7.10 [29] | 28.6 (17.3, 42.2) | 98.9(98.6, 99.2) |
| <7.17 (<5th percentile) | 42.9 (29.7, 58.8) | 95.5 (94.9, 96.1) |
| <7.25 (‘optimal’ cut-off from ROC curve) | 75.0 (61.6, 85.6) | 70.6 (69.3, 71.9) |
| Base excess | ||
| > 4.3 (‘optimal’ cut-off from ROC curve) | 71.4(57.8, 82.7) | 79.5 (78.3, 80.6) |
| > 7.2 (>95th percentile) | 46.4(33.0, 60.3) | 95.7(95.1, 96.3) |
| > 12.0 [30] | 19.6(10.2, 32.4) | 99.6(99.4, 99.8) |
| pH<7.0 and base excess >12 [1] | 8.9(3.0, 19.6) | 99.8(99.6, 99.9) |
When lactate was compared to arterial base excess the area under the ROC curve was higher for lactate, although the difference was not statistically significant (0.84 versus 0.81, p=0.33) (Figure 2). Sensitivity of the ‘optimal’ cut-off values was higher for lactate (83.8% versus 71.4%) while specificity was higher for base excess (74.1% versus 79.5%). Metabolic acidosis defined as pH<7.0 and base excess >12 based on cord gas analysis had sensitivity of only 8.9% for predicting the composite neonatal morbidity (Table 4).
DISCUSSION
We found that umbilical cord arterial lactate is a more discriminating measure of neonatal morbidity at term than pH. Lactate was both more sensitive and specific than pH for predicting neonatal morbidity.
The superiority of lactate over pH for predicting neonatal morbidity is biologically plausible. Lactate is a direct product of anerobic metabolism, and animal studies show it is produced earlier during hypoxia, and persists longer than low pH [5]. Further, low pH alone indicates respiratory acidosis which is less deleterious than metabolic acidosis as reflected by high umbilical arterial blood lactate. Although increased base excess is also a marker of metabolic acidosis, our data suggest that lactate may be a more discriminating predictor of neonatal morbidity. Even if base excess were equivalent to lactate for predicting outcomes, lactate still has the advantage of being measured directly as opposed to base excess that is calculated using algorithms that differ by the gas analyser used [17].
Prior studies comparing umbilical arterial lactate to pH for predicting neonatal morbidity showed either equal efficacy or superiority of lactate. Westgren et al. found that umbilical arterial lactate and acid base analysis predicted 5-minute Apgar score <4 with similar efficacy [6, 7]. Using a large cohort, Wiberg found umbilical arterial lactate to be at least as good as base deficit for predicting 5-minute Apgar <4 or 7. As in the present study, others found umbilical arterial lactate to be superior to pH or even base excess for predicting adverse neonatal outcomes [8–11].
Different studies have reported varying mean umbilical arterial lactate values in normal deliveries, ranging from 2.55 to 4.63 mmol/L [18–24]. The mean lactate concentration among the neonates in our cohort without the composite neonatal morbidity was 3.26 mmol/L. The differences may be attributable to studies utilizing hemolyzed or whole blood for lactate analysis and differences in lactate assays. Differences in mode of delivery may also affect lactate levels [6, 10]. The lactate cut-off values proposed for predicting adverse outcomes have also differed between studies, ranging from 3.2 to 10.0 mmol/L [6, 20, 23, 24]. In addition to differences in assays and study populations, these differences may be due to the varied outcome measures used in these studies.
The large prospective cohort design of the present study is a significant improvement over prior studies. The universal institutional cord gas and lactate policy is a critically important feature of this study. This eliminates the selection bias inherent in most studies. We measured cord gases and lactate from double clamped cord segments taken immediately after delivery. This is important because lactate, pH and base excess values change with delayed cord clamping [25]. We further objectively validated the samples as arterial. Finally, rather than using published cut-off values of lactate which may vary depending on the study population and assay used, we employed an objective method for estimating the ‘optimal’ cut-off values based on the maximal Youden index.
The study was limited to term births following labor. This controlled for variation of pH and lactate with gestational age and labor [10, 26]. However, our results may not be applicable to preterm births and deliveries occuring without labor. While the composite neonatal outcome may be seen as a weakness, it enabled us to perform detailed analysis, which would not be possible if the relatively rare individual outcomes such as hypoxic encephalopathy were used. We chose components of the composite carefully, including only neonatal morbidities that are directly or indirectly linked to intrauterine hypoxia. Operative deliveries were more common in subjects with the composite morbidity and may be considered a confounder. However, this would be expected to similarly affect lactate and pH values. Further, results of subgroup analysis in women undergoing operative delivery were comparable to our overall findings. Finally, we examined only short term neonatal outcomes in this study.
