Abstract
Tumor lysis syndrome (TLS) occurs in malignancies with high proliferative potential and tumor burden, such as lymphomas and leukemias. TLS syndrome is an oncologic emergency, requiring prompt intervention. The metabolic derangements cause acute kidney failure and may lead to cardiac arrhythmias, seizures, and death. With the advent of rasburicase, a recombinant urate oxidase, there has been a decline in the TLS-mediated renal failure and the need for dialysis. The recommended regimen and doses pose a heavy financial burden for patients in developing countries like India. With data and studies proving a similar efficacy for the reduced dose and lesser number of rasburicase, we report here a case series of seven children with acute leukemias, whose TLS was managed by a single dose of rasburicase. A retrospective analysis of case records of seven children with acute lymphoblastic leukemia and TLS, admitted to our Pediatric Oncology Unit of our Hospital between the period 2011 and 2013, was done. All our patients responded to a single dose, indicating that in appropriately monitored patients, single dose followed by as-needed dosing can be cost-saving.
Keywords: Single dose rasburicase, tumor lysis syndrome management, children
Introduction
Tumor lysis syndrome (TLS) occurs in malignancies that have high proliferative potential and high tumor burden, such as lymphomas and leukemias. TLS syndrome is an oncologic emergency, requiring prompt intervention. It is characterized by rapid release of intracellular metabolites from lysed malignant cells into the blood stream. The resulting metabolic derangements are hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia, causing acute renal failure and which may lead to cardiac arrhythmias, seizures, and death.[1]
The prevention and management of TLS include aggressive hydration and reduction in the plasma uric acid levels by drugs that decrease production or increase excretion of uric acid. With the advent of rasburicase, a recombinant urate oxidase, there has been a tremendous decline in the TLS-mediated renal failure and the need for dialysis. But the recommended regimen and doses pose a heavy financial burden for the poor patients in developing countries like India. With data and studies proving a similar efficacy with reduced dose and lesser number of rasburicase, it will be economical to devise a strategy that will help us to achieve reasonably comparable standards of efficiency, efficacy, and safety in TLS management. We report here a case series of seven children with acute leukemia, whose TLS was managed by a single dose of rasburicase.
Subjects and Methods
A retrospective analysis of case records of seven children with acute lymphoblastic leukemia (ALL) and TLS, admitted to our Pediatric Oncology Unit of our Hospital between the period 2011 and 2013, was done to evaluate the effectiveness of single-dose rasburicase (SDR) in the management of TLS. All of them had TLS with kidney failure at the time of presentation. The children were administered a single dose of rasburicase at the rate of 0.15 mg/kg. All patients were screened for glucose-6-phosphate dehydrogenase (G6PD) deficiency before the administration of rasburicase.
Hemoglobin, white cell count, differential count, platelets, serum electrolytes, serum calcium, phosphorous, uric acid, blood urea nitrogen, and creatinine tests were done at the time of admission and repeated daily until the renal parameters and uric acid levels returned to normal levels.
Results
The demographic details and clinical parameters of the children are enumerated in Table 1.
Table 1.
Demographic details and clinical parameters at the time of admission
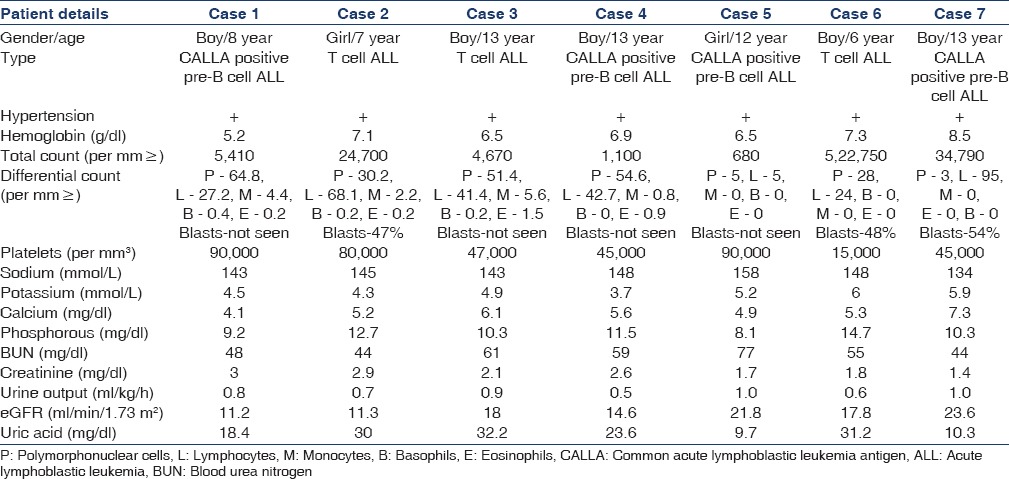
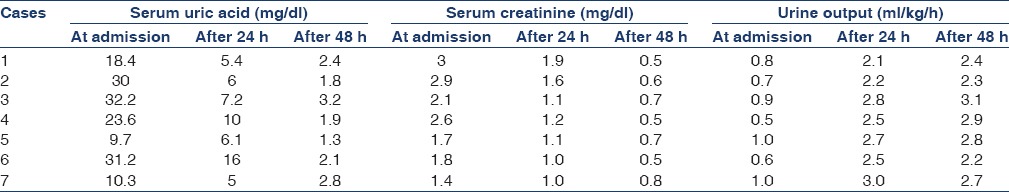
Among the seven children, five were males. Four had pre-B cell (common ALL) antigen-positive ALL and the other three had T-cell ALL determined by flow cytometry. The highest and lowest uric acid levels at admission were 32.2 mg/dl and 9.7 mg/dl, respectively. All of them had hypertension, hypocalcemia, hyperphosphatemia, and hyperuricemia and 3 had hyperkalemia. All were managed with hyperhydration, anti-hypertensives, allopurinol, and a single dose of rasburicase at 0.15 mg/kg. The dose was rounded off to the nearest available concentration of vials due to the financial constraints. A single dose of rasburicase produced a rapid and sustained therapeutic effect of lowering the plasma uric acid levels in all seven patients. Renal parameters normalized within 72 h [Table 2]. Uric acid levels remained below 4 mg/dl throughout the administration of chemotherapy until discharge.
Table 2.
Depicting the serum uric acid and, creatinine levels and urine output before and after administering a single dose of rasburicase
Discussion
Acute renal failure is one of the most immediate, serious, and costly TLS-related complication of treatment for hematologic malignancies.[2] Hyperuricemia results from rapid catabolism of purine-containing nucleic acids from tumor cells and can lead to renal insufficiency when uric acid precipitates into the renal tubules and distal collecting system.[3] This causes renal vasoconstriction, which in turn leads to impaired autoregulation, decreased renal flow, oxidation and inflammation, finally leading to acute kidney injury. Markedly increased phosphate levels compounded with calcium phosphate deposition in the renal tubules can also cause acute kidney injury. High concentrations of both uric acid and phosphate potentiate the risk of acute kidney injury because uric acid precipitates more readily in the presence of calcium phosphate and vice versa.
Allopurinol has been used for many years in the prevention and management of TLS-related hyperuricemia. Allopurinol decreases the new production of uric acid by inhibiting enzyme xanthine oxidase and blocking oxidation of xanthine and hypoxanthine into uric acid. Therefore, following allopurinol administration, there is a time lag for reduction in uric acid levels and hence should be administered for more than 3 days for the achievement of significant reduction in uric acid levels. An alternative to inhibiting uric acid formation by inhibiting xanthine oxidase with allopurinol is to promote the catabolism of existing pool of uric acid to allantoin, which is 5–10 times more soluble in urine by urate oxidase.[4]
Rasburicase offers potential advantage over allopurinol because of its rapid onset of action, reducing pre-existing pool of uric acid within few hours, and also helping in avoiding accumulation of xanthine and hypoxanthine, which has poor water solubility and can worsen renal function.[5]
Urate oxidase is an endogenous enzyme commonly found in many mammalian species but not in humans because of nonsense mutation I, the coding region in the gene during hominoid evolution. Rasburicase is a recombinant form of the urate oxidase enzyme.[6]
Rasburicase is indicated for the prophylactic management as well as treatment of high plasma uric acid levels (>7.5 mg/dl) in pediatric and adult patients with leukemia, lymphoma, and solid tumor malignancies, which have the highest risk of tumor lysis such as those with tumors that have a high proliferative rate(ALL, Burkitt's lymphoma), tumors with high sensitivity to cytotoxic therapy, patients with large tumor masses, and those with pre-existing renal insufficiency and high serum LDH levels.[7]
The cost of the drug is 15,000 rupees per 1.5 mg. It is administered as an intravenous (IV) infusion over 30 min and should never be given as a bolus. The reconstituted solution can be stored at 2–8°C for 24 h. Repeated use of rasburicase increases risk of hypersensitivity reactions.[8,9] It is contraindicated in individuals deficient in G6PD.