In conclusion, results of this large prospective cohort study show that umbilical cord arterial lactate is a more discriminating measure of neonatal morbidity at term than pH. Lactate was more sensitive and specific than pH and has the additional advantage of simplicity of measurement. Future studies should assess the comparative predictive ability of lactate and pH for long-term outcomes. Further, important institutional considerations before using lactate as the measure of choice for neonatal well being at term include standardizing lactate assays, defining neonatal outcome measures and calibrating the cut-off value of lactate to desired sensitivity and specificity.
Acknowledgments
Dr. Tuuli is supported by a Women’s Reproductive Health Research Career Development grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH/NICHD – 1K12HD063086-01). Dr. Cahill is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD061619-01, PI Cahill) and was a Robert Wood Johnson Foundation Faculty Physician Scholar, which partially supported this work.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of the NIH or Robert Wood Johnson Foundation.
Footnotes
Presented at the 34th Annual Meeting of the Society for Maternal-Fetal Medicine (SMFM), New Orleans, LA, and February 6, 2014.
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.Practice ACoO. ACOG Committee Opinion No. 348, November 2006: Umbilical cord blood gas and acid-base analysis. Obstet Gynecol. 2006;108(5):1319–1322. doi: 10.1097/00006250-200611000-00058. [DOI] [PubMed] [Google Scholar]
- 2.Uzan S, et al. Acid base balance in the fetus during labor: pathophysiology and exploration methods. J Gynecol Obstet Biol Reprod (Paris) 2003;32(1 Suppl):1S68–1S78. [PubMed] [Google Scholar]
- 3.Malin GL, Morris RK, Khan KS. Strength of association between umbilical cord pH and perinatal and long term outcomes: systematic review and meta-analysis. BMJ. 2010;340:c1471. doi: 10.1136/bmj.c1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordstrom L, et al. Fetal and maternal lactate increase during active second stage of labour. BJOG. 2001;108(3):263–268. doi: 10.1111/j.1471-0528.2001.00034.x. [DOI] [PubMed] [Google Scholar]
- 5.Engidawork E, et al. Effect of perinatal asphyxia on systemic and intracerebral pH and glycolysis metabolism in the rat. Exp Neurol. 1997;145(2 Pt 1):390–396. doi: 10.1006/exnr.1997.6482. [DOI] [PubMed] [Google Scholar]
- 6.Westgren M, et al. Routine measurements of umbilical artery lactate levels in the prediction of perinatal outcome. Am J Obstet Gynecol. 1995;173(5):1416–1422. doi: 10.1016/0002-9378(95)90627-4. [DOI] [PubMed] [Google Scholar]
- 7.Wiberg N, et al. Relation between umbilical cord blood pH, base deficit, lactate, 5-minute Apgar score and development of hypoxic ischemic encephalopathy. Acta Obstet Gynecol Scand. 2010;89(10):1263–1269. doi: 10.3109/00016349.2010.513426. [DOI] [PubMed] [Google Scholar]
- 8.Low JA, et al. Motor and cognitive deficits after intrapartum asphyxia in the mature fetus. Am J Obstet Gynecol. 1988;158(2):356–361. doi: 10.1016/0002-9378(88)90154-8. [DOI] [PubMed] [Google Scholar]
- 9.Low JA, Panagiotopoulos C, Derrick EJ. Newborn complications after intrapartum asphyxia with metabolic acidosis in the term fetus. Am J Obstet Gynecol. 1994;170(4):1081–1087. doi: 10.1016/s0002-9378(94)70101-6. [DOI] [PubMed] [Google Scholar]
- 10.Borruto F, et al. Screening of foetal distress by assessment of umbilical cord lactate. Clin Exp Obstet Gynecol. 2006;33(4):219–222. [PubMed] [Google Scholar]
- 11.Goldaber KG, et al. Pathologic fetal acidemia. Obstet Gynecol. 1991;78(6):1103–1107. [PubMed] [Google Scholar]