Rasburicase was initially approved by the US Food and Drug Administration for the management of TLS in the pediatric setting, based on a randomized controlled phase III clinical trial whereby rasburicase (0.15 or 0.2 mg/kg) was administered for 5 consecutive days.[10] The study by Goldman et al. and Pui et al. has confirmed the effectiveness and safety of rasburicase over allopurinol in children with hematologic malignancies.[11,12] Rasburicase was administered at a dose of 0.2 mg/kg IV over 30 min for 5–7 days in this study population.
Though the efficiency and advantage of rasburicase has been proved beyond doubt more than a decade ago, it's the cost of the product that has been the main inhibiting factor against its usage. In a developing country like India, where majority of the patients have to bear the brunt of the medical expenses with no insurance to support them, the use of rasburicase for 5 days is beyond the scope of the poor patients. So, when several noncontrolled studies involving retrospective case series suggested shorter duration of treatment to minimize the cost but without compromising the efficacy, it was boon for children in resource limited settings.[13]
Vadhan-Raj et al. evaluated the efficacy of a single dose rasburicase 0.15 mg/kg followed by as-needed dosing with a maximum of five doses instead of the standard 5-day regimen with 0.2 mg/kg in adult patients at risk for TLS and concluded that single dose was effective in most patients and only a subset of high-risk patients received a second dose.[7] A study in South Carolina depicted that a single dose of rasburicase 6 mg was effective in the management of TLS in adults.[14]
In California, SDR for adult cancer patients with hyperuricemia or at high risk for TLS demonstrated better response rate and stronger control of uric acid level compared with allopurinol. SDR response rate was not inferior to that of daily-dose rasburicase (DDR), and the standard-dose SDR generates more cost savings compared with the DDR.[15]
Though majority of the studies had been in adult patients, Lee et al. had proven the effectiveness of a single dose of rasburicase in three children with ALL and concluded that it is feasible and will improve the cost-effectiveness profile of the otherwise expensive compound.[16]
All the children received a single dose of rasburicase, and their electrolyte abnormalities, and renal parameters, hyperuricemia and hypertension normalized in 72 h. The children were started on chemotherapy as per Children's Oncology Group protocol. The uric acid levels were well below the normal levels during the entire period of chemo administration. The rasburicase was administered on the day of admission in all our patients at the earliest and within 12 h of admission. The time lag of 12 h in few patients was due to the financial constraints in arranging the drug.
Our experience suggests that in appropriately monitored patients single dose followed by dosing as needed can be cost-saving. None of our patients required dialysis, which otherwise would have added to the economic burden. Administration of rasburicase does not require any expertise like catheters, central lines, intensive care unit care unlike dialysis, and no dose modifications are needed in spite of deranged renal parameters.