- 12.Iams JD, et al. The Preterm Prediction Study: recurrence risk of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. American journal of obstetrics and gynecology. 1998;178(5):1035–1040. doi: 10.1016/s0002-9378(98)70544-7. [DOI] [PubMed] [Google Scholar]
- 13.Westgate J, Garibaldi JM, Greene KR. Umbilical cord blood gas analysis at delivery: a time for quality data. Br J Obstet Gynaecol. 1994;101(12):1054–1063. doi: 10.1111/j.1471-0528.1994.tb13581.x. [DOI] [PubMed] [Google Scholar]
- 14.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 15.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clinical chemistry. 1993;39(4):561–577. [PubMed] [Google Scholar]
- 17.Wiberg N, Kallen K, Olofsson P. Base deficit estimation in umbilical cord blood is influenced by gestational age, choice of fetal fluid compartment, and algorithm for calculation. Am J Obstet Gynecol. 2006;195(6):1651–1656. doi: 10.1016/j.ajog.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 18.Gjerris AC, et al. Umbilical cord blood lactate: a valuable tool in the assessment of fetal metabolic acidosis. Eur J Obstet Gynecol Reprod Biol. 2008;139(1):16–20. doi: 10.1016/j.ejogrb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Chanrachakul B, et al. Umbilical artery blood gas and lactate in healthy newborns. J Med Assoc Thai. 1999;82(4):388–393. [PubMed] [Google Scholar]
- 20.Linet T, et al. Microvolume dosage of lactate in cord blood for the evaluation of the neonatal well-being. J Gynecol Obstet Biol Reprod (Paris) 2002;31(4):352–357. [PubMed] [Google Scholar]
- 21.Nordstrom L, et al. Lactate in fetal scalp blood and umbilical artery blood measured during normal labor with a test strip method. Acta Obstet Gynecol Scand. 1994;73(3):250–254. doi: 10.3109/00016349409023449. [DOI] [PubMed] [Google Scholar]
- 22.Piquard F, et al. Are there two biological parts in the second stage of labor? Acta Obstet Gynecol Scand. 1989;68(8):713–718. doi: 10.3109/00016348909006144. [DOI] [PubMed] [Google Scholar]
- 23.Shirey T, St Pierre J, Winkelman J. Cord lactate, pH, blood gases from healthy neonates. Gynecol Obstet Invest. 1996;41(1):15–19. doi: 10.1159/000292027. [DOI] [PubMed] [Google Scholar]
- 24.Suidan JS, Young BK. Outcome of fetuses with lactic acidemia. Am J Obstet Gynecol. 1984;150(1):33–37. doi: 10.1016/s0002-9378(84)80105-2. [DOI] [PubMed] [Google Scholar]
- 25.Wiberg N, Kallen K, Olofsson P. Delayed umbilical cord clamping at birth has effects on arterial and venous blood gases and lactate concentrations. BJOG. 2008;115(6):697–703. doi: 10.1111/j.1471-0528.2008.01708.x. [DOI] [PubMed] [Google Scholar]
- 26.Wiberg N, et al. Lactate concentration in umbilical cord blood is gestational age-dependent: a population-based study of 17 867 newborns. BJOG. 2008;115(6):704–709. doi: 10.1111/j.1471-0528.2008.01707.x. [DOI] [PubMed] [Google Scholar]
- 27.Kruger K, et al. Predictive value of fetal scalp blood lactate concentration and pH as markers of neurologic disability. Am J Obstet Gynecol. 1999;181(5 Pt 1):1072–1078. doi: 10.1016/s0002-9378(99)70083-9. [DOI] [PubMed] [Google Scholar]
- 28.van den Berg PP, et al. Neonatal complications in newborns with an umbilical artery pH < 7.00. Am J Obstet Gynecol. 1996;175(5):1152–1157. doi: 10.1016/s0002-9378(96)70021-2. [DOI] [PubMed] [Google Scholar]
- 29.Dijxhoorn MJ, et al. Apgar score, meconium and acidaemia at birth in relation to neonatal neurological morbidity in term infants. Br J Obstet Gynaecol. 1986;93(3):217–222. doi: 10.1111/j.1471-0528.1986.tb07896.x. [DOI] [PubMed] [Google Scholar]
- 30.Low JA, Lindsay BG, Derrick EJ. Threshold of metabolic acidosis associated with newborn complications. Am J Obstet Gynecol. 1997;177(6):1391–1394. doi: 10.1016/s0002-9378(97)70080-2. [DOI] [PubMed] [Google Scholar]