Conclusion
From an economic point of view, especially in a developing country like India, the promising single low-dose approach of rasburicase administration achieved satisfactory results with a great reduction of cost per patient, which can result in better cost-effectiveness with no compromise in clinical efficacy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Hande KR, Garrow GC. Acute tumor lysis syndrome in patients with high-grade non-Hodgkin's lymphoma. Am J Med. 1993;94:133–9. doi: 10.1016/0002-9343(93)90174-n. [DOI] [PubMed] [Google Scholar]
- 2.Arrambide K, Toto RD. Tumor lysis syndrome. Semin Nephrol. 1993;13:273–80. [PubMed] [Google Scholar]
- 3.Shimada M, Johnson RJ, May WS, Jr, Lingegowda V, Sood P, Nakagawa T, et al. A novel role for uric acid in acute kidney injury associated with tumour lysis syndrome. Nephrol Dial Transplant. 2009;24:2960–4. doi: 10.1093/ndt/gfp330. [DOI] [PubMed] [Google Scholar]
- 4.Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: An evidence-based review. J Clin Oncol. 2008;26:2767–78. doi: 10.1200/JCO.2007.15.0177. [DOI] [PubMed] [Google Scholar]
- 5.Mughal TI, Ejaz AA, Foringer JR, Coiffier B. An integrated clinical approach for the identification, prevention, and treatment of tumor lysis syndrome. Cancer Treat Rev. 2010;36:164–76. doi: 10.1016/j.ctrv.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Legoux R, Delpech B, Dumont X, Guillemot JC, Ramond P, Shire D, et al. Cloning and expression in Escherichia coli of the gene encoding Aspergillus flavus urate oxidase. J Biol Chem. 1992;267:8565–70. [PubMed] [Google Scholar]
- 7.Vadhan-Raj S, Fayad LE, Fanale MA, Pro B, Rodriguez A, Hagemeister FB, et al. A randomized trial of a single-dose rasburicase versus five-daily doses in patients at risk for tumor lysis syndrome. Ann Oncol. 2012;23:1640–5. doi: 10.1093/annonc/mdr490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeha S, Kantarjian H, Irwin D, Shen V, Shenoy S, Blaney S, et al. Efficacy and safety of rasburicase, a recombinant urate oxidase (Elitek), in the management of malignancy-associated hyperuricemia in pediatric and adult patients: Final results of a multicenter compassionate use trial. Leukemia. 2005;19:34–8. doi: 10.1038/sj.leu.2403566. [DOI] [PubMed] [Google Scholar]
- 9.Pui CH, Relling MV, Lascombes F, Harrison PL, Struxiano A, Mondesir JM, et al. Urate oxidase in prevention and treatment of hyperuricemia associated with lymphoid malignancies. Leukemia. 1997;11:1813–6. doi: 10.1038/sj.leu.2400850. [DOI] [PubMed] [Google Scholar]
- 10.Smalley RV, Guaspari A, Haase-Statz S, Anderson SA, Cederberg D, Hohneker JA. Allopurinol: Intravenous use for prevention and treatment of hyperuricemia. J Clin Oncol. 2000;18:1758–63. doi: 10.1200/JCO.2000.18.8.1758. [DOI] [PubMed] [Google Scholar]
- 11.Goldman SC, Holcenberg JS, Finklestein JZ, Hutchinson R, Kreissman S, Johnson FL, et al. A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood. 2001;97:2998–3003. doi: 10.1182/blood.v97.10.2998. [DOI] [PubMed] [Google Scholar]
- 12.Pui CH, Jeha S, Irwin D, Camitta B. Recombinant urate oxidase (rasburicase) in the prevention and treatment of malignancy-associated hyperuricemia in pediatric and adult patients: Results of a compassionate-use trial. Leukemia. 2001;15:1505–9. doi: 10.1038/sj.leu.2402235. [DOI] [PubMed] [Google Scholar]
- 13.Hummel M, Reiter S, Adam K, Hehlmann R, Buchheidt D. Effective treatment and prophylaxis of hyperuricemia and impaired renal function in tumor lysis syndrome with low doses of rasburicase. Eur J Haematol. 2008;80:331–6. doi: 10.1111/j.1600-0609.2007.01013.x. [DOI] [PubMed] [Google Scholar]
- 14.McDonnell AM, Lenz KL, Frei-Lahr DA, Hayslip J, Hall PD. Single-dose rasburicase 6 mg in the management of tumor lysis syndrome in adults. Pharmacotherapy. 2006;26:806–12. doi: 10.1592/phco.26.6.806. [DOI] [PubMed] [Google Scholar]
- 15.Feng X, Dong K, Pham D, Pence S, Inciardi J, Bhutada NS. Efficacy and cost of single-dose rasburicase in prevention and treatment of adult tumour lysis syndrome: A meta-analysis. J Clin Pharm Ther. 2013;38:301–8. doi: 10.1111/jcpt.12061. [DOI] [PubMed] [Google Scholar]
- 16.Lee AC, Li CH, So KT, Chan R. Treatment of impending tumor lysis with single-dose rasburicase. Ann Pharmacother. 2003;37:1614–7. doi: 10.1345/aph.1D111. [DOI] [PubMed] [Google Scholar]


